Green Synthesis of TiO2-CeO2 Nanocomposites Using Plant Extracts for Efficient Organic Dye Photodegradation
Abstract
1. Introduction
2. Results and Discussion
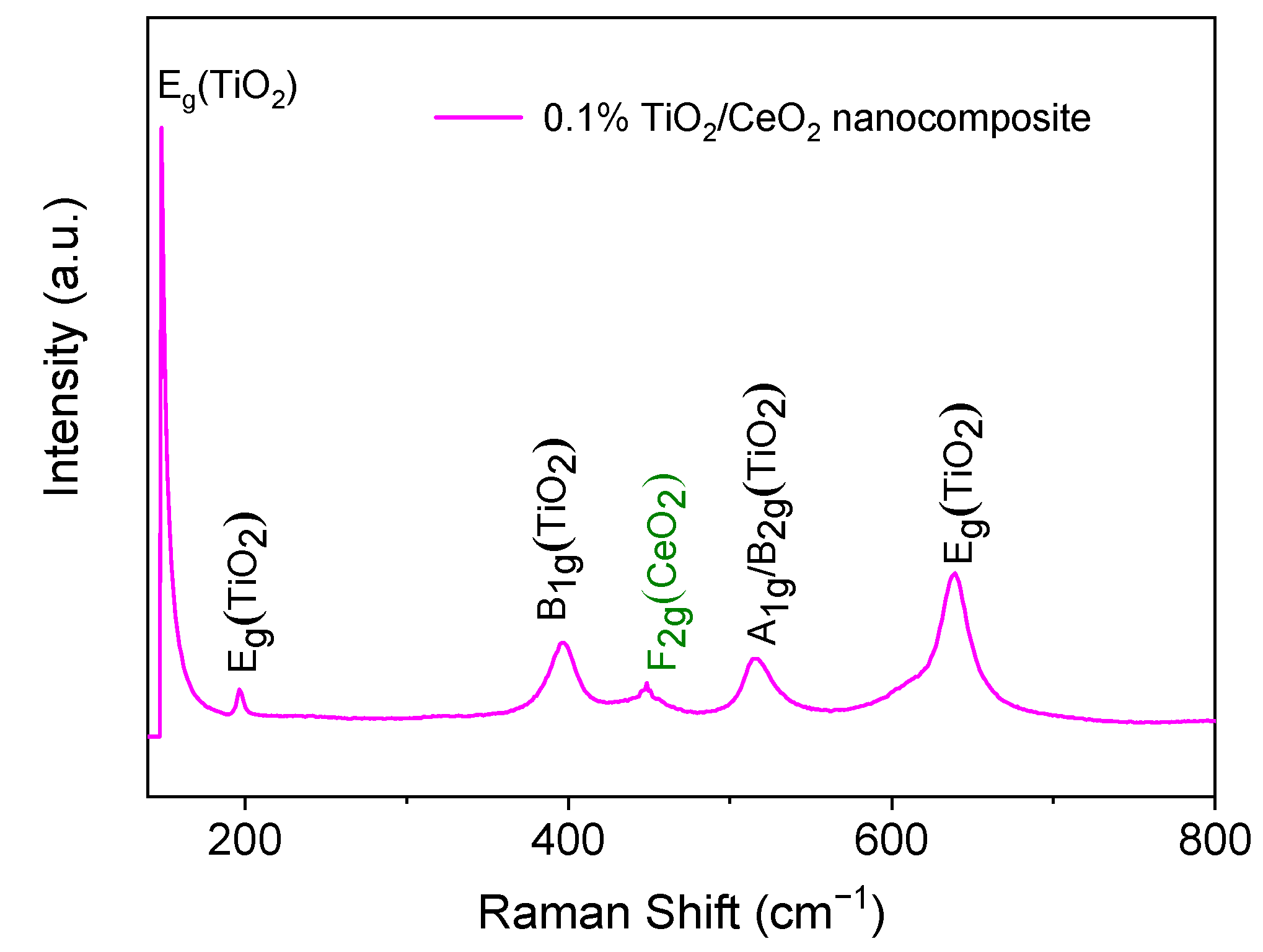
2.1. Morphological and Microstructural Characterization
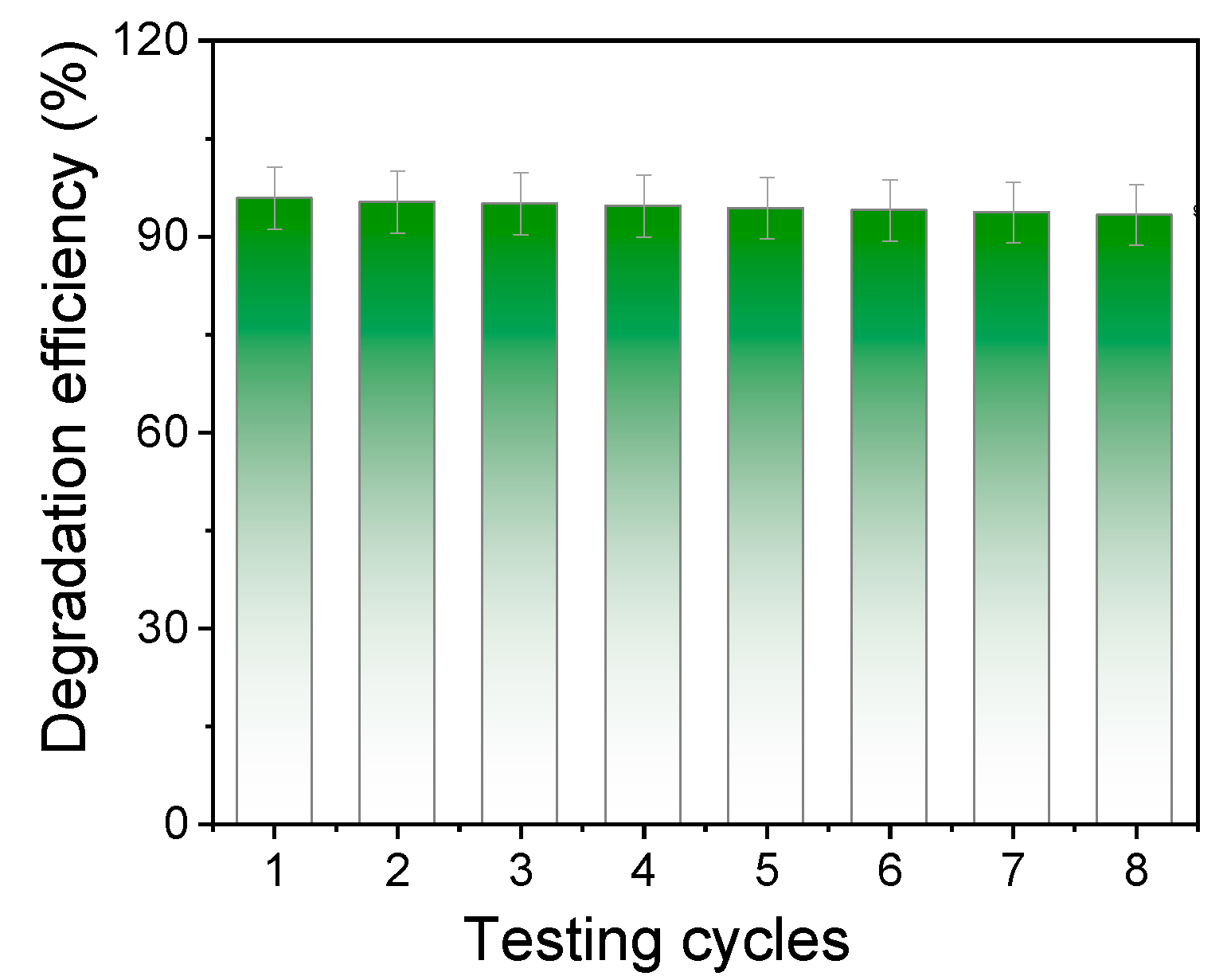
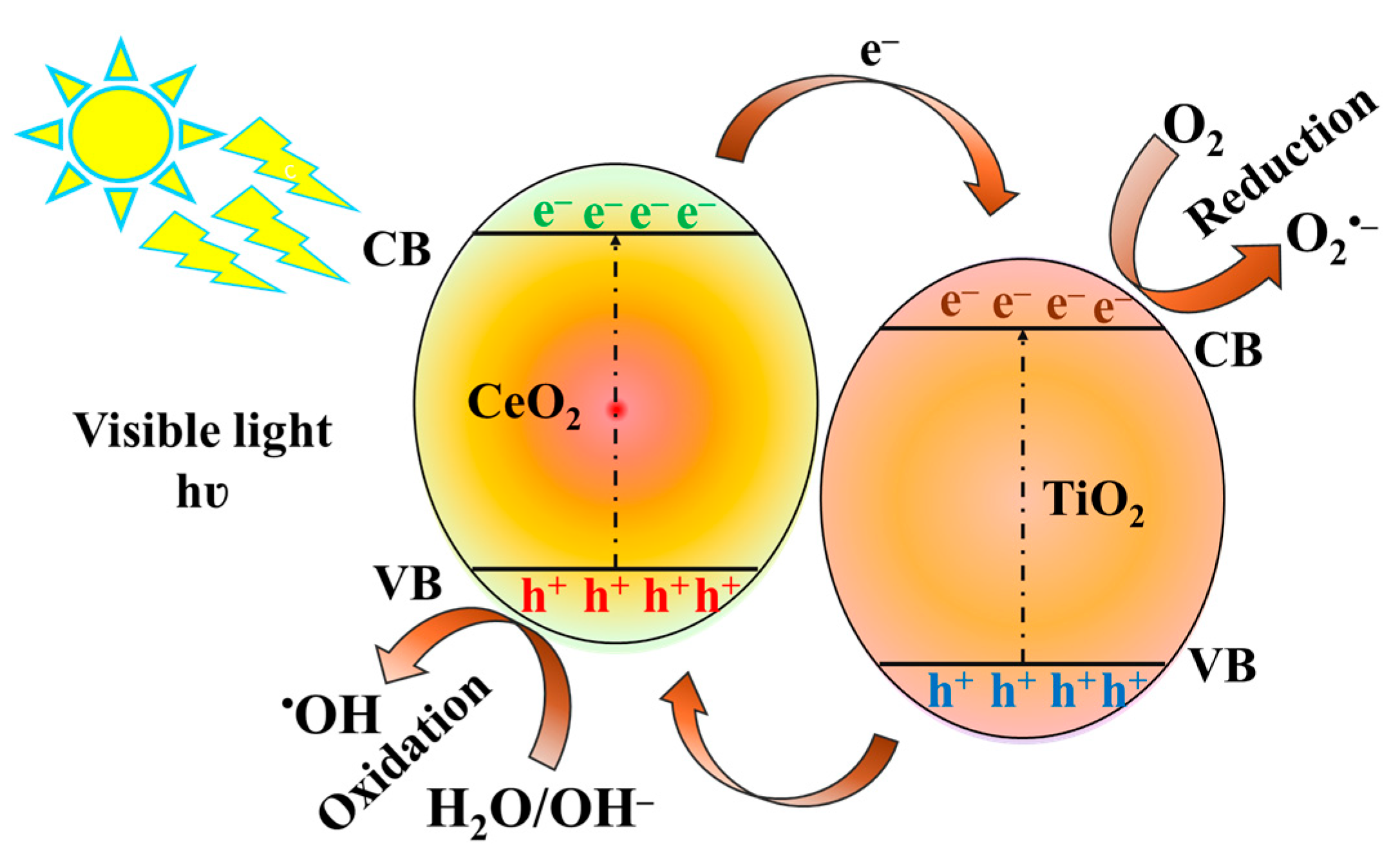
2.2. Evaluation of the Photocatalytic Degradation Efficiency of TiO2-CeO2 Nanoparticles on Methylene Blue
3. Materials and Methods
3.1. Materials
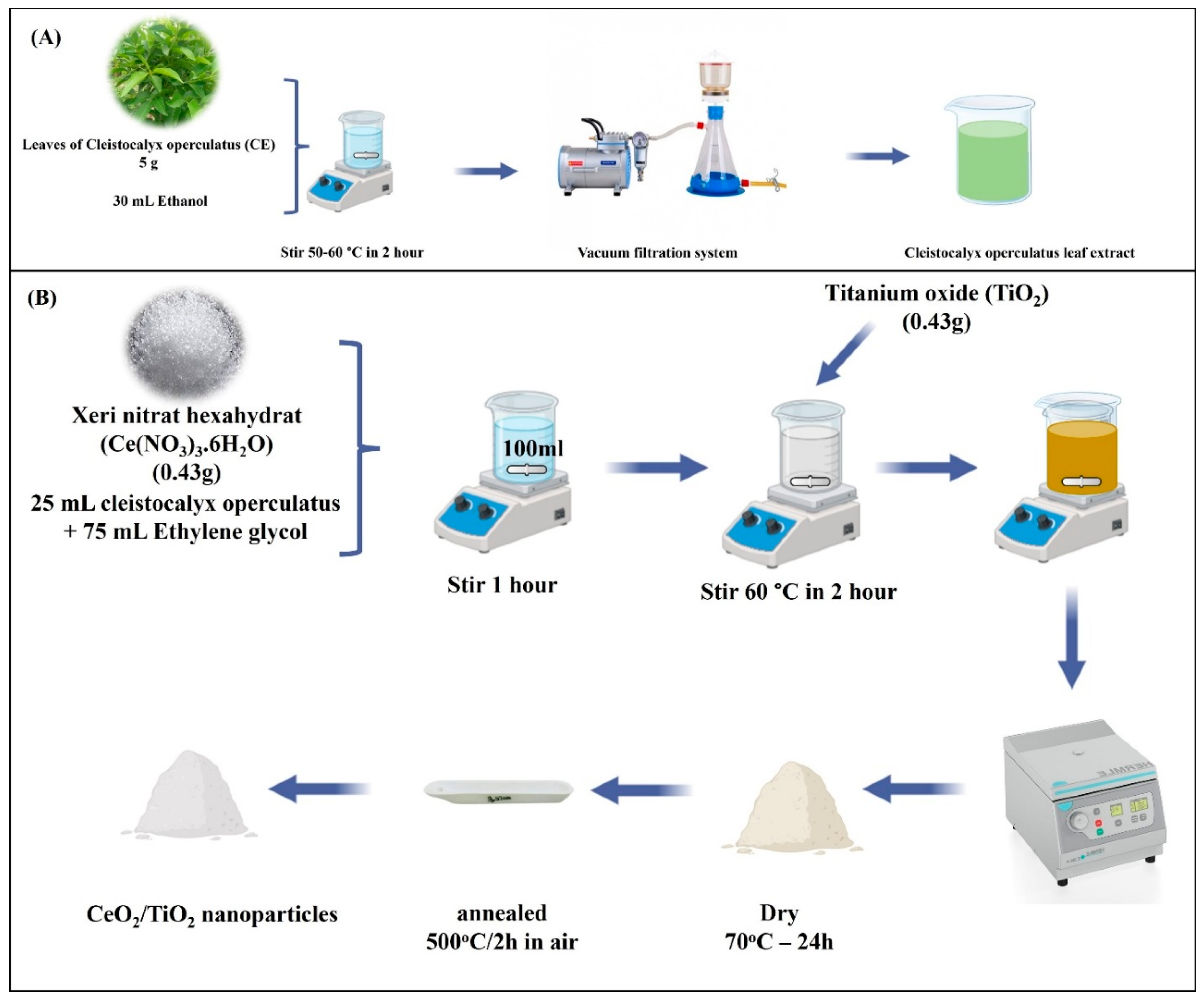
3.2. Synthesis of Cleistocalyx Operculatus Leaf Extract
3.3. Synthesis of TiO2-CeO2 Nanoparticles
3.4. Material Characterization
3.5. Photocatalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Din, M.I.; Khalid, R.; Najeeb, J.; Hussain, Z. Fundamentals and photocatalysis of methylene blue dye using various nanocatalytic assemblies- a critical review. J. Clean. Prod. 2021, 298, 126567. [Google Scholar] [CrossRef]
- Aruchamy, K.; Sudarsan, D.; Ajith, M.; Sreekumar, A.A.M.; Ayyasamy, U.M.; Manickam, S. Enhanced photocatalytic activity of V3O7/V2O5—Reduced graphene oxide nanocomposite towards methylene blue dye degradation. Environ. Sci. Pollut. Res. 2024, 31, 20983–20998. [Google Scholar] [CrossRef] [PubMed]
- Begum, R.; Najeeb, J.; Sattar, A.; Naseem, K.; Irfan, A.; Al-Sehemi, A.G.; Farooqi, Z.H. Chemical reduction of methylene blue in the presence of nanocatalysts: A critical review. Rev. Chem. Eng. 2020, 36, 749–770. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Ajiboye, T.O.; Omotola, E.O.; Oyewola, O.J. Methylene blue dye: Toxicity and potential elimination technology from wastewater. Results Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- Roguai, S.; Djelloul, A. Structural, microstructural and photocatalytic degradation of methylene blue of zinc oxide and Fe-doped ZnO nanoparticles prepared by simple coprecipitation method. Solid State Commun. 2021, 334–335, 114362. [Google Scholar] [CrossRef]
