Fabrication of a Novel Silica–Alumina-Based Photocatalyst Incorporating Carbon Nanotubes and Nanofiber Nanostructures Using an Unconventional Technique for Light-Driven Water Purification
Abstract
1. Introduction
2. Results
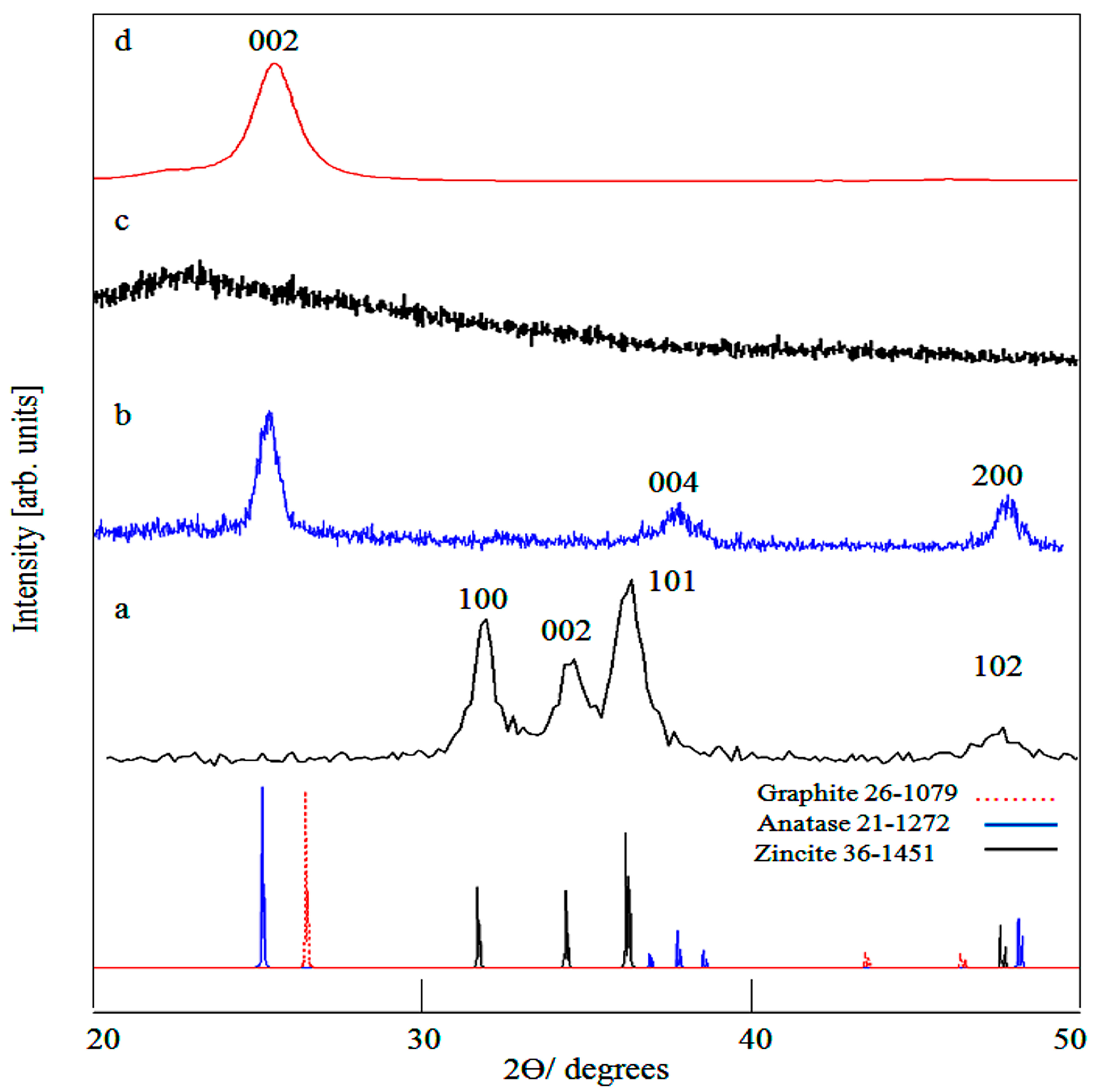
2.1. X-Ray Diffraction
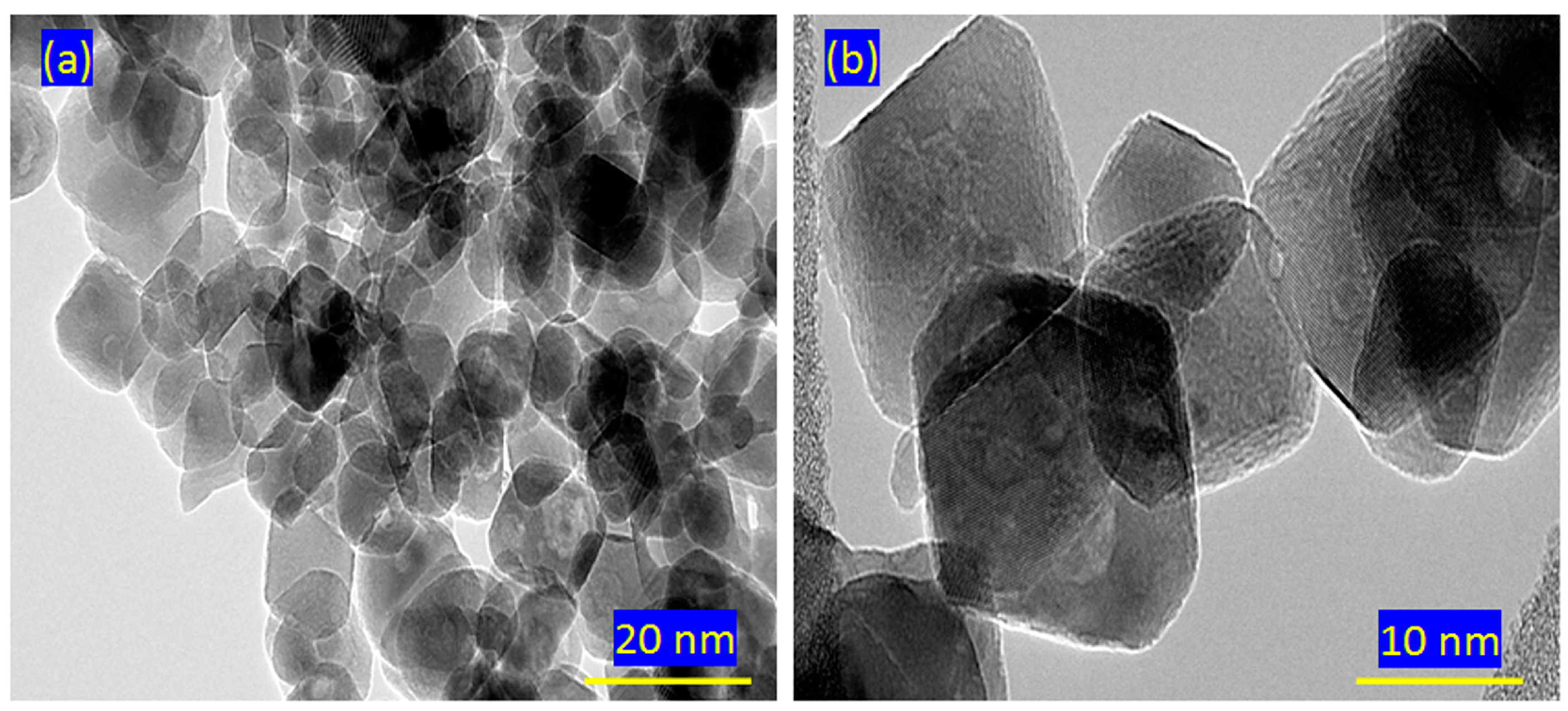
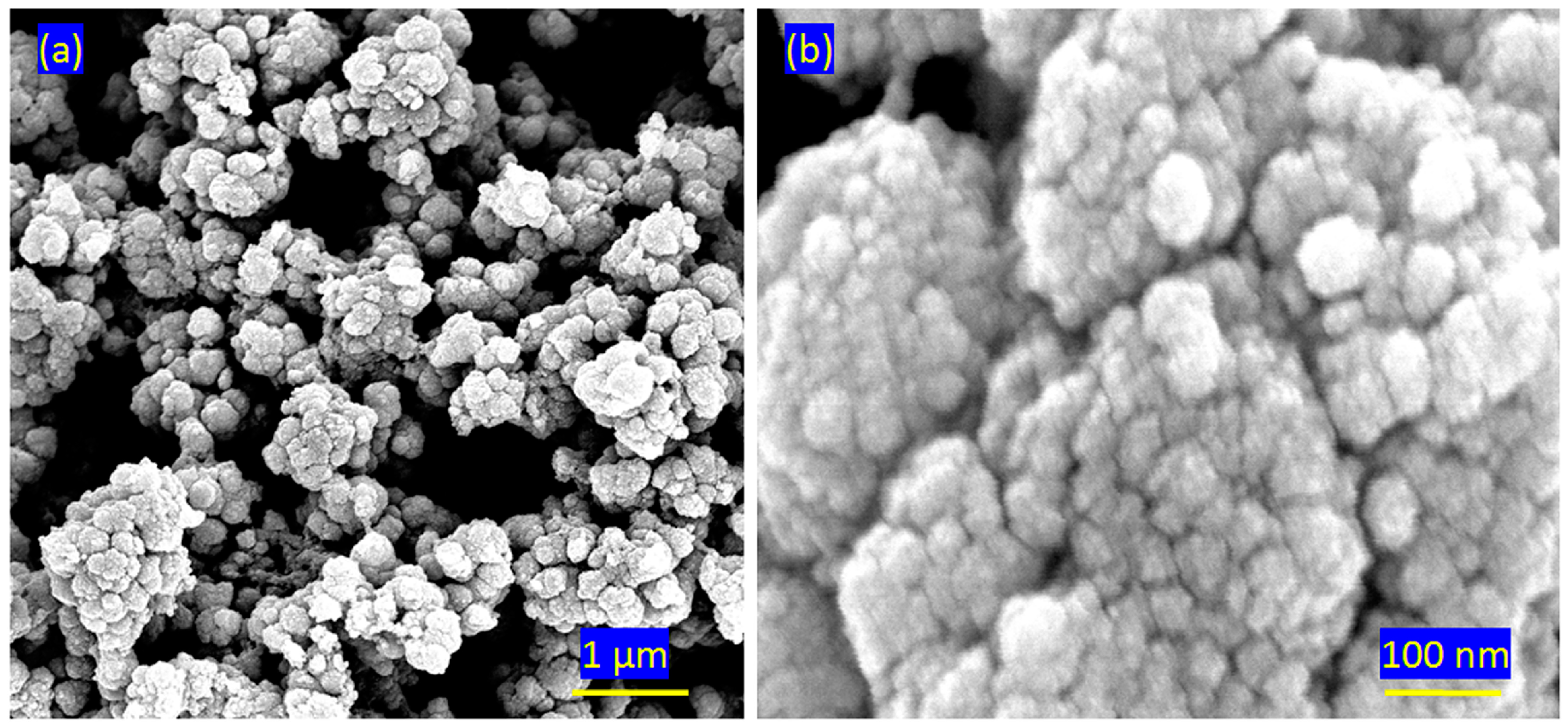
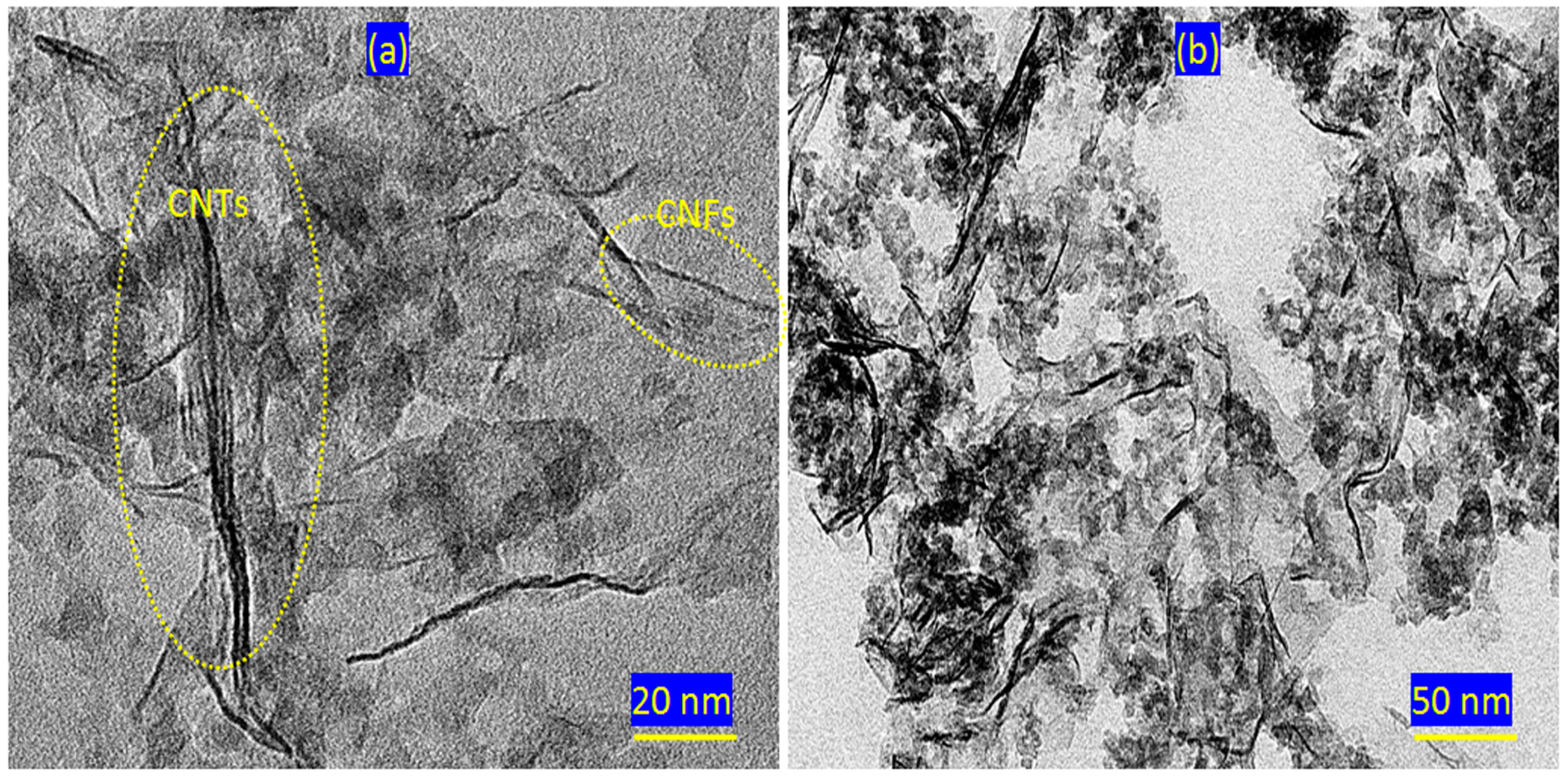
2.2. Scanning and Transmission Electron Microscopy
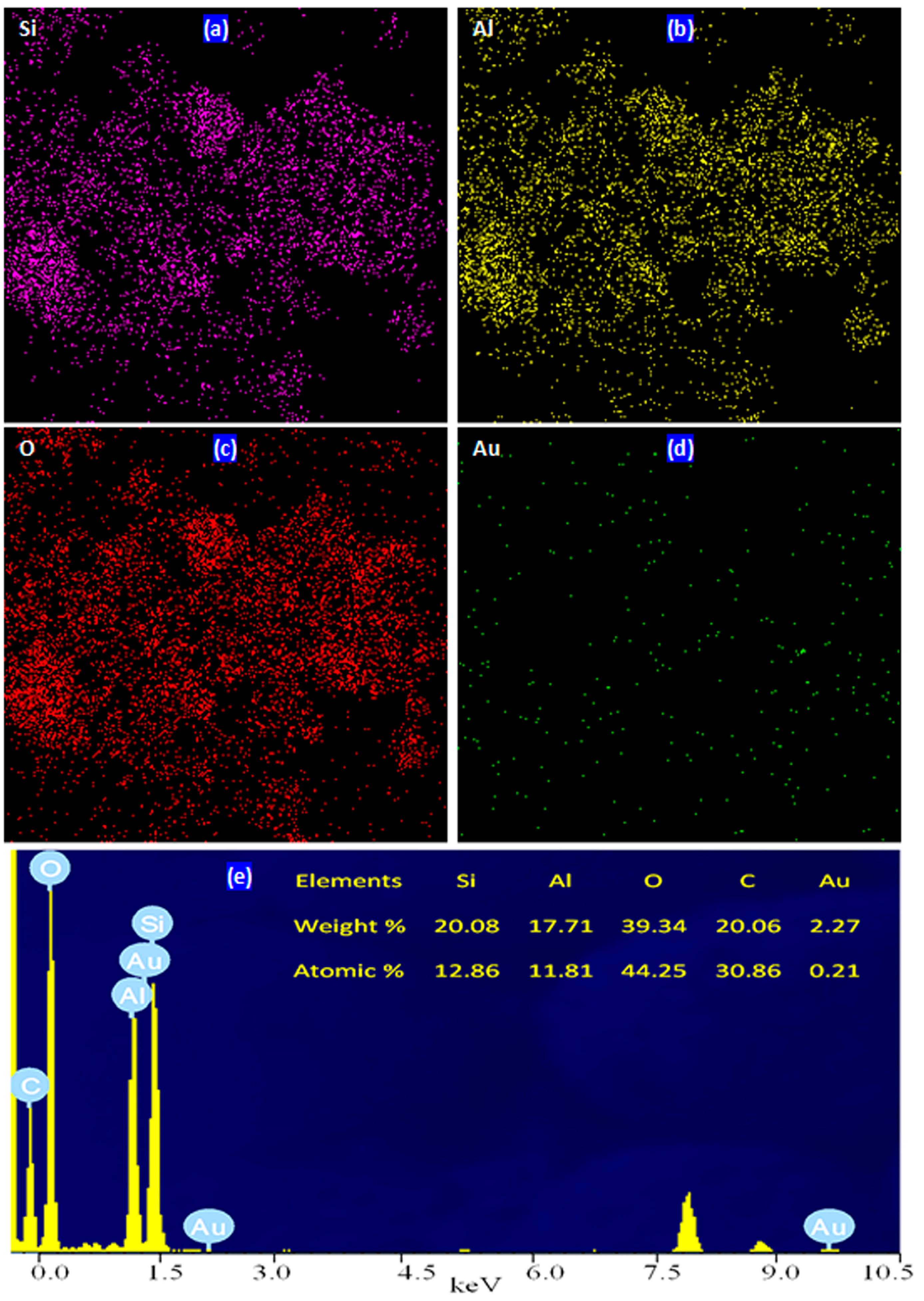
2.3. Mapping Images and EDX Analysis
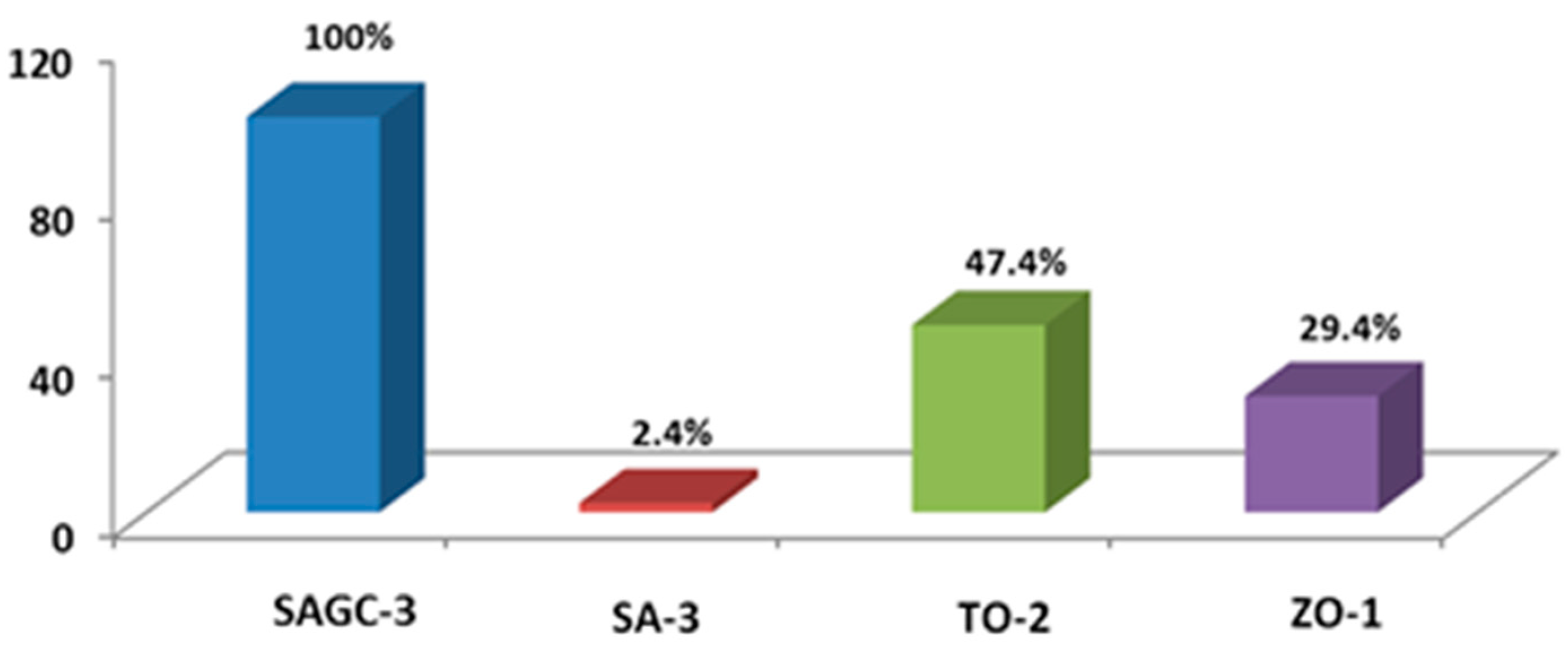
2.4. Surface Characteristics of Au/Silica–Alumina–CNT–CNF Nanoparticles
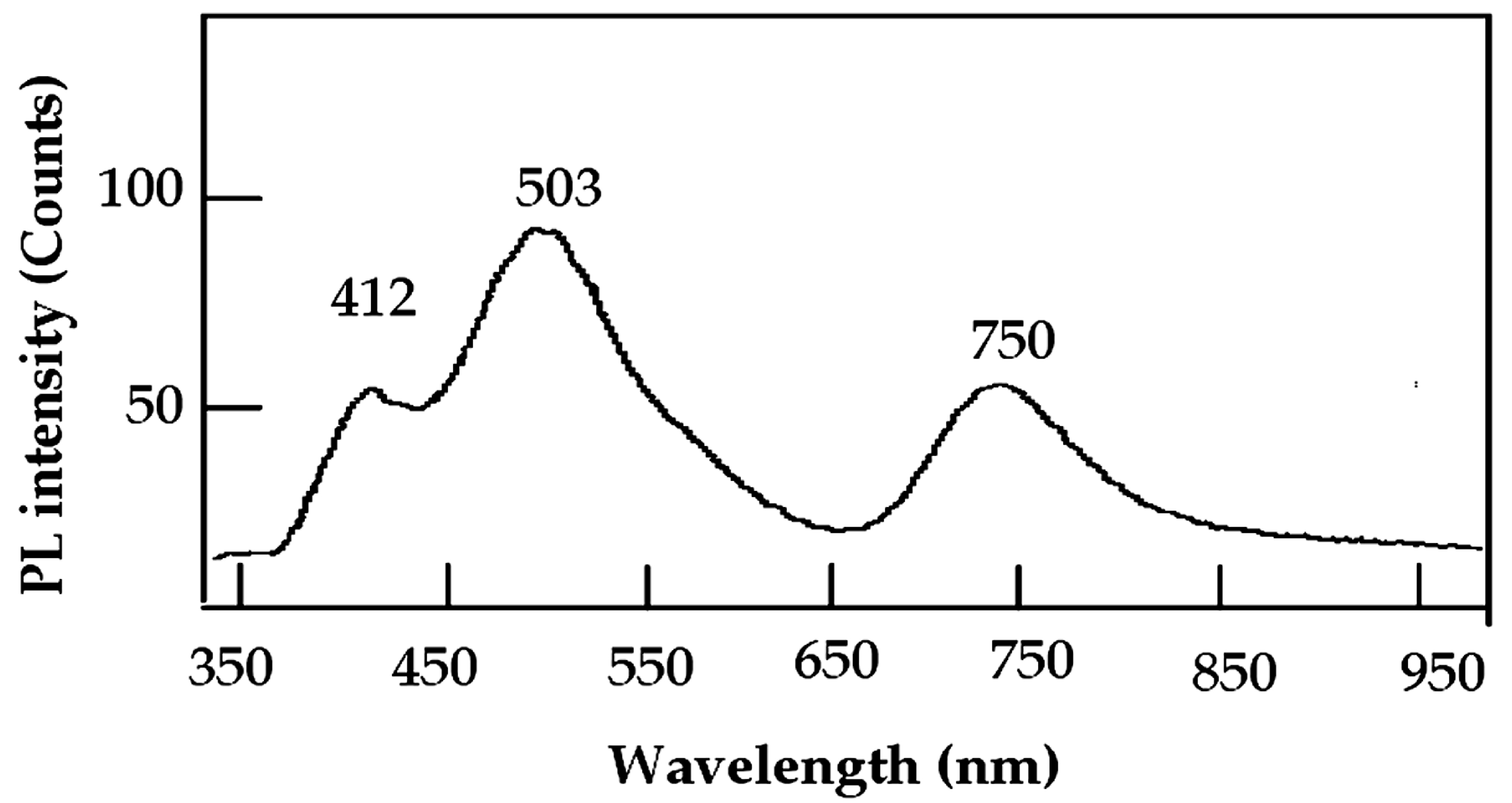
2.5. Optical Properties
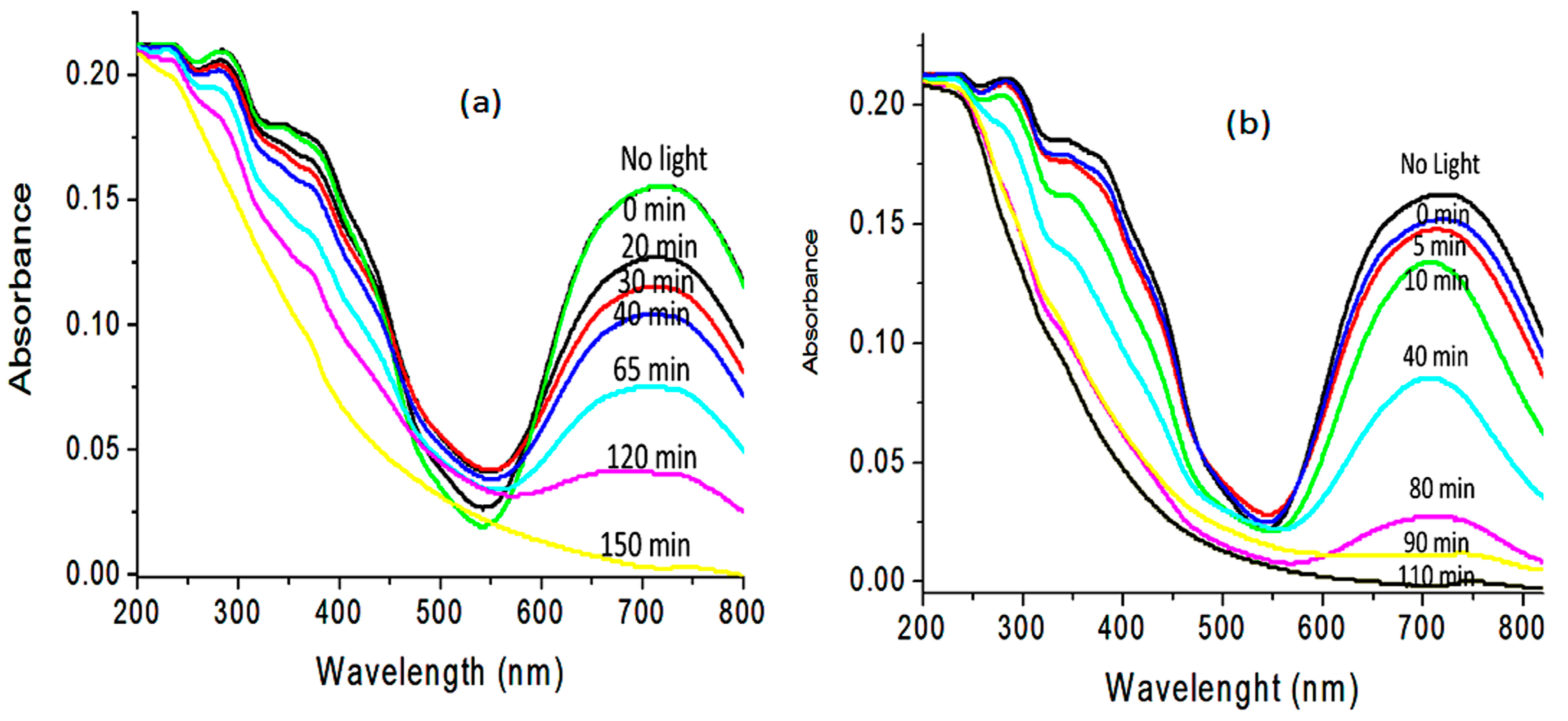
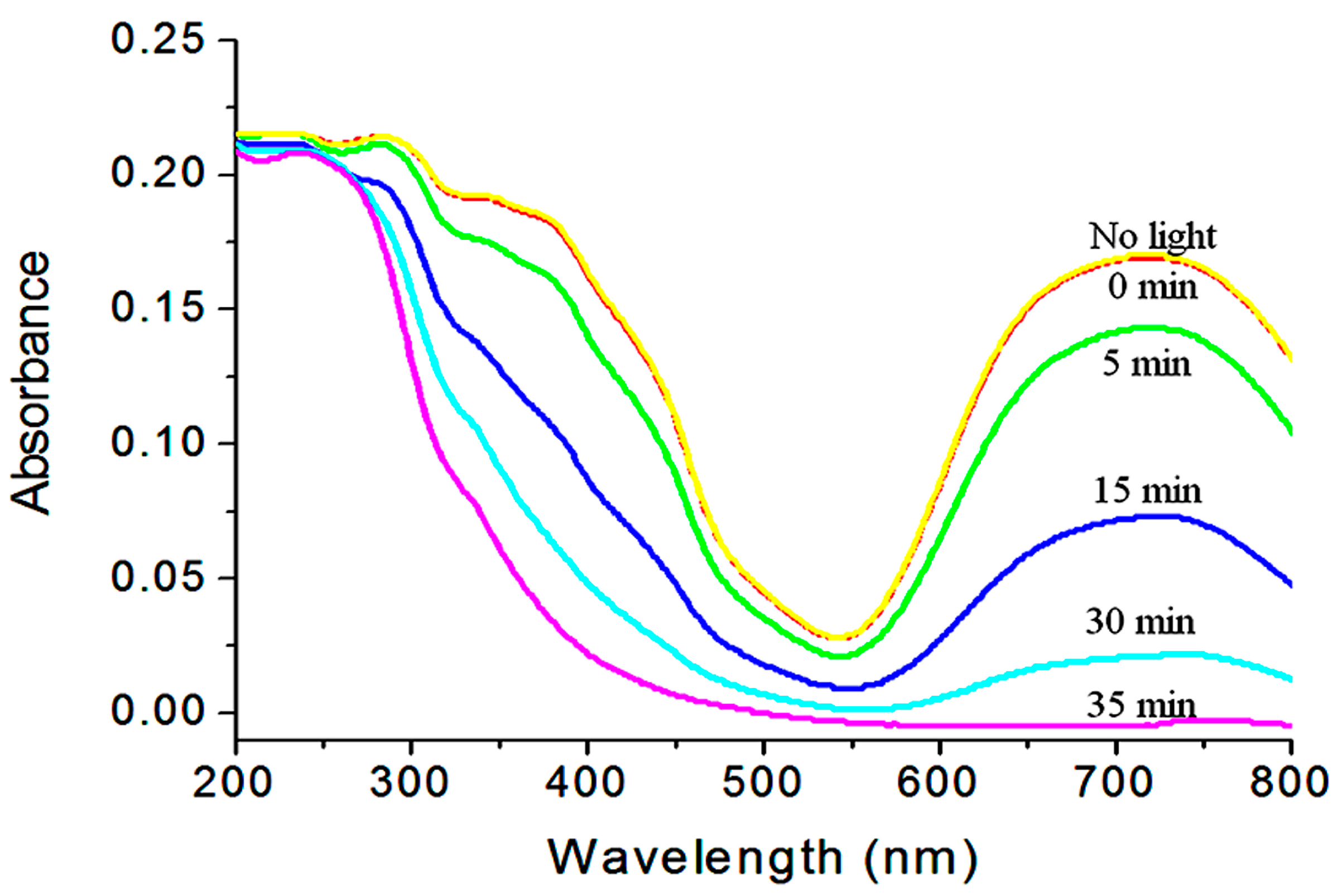
2.6. Water Purification
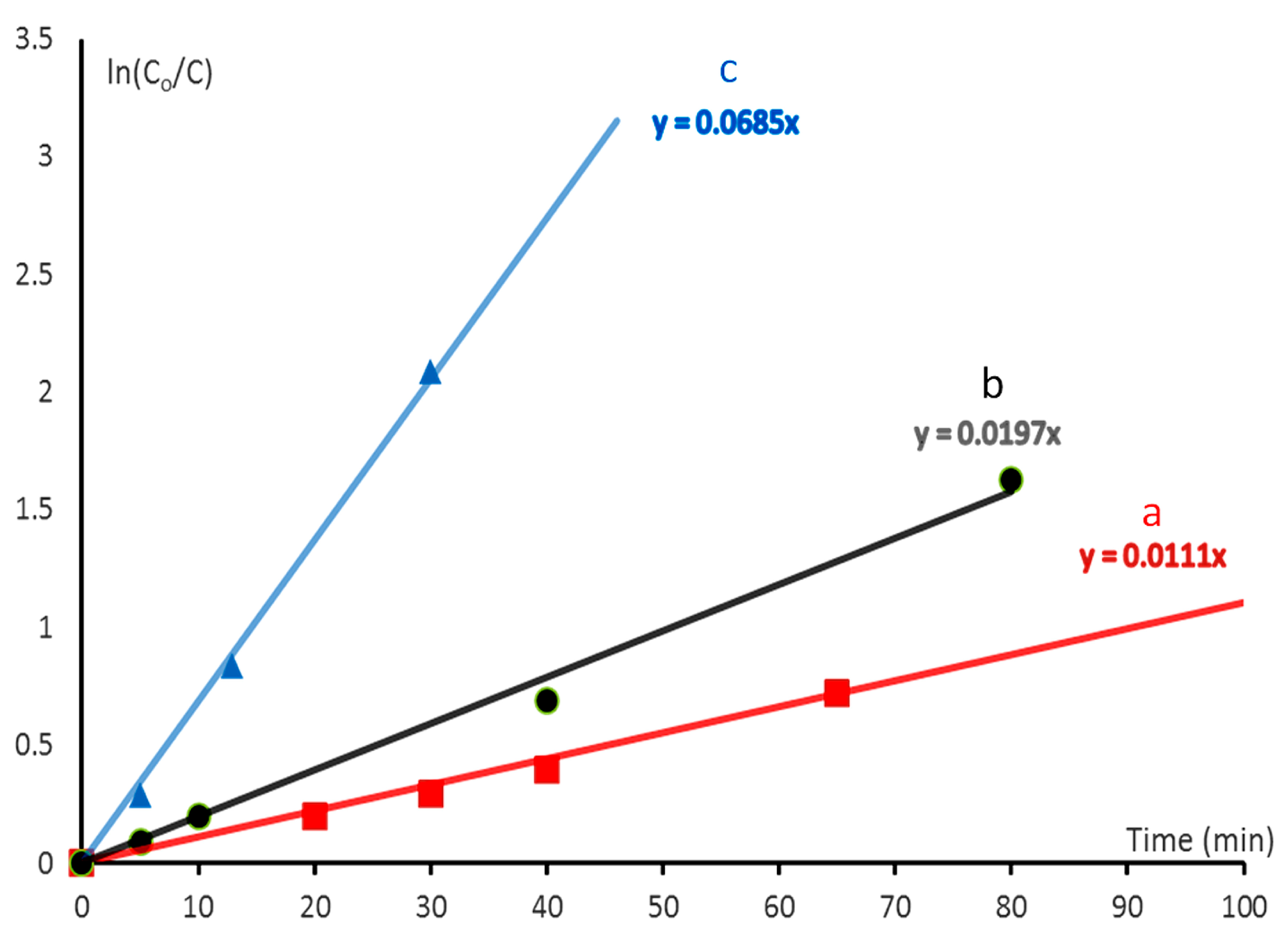
2.7. Kinetic Study
3. Discussion
4. Materials and Methods
4.1. Synthesis of the Modified Silica–Alumina
4.1.1. Synthesis of Explosive Compounds
4.1.2. Explosive Reactions for Silica–Alumina CNT and CNF Growth
4.1.3. Gold Support
4.2. Synthesis of Silica–Alumina Nanoparticles
4.3. Synthesis of Zinc Oxide Nanoparticles
4.4. Synthesis of Titanium Dioxide Nanoparticles
4.5. Characterization of the Prepared Samples
