The Impact of Sm Promoter on the Catalytic Performance of Ni/Al2O3-SiO2 in Methane Partial Oxidation for Enhanced H2 Production
Abstract
1. Introduction
2. Results
2.1. Brunauer–Emmett–Teller (BET) Investigations
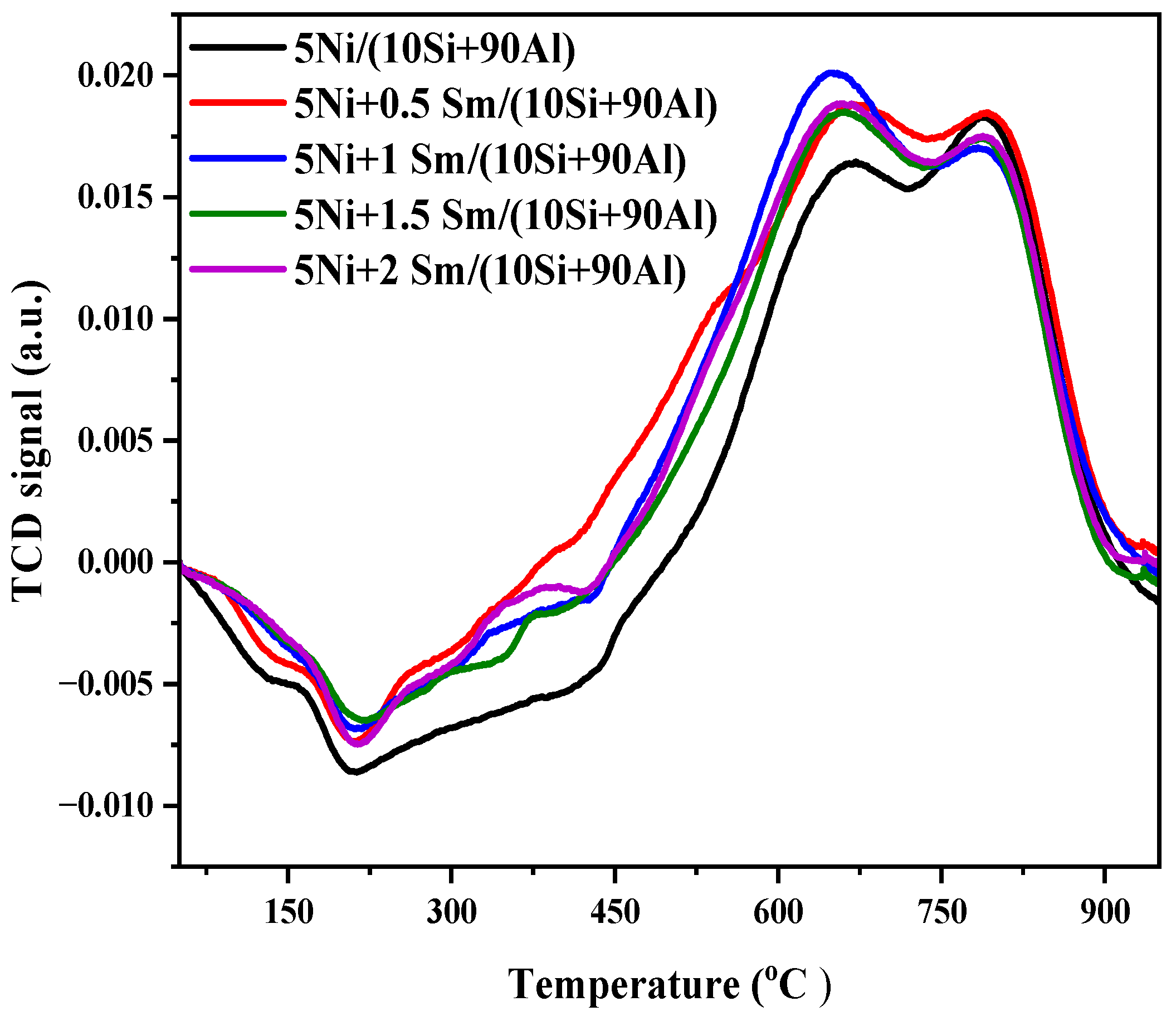
2.2. H2-TPR
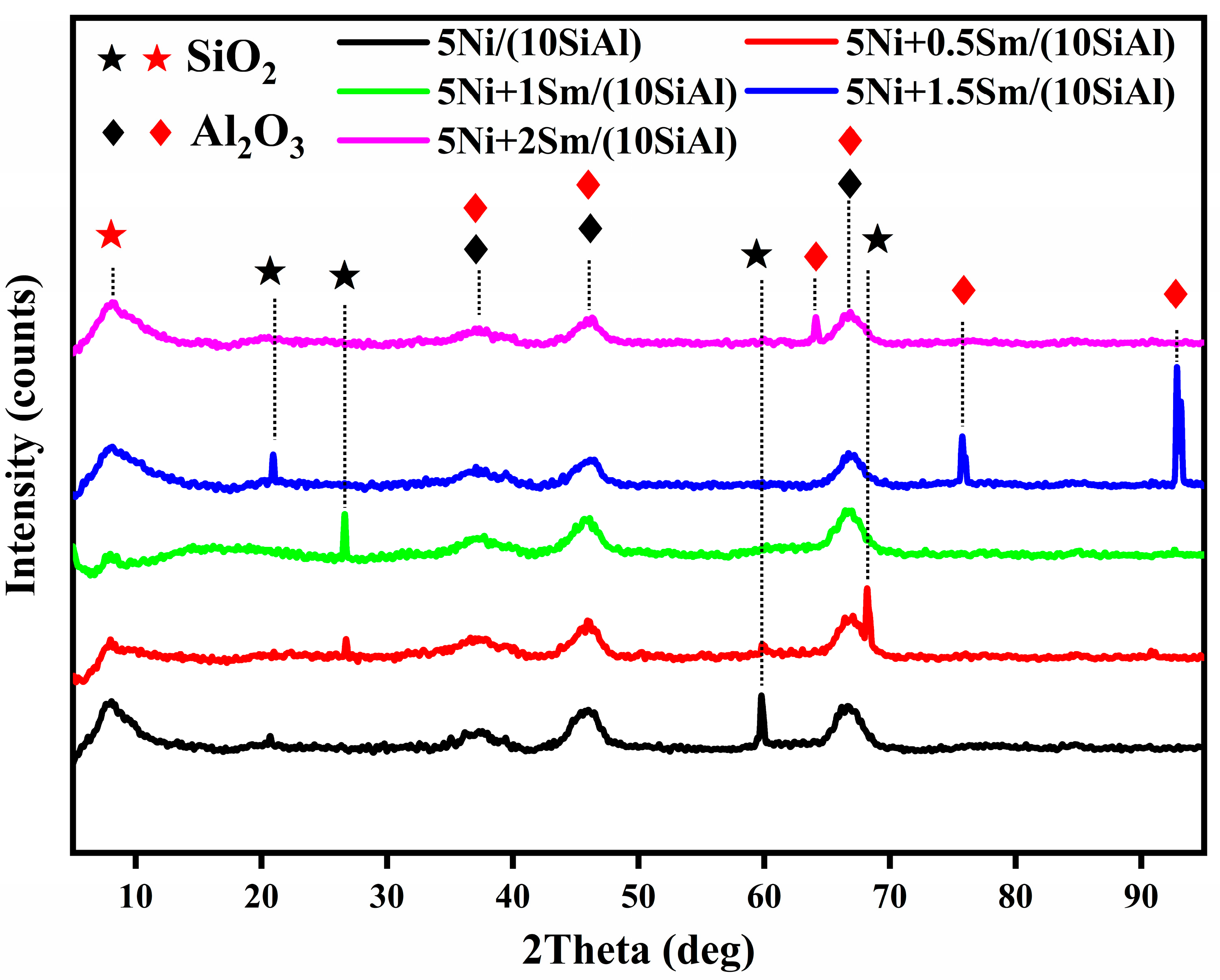
2.3. X-Ray Diffraction (XRD) Analysis
2.4. Fourier-Transform Infrared Spectroscopy (FTIR)
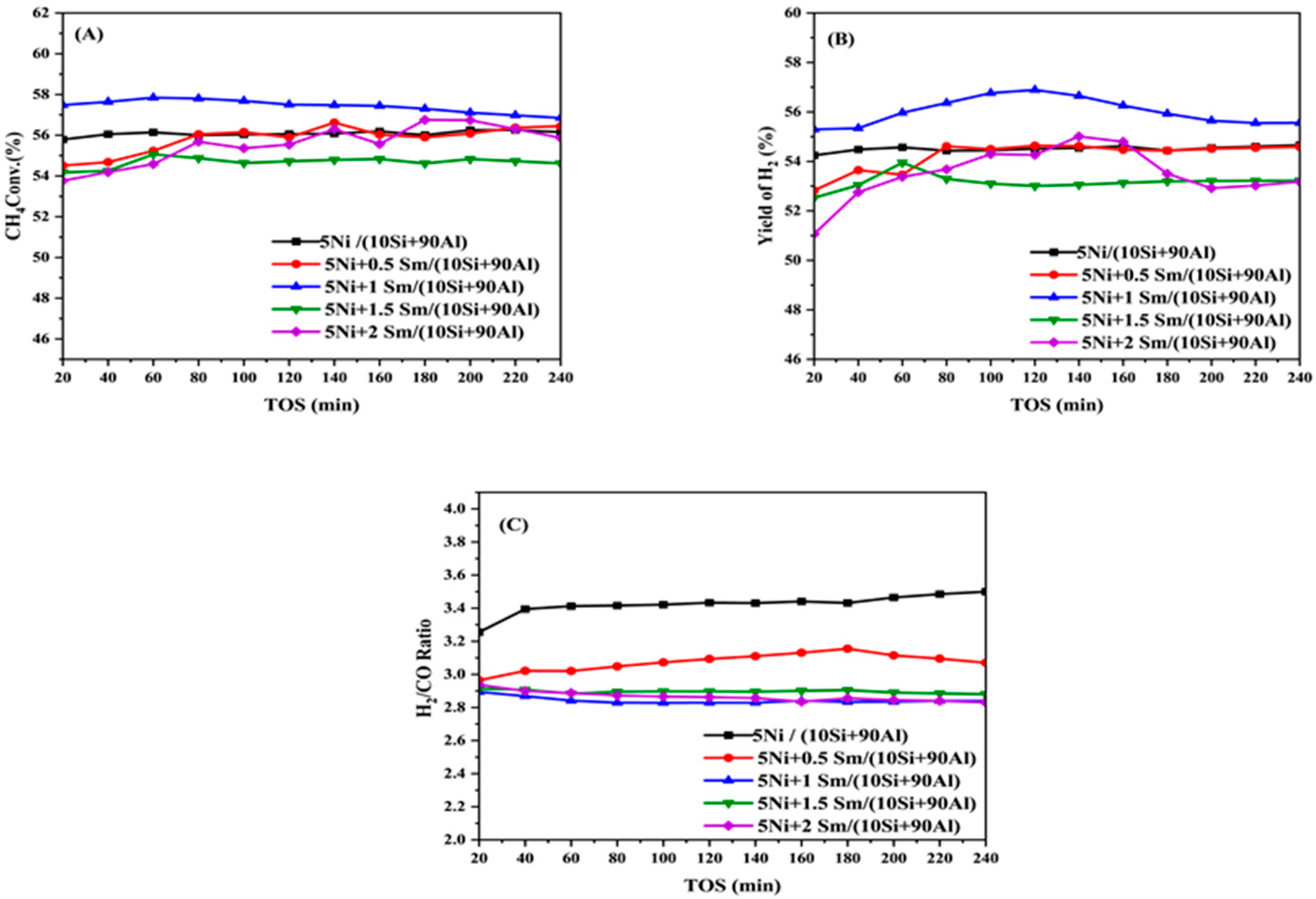
2.5. The Catalytic Activity Evaluation
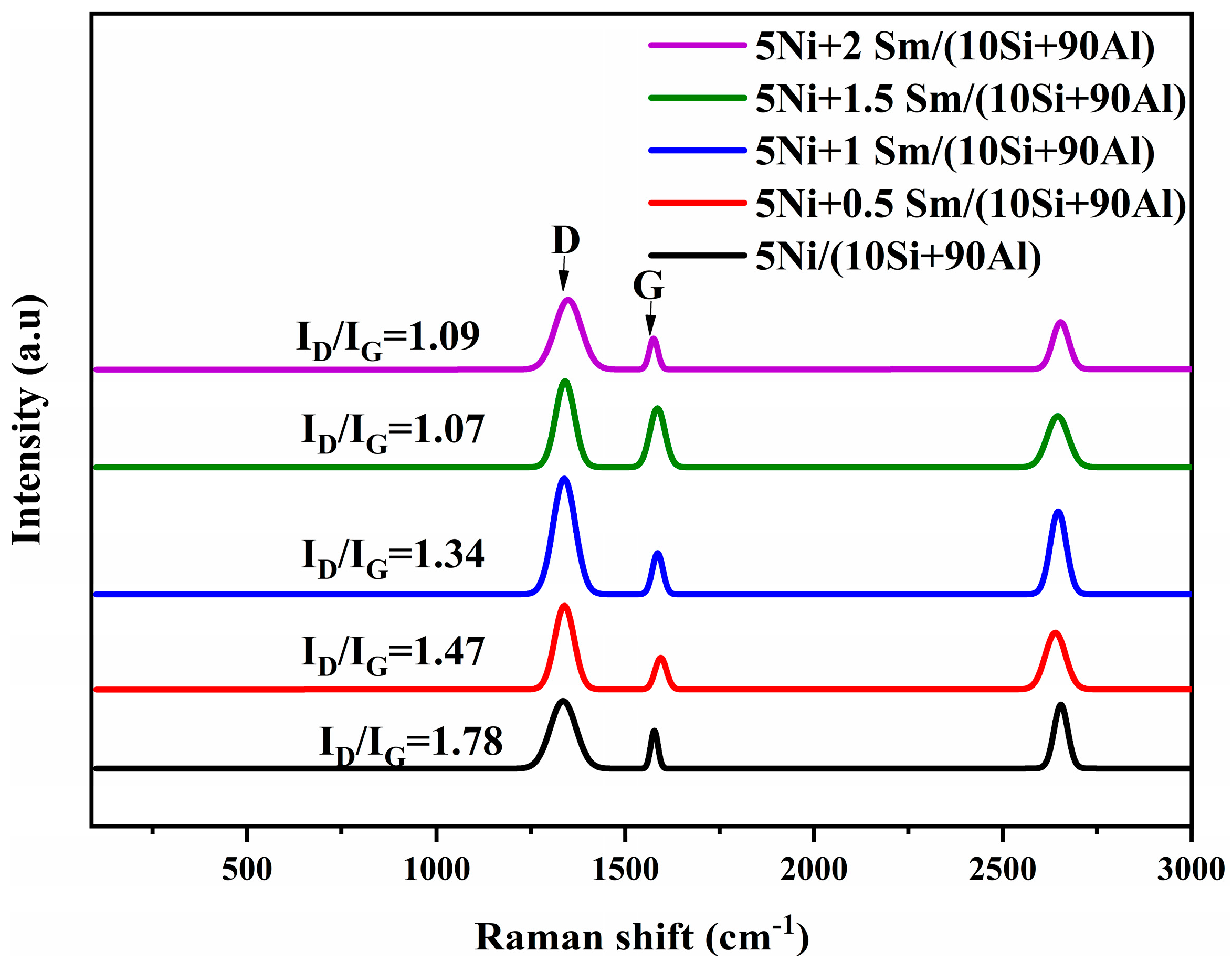
2.6. Spectra of Used Catalysts in Raman
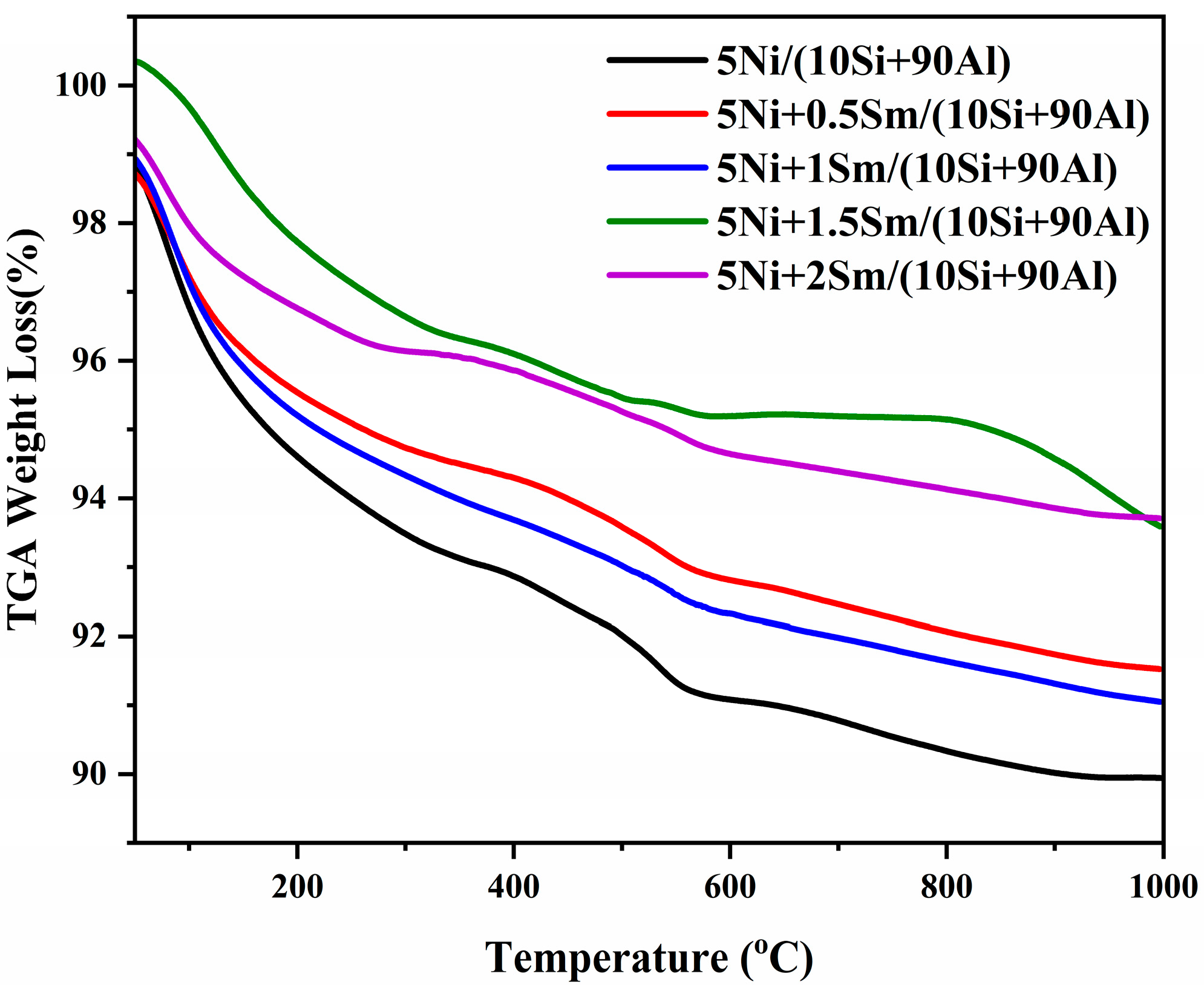
2.7. Thermogravimetric Analysis (TGA)
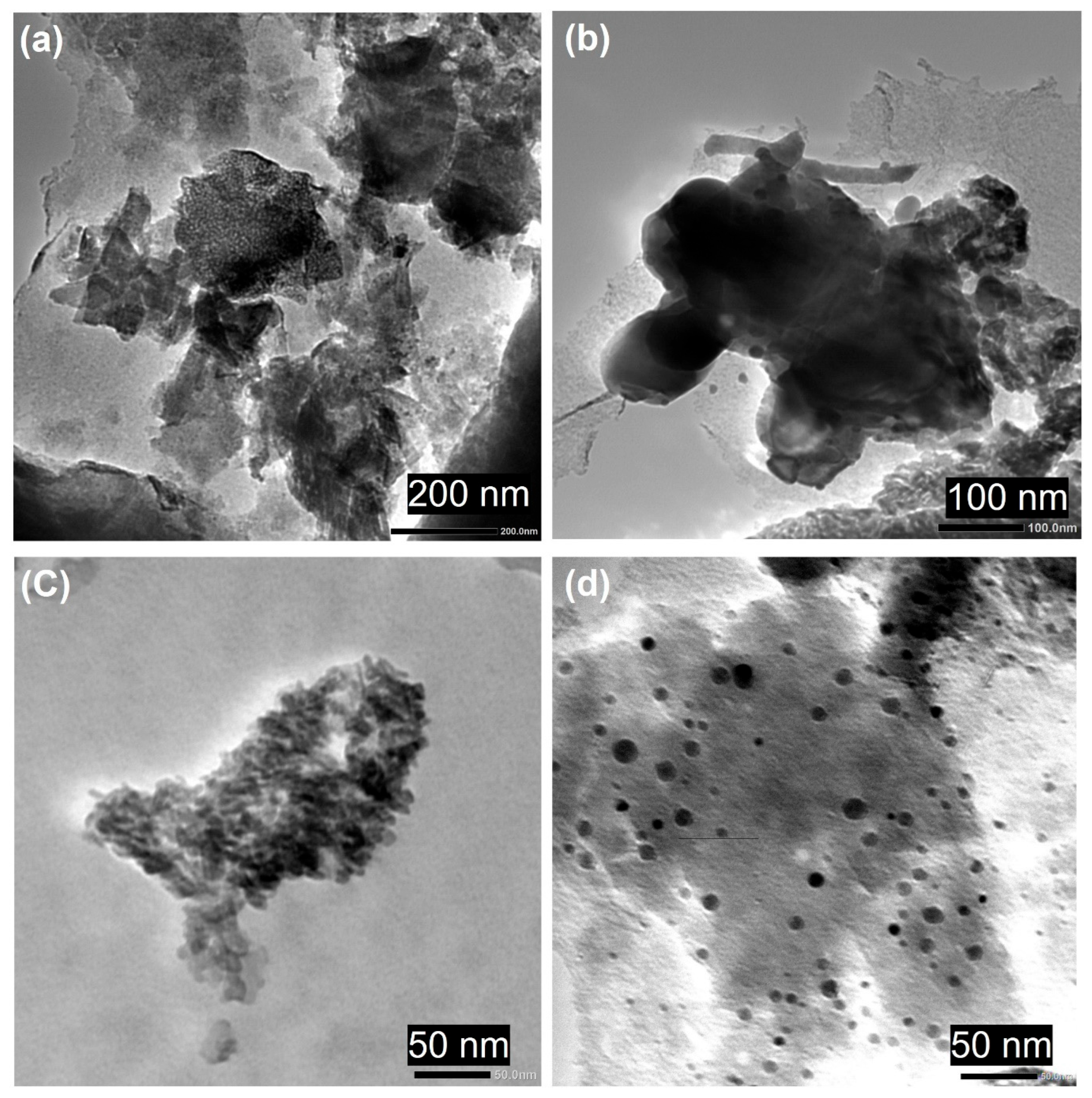
2.8. Analysis of TEM Morphology
2.9. Modeling and Simulation
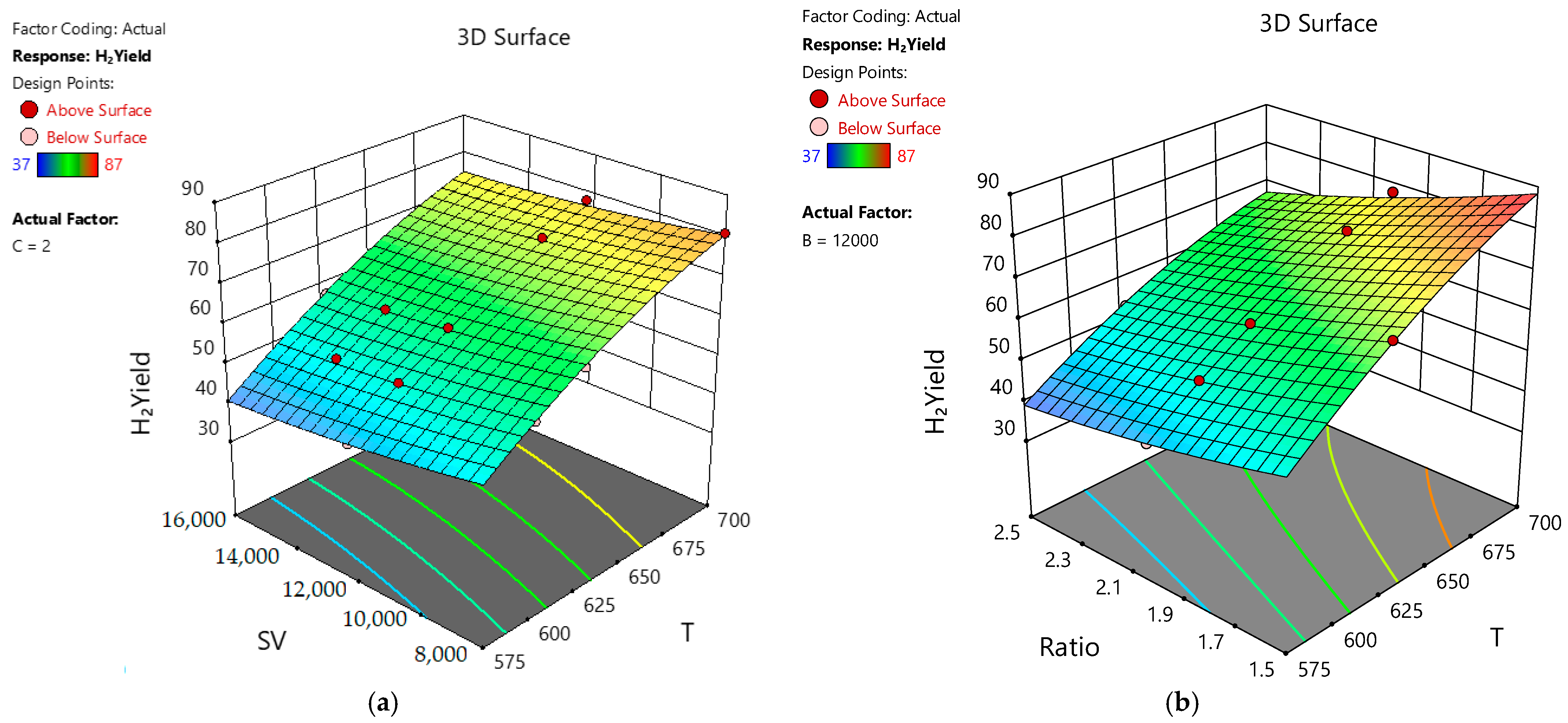
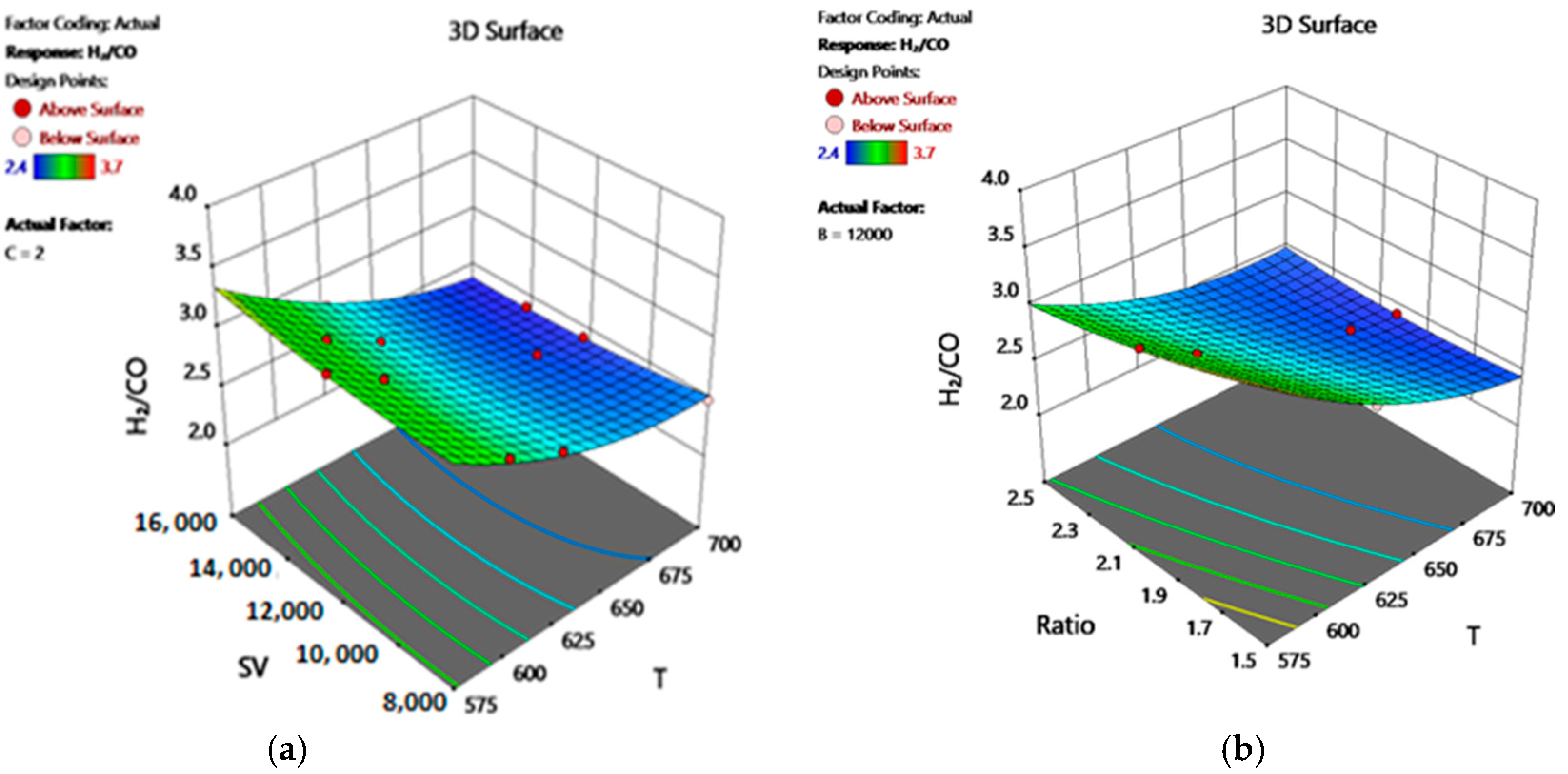
2.9.1. Response Surface Methodology Approach (RSM)
2.9.2. Process Modelling and Analysis of Variance
2.9.3. Final Formula About Real Factors
2.9.4. Simulation Using Design-Expert Software
2.9.5. Optimization Performance
3. Materials and Methods
3.1. Materials
3.2. Preparation of Promoted Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fakeeha, A.H.; Al-Fatesh, A.S.; Srivastava, V.K.; Ibrahim, A.A.; Abahussain, A.A.M.; Abu-Dahrieh, J.K.; Alotibi, M.F.; Kumar, R. Hydrogen Production from Gadolinium-Promoted Yttrium-Zirconium-Supported Ni Catalysts through Dry Methane Reforming. ACS Omega 2023, 8, 22108–22120. [Google Scholar] [CrossRef] [PubMed]