- Ho, Y.-C.; Chua, S.-C.; Chong, F.-K. Coagulation-Flocculation Technology in Water and Wastewater Treatment; IGI Global: Hershey, PA, USA, 2020. [Google Scholar] [CrossRef]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.N.; Pandith, A.H. Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ. Chem. Lett. 2018, 17, 729–754. [Google Scholar] [CrossRef]
- Santoso, E.; Ediati, R.; Kusumawati, Y.; Bahruji, H.; Sulistiono, D.O.; Prasetyoko, D. Review on recent advances of carbon based adsorbent for methylene blue removal from waste water. Mater. Today Chem. 2020, 16, 100233. [Google Scholar] [CrossRef]
- Li, N.; Lu, X.; He, M.; Duan, X.; Yan, B.; Chen, G.; Wang, S. Catalytic membrane-based oxidation-filtration systems for organic wastewater purification: A review. J. Hazard. Mater. 2021, 414, 125478. [Google Scholar] [CrossRef] [PubMed]
- Pronk, W.; Ding, A.; Morgenroth, E.; Derlon, N.; Desmond, P.; Burkhardt, M.; Wu, B.; Fane, A.G. Gravity-driven membrane filtration for water and wastewater treatment: A review. Water Res. 2019, 149, 553–565. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Varjani, S.; Jeevanantham, S.; Yaashikaa, P.R.; Thamarai, P.; Abirami, B.; George, C.S. A review on algal-bacterial symbiotic system for effective treatment of wastewater. Chemosphere 2021, 271, 129540. [Google Scholar] [CrossRef]
- Aditya, L.; Mahlia, T.M.I.; Nguyen, L.N.; Vu, H.P.; Nghiem, L.D. Microalgae-bacteria consortium for wastewater treatment and biomass production. Sci. Total Environ. 2022, 838, 155871. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.S.; Khoo, K.S.; Chew, K.W.; Ling, T.C.; Show, P.L. Recent advances biodegradation and biosorption of organic compounds from wastewater: Microalgae-bacteria consortium—A review. Bioresour. Technol. 2022, 344, 126159. [Google Scholar] [CrossRef] [PubMed]
