Current Developments in Ozone Catalyst Preparation Techniques and Their Catalytic Oxidation Performance
Abstract
1. Introduction
2. Heterogeneous Catalytic Ozone Oxidation Mechanism
3. Preparation and Application of Heterogeneous Catalyst
3.1. Impregnation
3.2. Coprecipitation
3.3. Sol–Gel Method
3.4. Hydrothermal Method
3.5. Ion Exchange Method
4. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AOP | Advanced oxidation process |
| ROS | Reactive oxygen species |
| BPA | Bisphenol A |
| DMP | Dimethyl phthalate |
| PCMC | 4-chloro-3-methylphenol |
| PA | Pyruvic acid |
| BAA | Bromaminic acid |
| SMT | Sulfamethizole |
| PTA | Purified terephthalic acid |
| SMT | Sulfamethazine |
| SMZ | Sulfadiazine |
| BP-4 | Benzophenone |
| RB5 | Reactive Black 5 |
| SMX | Sulfamethoxazole |
| AR88 | Acid Red 88 |
| HMIMBr | 1-Hexyl-3-methylimidazole bromide |
| CIP | Ciprofloxacin |
| SA | Salicylic acid |
| DMAC | N,N-Dimethylacetamide |
| DBP | Butyl phthalate |
| NB | Nitrobenzene |
| PNP | 4-Nitrophenol |
| MET | Metoprolol |
| IBU | Ibuprofen |
| PHBA | 4-Hydroxybenzoic acid |
| MB | Methylene Blue |
| DCF | Diclofenac |
| p-CBA | p-chlorobenzoic acid |
| CLX | Cefalexin |
| SSal | 5-Sulfosalicylic acid |
| XRD | X-ray diffraction |
| SEM | scanning electron microscopy |
| XPS | X-ray photoelectron spectroscopy |
| VSM | vibrating sample magnetometer |
| BET | Brunauer–Emmett–Teller |
| XRF | X-ray fluorescence |
| HRTEM | High Resolution Transmission Electron Microscope |
| H2-TPR | Hydrogen Temperature-Programmed Reduction |
References
- Connor, R.; United Nations Economic; South Commission for Women in Asia; South Commission in Asia; Pacific Task Force; United Nations Economic Commission for Latin America; Caribbean Task Force. The united nations world water development report 2017: Wastewater: The untapped resource. Future Food J. Food Agric. Soc. 2017, 5, 80–81. [Google Scholar]
- Ghuge, S.P.; Saroha, A.K. Catalytic ozonation for the treatment of synthetic and industrial effluents—Application of mesoporous materials: A review. J. Environ. Manag. 2018, 211, 83–102. [Google Scholar] [CrossRef]
- Malakootian, M.; Shahesmaeili, A.; Faraji, M.; Amiri, H.; Martinez, S.S. Advanced oxidation processes for the removal of organophosphorus pesticides in aqueous matrices: A systematic review and meta-analysis. Process. Saf. Environ. 2020, 134, 292–307. [Google Scholar] [CrossRef]
- Sun, X.Z.; Jin, L.L.; Zhou, F.Y.; Jin, K.; Wang, L.C.; Zhang, X.X.; Ren, H.Q.; Huang, H. Patent analysis of chemical treatment technology for wastewater: Status and future trends. Chemosphere 2022, 307, 135802. [Google Scholar] [CrossRef] [PubMed]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef]
- Wang, Y.J.; Yu, G. Challenges and pitfalls in the investigation of the catalytic ozonation mechanism: A critical review. J. Hazard. Mater. 2022, 436, 129157. [Google Scholar] [CrossRef]
- Real, F.J.; Acero, J.L.; Benitez, F.J.; Matamoros, E. Elimination of neonicotinoids by ozone-based advanced oxidation processes: Kinetics and performance in real water matrices. Sep. Purif. Technol. 2022, 301, 121975. [Google Scholar] [CrossRef]
- Esposito, S. “Traditional” Sol-Gel Chemistry as a Powerful Tool for the Preparation of Supported Metal and Metal Oxide Catalysts. Materials 2019, 12, 668. [Google Scholar] [CrossRef]
- Li, Z.X.; Jing, J.X.; Gao, K.C.; Ren, G.M.; Zhang, J.W.; Jiao, W.Z.; Liu, Y.Z. Degradation of nitrobenzene by high-gravity intensified heterogeneous catalytic ozonation with Mn-Fe/ZSM-5 catalysts. Chem. Eng. Process. 2021, 169, 108642. [Google Scholar] [CrossRef]
