Comprehensive Insights into Photoreforming of Waste Plastics for Hydrogen Production
Abstract
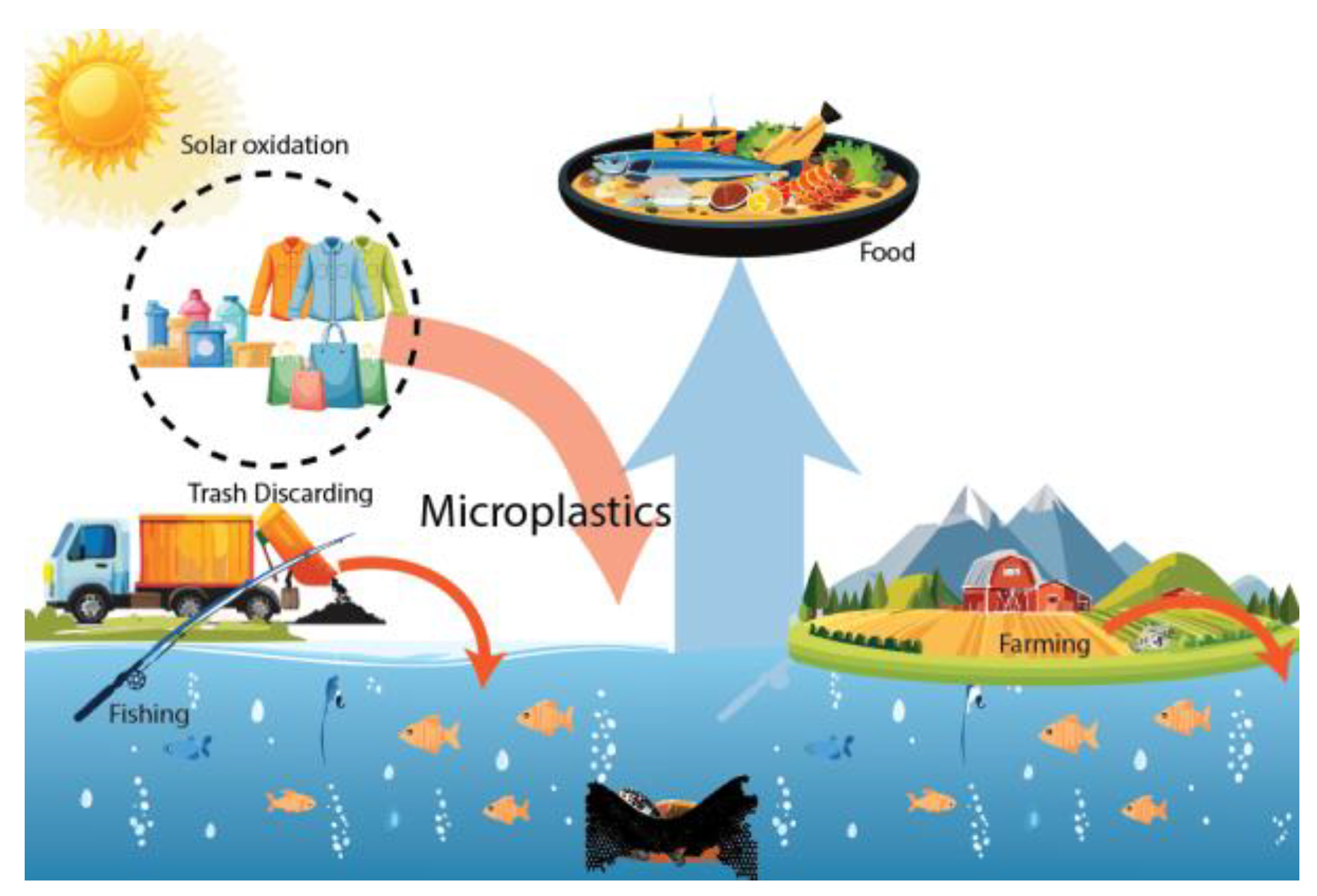
1. Introduction
2. Fundamentals of Plastic Photoreforming
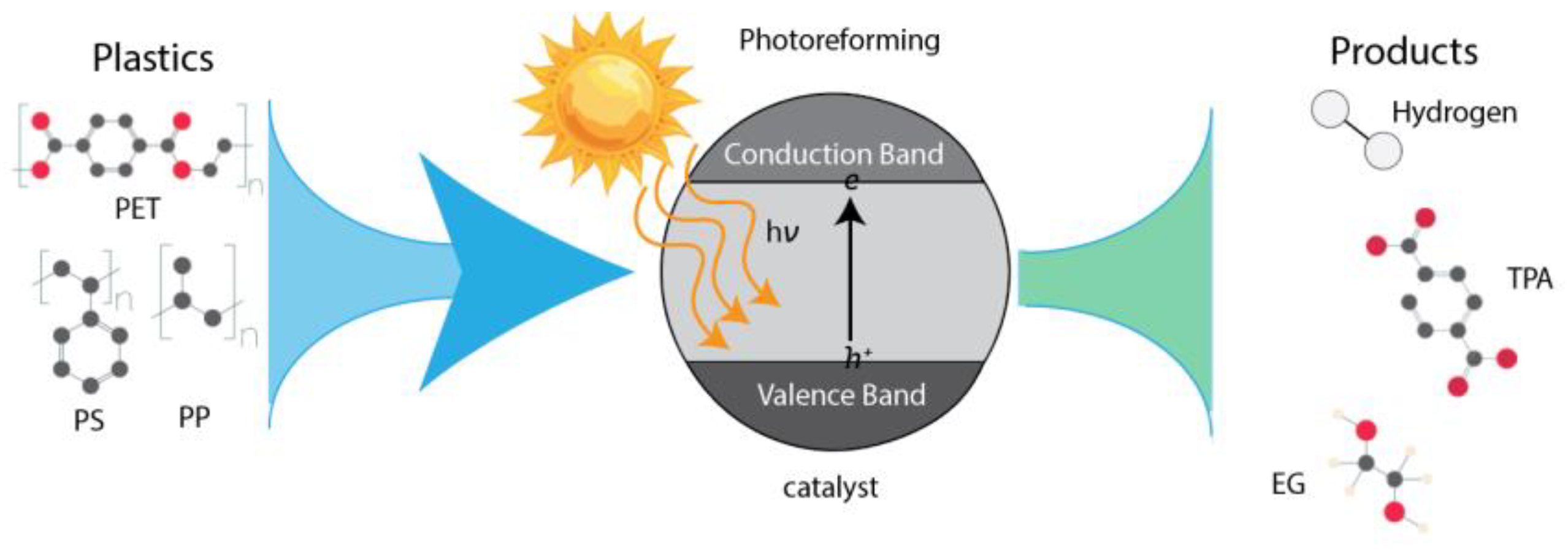
2.1. Overview of Photoreforming
- The catalyst absorbs photons, exciting electrons from the highest occupied molecular orbital to the lowest unoccupied molecular orbital, resulting in an activated catalyst.
- The excited catalyst interacts with reactants, generating active intermediates through H2 atom transfer or electron transfer, thereby selectively facilitating plastic conversion.
2.2. Reaction Pathways and Mechanisms
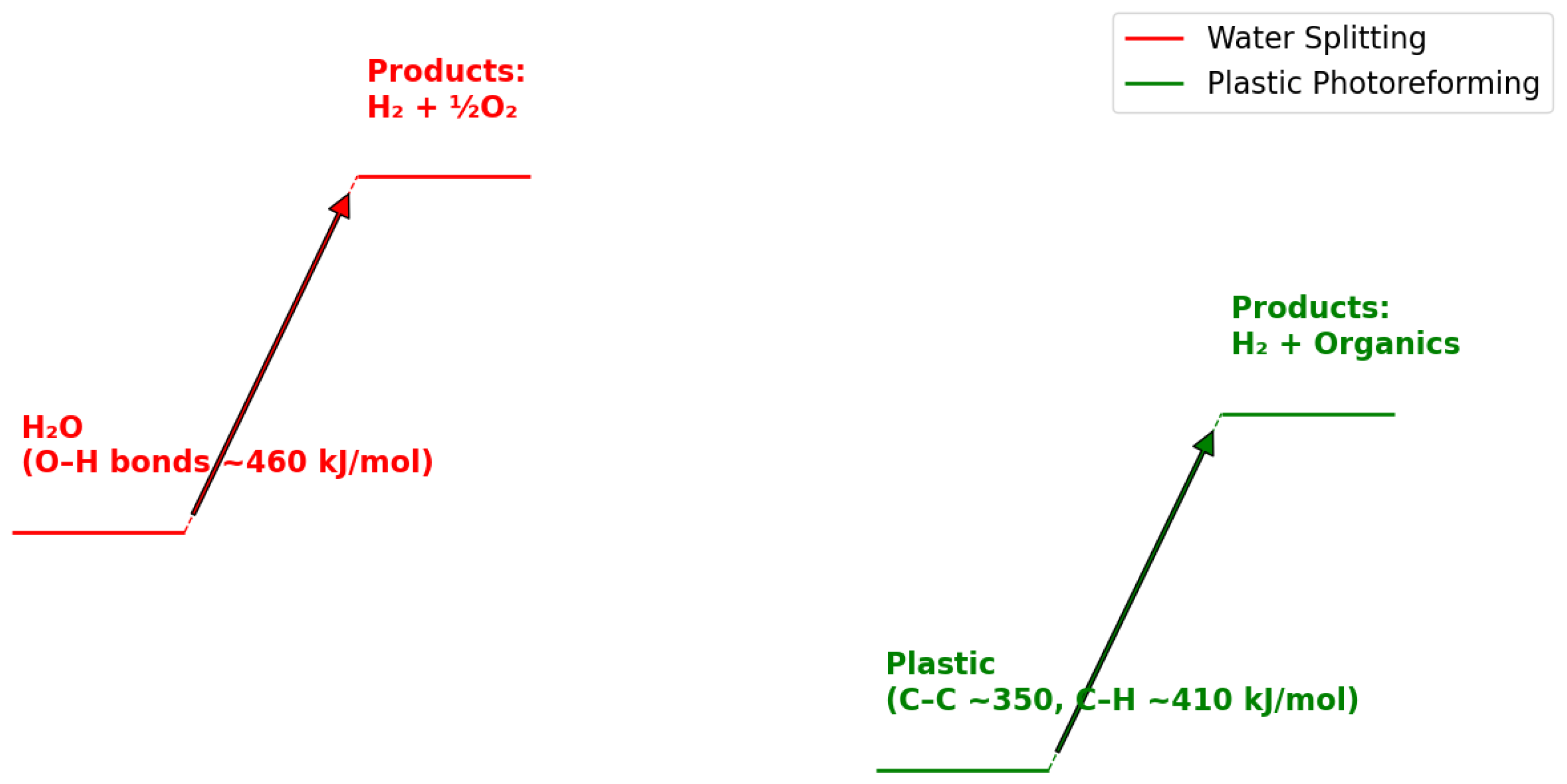
2.3. Thermodynamic and Kinetic Considerations
3. Types of Plastics and Their Suitability for Photoreforming
3.1. Common Plastics
3.2. Characteristics Affecting Photoreforming
4. Photocatalysts and Catalyst Engineering
4.1. Semiconductor Photocatalysts
4.2. Metal–Organic Frameworks (MOFs)
4.3. Carbon-Based and Heterojunction Composites
4.4. Noble Metal and Transition Metal Co-Catalysts
4.5. Design and Fabrication Strategies
4.6. Catalyst Characterization Techniques
5. Reactor Configurations and Photoreforming Conditions
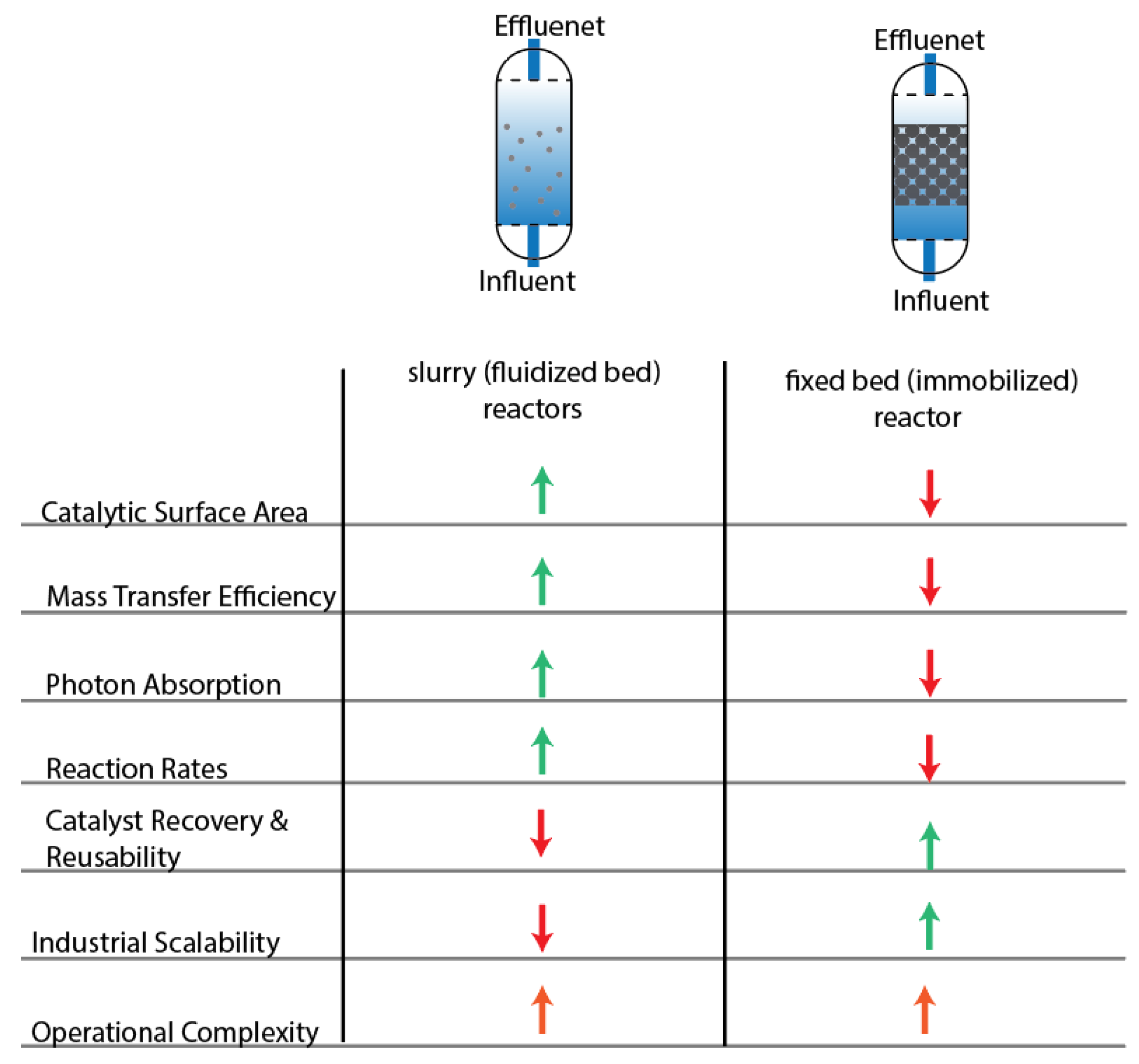
5.1. Bench-Scale Reactors and Scale-Up Challenges
5.2. Light Sources and Illumination Strategies
5.3. Operational Parameters
6. Performance Metrics and Process Evaluation
6.1. Hydrogen Yield and Production Rates
| Catalyst | Experimental Conditions | Hydrogen Yield (µmol g−1 h−1) | Reference |
|---|---|---|---|
| Pt/TiO2 | 500 W Xe lamp, 5 M NaOH, PET | 1130 | [134] |
| CNx|Ni2P | AM 1.5 sunlight, ambient temperature, PET | 82 | [58] |
| CNx|Ni2P | AM 1.5 sunlight, ambient temperature, PLA | 178 | |
| Zn|n2S4 (mesoporous) | Simulated sunlight, PET | 23.6 | [139] |
| Zn|n2S4 (conventional) | Simulated sunlight, PET | 8.9 | |
| CdS/CdOx | Simulated sunlight, PET | 6.6 | [140] |
| NiCr2O4/TiO2-Zn0.5Cd0.5S | Visible light, mixed plastics | 81.4 × 103 | [141] |
| NiPS3/CdS | Solar-driven, PET | 31.4 × 103 | [142] |
| NiPS3/CdS | Solar-driven, PLA | 39.8 × 103 | |
| g-C3N4/Pt | With NaOH pre-treatment, PET | 533 | [136] |
| Au0.28Pd0.72/TiO2 HS | 5 M NaOH, 300 W Xe, PET | 0.85 × 103 | [134] |
| MoS2/CdS | 300 W Xe lamp AM 1.5, 10 M KOH | PLA: 6.68 × 103 PET: 3.90 × 103 PE: 1.13 × 103 | |
| Co–Ga2O3 | 300 W Xe lamp, AM 1.5, 10 M KOH, PE | 692 | [143] |
| d-NiPS3/CdS | 43× and 1.5 × 300 W xenon lamp (PLS-SXE 300), λ > 400 nm, 2 M KOH, PLA | 39.76 × 103 | [137] |
6.2. Selectivity and Byproduct Formation
6.2.1. Liquid-Phase Organics
6.2.2. Gas-Phase Organics
6.2.3. Solid Residues
6.2.4. Treatment and Opportunities with Byproducts
6.3. Life Cycle Assessment (LCA) and Techno-Economic Analyses (TEA)
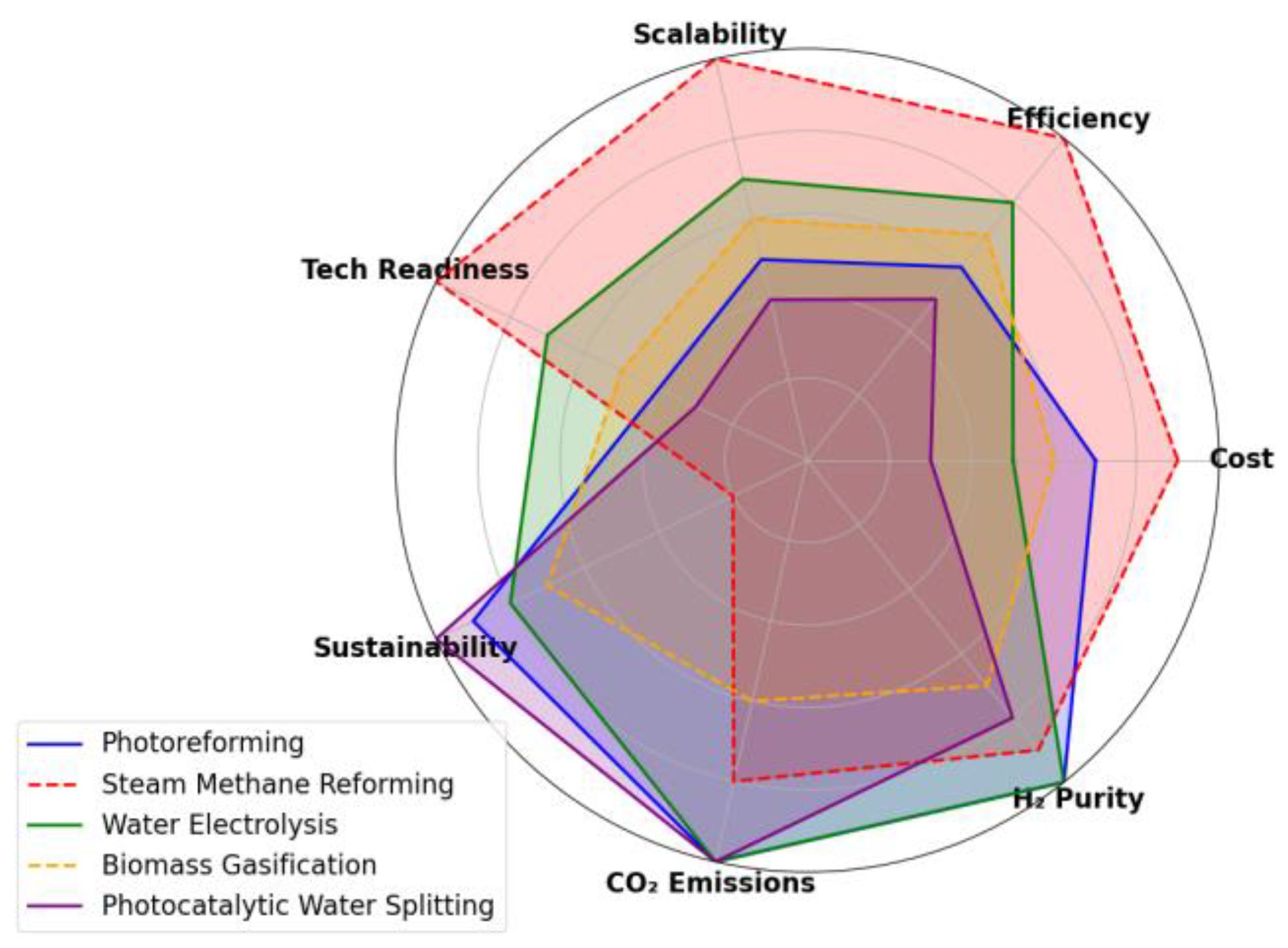
6.4. Comparative Benchmarking
7. Environmental and Sustainability Perspectives
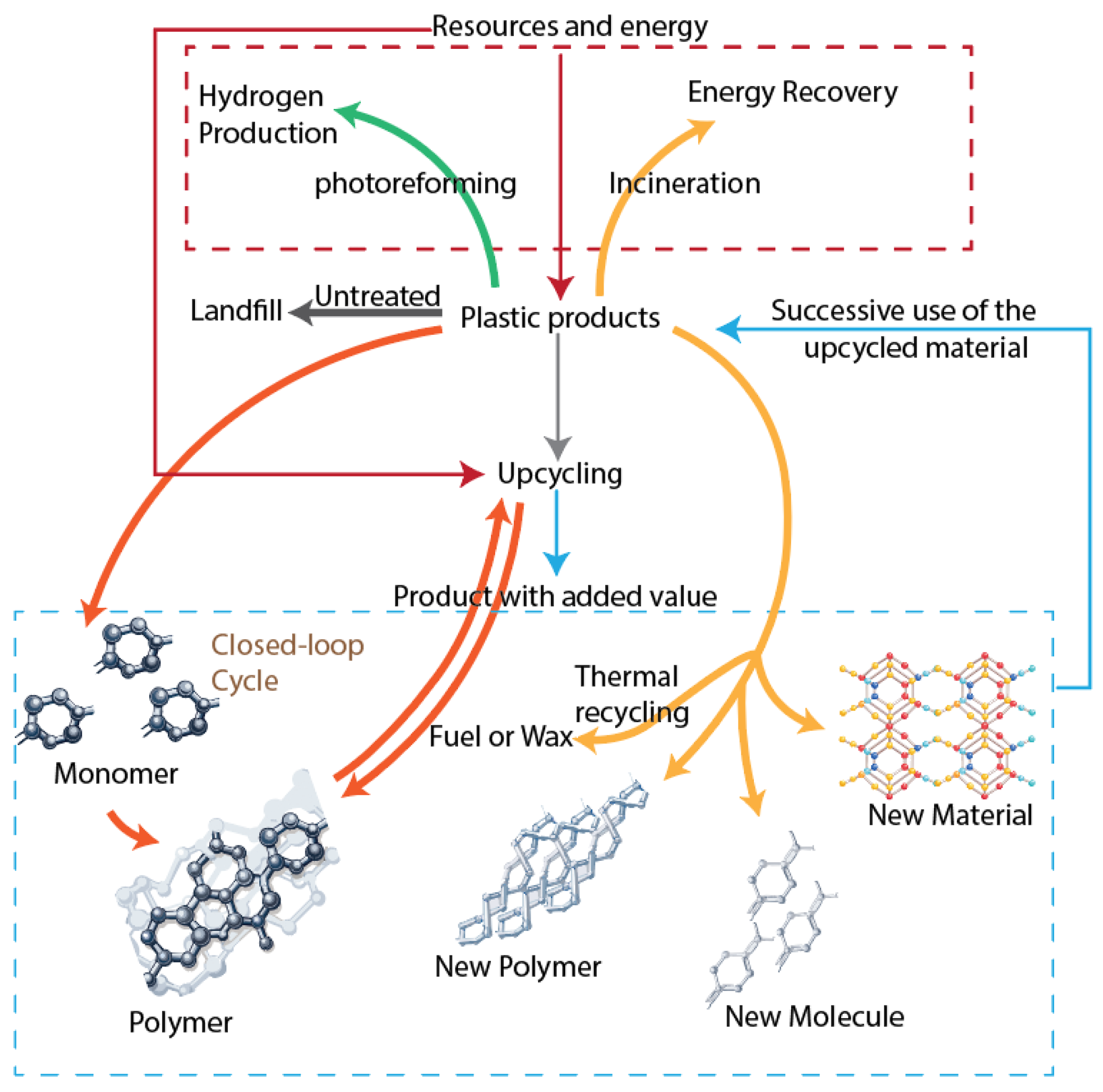
7.1. Waste Management and the Circular Economy Context
7.2. Policy and Regulatory Landscape
- Increased R&D Funding: Governments could allocate research grants to accelerate the development of high efficiency photocatalysts and scalable reactor designs.
- Market-Based Incentives: Carbon credits or renewable energy credits could be extended to photoreforming projects.
- Mandated Recycled Content: Setting minimum thresholds for recycled plastic usage in industrial applications could further drive investment in advanced recycling technologies.
8. Challenges and Future Directions
8.1. Technical Hurdles
8.2. Materials Innovation
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Market Trends in the Plastics Industry: An Analysis of Developments by Key Plastics Manufacturers. 2023. Available online: https://www.bccresearch.com/market-research/plastics/plastic-industry-market-trends.html (accessed on 3 May 2025).
- Plastic Production and Industry|Plastics and the Environment Series. Geneva Environment Network. Available online: https://www.genevaenvironmentnetwork.org/resources/updates/plastic-production-and-industry/ (accessed on 3 May 2025).
- Kumar, M.; Chen, H.; Sarsaiya, S.; Qin, S.; Liu, H.; Awasthi, M.K.; Kumar, S.; Singh, L.; Zhang, Z.; Bolan, N.S. Current research trends on micro-and nano-plastics as an emerging threat to global environment: A review. J. Hazard. Mater. 2021, 409, 124967. [Google Scholar] [CrossRef] [PubMed]
- Plastics—The Fast Facts 2024. Plastic Europe. 2024. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2024/ (accessed on 3 May 2025).
- Department of Energy Hydrogen Program Plan. U.S. Department of Energy 2024. Available online: https://www.hydrogen.energy.gov/docs/hydrogenprogramlibraries/pdfs/hydrogen-program-plan-2024.pdf?sfvrsn=bfc739dd_1 (accessed on 3 May 2025).
- Lan, K.; Yao, Y. Feasibility of gasifying mixed plastic waste for hydrogen production and carbon capture and storage. Commun. Earth Environ. 2022, 3, 300. [Google Scholar] [CrossRef]
- Jehanno, C.; Alty, J.W.; Roosen, M.; De Meester, S.; Dove, A.P.; Chen, E.Y.X.; Leibfarth, F.A.; Sardon, H. Critical advances and future opportunities in upcycling commodity polymers. Nature 2022, 603, 803–814. [Google Scholar] [CrossRef] [PubMed]
- National Overview: Facts and Figures on Materials, Wastes and Recycling. US EPA. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/national-overview-facts-and-figures-materials#Recycling/Composting (accessed on 3 May 2025).
- Anh Nguyen, T.K.; Tran-Phu, T.; Daiyan, R.; Minh Chau Ta, X.; Amal, R.; Tricoli, A. From Plastic Waste to Green Hydrogen and Valuable Chemicals Using Sunlight and Water. Angew. Chem. Int. Ed. Engl. 2024, 63, e202401746. [Google Scholar] [CrossRef]
- Edirisooriya, E.M.N.T.; Senanayake, P.S.; Wang, H.B.; Talipov, M.R.; Xu, P.; Wang, H. Photo-reforming and degradation of waste plastics under UV and visible light for H2 production using nanocomposite photocatalysts. J. Environ. Chem. Eng. 2023, 11, 109580. [Google Scholar] [CrossRef]
- Hai, H.T.N.; Nguyen, T.T.; Nishibori, M.; Ishihara, T.; Edalati, K. Photoreforming of plastic waste into valuable products and hydrogen using a high-entropy oxynitride with distorted atomic-scale structure. Appl. Catal. B Environ. Energy 2025, 365, 124968. [Google Scholar] [CrossRef]
- Ashraf, M.; Ullah, N.; Khan, I.; Tremel, W.; Ahmad, S.; Tahir, M.N. Photoreforming of Waste Polymers for Sustainable Hydrogen Fuel and Chemicals Feedstock: Waste to Energy. Chem. Rev. 2023, 123, 4443–4509. [Google Scholar] [CrossRef]