- Kurdi, A.N.; Ibrahim, A.A.; Al-Fatesh, A.S.; Alquraini, A.A.; Abasaeed, A.E.; Fakeeha, A.H. Hydrogen Production from CO2 Reforming of Methane Using Zirconia Supported Nickel Catalyst. RSC Adv. 2022, 12, 10846–10854. [Google Scholar] [CrossRef] [PubMed]
- Therdthianwong, S.; Siangchin, C.; Therdthianwong, A. Improvement of Coke Resistance of Ni/Al2O3 Catalyst in CH4/CO2 Reforming by ZrO2 Addition. Fuel Process. Technol. 2008, 89, 160–168. [Google Scholar] [CrossRef]
- Teh, L.P.; Setiabudi, H.D.; Timmiati, S.N.; Aziz, M.A.A.; Annuar, N.H.R.; Ruslan, N.N. Recent Progress in Ceria-Based Catalysts for the Dry Reforming of Methane: A Review. Chem. Eng. Sci. 2021, 242, 116606. [Google Scholar] [CrossRef]
- Fazlikeshteli, S.; Vendrell, X.; Llorca, J. Low-Temperature Methane Partial Oxidation over Pd Supported on CeO2: Effect of the Preparation Method and Precursors. Reactions 2021, 2, 30–42. [Google Scholar] [CrossRef]
- Fazlikeshteli, S.; Vendrell, X.; Llorca, J. Bimetallic Ru–Pd Supported on CeO2 for the Catalytic Partial Oxidation of Methane into Syngas. Fuel 2023, 334, 126799. [Google Scholar] [CrossRef]
- Hasanat, A.U.; Khoja, A.H.; Naeem, N.; Al-Anazi, A.; Liaquat, R.; Khan, B.A.; Din, I.U. Thermocatalytic Partial Oxidation of Methane to Syngas (H2, CO) Production Using Ni/La2O3 Modified Biomass Fly Ash Supported Catalyst. Results Eng. 2023, 19, 101333. [Google Scholar] [CrossRef]
- Dissanayake, D.; Rosynek, M.P.; Kharas, K.C.C.; Lunsford, J.H. Partial Oxidation of Methane to Carbon Monoxide and Hydrogen over a Ni/Al2O3 Catalyst. J. Catal. 1991, 132, 117–127. [Google Scholar] [CrossRef]
