Efficient Degradation of Tetracycline via Cobalt Phosphonate-Activated Peroxymonosulfate: Mechanistic Insights and Catalytic Optimization
Abstract
1. Introduction
2. Results and Discussion
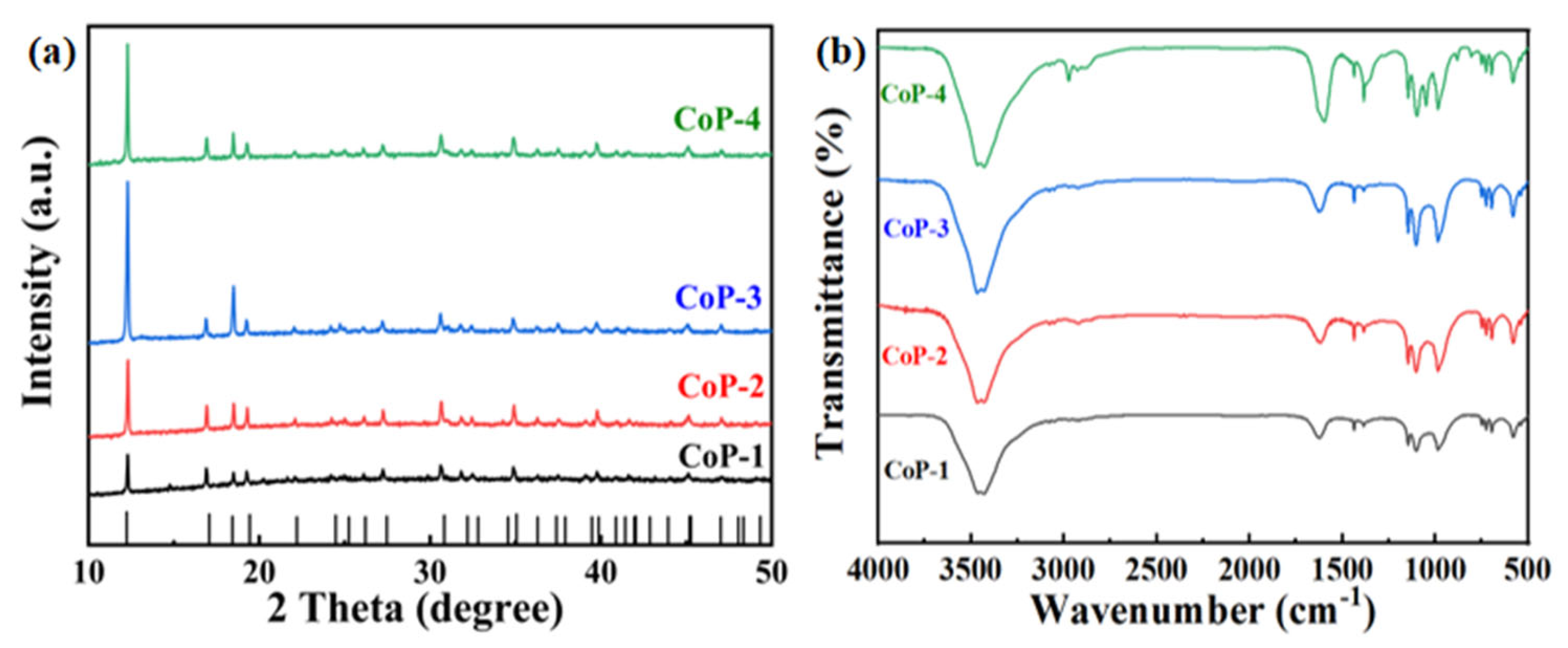
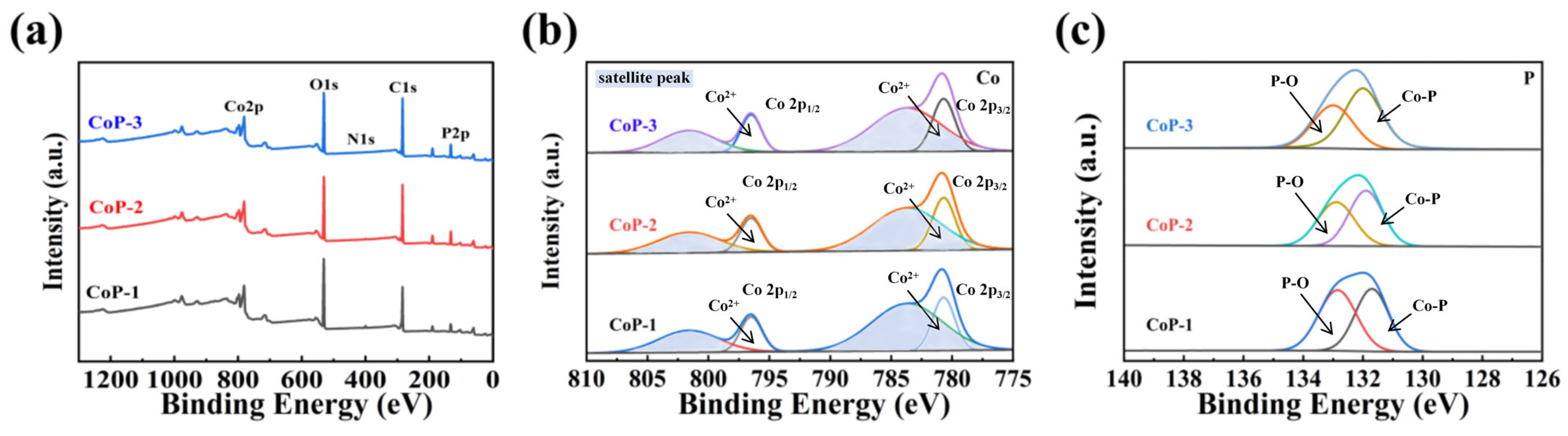
2.1. Characterization of Catalysts
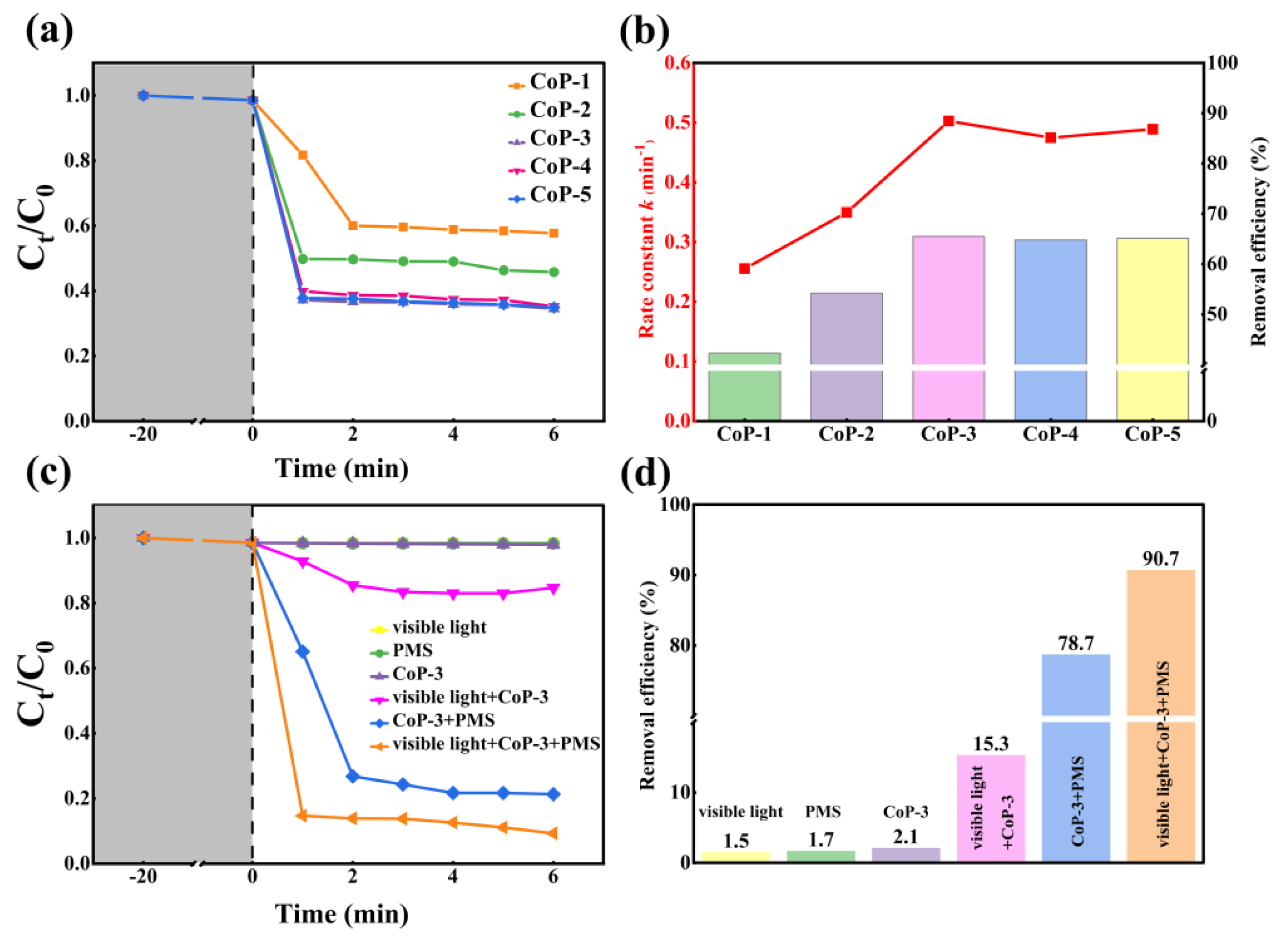
2.2. Photocatalytic Activity of CoP Catalysts with Different Proportions
2.3. Catalytic Activity of CoP-3 Catalyst
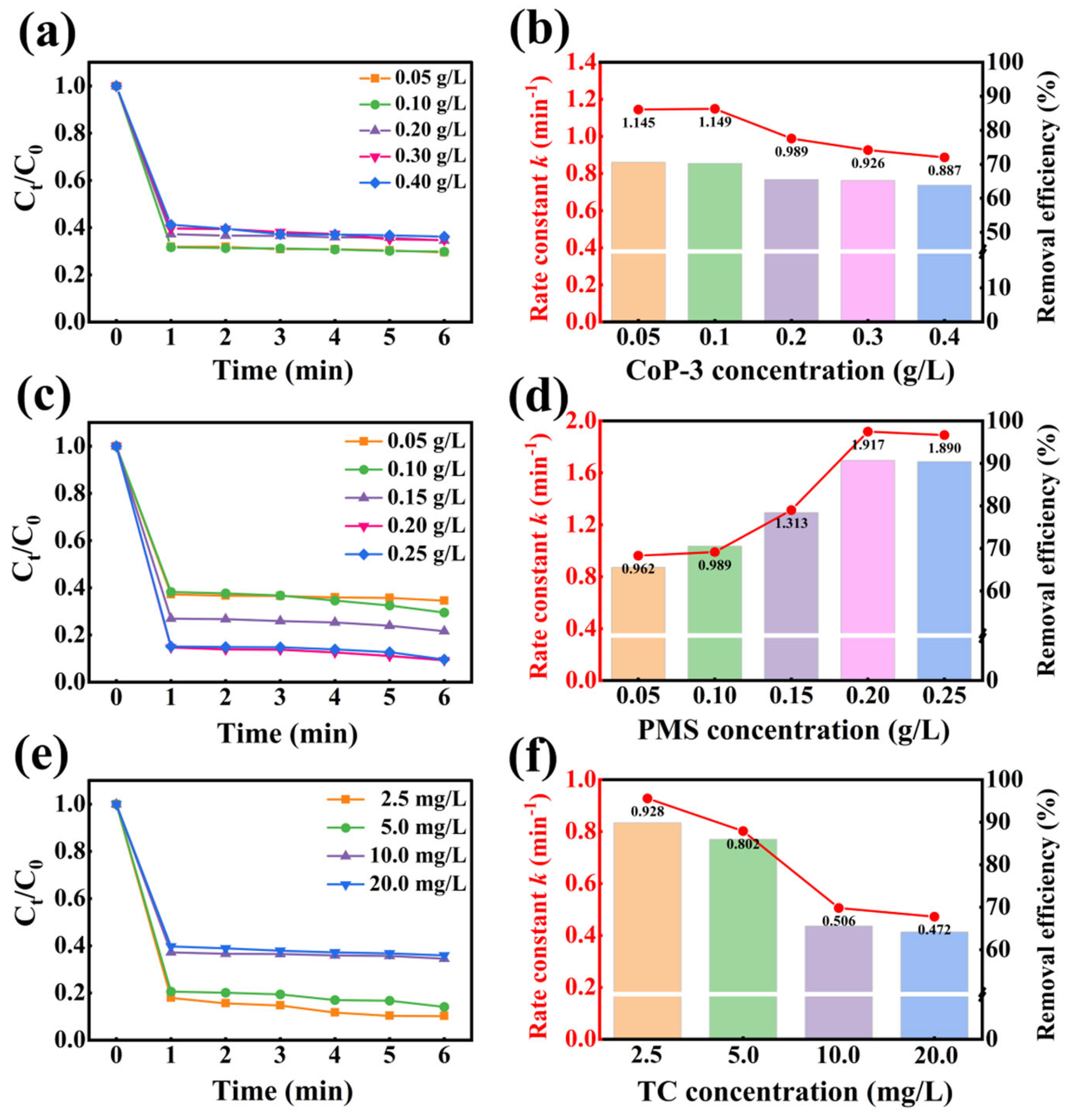
2.4. Optimization of Experimental Parameters in the CoP-3/PMS/Vis System
2.4.1. Influence of Catalyst Dosage
2.4.2. Influence of PMS Concentration
2.4.3. Influence of TC Concentration
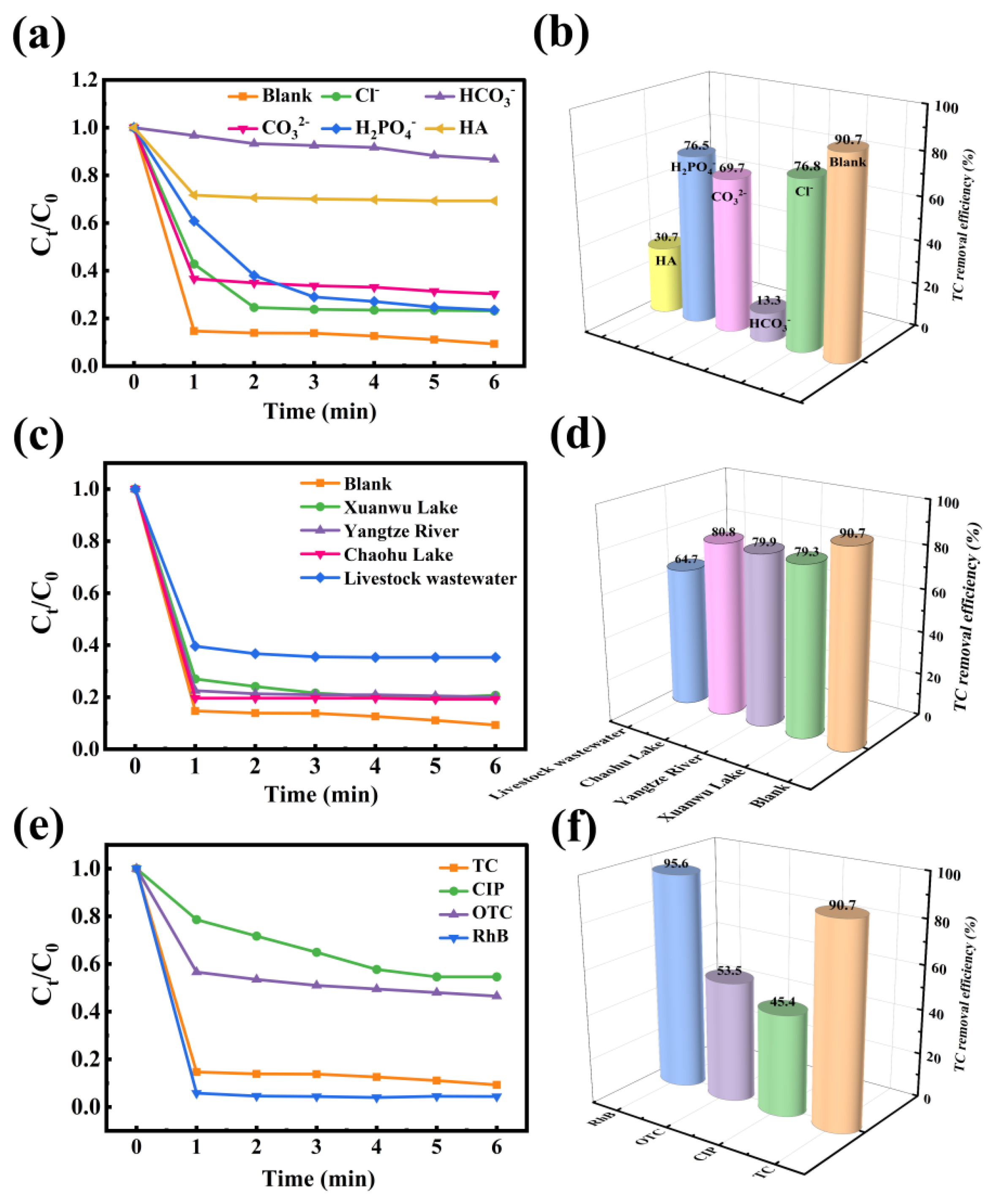
2.5. Response of the CoP-3/PMS/Vis Degradation System to External Disturbances
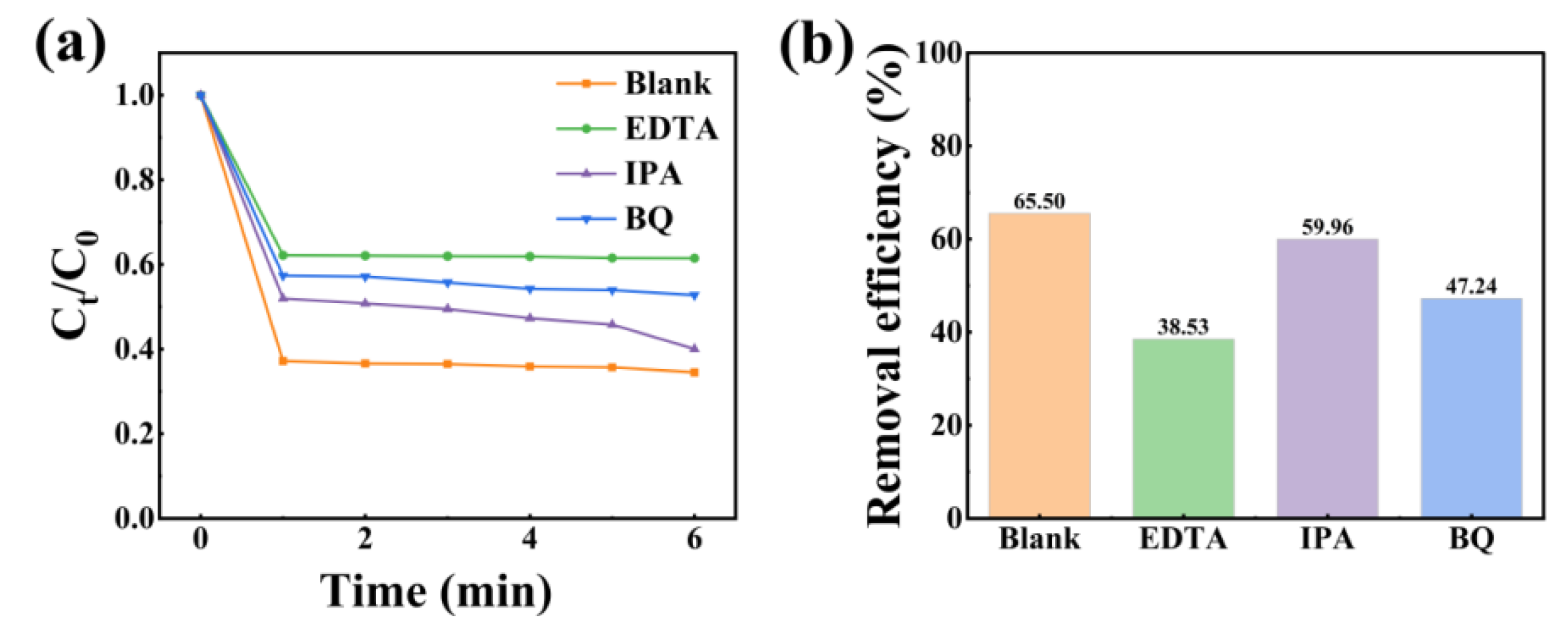
2.6. Free Radical Analysis
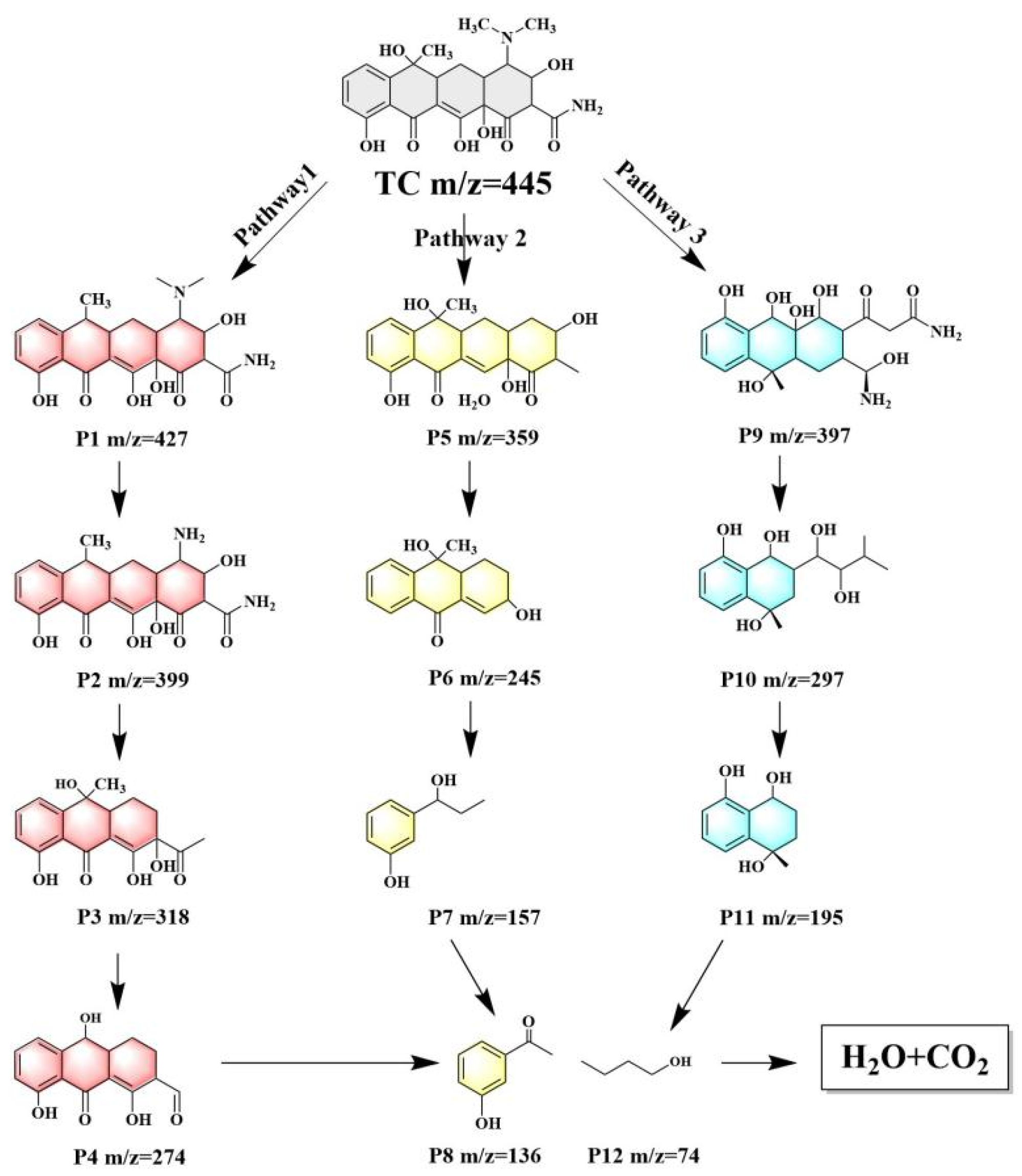
2.7. Transformation Pathways of TC Degradation
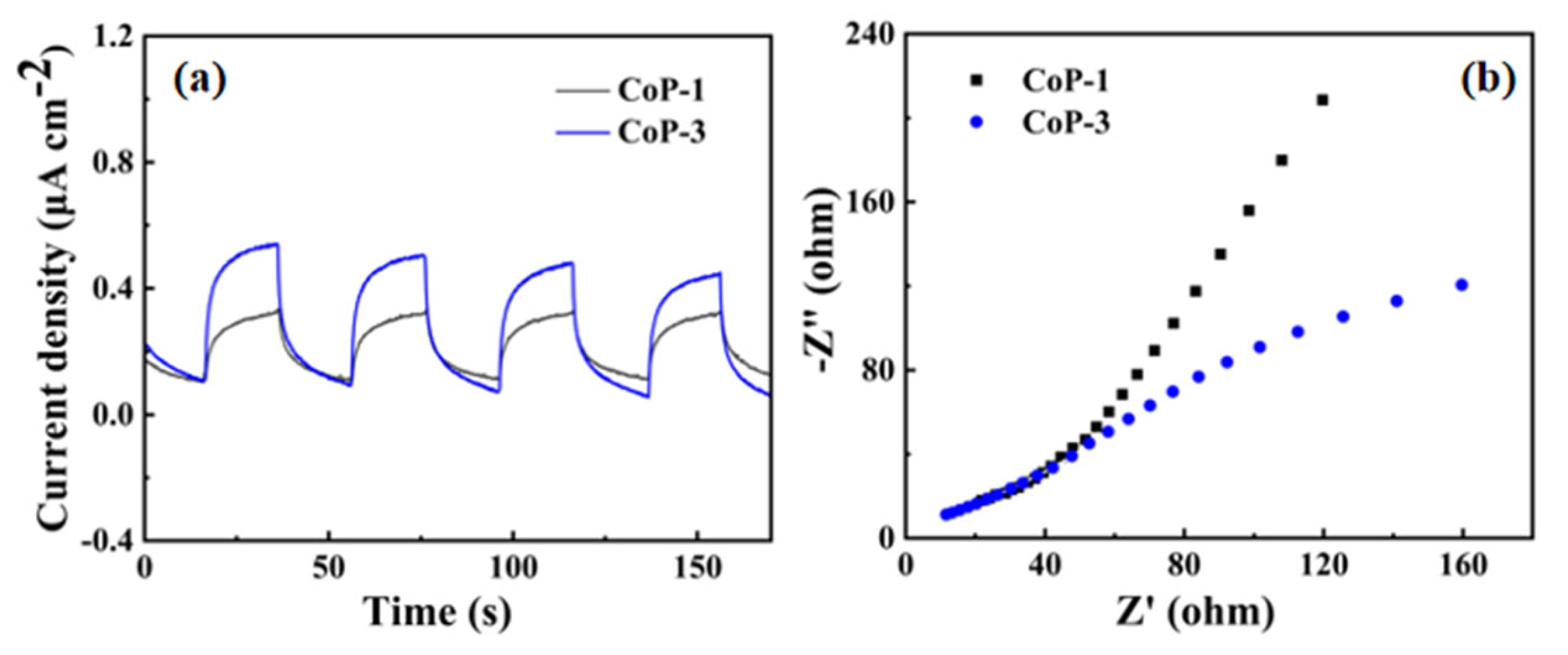
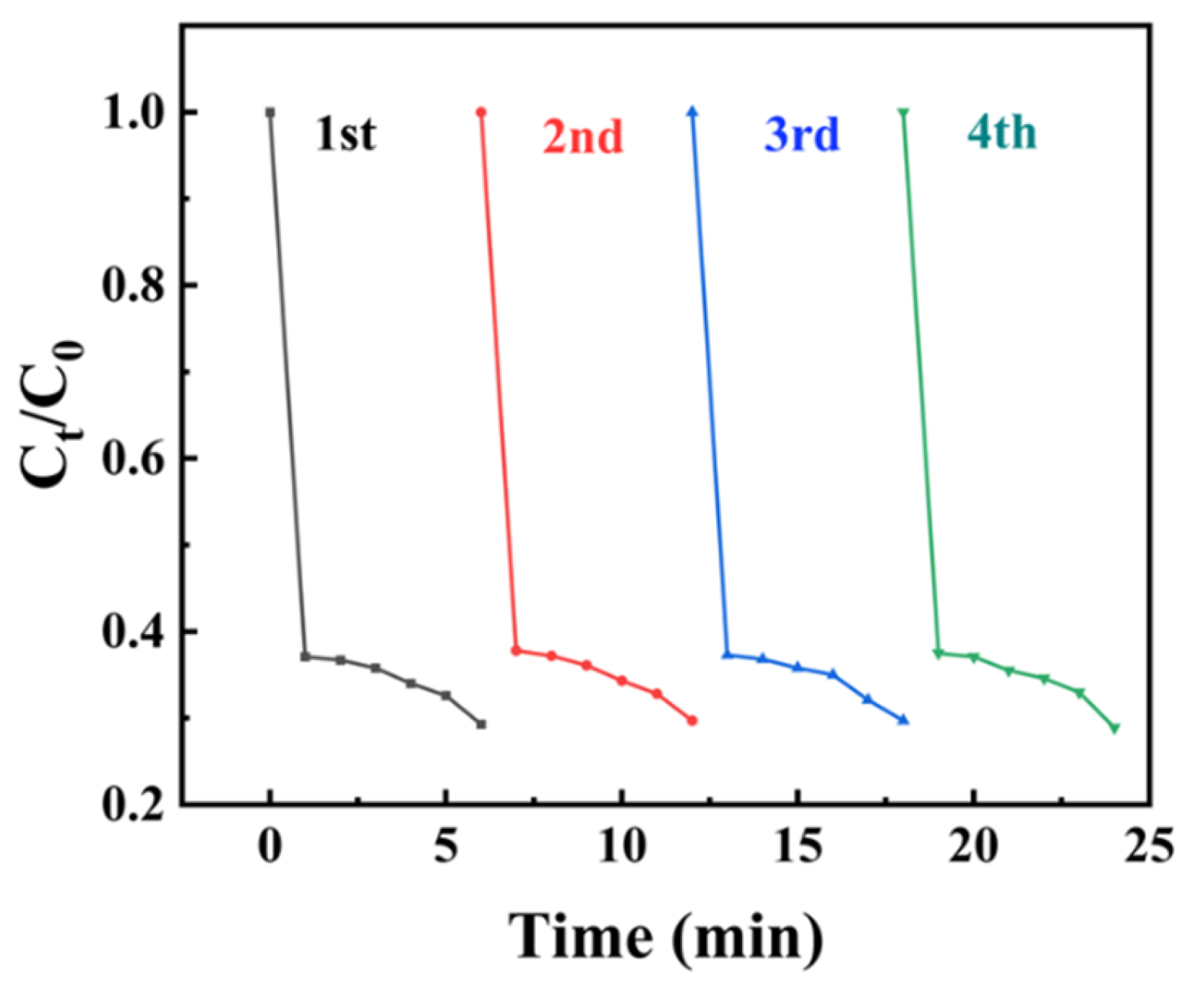
2.8. Catalyst Stability
2.9. Catalyst Performance Comparison
3. Materials and Methods
3.1. Chemicals and Reagents
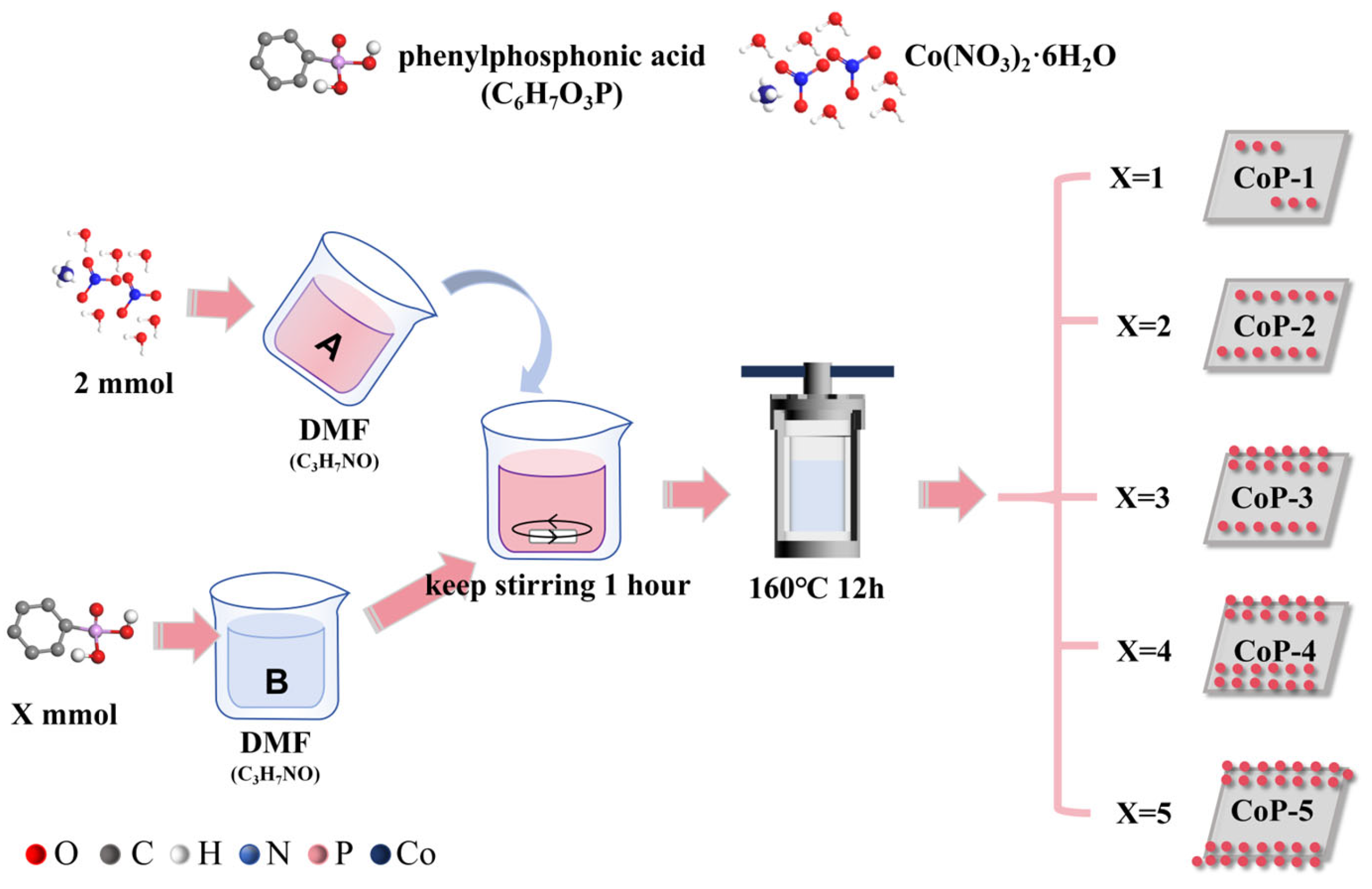
3.2. Synthesis of Catalysts
3.3. Characterization Techniques and Degradation Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, X.Y.; Lai, C.Y.; Zhao, H.P. A Novel Oxygen-Based Manganese Oxides Modified Membrane Reactor for TC Oxidative Degradation. J. Water Process Eng. 2024, 59, 104978. [Google Scholar] [CrossRef]
- Li, Y.; Jia, Z.; Xu, L.; Sheng, G.; Wan, C. Synergistic Removal of Tetracycline Hydrochloride by Nano-MnOx/NCF Composite Fiber (MNCs) Catalysts via Heterogeneous Peroxymonosulfate (PMS) Activation. Inorg. Chem. Commun. 2025, 172, 113682. [Google Scholar] [CrossRef]
- Lyu, J.; Yang, L.; Zhang, L.; Ye, B.; Wang, L. Antibiotics in Soil and Water in China–a Systematic Review and Source Analysis. Environ. Pollut. 2020, 266, 115147. [Google Scholar] [CrossRef]
- Chu, G.; Qi, W.; Chen, W.; Zhang, Y.; Gao, S.; Wang, Q.; Gao, C.; Gao, M. Metagenomic Insights into the Nitrogen Metabolism, Antioxidant Pathway, and Antibiotic Resistance Genes of Activated Sludge from a Sequencing Batch Reactor under Tetracycline Stress. J. Hazard. Mater. 2024, 462, 132788. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Johnson, T.A.; Su, J.Q.; Qiao, M.; Guo, G.X.; Stedtfeld, R.D.; Hashsham, S.A.; Tiedje, J.M. Diverse and Abundant Antibiotic Resistance Genes in Chinese Swine Farms. Proc. Natl. Acad. Sci. USA 2013, 110, 3435–3440. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.F. Antibiotic Resistance in the Environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- Ding, Y.; Liang, B.; Jiang, W.; Han, J.; Guadie, A.; Yun, H.; Cheng, H.; Yang, R.; Liu, S.J.; Wang, A.; et al. Effect of Preferential UV Photolysis on the Source Control of Antibiotic Resistome during Subsequent Biological Treatment Systems. J. Hazard. Mater. 2021, 414, 125484. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Shen, X.; Lu, X.; Guo, Y.; Li, Y.; Han, X.; Liu, R.; Chen, F.; Sun, C. Research Status of Membrane Separation Technology in the Treatment of Antibiotic Wastewater. Environ. Sci. Water Res. Technol. 2025, 11, 1386–1400. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, G.; Xiao, B.; Geng, J.; Yang, Y.; Wang, D.; Li, J.; Wang, J.; Zhu, Y. Iron-Based Resin Heterogeneous Photo-Self-Fenton System for Efficient Photocatalytic Degradation of Antibiotic Wastewater. Sep. Purif. Technol. 2024, 330, 125338. [Google Scholar] [CrossRef]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.d.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic Resistance in Microbes: History, Mechanisms, Therapeutic Strategies and Future Prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef]
- Sun, Y.; Yi, F.; Li, R.; Min, X.; Qin, H.; Cheng, S.; Liu, Y. Inorganic–Organic Hybrid Membrane Based on Pillararene-intercalated MXene Nanosheets for Efficient Water Purification. Angew. Chem. Int. Ed. 2022, 61, e202200482. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Z.; Huang, F.; Liu, Y.; Feng, L.; Jiang, J.; Zhang, L.; Qi, F.; Liu, C. Carbonized Polyaniline Activated Peroxymonosulfate (PMS) for Phenol Degradation: Role of PMS Adsorption and Singlet Oxygen Generation. Appl. Catal. B 2021, 286, 119921. [Google Scholar] [CrossRef]
- Feng, S.; Xie, T.; Wang, J.; Yang, J.; Kong, D.; Liu, C.; Chen, S.; Yang, F.; Pan, M.; Yang, J.; et al. Photocatalytic Activation of PMS over Magnetic Heterojunction Photocatalyst SrTiO3/BaFe12O19 for Tetracycline Ultrafast Degradation. Chem. Eng. J. 2023, 470, 143900. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, M.; Ge, C.; Zhang, P.; Xu, W.; Zhang, L.; Zhou, S.; Liao, J. Cu Single Atoms/O Doping g-C3N4 Mediated Photocatalytic Activation of Peroxymonosulfate for Ultrafast Carbamazepine Removal via High 1O2 Yield. Appl. Surf. Sci. 2023, 640, 158290. [Google Scholar] [CrossRef]
- Shi, W.; Hao, C.; Fu, Y.; Guo, F.; Tang, Y.; Yan, X. Enhancement of Synergistic Effect Photocatalytic/Persulfate Activation for Degradation of Antibiotics by the Combination of Photo-Induced Electrons and Carbon Dots. Chem. Eng. J. 2022, 433, 133741. [Google Scholar] [CrossRef]