- Mehtab, A.; Ali, S.A.; Sadiq, I.; Shaheen, S.; Khan, H.; Fazil, M.; Pandit, N.A.; Naaz, F.; Ahmad, T. Hydrogen Energy as Sustainable Energy Resource for Carbon-Neutrality Realization. ACS Sustain. Resour. Manag. 2024, 1, 604–620. [Google Scholar] [CrossRef]
- Chang, L.-H.; Yong, S.-T.; Chai, S.P.; Putri, L.K.; Tan, L.L.; Mohamed, A.R. A review of methanol photoreforming: Elucidating the mechanisms, photocatalysts and recent advancement strategies. Mater. Today Chem. 2023, 27, 101334. [Google Scholar] [CrossRef]
- Tang, X.; Han, X.; Sulaiman, N.H.M.; He, L.; Zhou, X. Recent Advances in the Photoreforming of Plastic Waste: Principles, Challenges, and Perspectives. Ind. Eng. Chem. Res. 2023, 62, 9032–9045. [Google Scholar] [CrossRef]
- Toe, C.Y.; Tsounis, C.; Zhang, J.; Masood, H.; Gunawan, D.; Scott, J.; Amal, R. Advancing photoreforming of organics: Highlights on photocatalyst and system designs for selective oxidation reactions. Energy Environ. Sci. 2021, 14, 1140–1175. [Google Scholar] [CrossRef]
- Shelake, S.P.; Sutar, D.N.; Abraham, B.M.; Banerjee, T.; Sainath, A.V.S.; Pal, U. Emerging Photoreforming Process to Hydrogen Production: A Future Energy. Adv. Funct. Mater. 2024, 34, 2403795. [Google Scholar] [CrossRef]
- Thiloka Edirisooriya, E.M.N.; Senanayake, P.S.; Xu, P.; Wang, H. Hydrogen production and value-added chemical recovery from the photo-reforming process using waste plastics. J. Environ. Chem. Eng. 2023, 11, 111429. [Google Scholar] [CrossRef]
- Praus, P. Photoreforming for microplastics recycling: A critical review. J. Environ. Chem. Eng. 2024, 12, 112525. [Google Scholar] [CrossRef]
- Nguyen, T.K.A.; Trần-Phú, T.; Ta, X.M.C.; Truong, T.N.; Leverett, J.; Daiyan, R.; Amal, R.; Tricoli, A. Understanding Structure-Activity Relationship in Pt-loaded g-C3N4 for Efficient Solar- Photoreforming of Polyethylene Terephthalate Plastic and Hydrogen Production. Small Methods 2024, 8, e2300427. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Sun, Y.; Sun, S.; Ge, L. Photogenerated charge carriers’ regulation strategies: Structure design, mechanism, and characterization technology. Int. J. Hydrogen Energy 2024, 69, 1341–1365. [Google Scholar] [CrossRef]
- Morshedy, A.S.; El-Fawal, E.M.; Zaki, T.; El-Zahhar, A.A.; Alghamdi, M.M.; El Naggar, A.M.A. A review on heterogeneous photocatalytic materials: Mechanism, perspectives, and environmental and energy sustainability applications. Inorg. Chem. Commun. 2024, 163, 112307. [Google Scholar] [CrossRef]
- Goodarzi, N.; Ashrafi-Peyman, Z.; Khani, E.; Moshfegh, A.Z. Recent Progress on Semiconductor Heterogeneous Photocatalysts in Clean Energy Production and Environmental Remediation. Catalysts 2023, 13, 1102. [Google Scholar] [CrossRef]
- Tahir, M.B.; Riaz, K.N. Fundamentals of Photocatalysis for Environmental Remediation. In Nanomaterials and Photocatalysis in Chemistry: Mechanistic and Experimental Approaches; Tahir, M.B., Riaz, K.N., Eds.; Springer: Singapore, 2021; pp. 19–41. [Google Scholar]
- Khan, M.M. Chapter 4—Semiconductors as photocatalysts: Visible-light active materials. In Theoretical Concepts of Photocatalysis; Mansoob Khan, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 53–75. [Google Scholar]
- Huang, K.; Liang, G.; Sun, S.; Hu, H.; Peng, X.; Shen, R.; Li, X. Interface-induced charge transfer pathway switching of a Cu2O-TiO2 photocatalyst from p-n to S-scheme heterojunction for effective photocatalytic H2 evolution. J. Mater. Sci. Technol. 2024, 193, 98–106. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, F.; Bi, C.; Shi, J.; Gao, G.; An, Y.; Huang, Z. Bandgap engineering control bifunctional MnxCd1-xS photocatalysts selectively reforming xylose to C3 organic acids and efficient hydrogen production. J. Colloid Interface Sci. 2023, 652, 2066–2075. [Google Scholar] [CrossRef]
- Mothika, V.S.; Sutar, P.; Verma, P.; Das, S.; Pati, S.K.; Maji, T.K. Regulating Charge-Transfer in Conjugated Microporous Polymers for Photocatalytic Hydrogen Evolution. Chem.–A Eur. J. 2019, 25, 3867–3874. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Jing, Y. Band Structure Engineering of 2D Heterotriangulene Polymers by Incorporating Acetylenic Linkages for Photocatalytic Hydrogen Production. J. Phys. Chem. C 2022, 126, 17836–17843. [Google Scholar] [CrossRef]
- Tuan Van, N.; Mahider, T.; Quyet Van, L.; Chau Van, T.; Sang Hyun, A.; Soo Young, K. Recent progress and strategies of non-noble metal electrocatalysts based on MoS2/MOF for the hydrogen evolution reaction in water electrolysis: An overview. Microstructures 2024, 4, 2024046. [Google Scholar] [CrossRef]
- Wang, X.; Yu, M.; Feng, X. Electronic structure regulation of noble metal-free materials toward alkaline oxygen electrocatalysis. eScience 2023, 3, 100141. [Google Scholar] [CrossRef]
- Ni, J.; Shi, Z.; Wang, Y.; Yang, J.; Wu, H.; Wang, P.; Xiao, M.; Liu, C.; Xing, W. Development of noble metal-free electrocatalysts towards acidic water oxidation: From fundamental understanding to state-of-the-art catalysts. eScience 2025, 5, 100295. [Google Scholar] [CrossRef]
- Lin, Y.; Cao, Y.; Yao, Q.; Chai, O.J.H.; Xie, J. Engineering Noble Metal Nanomaterials for Pollutant Decomposition. Ind. Eng. Chem. Res. 2020, 59, 20561–20581. [Google Scholar] [CrossRef]
- Edirisooriya, E.M.N.T.; Senanayake, P.S.; Xu, P.; Wang, H. Enhanced H2 Production Efficiency in Photo-Reforming of PET Waste Plastic Using Dark-Deposited Atom/Nanocomposite Pt/TiO2 Photocatalysts. Catalysts 2025, 15, 334. [Google Scholar] [CrossRef]
- Wang, J.; Kumar, P.; Zhao, H.; Kibria, M.G.; Hu, J. Polymeric carbon nitride-based photocatalysts for photoreforming of biomass derivatives. Green Chem. 2021, 23, 7435–7457. [Google Scholar] [CrossRef]
- Yue, S.; Zhao, Z.; Zhang, T.; Li, F.; Wang, P.; Zhan, S. Photoreforming of Plastic Waste to Sustainable Fuels and Chemicals: Waste to Energy. Environ. Sci. Technol. 2024, 58, 22865–22879. [Google Scholar] [CrossRef]
- Gautam, A.; Das, S.; Ahmad, M.I. Band gap engineering through calcium addition in (Mg, Co, Ni, Cu, Zn)O high entropy oxide for efficient photocatalysis. Surf. Interfaces 2024, 46, 104054. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, Z.; Huang, H. Surface engineered single-atom systems for energy conversion. Adv. Mater. 2024, 36, 2311148. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Gao, T.; Cui, Y.; Dong, Q.; Li, X.; Labidi, A.; Lichtfouse, E.; Li, F.; Yu, F.; Wang, C. Photoreforming of poly(ethylene-terephthalate) plastic into valuable chemicals and hydrogen over BiVO4/MoOx: Synergistic promotion of oxidation and reduction processes. Appl. Catal. B Environ. Energy 2024, 357, 124326. [Google Scholar] [CrossRef]