- Hickman, D.A.; Schmidt, L.D. The Role of Boundary Layer Mass Transfer in Partial Oxidation Selectivity. J. Catal. 1992, 136, 300–308. [Google Scholar] [CrossRef]
- Zhu, H.; Dong, K.; Huang, J.; Li, J.; Wang, G.; Xie, Z. Reaction Mechanism and Mechanical Properties of an Aluminum-Based Composite Fabricated in-Situ from Al-SiO2 System. Mater. Chem. Phys. 2014, 145, 334–341. [Google Scholar] [CrossRef]
- Shah, M.; Al Mesfer, M.K.; Danish, M.; Mondal, P.; Goyal, H.; Das, S. Study on the Effect of Promotors in CO2 Utilization for Syngas Production via Dry Reforming of Methane over Co-MOX/TiO2-Al2O3 (MOX = La, Ce, Mg, and K) Catalysts. Fuel Process. Technol. 2024, 257, 108089. [Google Scholar] [CrossRef]
- Sun, K.; Jiang, J.; Liu, Z.; Geng, S.; Liu, Z.; Yang, J.; Li, S. Research Progress on Metal-Support Interactions over Ni-Based Catalysts for CH4-CO2 Reforming Reaction. Ranliao Huaxue Xuebao/J. Fuel Chem. Technol. 2025, 53, 434–451. [Google Scholar] [CrossRef]
- Vahid Shahed, G.; Taherian, Z.; Khataee, A.; Meshkani, F.; Orooji, Y. Samarium-Impregnated Nickel Catalysts over SBA-15 in Steam Reforming of CH4 Process. J. Ind. Eng. Chem. 2020, 86, 73–80. [Google Scholar] [CrossRef]
- Jing, Z.; Zhang, T.; Shang, J.; Zhai, M.; Yang, H.; Qiao, C.; Ma, X. Influence of Cu and Mo Components of γ-Al2O3 Supported Nickel Catalysts on Hydrodeoxygenation of Fatty Acid Methyl Esters to Fuel-like Hydrocarbons. J. Fuel Chem. Technol. 2018, 46, 427–440. [Google Scholar] [CrossRef]
