Abstract
Photocatalysis is considered to be a very promising method for the degradation of organic matter, because its process of degrading organic matter is safe. However, some problems such as weak absorption of visible light and electronic-hole recombination easily are obviously drawbacks. In this paper, three different morphologies of Bi5O7I (nanoball, nanosheet, and nanotube) were successfully prepared by solvothermal method, which was used for the degradation of Rhodamine B (RhB). Comparing the photocatalytic effect of three different morphologies and concluding that the optimal morphology was the Bi5O7I nanoball (97.8% RhB degradation within 100 min), which was analysed by the characterisation tests. Free radical trapping experiments were tested, which revealed that the main roles in the degradation process were singlet oxygen (1O2) and holes (h+). The degradation pathways of RhB were analyzed in detail. The photo/electrochemical parts of the three materials were analysed and explained the degradation mechanism of RhB degradation. This investigate provides a very valuable guide for the development of multiple morphologies of bismuth-based photocatalysts for removing organic dyes in aquatic environment.
1. Introduction
Currently, organic pollution is becoming one of the major problems in the world [1]. The diversity of intrinsic characteristics of different organic molecules makes it difficult for them to be naturally degraded in nature [2,3], which leads to significant economic losses. Environmental pollution has a huge impact on human society, seriously threatening human health and leading to a variety of diseases [4]. Especially the discharge of industrial wastewater, which contains a variety of chemical substances that harm the water environment and human life all the time [5]. Rhodamine B (RhB) [6], which is a red synthetic dye that is mainly used in laboratory cells for fluorescent dyes, colourants, paper industry, and manufacturing of paints and pigments. RhB is toxic, difficult to biodegrade, and also reacts with other chemicals to form more hazardous substances that not only cause damage to plants and animals, but also pose a threat to human health and the International Agency for Research on Cancer of the World Health Organization has listed RhB as a group 3 carcinogen [7,8]. Therefore, it is necessary to find a way to reduce its harmful effects.
Among the current research solutions, there are a series of methods such as biodegradation [9,10,11], electro-oxidation [12,13,14], adsorption [15,16], PMS activation [17,18,19,20] etc. However, long degradation time, high cost consumption, low removal efficiency, and inconvenient operation limited their widely used in industrialization. In recent years, with the continuous progress of technology, a new degradation method was founded, which is photocatalytic technology [21,22,23,24,25]. The principle of photocatalytic technology is to use the irradiation of visible light to promote the excitation of valence band electrons [26], which undergoes a series of redox reactions to produce charge carriers, in which these reactions will cause the appearance of various types of free radicals in the solution, which are eliminated by oxidative reactions with RhB [27,28]. The common photocatalysts available on the market today are TiO2, ZnO, SnS/TiO2 and so on, but there are some problems, such as low light utilisation [29], very small specific surface area [26], too few active sites on the surface of the catalysts [30]. Therefore, it is necessary to investigate new visible light-driven photocatalysts.
In this context, bismuth-based photocatalytic materials began to enter within the performance [31,32]. The structure of bismuth halide oxide is such that it can present a different types of morphology, which greatly enhances its degradation efficiency [33,34]. The very weak bond strength of bismuth halide oxide leads to the easy deformation of its structure, coupled with its layered structure of the intramolecular electric field can greatly impede the photogenerated electron-hole recombination, which allows enough space to polarise the corresponding atoms and atomic orbitals and, thus, effectively separates the photogenerated carriers to promote the photocatalytic redox reaction [35,36]. Bi5O7I, which currently has a typically low photoconversion efficiency. The ability of the material to effectively absorb and excite light energy is limited, its reaction rate is slow for RhB and its long-term stability is limited under the influence of the external environment. Controlling various conditions, such as temperature is a useful way to accelerate the reaction rate and enhance its stability. In this process, it was found that the morphology of the material can be altered by controlling the duration of high-temperature treatment, and the degradation effect will vary with different morphologies. Currently, the common modification methods include elemental doping [37], composite [38], and other methods, but there are more complex synthesis and preparation, the cost of higher cost drawbacks. Some BiOX photocatalysts for the degradation of RhB, such as BiOX (X = Cl, Br, I)/WO3/Polyacrylonitrile Nanofibrous Membranes [39], showed a degradation rate of 97.25% of RhB in 120 min; the g-C3N4-loaded BiOCl0.5I0.5 dual-Z-scheme heterojunction degraded only 92.32% in 120 min [40]. In contrast, the material synthesised in this paper degraded 98.00% of RhB in 100 min, demonstrating the novelty of this experiment.
In this paper, we modulate the morphological structure of Bi5O7I using a one-step method and compare their characterization and properties to investigate their photocatalytic performance with the aim of identifying the optimal photocatalyst. At the same time, the experience will optimise them by adjusting the pollutant concentration, pH, photocatalyst content and other conditions to test them and compare the degradation efficiency and degradation rate curves, which generally follow the quasi-primary kinetic model.
2. Results and Discussion
2.1. Characterization of Catalysts
The morphology and size of the prepared samples were characterised by Scanning electron microscope (SEM) and Transmission electron microscope (TEM) and the records are shown in Figure 1. It could be observed from Figure 1a that the Bi5O7I NB exist in the form of microspheres with diameters in the range of 1.0–2.0 μm and the surface of the microspheres shows a three-dimensional nanorodular structure consisting of rough nanosheets tightly bonded together. Images of the Bi5O7I NB TEM (Figure 1b,c) show a dense aggregation of limb-like nanosheets in the Bi5O7I NB [41]. The distribution of Bi5O7I NB is regular, each ball is closely connected with each other and there is some order arrangement. Bi5O7I NB are uniform in size and the difference is not big and this uniformity lays a solid foundation for the stableility of its performance in subsequent applications. As can be seen in Figure 1d the average size of Bi5O7I NS is about 1.0 μm, which is similar to previous publications [42]. Although Bi5O7I NS are similar to Bi5O7I NB in that they are composed of lamellar structures, the Bi5O7I NS are made up of irregular lamellar stacks with irregular distribution. A deeper analysis of the TEM images reveals that the Bi5O7I NS (Figure 1e,f) present a lamellar structure with obvious angles and these Bi5O7I NS are stacked and crowded together without any regularity. This structural disorder and size non-uniformity further weaken the stability and consistency of their overall properties. In contrast, Bi5O7I NT (Figure 1g–i) were in the form of tubes and short rods, with variable lengths, averaging 5.0 μm, and diameters ranging from 200–300 nm. They had smooth and clean outer walls, as well as disordered and interlaced distributions, which will lead to the formation of effective degradation sites during the degradation process, resulting in a relatively weak degradation effect. As a result, the overall processing effect is poor, and it is difficult to meet the needs of some applications that require high degradation efficiency.
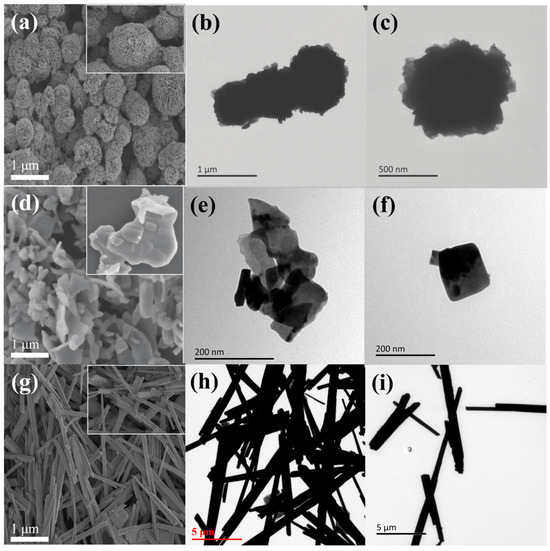
Figure 1.