- Kolosov, P.; Peyot, M.L.; Yargeau, V. Novelmaterials for catalytic ozonation of wastewater for disinfection and removal of micropollutants. Sci. Total Environ. 2018, 644, 1207–1218. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, P.; Li, J.; Hou, D.Y.; Wang, J.; Liu, H.J. A hybrid process combining homogeneous catalytic ozonation and membrane distillation for wastewater treatment. Chemosphere 2016, 160, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.Y.; Shao, S.J.; Ding, X.; Jiao, W.Z.; Liu, Y.Z. Degradation of phenol with heterogeneous catalytic ozonation enhanced by high gravity technology. J. Clean. Prod. 2020, 248, 119179. [Google Scholar] [CrossRef]
- Yu, G.F.; Wang, Y.X.; Cao, H.B.; Zhao, H.; Xie, Y.B. Reactive Oxygen Species and Catalytic Active Sites in Heterogeneous Catalytic Ozonation for Water Purification. Environ. Sci. Technol. 2020, 54, 5931–5946. [Google Scholar] [CrossRef] [PubMed]
- Orge, C.A.; Orfao, J.J.M.; Pereira, M.F.R.; de Farias, A.M.D.; Fraga, M.A. Ceria and cerium-based mixed oxides as ozonation catalysts. Chem. Eng. J. 2012, 200, 499–505. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, D.L.; Peng, S.H.; Feng, Y.; Liu, Z.G. Enhanced mineralization of dimethyl phthalate by heterogeneous ozonation over nanostructured Cu-Fe-O surfaces: Synergistic effect and radical chain reactions. Sep. Purif. Technol. 2019, 209, 588–597. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, P.; Chen, L. Catalytic decomposition of gaseous ozone over manganese dioxides with different crystal structures. Appl. Catal. B Environ. 2016, 189, 210–218. [Google Scholar] [CrossRef]
- Huang, Y.X.; Sun, Y.R.; Xu, Z.H.; Luo, M.Y.; Zhu, C.L.; Li, L. Removal of aqueous oxalic acid by heterogeneous catalytic ozonation with MnOx/sewage sludge-derived activated carbon as catalysts. Sci. Total Environ. 2017, 575, 50–57. [Google Scholar] [CrossRef]
- Zhang, H.F.; Wang, J.Y. Catalytic Ozonation of Humic Acids by Ce-Ti Composite Catalysts. Kinet. Catal. 2017, 58, 734–740. [Google Scholar] [CrossRef]
- Nawrocki, J.; Kasprzyk-Hordern, B. The efficiency and mechanisms of catalytic ozonation. Appl. Catal. B Environ. 2010, 99, 27–42. [Google Scholar] [CrossRef]
- Wang, Y.X.; Chen, L.L.; Chen, C.M.; Xi, J.X.; Cao, H.B.; Duan, X.G.; Xie, Y.B.; Song, W.Y.; Wang, S.B. Occurrence of both hydroxyl radical and surface oxidation pathways in N-doped layered nanocarbons for aqueous catalytic ozonation. Appl. Catal. B Environ. 2019, 254, 283–291. [Google Scholar] [CrossRef]
- Li, X.F.; Chen, W.Y.; Ma, L.M.; Wang, H.W.; Fan, J.H. Industrial wastewater advanced treatment via catalytic ozonation with an Fe-based catalyst. Chemosphere 2018, 195, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; He, Y.L.; Lai, L.D.; Yao, G.; Lai, B. Catalytic ozonation of Bisphenol A in aqueous solution by Fe3O4-MnO2 magnetic composites: Performance, transformation pathways and mechanism. Sep. Purif. Technol. 2020, 245, 116449. [Google Scholar] [CrossRef]
- Li, X.Y.; Li, G.; He, R.F. Degradation of quinoline by ozone oxidation using Fe3Ce2/NaY catalyst. Microporous Mesoporous Mater. 2025, 392, 113630. [Google Scholar] [CrossRef]
- Wang, Y.C.; Liu, H.Q.; Zhang, Y.; Sun, W.Q.; Liu, Z.Y.; Lu, X.; Sun, Y.J.; Xu, Y.H. Efficient catalytic ozonation of 4-chloro-3-methylphenol by Mn-Cu/Al2O3: Performance, mechanism, and degradation pathways. J. Water Process. Eng. 2025, 71, 107325. [Google Scholar] [CrossRef]
- Alvarez, P.M.; Beltrán, F.J.; Pocostales, J.P.; Masa, F.J. Preparation and structural characterization of Co/Al2O3 catalysts for the ozonation of pyruvic acid. Appl. Catal. B Environ. 2007, 72, 322–330. [Google Scholar] [CrossRef]