- Malekkiani, M.; Ravari, F.; Heshmati Jannat Magham, A.; Dadmehr, M.; Groiss, H.; Hosseini, H.A.; Sharif, R. Fabrication of Graphene-Based TiO2@CeO2 and CeO2@TiO2 Core–Shell Heterostructures for Enhanced Photocatalytic Activity and Cytotoxicity. ACS Omega 2022, 7, 30601–30621. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Thampi, K.R.; Tayade, R.J. Visible light driven redox-mediator-free dual semiconductor photocatalytic systems for pollutant degradation and the ambiguity in applying Z-scheme concept. Appl. Catal. B Environ. 2018, 227, 296–311. [Google Scholar] [CrossRef]
- Sari, Y.; Gareso, P.L.; Armynah, B.; Tahir, D. A review of TiO2 photocatalyst for organic degradation and sustainable hydrogen energy production. Int. J. Hydrog. Energy 2024, 55, 984–996. [Google Scholar] [CrossRef]
- Güell, F.; Galdámez-Martínez, A.; Martínez-Alanis, P.R.; Catto, A.C.; da Silva, L.F.; Mastelaro, V.R.; Santana, G.; Dutt, A. ZnO-based nanomaterials approach for photocatalytic and sensing applications: Recent progress and trends. Mater. Adv. 2023, 4, 3685–3707. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Metal oxides (ZnO, CuO and NiO)-based nanostructured materials for photocatalytic remediation of organic contaminants. Nanotechnol. Environ. Eng. 2022, 8, 219–235. [Google Scholar] [CrossRef]
- Liu, W.; Liu, N.; Song, K.; Zeng, M.; Lu, Z. Fabrication of WO3 photocatalyst by plasma assisted ball milling under different discharge atmospheres. Vacuum 2024, 220, 112809. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, C.; Yuan, J.; Wang, Z.; Wang, Y.; Zhou, S.; Gu, P.; Li, Y. Extended construction strategies of Ag3PO4-based heterojunction photocatalysts for robust environmental applications. J. Environ. Chem. Eng. 2023, 11, 110705. [Google Scholar] [CrossRef]
- Ren, Y.; Li, Y.; Pan, G.; Wang, N.; Xing, Y.; Zhang, Z. Recent progress in CdS-based S-scheme photocatalysts. J. Mater. Sci. Technol. 2024, 171, 162–184. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Sharma, P.K.; Cortes, M.A.L.R.M.; Hamilton, J.W.J.; Han, Y.; Byrne, J.A.; Nolan, M. Surface modification of TiO2 with copper clusters for band gap narrowing. Catal. Today 2019, 321–322, 9–17. [Google Scholar] [CrossRef]