- Hu, J.; Tian, J.; Yang, Y.; Li, S.; Lu, J. Enhanced Antibiotic Degradation via Photo-Assisted Peroxymonosulfate over Graphitic Carbon Nitride Nanosheets/CuBi2O4: Highly Efficiency of Oxygen Activation and Interfacial Charge Transfer. J. Colloid Interface Sci. 2024, 661, 68–82. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Y.; Wang, X.; Zhou, Y.; Wu, W.D.; Chen, X.D.; Wu, Z. Unraveling the Photocatalytic Activation of Peroxymonosulfate on Anatase/Rutile Homojunctions: Efficacy and Mechanism Study. Chem. Eng. J. 2023, 476, 146804. [Google Scholar] [CrossRef]
- Jiang, X.; Li, J.; Li, J.; Xu, W.; Liu, Z. Visible-Light-Driven Peroxymonosulfate Activation for Accelerating Tetracycline Removal Using Co-TiO2 Nanospheres. Catalysts 2023, 13, 836. [Google Scholar] [CrossRef]
- Cui, M.; Cui, K.; Liu, X.; Zhang, Y.; Chen, X.; Chen, Y.; Sun, S.; Liu, J.; Hu, Z. Peroxymonosulfate Conversion to Singlet Oxygen over π-Electron Delocalized Carbon Nitride for Selective Degradation of Emerging Contaminants. J. Environ. Chem. Eng. 2023, 11, 111494. [Google Scholar] [CrossRef]
- Xiang, W.; Liao, Y.; Cui, J.; Fang, Y.; Jiao, B.; Su, X. Room-Temperature Synthesis of Fe/Cu-BTC for Effective Removal of Tetracycline Antibiotic from Aquatic Environments. Inorg. Chem. Commun. 2024, 167, 112728. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, Y.; Han, C.; Li, Z.; Lun, X.; Zhang, C. Photocatalysis-PMS Oxidation System Based on CQDs-Doped Carbon Nitride Nanosheets for Degradation of Residual Drugs in Water. Environ. Sci. Pollut. Res. 2023, 30, 108538–108552. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Shen, Q.; Nan, T.; Zhou, M.; Xia, Y.; Hu, G.; Zheng, Q.; Wu, Y.; Bian, T.; Wei, T.; et al. Cobalt-Based Catalysts for Heterogeneous Peroxymonosulfate (PMS) Activation in Degradation of Organic Contaminants: Recent Advances and Perspectives. J. Alloys Compd. 2023, 958, 170370. [Google Scholar] [CrossRef]
- Xian, L.; Fan, G. N,S,O Triply-Doped Carbon with Nanotubes-Interwoven Nanosheets Encapsulated Co Nanoparticles for Robust Antibiotic Destruction via Activating Peroxymonosulfate. Environ. Res. 2024, 248, 118259. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Feng, C.; Ding, D.; Chen, N.; Yang, S.; Chen, H.; Wang, X.; Chen, R. Oxygen Vacancies-Enriched CoFe2O4 for Peroxymonosulfate Activation: The Reactivity between Radical-Nonradical Coupling Way and Bisphenol a. J. Hazard. Mater. 2021, 418, 126357. [Google Scholar] [CrossRef]