- Wu, Z.W.; Zhang, G.Q.; Zhang, R.Y.; Yang, F.L. Insights into Mechanism of Catalytic Ozonation over Practicable Mesoporous Mn-CeOx/γ-Al2O3 Catalysts. Ind. Eng. Chem. Res. 2018, 57, 1943–1953. [Google Scholar] [CrossRef]
- Chen, J.J.; Qin, J.H.; Tu, Y.M.; Shao, G.Y.; Liu, F.; Zhou, Z.Y.; Tian, S.C.; Ren, Z.Q. Catalytic ozonation with carbon-coated copper-based core-shell catalysts (C/Cu-Al2O3) for the treatment of high-salt petrochemical wastewater. J. Environ. Chem. Eng. 2024, 12, 112303. [Google Scholar] [CrossRef]
- Bai, Z.Y.; Yang, Q.; Wang, J.L. Catalytic ozonation of sulfamethazine antibiotics using Fe3O4/multiwalled carbon nanotubes. Environ. Prog. Sustain. Energy 2018, 37, 678–685. [Google Scholar] [CrossRef]
- Lu, X.; Xie, S.Q.; Li, S.; Zhou, J.; Sun, W.Q.; Xu, Y.H.; Sun, Y.J. Treatment of Purified Terephthalic Acid Wastewater by Ozone Catalytic Oxidation Method. Water 2021, 13, 1906. [Google Scholar] [CrossRef]
- Huang, G.D.; Pan, F.; Fan, G.F.; Liu, G.G. Application of heterogeneous catalytic ozonation as a tertiary treatment of effluent of biologically treated tannery wastewater. J. Environ. Sci. Health A 2016, 51, 626–633. [Google Scholar] [CrossRef]
- Alhassan, S.I.; He, Y.J.; Huang, L.; Wu, B.C.; Yan, L.J.; Deng, H.Y.; Wang, H.Y. A review on fluoride adsorption using modified bauxite: Surface modification and sorption mechanisms perspectives. J. Environ. Chem. Eng. 2020, 8, 104532. [Google Scholar] [CrossRef]
- He, H.P.; Wu, D.L.; Lv, Y.P.; Ma, L.M. Enhanced mineralization of aqueous Reactive Black 5 by catalytic ozonation in the presence of modified GAC. Desalination Water Treat. 2016, 57, 14997–15006. [Google Scholar] [CrossRef]
- Wei, K.J.; Cao, X.X.; Gu, W.C.; Liang, P.; Huang, X.; Zhang, X.Y. Ni-Induced C-Al2O3-Framework (NiCAF) Supported Core-Multishell Catalysts for Efficient Catalytic Ozonation: A Structure-to-Performance Study. Environ. Sci. Technol. 2019, 53, 6917–6926. [Google Scholar] [CrossRef]
- Sun, W.Q.; Wang, Y.; Yang, X.W.; Wang, Z.R.; Zhu, H.Q.; Zhou, J.; Sun, Y.J. Catalytic ozone oxidation of chemical RO membrane concentrate wastewater by a Cu-Ce@γ-Al2O3 ozone catalyst. Water Environ. Res. 2025, 97, e70034. [Google Scholar] [CrossRef]
- Bai, Z.Y.; Yang, Q.; Wang, J.L. Catalytic ozonation of sulfamethazine using Ce0.1Fe0.9OOH as catalyst: Mineralization and catalytic mechanisms. Chem. Eng. J. 2016, 300, 169–176. [Google Scholar] [CrossRef]
- Yan, P.W.; Shen, J.M.; Zhou, Y.C.; Yuan, L.; Kang, J.; Wang, S.Y.; Chen, Z.L. Interface mechanism of catalytic ozonation in an α-Fe0.9Mn0.1OOH aqueous suspension for the removal of iohexol. Appl. Catal. B Environ. 2020, 277, 119055. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhu, W.X.; Yang, Z.G.; Yang, Y.; Li, H.P. Efficient ozone catalysis by manganese iron oxides/activated carbon for sulfamerazine degradation. J. Water Process. Eng. 2022, 49, 103050. [Google Scholar] [CrossRef]
- Liu, Y.; Song, Z.L.; Wang, W.H.; Wang, Z.B.; Zhang, Y.T.; Liu, C.; Wang, Y.P.; Li, A.; Xu, B.B.; Qi, F. A CuMn2O4/g-C3N4 catalytic ozonation membrane reactor used for water purification: Membrane fabrication and performance evaluation. Sep. Purif. Technol. 2021, 265, 118268. [Google Scholar] [CrossRef]
- Chokshi, N.P.; Ruparelia, J.P. Synthesis of Nano Ag-La-Co Composite Metal Oxide for Degradation of RB 5 Dye Using Catalytic Ozonation Process. Ozone Sci. Eng. 2021, 44, 182–195. [Google Scholar] [CrossRef]
- Zuo, X.T.; Ma, S.L.; Wu, Q.Y.; Xiong, J.; He, J.J.; Ma, C.; Chen, Z.B. Nanometer CeO2 doped high silica ZSM-5 heterogeneous catalytic ozonation of sulfamethoxazole in water. J. Hazard. Mater. 2021, 411, 125072. [Google Scholar] [CrossRef]