- Alanazi, R.S.A.; Alreshaidan, S.B.; Ibrahim, A.A.; Wazeer, I.; Alarifi, N.; Bellahwel, O.A.; Abasaeed, A.E.; Al-Fatesh, A.S. Influence of Alumina and Silica Supports on the Performance of Nickel Catalysts for Methane Partial Oxidation. Catalysts 2025, 15, 102. [Google Scholar] [CrossRef]
- Mageed, A.K.; Ayodele, B.V.; Mustapa, S.I. Response Surface Optimization of Hydrogen-Rich Syngas Production by Greenhouse Gases Reforming. Chem. Eng. Technol. 2020, 43, 742–751. [Google Scholar] [CrossRef]
- Nelson, B.L.; Pei, L. Experiment Design and Analysis. Int. Ser. Oper. Res. Manag. Sci. 2021, 316, 199–229. [Google Scholar] [CrossRef]
- Hossain, M.A.; Ayodele, B.V.; Cheng, C.K.; Khan, M.R. Optimization of Renewable Hydrogen-Rich Syngas Production from Catalytic Reforming of Greenhouse Gases (CH4 and CO2) over Calcium Iron Oxide Supported Nickel Catalyst. J. Energy Inst. 2019, 92, 177–194. [Google Scholar] [CrossRef]
- Whitman, W. Section 1. In Song Myself; University of Iowa Press: Iowa City, IA, USA, 2018; pp. 7–9. [Google Scholar] [CrossRef]
- Yang, L.; Yin, P.; Fan, H.; Xue, Q.; Li, K.; Li, X.; Sun, L.; Liu, Y. Response Surface Methodology Optimization of Ultrasonic-Assisted Extraction of Acer Truncatum Leaves for Maximal Phenolic Yield and Antioxidant Activity. Molecules 2017, 22, 232. [Google Scholar] [CrossRef]