- Etshindo, L.A.; Sousa, C.; Tamiasso-Martinhon, P.; Colaço, M.V.; Camara, A.R.; Rocha, A.S. SnO2-TiO2 materials for photocatalytic degradation of cationic dye under UV and visible light and a chitosan composite film investigation. Catal. Today 2025, 444, 114995. [Google Scholar] [CrossRef]
- Ghamarpoor, R.; Fallah, A.; Jamshidi, M. A Review of Synthesis Methods, Modifications, and Mechanisms of ZnO/TiO2-Based Photocatalysts for Photodegradation of Contaminants. ACS Omega 2024, 9, 25457–25492. [Google Scholar] [CrossRef]
- Ma, B.; Zha, Y.; Xu, R.; Li, J.; Guo, Y.; Liu, J.; Wang, S.; Yan, B.; Lan, Y.; Zhao, B.; et al. Hollow α-Bi2O3/TiO2 nanotube arrays hierarchical heterojunction with strong interface interaction for efficient photocatalytic ciprofloxacin degradation. J. Water Process Eng. 2024, 60, 105134. [Google Scholar] [CrossRef]
- Alamgir; Ullah, R.; Talha, K.; Yang, H.; Mushtaq, N.; Ahmad, A.; Fan, L.; Li, L.; Zhao, G.; Wang, X.; et al. MOF-derived In2O3/TiO2 S-scheme heterojunction for efficient photocatalytic degradation of tetracycline. J. Alloys Compd. 2024, 1002, 175398. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, S.; Wen, T.; Gu, P.; Li, L.; Zhao, G.; Niu, F.; Huang, Q.; Tang, Z.; Wang, X. A critical review on visible-light-response CeO2-based photocatalysts with enhanced photooxidation of organic pollutants. Catal. Today 2019, 335, 20–30. [Google Scholar] [CrossRef]
- Hoque, K.A.; Sathi, S.A.; Akter, F.; Akter, T.; Ahmed, T.; Ullah, W.; Arafin, K.; Rahaman, M.S.; Shahadat, H.M.; Imran, A.B.; et al. Recent advances on photocatalytic CO2 reduction using CeO2-based photocatalysts: A review. J. Environ. Chem. Eng. 2024, 12, 113487. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, H.; Liu, J.; Liu, X.; Song, X.; Zhou, W.; Zhang, J.; Huo, P. Enhanced degradation of polyethylene terephthalate plastics by CdS/CeO2 heterojunction photocatalyst activated peroxymonosulfate. J. Hazard. Mater. 2023, 452, 131375. [Google Scholar] [CrossRef]