- Fahadi, M.; Nabavi, S.R.; Chaichi, M.J. Mesoporous Fe3O4/graphene oxide nanohybrid for catalytic Ozonation: Preparation, characterization and process modeling by neural network. J. Taiwan Inst. Chem. E 2022, 134, 104278. [Google Scholar] [CrossRef]
- Tian, J.; Wei, J.Y.; Liang, Y.P.; Guo, R.X.; Li, B.B.; Qu, R.J.; Zhou, D.M.; Wang, Z.Y.; Sun, P. Catalytic ozonation of an imidazole ionic liquid via Fe3O4/ZnO nanocomposites: Performance, products and reaction mechanism. J. Environ. Chem. Eng. 2022, 10, 108726. [Google Scholar] [CrossRef]
- Xu, R.J.; Li, L.; Fu, X.J.; Yu, L.; Jin, Y.J.; Li, L.D. Catalytic Ozonation of Ciprofloxacin with Cu-Al Layered Double Hydroxides Based on Response Surface Analysis. J. Environ. Eng. 2022, 148, 04022010. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, R.; Li, J.; Cheng, Y.Z.; Wang, C.M.; Wang, W.Q.; Zheng, H.F. Enhanced Ciprofloxacin Ozonation Degradation by an Aqueous Zn-Cu-Ni Composite Silicate: Degradation Performance and Surface Mechanism. Separations 2025, 12, 15. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Brown, D.R.; Kasprzyk-Hordern, B. Catalytic ozonation for the removal of organic contaminants in water on ZSM-5 zeolites. Appl. Catal. B Environ. 2014, 154, 110–122. [Google Scholar] [CrossRef]
- Malakootian, M.; Shahamat, Y.D.; Mahdizadeh, H. Optimization and modeling of p-nitroaniline removal from aqueous solutions in heterogeneous catalytic ozonation process using MgAl-layered double hydroxides (MgAl-LDH COP). Desalination Water Treat. 2021, 223, 114–127. [Google Scholar] [CrossRef]
- Dong, H.X.; Koenig, G.M. A review on synthesis and engineering of crystal precursors produced via coprecipitation for multicomponent lithium-ion battery cathode materials. Crystengcomm 2020, 22, 1514–1530. [Google Scholar] [CrossRef]
- Jothinathan, L.; Cai, Q.Q.; Ong, S.L.; Hu, J.Y. Fe-Mn doped powdered activated carbon pellet as ozone catalyst for cost-effective phenolic wastewater treatment: Mechanism studies and phenol by-products elimination. J. Hazard. Mater. 2022, 424, 127483. [Google Scholar] [CrossRef]
- Chen, W.R.; Bao, Y.X.; Li, X.K.; Huang, J.; Tang, Y.M.; Li, L.S. Mineralization of salicylic acid via catalytic ozonation with Fe-Cu@SiO2 core-shell catalyst: A two-stage first order reaction. Chemosphere 2019, 235, 470–480. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, F.Z.; Zhang, Y.H.; Pan, Z.C.; Lai, B. Catalytic ozonation of N,N-dimethylacetamide (DMAC) in aqueous solution using nanoscaled magnetic CuFe2O4. Sep. Purif. Technol. 2018, 193, 368–377. [Google Scholar] [CrossRef]
- Wang, Z.M.; Ma, H.; Zhang, C.; Feng, J.; Pu, S.Y.; Ren, Y.M.; Wang, Y. Enhanced catalytic ozonation treatment of dibutyl phthalate enabled by porous magnetic Ag-doped ferrospinel MnFe2O4 materials: Performance and mechanism. Chem. Eng. J. 2018, 354, 42–52. [Google Scholar] [CrossRef]
- Yang, Y.X.; Ma, J.; Qin, Q.D.; Zhai, X.D. Degradation of nitrobenzene by nano-TiO2 catalyzed ozonation. J. Mol. Catal. A Chem. 2007, 267, 41–48. [Google Scholar] [CrossRef]
- Zhang, L.H.; Zhou, J.; Liu, Z.Q.; Guo, J.B. Mesoporous CeO2 Catalyst Synthesized by Using Cellulose as Template for the Ozonation of Phenol. Ozone Sci. Eng. 2019, 41, 166–174. [Google Scholar] [CrossRef]
- Afzal, S.; Quan, X.; Lu, S. Catalytic performance and an insight into the mechanism of CeO2 nanocrystals with different exposed facets in catalytic ozonation of p-nitrophenol. Appl. Catal. B Environ. 2019, 248, 526–537. [Google Scholar] [CrossRef]
- Wang, J.; Quan, X.; Chen, S.; Yu, H.T.; Liu, G.B. Enhanced catalytic ozonation by highly dispersed CeO2 on carbon nanotubes for mineralization of organic pollutants. J. Hazard. Mater. 2019, 368, 621–629. [Google Scholar] [CrossRef]
