Abstract
Microplastics (MPs), defined as synthetic polymer particles ranging from 1 μm to 5 mm, originate from various sources, including synthetic textiles, tire wear, degraded plastic waste, etc. Their small size and chemical stability make them challenging to remove, collect and degrade, posing significant adverse effects to both ecosystems and human health. While efforts to develop sustainable alternatives and removal methods are ongoing, effective solutions remain limited. Catalytic degradation and upcycling present a promising route to mitigate MP pollution by enabling efficient breakdown into less harmful molecules and potential upcycling into valuable products with lower energy requirements. This review provides a comprehensive overview of recent advances in catalyst design and development specifically for MP degradation, highlighting photochemical, thermal, biological, electrochemical, and hybrid approaches. Key challenges, reaction mechanisms, and future directions are discussed, offering a timely reference for researchers in this emerging field.
1. Introduction
Since the term MPs was first introduced in 2004 [1], its usage has been boosted due to increased awareness of MPs’ adverse effects on ecosystems and human beings [2]. As shown in Figure 1a, the number of papers published related to MPs has increased from 9 in 2011 to 3787 in 2024, showcasing a nearly 400 times increment. Increased attention has also been paid to the efficient removal of MPs using catalysts (Figure 1b). MPs are an emergent yet critical issue for the environment because of their high degradation resistance and bioaccumulation [3,4]. Unfortunately, the current technologies to remove, recycle, or degrade MPs are insufficient for their complete elimination [5,6]. In addition, the fragmentation and degradation of mismanaged plastic wastes in the environment have also been identified as a significant source of MPs [7], making the sustainable management of MPs an urgent need. Thus, the development of effective removal methods, as well as plastic recycling strategies, is crucial to building an MP-free environment.
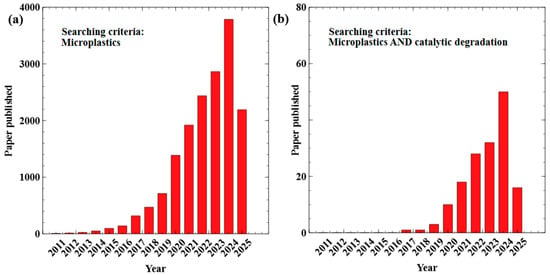
Figure 1.
The number of papers published each year in the past 15 years related to (a) microplastics in general and (b) the catalytic degradation of microplastics. Source: Web of Science. Data was collected on 19 August 2025. The search criteria are listed in the plot.
MPs refer to plastics with a size of ≤5 mm and can be further divided into more defined categories, including micro-size, nano-size, and even pico-size, as listed in Table 1 [8]. Until now, there is still no consensus on the upper and lower limits of an MP’s size [9]. Based on the origin, MPs could be generated from two sources. One is primary MPs, which are from cosmetics, paint, or pellets and flakes for plastic product generation. Primary MPs are intentionally manufactured and added to products. Common examples include microbeads in cosmetics, plastic pellets used in manufacturing, microfibers in textiles, etc. The other is secondary MPs that are produced from larger plastic products during use and under weathering, such as the wearing of textiles and tires, fragmentation of larger pieces under ultraviolet (UV) light, and biological interaction [3,10]. MPs can be redistributed by wind, rain, and water, and finally end up in large water bodies (lakes and oceans), as shown in the life cycle of MPs in Figure 2. The small size of MPs makes them easily enter living organisms from the top to the bottom of the food chain, while their pervasive nature further increases their bioaccumulation [11]. The toxicity of MPs has been widely observed and identified across terrestrial and aquatic ecosystems. Behavioral, metabolic, and developmental changes could happen with MP exposure [12]. Adverse effects, including the survival rate of zooplankton, altered finfish behavior [13], clam eggs hatching [14], and urchin physical abnormalities [15] have been reported. With prolonged consumption of MPs, digestive tract blocking, digestive behavior disorder, and even reproductive capability impairment could happen for whole organisms in the food chain [16].

Table 1.
MP size classification nomenclature with the respective size ranges and organisms of equivalent size. Modified from reference [17].
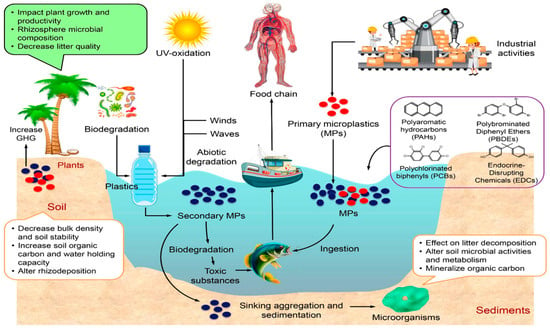
Figure 2.