4.6. Photocatalytic Processes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saber, O.; El-Brolossy, T.; Al Jaafari, A.A. Improvement of Photocatalytic Degradation of Naphthol Green B Under Solar Light Using Aluminum Doping of Zinc Oxide Nanoparticles. Water Air Soil Pollut. 2012, 223, 4615. [Google Scholar] [CrossRef]
- Gupta, V.K.; Gupta, B.; Rastogi, A.; Agarwal, S.; Nayak, A. A comparative investigation on adsorption performances of mesoporous activated carbon prepared from waste rubber tire and activated carbon for a hazardous azo dye Acid Blue. J. Hazard. Mater. 2011, 186, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Jain, R.; Nayak, A.; Agarwal, S.; Shrivastava, M. Removal of the hazardous dye—Tartrazine by photodegradation on titanium dioxide surface. Mater. Sci. Eng. C 2011, 31, 1062–1067. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, R.; Agarwal, S.; Shrivastava, M. Kinetics of photo-catalytic degradation of hazardous dye Tropaeoline 000 using UV/TiO2 in a UV reactor. Colloids Surf. A Physicochem. Eng. Asp. 2011, 378, 22–26. [Google Scholar] [CrossRef]
- Lorimer, J.P.; Mason, T.J.; Plattes, M.; Phull, S.S.; Walton, D.J. Degradation of dye effluent. Pure Appl. Chem. 2001, 12, 1957–1968. [Google Scholar] [CrossRef]
- Chen, N.; Xia, J.; Li, L.; Lv, Q.; Zhao, K.; Ahmad, M.; Xiao, Z.; Wang, S.; Ye, F.; Zhang, Q. Comprehensive enhancement of photocatalytic H2O2 generation and antibacterial efficacy on carbon nitride through a straightforward polydopamine coating strategy. Surf. Interfaces 2025, 56, 105566. [Google Scholar] [CrossRef]