- Ren, T.; Yin, M.; Chen, S.; Ouyang, C.; Huang, X.; Zhang, X. Single-Atom Fe-N4 Sites for Catalytic Ozonation to Selectively Induce a Nonradical Pathway toward Wastewater Purification. Environ. Sci. Technol. 2023, 57, 3623–3633. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, X.; Zhao, S.; Wang, S. gCN-P: A Coupled g-C3N4/Persulfate System for Photocatalytic Degradation of Organic Pollutants under Simulated Sunlight. Environ. Sci. Pollut. Res. 2022, 29, 23280–23291. [Google Scholar] [CrossRef]
- Du, M.; Li, D.; Liu, S.F.; Yan, J. Transition Metal Phosphides: A Wonder Catalyst for Electrocatalytic Hydrogen Production. Chin. Chem. Lett. 2023, 34, 108156. [Google Scholar] [CrossRef]
- Yan, X.; An, H.; Chen, Z.; Yang, G. Significantly Enhanced Charge Transfer Efficiency and Surface Reaction on NiP2/g-C3N4 Heterojunction for Photocatalytic Hydrogen Evolution. Chin. J. Chem. Eng. 2022, 43, 31–39. [Google Scholar] [CrossRef]
- Xu, L.H.; Che, P.C.; Zhang, X.J.; Cosnier, S.; Shan, D. FeP Nanoparticles Highly Dispersed on N,P-Doped Petaloid Carbon Nanosheet: Interface Engineering and Boosted Intrinsic ORR Activity. Appl. Surf. Sci. 2023, 620, 156770. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Y.; Ma, Z.; Sun, Z.; Wang, J.; Feng, J.; Yan, W.; Wang, H. Challenges and Strategies of Transition Metal Phosphides Applied in Oxygen Evolution Reaction of Electrocatalytic Water Splitting: A Review. Fuel 2024, 369, 131741. [Google Scholar] [CrossRef]
- Sushma; Kumari, M.; Saroha, A.K. Performance of Various Catalysts on Treatment of Refractory Pollutants in Industrial Wastewater by Catalytic Wet Air Oxidation: A Review. J. Environ. Manag. 2018, 228, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhai, Y.; Wang, X.; Cao, X.; Guan, D.; Zhang, H.; Zhu, Q.; Fang, S.; Wang, L. Polar Solvent-Mediated Direct Room Temperature Synthesis of Pure-Red-Emitting Perovskite Nanocrystals. J. Lumin. 2024, 271, 120610. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, G.; Ji, Q.; Qu, J.; Liu, H. Arrayed Cobalt Phosphide Electrocatalyst Achieves Low Energy Consumption and Persistent H2 Liberation from Anodic Chemical Conversion. Nano-Micro Lett. 2020, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.B.; Ying, J.; Xiao, Y.X.; Tian, G.; Dong, Y.; Shen, L.; Córdoba De Torresi, S.I.; Symes, M.D.; Janiak, C.; Yang, X.-Y. Directed Mass and Electron Transfer Promoted by Hierarchical Porous Co–P–O Leads to Enhancement of the Overall Water Splitting Efficiency. ACS Catal. 2023, 13, 14802–14812. [Google Scholar] [CrossRef]
- Zhang, Y.; Diao, Z.; Wei, J.; Luo, M.; Xie, L.; Huang, L.; Ren, J.; Ai, N.; Li, L.; Guo, H.; et al. Bifunctional Synergistic CoP/Coral-like g-C3N4 Catalyst: Boosting the Photocatalytic Water Splitting Hydrogen Evolution and Appreciation of Anisalcohol at Same Time. Appl. Surf. Sci. 2023, 614, 156187. [Google Scholar] [CrossRef]