SEM images of Bi5O7I NB (a), Bi5O7I NS (d), Bi5O7I NT (g); TEM images of Bi5O7I NB (b,c), Bi5O7I NS (e,f), Bi5O7I NT (h,i).
X-ray diffractometers (XRD) were used the principle of diffraction to accurately determine the crystal structure of a substance and to accurately perform physical phase analysis. The XRD of the synthesised materials is shown in Figure 2 and the detectable peaks of the three materials are in good agreement with the Bi5O7I standard card (JCPDS Card No. 40-0548), which means the materials were synthesised successful. The characteristic diffraction peaks of the three materials are located at about 7.7°, 28.2°, 31.1° and 33.1°, indexed to the (001), (312), (004) and (204) diffraction planes, respectively [43]. When it can be found through Figure 2 that spurious peaks appear on the sides of the three characteristic peaks of Bi5O7I NB and Bi5O7I NS with a large influence, it can be analysed that the three materials belong to the same compound, but the internal crystal structure is different. The XRD images of Bi5O7I NB and Bi5O7I NS are close to each other because Bi5O7I NB are formed by stacking nanosheets in spherical shape (results of SEM and TEM analyses). While the other two materials compared to Bi5O7I NT, presenting peaks at 31.1° and 33.1°, probably because Bi5O7I NT belonging to the orthorrombic alpha phase based on the different synthesis conditions, the phase structure of nanorods and nanosheets undergoes a transition from orthorhombic to monoclinic beta phase during synthesis [44].

Figure 2.
XRD patterns of Bi5O7I NB, Bi5O7I NS and Bi5O7I NT.
The elemental composition and chemical state of Bi5O7I NB were observed by X-ray photoelectron spectroscopy (XPS). From the investigation of the XPS spectra, it can be seen that the Bi5O7I NB contain elements such as Bi, O and I (Figure 3a), respectively. Figure 3b demonstrates the XPS spectrum of O 1s, showing that oxygen exists in some kinds of chemical environments with binding energies of 530.04 eV and 531.50 eV for Bi-O and hydroxyl oxygen, respectively [45]. Observation of Figure 3c shows that the binding energies of I 3d5/2 and I 3d3/2 are 619.29 eV and 630.74 eV, respectively, and the difference in spin-orbital binding energies is 11.45 eV, which is similar to that of the standard binding energy [46]. As shown in Figure 3d, the binding energies of Bi at Bi 4f7/2 and Bi 4f5/2 are 158.99 eV and 164.30 eV, which are all in agreement with the data reviewed in the literature [47].
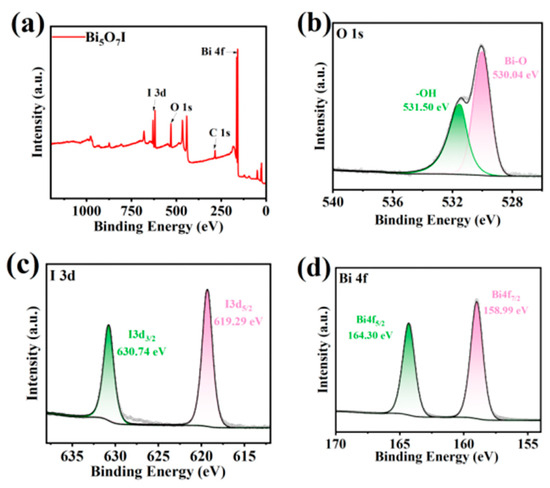
Figure 3.
XPS survey of Bi5O7I NB (a); corresponding to high-resolution spectra of C 1s (b), I 3d (c), and Bi 4f (d).
2.2. Comparison of Photocatalytic Properties of Bi5O7I NB, Bi5O7I NS and Bi5O7I NT
The RhB was selected as target pollutant to evaluate the photocatalysis ability based on Bi5O7I NT, Bi5O7I NS, and Bi5O7I NB. In Figure 4a, it can be found that the degradation of the Bi5O7I NB is better and the rate is faster at 100 min, the degradation rate reached 98.0% with a rate constant of 0.041 min−1. While Bi5O7I NS and Bi5O7I NT had degradation rates of only 88.1% and 12.0%, with degradation rates of 0.021 min−1 and 0.001 min−1, so for the subsequent experiments the Bi5O7I NB will be optimised. At the same time, dark adsorption treatment was carried out and the absorbance did not show a significant decrease in 30 min (Figure S1), which can prove that the material removes pollutants because of the photocatalytic effect, not the adsorption effect. And the low adsorption in the dark indicates that in the absence of light, the generation of electrons and holes in materials is inhibited, leading to a lack of necessary catalytic activity, which prevents effective adsorption of RhB. Furthermore, the absence of light energy can create thermodynamic barriers to the adsorption process, resulting in a reduced reaction rate that hinders the effective approach and attachment of dye molecules to the material surface.

Figure 4.
Comparison of photocatalytic properties of Bi5O7I NB, Bi5O7INS and Bi5O7I NT (a); pseudo-first-order kinetic fitting of three materials (b), comparison of different amounts of Bi5O7I NB (c), and pseudo-first-order kinetic fitting of different amounts (d).
After comparing the Bi5O7I NB with the best degradation effect, further exploration of the optimal mass of Bi5O7I NB was be put into the RhB solution at a concentration of 10 mg/L. According to the data presented in Figure 4b,c, the respective degradation rates were 0.018, 0.032, 0.041, and 0.032 min−1 for Bi5O7I NB of 10, 20, 25, and 30 mg, respectively. It was found that the best degradation effect and high degradation rate of 0.041 min−1 were achieved with 25 mg. An amount of 30 mg was also effective, but the research chose the better 25 mg for the industrialisation cost and material saving. A total of 25 mg Bi5O7I NB was used in all the subsequent optimisation experiments. Also, the degradation of RhB remained 76.8% after three cycles (Figure S2). Table S1 presented the characteristics of other photocatalysts that have been reported before. The Bi5O7I NB shows excellent photodegrade ability better than the other materials, which showed that it could be used to remove RhB from wastewater.
2.3. Optimisation of Reaction Conditions for Degradation of Bi5O7I NB
2.3.1. Effect of RhB Concentration
Firstly, experience was started to explore the catalytic effect of Bi5O7I NB for different concentrations by varying the concentration of RhB solution. According to the data in Figure 5a,b, the degradation efficiencies were 96.1%, 97.8%, and 88.3% at the concentrations of 5 mg/L, 10 mg/L and 20 mg/L of RhB, and the reaction rate constants were 0.035, 0.040 and 0.021 min−1, respectively, and the Bi5O7I NB was able to maintain a high degradation efficiency close to or even exceeding 90% in both the low and the high concentrations of RhB solutions. Bi5O7I NB can maintain the ultra-high degradation efficiency close to or even more than 90%, and have a faster degradation rate compared with other removal methods [48,49]. From the above Figures, it could be seen that the degradation efficiency is close to 98% under the condition of RhB concentration lower than 10 mg/L, and the rate is similar, which can be inferred that there are still a huge number of reaction sites for photocatalysts to attach. When the concentration increased to 20 mg/L, the degradation efficiency and the rate of explanation decreased slightly, and the reaction attachment sites of the catalyst in the solution were gradually insufficient.