- Ahmadi Azqhandi, M.H.; Ghaedi, M.; Yousefi, F.; Jamshidi, M. Application of Random Forest, Radial Basis Function Neural Networks and Central Composite Design for Modeling and/or Optimization of the Ultrasonic Assisted Adsorption of Brilliant Green on ZnS-NP-AC. J. Colloid Interface Sci. 2017, 505, 278–292. [Google Scholar] [CrossRef]
- Dong, W.S.; Jun, K.W.; Roh, H.S.; Liu, Z.W.; Park, S.E. Comparative Study on Partial Oxidation of Methane over Ni/ZrO2, Ni/CeO2 and Ni/Ce-ZrO2 Catalysts. Catal. Letters 2002, 78, 215–222. [Google Scholar] [CrossRef]
- Wang, H.Y.; Ruckenstein, E. Partial Oxidation of Methane to Synthesis Gas over MgO- and SiO2-Supported Rhodium Catalysts. J. Catal. 1999, 186, 181–187. [Google Scholar] [CrossRef]
- Zhu, J.; Van Ommen, J.G.; Lefferts, L. Reaction Scheme of Partial Oxidation of Methane to Synthesis Gas over Yttrium-Stabilized Zirconia. J. Catal. 2004, 225, 388–397. [Google Scholar] [CrossRef]
- Costa, D.S.; Gomes, R.S.; Rodella, C.B.; da Silva, R.B.; Fréty, R.; Teixeira Neto, É.; Brandão, S.T. Study of Nickel, Lanthanum and Niobium-Based Catalysts Applied in the Partial Oxidation of Methane. Catal. Today 2020, 344, 15–23. [Google Scholar] [CrossRef]
- Cheephat, C.; Daorattanachai, P.; Devahastin, S.; Laosiripojana, N. Partial Oxidation of Methane over Monometallic and Bimetallic Ni-, Rh-, Re-Based Catalysts: Effects of Re Addition, Co-Fed Reactants and Catalyst Support. Appl. Catal. A Gen. 2018, 563, 1–8. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Y.; Chen, Y.; Ma, S.; Li, Q.; Hu, X.; Wang, Z.; Buckley, C.E.; Dong, D. Highly Stable Nanofibrous La2NiZrO6 Catalysts for Fast Methane Partial Oxidation. Fuel 2020, 265, 116861. [Google Scholar] [CrossRef]