- Luo, R.; Liu, C.; Li, J.; Wang, J.; Hu, X.; Sun, X.; Shen, J.; Han, W.; Wang, L. Nanostructured CoP: An Efficient Catalyst for Degradation of Organic Pollutants by Activating Peroxymonosulfate. J. Hazard. Mater. 2017, 329, 92–101. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Giannakis, S.; Moussavi, G.; Bensimon, M.; Gholami, M.R.; Pulgarin, C. An Innovative, Highly Stable Ag/ZIF-67@GO Nanocomposite with Exceptional Peroxymonosulfate (PMS) Activation Efficacy, for the Destruction of Chemical and Microbiological Contaminants under Visible Light. J. Hazard. Mater. 2021, 413, 125308. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Chen, X.; Huang, Z.; Zou, Z.; Zheng, H. Co3O4-ZnO/rGO Catalyst Preparation and Rhodamine B Degradation by Sulfate Radical Photocatalysis. J. Zhejiang Univ.-Sci. A 2023, 24, 710–721. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, H.; Ma, H.; Ko, J.; Sun, W.; Andrew Lin, K.; Zhan, S.; Wang, H. Highly Efficient Activation of Peroxymonosulfate by MOF-Derived CoP/CoOx Heterostructured Nanoparticles for the Degradation of Tetracycline. Chem. Eng. J. 2022, 430, 132816. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, J.; Li, Z.; Zhu, M.; Yin, R. Activation of Peroxymonosulfate by Co2P via Interfacial Radical Pathway for the Degradation and Mineralization of Carbamazepine. Surf. Interfaces 2023, 40, 103045. [Google Scholar] [CrossRef]
- Li, Y.; Chen, L.; Zhang, J.; Zhu, C.; Liu, L. Synergistic Photocatalytic Degradation of TC-HCl by Mn3+/Co2+/Bi2O3 and PMS. Inorg. Chem. Commun. 2023, 150, 110468. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Xu, S.; Du, X.; Zhang, K.; Hu, C.P.; Zhan, S.; Mi, X.; Oh, W.D.; Hu, X.; et al. A Recyclable Co-Fe Bimetallic Immobilized Cellulose Hydrogel Bead (CoFeO@CHB) to Boost Singlet Oxygen Evolution for Tetracycline Degradation. Catalysts 2023, 13, 1150. [Google Scholar] [CrossRef]
- Ren, X.; Xu, P.; Tian, K.; Cao, M.; Shi, F.; Zhang, G. Peroxymonosulfate Activation by CuO-Fe2O3-Modified Ni Foam: A 1O2 Dominated Process for Efficient and Stable Degradation of Tetracycline. Catalysts 2023, 13, 329. [Google Scholar] [CrossRef]
- Xu, D.; Yang, T.; Dong, Y.; Wang, Q.; Zhu, J.; Zhang, G.; Lv, L.; Xia, Y.; Ren, Z.; Wang, P. Activation of Peroxymonosulfate by CoP@Co2P Heterostructures via Radical and Non-Radical Pathways for Antibiotics Degradation. Ceram. Int. 2023, 49, 16999–17007. [Google Scholar] [CrossRef]