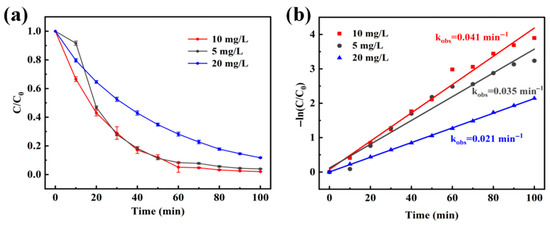
Figure 5.
Effects of RhB concentration (a), pseudo-first-order kinetic fitting (b).
2.3.2. Effects of pH Value of Initial Solution on Degradation Effect
At the same time, the pH of the initial RhB solution was changed to test whether the catalytic effect of the Bi5O7I NB would be affected under different acidic and alkaline conditions. As shown in Figure 6a,b, Bi5O7I NB could still achieve a high degradation effect under the conditions of strong acid, weak acid, neutral and weak alkali, and the degradation percentage could still be maintained at about 98%, and the degradation rates reached 0.035, 0.041, 0.048 and 0.039 min−1 at pH = 3, 5, 7 and 9, with no significant change in the degradation performance. There was no significant change, indicating that the Bi5O7I NB can play a good degradation effect in acidic, neutral and weak alkaline environments with a wide range of applications, whereas, the degradation effect decreasing rapidly when pH = 11, the degradation percentage is only 65.4% and the degradation rate is only 0.011 min−1. It can be seen that the number of free radicals produced decreases under strong alkaline conditions, while their lifetimes are shortened dramatically [50].

Figure 6.
Effect of initial solution pH (a), pseudo-first-order kinetic fitting (b).
2.3.3. Effects of Ion Strength on Degradation
In order to further investigate the degradation performance of the Bi5O7I NB, this experiment continued to investigate the effects of various types of ions and organic acid on the catalysis of materials. Five anions commonly found in surface water were used in the study, namely chloride (Cl−), bicarbonate (HCO3−), dihydrogen phosphate (H2PO42−), sulphate (SO42−) and humic acid. As shown in Figure 7a,b, the inhibition effect of H2PO42− was the most obvious, with no degradation effect at all in the first 60 min and the degradation rate reached only 18.9% after 100 min, which was a negligible degradation effect. The inhibition effect of bicarbonate was also very obvious and the degradation rate was only 33.8% after 100 min. The inhibition effect of adding sulphate and humic acid was the next most effective, with degradation rates of 80.1% and 89.0%, respectively, which had an inhibitory effect, but the overall effect was not significant. Chloride ions had almost no bad effect, and the degradation rate was even faster than that of the blank control group in the early stage, and the final degradation rate reached 97.9%, so it can be deduced that chlorine ions played a certain role in promoting the degradation of RhB (Equation (1)). In the early stages of the degradation process, RhB was also degraded faster than the blank control group. The following reactions may have occurred:
h+ + Cl− → Cl•

Figure 7.
Effects of anion on degradation part (a), pseudo-first-order kinetic fitting (b).
As this reaction occurs thereby reducing recombination and extending the lifetime of the photogenerated electrons [51]. Humic acid because when adsorbed onto the photocatalyst, it causes light attenuation, removes holes and occupies the active sites of the photocatalyst, thus reducing the photocatalytic activity. And bicarbonate, dihydrogen phosphate, and sulphate all remove holes and hydroxyl radicals generated during photocatalysis, etc., so that the photocatalytic effect is greatly reduced [52].
2.4. Photochemical Analysis and Investigation of Photocatalytic Mechanisms
2.4.1. Optical and Electrochemical Properties
As shown in Figure S3, the PL strength of Bi5O7I NB is significantly weaker than that of Bi5O7I NS and Bi5O7I NT, indicating that the heterogeneous structure inhibits electron-hole pair complexation. In Figure 8a, the transient photocurrent response signals of Bi5O7I NB, Bi5O7I NS and Bi5O7I NT show their performance during repetitive light illumination and shutdown. When the photocurrent is higher, the separation efficiency of photogenerated electron holes is higher. The results of these experiments show that the photocurrent response of the Bi5O7I NB is the strongest and much higher than that of the Bi5O7I NT and Bi5O7I NS, suggesting that the material has a lower carrier complexation rate, which is correspond with the records in our previous comparative experiments were obtained. In addition, the photocurrent density remained stable and did not decrease significantly after several cycles, indicating that the material has excellent photophysical stability. Electrochemical impedance spectroscopy (EIS) is an effective method to observe the separation and transfer of photogenerated electron and hole pairs. In general, the smaller radius of the circle in the EIS curve indicates a smaller electron transfer resistance, which means a faster charge transfer at the photoelectrode interface [53]. In Figure 8b, the Nyquist plots for Bi5O7I NT and Bi5O7I NB show smaller arc radii, indicating that Bi5O7I NB are more efficient in the transport of photogenerated carriers. The results of the study suggest that Bi5O7I NB can more efficiently facilitate the transfer of photogenerated carriers to the surface for subsequent reactions, leading to a significant enhancement in their photocatalytic performance. The above photoelectrochemical measurements indicate that the Bi5O7I NB have the best performance in photocatalytic degradation of RhB [54].
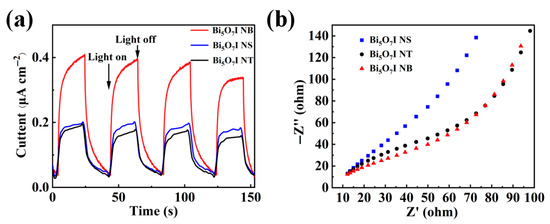
Figure 8.
Transient photocurrent response curves (a,b) Nyquist plots of electrochemical impedance spectroscopy of Bi5O7I NS, Bi5O7I NT and Bi5O7I NB.