- Wu, T.; Yan, Q.; Wan, H. Partial Oxidation of Methane to Hydrogen and Carbon Monoxide over a Ni/TiO2 Catalyst. J. Mol. Catal. A Chem. 2005, 226, 41–48. [Google Scholar] [CrossRef]
- Singha, R.K.; Shukla, A.; Yadav, A.; Sivakumar Konathala, L.N.; Bal, R. Effect of Metal-Support Interaction on Activity and Stability of Ni-CeO2 Catalyst for Partial Oxidation of Methane. Appl. Catal. B Environ. 2017, 202, 473–488. [Google Scholar] [CrossRef]
- Chu, Y.; Li, S.; Lin, J.; Gu, J.; Yang, Y. Partial Oxidation of Methane to Carbon Monoxide and Hydrogen over NiO/La2O3/γ-Al2O3 Catalyst. Appl. Catal. A Gen. 1996, 134, 67–80. [Google Scholar] [CrossRef]
- Morioka, H.; Shimizu, Y.; Sukenobu, M.; Ito, K.; Tanabe, E.; Shishido, T.; Takehira, K. Partial Oxidation of Methane to Synthesis Gas over Supported Ni Catalysts Prepared from Ni-Ca/Al-Layered Double Hydroxide. Appl. Catal. A Gen. 2001, 215, 11–19. [Google Scholar] [CrossRef]
- Yan, Q.G.; Wu, T.H.; Weng, W.Z.; Toghiani, H.; Toghiani, R.K.; Wan, H.L.; Pittman, C.U. Partial Oxidation of Methane to H2 and CO over Rh/SiO2 and Ru/SiO2 Catalysts. J. Catal. 2004, 226, 247–259. [Google Scholar] [CrossRef]
- Cesar, D.V.; Baldanza, M.A.S.; Henriques, C.A.; Pompeo, F.; Santori, G.; Múnera, J.; Lombardo, E.; Schmal, M.; Cornaglia, L.; Nichio, N. Stability of Ni and Rh-Ni Catalysts Derived from Hydrotalcite-like Precursors for the Partial Oxidation of Methane. Int. J. Hydrog. Energy 2013, 38, 5616–5626. [Google Scholar] [CrossRef]