- Saber, O.; Osama, A.; Shaalan, N.M.; Osama, M. Engineering the Integration of Titanium and Nickel into Zinc Oxide Nanocomposites through Nanolayered Structures and Nanohybrids to Design Effective Photocatalysts for Purifying Water from Industrial Pollutants. Catalysts 2024, 14, 340. [Google Scholar] [CrossRef]
- Saber, O.; Osama, A.; Shaalan, N.M.; Osama, M. Nanolayered Structures and Nanohybrids Based on a Ternary System Co/Ti/Zn for Production of Photo-Active Nanocomposites and Purification of Water Using Light. Nanomaterials 2024, 14, 93. [Google Scholar] [CrossRef]
- Saber, O.; Osama, M.; Alshoaibi, A.; Shaalan, N.M. Doaa Osama Designing inorganic–magnetic–organic nanohybrids for producing effective photocatalysts for the purification of water. RSC Adv. 2022, 12, 18282–18295. [Google Scholar] [CrossRef]
- Saber, O.; Awada, C.; Osama, A.; Shaalan, N.M.; Alshoaibi, A.; Mansour, S.A.; Osama, M.; Farha, A.H. Boosting the Efficiency of Titanium Oxides and Accelerating Industrial Pollutant Removal Through Triple-Action Effects to Purify Water by Light. Catalysts 2024, 14, 772. [Google Scholar] [CrossRef]
- Albeladi, A.; Khan, Z.; Al-Thabaiti, S.A.; Patel, R.; Malik, M.A.; Meht, S. A Fe3O4-CdO Nanocomposite for Organic Dye Photocatalytic Degradation: Synthesis and Characterization. Catalysts 2024, 14, 71. [Google Scholar] [CrossRef]