A life cycle demonstration of MPs, including the formation of primary and secondary MPs and the adverse effects of their formation on aquatic flora and humans through the food chain [16].
Therefore, identifying effective strategies to mitigate the accumulation of environmental MPs is crucial, given its direct implications for both human health and ecosystem sustainability. In this regard, catalytic MP handling shows great promise. Recent studies have highlighted several advantages of catalytic MP degradation over conventional treatment methods (filtration, coagulation–flocculation, sedimentation, etc.). According to reference [18], the estimated half-lives for high density polyethylene (HDPE) can range from 58 years (bottles) to 1200 years (pipes) in the natural marine environment. To tackle the bottleneck of reaction rate, catalytic processes demonstrate high efficiency in accelerating polymer breakdown under relatively mild and environmentally compatible conditions, including ambient temperature [19], atmospheric pressure [20], and visible light irradiation [21]. In addition, catalysts offer improved selectivity and control, allowing for targeted degradation pathways while minimizing the formation of undesirable by-products [22]. A further benefit lies in the potential conversion of MPs into value-added products, such as fuels or chemical feedstocks [23], coupling environmental remediation with resource recovery. Moreover, catalytic approaches reduce the likelihood of generating secondary pollutants and show strong potential for scalability through advances in nanocatalyst design and reactor engineering. Collectively, these advantages underscore the promise of catalytic degradation as a sustainable strategy for mitigating MP pollution.
This work centers on catalyst development and engineering specifically aimed at MP degradation and upcycling. While extensive research has been devoted to catalyst design for bulk plastic recycling and upcycling, studies on catalytic processes for MPs remain relatively limited due to the unique physicochemical properties of MPs and the challenges associated with their removal and collection. Accordingly, this review focuses exclusively on studies involving catalytic degradation of MPs, whereas research on macro-scale plastic catalytic degradation is either excluded or used only as a comparative reference. To establish a foundation for catalyst development in this field, the review first outlines the key differences between MPs and bulk plastics. It then briefly discusses methods for MP identification and quantification, given that accurate evaluation of catalyst performance depends closely on these techniques. Section 4 provides a detailed overview of current catalytic degradation approaches for MPs—including photochemical, biological, electrochemical, thermochemical, and hybrid chemical pathways—with particular emphasis on catalyst-assisted processes that enhance degradation rates and performance. Building on this, the underlying mechanisms are examined to highlight the fundamental principles of catalyst design and engineering. Finally, the review lists current challenges and explores future opportunities in catalytic MP degradation, with the aim of offering insights and inspiration for subsequent research in this emerging area.
2. Difference Between MPs and Bulk Plastics
Before getting into the catalyst’s development for efficient MP degradation, it is important to understand the differences between bulk plastics and MPs. Degradation, recycling, and upcycling of bulk plastics have been extensively studied through pyrolysis, gasification, reprocessing, and photo-reforming [24,25,26]. Theoretically, the catalysts that have been developed and tested for bulk plastic treatment could be extended to MPs. While there are still many questions remaining to be answered, many differences exist between bulk materials and their size-reduced counterparts. As the name implies, the major difference between bulk plastics and MPs is the size. The size of MPs can span a wide range, from nm to mm (as shown in Table 1—MP size categories based on biological application), making them hard to detect, observe, collect, and study, especially with current commercially available methods. Due to the small size of MPs, they can be easily taken in by living organisms and widely distributed from organic to inorganic substances, from soil to water, and from deep in the sea to the top of mountains [27]. Therefore, mixed methodologies need to be applied for catalyst performance evaluation based on MPs under different conditions.
Besides the size, the second difference of MPs is their complexity, both in physical and chemical properties. MPs can originate from numerous sources and are usually treated as heterogeneous materials, including synthetic clothing, microbeads, and the breakdown of larger plastic items. Their variation in size, shape, density, and chemical composition makes their studies complicated not only in detection but also in the development of removal methodologies. In a work on MP identification on beaches in Plymouth, UK, by Richard Thompson et al. [1], eight polymers were identified, including acrylic, alkyd, polyethylene, polypropylene, polyamide, polyester, polymethylacrylate, and polyvinyl alcohol, indicating that the MPs come from different bulk plastic sources. Furthermore, for the origin of MP toxicity, it can be a result of the plastic chemicals or the particle itself, while different MPs can show significant differences in toxicity. According to the work by Lisa Zimmermann et al. [28], the authors show that chemicals in plastics contribute more to toxicity in the case of polyvinyl chloride (PVC), but not in the case of polyurethane (PUR) and polylactic acid (PLA) plastics. Interestingly, they observed that bioplastics show similar toxicity to their conventional plastic counterparts. Therefore, treating MPs as a homogeneous entity is not suitable for environmental impact assessment. Instead, the chemical compositions and physical conditions of different MPs should be considered. Therefore, it will be much more challenging to develop efficient catalysts for the heterogeneous MPs treatment compared to their bulk counterparts. For a better view, the property changes of macroplastics to MPs after degradation are summarized and shown in Figure 3. A detailed property comparison between bulk plastic and MPs is also provided in Table 2.