- Zhang, X.; Zu, W.; Lee, L.Y.S. Crucial role of pre-treatment in plastic photoreforming for precision upcycling. NPJ Mater. Sustain. 2025, 3, 3. [Google Scholar] [CrossRef]
- Xiao, J. Catalyzing photo-degradation of waste plastics with a uranium complex. Sci. Bull. 2023, 68, 2498–2499. [Google Scholar] [CrossRef]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef]
- Scott, G. Degradable Polymers: Principles and Applications; Springer: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Pritchard, G. Plastics Additives; Springer: Dordrecht, The Netherlands, 1998. [Google Scholar]
- Wu, Y.; Sakurai, T.; Adachi, T.; Wang, Q. Alternatives to water oxidation in the photocatalytic water splitting reaction for solar hydrogen production. Nanoscale 2023, 15, 6521–6535. [Google Scholar] [CrossRef]
- Sperling, L.H. Introduction to Physical Polymer Science; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- da Silva, P.L.; Guimarães, L.; Pliego, J.R., Jr. Revisiting the Mechanism of Neutral Hydrolysis of Esters: Water Autoionization Mechanisms with Acid or Base Initiation Pathways. J. Phys. Chem. B 2013, 117, 6487–6497. [Google Scholar] [CrossRef]
- Norman, S.A.; Michael, E.; Mehrdad, M.; Ken, J. Hydrolytic degradation of poly(ethylene terephthalate): Importance of chain scission versus crystallinity. Eur. Polym. J. 1991, 27, 1373–1378. [Google Scholar] [CrossRef]
- Fink, J.K.; Thomas, S.; Visakh, P.M. Handbook of Engineering and Specialty Thermoplastics; WILEY: Hoboken, NJ, USA, 2011. [Google Scholar]
- Uekert, T.; Kuehnel, M.F.; Wakerley, D.W.; Reisner, E. Plastic waste as a feedstock for solar-driven H2 generation. Energy Environ. Sci. 2018, 11, 2853–2857. [Google Scholar] [CrossRef]
- Jiao, X.; Zheng, K.; Hu, Z.; Zhu, S.; Sun, Y.; Xie, Y. Conversion of waste plastics into value-added carbonaceous fuels under mild conditions. Adv. Mater. 2021, 33, 2005192. [Google Scholar] [CrossRef]
- Jiao, X.; Zheng, K.; Chen, Q.; Li, X.; Li, Y.; Shao, W.; Xu, J.; Zhu, J.; Pan, Y.; Sun, Y.; et al. Photocatalytic Conversion of Waste Plastics into C2 Fuels under Simulated Natural Environment Conditions. Angew. Chem. Int. Ed. 2020, 59, 15497–15501. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Andrei, V.; Pornrungroj, C.; Rahaman, M.; Pichler, C.M.; Reisner, E. Reforming of Soluble Biomass and Plastic Derived Waste Using a Bias-Free Cu30Pd70|Perovskite|Pt Photoelectrochemical Device. Adv. Funct. Mater. 2022, 32, 2109313. [Google Scholar] [CrossRef]
- Rakowski DuBois, M.; DuBois, D.L. Development of molecular electrocatalysts for CO2 reduction and H2 production/oxidation. Acc. Chem. Res. 2009, 42, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Stache, E.E. Recent advances in oxidative degradation of plastics. Chem. Soc. Rev. 2024, 53, 7309–7327. [Google Scholar] [CrossRef]
- Oh, S.; Stache, E.E. Chemical Upcycling of Commercial Polystyrene via Catalyst-Controlled Photooxidation. J. Am. Chem. Soc. 2022, 144, 5745–5749. [Google Scholar] [CrossRef] [PubMed]
- Díez, A.M.; Licciardello, N.; Kolen´ko, Y.V. Photocatalytic processes as a potential solution for plastic waste management. Polym. Degrad. Stab. 2023, 59, 110459. [Google Scholar] [CrossRef]
- Uekert, T.; Kasap, H.; Reisner, E. Photoreforming of Nonrecyclable Plastic Waste over a Carbon Nitride/Nickel Phosphide Catalyst. J. Am. Chem. Soc. 2019, 141, 15201–15210. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, H.; Liu, J.; Liu, X.; Song, X.; Zhou, W.; Zhang, J.; Huo, P. Enhanced degradation of polyethylene terephthalate plastics by CdS/CeO2 heterojunction photocatalyst activated peroxymonosulfate. J. Hazard. Mater. 2023, 452, 131375. [Google Scholar] [CrossRef]
- Ippolito, F.; Hübner, G.; Claypole, T.; Gane, P. Calcium Carbonate as Functional Filler in Polyamide 12-Manipulation of the Thermal and Mechanical Properties. Processes 2021, 9, 937. [Google Scholar] [CrossRef]
- Siraj, S.; Al-Marzouqi, A.H.; Iqbal, M.Z.; Ahmed, W. Impact of Micro Silica Filler Particle Size on Mechanical Properties of Polymeric Based Composite Material. Polymers 2022, 14, 4830. (In English) [Google Scholar] [CrossRef]
- Cazan, C.; Enesca, A.; Andronic, L. Synergic Effect of TiO(2) Filler on the Mechanical Properties of Polymer Nanocomposites. Polymers 2021, 13, 2017. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, G.L.; Di Paola, A.; Palmisano, L.; Selli, E. Effect of titanium dioxide crystalline structure on the photocatalytic production of hydrogen. Photochem. Photobiol. Sci. 2011, 10, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Zhao, Y.; Gao, J.; Wang, J.; Zhang, T. Direct Photoreforming of Real-World Polylactic Acid Plastics into Highly Selective Value-Added Pyruvic Acid under Visible Light. J. Am. Chem. Soc. 2024, 146, 4842–4850. [Google Scholar] [CrossRef]
- Li, H.; Cheng, B.; Xu, J.; Yu, J.; Cao, S. Crystalline carbon nitrides for photocatalysis. EES Catal. 2024, 2, 411–447. [Google Scholar] [CrossRef]
- Pita, F.; Castilho, A. Separation of PET from other plastics by flotation combined with alkaline pretreatment. Polímeros 2020, 30, e2020026. [Google Scholar] [CrossRef]
- Nagakawa, H.; Nagata, M. Photoreforming of Organic Waste into Hydrogen Using a Thermally Radiative CdO(x)/CdS/SiC Photocatalyst. ACS Appl. Mater. Interfaces 2021, 13, 47511–47519. [Google Scholar] [CrossRef]
- Edirisooriya, E.M.N.T.; Senanayake, P.S.; Xu, P.; Talipov, M.R.; Wang, H. Optimization of green hydrogen evolution from low-density plastics using TiO2-based nano-photocatalysts with techno-economic and carbon footprint assessment. Nanotechnol. Environ. Eng. 2024, 9, 817–832. [Google Scholar] [CrossRef]
- Pan, H.; Li, J.; Wang, Y.; Xia, Q.; Qiu, L.; Zhou, B. Solar-Driven Biomass Reforming for Hydrogen Generation: Principles, Advances, and Challenges. Adv. Sci. (Weinh.) 2024, 11, e2402651. (In English) [Google Scholar] [CrossRef]
- Sutar, D.N.; Shelake, S.P.; Indla, N.R.; Varangane, S.; Sesha Sainath, A.V.; Pal, U. Visible light-driven photoreforming of polystyrene segmented glycopolymer architectures for enhanced hydrogen generation. Mater. Today Energy 2024, 45, 101667. [Google Scholar] [CrossRef]
- He, J.; Han, L.; Ma, W.; Chen, L.; Ma, C.; Xu, C.; Yang, Z. Efficient photodegradation of polystyrene microplastics integrated with hydrogen evolution: Uncovering degradation pathways. iScience 2023, 26, 106833. [Google Scholar] [CrossRef]