- Lau, G.E.; Abdullah, C.A.C.; Ahmad, W.A.N.; Assaw, W.S.; Zheng, A.L.T. Eco-Friendly Photocatalysts for Degradation of Dyes. Catalysts 2020, 10, 1129. [Google Scholar] [CrossRef]
- Hauser, K.; Hassou, S.O.; Riaz, U. Ultrasound-Assisted Photocatalytic Degradation of Azo Dyes under Visible Light Irradiation Using Polythiophene-Decorated CoFe2O4 Nanohybrids. Catalysts 2024, 14, 495. [Google Scholar] [CrossRef]
- Palma Soto, E.; Rodriguez Gonzalez, C.A.; Luque Morales, P.A.; Reyes Blas, H.; Carrillo Castillo, A. Degradation of Organic Dye Congo Red by Heterogeneous Solar Photocatalysis with Bi2S3, Bi2S3/TiO2, and Bi2S3/ZnO Thin Films. Catalysts 2024, 14, 589. [Google Scholar] [CrossRef]
- Mbuyazi, T.B.; Ajibade, P.A. Photocatalytic Degradation of Organic Dyes by Magnetite Nanoparticles Prepared by Co-Precipitation. Int. J. Mol. Sci. 2024, 25, 7876. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Ozuğur Uysal, B.; Pekcan, O.; Erim, F.B. Efficient Photocatalytic Degradation of Methylene Blue Dye from Aqueous Solution with Cerium Oxide Nanoparticles and Graphene Oxide-Doped Polyacrylamide. ACS Omega 2023, 8, 13004–13015. [Google Scholar] [CrossRef]
- Khan, S.; Noor, T.; Iqbal, N.; Yaqoob, L. Photocatalytic Dye Degradation from Textile Wastewater: A Review. ACS Omega 2024, 9, 21751–21767. [Google Scholar] [CrossRef]
- Long, Z.; Li, Q.; Wei, T.; Zhang, G.; Ren, Z. Historical development and prospects of photocatalysts for pollutant removal in water. J. Hazard. Mater. 2020, 395, 122599. [Google Scholar] [CrossRef]