- He, Y.; Wang, L.J.; Chen, Z.; Shen, B.; Wei, J.S.; Zeng, P.; Wen, X.H. Catalytic ozonation for metoprolol and ibuprofen removal over different MnO2 nanocrystals: Efficiency, transformation and mechanism. Sci. Total Environ. 2021, 785, 147328. [Google Scholar] [CrossRef]
- Wang, Y.X.; Xie, Y.B.; Sun, H.Q.; Xiao, J.D.; Cao, H.B.; Wang, S.B. Efficient Catalytic Ozonation over Reduced Graphene Oxide for p-Hydroxylbenzoic Acid (PHBA) Destruction: Active Site and Mechanism. ACS Appl. Mater. Interfaces 2016, 8, 9710–9720. [Google Scholar] [CrossRef]
- Luo, K.; Zhao, S.X.; Wang, Y.F.; Zhao, S.J.; Zhang, X.H. Synthesis of petal-like δ-MnO2 and its catalytic ozonation performance. New J. Chem. 2018, 42, 6770–6777. [Google Scholar] [CrossRef]
- Zhu, B.J.; Jiang, G.F.; Chen, S.H.; Liu, F.; Wang, Y.Q.; Zhao, C.C. Multifunctional Cl-S double-doped carbon nitride nanotube unit in catalytic ozone oxidation synergistic photocatalytic system: Generation of ROS-rich region and effective treatment of organic wastewater. Chem. Eng. J. 2022, 430, 132843. [Google Scholar] [CrossRef]
- de Oliveira, J.S.; Salla, J.D.; Kuhn, R.C.; Jahn, S.L.; Foletto, E.L. Catalytic Ozonation of Melanoidin in Aqueous Solution over CoFe2O4 Catalyst. Mater. Res. Ibero Am. J. Mater. 2019, 22, e20180405. [Google Scholar] [CrossRef]
- Xiang, T.X.; Zhong, D.J.; Zhou, Y.X.; Xu, Y.L.; Tang, D.L.; Li, W.T.; Yang, Y.F.; Fan, C.M.; Chen, J.S. Degradation of Methylene Blue by Ozone Oxidation Catalyzed by the Magnetic MnFe2O4@Co3S4 Nanocomposite. Langmuir 2025, 41, 2699–2713. [Google Scholar] [CrossRef]
- Li, X.K.; Chen, W.R.; Tang, Y.M.; Li, L.S. Relationship between the structure of Fe-MCM-48 and its activity in catalytic ozonation for diclofenac mineralization. Chemosphere 2018, 206, 615–621. [Google Scholar] [CrossRef]
- Bing, J.S.; Hu, C.; Nie, Y.L.; Yang, M.; Qu, J.H. Mechanism of Catalytic Ozonation in Fe2O3/Al2O3@SBA-15 Aqueous Suspension for Destruction of Ibuprofen. Environ. Sci. Technol. 2015, 49, 1690–1697. [Google Scholar] [CrossRef]
- Pan, Z.Q.; Zeng, J.Y.; Lan, B.Y.; Li, L.S. Catalytic Activity of Argentum-loaded MCM-41 for Ozonation of p-Chlorobenzoic Acid (p-CBA) in Aqueous Solution. J. Adv. Oxid. Technol. 2015, 18, 139–146. [Google Scholar] [CrossRef]
- Zhu, L.J.; Zhou, S.Y.; Cheng, H.; Ma, J.F.; Imanova, G.; Komarneni, S. Cu2S/Ni3S2 nanosheets combined with nickel foam substrate for efficient catalytic ozonation of p-nitrophenol in wastewater. J. Environ. Chem. Eng. 2024, 12, 113591. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Qian, M.Q.; Pan, J.; Ding, J.; Guan, B.H. Amino-functionalized synthesis of MnO2-NH2-GO for catalytic ozonation of cephalexin. Appl. Catal. B Environ. 2019, 256, 117797. [Google Scholar] [CrossRef]
- Chen, J.; Tian, S.H.; Lu, J.; Xiong, Y. Catalytic performance of MgO with different exposed crystal facets towards the ozonation of 4-chlorophenol. Appl. Catal. A Gen. 2015, 506, 118–125. [Google Scholar] [CrossRef]
- Athalathil, S.; Erjavec, B.; Kaplan, R.; Stüber, F.; Bengoa, C.; Font, J.; Fortuny, A.; Pintar, A.; Fabregat, A. TiO2-sludge carbon enhanced catalytic oxidative reaction in environmental wastewaters applications. J. Hazard. Mater. 2015, 300, 406–414. [Google Scholar] [CrossRef]
- Dai, Q.Z.; Zhang, Z.; Zhan, T.T.; Hu, Z.T.; Chen, J.M. Catalytic Ozonation for the Degradation of 5-Sulfosalicylic Acid with Spinel-Type ZnAl2O4 Prepared by Hydrothermal, Sol-Gel, and Coprecipitation Methods: A Comparison Study. ACS Omega 2018, 3, 6506–6512. [Google Scholar] [CrossRef]