The optical properties of the three samples were also investigated by UV-vis Diffuse Reflectance Spectroscopy (UV-vis DRS) and Mott–Schottky spectroscopy. For crystalline semiconductors, the optical bandgap is determined by Equation (2) using the optical absorption data near the band edges [55]. According to Figure 9a, it can be seen that in the UV and visible part of the spectrum, the Bi5O7I NB has better light-collecting properties than the Bi5O7I NS and Bi5O7I NT, and this strong light absorption capability is highly advantageous for promoting the electron delocalization range and stabilizing photoinduced electrons. Meanwhile, the band gaps of the three materials can be calculated as shown in Figure 9b. The band gaps Eg of Bi5O7I NB, Bi5O7I NS, and Bi5O7I NT are 2.40, 2.63, and 3.08 eV, respectively, where υ, h, α, A and Eg correspond to the optical frequencies, Planck’s constant, absorption coefficients, constants, and band gaps, respectively. The band gaps of Bi5O7I NB and Bi5O7I NS, calculated to be less than 3 eV, allow them to absorb visible light, making them suitable for photocatalytic reactions under sunlight. Moreover, an optimal band gap enhances the absorption of photons and facilitates the generation of a greater number of photoinduced electrons and holes. The flat band potential (EFB) of the three samples were investigated by Mott–Schottky plots using Ag/AgCl electrodes. The EFB of Bi5O7I NB is derived as −0.76 V in Figure 9c. Correspondingly, the EFB of Bi5O7I NS and Bi5O7I NT can be derived as −0.64 and −0.65 V based on Figure 9d,e. The pH of the electrolyte solution of 0.5 M NaSO4 is about 6.8, which can be converted to the standard hydrogen electrode (NHE) potential by Equation (3), and, thus, the Ef for Bi5O7I NB, Bi5O7I NS and Bi5O7I NT are −0.16, −0.04, and −0.05 V vs. NHE, because Bi5O7I is an n-type semiconductor [56] and the Ef of n-type semiconductors is 0.20 V higher than the ECB [57], so the ECB of the three materials are −0.36, −0.24, and −0.25 V, respectively. According to Equation (4), the valence (VB) potentials of the Bi5O7I NB, Bi5O7I NS, and Bi5O7I NT (EVB) could be calculated as 2.04, 2.39, and 2.83 V, respectively (Figure 9f). The conduction band position should be higher than the reduction potential of the reactants, allowing photogenerated electrons to effectively participate in the reduction reactions. Furthermore, appropriately positioned energy levels can minimise the recombination of electron-hole pairs, thereby enhancing photocatalytic activity. Based on the images, what can be seen is that the redox potential of OH−/•OH is higher than the valence band of the Bi5O7I NB, whereas that of O2/O2•− is lower than the conduction band of the Bi5O7I NB, which suggests that two formations of •OH and the formation of O2•− through the reaction between the photogenerated electrons and the O2 are thermodynamically favourable [58]. The conduction band of Bi5O7I NS and Bi5O7I NT is lower than the redox potential of O2/O2•−, so it must be going to be difficult to form O2•−, and its degradation is inferior to that of Bi5O7I NB, which is in line with the results done in our previous comparative experiments.
αhυ = A(hυ − Eg)2/n
E (vs. NHE) = E (vs. Ag/AgCl) + 0.0591 × pH + 0.198 V
EVB = ECB + Eg
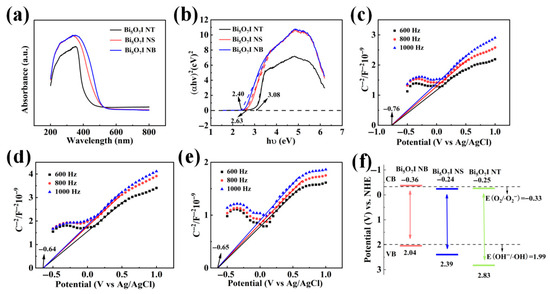
Figure 9.
UV-vis DRS spectra (a) and plot of (αhυ)2 vs. photon energy of Bi5O7I NB, Bi5O7I NS and Bi5O7I NT (b); Mott–Schottky plots of Bi5O7I NB (c), Bi5O7I NS (d) and Bi5O7I NT (e); schematic of the energy band structure of Bi5O7I NS, Bi5O7I NT, and Bi5O7I NB (f).
2.4.2. Free Radical Quenching Experiment and Photocatalytic Mechanism Investigation
In order to investigate the degradation mechanism of Bi5O7I NB, free radical quenching experiments using tert-butanol (TBA), p-benzoquinone (BQ), L-histidine (L-his) and triethanolamine (TEOA) as quenching agents for hydroxyl radicals (•OH), superoxide radicals (O2•−), singlet oxygen (1O2) and holes (h+), respectively. As shown in Figure 10a,b, the blank degradation rate was 97.8% without the quencher and 97.7%, 95.7%, 25.8%, and 5.7% with the addition of the quencher. After the addition of L-his and TEOA, the degradation rates were only 25.8% and 5.7%, which could be sure that h+ and 1O2 play dominant roles in the degradation of RhB. It is also proved that, as a main active material, the photogenerated cavities on Bi5O7I can be directly involved in the degradation of RhB [59]. The degradation rate was 25.8% after the addition of L-his, which still had a degradation effect, while the degradation rates were 97.7% and 95.7% after the addition of TBA and BQ, which can be inferred that hydroxyl radicals or superoxide radicals likewise played a role in the degradation process, although they did not occupy a dominant role, and also it is possible that the two kinds of radicals were converted to 1O2. Based on the premise that h+ and 1O2 dominate the degradation process and the activator is hydrogen peroxide, following reaction mechanism was proposed Equation (5)–(9).
Photocatalyst + hυ → h+ + e−
O2•− + h+ → 1O2
O2•− + H2O2 → 1O2 + •OH + OH−
O2•− + •OH → 1O2 + OH−
RhB + 1O2/O2•−/•OH/h+ → CO2 + H2O
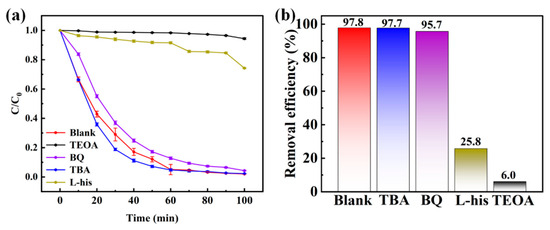
Figure 10.
Influences of (a), degradation ratio of (b) different quenchers of Bi5O7I NB.
The degradation mechanism is shown in Figure 11. In the presence of light, the nanospheres can induce the transfer of photogenerated carriers to the surface for further reactions. Because the valence band of the nanospheres is situated below the redox potential of OH−/•OH, while the conduction band is above the redox potential of O2/O2•−, both O2 and OH− in solution can be readily converted into the radicals O2•− and •OH. Simultaneously, under the catalytic influence of the Bi5O7I NB, along with the synergistic effects of h+ and H2O2, O2•− and •OH are rapidly transformed into 1O2, which plays a significant role in the degradation of RhB to CO2 and H2O.

Figure 11.
The possible mechanism for RhB degradation with Bi5O7I NB.
2.5. Possible Transformation Pathways of the RhB Degradation
The results of the intermediate products of RhB degradation are shown in Table S2. By combining the experimental results with the previous literature, we divided the degradation process of RhB into the four steps of N-ethylation, chromophore cleavage, ring-opening, and mineralization [60], which generated a series of intermediates (Figure 12), such as C26H27O3N2+ (m/z = 415), C24H23O3N2+ (m/z = 387), C22H19O3N2+ (m/z = 359) and C20H15O3N2+ (m/z = 331). And then, C20H15O3N2+ was fractured to generate C20H14O3N+ (m/z = 316) and C19H15ON2+ (m/z = 287), it proceeds with decarboxylation, deaminations and benzene ring opening to produce a series of small molecules that are eventually mineralised to H2O and CO2 [61].

Figure 12.
Possible transformation pathways of the RhB degradation.
3. Materials and Methods
3.1. Chemicals and Reagents
The materials in this paper are shown in Supporting Information.