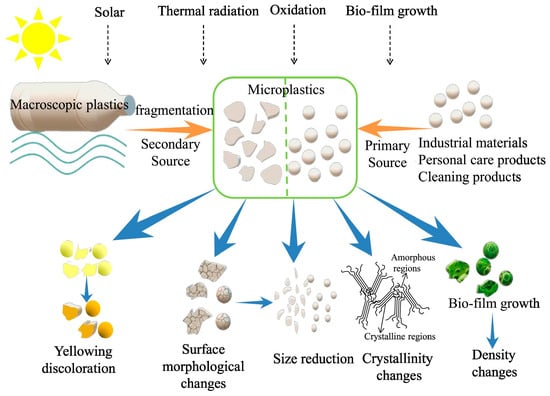
Figure 3.
Property changes of microplastics after degradation [29].

Table 2.
Property comparison between bulk plastic and MP.
Moreover, MPs present varied chemical properties due to their high-energy surface through degradation processes. With a large surface area-to-volume ratio, MPs can readily adsorb pollutants from the surrounding environment through hydrophobic interactions, sorption mechanisms, electrostatic forces, van der Waals forces, hydrogen bonding, and pi–pi interactions [30]. These adsorbed chemicals can include persistent organic pollutants (POPs), heavy metals, and other toxins, not only increasing the harmful potential of the ingested plastics to the ecosystem but also affecting their catalytic recycling. The aging process of plastics, leading to MPs formation, can also modify their surface properties and crystallinity, affecting their ability to adsorb and desorb pollutants. Since MPs are usually generated from bulk plastic degradation, the physical and chemical properties of MPs, such as color, surface morphology, crystallinity, particle size, and density, are usually different compared to the bulk materials [29].
3. Microplastic Identification and Quantification
Precise identification and quantification of MPs before and after the catalytic process are vital for the catalyst’s performance evaluation. However, this is not an easy task due to the complicated chemical and physical properties of MPs and interference from other similar micro-substances. In this section, techniques that can be used for MP identification and quantification are summarized and discussed.
The selection of MP identification and quantification methods depends largely on particle size and matrix complexity. For particles >100 µm, optical microscopy enables rapid visualization of morphology, size distribution, and color, though often requiring sediment and physical sieving [31,32]. For <100 µm particles, the resolution limits of optical microscopy reduce reliability, and electron microscopy (SEM) provides detailed structural insights with the help of Energy Dispersive X-ray Spectroscopy (EDS). It is worth mentioning that unlike the traditional processing methods for non-conductive samples, wet-mode imaging avoided the introduction of other elements during the coating process, thereby enhancing the identification capability of SEM/EDS for microplastics [33].
Among chemical methods, Fourier Transform Infrared (FTIR) remains the most widely applied, with focal plane array (FPA)-based imaging enabling large-scale mapping. It is effective for >20 µm particles but less sensitive to nanoplastics and prone to spectral fouling [34,35]. Raman spectroscopy is effective for <20 µm particles and is less affected by water interference, though fluorescence background remains a challenge [36]. 1H NMR provides quantitative compositional and degradation information of microplastic particles that are <300 µm but requires large sample quantities, restricting routine use [37,38,39].
Thermal and mass-based approaches, including Py-GC/MS and thermal gravimetric analysis (TGA), are powerful for compositional analysis. In principle, these techniques are not fundamentally constrained by particle size because they rely on the complete thermal degradation of polymers. However, in practical applications, excessive particle size or non-uniform heat transfer can lead to incomplete decomposition, reduced signal efficiency, or altered degradation profiles [40]. Hence, while theoretically particle-size-independent, both Py-GC/MS and TGA exhibit a practical upper limit governed by heat and mass transfer efficiency, loading capacity, and signal linearity. But even under such restrictions, by identifying certain additives or molecular markers, they can still indicate the origin of specific plastics, providing evidence for analyzing samples with complex microplastic components [41]. Py-GC/MS identifies polymers via characteristic pyrolytic fragments, while TGA provides thermal degradation profiles. Both require dried, homogenized samples and offer robust quantitative data, although they are destructive and cannot preserve morphology [42].