- Xu, T.; Jiang, M.H.; Mao, X.H.; Sher, F.; Ren, T.L.; Xin, K.; Hussain, I.; Hua, H.L.; Wang, X. Design of high-performance ozone catalysts by identifying and modulating anchoring sites for metal on γ-Al2O3 spheres. Appl. Catal. B Environ. 2025, 372, 125321. [Google Scholar] [CrossRef]
- Wang, H.; Yu, Z.; Sun, Y.J.; Zhou, J.H.; Yang, Y.X.; Yang, Y.M.; Chen, X.F.; Chen, F.T.; Lu, W.Y. Preparation of porous La0.9Ce0.1CoO3 and its application in enhancing catalytic ozonation for nitrobenzene degradation in aqueous solution. J. Environ. Chem. Eng. 2025, 13, 116814. [Google Scholar] [CrossRef]
- Rutkowska, M.; Chmielarz, L.; Jablonska, M.; Van Oers, C.J.; Cool, P. Iron exchanged ZSM-5 and Y zeolites calcined at different temperatures: Activity in N2O decomposition. J. Porous Mater. 2014, 21, 91–98. [Google Scholar] [CrossRef]
- Zhang, N.; Ouyang, S.X.; Kako, T.; Ye, J.H. Synthesis of hierarchical Ag2ZnGeO4 hollow spheres for enhanced photocatalytic property. Chem. Commun. 2012, 48, 9894–9896. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, X.J.; Dong, H.Y.; Sans, C. Progress in MnO2/MnO2-based materials catalytic ozonation process for water and wastewater treatment. J. Environ. Manag. 2025, 383, 125493. [Google Scholar] [CrossRef]
- Yan, C.L.; Rosei, F. Hollow micro/nanostructured materials prepared by ion exchange synthesis and their potential applications. New J. Chem. 2014, 38, 1883–1904. [Google Scholar] [CrossRef]
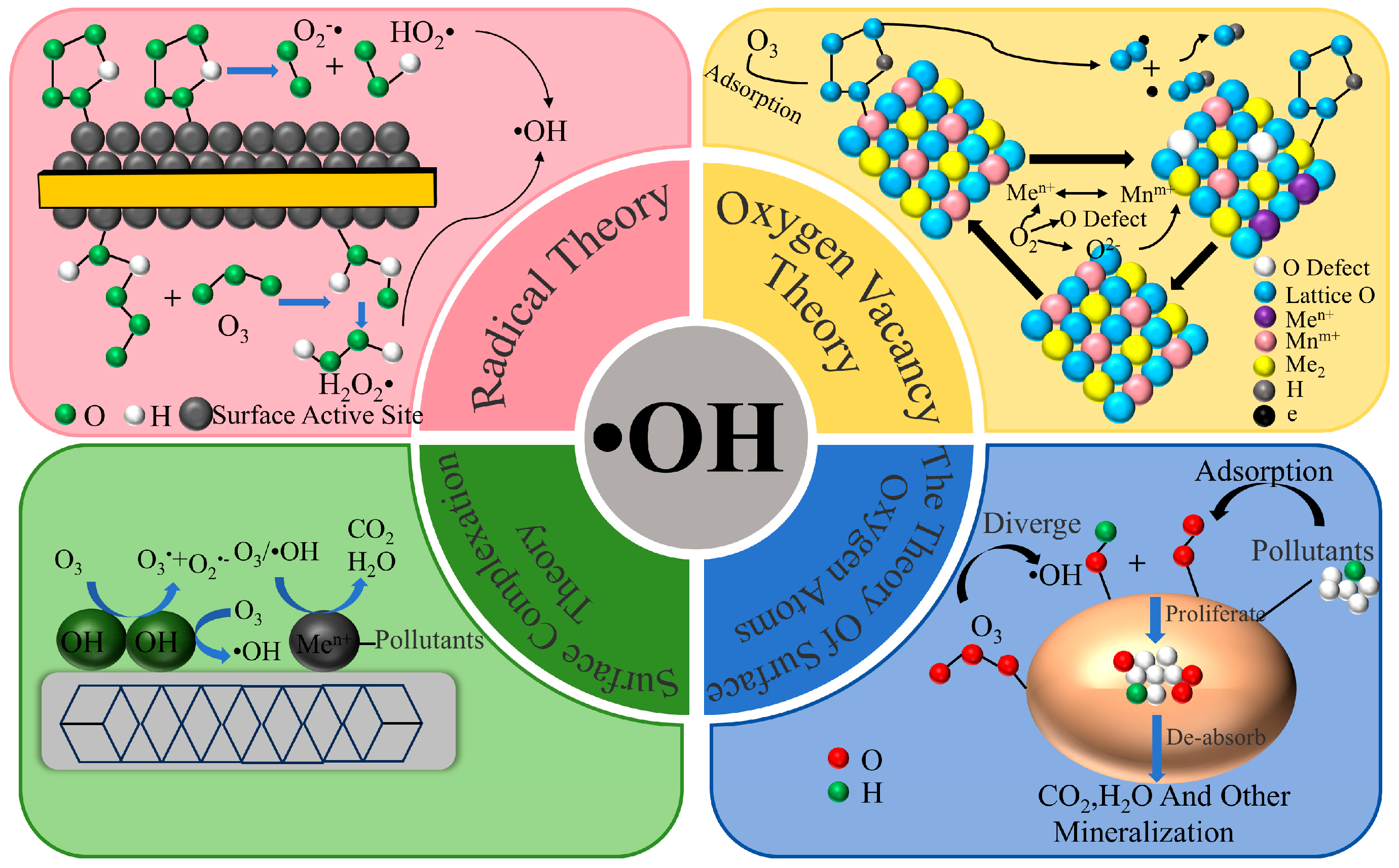
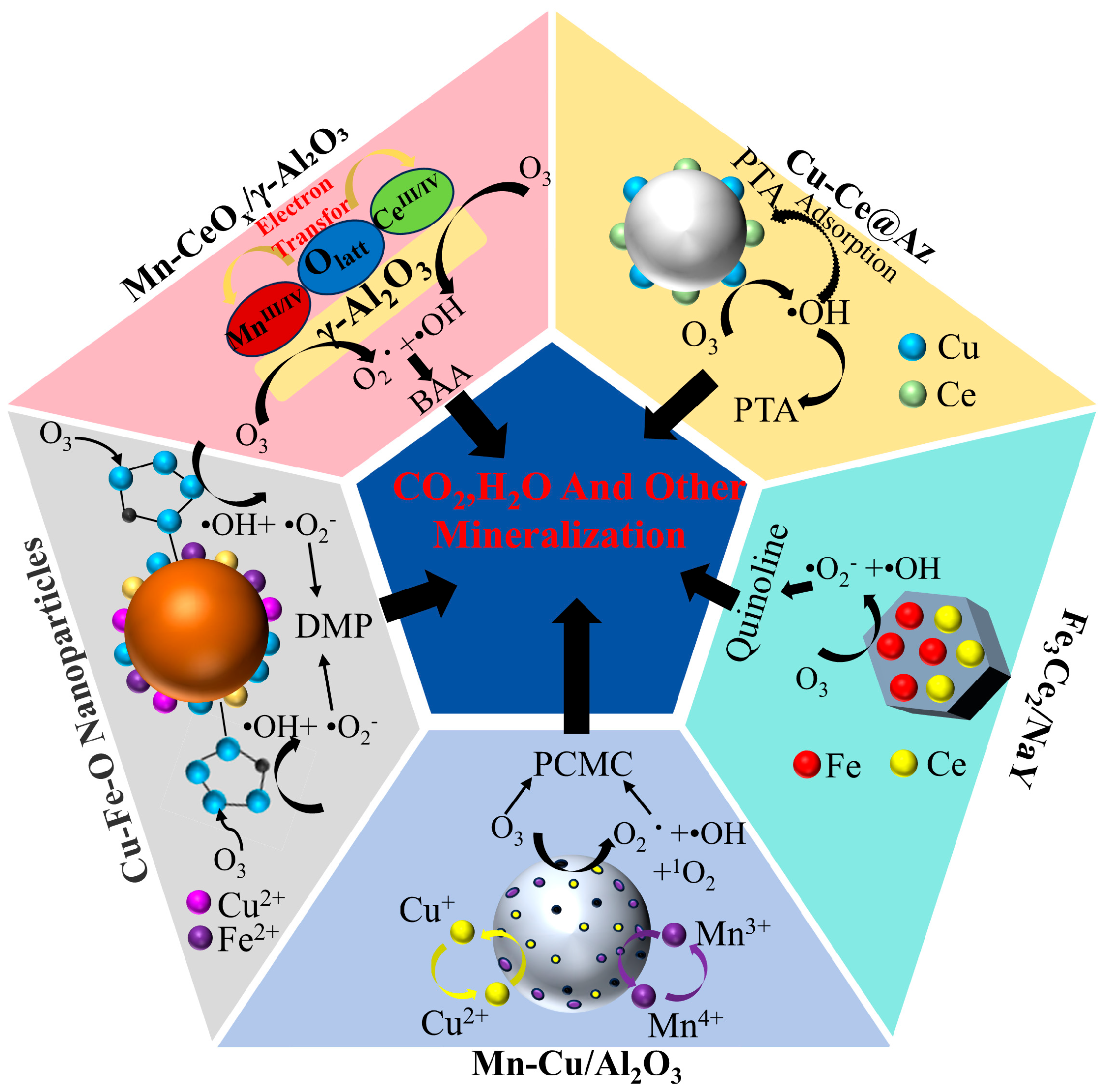
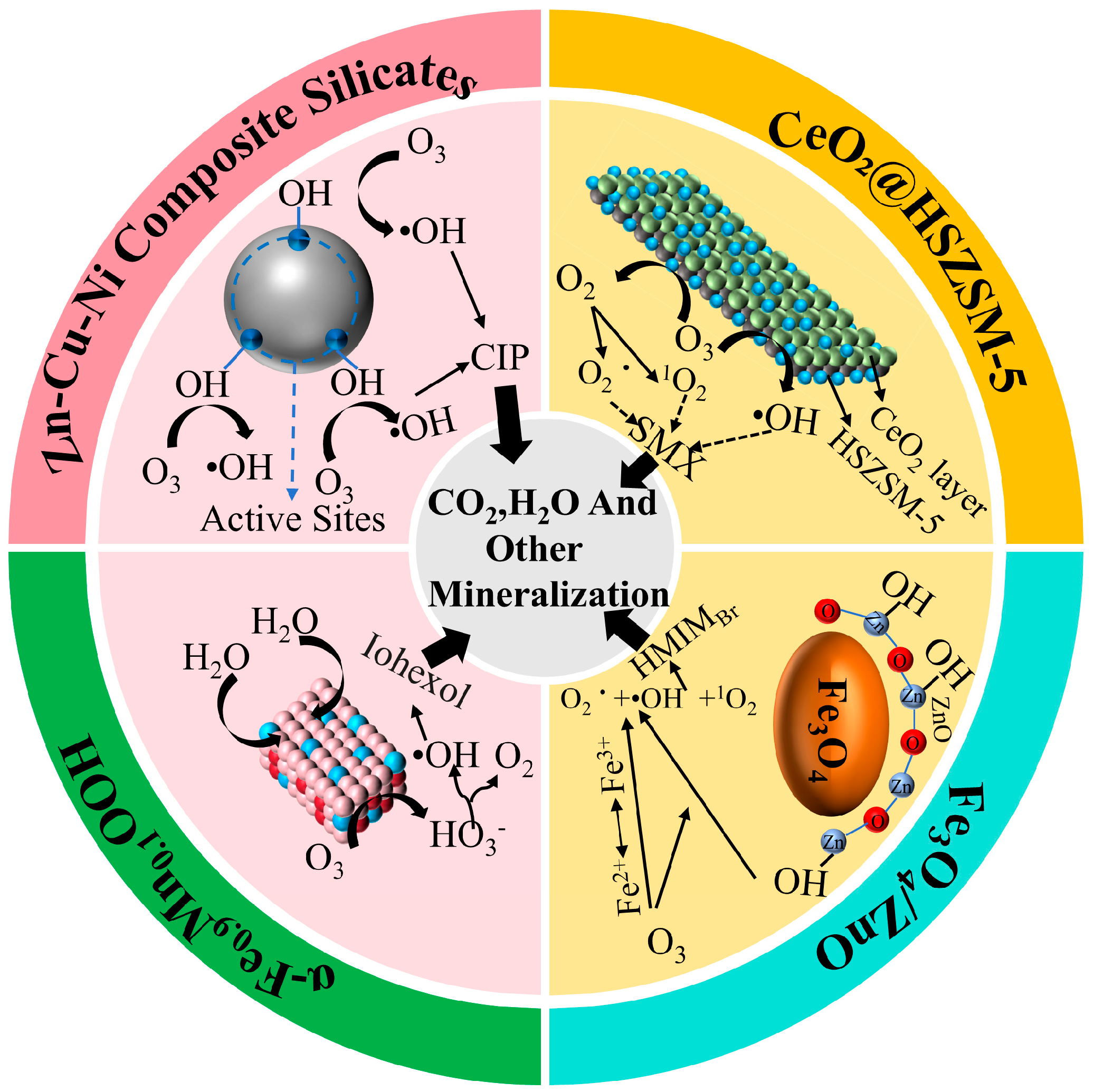

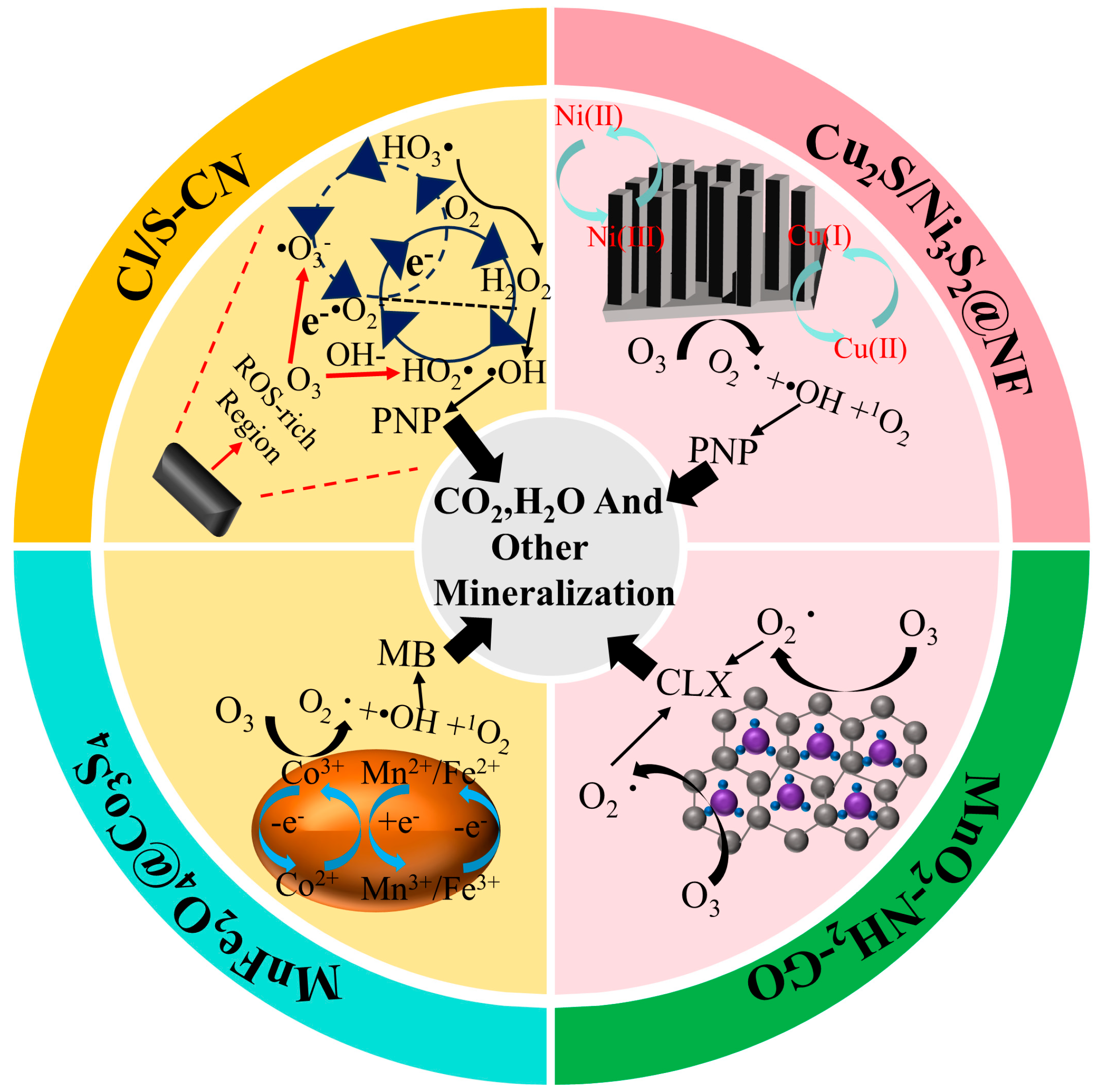

| Catalyst | Pollutants | Experimental Condition | Catalytic Performance | Reusability | References |
|---|---|---|---|---|---|
| Iron-based catalyst | Industrial wastewater | [Cat] = 200 g/L [O3] = 5 mg/min | TOC removal rate 78.7% | After 50 uses, the TOC above 60% | [21] |
| [Pull]0 = 69.3 mg/L pH = 6.8 | |||||
| Fe3O4-MnO2 magnetic composite | Bisphenol A | [Cat] = 0.1 g/L [O3] = 0.1 L/min | BPA removal rate was 97% | After five cycles, the degradation efficiency of BPA was 88.8%. | [22] |
| (BPA) | [Pull]0 = 0.05 mg/L pH = 7.0 | ||||
| Fe3Ce2/NaY | Quinoline | [Cat] = 0.5 g/L [O3] = 2.19 g/h [Pull]0 = 0.05 mg/L pH = 7.0 | Quinoline removal rate was 99.14% | The COD removal efficiencies were nearly same during four cycles | [23] |
| Cu-Fe-O nanoparticles | Dimethyl phthalate (DMP) | [Cat] = 75 mg/L [O3] = 1.8 mg/min | DMP degradation rate close to 100% | After three cycles, the degradation rate of DMP was close to 100%. | [15] |
| [Pull]0 = 50 mg/L pH = 5.7 | |||||
| Mn-Cu/Al2O3 | 4-chloro-3-methylphenol (PCMC) | [Cat] = 15 mg/L [O3] = 4.0 mg/min | PCMC removal rate near 100% | After five reuses, the PCMC removal rate decreased from 100% to 90.8%. | [24] |
| [Pull]0 = 100 mg/L pH = 7.0 | |||||
| Co/Al2O3 | Pyruvic acid | [Cat] = 5 g/L [O3] = 40 mg/min | PA removal rate was 94.4% | / | [25] |
| (PA) | [Pull]0 = 0.1 g/L pH = 4.0 | ||||
| Mn-CeOx/γ-Al2O3 | Bromaminic acid | [Cat] = 1 g/L [O3] = 20 mg/L | BAA almost completely degraded | After three reuses, the TOC removal rate dropped to 57.2%. | [26] |
| (BAA) | [Pull]0 = 50 mg/L pH = 6.8 | ||||
| C/Cu-Al2O3 | High-salt petrochemical wastewater | [Cat] = 400 g/L [O3] = 12 mg/L | COD removal of pollutants was 62.5% | After 20 reuses, its COD removal rate for pollutants exceeds 53%. | [27] |
| [Pull]0 = 100 mg/L pH = 7.8 | |||||
| Fe3O4/MWCNTs | Sulfamethizole (SMT) | [Cat] = 0.5 g/L [O3] = 9 mg/min | TOC removal rate of SMT was 39.1% | / | [28] |
| [Pull]0 = 20 mg/L pH = 4.0 | |||||
| Cu-Ce@Az | Purified terephthalic acid | [Cat] = 86 g/L [O3] = 2 g/h | COD removal rate of PAT was 84.2% | After 30 reuses, the COD removal rate of PTA wastewater was 68.2%. | [29] |