- Tahir, D.; Kwon, H.; Shin, H.; Oh, S.; Kang, H.; Heo, S.; Chung, J.; Lee, J.; Tougaard, S. Electronic and optical properties of Al2O3/SiO2 thin films grown on Si substrate. J. Phys. D Appl. Phys. 2010, 43, 255301. [Google Scholar] [CrossRef]
- Baha, A.A.; Ait-Karra, A.; Idouhli, R.; Tabit, K.; Zakir, O.; Dikici, B.; Khadiri, M.E.; Abouelfida, A. Synergistic Photocatalysis of Bayerite/Zeolite Loaded TiO2 Nanocomposites for Highly Efficient Degradation of Organic Pollutants in Aqueous Environments. Silicon 2024, 16, 4843–4856. [Google Scholar] [CrossRef]
- Stojanović, S.; Vranješ, M.; Šaponjić, Z.; Rac, V.; Rakić, V.; Ignjatović, L.; Damjanović-Vasilić, L. Photocatalytic performance of TiO2/zeolites under simulated solar light for removal of atenolol from aqueous solution. Int. J. Environ. Sci. Technol. 2023, 20, 1–16. [Google Scholar] [CrossRef]
- Hutsul, K.; Ivanenko, I.; Patrylak, L.; Pertko, O.; Kamenskyh, D. ZnO/Zeolite composite photocatalyst for dyes degradation. Appl. Nanosci. 2023, 13, 7601–7609. [Google Scholar] [CrossRef]
- John, A.; Rajan, M.S.; Thomas, J. Sunlight Driven Degradation of Drug Residues Using CuO Incorporated- Zeolite Supported- Graphitic Carbon Nitride. Catal. Lett. 2025, 155, 23. [Google Scholar] [CrossRef]
- Zarazúa-Morín, M.E.; Galindo-Luna, A.S.; Gallegos-Sánchez, V.J.; Juárez-Ramírez, I. Chemical exfoliation of silica filters used on methylene blue degradation by photocatalysis. Chem. Pap. 2022, 76, 5627–5642. [Google Scholar] [CrossRef]