- Fu, X.; Lin, Y.; Yang, C.; Wu, S.; Wang, Y.; Li, X. Peroxymonosulfate Activation via CoP Nanoparticles Confined in Nitrogen-Doped Porous Carbon for Enhanced Degradation of Sulfamethoxazole in Wastewater with High Salinity. J. Environ. Chem. Eng. 2022, 10, 107734. [Google Scholar] [CrossRef]
- Huang, X.; Li, G.; Liu, L.; He, Y.; Su, X.; Pan, Y.; Xing, W.; Wu, G.; Zhang, M. Boosting Peroxymonosulfate Activation over Co-N-C@Co9S8 Double-Shelled Nanocages for Ciprofloxacin Degradation: Insights into Catalytic Performance, Degradation Mechanism and Routes. Sep. Purif. Technol. 2025, 359, 130662. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, J.; Yuan, Y.; Guo, Y.; Tan, X.; Wang, H.; Hu, X.; Tang, C. Highly Conductive Ti3C2 MXene Reinforced G-C3N4 Heterojunction Photocatalytic for the Degradation of Ciprofloxacin: Mechanism Insight. Sep. Purif. Technol. 2024, 330, 125520. [Google Scholar] [CrossRef]
- Meng, X.; Yang, J.; Xu, S.; Zhang, C.; Ma, B.; Ding, Y. Integrating Mo2Bx (x = 1, 4) with CdS for Efficient Photocatalytic Hydrogen Production. Chem. Eng. J. 2021, 410, 128339. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, J.F.; Yang, J.-C.E.; Li, D.; Zhong, L.B.; Zheng, Y.M. Ultra-Fast Degradation of Ciprofloxacin by the Peroxymonosulfate Activation Using a Co/Al-LDH Decorated Magnetic Hydrochar: Structural Design, Catalytic Performance and Synergistic Effects. Chem. Eng. J. 2023, 477, 146961. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Peng, Y.; Xiao, W.; Yu, W.; Wang, H.; Bian, Z. Continuous Peroxymonosufate Activation for Antibiotics Degradation via Fluorine-Free-Ti3C2Tx-CoFe2O4 Hydrogel Beads: Performance, Mechanism and Application. Appl. Catal. B 2024, 358, 124441. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, X.; Liu, Y.; Zhang, Y.; Xia, S.; Zhao, J. Visible-Light-Driven g-C3N4 Doped CuFe2O4 Floating Catalyst Enhanced Peroxymonosulfate Activation for Sulfamethazine Removal via Singlet Oxygen and High-Valent Metal-Oxo Species. Chem. Eng. J. 2023, 455, 140198. [Google Scholar] [CrossRef]
- Shen, Q.; Song, X.; Fan, J.; Chen, C.; Guo, Z. Degradation of Humic Acid by UV/PMS: Process Comparison, Influencing Factors, and Degradation Mechanism. RSC Adv. 2024, 14, 22988–23003. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Impalli, I.; Poleon, S.; Denoel, P.; Cipriano, M.; Van Boeckel, T.P.; Pecetta, S.; Bloom, D.E.; Nandi, A. Global Trends in Antibiotic Consumption during 2016–2023 and Future Projections through 2030. Proc. Natl. Acad. Sci. USA 2024, 121, e2411919121. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.H.; Li, Y.Q.; Hayat, F.; Liu, C.; Li, J.; Chen, M. Multi-Targeted Removal of Coexisted Antibiotics in Water by the Synergies of Radical and Non-Radical Pathways in PMS Activation. Sep. Purif. Technol. 2023, 305, 122475. [Google Scholar] [CrossRef]