- Lan, L.; Chen, H.; Lee, D.; Xu, S.; Skillen, N.; Tedstone, A.; Robertson, P.; Garforth, A.; Daly, H.; Hardacre, C.; et al. Effect of Ball-Milling Pretreatment of Cellulose on Its Photoreforming for H(2) Production. ACS Sustain. Chem. Eng. 2022, 10, 4862–4871. (In English) [Google Scholar] [CrossRef] [PubMed]
- Samadi, M.; Zirak, M.; Naseri, A.; Khorashadizade, E.; Moshfegh, A.Z. Recent progress on doped ZnO nanostructures for visible-light photocatalysis. Thin Solid Film. 2016, 605, 2–19. [Google Scholar] [CrossRef]
- Khan, M.A.; Mutahir, S.; Shaheen, I.; Qunhui, Y.; Bououdina, M.; Humayun, M. Recent advances over the doped g-C3N4 in photocatalysis: A review. Coord. Chem. Rev. 2025, 522, 216227. [Google Scholar] [CrossRef]
- Rasheed, H.M.; Aroosh, K.; Meng, D.; Ruan, X.; Akhter, M.; Cui, X. A review on modified ZnO to address environmental challenges through photocatalysis: Photodegradation of organic pollutants. Mater. Today Energy 2025, 48, 101774. [Google Scholar] [CrossRef]
- Jeyavani, J.; Al-Ghanim, K.A.; Govindarajan, M.; Malafaia, G.; Vaseeharan, B. A convenient strategy for mitigating microplastics in wastewater treatment using natural light and ZnO nanoparticles as photocatalysts: A mechanistic study. J. Contam. Hydrol. 2024, 267, 104436. [Google Scholar] [CrossRef]
- Ma, Y.; Hai, G.; Liu, J.; Bao, J.; Li, Y.; Wang, G. Enhanced visible light photocatalytic hydrogen evolution by intimately contacted Ni2P decorated Ni-doped CdS nanospheres. Chem. Eng. J. 2022, 441, 136002. [Google Scholar] [CrossRef]
- Wang, J.R.; Song, K.; Luan, T.X.; Cheng, K.; Wang, Q.; Wang, Y.; Yu, W.W.; Li, P.Z.; Zhao, Y. Robust links in photoactive covalent organic frameworks enable effective photocatalytic reactions under harsh conditions. Nat. Commun. 2024, 15, 1267. [Google Scholar] [CrossRef]
- Sun, N.; Si, X.; He, L.; Zhang, J.; Sun, Y. Strategies for enhancing the photocatalytic activity of semiconductors. Int. J. Hydrogen Energy 2024, 58, 1249–1265. [Google Scholar] [CrossRef]
- Downs, N.; Parisi, A.V.; Galligan, L.; Turner, J.; Amar, A.; King, R.; Ultra, F.; Butler, H. Solar radiation and the UV index: An application of numerical integration, trigonometric functions, online education and the modelling process. Int. J. Res. Educ. Sci. 2016, 2, 179–189. [Google Scholar] [CrossRef]
- Graham, J.D.; Hammer, N.I. Photocatalytic Water Splitting and Carbon Dioxide Reduction. In Handbook of Climate Change Mitigation; Chen, W.-Y., Seiner, J., Suzuki, T., Lackner, M., Eds.; Springer: New York, NY, USA, 2012; pp. 1755–1780. [Google Scholar]
- Ma, W.; Yu, L.; Kang, P.; Chu, Z.; Li, Y. Modifications and Applications of Metal-Organic-Framework-Based Materials for Photocatalysis. Molecules 2024, 29, 5834. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Li, Y.; Pang, J.; Bu, X. The Advanced Synthesis of MOFs-Based Materials in Photocatalytic HER in Recent Three Years. Catalysts 2022, 12, 1350. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Huang, F.; Song, S.; Ai, G.; Xin, X.; Zhao, B.; Zheng, Y.; Zhang, Z. Recent Advances in g-C(3)N(4)-Based Materials and Their Application in Energy and Environmental Sustainability. Molecules 2023, 28, 432. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Youn, D.H. Nanomaterials for Advanced Photocatalytic Plastic Conversion. Molecules 2023, 28, 6502. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Hidalgo-Jiménez, J.; Sauvage, X.; Saito, K.; Guo, Q.; Edalati, K. Phase and sulfur vacancy engineering in cadmium sulfide for boosting hydrogen production from catalytic plastic waste photoconversion. Chem. Eng. J. 2025, 504, 158730. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Mahmoud, S.A.; Mohamed, A.A. Unveiling the photocatalytic potential of graphitic carbon nitride (g-C(3)N(4)): A state-of-the-art review. RSC Adv. 2024, 14, 25629–25662. [Google Scholar] [CrossRef]
- Ya, Z.; Tang, L.; Xu, D.; Wang, H.; Zhang, S. Photoreforming of waste plastic by B-doped carbon nitride nanotube: Atomic-level modulation and mechanism insights. Am. Inst. Chem. Eng. 2025, 71, e18740. [Google Scholar] [CrossRef]
- Liu, D.; Yao, J.; Chen, S.; Zhang, J.; Li, R.; Peng, T. Construction of rGO-coupled C3N4/C3N5 2D/2D Z-scheme heterojunction to accelerate charge separation for efficient visible light H2 evolution. Appl. Catal. B Environ. 2022, 318, 121822. [Google Scholar] [CrossRef]
- Yan, J.-Q.; Sun, D.-W.; Huang, J.-H. Synergistic poly(lactic acid) photoreforming and H2 generation over ternary NixCo1-xP/reduced graphene oxide/g-C3N4 composite. Chemosphere 2022, 286, 131905. [Google Scholar] [CrossRef]
- Chen, S.; Takata, T.; Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2017, 2, 17050. [Google Scholar] [CrossRef]
- Xi, Z. Nanostructures Design: The Role of Cocatalysts for Hydrogen and Oxygen Generation in Photocatalytic Water Splitting. arXiv 2021, arXiv:2103.09798. [Google Scholar]
- Al-Madanat, O.; AlSalka, Y.; Ramadan, W.; Bahnemann, D.W. TiO2 Photocatalysis for the Transformation of Aromatic Water Pollutants into Fuels. Catalysts 2021, 11, 317. [Google Scholar] [CrossRef]
- Exner, K.S. On the optimum binding energy for the hydrogen evolution reaction: How do experiments contribute? Electrochem. Sci. Adv. 2021, 2, e2100101. [Google Scholar] [CrossRef]
- Cha, G.; Hwang, I.; Hejazi, S.; Dobrota, A.S.; Pašti, I.A.; Osuagwu, B.; Kim, H.; Will, J.; Yokosawa, T.; Badura, Z.; et al. As a single atom Pd outperforms Pt as the most active co-catalyst for photocatalytic H2 evolution. iScience 2021, 24, 102938. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Yang, M.-Q.; Wang, S.; Qian, Q. Recent Advancements in Photocatalytic Valorization of Plastic Waste to Chemicals and Fuels. Front. Nanotechnol. 2021, 3, 723120. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, L.; Peng, D.; Wang, X.; Wu, M.; Bian, Z. Advancements in catalysis for plastic resource utilization. Environ. Sci. Adv. 2023, 2, 1151–1166. [Google Scholar] [CrossRef]
- Pan, Y.; Lin, Y.; Chen, Y.; Liu, Y.; Liu, C. Cobalt phosphide-based electrocatalysts: Synthesis and phase catalytic activity comparison for hydrogen evolution. J. Mater. Chem. A 2016, 4, 4745–4754. [Google Scholar] [CrossRef]
- Chu, S.; Zhang, B.; Zhao, X.; Soo, H.S.; Wang, F.; Xiao, R.; Zhang, H. Photocatalytic Conversion of Plastic Waste: From Photodegradation to Photosynthesis. Adv. Energy Mater. 2022, 12, 2200435. [Google Scholar] [CrossRef]