- Tuyen, L.T.T.; Quang, D.A.; Tam Toan, T.T.; Tung, T.Q.; Hoa, T.T.; Mau, T.X.; Khieu, D.Q. Synthesis of CeO2/TiO2 nanotubes and heterogeneous photocatalytic degradation of methylene blue. J. Environ. Chem. Eng. 2018, 6, 5999–6011. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Y.; Szeto, W.; Pan, W.; Huang, H.; Leung, D.Y.C. Vacuum ultraviolet (VUV)-based photocatalytic oxidation for toluene degradation over pure CeO2. Chem. Eng. Sci. 2019, 200, 203–213. [Google Scholar] [CrossRef]
- Stefa, S.; Lykaki, M.; Fragkoulis, D.; Binas, V.; Pandis, P.K.; Stathopoulos, V.N.; Konsolakis, M. Effect of the Preparation Method on the Physicochemical Properties and the CO Oxidation Performance of Nanostructured CeO2/TiO2 Oxides. Processes 2020, 8, 847. [Google Scholar] [CrossRef]
- Kumar Mandal, R.; Kumar Pradhan, S. Enhanced photocatalytic performance of cauliflower-like CeO2-TiO2 nanocomposite for the RhB dye degradation under visible light. Mater. Today Proc. 2022, 66, 3307–3314. [Google Scholar] [CrossRef]
- SenthilKumar, S.; Lellala, K.; Ashok, M.; Priyadharsan, A.; Sanjeeviraja, C.; Rajendran, A. Green synthesis of CeO2–TiO2 compound using Cleome chelidonii leaf extract for excellent photocatalytic activity. J. Mater. Sci. Mater. Electron. 2018, 29, 14022–14030. [Google Scholar] [CrossRef]
- Ramakrishnan, K.; Gayathri, V.; Aravinthkumar, K.; Ramachandran, K.; Ajitha, B.; Rameshbabu, M.; Sasiflorence, S.; Karazhanov, S.; Praba, K.; Raja Mohan, C. TiO2/CeO2 Core/Shell nanostructures for photocatalytic and photo electrochemical applications. Inorg. Chem. Commun. 2022, 144, 109842. [Google Scholar] [CrossRef]