- Lv, H.; Yu, W.; Li, Y.; Sun, X.; Hou, X.; Bian, Z.; Wang, H.; Wei, Y. Cyclodextrin-Supported Sulfide Zero-Valent Iron as PMS Activator for Simultaneous Removing Norfloxacin and ARGs in Reclaimed Water: Activation and Controlled Release of Active Components. Chem. Eng. J. 2025, 505, 159656. [Google Scholar] [CrossRef]
- Chen, L.; Fu, W.; Hou, C.; Yang, Y.; Zhang, X. A Comprehensive Assessment of Catalytic Performances of Mn2O3 Nanoparticles for Peroxymonosulfate Activation during Bisphenol A Degradation. Catalysts 2021, 11, 993. [Google Scholar] [CrossRef]
- Wang, C.; Chang, L.; Zhang, X.; Chai, H.; Huang, Y. Promoting Oxygen Vacancies Utility for Tetracycline Degradation via Peroxymonosulfate Activation by Reduced Mg-Doped Co3O4: Kinetics and Key Role of Electron Transfer Pathway. Environ. Res. 2024, 252, 118892. [Google Scholar] [CrossRef]
- Ma, R.; Chen, Z.; Xu, W.; Yu, R.; Zhang, Y.; Chen, F.; Peng, X.; Ni, B.-J.; Qian, J. Fe-MOFs/CuS Nanocomposite-Mediated Peroxymonosulfate Activation for Tetracycline Degradation: Boosted Dual Redox Cycles. J. Clean. Prod. 2024, 442, 140885. [Google Scholar] [CrossRef]
- Huo, Y.; Zhou, G.; Guan, Y.; Meng, X.; Yan, W.; Hu, J.; Jiang, Y.; Xu, Y.; Yin, J.; Zhang, L. Inducing Oxygen Vacancies in ZnO/Co3O4 via g-C3N4 Carrier for Enhanced Universality and Stability in TC Degradation. Colloids Surf. A 2024, 683, 132974. [Google Scholar] [CrossRef]
- Li, Z.; He, J.; Ma, H.; Zang, L.; Li, D.; Guo, S.; Ci, Y. Preparation of Heterogeneous TiO2/g-C3N4 with a Layered Mosaic Stack Structure by Use of Montmorillonite as a Hard Template Approach: TC Degradation, Kinetic, Mechanism, Pathway and DFT Investigation. Appl. Clay Sci. 2021, 207, 106107. [Google Scholar] [CrossRef]
- Jahanshahi, M.; Afsharipour, R.; Mirhosseini Rayen, N.A. Fabrication of a Spirulina Algae-CuFeS2/CuWO4 Composite for Effective Photo-Fenton Degradation of TC and RhB with Environmental Assessment. J. Environ. Manag. 2025, 381, 125340. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Song, L.; Guo, Y.; Zhang, X.; Hu, S. Coupled Homogeneous-Heterogeneous Fenton-like System to Highly Efficiently Degrade Tetracycline in Water at Circumneutral pH: Mechanism and Toxicity Evaluation. J. Water Process Eng. 2025, 72, 107569. [Google Scholar] [CrossRef]
- Chen, P.; Cheng, Z.; Zhang, X.; Yan, C.; Wei, J.; Qiu, F.; Liu, Y. Fe–Mn Bimetallic Catalyst to Activate Peroxymonosulfate (PMS) for Efficient Degradation of Tetracycline: Mechanism Insights and Application for Pharmaceutical Wastewater. J. Clean. Prod. 2024, 445, 141365. [Google Scholar] [CrossRef]