- Pompeo, F.; Nichio, N.N.; Ferretti, O.A.; Resasco, D. Study of Ni Catalysts on Different Supports to Obtain Synthesis Gas. Int. J. Hydrog. Energy 2005, 30, 1399–1405. [Google Scholar] [CrossRef]
- Shishido, T.; Sukenobu, M.; Morioka, H.; Kondo, M.; Wang, Y.; Takaki, K.; Takehira, K. Partial Oxidation of Methane over Ni/Mg-Al Oxide Catalysts Prepared by Solid Phase Crystallization Method from Mg-Al Hydrotalcite-like Precursors. Appl. Catal. A Gen. 2002, 223, 35–42. [Google Scholar] [CrossRef]



| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| 5Ni/(10Si+90Al) | 192 | 0.58 | 11.41 |
| 5Ni+0.5Sm/(10Si+90Al) | 187 | 0.55 | 11.00 |
| 5Ni+1Sm/(10Si+90Al) | 186 | 0.56 | 11.01 |
| 5Ni+1.5Sm/(10Si+90Al) | 186 | 0.55 | 10.91 |
| 5Ni+2Sm/(10Si+90Al) | 177 | 0.53 | 11.14 |
| Sample | Quantity (cm3/g STP) | DR a (%) |
|---|---|---|
| 5Ni/(10Si+90Al) | 19.3 | 101 |
| 5Ni+0.5Sm/(10Si+90Al) | 18.5 | 97 |
| 5Ni+1Sm/(10Si+90Al) | 18.0 | 95 |
| 5Ni+1.5Sm/(10Si+90Al) | 17.1 | 90 |
| 5Ni+2Sm/(10Si+90Al) | 17.3 | 91 |
| Process Parameter | Levels | |
|---|---|---|
| −1 (Low) | +1 (High) | |
| SV: Gas Hour Space velocity (ccg−1h−1) | 8000 | 16,000 |
| T: Temperature (°C) | 575 | 700 |
| ratio | 1. 5 | 2.5 |
| Variables | Goals Function | |||
|---|---|---|---|---|
| T | SV | CH4/O2 | H2 Yield | |
| Criteria | 575–700 | 8000–16,000 | 1.5–2.5 | Max. |
| Theoretical conditions | 696.225 | 8090.505 | 1.663 | 87.563 |