- Cano-Franco, J.C.; Álvarez-Láinez, M. Effect of CeO2 content in morphology and optoelectronic properties of TiO2-CeO2 nanoparticles in visible light organic degradation. Mater. Sci. Semicond. Process. 2019, 90, 190–197. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Wandre, T.M.; Gaikwad, P.N.; Tapase, A.S.; Garadkar, K.M.; Vanalakar, S.A.; Lokhande, P.D.; Sasikala, R.; Hankare, P.P. Sol–gel synthesized TiO2-CeO2 nanocomposite: An efficient photocatalyst for degradation of methyl orange under sunlight. J. Mater. Sci. Mater. Electron. 2015, 27, 825–833. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, G.; He, R.; Huang, W.; Zhu, J.; Mao, C.; Wu, C.; Lu, G. Experimental preparation and optical properties of CeO2/TiO2 heterostructure. J. Mater. Res. Technol. 2020, 9, 9920–9928. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.M.; Cisneros, S.; Mosrati, J.; Atia, H.; Vuong, T.H.; Rockstroh, N.; Wohlrab, S.; Brückner, A.; Rabeah, J. Controlling Activity of Heterogeneous Cu Single-Atom Catalysts by Fine-Tuning the Redox Properties of CeO2-TiO2 Supports. ChemCatChem 2023, 15, e202201669. [Google Scholar] [CrossRef]
- Kumar, P.; Kaur, R.; Verma, S.; Singh, S.; Štangar, U.L. Tuning the catalytic active sites of TiO2/CeO2 interactions to improve oxygen vacancy and lattice parameters for CO2 conversion to green fuel additive. Catal. Today 2024, 433, 114652. [Google Scholar] [CrossRef]
- Wang, J.; Meng, F.; Xie, W.; Gao, C.; Zha, Y.; Liu, D.; Wang, P. TiO2/CeO2 composite catalysts: Synthesis, characterization and mechanism analysis. Appl. Phys. A 2018, 124, 645. [Google Scholar] [CrossRef]
- Sarvari, N.; Mohammadi, M.R. Influence of photoanode architecture on light scattering mechanism and device performance of dye-sensitized solar cells using TiO2 hollow cubes and nanoparticles. J. Taiwan Inst. Chem. Eng. 2018, 86, 81–91. [Google Scholar] [CrossRef]