Pretreatment strategies vary across methods: microscopy relies mainly on physical separation, such as sedimentation [31]; FTIR and Raman require density separation and drying on the anodisc filters [35]; nuclear magnetic resonance (NMR) demands bulk concentration [38]; and thermal/mass-based methods depend on drying and homogenization, which are usually more complex depending on the specific situation [43]. In terms of MP identification and quantification, no single technique is sufficient for a comprehensive characterization. Table 3 lists a simple comparison between different techniques used for MP identification and quantification. Figure 4 presents current and novel technologies that can be used for the detection of MPs in different environments.

Table 3.
Comparison summary between different techniques used for MP identification and quantification.
Often, all the aforementioned methods need to be paired and cross-verified to achieve a robust evaluation for the study of MPs. In the work of Thava Palanisami et al. [44], a combined technique using SEM, Brunauer-Emmett-Teller (BET), X-ray Diffraction (XRD), Attenuated Total Reflectance—Fourier Transform Infrared (ATR-FTIR), and TGA is applied to study the long-term degradation of MPs in the marine environment in terms of their surface morphology, surface area, surface chemical species, crystallinity, and stability changes. In another work of measuring fragmentation rates of MPs [45], the authors include the analytical ultracentrifugation with a refractive index detector (AUC-RI), a single-particle counter, for particle size distribution, and concentration quantification. The particle shape is characterized using SEM, while ultraviolet–visible spectroscopy (UV-Vis) is applied for monitoring the content of released water-soluble organics (total organic carbon (TOC)).

Figure 4.
Summary of current and novel technologies that can be used for MP detection in different environments [46].
4. Enhanced Microplastic Degradation Using a Catalyst
MP degradation includes transforming MPs into smaller molecules that are less harmful or useful components, such as CO2 [47], light hydrocarbons [48], carbon-based materials for catalyst preparation [49], fuel, chemicals [50], etc. Under natural environmental conditions, the MP degradation process is slow and results in a loss of carbon stock due to its persistent nature and a lack of reaction control. To mitigate the challenges and potentially turn wasted MPs into valuable feedstock in the carbon cycle, catalysts play a vital role in speeding up the degradation process and controlling the reaction direction. In the review, depending on the working mechanism and materials type used, catalysts for MP degradation are divided into photocatalysts, Fenton and Fenton-like catalysts, thermochemical catalysts, bio and bio-inspired catalysts, electrocatalysts, and hybrid catalysts, aiming to provide a clear and systematic overview of currently applied MP catalysts. Based on the category, novel catalyst design and engineering methods used to achieve improved MP degradation will be summarized and discussed based on the most recent publications.
4.1. Photocatalysts
Currently, the most often used photocatalysts for MP degradation include TiO2 [51], ZnO [52], graphite carbon nitride (g-C3N4) [53], metal–organic frameworks (MOFs), etc. These materials work by generating reactive oxygen species (ROS) when activated by light, which then break down the polymer chains of MPs into smaller, less harmful components, ultimately aiming for mineralization into CO2 and H2O. Besides the conventional materials, bismuth-based catalysts [54], Mxene-based materials, and plasmonic metal nanoparticles like Au and Ag can enhance light absorption and catalytic activity, resulting in superior performance. A more detailed summary of photocatalysts used for MP degradation can be found in the review paper [55].
Mechanism: Usually, three steps are involved in a general photocatalytic process: (1) charge carrier are generated from the semiconductor photocatalyst through photon energy activation, (2) charge carriers transfer from bulk to the surface of photocatalysts, and (3) photogenerated charge carriers induce surface redox reaction on the target [56]. In photocatalysis, semiconductors are usually used and act as photocatalysts by absorbing photon energy, becoming excited, and generating electron–hole pairs and ROS. These ROS, including hydroxyl radicals (·OH) and superoxide ions (O2·−), play a crucial role in the degradation of MPs [57]. In terms of MP photocatalytic degradation mechanisms, the process includes mainly four steps: (1) chain initiation, (2) chain growth, (3) chain branching, and (4) chain termination. For the initiation of MP degradation, two pathways have been proposed. In the first route, the strong oxidative holes (h+) in the catalyst valence band (VB) can oxidize MPs, leading to CO2 and H2O generation. Alternatively, the excited e− and h+ can react with O2 and H2O on the surface of the photocatalyst, generating primarily ·OH and O2·−.