3.2. Synthesis of Catalysts
3.2.1. Synthesis of Bi5O7I Nanotubes (Bi5O7I NT) Photocatalyst
Bi5O7I NT was Synthesised by using hydrothermal method. Firstly, 7.200 g of Bi(NO3)3•5H2O was added in 50 mL of deionised water to obtain solution A. Then, 1.800 g of NaOH was dissolved in 25 mL of deionised water to obtain solution B. Then, 1.000 g of KI was added in 6 mL of deionised water to get solution C. Next, the prepared solution B was added to solution A and stir until a milky white suspension was formed. And solution C dropwise was added. After stirring the mixed solution at room temperature for 30 min, the suspension was transferred to a 150 mL PTFE high-pressure reactor and it was kept at 180 °C for 24 h in an oven. After cooling, the Bi5O7I NT photocatalyst was obtained by high-speed centrifugation with deionised water for 4 times, and then dried in a 60 °C oven for 12 h. It was ground and collected for use in subsequent experiments.
3.2.2. Synthesis of Bi5O7I Nanosheets (Bi5O7I NS) Photocatalyst
Bi(NO3)3•5H2O (4.000 g) was added to 60 mL of ethylene glycol and dissolved for half an hour to obtain solution A. A total of 1.400 g of KI was dissolved in 60 mL of ethylene glycol to solution B. The prepared solution B was slowly added to solution A to obtain a mixed solution, which was stirred for 30 min. The suspension was transferred to a 150 mL polytetrafluoroethylene autoclave and kept at 180 °C for 18 h. After the solution was cooled down, it was washed by centrifugation alternately with hot water and ethanol at high speed for two times, and then dried in an oven at 60 °C for 12 h to obtain the Bi5O7I NS photocatalyst precursor. The dried solid powder of Bi5O7I NS photocatalyst precursor was put into a tube furnace for secondary calcination. The tube furnace was heated up to 450 °C at a rate of 5 °C/min, and the solid was held at 450 °C for 3 h. After cooling, it was taken out of the furnace, and the prepared Bi5O7I NS were milled and collected for use in subsequent experiments.
3.2.3. Synthesis of Bi5O7I Nanoballs (Bi5O7I NB) Photocatalyst
Bi(NO3)3•5H2O (1.382 g) was weighed and dissolved in 25 mL of ethylene glycol and stirred for 30 min to obtain solution A. At the same time, 0.498 g of KI was also dissolved in 25 mL of ethylene glycol and stirred for 30 min to obtain solution B. Prepared solution B was also added slowly into solution A to obtain a mixed solution, which was stirred for 1 h. After 60 min, the suspension was transferred to a 150 mL polytetrafluoroethylene autoclave and laid in an oven at 180 °C for 12 h. After being left to cool, it was washed by centrifugation alternately with deionised water and ethanol for three times at high speed, and then dried in an oven at 60 °C for 12 h to obtain the Bi5O7I NB photocatalyst precursor. The dried solid powder of Bi5O7I NB photocatalyst precursor was put into a muffle furnace for secondary calcination, which was held at 450 °C for 2 h at a temperature increase rate of 5 °C/min, and then taken out after cooling, and the prepared Bi5O7I NB were milled and collected for use in subsequent experiments.
The synthesis pathways for the three materials are shown in Scheme 1.

Scheme 1.
Scheme of preparation of Bi5O7I NB, Bi5O7I NT, and Bi5O7I NS.
3.3. Characterization Techniques and Degradation Testing
In this experiment, XRD, XPS, SEM, TEM, UV-Vis, PL, and I-t were used to compare the morphological characteristics as well as photochemical properties of Bi5O7I NB, Bi5O7I NT, and Bi5O7I NS. The specific characterisation tests in this paper were showed in Supporting Information.
4. Conclusions
In conclusion, Bi5O7I NB, Bi5O7I NS, and Bi5O7I NT photocatalysts were successfully synthesised using a very facile experimental synthesis method. Compared with Bi5O7I NS and Bi5O7I NT, the Bi5O7I NB possessed better photocatalytic properties and showed good degradation under visible light irradiation, reaching a very high photodegradation rate of 98.00% for RhB after 100 min. At the same time, the degradation rate can be maintained at a high level after several cycles, with high stability and recyclability, which is conducive to our industrial use. With the solution of RhB (10 mg/L) at a concentration of 50 mL, 25 mg Bi5O7I NB and 50 mL 30% H2O2 solution were added as an activator, optimisation experiments were also carried out. In free radical trapping experiments, it was founded that the main catalytic roles were h+ and 1O2. The mechanism and pathways of the RhB degradation were discussed. In this research, Bi5O7I NB has the potential to be an effective catalyst for organic pollutants degradation and environmental remediation. Bi5O7I NB has great potential for future applications in wastewater treatment, particularly for degrading organic pollutants such as RhB. Their unique properties, such as high photocatalytic activity and stability under light irradiation, make them effective in breaking down these contaminants, which offers a sustainable method for purifying wastewater.
Supplementary Materials
The following are available online at: https://www.mdpi.com/article/10.3390/catal15080714/s1. The reuse studies, Figure S1: influences of dark adsorption, degradation ratio of Bi5O7I NT, Bi5O7I NS and Bi5O7I NB in 30 min; Figure S2: reuse performance of Bi5O7I NB in four cycles; Table S1: the degradation time and degradation rate in this experiment compared with previous papers; Figure S3: UV-vis DRS of Bi5O7I NT, Bi5O7I NS and Bi5O7I NB; Table S2: the possible intermediates of RhB degradation. References [62,63,64,65,66,67] are cited in the supplementary materials.
Author Contributions