- Mokhtar, B.; Ahmed, M.G.; Alqahtani, H.S.; Kandiel, T.A. Biomass and Plastic Photoreforming for Hydrogen and Valuable Chemicals Production; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–31. [Google Scholar]
- Uekert, T.; Bajada, M.A.; Schubert, T.; Pichler, C.M.; Reisner, E. Scalable Photocatalyst Panels for Photoreforming of Plastic, Biomass and Mixed Waste in Flow. ChemSusChem 2021, 14, 4190–4197. (In English) [Google Scholar] [CrossRef]
- Islam, M.T.; Roni, M.N.P.; Ali, M.Y.; Islam, M.R.; Hossan, M.S.; Rahman, M.H.; Zahid, A.; Alam, M.N.E.; Hanif, M.A.; Akhtar, M.S. Selectivity of Sol-Gel and Hydrothermal TiO(2) Nanoparticles towards Photocatalytic Degradation of Cationic and Anionic Dyes. Molecules 2023, 28, 6834. [Google Scholar] [CrossRef]
- Quispe Cohaila, A.B.; Sacari Sacari, E.J.; Lanchipa Ramos, W.O.; Canahua Loza, H.B.; Tamayo Calderón, R.M.; Medina Salas, J.P.; Gamarra Gómez, F.; Mangalaraja, R.V.; Rajendran, S. Improving Photocatalytic Hydrogen Production with Sol–Gel Prepared NiTiO₃/TiO₂ Composite. Energies 2024, 17, 5830. [Google Scholar] [CrossRef]
- Kadiyala, N.; Tirukkovalluri, S.R.; Gorli, D.; Genji, J.; Raffiunnisa; Matangi, R.; Singupilla, S.S. Ionic Liquid Mediated Sol Gel Method for Fabrication of Nanostructured Cerium and Phosphorus Doped TiO(2)—A Benign Photocatalyst: Diversified Applications in Degradation of Dyes and Microbes. ACS Omega 2025, 10, 2658–2678. (In English) [Google Scholar] [CrossRef]
- Niu, X.; Du, Y.; He, J.; Li, X.; Wen, G. Hydrothermal Synthesis of Co-Exposed-Faceted WO3 Nanocrystals with Enhanced Photocatalytic Performance. Nanomaterials 2022, 12, 2879. Available online: https://www.mdpi.com/2079-4991/12/16/2879 (accessed on 29 April 2025). [CrossRef] [PubMed]
- Xu, F.; Hu, C.; Zhu, D.; Wang, D.; Zhong, Y.; Tang, C.; Zhou, H. One-Step Hydrothermal Synthesis of Nanostructured MgBi(2)O(6)/TiO(2) Composites for Enhanced Hydrogen Production. Nanomaterials 2022, 12, 1302. (In English) [Google Scholar] [CrossRef] [PubMed]
- Zindrou, A.; Psathas, P.; Deligiannakis, Y. Flame Spray Pyrolysis Synthesis of Vo-Rich Nano-SrTiO3-x. Nanomaterials 2024, 14, 346. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.K.; Dash, P.; Bariki, R.; Pradhan, S.K.; Panda, S.; Nayak, B.; Mishra, B.G. Metal–Organic Framework-Derived Hierarchical Ag/Sr6Bi2O9-α-Bi2O3 Ternary Photocatalyst for Micropollutant Remediation and Bacterial Photoinactivation. ACS Appl. Eng. Mater. 2024, 2, 179–194. [Google Scholar] [CrossRef]
- Wang, C.; Ghazzal, M.N. Nanostructured TiO2 for improving the solar-to-hydrogen conversion efficiency. Energy Adv. 2023, 2, 965–979. [Google Scholar] [CrossRef]
- Ma, Z.; Zhan, S.; Zhang, Y.; Kuklin, A.; Chen, Y.; Lin, Y.; Zhang, H.; Ren, X.; Agren, H.; Zhang, Y. An Electron Transfer Mediated Mechanism for Efficient Photoreforming of Waste Plastics Using a Ni(3)S(4)/ZnCdS Heterojunction. Adv. Mater. 2025, 37, e2416581. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, B.; Xiong, L.; Liu, W.; Wang, D.; Ma, W.; Jiang, L.; Yang, J.; Wang, P.; Xiao, T.; et al. Highly selective upcycling of plastic mixture waste by microwave-assisted catalysis over Zn/b-ZnO. Nat. Commun. 2025, 16, 1726. [Google Scholar] [CrossRef]
- Hentschel, S.; Dittmar, M.; Strelow, C.; Ruhmlieb, C.; Hackl, T.; Mews, A. Activation and Deactivation of the Photocatalytic Hydrogen Production Activity of Pt-Tipped CdSe/CdS Nanorods. Adv. Sustain. Syst. 2025, 9, 2400782. [Google Scholar] [CrossRef]
- Nevárez Martínez, M.C.; Cavdar, O.; Haliński, Ł.P.; Miodyńska, M.; Parnicka, P.; Bajorowicz, B.; Kobylański, M.; Lewandowski, Ł.; Zaleska-Medynska, A. Hydrogen detection during photocatalytic water splitting: A tutorial. Int. J. Hydrogen Energy 2022, 47, 15783–15788. [Google Scholar] [CrossRef]
- Talledo, S.; Kubaney, A.; Baumer, M.A.; Pietrak, K.; Bernhard, S. High throughput methodology for investigating green hydrogen generating processes using colorimetric detection films and machine vision. Digit. Discov. 2024, 3, 1430–1440. [Google Scholar] [CrossRef]
- Abdel-Maksoud, Y.; Imam, E.; Ramadan, A. TiO2 Solar Photocatalytic Reactor Systems: Selection of Reactor Design for Scale-up and Commercialization—Analytical Review. Catalysts 2016, 6, 138. [Google Scholar] [CrossRef]
- Damodar, R.A.; You, S.-J.; Ou, S.-H. Coupling of membrane separation with photocatalytic slurry reactor for advanced dye wastewater treatment. Sep. Purif. Technol. 2010, 76, 64–71. [Google Scholar] [CrossRef]
- Xi, W.; Geissen, S.-U. Separation of titanium dioxide from photocatalytically treated water by cross-flow microfiltration. Water Res. 2001, 35, 1256–1262. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, G.; Huang, T.; Chen, J.; Wang, Q.; Meng, Q. Photocatalytic membrane reactor for degradation of acid red B wastewater. Chem. Eng. J. 2010, 156, 571–577. [Google Scholar] [CrossRef]
- Yang, R.; Xu, J.; Wu, J.; Lu, D.; Wang, F.; Nie, K. Enzyme Immobilization on Stainless Steel Fleece and Its Mass Transfer Enhancement of Enzymatic Catalysis in a Rotating Packed Bed Reactor. Catalysts 2023, 13, 1501. [Google Scholar] [CrossRef]
- Kudaibergenov, S.E.; Dzhardimalieva, G.I. Flow-Through Catalytic Reactors Based on Metal Nanoparticles Immobilized within Porous Polymeric Gels and Surfaces/Hollows of Polymeric Membranes. Polymers 2020, 12, 572. [Google Scholar] [CrossRef]
- Khodadadian, F. Optimizing Photon Utilization in LED-Based Photocatalytic Reactors. Ph.D. Thesis, Delft University of Technology, Dissertation (TU Delft), Delft, The Netherlands, 2019. Available online: https://resolver.tudelft.nl/uuid:68bc7aa0-914c-4d2d-8e32-9d0ef996fdcc (accessed on 29 April 2025).
- Fu, H.; Pan, Z.; Lai, Y.-J.S.; Ananpattarachai, J.; Serpa, M.; Shapiro, N.; Zhao, Z.; Westerhoff, P. Green hydrogen production via a photocatalyst-enabled optical fiber system: A promising route to net-zero emissions. Energy Clim. Change 2025, 6, 100175. [Google Scholar] [CrossRef]
- Kant, P.P. Optimizing Photocatalysts and Photoreactors for Solar Fuel Synthesis. Ph.D. Thesis, Faculty of Chemical and Process Engineering, Institute of Micro Process Engineering, Eggenstein-Leopoldshafen, Germany, 2023. Available online: https://publikationen.bibliothek.kit.edu/1000162170 (accessed on 3 May 2025).
- Degerli, S.N.; Gramegna, A.; Tommasi, M.; Ramis, G.; Rossetti, I. Reactor and Plant Designs for the Solar Photosynthesis of Fuels. Energies 2024, 17, 3112. [Google Scholar] [CrossRef]
- Ramis, G.; Bahadori, E.; Rossetti, I. Photoreactors Design for Hydrogen Production. Chem. Eng. Trans. 2019, 74, 481–486. [Google Scholar] [CrossRef]