- Nie, L.; Fang, C.; Xin, S.; Yang, Y.; Chen, H.; Chen, X.; Li, X. Effective Removal of Tetracycline Hydrochloride from Wastewater over Porous Co3O4@NC/Honeycomb Ceramics by Fenton-like Catalysis. Inorg. Chem. Commun. 2025, 171, 113598. [Google Scholar] [CrossRef]
- Xing, H.; Gao, S.; Zhang, J.; Xu, Y.; Du, H.; Zhu, Z.; Wang, J.; Yao, Y.; Zhang, S.; Ren, L. Ultrasound-Assisted Synthesized BiFeO3 as FeOH+ Promoted Peroxymonosulfate Activator for Highly Efficient Degradation of Tetracycline. J. Alloys Compd. 2021, 854, 157281. [Google Scholar] [CrossRef]
- Su, L.; Long, L.; Wang, W.; Kuang, Z.; Zhou, X.; Chen, C.; Xu, M.; He, Y.; Yang, G. Mn-Enhanced Cobalt Silicate-Activated Peroxymonosulfate for Tetracycline Degradation: Inheriting Merit and Offsetting Deficiency of Oxygen Vacancies. ACS EST Eng. 2023, 3, 1614–1625. [Google Scholar] [CrossRef]
- Lu, S.; You, S.; Hu, J.; Li, X.; Li, L. Magnetic MnFe2O4 /ZIF-67 Nanocomposites with High Activation of Peroxymonosulfate for the Degradation of Tetracycline Hydrochloride in Wastewater. RSC Adv. 2024, 14, 7528–7539. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, B.; Wang, S.; Li, X.; Wang, C.; Liu, B.; Han, F.; Xu, Y.; Yu, P.; Sun, Y. Tetracycline Degradation by Peroxymonosulfate Activated with CoNx Active Sites: Performance and Activation Mechanism. Chem. Eng. J. 2022, 431, 133477. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Sun, W.; Bai, R.; He, Y.; Li, J.; Pan, Y.; Zhang, M.; Wu, G. Efficient Degradation of Tetracycline via Cobalt Phosphonate-Activated Peroxymonosulfate: Mechanistic Insights and Catalytic Optimization. Catalysts 2025, 15, 580. https://doi.org/10.3390/catal15060580
Huang X, Sun W, Bai R, He Y, Li J, Pan Y, Zhang M, Wu G. Efficient Degradation of Tetracycline via Cobalt Phosphonate-Activated Peroxymonosulfate: Mechanistic Insights and Catalytic Optimization. Catalysts. 2025; 15(6):580. https://doi.org/10.3390/catal15060580
Chicago/Turabian StyleHuang, Xinlin, Wenting Sun, Rong Bai, Yuchen He, Jingdan Li, Yuwei Pan, Ming Zhang, and Guangyu Wu. 2025. "Efficient Degradation of Tetracycline via Cobalt Phosphonate-Activated Peroxymonosulfate: Mechanistic Insights and Catalytic Optimization" Catalysts 15, no. 6: 580. https://doi.org/10.3390/catal15060580
APA StyleHuang, X., Sun, W., Bai, R., He, Y., Li, J., Pan, Y., Zhang, M., & Wu, G. (2025). Efficient Degradation of Tetracycline via Cobalt Phosphonate-Activated Peroxymonosulfate: Mechanistic Insights and Catalytic Optimization. Catalysts, 15(6), 580. https://doi.org/10.3390/catal15060580