Conceptualization, methodology, formal analysis, and writing—original draft preparation were done by X.Y.; material preparation and characterization were done by J.L., L.Z., Q.W., F.W., Y.P., and M.Z.; manuscript review, supervision, and funding acquisition were done by G.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Opening Project of Guangxi Key Laboratory of Petrochemical Resource Processing and Process Intensification Technology (2023K004), Natural Science Foundation of Jiangsu Province in China (BK20230410), Natural Science Research of Jiangsu Higher Education Institution of China (23KJB610010), The National College Students Innovation and Entrepreneurship Training Program (2023NFUSPITP0614). Thanks Shiyanjia Lab (https://www.shiyanjia.com, access on 25 June 2025) for help with testing.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Rojas, S.; Horcajada, P. Metal–Organic Frameworks for the Removal of Emerging Organic Contaminants in Water. Chem. Rev. 2020, 120, 8378–8415. [Google Scholar] [CrossRef]
- Hu, A.; Jang, K.-S.; Tanentzap, A.J.; Zhao, W.; Lennon, J.T.; Liu, J.; Li, M.; Stegen, J.; Choi, M.; Lu, Y.; et al. Thermal Responses of Dissolved Organic Matter under Global Change. Nat. Commun. 2024, 15, 576. [Google Scholar] [CrossRef]
- Sousa, J.C.G.; Ribeiro, A.R.; Barbosa, M.O.; Pereira, M.F.R.; Silva, A.M.T. A Review on Environmental Monitoring of Water Organic Pollutants Identified by EU Guidelines. J. Hazard. Mater. 2018, 344, 146–162. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jia, Y.; Sun, Z.; Su, J.; Liu, Q.S.; Zhou, Q.; Jiang, G. Environmental Pollution, a Hidden Culprit for Health Issues. Eco-Environ. Health 2022, 1, 31–45. [Google Scholar] [CrossRef]
- Shobier, A.H.; Shabaka, S.H.; El-Sayed, A.A.M.; Shreadah, M.A.; Abdel Ghani, S.A. Assessment of Persistent and Emerging Pollutants Levels in Marine Bivalves in the Gulf of Suez, Egypt. Mar. Pollut. Bull. 2024, 208, 117000. [Google Scholar] [CrossRef]
- Lops, C.; Ancona, A.; Di Cesare, K.; Dumontel, B.; Garino, N.; Canavese, G.; Hérnandez, S.; Cauda, V. Sonophotocatalytic Degradation Mechanisms of Rhodamine B Dye via Radicals Generation by Micro- and Nano-Particles of ZnO. Appl. Catal. B Environ. 2019, 243, 629–640. [Google Scholar] [CrossRef]
- Oviedo, L.R.; Druzian, D.M.; Nora, L.D.D.; Da Silva, W.L. Study of Machine Learning on the Photocatalytic Activity of a Novel Nanozeolite for the Application in the Rhodamine B Dye Degradation. Catal. Today 2025, 443, 114986. [Google Scholar] [CrossRef]
- Mei, J.; Tao, Y.; Gao, C.; Zhu, Q.; Zhang, H.; Yu, J.; Fang, Z.; Xu, H.; Wang, Y.; Li, G. Photo-Induced Dye-Sensitized BiPO4/BiOCl System for STablely Treating Persistent Organic Pollutants. Appl. Catal. B Environ. 2021, 285, 119841. [Google Scholar] [CrossRef]
- Saravanan, S.; Carolin C, F.; Kumar, P.S.; Chitra, B.; Rangasamy, G. Biodegradation of Textile Dye Rhodamine-B by Brevundimonas Diminuta and Screening of Their Breakdown MeTableolites. Chemosphere 2022, 308, 136266. [Google Scholar] [CrossRef]
- Tirkey, N.; Mishra, S. Evaluation of Neem Gum-Poly(Acrylic Acid) Based Adsorbent for Cationic Dye Removal Using Adsorption Isotherm, Kinetics and Thermodynamics: Linear Regression Models. Int. J. Biol. Macromol. 2025, 307, 142059. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wu, Y.; Jiang, Q.; Sun, S.; Wang, J.; Gao, Y.; Zhang, W.; Du, Q.; Song, X. Pyrolyzed Sediment Accelerates Electron Transfer and Regulates Rhodamine B Biodegradation. Sci. Total Environ. 2023, 905, 167126. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Guo, H.; Chai, C.; Li, N.; Lin, X.; Xu, W. Anodized Graphite Felt as an Efficient Cathode for In-Situ Hydrogen Peroxide Production and Electro-Fenton Degradation of Rhodamine B. Chemosphere 2022, 286, 131936. [Google Scholar] [CrossRef]
- Wang, T.; Wang, W.; Zhu, Y.; Zhang, B. Electro-Fenton Degradation of Rhodamine B with in-Situ H2O2 Generation by Au Nanoparticles Modified Reduced Graphene Oxide. J. Environ. Chem. Eng. 2025, 13, 115685. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, T.; Wang, X.; Zhang, L.; Zhao, C.; Wu, W.; Zhu, G.; Jia, Y. Natural Tourmaline for Pyroelectric Dye Decomposition under 25–60 °C Room-Temperature Cold-Hot Fluctuation. Sep. Purif. Technol. 2023, 327, 124971. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, L.; Lyu, Y.; Zhang, J.; Wu, F.; Zhang, X.; Xiong, W.; He, B.; Yi, H. Adsorption Mechanism of Rhodamine Wastewater in the MOFs Materials: Effect of Metal Center and Organic Ligand. Sep. Purif. Technol. 2025, 363, 132056. [Google Scholar] [CrossRef]
- Pedebos, M.E.S.; Druzian, D.M.; Oviedo, L.R.; Ruiz, Y.P.M.; Galembeck, A.; Pavoski, G.; Espinosa, D.C.R.; Da Silva, W.L. Removal of Rhodamine B Dye by Adsorption onto an Eco-Friendly Zeolite and Machine Learning Modeling. J. Photochem. Photobiol. Chem. 2024, 449, 115404. [Google Scholar] [CrossRef]
- Li, G.; Cai, Z.; Su, K.; Zhao, Y.; Zhu, Y.; Han, J.; Pan, Y.; Xing, W.; Wu, G. Peroxymonosulfate Activation with Co3O4 by Microstructure Engineering for Efficient Degradation of Tetracycline: Efficiency, Mechanism and Stableility. Colloids Surf. Physicochem. Eng. Asp. 2023, 677, 132353. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Li, G.; Han, F.; Hu, D.; Huang, X.; Yuan, H.; Tan, Y. Hollow Co/CoO/Carbon Nanofibers Promoted PMS Decomposition for the Degradation of Rhodamine B. J. Mater. Sci. Technol. 2023, 157, 120–129. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, X.; Cheng, Z.; Xu, Q.; Zhang, X.; Liu, Y.; Qiu, F. Peroxymonosulfate (PMS) Activated by Magnetic Fe3O4 Doped Carbon Quantum Dots (CQDs) for Degradation of Rhodamine B (RhB) under Visible Light: DFT Calculations and Mechanism Analysis. J. Clean. Prod. 2023, 426, 139202. [Google Scholar] [CrossRef]
- Song, T.; Li, G.; Hu, R.; Liu, Y.; Liu, H.; Gao, Y. Degradation of Antibiotics via UV-Activated Peroxodisulfate or Peroxymonosulfate: A Review. Catalysts 2022, 12, 1025. [Google Scholar] [CrossRef]
- Zhang, F.; Cheng, W.; Yu, Z.; Ge, S.; Shao, Q.; Pan, D.; Liu, B.; Wang, X.; Guo, Z. Microwave Hydrothermally Synthesized WO3/UiO-66 Nanocomposites toward Enhanced Photocatalytic Degradation of Rhodamine B. Adv. Compos. Hybrid Mater. 2021, 4, 1330–1342. [Google Scholar] [CrossRef]