The photocatalytic MP treatment can be divided into three categories: photo-degradation, photo-conversion, and photo-reforming [58], as shown in Figure 5. For photo-degradation, CO2, as a mineralization product, is produced through a non-selective oxidation process, which occurs in an aerobic environment, while value-added chemicals and fuels are generated through photo-reforming and photo-conversion processes, which are typically in an anoxic condition. At the same time, side products such as H2, formate, acetic acid, and other valuable chemicals [53,59,60] can also be produced through photo-reforming and photo-conversion using the remaining electrons in the system.
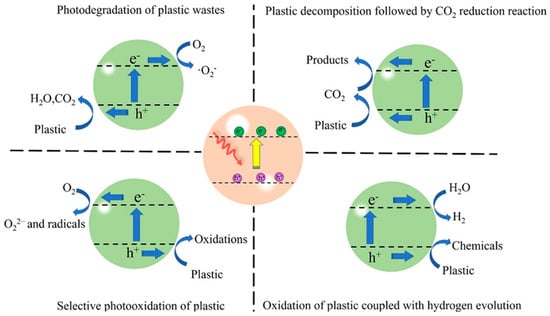
Figure 5.
Mechanism summary of photocatalytic plastic conversion pathways, including photo-conversion, photo-degradation, and photo-reforming with different products generated [58].
To improve photocatalytic performance, one of the effective ways is to construct heterostructures with other semiconductors, activated carbon, graphene, or noble metals. Slamet et al. [61] prepared Ag/TiO2 nanocomposites. The catalyst is used for simulated MP particle degradation in water under UV light. A total of 50 mg of the catalyst is added for 100 ppm of polyethylene (PE) MP decomposition. The results show that 100% MP degradation within 120 min can be achieved, while MPs with particle sizes between 125 and 150 μm show the fastest degradation rate, with 100% degradation efficiency in 90 min irradiation. Similarly, a g-C3N4/TiO2/WCT-AC composite was prepared using waste cotton-based activated carbon (WCT-AC)-loaded TiO2 as a precursor [59]. Through the heterojunction construction, improved visible light absorption and photoelectron–hole separation can be achieved, resulting in 67.58% PE degradation in 200 h under visible light conditions. The same engineering approach has also been applied by synthesizing a Pt-ZnO nanocomposite [62] for LDPE film photodegradation. The photocatalysis testing of LDPE film (1 cm × 1 cm) is conducted in deionized (DI) water under visible light for 175 h. Through FTIR characterizations, the authors indirectly quantify the degradation content using carbonyl and vinyl indices (CI and VI). On average, there is a 13% and 15% increase, respectively, for CI and VI, indicating an increased degradation performance using the Pt-ZnO combination. Furthermore, LDPE showcased deeper cavities, and wrinkles formed after 175 h of visible light irradiation compared to the original smooth surface. Heterojunctions of MXene-based photocatalyst composites have also been developed for H2 evolution integrated with enhanced polyethylene terephthalate (PET) degradation [60]. By successfully constructing MXene/ZnxCd1−xS photocatalysts through a band structure engineering approach, simultaneously, H2 production with a rate of 14.17 mmol·g−1·h−1 and PET degradation can be achieved. What is more, organically valuable micro-molecule compounds, such as glycolate, acetate, methanol, etc., can also be produced, demonstrating a superb photocatalyst development scheme. WO3/g-C3N4 as a composite photocatalyst has also been synthesized via a hydrothermal method to do the same work [53]. With a 30% WO3/g-C3N4 formula, a high H2 evolution rate of 14.21 mM and PET degradation by formate, methanol, acetic acid, and ethanol can be realized under visible light. By creating a heterojunction between WO3 and g-C3N4, improved photocatalytic performance can be achieved due to the reduced recombination of charge carriers and the enhanced redox capability. Catalyst structure can be another factor to tune. A core–shell BiO2−x/CuBi2O4 heterojunction is constructed [63]. Increased PE and polystyrene (PS) MP degradation can be achieved due to an elevated electron transfer from CuBi2O4 to BiO2 with a built-in electric field, facilitating efficient electron flow.
Besides heterojunction construction, doping offers another promising strategy to alter the photoelectron–hole pair recombination. To mitigate the issue facing bismuth-based photocatalysts, a novel Fe-doped BiO2−x/BiOI heterojunction photocatalyst was developed through a chemical precipitation method [54]. Due to a Z-scheme heterojunction construction and impure Fe introduction, increased redox abilities and electron–hole pair separation can be achieved, resulting in elevated PET degradation within 10 h under full-spectrum light. In another work, Nb-doped SnO2 quantum dots (QDs) were prepared by a hydrothermal process [64], aiming to generate a synergistic effect using dual-defect engineering. Nb impurities and the introduced oxygen vacancy extend the conduction band edge to the Fermi level, narrowing the bandgap and providing abundant defect states for electron transitions. Finally, a 28.9% PE MPs weight loss can be achieved after 7 h photo-irradiation. Using doped photocatalysts, elevated MP degradation can be acquired by coupling photos and electrical methods. TiO2-modified boron-doped diamond photoanode has been prepared for the purpose [65]. HDPE MP degradation was performed using electrochemical oxidation and photo-electrocatalysis (PEC) on bare and modified boron-doped diamond (BDD) electrodes, both under dark and UV light conditions. The results show that 89.91 ± 0.08% of HDPE MP degradation in 10 h can be achieved and is more efficient at a low current density (6.89 mA/cm).