- Jaafar, N.F.; Jalil, A.A.; Triwahyono, S.; Ripin, A. Significant Effect of pH on Photocatalytic Degradation of Organic Pollutants Using Semiconductor Catalysts. J. Teknol. 2016, 78, 7–12. [Google Scholar] [CrossRef]
- Azeez, F.; Al-Hetlani, E.; Arafa, M.; Abdelmonem, Y.; Nazeer, A.A.; Amin, M.O.; Madkour, M. The effect of surface charge on photocatalytic degradation of methylene blue dye using chargeable titania nanoparticles. Sci. Rep. 2018, 8, 7104. (In English) [Google Scholar] [CrossRef] [PubMed]
- Wenk, J.; Graf, C.; Aeschbacher, M.; Sander, M.; Canonica, S. Effect of Solution pH on the Dual Role of Dissolved Organic Matter in Sensitized Pollutant Photooxidation. Environ. Sci. Technol. 2021, 55, 15110–15122. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.Y.; Mortelliti, M.J.; Dempsey, J.L. A compendium and meta-analysis of flatband potentials for TiO2, ZnO, and SnO2 semiconductors in aqueous media. Chem. Phys. Rev. 2022, 3, 011303. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Zondag, S.D.A.; Schuurmans, J.H.A.; van der Schaaf, J.; Noël, T. Scale-Up of a Heterogeneous Photocatalytic Degradation Using a Photochemical Rotor–Stator Spinning Disk Reactor. Org. Process Res. Dev. 2022, 26, 1279–1288. [Google Scholar] [CrossRef]
- García-Prieto, J.C.; González-Burciaga, L.A.; Proal-Nájera, J.B.; García-Roig, M. Study of Influence Factors in the Evaluation of the Performance of a Photocatalytic Fibre Reactor (TiO2/SiO2) for the Removal of Organic Pollutants from Water. Catalysts 2022, 12, 122. [Google Scholar] [CrossRef]
- Akach, J.; Kabuba, J.; Ochieng, A. Simulation of the Light Distribution in a Solar Photocatalytic Bubble Column Reactor Using the Monte Carlo Method. Ind. Eng. Chem. Res. 2020, 59, 17708–17719. [Google Scholar] [CrossRef]
- Jamil, Q.; Žener, B.; Putar, U.; Matoh, L. Continuous flow photocatalytic reactor for degradation of selected pollutants: Modeling, kinetics, mineralization rate, and toxicity assessment. Heliyon 2024, 10, e40019. [Google Scholar] [CrossRef]
- Sulaiman, N.H.M.; Wang, S.; Yue, H.; Wei, J.; Schmuki, P.; Zhou, X. Hydrogen evolution using alloyed AuPd/TiO2 hollow spheres by photoreforming of polyethylene terephthalate waste. J. Mater. Chem. A 2025, 13, 12545–12552. [Google Scholar] [CrossRef]
- Zheng, Y.; Fan, P.; Guo, R.; Liu, X.; Zhou, X.; Xue, C.; Ji, H. Visible light driven reform of wasted plastics to generate green hydrogen over mesoporous ZnIn(2)S(4). RSC Adv. 2023, 13, 12663–12669. [Google Scholar] [CrossRef]
- Zhang, Q.; Yue, M.; Chen, P.; Ren, Q.; Kong, W.; Jia, C.; Lu, Q.; Wu, J.; Li, Y.; Liu, W.; et al. Accelerating photocatalytic hydrogen production by anchoring Pt single atoms on few-layer g-C3N4 nanosheets with Pt–N coordination. J. Mater. Chem. C 2024, 12, 3437–3449. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, H.; Fu, S.; Jaroniec, M.; Shan, J.; Xia, B.; Qu, Y.; Qu, J.; Chen, S.; Song, L.; et al. NiPS(3) ultrathin nanosheets as versatile platform advancing highly active photocatalytic H(2) production. Nat. Commun. 2022, 13, 4600. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, H.-P.; Zhao, G.; Huang, G.; Sun, W.; Peng, C. Plastic Waste Conversion by Leveraging Renewable Photo/Electro-Catalytic Technologies. ChemSusChem 2024, 17, e202301352. [Google Scholar] [CrossRef]
- Tang, J.; Li, X.; Ma, Y.; Wang, K.; Liu, Z.; Zhang, Q. Boosting exciton dissociation and charge transfer by regulating dielectric constant in polymer carbon nitride for CO2 photoreduction. Appl. Catal. B Environ. 2023, 327, 122417. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, H.; Huo, P. Plastic Degradation and Conversion by Photocatalysis. In Plastic Degradation and Conversion by Photocatalysis (Volume 2): From Waste to Wealth; ACS Symposium Series, no. 1490; American Chemical Society: Washington, DC, USA, 2024; Volume 1490, Chapter 1; pp. 1–22. [Google Scholar]
- Qin, N.; Mao, A.; Zou, J.; Mi, L.; Wu, L. Visible-light-driven H2 production from heterostructured Zn0.5Cd0.5S–TiO2 photocatalysts modified with reduced graphene oxides. New J. Chem. 2021, 45, 21415–21422. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Wang, L.; Liu, J.; Liang, G.; Davey, K.; Ran, J.; Qiao, S.-Z. Boosted Photoreforming of Plastic Waste via Defect-Rich NiPS3 Nanosheets. J. Am. Chem. Soc. 2023, 145, 6410–6419. [Google Scholar] [CrossRef]
- Xu, J.; Jiao, X.; Zheng, K.; Shao, W.; Zhu, S.; Li, X.; Zhu, J.; Pan, Y.; Sun, Y.; Xie, Y. Plastics-to-syngas photocatalysed by Co–Ga2O3 nanosheets. Natl. Sci. Rev. 2022, 9, nwac011. [Google Scholar] [CrossRef]
- Schanze, J.M.B.V.K.S. Best Practices for Reporting on Heterogeneous Photocatalysis. ACS Appl. Mater. Interfaces 2014, 6, 11815–11816. [Google Scholar] [CrossRef]
- Nees, M.; Adeel, M.; Pazdur, L.; Porters, M.; Vande Velde, C.M.L.; Billen, P. Polyurethane Waste Recycling: Thermolysis of the Carbamate Fraction. ACS Omega 2024, 9, 43438–43446. (In English) [Google Scholar] [CrossRef]
- Fawzi, S.; Yousif, E.; Zainulabdeen, K.; Bufaroosha, M.; Ahmed, D. Highly effective photostabilization of polyvinyl chloride films using omeprazole-tin additive complexes. J. Umm Al-Qura Univ. Appl. Sci. 2025. [Google Scholar] [CrossRef]
- Yang, R.; Cao, H.; Dong, H.; Wang, X. The mechanism of UV accelerated aging of polyvinyl chloride in marine environment: The role of free radicals. Mar. Pollut. Bull. 2024, 207, 116736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, C.; Zhao, G.; Gao, Y.; Zhuang, T.; Lv, Z. Single-atom Pt supported on defective graphitic carbon nitride for efficient photocatalytic hydrogen production. Chem. Eng. J. 2025, 505, 159567. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, H.; Hong, L.; Shao, J.; Zhang, B.; Yu, J.; Chu, S. An Integrated Plasma–Photocatalytic System for Upcycling of Polyolefin Plastics. ChemSusChem 2023, 16, e202300106. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Guo, C.; Lam, E.; Holstein, J.M.; Rangel Pereira, M.; Pichler, C.M.; Pornrungroj, C.; Rahaman, M.; Uekert, T.; Hollfelder, F.; et al. Chemoenzymatic Photoreforming: A Sustainable Approach for Solar Fuel Generation from Plastic Feedstocks. J. Am. Chem. Soc. 2023, 145, 20355–20364. [Google Scholar] [CrossRef]
- Ng, K.S.; Phan, A.N. Evaluating the Techno-economic Potential of an Integrated Material Recovery and Waste-to-Hydrogen System. Resour. Conserv. Recycl. 2021, 167, 105392. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, W.; Chen, X.; Liu, Y.; Shen, Y.; Ni, B.-J. Upcycling of plastic wastes for hydrogen production: Advances and perspectives. Renew. Sustain. Energy Rev. 2024, 195, 114333. [Google Scholar] [CrossRef]