- Asgari, S.; Mohammadi Ziarani, G.; Badiei, A.; Vasseghian, Y. Zr-UiO-66, Ionic Liquid (HMIM+TFSI−), and Electrospun Nanofibers (Polyacrylonitrile): All in One as a Piezo-Photocatalyst for Degradation of Organic Dye. Chem. Eng. J. 2024, 487, 150600. [Google Scholar] [CrossRef]
- Qaraah, F.A.; Mahyoub, S.A.; Hezam, A.; Qaraah, A.; Drmosh, Q.A.; Xiu, G. Construction of 3D Flowers-like O-Doped g-C3N4-[N-Doped Nb2O5/C] Heterostructure with Direct S-Scheme Charge Transport and Highly Improved Visible-Light-Driven Photocatalytic Efficiency. Chin. J. Catal. 2022, 43, 2637–2651. [Google Scholar] [CrossRef]
- Qiu, L.; Li, H.; Xu, W.; Zhu, R.; Ouyang, F. TiO2 Catalysts Co-Modified with Bi, F, SnO2, and SiO2 for Photocatalytic Degradation of Rhodamine B Under Simulated Sunlight. Catalysts 2024, 14, 735. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.W. Photocatalytic Degradation of Eriochrome Black-T Using BaWO4/MoS2 Composite. Catalysts 2022, 12, 1290. [Google Scholar] [CrossRef]
- Akti, F. Photocatalytic Degradation of Remazol Yellow Using Polyaniline–Doped Tin Oxide Hybrid Photocatalysts with Diatomite Support. Appl. Surf. Sci. 2018, 455, 931–939. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, S.; Liu, J.; Zhang, Y.; Wang, Y.; Yu, J.; Yuan, M.; Zhang, P.; Liu, W.; Zhang, J. C, F Co-Doping Ag/TiO2 with Visible Light Photocatalytic Performance toward Degrading Rhodamine B. Environ. Res. 2023, 232, 116311. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Chen, B.B.; Chang, S.; Lv, J.; Li, D.W. Room-Temperature Growth of Morphoplastic Scandium Nanowires through Coordination-Induced Self-Assembly for White-Light-Driven Photocatalytic Degradation of Rhodamine. ACS Appl. Nano Mater. 2023, 6, 11747–11753. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Huang, J.; Li, S.; Meng, A.; Li, Z. Interfacial Chemical Bond and Internal Electric Field Modulated Z-Scheme Sv-ZnIn2S4/MoSe2 Photocatalyst for Efficient Hydrogen Evolution. Nat. Commun. 2021, 12, 4112. [Google Scholar] [CrossRef]
- Hassan, G.K.; Mahmoud, W.H.; Al-sayed, A.; Ismail, S.H.; El-Sherif, A.A.; Abd El Wahab, S.M. Multi-Functional of TiO2@Ag Core–Shell Nanostructure to Prevent Hydrogen Sulfide Formation during Anaerobic Digestion of Sewage Sludge with Boosting of Bio-CH4 Production. Fuel 2023, 333, 126608. [Google Scholar] [CrossRef]
- Viruthagiri, G.; Kannan, P.; Shanmugam, N. Photocatalytic Rendition of Zn2+-Doped Bi2O3 Nanoparticles. Photonics Nanostruct.-Fundam. Appl. 2018, 32, 35–41. [Google Scholar] [CrossRef]
- Shang, J.; Chen, T.; Wang, X.; Sun, L.; Su, Q. Facile Fabrication and Enhanced Photocatalytic Performance: From BiOCl to Element-Doped BiOCl. Chem. Phys. Lett. 2018, 706, 483–487. [Google Scholar] [CrossRef]
- Yang, J.; Xu, L.; Liu, C.; Xie, T. Preparation and Photocatalytic Activity of Porous Bi5O7I Nanosheets. Appl. Surf. Sci. 2014, 319, 265–271. [Google Scholar] [CrossRef]
- Ding, H.; Guan, Y.; Wang, Z.; Yamauchi, Y.; Asakura, Y.; Han, Q. Effect of Phase Structure of Bi5O7I on the Photocatalytic Activity of S-Scheme AgBr/Ag/Bi5O7I. J. Alloys Compd. 2024, 1002, 175215. [Google Scholar] [CrossRef]
- Li, X.; Chen, T.; Lin, H.; Cao, J.; Huang, H.; Chen, S. Intensive Photocatalytic Activity Enhancement of Bi5O7I via Coupling with Band Structure and Content AdjusTablele BiOBrxI1-x. Sci. Bull. 2018, 63, 219–227. [Google Scholar] [CrossRef]
- Yu, H.; He, Z.; Zhang, Y.; Ng, L.S.; Ni, J.; Guo, F.; Hu, J.; Lee, H.K.; Han, J. In Situ Reversible Assembly of Atomic Interfacial Structure in BiOI/Bi5O7I p-n Heterojunctions to Promote Visible-Light Photocatalysis. Chem. Eng. J. 2024, 481, 148350. [Google Scholar] [CrossRef]
- Wu, H.; Peng, J.; Sun, H.; Ruan, Q.; Dong, H.; Jin, Y.; Sun, Z.; Hu, Y. Surface Activation of Calcium Tungstate by Europium Doping for Improving Photocatalytic Performance: Towards Lanthanide Site Photocatalysis. Chem. Eng. J. 2022, 432, 134339. [Google Scholar] [CrossRef]
- Siao, C.W.; Chen, H.L.; Chen, L.W.; Chang, J.L.; Yeh, T.W.; Chen, C.C. Controlled Hydrothermal Synthesis of Bismuth Oxychloride/Bismuth Oxybromide/Bismuth Oxyiodide Composites Exhibiting Visible-Light Photocatalytic Degradation of 2-Hydroxybenzoic Acid and Crystal Violet. J. Colloid Interface Sci. 2018, 526, 322–336. [Google Scholar] [CrossRef]
- He, D.; Wang, C.; Zhao, R.; Lu, X.; Yang, M.; Qiu, J.; Wang, K.; Wang, C. BiOX (X = Cl, Br, I)/WO3/Polyacrylonitrile Nanofibrous Membranes for Diagnostic X-Ray Shielding and Visible-Light Photocatalysis. ACS Appl. Nano Mater. 2022, 5, 4157–4169. [Google Scholar]
- Zhou, Y.; Liang, Z.; Zheng, W.; Dong, J.; Chang, C.; Wang, Q.; Li, Y.; Liu, T.; Wen, J.; Zheng, X. Construction of G-C3N4-Loaded BiOCl0.5I0.5 Dual-Z-Scheme Heterojunction for Photocatalytic Reduction of CO2 and RhB Degradation. Colloids Surf. Physicochem. Eng. Asp. 2024, 702, 134968. [Google Scholar] [CrossRef]
- Wang, L.; Wang, P.; Huang, B.; Ma, X.; Wang, G.; Dai, Y.; Zhang, X.; Qin, X. Synthesis of Mn-Doped ZnS Microspheres with Enhanced Visible Light Photocatalytic Activity. Appl. Surf. Sci. 2017, 391, 557–564. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.; Lin, H.; Xu, B.; Luo, B.; Chen, S. Low Temperature Synthesis of Novel Rodlike Bi5O7I with Visible Light Photocatalytic Performance. Mater. Lett. 2012, 76, 181–183. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, Y.; Lin, S.; Hu, Y.; Tian, N.; Huang, H. Room-Temperature Controllable Synthesis of Bi5O7INanostrips for Improved Photocatalytic Activity. Colloids Surf. Physicochem. Eng. Asp. 2020, 594, 124642. [Google Scholar] [CrossRef]
- Hojamberdiev, M.; Zhu, G.; Li, S.; Zhang, Y.; Gao, J.; Zhu, R.; Zhang, F. Er3+-Doping Induced Formation of Orthorhombic/Monoclinic Bi5O7I Heterostructure with Enhanced Visible-Light Photocatalytic Activity for Removal of Contaminants. Mater. Res. Bull. 2020, 123, 110701. [Google Scholar] [CrossRef]