- Kusuma, K.B.; Manju, M.; Ravikumar, C.R.; Raghavendra, N.; Amulya, M.A.S.; Nagaswarupa, H.P.; Murthy, H.C.A.; Kumar, M.R.A.; Shashi Shekhar, T.R. Photocatalytic degradation of Methylene Blue and electrochemical sensing of paracetamol using Cerium oxide nanoparticles synthesized via sonochemical route. Appl. Surf. Sci. Adv. 2022, 11, 100304. [Google Scholar] [CrossRef]
- Hkiri, K.; Elsayed Ahmed Mohamed, H.; Ghotekar, S.; Maaza, M. Green synthesis of cerium oxide nanoparticles using Portulaca oleracea Extract: Photocatalytic activities. Inorg. Chem. Commun. 2024, 162, 112243. [Google Scholar] [CrossRef]
- Ahmad, A.; Javed, M.S.; Khan, S.; Almutairi, T.M.; Mohammed, A.A.A.; Luque, R. Green synthesized Ag decorated CeO2 nanoparticles: Efficient photocatalysts and potential antibacterial agents. Chemosphere 2023, 310, 136841. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Özuğur Uysal, B.; Pekcan, Ö.; Erim, F.B. Efficient Photocatalytic Degradation of Methylene Blue Dye from Aqueous Solution with Cerium Oxide Nanoparticles and Graphene Oxide-Doped Polyacrylamide. ACS Omega 2023, 8, 13004–13015. [Google Scholar] [CrossRef]
- Kılıç Dokan, F.; Kuru, M. A new approach to optimize the synthesis parameters of TiO2 microsphere and development of photocatalytic performance. J. Mater. Sci. Mater. Electron. 2020, 32, 640–655. [Google Scholar] [CrossRef]
- Paramarta, V.; Taufik, A.; Saleh, R. Photocatalytic degradation of methylene blue (MB) by UV-light irradiation using SnO2/CeO2 modified nanographene platelets (NGP). J. Phys. Conf. Ser. 2020, 1442, 012005. [Google Scholar] [CrossRef]
- Husna, R.A.; Suherman; Natsir, T.A. Enhancing photocatalytic degradation of methylene blue by mixed oxides TiO2/SnO2/CeO2 under visible light. Results Eng. 2023, 19, 101253. [Google Scholar] [CrossRef]
- Khatibnezhad, H.; Ettouil, F.B.; Moreau, C. Photoactive Ce-doped TiO2 and CeO2-TiO2 composite coatings deposited by suspension/solution plasma spray. Mater. Res. Bull. 2023, 166, 112346. [Google Scholar] [CrossRef]






| Photocatalyst | Weight of Catalyst | Volume of MB Solution | Initial MB Solution Concentration | Degr. Time (min) | Light Source | Photo Degr. Effic. (%) | Ref. |