- Balani, K.; Agarwal, A. Wetting of carbon nanotubes by aluminum oxide. Nanotechnology 2008, 19, 165701. [Google Scholar] [CrossRef]
- Keshri, A.K.; Huang, J.; Singh, V.; Choi, W.; Seal, S.; Agarwal, A. Synthesis of aluminum oxide coating with carbon nanotube reinforcement produced by chemical vapor deposition for improved fracture and wear resistance. Carbon 2010, 48, 431–442. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Li, F.; Cheng, H.M.; Su, G.; Yu, Y.D.; Shen, Z.H. Preparation, morphology, and microstructure of diameter-controllable vapor-grown carbon nanofibers. J. Mater. Res. 1998, 8, 13. [Google Scholar] [CrossRef]
- Luhrs, C.C.; Moberg, M.; Maxson, A.; Brewer, L.; Menon, S. IF-WS2/Nanostructured Carbon Hybrids Generation and Their Characterization. Inorganics 2014, 2, 211–232. [Google Scholar] [CrossRef]
- Toyama, N.; Inoue, H.; Ohki, S.; Tansho, M.; Shimizu, T.; Umegaki, T.; Kojima, Y. Influence of hollow silica-alumina composite spheres prepared using various amount of L(+)-arginine on their activity for hydrolytic dehydrogenation of ammonia borane. Adv. Mater. Lett. 2016, 7, 339–343. [Google Scholar] [CrossRef]
- Sethi, R.; Ahmad, S.; Aziz, A.; Siddiqui, A.M. Structural, optical and electrical properties of tin oxide thin films for application as a wide band gap semiconductor. AIP Conf. Proc. 2015, 1675, 030039. [Google Scholar]