Morphological modification and surface sensitization represent another effective design approach for promoting photocatalytic MP degradation efficiency. Spherical and nanosheet bismuth oxychloride (BiOCl) have been prepared, aiming to study the catalyst morphology effect on MP degradation [66]. The results showed that BOC-N nanosheets achieved a 44.33% degradation of PET MPs within 5 h, which was ascribed to the easy transfer of photogenerated carriers to the surface. Titania-coated 3D ZnO tetrapods were also prepared for photocatalytic degradation of PE and polyester (PES) MPs [67]. Complete PE and PES degradation is achieved under UV illumination for 480 h and 624 h, respectively. The authors found that MP morphology can affect the degradation rate, and the use of electron scavengers is necessary. MP surface modification can also play an important role. In the work of Jiang et al. [68], PS MPs were modified by different functional groups, including –OH, –NH2, -SO3H, –COOH, and epoxy. Photocatalytic degradation was conducted using a BiOBr-OH semiconductor–organic framework. The results showed that surface modification increased the polarity of the MPs’ surfaces, resulting in a sluggish degradation rate. Therefore, the plain PS MPs without any treatment showed the highest degradation rate. Via phase transformation and sulfur vacancy engineering, Edalati and colleagues introduce a highly efficient cadmium sulfide (CdS) photocatalyst that dramatically improves H2 production and PET MP degradation [69]. Using hydrothermal treatment and high-pressure processing, parent CdS can be converted into a thermodynamically stable wurtzite (hexagonal) phase with abundant sulfur vacancies generated at the same time. These sulfur vacancies serve as active catalytic sites that facilitate enhanced photocatalytic performance. A 23-time increase in both H2 production and PET degradation can be achieved compared to the commercial CdS.
A table summary of catalyst materials, reaction conditions, and the performance of photocatalytic MP degradation is shown in Table 4. It can be shown that semiconductors and their modified counterparts are mostly used as photocatalysts for MP degradation. Current photocatalytic MP degradation focuses on lab-scale demonstrations with single and controlled MP environments. Furthermore, in most of the work, MP degradation efficiency is calculated by using a weight loss measurement. However, based on the low MP concentration used, it is questionable how accurate the quantification method can be, raising concerns for the final performance evaluation. The majority of the work does not consider the formation of nanoplastics, resulting in an overestimated degradation efficiency and possibly secondary pollution. Finally, the use of a lab-controlled environment in photocatalytic testing is suitable for scientific research but lacks relevance to real-world applications. This is extremely important, considering that a practical solution to tackle the existing MPs’ adverse effects is urgently needed. For more information about the current status, challenges, and future direction for MP photocatalytic degradation, the following reviews [52,70,71] can be referred to.

Table 4.
Summary of catalyst, reaction conditions, and performance of photocatalytic MP degradation.
4.2. Fenton and Fenton-like Catalysts
Beyond photocatalysis, Fenton and Fenton-like reactions represent the second most widely applied approaches for the degradation of MPs and bulk plastics. Before getting into the details of Fenton and Fenton-like reactions. It is important to distinguish between Fenton and Fenton-like reactions and advanced oxidation processes (AOPs), as many studies tend to use these terms interchangeably. AOP is a broad category of techniques designed to oxidize and mineralize persistent organic pollutants. It includes processes where chemical oxidants like ozone, Fenton’s reagents, and electrochemical or photochemical methods are applied to produce ROS. The core principle of AOPs is the in situ generation of highly reactive, short-lived radical species, such as ·OH, sulfate radicals (SO4·−), or others, which are strong oxidizers and can chemically break down organic contaminants, while the Fenton reaction uses ferrous iron (Fe2+) or other multivalent metal ions to activate H2O2 and produce ·OH or other ROSs. A detailed reaction mechanism is listed below.