|---|---|---|---|---|---|---|---|
| CeO2 NPs | 60 mg | 250 mL | 20 mg/L | 90 | UV light | 90.84 | [46] |
| CeO2 NPs | 0.10 g | 50 mL | 20 mg/L | 90 | Visible light, (Xenon arc lamp, 300 W) | 98 | [47] |
| Ag/CeO2 | 10 mg | 100 mL | 30 mg/L | 120 | Visible light (Xenon lamp, 400 nm) | 94 | [48] |
| CeO2-NPs/GO | 2.5 mg | 10 mL | 5 mg/L | 90 | UV-A light | 90 | [49] |
| 0.1% CeO2–TiO2 | 3 g | 100 mL | 10 μmol/L | 60 | UV light (15 W) | 95 | [50] |
| CeO2–TiO2 (5CeTiO) | 10 mg | 20 mg/L | 150 | UV–Vis light | 95 | [36] | |
| TiO2/CeO2 | 40 mg | 40 mg/L | 40 | UV light | 71 | [37] | |
| SnO2/CeO2 | 100 mL | 20 mg/L | 120 | UV light (40 W) | 80 | [51] | |
| TiO2/SnO2/CeO2 | 200 mg | 50 mL | 10 mg/L | 120 | UV lamp (254 nm) | 85.5 | [52] |
| 0.1% TiO2-CeO2 nanocomposite | 30 mg | 20 mL | 10 mg/L | 150 | Xenon lamp (350 W) | 95.06 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, D.Q.; Lai, V.D.; Nguyen, Q.A.; Nguyen, D.D.; La, D.D. Green Synthesis of TiO2-CeO2 Nanocomposites Using Plant Extracts for Efficient Organic Dye Photodegradation. Catalysts 2025, 15, 583. https://doi.org/10.3390/catal15060583
Ho DQ, Lai VD, Nguyen QA, Nguyen DD, La DD. Green Synthesis of TiO2-CeO2 Nanocomposites Using Plant Extracts for Efficient Organic Dye Photodegradation. Catalysts. 2025; 15(6):583. https://doi.org/10.3390/catal15060583
Chicago/Turabian StyleHo, Dinh Quang, Van Duy Lai, Quynh Anh Nguyen, D. Duc Nguyen, and Duong Duc La. 2025. "Green Synthesis of TiO2-CeO2 Nanocomposites Using Plant Extracts for Efficient Organic Dye Photodegradation" Catalysts 15, no. 6: 583. https://doi.org/10.3390/catal15060583
APA StyleHo, D. Q., Lai, V. D., Nguyen, Q. A., Nguyen, D. D., & La, D. D. (2025). Green Synthesis of TiO2-CeO2 Nanocomposites Using Plant Extracts for Efficient Organic Dye Photodegradation. Catalysts, 15(6), 583. https://doi.org/10.3390/catal15060583