| Experimental conditions | 696 | 8091 | 1.663 | 88.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alreshaidan, S.B.; Alanazi, R.S.A.; Odhah, O.H.; Ibrahim, A.A.; Ali, F.A.A.; Alarifi, N.; Banabdwin, K.M.; Ramesh, S.; Al-Fatesh, A.S. The Impact of Sm Promoter on the Catalytic Performance of Ni/Al2O3-SiO2 in Methane Partial Oxidation for Enhanced H2 Production. Catalysts 2025, 15, 721. https://doi.org/10.3390/catal15080721
Alreshaidan SB, Alanazi RSA, Odhah OH, Ibrahim AA, Ali FAA, Alarifi N, Banabdwin KM, Ramesh S, Al-Fatesh AS. The Impact of Sm Promoter on the Catalytic Performance of Ni/Al2O3-SiO2 in Methane Partial Oxidation for Enhanced H2 Production. Catalysts. 2025; 15(8):721. https://doi.org/10.3390/catal15080721
Chicago/Turabian StyleAlreshaidan, Salwa B., Rasha S. A. Alanazi, Omalsad H. Odhah, Ahmed A. Ibrahim, Fekri Abdulraqeb Ahmed Ali, Naif Alarifi, Khaled M. Banabdwin, Sivalingam Ramesh, and Ahmed S. Al-Fatesh. 2025. "The Impact of Sm Promoter on the Catalytic Performance of Ni/Al2O3-SiO2 in Methane Partial Oxidation for Enhanced H2 Production" Catalysts 15, no. 8: 721. https://doi.org/10.3390/catal15080721
APA StyleAlreshaidan, S. B., Alanazi, R. S. A., Odhah, O. H., Ibrahim, A. A., Ali, F. A. A., Alarifi, N., Banabdwin, K. M., Ramesh, S., & Al-Fatesh, A. S. (2025). The Impact of Sm Promoter on the Catalytic Performance of Ni/Al2O3-SiO2 in Methane Partial Oxidation for Enhanced H2 Production. Catalysts, 15(8), 721. https://doi.org/10.3390/catal15080721