Mechanism: The Fenton reaction involves the reaction of Fe2+ with H2O2 to produce ·OH, which are strong oxidizers, and ferric iron (Fe3+). Fe3+ is then reduced back to Fe2+ by another molecule of H2O2, creating a chain reaction, as shown in Equations (1) and (2). The overall effect is the generation of ·OH, which are highly reactive and capable of oxidizing and degrading organic pollutants [74], while for Fenton-like reactions, different metal catalysts, ligands, or peroxides operating under modified conditions, such as different pH levels or the application of light or electricity, can be applied, leading to variations in the reaction mechanism and the production of various ROS. The Fenton/Fenton-like reaction belongs to one type of AOP. In Fenton/Fenton-like oxidation systems, multivalent metals, such as Fe, Cu, Co, Mn, Ce, Ag, Cr, Ru, W, Mo, V, Ti, etc., are commonly applied as catalysts that can activate H2O2 and generate ROS. The mechanism is through changes of low-valent metal (Mn+) with reduction properties and high-valent metal (M(n+z)+) with oxidation properties due to the reductant and oxidant properties of H2O2. Besides H2O2, organic peroxides (ROOH) or peroxydisulfate (S2O82−) and persulfate (HSO5−) can also be used. Fenton/Fenton-like reactions can be carried out in homogeneous and non-homogeneous systems, while in terms of the driving force used, photo-, electrically, ultrasonically, and piezoelectrically triggered Fenton reactions can be divided as shown in Figure 6. For detailed information on Fenton and Fenton-like reactions, including mechanisms, application systems, and materials, review papers [75,76,77] can provide ample information.
Fe2+ + H2O2 → Fe3+ + ·OH + OH
Fe3+ + H2O2 → Fe2+ + ·HO2 + H+
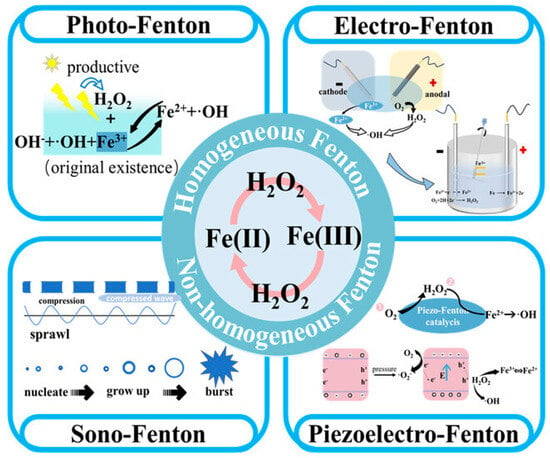
Figure 6.
Schematic illustration of MP degradation mechanisms for different types of Fenton and Fenton-like reactions [77].
Toward a green MP Fenton degradation, sustainable cuttlefish bone (CFB)-supported CoFe2O4 nanoparticles have been prepared with a persulfate Fenton-like strategy for the efficient degradation of PS-NPs [78]. In total, an 88.27% removal can be performed in 30 min due to fast persulfate activation through the Co3+/Co2+ redox cycle. Based on a Fenton-like reaction utilizing peroxymonosulfate (PMS) as an oxidant, CuMg co-doped carbonized wood sponge catalysts (CuMgCWS) with concentrated loading and dispersed distribution of Cu/Mg nanoparticles are synthesized [79]. Furthermore, 80 wt% selectivity for MPs to hydrocarbons and ketones can be realized due to Mg-O sites in Cu/C sponges, which controlled the interfacial charge distribution and promoted adsorption and activation of PMS through Cu-centered sites. Cobalt/carbon quantum dot (Co/CQD) core–shell nanoparticles with regulated positions and close intimacy are also designed for Fenton-like polypropylene (PP) degradation [80]. Through CQD, a strong reduction effect accelerated the Co2+/Co3+ cycle, and a significantly higher degradation activity was realized.