| (PTA) | [Pull]0 = 178.6 mg/L pH = 8.0 | ||||
| Mn-Cu/γ-Al2O3 | Tannery wastewater | [Cat] = 2 g/L [O3] = 0.3 g/h | COD removal rate of tannery wastewater was 88% | / | [30] |
| [Pull]0 = 5200 mg/L pH = 7.0 |
| Catalyst | Pollutants | Experimental Condition | Catalytic Performance | Reusability | References |
|---|---|---|---|---|---|
| Ce0.1Fe0.9OOH | Sulfamethazine (SMT) | [Cat] = 0.4 g/L [O3] = 15 mg/L | SMT removal rate was 42% | / | [35] |
| [Pull]0 = 40 mg/L pH = 3.0 | |||||
| α-Fe0.9Mn0.1OOH | Iohexol | [Cat] = 100 mg/L [O3] = 0.8 mg/L | Iohexol removal rate was 87.6% | After three cycles, the mineralization rate of TOC still reached 71.7%. | [36] |
| [Pull]0 = 1 mg/L pH = 7.0 | |||||
| MnxFeyOz/AC | Sulfadiazine (SMZ) | [Cat] = 0.05 g/L [O3] = 50 mL/min [Pull]0 = 10 mg/L pH = 6.1 | SMZ removal rate was 90.5% | After five reuses, the removal rate of SMZ decreased from 90.5% to 77.5%. | [37] |
| CuMn2O4/g-C3N | Benzophenone (BP-4) | [Cat] = 0.25 g/L [O3] = 20 mg/L | BP-4 removal rate was 87% | / | [38] |
| [Pull]0 = 0.084 mmol/L pH = 6.4 | |||||
| Ag-La-Co nano-metal oxide | Reactive Black 5 (RB5) | [Cat] = 1 g/L [O3] = 30 L/h | TOC removal rate of RB5 was 95% | After three reuses, the catalytic activity does not decrease significantly. | [39] |
| [Pull]0 = 100 mg/L pH = 7.0 | |||||
| CeO2@HSZSM-5 | Sulfamethoxazole | [Cat] = 0.4 g/L [O3] = 9.3 mg/min | TOC removal rate of SMX was 80.4% | After five cycles, the TOC removal rate decreased by only 7.3%. | [40] |
| (SMX) | [Pull]0 = 20 mg/L pH = 7.0 | ||||
| Fe3O4/GO nanohybrid material | Acid Red 88 | [Cat] = 0.25 g/L [O3] = 6 mg/min | COD removal rate of AR88 was 69% | After five cycles of reuse, the degradation efficiency of AR88 remains high. | [41] |
| (AR88) | [Pull]0 = 150 mg/L pH = 4.5 | ||||
| Fe3O4/ZnO | 1-Hexyl-3-methylimidazole bromide (HMIMBr) | [Cat] = 0.25 g/L [O3] = 36 mL/min | HMIMBr removal rate was 90.5% | After five cycles of reuse, the degradation efficiency of [HMIM]Br can still reach 90%. | [42] |
| [Pull]0 = 150 mg/L pH = 9.0 | |||||
| Cu-Al LDHs | Ciprofloxacin (CIP) | [Cat] = 0.79 g/L [O3] = 62 mg/min | CIP removal rate was 96% | After five cycles, the TOC removal rate remained at approximately 70%. | [43] |
| [Pull]0 = 10 mg/L pH = 9.0 | |||||
| Zn-Cu-Ni composite silicate | Ciprofloxacin | [Cat] = 0.5 g/L [O3] = 1.5 mg/L | COD removal of PAT was 84.2% | / | [44] |
| (CIP) | [Pull]0 = 3 mg/L pH = 7.0 |
| Catalyst | Pollutants | Experimental Condition | Catalytic Performance | Reusability | References |
|---|---|---|---|---|---|
| Fe-Mn/PAC | phenolic compounds | [Cat] = 4 g/L [O3] = 50 mg/L [Pull]0 = 700 mg/L pH = 7.5 | Phenol removal rate was 95% | Over 50 operating days, the COD removal rate remained stable at around 74%. | [48] |
| Fe-Cu@SiO2 | Salicylic acid (SA) | [Cat] = 0.1 g/L [O3] = 4.6 mg/L | TOC removal rate of SA was 88% | / | [49] |
| [Pull]0 = 11.2 mg/L pH = 7.0 | |||||
| CuFe2O4 | N,N-Dimethylacetamide (DMAC) | [Cat] = 30 g/L [O3] = 0.06 L/min [Pull]0 = 200 mg/L pH = 6.8 | DMAC removal rate was 95.4% | After five reuses, the removal rate of DMAC did not decrease. | [50] |
| Ag/MnFe2O4 | Butyl phthalate (DBP) | [Cat] = 10 mg/L [O3] = 20 mg/L | DBP removal rate was 75.3% | / | [51] |
| [Pull]0 = 0.5 mg/L pH = 7.3 | |||||
| Nano TiO2 | Nitrobenzene (NB) | [Cat] = 0.1 g/L [O3] = 15 mg/L | NB removal rate was 75.3% | / | [52] |
| [Pull]0 = 20 mg/L pH = 7.0 | |||||
| CeO2-T | Phenol | [Cat] = 0.1 g/L [O3] = 172.8 mg/L | Phenol removal rate was 91.7% | / | [53] |
| [Pull]0 = 100 mg/L pH = 7.8 | |||||