- Li, P.; Wu, Q.; Ji, Q.; Abdeta, A.B.; Kuo, D.-H.; Huang, T.; Zhang, H.; Zelekew, O.A.; Lin, J.; Chen, X. Sulfur-Doped Sb4Mo10O31 Bimetallic Sulfur-Oxide Catalyst for Highly Efficient Reduction of Toxic Organic and Hexavalent Chromium under Dark. J. Environ. Chem. Eng. 2023, 11, 110700. [Google Scholar] [CrossRef]
- Chen, C.C.; Fu, J.Y.; Chang, J.L.; Huang, S.T.; Yeh, T.W.; Hung, J.T.; Huang, P.H.; Liu, F.Y.; Chen, L.W. Bismuth Oxyfluoride/Bismuth Oxyiodide Nanocomposites Enhance Visible-Light-Driven Photocatalytic Activity. J. Colloid Interface Sci. 2018, 532, 375–386. [Google Scholar] [CrossRef]
- Su, Z.; Ye, C.; Xu, Y.; Wu, B.; Kuo, D.-H.; Wu, X.; Yang, B.; Zhang, P.; Chen, L.; Lu, D.; et al. Synergistic Vacancy Defects and the Surface Hydrophobic-to-Superhydrophilic Wetting Engineering in W/S Co-Doped BiOI for Enhanced Photocatalytic Hydrogen Evolution. Chem. Eng. J. 2024, 496, 154282. [Google Scholar] [CrossRef]
- Ptaszkowska-Koniarz, M.; Goscianska, J.; Pietrzak, R. Removal of Rhodamine B from Water by Modified Carbon Xerogels. Colloids Surf. A Physicochem. Eng. Asp. 2018, 543, 109–117. [Google Scholar] [CrossRef]
- Gu, J.; Luo, C.; Zhou, W.; Tong, Z.; Zhang, H.; Zhang, P.; Ren, X. Degradation of Rhodamine B in Aqueous Solution by Laser Cavitation. Ultrason. Sonochem. 2020, 68, 105181. [Google Scholar] [CrossRef] [PubMed]
- Hovan, A.; Sedláková, D.; Lee, O.S.; Bánó, G.; Sedlák, E. pH Modulates Efficiency of Singlet Oxygen Production by Flavin Cofactors. RSC Adv. 2024, 14, 28783–28790. [Google Scholar] [CrossRef]
- Mao, X.; Fan, C.; Zhang, X.; Wang, Y.; Wang, Y.; Ding, G. Effect of Chlorine Ion on the Crystalline and Photocatalytic Activity of BiOCl for the Degradation of Rhodamine B. Cryst. Res. Technol. 2013, 48, 496–504. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Huang, C.P.; Doong, R. Photocatalytic Degradation of Bisphenol A over a ZnFe2O4/TiO2 Nanocomposite under Visible Light. Sci. Total Environ. 2019, 646, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Zhang, J.B.; Gharbi, O.; Vivier, V.; Gao, M.; Orazem, M.E. Electrochemical Impedance Spectroscopy. Nat. Rev. Methods Primers 2021, 1, 42. [Google Scholar] [CrossRef]
- Manohar, A.; Suvarna, T.; Vattikuti, S.V.P.; Almunyif, A.A.; Sangaraju, S.; Kim, K.H. Characterization of a Synthesized Mg0.7Ni0.3Fe2O4/CeO2/MgFe2O4 Nanocomposite for Magnetic and Electrochemical Applications. Colloids Surf. Physicochem. Eng. Asp. 2025, 722, 137269. [Google Scholar] [CrossRef]
- Tang, J.; Zou, Z.; Ye, J. Photophysical and Photocatalytic Properties of AgInW2O8. J. Phys. Chem. B 2003, 107, 14265–14269. [Google Scholar] [CrossRef]
- Song, M.; Li, M.; Yang, X.; Zhang, K.; Qu, X.; Liu, P.; Wu, Y.; Li, L. Dual Transfer Channels in NFS-Supported Sandwich-like Bi4O5I2/Bi5O7I/g-C3N4 Z-Scheme Heterojunctions Facilitated Efficient Degradation of Diclofenac. J. Environ. Chem. Eng. 2025, 13, 116867. [Google Scholar] [CrossRef]
- Almenia, S.H.; Ismail, A.A.; Alzahrani, K.A.; Aljahdali, M. Li2MnO3 Nanoparticles Decorated with Co3O4 as Functional Visible-Light-Induced Heterojunction Photocatalysts for the Degradation of Tetracycline. J. Alloys Compd. 2023, 953, 170127. [Google Scholar] [CrossRef]
- Shi, H.; He, Y.; Li, Y.; He, T.; Luo, P. Efficient Degradation of Tetracycline in Real Water Systems by Metal-Free g-C3N4 Microsphere through Visible-Light Catalysis and PMS Activation Synergy. Sep. Purif. Technol. 2022, 280, 119864. [Google Scholar] [CrossRef]
- Mai, X.; Lin, W.; Chen, J.; Yang, Q.; Gao, R. Synthesis of Z-Scheme (001)-TiO2/Bi5O7I Heterojunctions with Enhanced Interfacial Charge Separation and Photocatalytic Degradation of Rhodamine B. React. Kinet. Mech. Catal. 2022, 135, 3447–3459. [Google Scholar] [CrossRef]
- Tan, J.; Xu, C.; Zhang, X.; Huang, Y. MOFs-Derived Defect Carbon Encapsulated Magnetic Metallic Co Nanoparticles Capable of Efficiently Activating PMS to Rapidly Degrade Dyes. Sep. Purif. Technol. 2022, 289, 120812. [Google Scholar] [CrossRef]
- Zhou, W.; Hong, C.; Wang, W.; Long, S.; Jing, C.; Lin, Y.; Wu, L. Novel MIL-53(Fe)/SnS2 Z-Scheme Heterojunction as Photoactivator of Peroxymonosulfate for Efficient Degradation of RhB. J. Alloys Compd. 2025, 1027, 180627. [Google Scholar] [CrossRef]
- Chandrapal, R.R.; Bharathi, K.; Bakiyaraj, G.; Bharathkumar, S.; Priyajanani, Y.; Manivannan, S.; Archana, J.; Navaneethan, M. Harnessing ZnCr2O4/g-C3N4 nanosheet heterojunction for enhanced photocatalytic degradation of rhodamine B and ciprofloxacin. Chemosphere 2024, 350, 141094. [Google Scholar] [CrossRef]
- Wei, J.; Liang, Z.; Hao, L.; Yu, Y.; Hou, H.; Chen, C.; Qian, G.; Min, D. Oxygen vacancies and heterojunction synergistically enhancing degradation of rhodamine B by UiO-66-NH2 derived photocatalyst. J. Water Process Eng. 2025, 69, 106830. [Google Scholar] [CrossRef]
- Osanloo, M.; Khorasheh, F.; Larimi, A. Fabrication of nano-dandelion magnetic TiO2/CuFe2O4 doped with silver as a highly visible-light-responsive photocatalyst for degradation of Naproxen and Rhodamine B. J. Mol. Liq. 2024, 407, 125242. [Google Scholar] [CrossRef]
- Xi, H.; Wang, H.; Liu, D.; Mu, Q.; Xu, X.; Kang, Q.; Yang, Y.; Yang, Z.; Lei, Z. Boosting the photocatalytic benzylamine oxidation and rhodamine B degradation using Z-scheme heterojunction of NiFe2O4/rGO/Bi2WO6. J. Alloys Compd. 2025, 1010, 177818. [Google Scholar] [CrossRef]
- Essenni, S.; Khan, M.A.; El Kaim Billah, R.; Jeon, B.H.; Sundaramurthy, S.; Agunaou, M. Template assisted hydrothermal synthesis of bismuth vanadate for Rhodamine B photodegradation. J. Mol. Liq. 2024, 398, 124270. [Google Scholar] [CrossRef]
- Shi, K.; Li, X.; Tian, Z.; Luo, Y.; Ding, R.; Zhu, Y.; Yao, H. Synergistic and efficient photocatalytic degradation of rhodamine B and tetracycline in wastewater based on novel S-scheme heterojunction phosphotungstic Acid@MIL-101(Cr). J. Environ. Manag. 2025, 373, 123716. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).