- Edirisooriya, E.M.N.T.; Senanayake, P.S.; Xu, P.; Wang, H. Recyclability and regeneration of Au/TiO2 nanocomposite and Pt/TiO2 atom-nano composite catalysts in photo-reforming plastics for hydrogen production. J. Environ. Chem. Eng. 2025, 13, 116467. [Google Scholar] [CrossRef]
- Palma, V.; Ruocco, C.; Martino, M.; Barba, D.; Meloni, E. Chapter 14—General catalyst-related issues. In Current Trends and Future Developments on (Bio-) Membranes; Figoli, A., Li, Y., Basile, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 303–324. [Google Scholar]
- Wiedemann, B.; Carogher, K.M.; Kuhn, S.; Ziegenbalg, D. The interplay of irradiated- and shaded zones in photoreactor scale-up. Chem. Eng. J. 2025, 503, 157987. [Google Scholar] [CrossRef]
- Gunawan, D.; Zhang, J.; Li, Q.; Toe, C.Y.; Scott, J.; Antonietti, M.; Guo, J.; Amal, R. Materials Advances in Photocatalytic Solar Hydrogen Production: Integrating Systems and Economics for a Sustainable Future. Adv. Mater. 2024, 36, 2404618. [Google Scholar] [CrossRef]
- Ouyang, D.; Wang, F.; Gao, D.; Han, W.; Hu, X.; Qiao, D.; Zhao, X. Light-driven lignocellulosic biomass conversion for production of energy and chemicals. iScience 2022, 25, 105221. (In English) [Google Scholar] [CrossRef]
- Tian, J.; Guan, C.; Hu, H.; Liu, E.; Yang, D. Waste plastics promoted photocatalytic H2 evolution over S-scheme NiCr2O4/twinned-Cd0.5Zn0.5S homo-heterojunction. Acta Phys.-Chim. Sin. 2025, 41, 100068. [Google Scholar] [CrossRef]
- Balu, R.; Dutta, N.K.; Roy Choudhury, N. Plastic Waste Upcycling: A Sustainable Solution for Waste Management, Product Development, and Circular Economy. Polymers 2022, 14, 4788. (In English) [Google Scholar] [CrossRef] [PubMed]
- Bratovcic, A. Photocatalytic Degradation of Plastic Waste: Recent Progress and Future Perspectives. Adv. Nanoparticles 2024, 13, 61–78. [Google Scholar] [CrossRef]
- Biale, G.; La Nasa, J.; Mattonai, M.; Corti, A.; Vinciguerra, V.; Castelvetro, V.; Modugno, F. A Systematic Study on the Degradation Products Generated from Artificially Aged Microplastics. Polymers 2021, 13, 1997. [Google Scholar] [CrossRef]
- Li, T.; Vijeta, A.; Casadevall, C.; Gentleman, A.S.; Euser, T.; Reisner, E. Bridging Plastic Recycling and Organic Catalysis: Photocatalytic Deconstruction of Polystyrene via a C–H Oxidation Pathway. ACS Catal. 2022, 12, 8155–8163. [Google Scholar] [CrossRef]
- Chu, D.; Weng, J.; Liu, X.; Wang, H.; Cui, Y.; Nie, L.; Li, Z. Photocatalytic Oxidation of Polyethylene to Dicarboxylic Acid over BiOI/BiVO4 p–n Heterojunction Under Visible Light. CCS Chem. 2025, 1–11. [Google Scholar] [CrossRef]
- Costa, J.P.d.; Avellan, A.; Mouneyrac, C.; Duarte, A.; Rocha-Santos, T. Plastic additives and microplastics as emerging contaminants: Mechanisms and analytical assessment. TrAC Trends Anal. Chem. 2023, 158, 116898. [Google Scholar] [CrossRef]
- Zhang, X.; Jun, M.; Zu, W.; Kim, M.; Lee, K.; Lee, L.Y.S. Photoreforming of Microplastics: Challenges and Opportunities for Sustainable Environmental Remediation. Small 2024, 20, e2403347. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Shinnur, M.V.; Pedeferri, M.; Ferrari, A.M.; Rosa, R.; Meroni, D. Toward Sustainable Photocatalysis: Addressing Deactivation and Environmental Impact of Anodized and Sol–Gel Photocatalysts. Adv. Sustain. Syst. 2025, 2401017. [Google Scholar] [CrossRef]
- He, Y.; Rehman, A.U.; Xu, M.; Not, C.A.; Ng, A.M.C.; Djurišić, A.B. Photocatalytic degradation of different types of microplastics by TiO(x)/ZnO tetrapod photocatalysts. Heliyon 2023, 9, e22562. (In English) [Google Scholar] [CrossRef]
- Bora, L.V.; Bhatt, M.; Patel, A.; Bora, N.V. Plastic Degradation by Photocatalysis: Basic Concepts and General Mechanisms. In Plastic Degradation and Conversion by Photocatalysis (Volume 1): A Sustainable Approach; ACS Symposium Series, no. 1489; American Chemical Society: Washington, DC, USA, 2024; Volume 1489, Chapter 1; pp. 1–22. [Google Scholar]
- Du, H.; Xie, Y.; Wang, J. Microplastic degradation methods and corresponding degradation mechanism: Research status and future perspectives. J. Hazard. Mater. 2021, 418, 126377. [Google Scholar] [CrossRef] [PubMed]
- Li, D.T.; Yu, H.; Huang, Y. Facile H(2)PdCl(4)-induced photoreforming of insoluble PET waste for C1-C3 compound production. Front. Chem. 2023, 11, 1265556. [Google Scholar] [CrossRef] [PubMed]
- Bule Možar, K.; Miloloža, M.; Martinjak, V.; Radovanović-Perić, F.; Bafti, A.; Ujević Bošnjak, M.; Markić, M.; Bolanča, T.; Cvetnić, M.; Kučić Grgić, D.; et al. Evaluation of Fenton, Photo-Fenton and Fenton-like Processes in Degradation of PE, PP, and PVC Microplastics. Water 2024, 16, 673. [Google Scholar] [CrossRef]
- Surana, M.; Pattanayak, D.S.; Yadav, V.; Singh, V.K.; Pal, D. An insight decipher on photocatalytic degradation of microplastics: Mechanism, limitations, and future outlook. Environ. Res. 2024, 247, 118268. [Google Scholar] [CrossRef]
- Pan, Y.; Gao, S.H.; Ge, C.; Gao, Q.; Huang, S.; Kang, Y.; Luo, G.; Zhang, Z.; Fan, L.; Zhu, Y.; et al. Removing microplastics from aquatic environments: A critical review. Environ. Sci. Ecotechnol. 2023, 13, 100222. (In English) [Google Scholar] [CrossRef]
- Xie, A.; Jin, M.; Zhu, J.; Zhou, Q.; Fu, L.; Wu, W. Photocatalytic Technologies for Transformation and Degradation of Microplastics in the Environment: Current Achievements and Future Prospects. Catalysts 2023, 13, 846. [Google Scholar] [CrossRef]
- Chattopadhyay, P.; Ariza-Tarazona, M.C.; Cedillo-Gonzalez, E.I.; Siligardi, C.; Simmchen, J. Combining photocatalytic collection and degradation of microplastics using self-asymmetric Pac-Man TiO(2). Nanoscale 2023, 15, 14774–14781. [Google Scholar] [CrossRef]
- Llorente-García, B.E.; Hernández-López, J.M.; Zaldívar-Cadena, A.A.; Siligardi, C.; Cedillo-González, E.I. First Insights into Photocatalytic Degradation of HDPE and LDPE Microplastics by a Mesoporous N–TiO2 Coating: Effect of Size and Shape of Microplastics. Coatings 2020, 10, 658. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Tan, J.; Chellappan, V.; Ramakrishna, S. Recent Advances in Extended Producer Responsibility Initiatives for Plastic Waste Management in Germany and UK. Mater. Circ. Econ. 2023, 5, 6. [Google Scholar] [CrossRef]
- U.S. Department of Energy. Hydrogen Laws and Incentives in the United States. Available online: https://afdc.energy.gov/fuels/laws/HY?state=US (accessed on 1 May 2025).