| CeO2 | 4-Nitrophenol | [Cat] = 0.4 g/L [O3] = 1.6 mg/min | TOC removal rate of PNP was 86% | After three reuses, the TOC removal rate decreased from 86.14% to 75.38%. | [54] |
| (PNP) | [Pull]0 = 25 mg/L pH = 5.7 |
| Catalyst | Pollutants | Experimental Condition | Catalytic Performance | Reusability | References |
|---|---|---|---|---|---|
| α-MnO2 | Metoprolol (MET) Ibuprofen (IBU) | [Cat] = 0.1 g/L [O3] = 1 mg/min [Pull]0 = 10 mg/L pH = 7.0 | MET removal rate was 99.62%; IBU removal rate was 99.51% | After four reuses, there was no significant decrease in pollutant removal efficiency. | [56] |
| rGO | 4-Hydroxybenzoic acid (PHBA) | [Cat] = 0.05 g/L [O3] = 20 mg/L | PHBA removal rate close to 100% | After five reuses, the inactivation phenomenon continues. | [57] |
| [Pull]0 = 20 mg/L pH = 3.5 | |||||
| CoFe2O4 | Melanoidin | [Cat] = 0.1 g/L [O3] = 10 mL/min [Pull]0 = 300 mg/L pH = 6.85 | TOC removal rate of Melanoidin was 80% | / | [60] |
| MnFe2O4@Co3S4 | Methylene Blue (MB) | [Cat] = 0.6 g/L [O3] = 2.5 mg/L | MB removal rate was 93.55% | After five cycles of reuse, the degradation rate of MB dropped to 90.02%. | [61] |
| [Pull]0 = 50 mg/L pH = 6.6 | |||||
| Fe-MCM-48 | Diclofenac (DCF) | [Cat] = 0.15 g/L [O3] = 100 mg/h | TOC removal rate of DCF was 49.9% | / | [62] |
| [Pull]0 = 15 mg/L pH = 7.0 | |||||
| Fe2O3/Al2O3@SBA-15 | Ibuprofen (IBU) | [Cat] = 1.5 g/L [O3] = 30 mg/L | TOC removal rate of IBU was 49.9% | / | [63] |
| [Pull]0 = 10 mg/L pH = 7.0 | |||||
| Ag/MCM-41 | p-chlorobenzoic acid (p-CBA) | [Cat] = 1 g/L [O3] = 100 mg/h | TOC removal rate of p-CBA was 84% | The TOC removal rate decreased by only 4% after four reuses. | [64] |
| [Pull]0 = 10 mg/L pH = 4.2 | |||||
| Cu2S/Ni3S2@NF | 4-Nitrophenol (PNP) | [Cat] = 3 pcs [O3] = 2 mg/min | PNP removal rate was 99.9% | After five cycles of reuse, the removal rate of PNP decreased to only 90.1%. | [65] |
| [Pull]0 = 100 mg/L pH = 7.8 | |||||
| MnO2-NH2-GO | Cefalexin (CLX) | [Cat] = 25 g/L [O3] = 0.12 mg/L [Pull]0 = 1 mg/L pH = 4.2 | CLX removal rate was 55.6% | / | [66] |
| MgO | P-chlorophenol (4-CP) | [Cat] = 1 g/L [O3] = 5 mg/L [Pull]0 = 100 mg/L pH = 6.2 | 4-CP removal rate was 95.1% | During the 7.5 h of operation, the removal rate remained at around 99.0%. | [67] |
| Sludge carbon/TiO2 | Bisphenol A (BPA) | [Cat] = 0.2 g/L [O3] = 40 mL/min [Pull]0 = 10 mg/L pH = 7.0 | BPA removal rate was 75% | / | [68] |
| ZnAl2O4 | 5-Sulfosalicylic acid (SSal) | [Cat] = 0.2 g/L [O3] = 10 mg/min [Pull]0 = 500 mg/L pH = 7.0 | SSal removal rate was 64.8% | After three reuses, the removal rate of SSal was 64.8–59.7%. | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Chen, S.; Gao, Y.; Sun, W.; Zhou, J.; Shah, K.J.; Sun, Y. Current Developments in Ozone Catalyst Preparation Techniques and Their Catalytic Oxidation Performance. Catalysts 2025, 15, 671. https://doi.org/10.3390/catal15070671
Gao J, Chen S, Gao Y, Sun W, Zhou J, Shah KJ, Sun Y. Current Developments in Ozone Catalyst Preparation Techniques and Their Catalytic Oxidation Performance. Catalysts. 2025; 15(7):671. https://doi.org/10.3390/catal15070671
Chicago/Turabian StyleGao, Jiajia, Siqi Chen, Yun Gao, Wenquan Sun, Jun Zhou, Kinjal J. Shah, and Yongjun Sun. 2025. "Current Developments in Ozone Catalyst Preparation Techniques and Their Catalytic Oxidation Performance" Catalysts 15, no. 7: 671. https://doi.org/10.3390/catal15070671
APA StyleGao, J., Chen, S., Gao, Y., Sun, W., Zhou, J., Shah, K. J., & Sun, Y. (2025). Current Developments in Ozone Catalyst Preparation Techniques and Their Catalytic Oxidation Performance. Catalysts, 15(7), 671. https://doi.org/10.3390/catal15070671