- Soumya, S.S. Optical, Dielectric and Structural Properties of SnO2 Nanoparticles via Sol-Gel Method. J. Phys. Conf. Ser. 2023, 2426, 012039. [Google Scholar] [CrossRef]
- Sun, P.; Bai, L.; Kripalani, D.R.; Zhou, K. A new carbon phase with direct bandgap and high carrier mobility as electron transport material for perovskite solar cells. Comput. Mater. 2019, 5, 9. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, F.; Liu, J.; Zhou, G.; Chen, D.; Liu, Z.; Fang, J. Design and Architecture of P-O Co-Doped Porous g-C3N4 by Supramolecular Self-Assembly for Enhanced Hydrogen Evolution. Catalysts 2022, 12, 1583. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Hou, H.; Zhou, M. Facile Synthesis of Anatase–Rutile Diphase N-doped TiO2 Nanoparticles with Excellent Visible Light Photocatalytic Activity. Catalysts 2020, 10, 1126. [Google Scholar] [CrossRef]
- Xiang, D.; Wang, Z.; Xu, J.; Shen, H.; Zhang, X.; Liu, N. Defective TiO2/MIL-88B(Fe) Photocatalyst for Tetracycline Degradation: Characterization and Augmented Photocatalytic Efficiency. Catalysts 2024, 14, 528. [Google Scholar] [CrossRef]

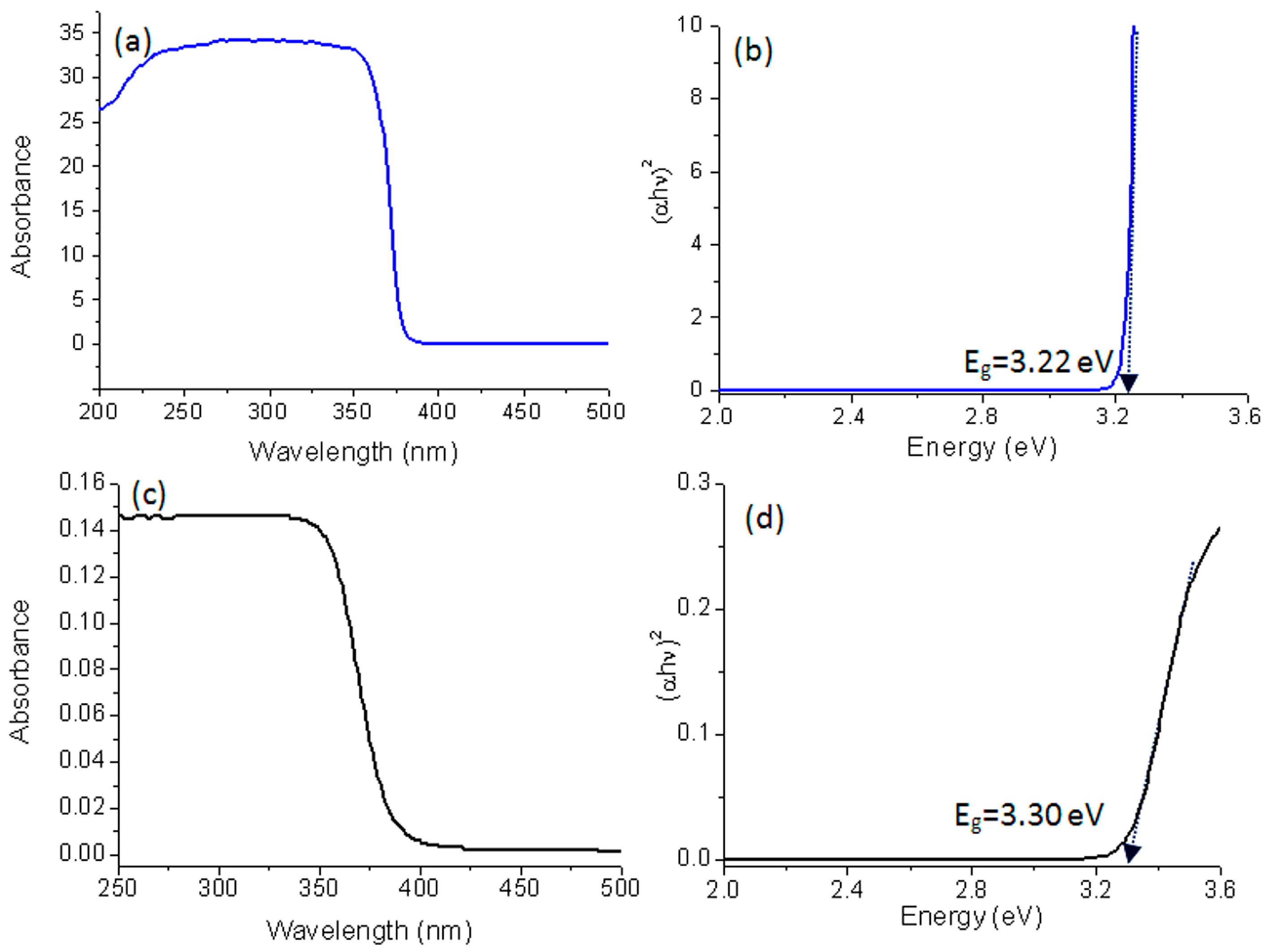
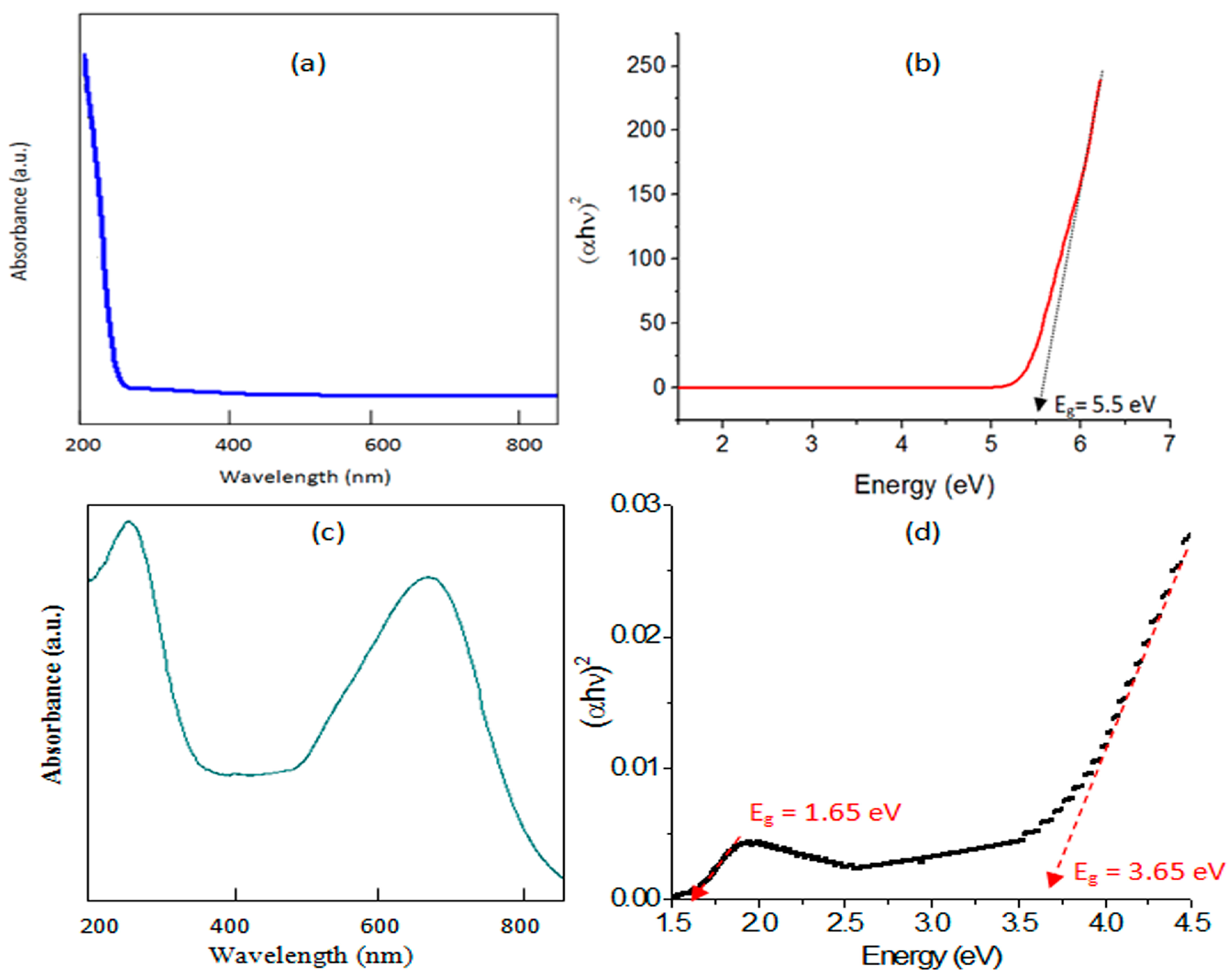


| Sample | Bang Gap Energy (eV) | Cut-off Wavelength (nm) |
|---|---|---|
| ZO-1 | 3.22 | 386.0 |
| TO-2 | 3.30 | 376.7 |
| SA-3 | 5.50 | 226.0 |