- Ran, J.; Talebian-Kiakalaieh, A.; Zhang, S.; Hashem, E.M.; Guo, M.; Qiao, S.Z. Recent advancement on photocatalytic plastic upcycling. Chem. Sci. 2024, 15, 1611–1637. [Google Scholar] [CrossRef]
- Kumar, S.; Karthikeyan, S.; Lee, A.F. g-C3N4-Based Nanomaterials for Visible Light-Driven Photocatalysis. Catalysts 2018, 8, 74. Available online: https://www.mdpi.com/2073-4344/8/2/74 (accessed on 29 April 2025). [CrossRef]
- Wei, R.; Shi, Y.; Zhang, S.; Diao, X.; Ya, Z.; Xu, D.; Zheng, Y.; Yan, C.; Cao, K.; Ma, Y.; et al. Photocatalytic Upgrading of Plastic Waste into High-Value-Added Chemicals and Fuels: Advances and Perspectives. ACS Sustain. Chem. Eng. 2025, 13, 2615–2632. [Google Scholar] [CrossRef]
- Carceller, J.M.; Arias, K.S.; Climent, M.J.; Iborra, S.; Corma, A. One-pot chemo- and photo-enzymatic linear cascade processes. Chem. Soc. Rev. 2024, 53, 7875–7938. [Google Scholar] [CrossRef]
- Daboczi, M. Virtually free clean hydrogen generation by photoelectrochemical devices? Matter 2023, 6, 2594–2596. [Google Scholar] [CrossRef]
- Khan, M.M.; Rahman, A.; Matussin, S.N. Recent Progress of Metal-Organic Frameworks and Metal-Organic Frameworks-Based Heterostructures as Photocatalysts. Nanomaterials 2022, 12, 2820. (In English) [Google Scholar] [CrossRef]
- Yang, H.; Xu, J.; Cao, H.; Wu, J.; Zhao, D. Recovery of homogeneous photocatalysts by covalent organic framework membranes. Nat. Commun. 2023, 14, 2726. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Y.; Zhang, C.; Fan, W.; Li, G.; Fang, J.; Lu, L. Atomically engineering the metal-support interaction of single-atom Cu/TiO2 for efficient polyethylene terephthalate plastic photoreforming. Chem Catal. 2024, 4, 100902. [Google Scholar] [CrossRef]
- Kavanagh, S.R. Accurately Modelling Point Defects in Semiconductors: The Case of CdTe. Ph.D. Thesis, Computational Materials Science, Department of Chemistry (UCL) & Department of Materials (ICL), University College London & Imperial College London, London, UK, 2024. [Google Scholar]
- Frieden, F.; Leker, J. Future costs of hydrogen: A quantitative review. Sustain. Energy Fuels 2024, 8, 1806–1822. [Google Scholar] [CrossRef]
- Ahasan, T.; Edirisooriya, E.M.N.T.; Senanayake, P.S.; Xu, P.; Wang, H. Advanced TiO2-Based Photocatalytic Systems for Water Splitting: Comprehensive Review from Fundamentals to Manufacturing. Molecules 2025, 30, 1127. [Google Scholar] [CrossRef]




| Heterojunction Type | Catalyst System | Function | Ref. |
|---|---|---|---|
| Type-II | ZnO/UiO-66-NH2 | Enhanced charge separation via staggered bands | [82] |
| Type-II | g-C3N4/rGO | Improved e− transport, recombination suppression | [84] |
| Z-scheme | CdS/MOF hybrid | High redox potential with suppressed recombination | [83] |
| Z-scheme | C3N5/g-C3N4/graphene | Dual heterojunction with enhanced light absorption | [86] |
| Multi-junction | NiCoP/rGO/g-C3N4 | Synergistic multi-phase charge mediation | [87] |
| Category | Noble Metal Co-Catalysts (Pt, Pd, Au) | Non-Noble Metal Co-Catalysts (Ni, Co, Fe, Cu, Mo, etc.) |
|---|---|---|
| H2 Evolution Efficiency | High activity, excellent electron trapping, and fast proton reduction | Moderate to high activity dependent on material engineering (doping, alloying, or structuring) |
| Charge Separation | Forms Schottky junctions, effectively suppressing electron–hole recombination | Requires heterojunctions or defect engineering to achieve similar charge separation efficiency |
| Stability and Durability | Chemically stable under reaction conditions, low corrosion rate | Some non-noble metals (e.g., Fe) can corrode or deactivate over time unless properly stabilized. |
| Cost and Scalability | Very expensive and scarce, limiting large-scale applications | Earth-abundant and low-cost, catalysts are highly scalable for industrial use |
| Environmental Impact | Mining and refining noble metals have significant environmental impacts | More sustainable, widely available, and eco-friendly |
| Versatility | Effective across a range of photocatalytic systems (e.g., plastic reforming, water splitting) | Catalysts can be engineered into various alloys and oxides to enhance versatility and selectivity |
| Activation Energy and Reaction Kinetics | Low activation energy, enabling rapid reaction kinetics | Requires co-doping (e.g., Ni2P, CoP, MoS2) to reduce activation energy for HER |
| Long-Term Performance | Maintains high catalytic performance over extended use | Some non-noble metals may degrade or lose efficiency over prolonged cycles |
| Engineering Potential | Limited modification potential due to intrinsic properties of noble metals | High tunability is achieved through the capabilities of doping, alloying, or nanostructuring for enhanced photocatalytic properties |
| Characterization Technique | Key Findings | Advantages | Limitation |
|---|---|---|---|
| X-ray Diffraction (XRD) | Determines the crystalline structure and phase composition of catalysts | Identifies crystalline phases | Limited to crystalline materials |
| X-ray Absorption Spectroscopy (XAS) | Probes local electronic and structural environment of specific elements | Elucidates oxidation states, coordination numbers, and bond distances | Requires synchrotron radiation sources |
| Electron Microscopy (SEM and TEM) | Provides high-resolution images of catalyst morphology and nanostructure | Observes particle size, shape, and dispersion | Sample preparation can be intricate |
| Surface Area and Porosity Analysis (BET Method) | Assesses surface area and porosity of catalysts | Determines the availability of active sites | Assumes idealized models that may not fit all materials |
| UV-Vis Diffuse Reflectance Spectroscopy (DRS) | Investigates optical properties and bandgap energies | Assesses light absorption capabilities | Interpretation can be challenging for complex materials |
| Photoluminescence (PL) Spectroscopy | Measures recombination rate of photogenerated electron–hole pairs | Indicates efficiency of charge separation | PL signals can be weak and require sensitive detection |
| Fourier Transform Infrared (FTIR) Spectroscopy | Identifies functional groups and chemical bonds on catalyst surfaces | Provides insights into surface modifications and interactions with reactants | Surface sensitivity can be limited |
| Raman Spectroscopy | Offers information about molecular vibrations and crystal structures | Identifies structural defects and phase compositions | Fluorescence interference can obscure Raman signals |
| X-ray Photoelectron Spectroscopy (XPS) | Provides information on elemental composition and chemical states of surface elements | Surface-sensitive technique | Limited to surface analysis (typically 1–10 nm depth) |
| Mass Spectrometry (MS) | Analyze reaction intermediates and products | Offers insights into catalytic processes and efficiency | Requires coupling with other techniques for comprehensive analysis |
| Cost Factor | Key Points |
|---|---|
| Catalyst Synthesis |
|
| Photoreactor Setup |
|
| Operational Costs |
|
| Feedstock Pre-treatment |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edirisooriya, E.M.N.T.; Senanayake, P.S.; Ahasan, T.; Xu, P.; Wang, H. Comprehensive Insights into Photoreforming of Waste Plastics for Hydrogen Production. Catalysts 2025, 15, 453. https://doi.org/10.3390/catal15050453
Edirisooriya EMNT, Senanayake PS, Ahasan T, Xu P, Wang H. Comprehensive Insights into Photoreforming of Waste Plastics for Hydrogen Production. Catalysts. 2025; 15(5):453. https://doi.org/10.3390/catal15050453
Chicago/Turabian StyleEdirisooriya, E. M. N. Thiloka, Punhasa S. Senanayake, Tarek Ahasan, Pei Xu, and Huiyao Wang. 2025. "Comprehensive Insights into Photoreforming of Waste Plastics for Hydrogen Production" Catalysts 15, no. 5: 453. https://doi.org/10.3390/catal15050453
APA StyleEdirisooriya, E. M. N. T., Senanayake, P. S., Ahasan, T., Xu, P., & Wang, H. (2025). Comprehensive Insights into Photoreforming of Waste Plastics for Hydrogen Production. Catalysts, 15(5), 453. https://doi.org/10.3390/catal15050453