| SAGC-3 | 1.65, 3.65 | 753.3, 340.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saber, O.; Alshehab, A.; Shaalan, N.M.; Hegazy, A.M.; Aljasem, F.K.; Osama, A. Fabrication of a Novel Silica–Alumina-Based Photocatalyst Incorporating Carbon Nanotubes and Nanofiber Nanostructures Using an Unconventional Technique for Light-Driven Water Purification. Catalysts 2025, 15, 452. https://doi.org/10.3390/catal15050452
Saber O, Alshehab A, Shaalan NM, Hegazy AM, Aljasem FK, Osama A. Fabrication of a Novel Silica–Alumina-Based Photocatalyst Incorporating Carbon Nanotubes and Nanofiber Nanostructures Using an Unconventional Technique for Light-Driven Water Purification. Catalysts. 2025; 15(5):452. https://doi.org/10.3390/catal15050452
Chicago/Turabian StyleSaber, Osama, Abdullah Alshehab, Nagih M. Shaalan, Asmaa M. Hegazy, Fatimah K. Aljasem, and Aya Osama. 2025. "Fabrication of a Novel Silica–Alumina-Based Photocatalyst Incorporating Carbon Nanotubes and Nanofiber Nanostructures Using an Unconventional Technique for Light-Driven Water Purification" Catalysts 15, no. 5: 452. https://doi.org/10.3390/catal15050452
APA StyleSaber, O., Alshehab, A., Shaalan, N. M., Hegazy, A. M., Aljasem, F. K., & Osama, A. (2025). Fabrication of a Novel Silica–Alumina-Based Photocatalyst Incorporating Carbon Nanotubes and Nanofiber Nanostructures Using an Unconventional Technique for Light-Driven Water Purification. Catalysts, 15(5), 452. https://doi.org/10.3390/catal15050452