Limited oxidation efficiency constrains the wide application of Fenton and Fenton-like oxidation for MP degradation. To increase the performance of the process, a combined Fenton reaction system is usually constructed. For instance, heterogeneous electro-Fenton (EF) PS MP degradation processes have been demonstrated through constructing a copper–cobalt–carbon aerogel (CuCo-CA) bifunctional cathode [81]. The CuCo-CA possesses H2O2 electro-synthesis and in situ activation of ·OH generation capability (without the need for external aeration) for oxidative decomposition of PS-NPs. In total, 94.8% of PS NP removal efficiency can be achieved in 6 h. Similarly, a piezo-Fenton process is also developed using ferroelectric Bi12(Bi0.5Fe0.5)O19.5 catalysts [82]. Bi12(Bi0.5Fe0.5)O19.5 with exceptional piezoelectric properties can ensure efficient polarization under ultrasonic fields to generate a substantial amount of ROSs, resulting in the degradation of PET-MPs via piezocatalysis/Fenton-like mechanisms. Additionally, a 28.9% removal rate of PET MPs can be achieved in 72 h. Besides coupling the Fenton reaction with electro and ultrasonic fields, a visible-light-driven photocatalysis and photothermal Fenton-like reaction has been formulated utilizing an α-Fe2O3 nanoflower on hierarchical TiO2 (opal-like layer/nanotube arrays, denoted as α-Fe2O3/TiO2HNTAs film) [83]. Under light initiation and photothermal effects, without external H2O2 input, a nearly 100% degradation of 310 nm PS spheres can be obtained after 4 h at 75 °C. It is found that PS can melt at a temperature much lower than its melting point in the process, and TiO2HNTAs promote PS degradation efficiency on α-Fe2O3 (active sites) through electron population. Interestingly, without using a catalyst, Hu et al. [84] demonstrate that a hydrothermally coupled Fenton reactions can achieve up to 95.9% mass loss and 75.6% mineralization of PE MPs within hours due to synergistic thermal hydrolysis and hydroxyl radical oxidation. A summary of catalysts, reaction conditions, and the performance of Fenton and Fenton-like MP degradation is shown in Table 5.

Table 5.
Summary of catalysts, reaction conditions, and the performance of Fenton and Fenton-like MP degradation.
Developing Fenton-like reactions coupled with other activation methods shows great promise for enhanced MP degradation due to the technology’s maturity, ease of scaling up, and low cost. However, there are many more to be studied, especially in terms of the mechanism of understanding in the complicated system. As demonstrated in the recent work [86], Fenton, photo-Fenton, and Fenton-like processes show polymer-specific efficacy—some microplastics respond to light-assisted oxidation while others remain refractory. Consequently, a one-size-fits-all Fenton treatment is unlikely; but a tailored or hybrid strategy matched to polymer type is preferable. Furthermore, MP degradation using Fenton and Fenton-like reactions is usually carried out in an aqueous system on a laboratory scale. Its wide application and implementation are limited due to the long reaction time, low liquid-phase degradation efficiency, and the formation of secondary nanoplastics [87]. Therefore, future catalyst developments should focus on real condition-related environments and more effective and faster reaction pathways.
4.3. Thermal Catalytic Process
Thermal catalytic treatment of MPs offers a super-efficient way of transforming MPs into nonhazardous products (CO2 and H2O), value-added chemicals, and fuels through oxidation, pyrolysis, and reforming mechanisms. With the use of a catalyst, the process efficiency can be improved, especially for lowering the reaction temperature and elevating the product selectivity. With careful design of the catalysts, using composite, constructing bifunctionality, controlling structure and surface properties, enhanced MP transformation and degradation can be realized.
Mechanism: Thermal catalytic microplastic treatment couples heat with a solid or homogeneous catalyst to depolymerize MPs into smaller intermediates or convert them into value-added products (e.g., fuels or carbon materials). In this approach, thermal energy drives bond cleavage, while the catalyst lowers activation barriers and improves selectivity, enabling effective conversion at lower temperatures than thermal cracking alone. Reported catalysts include biochar, metal-modified biochar, and Fenton-like systems, applied in both liquid- and gas-phase processes to accelerate degradation and steer product distributions. To be noted, the thermal degradation mechanism of MPs is highly dependent on the catalyst types. For a detailed reaction mechanism illustration, the following work [88,89] can be referred to.
An intensified PS MP adsorption and pyrolysis degradation process is formulated by modifying magnetic biochar (MBC) with Mg/Zn [90]. Through the magnetic catalyst design, the MPs and adsorbents can be easily separated using a simple magnet. What is more, MP degradation and adsorbent regeneration can be accomplished by thermal treatment simultaneously. The authors found that Mg/Zn modification improves MP adsorption through increased electrostatic interaction and chemical bonding. A bimetallic Ni-Pd/TiO2 is formulated by adding Pd into Ni [91] to increase catalyst redox properties and basic sites for MP transformation into useful H2 and liquid hydrocarbons. The process is based on Ni-Pd/TiO2 as a cracking and steam reforming catalyst. At 700 °C, a H2 yield of 93% and a phenol conversion of 77% can be achieved. The superior H2 selectivity is due to the increased catalyst redox properties and basic sites introduced by Pd.
Catalytic thermal treatment is an effective way of upcycling MPs by converting them into novel carbon-based catalysts that can be used for other catalytic processes. For instance, Shengjia Ma et al. [92] turn PS MPs into a Fe/Mo-doped carbon sponge (Fe/Mo-N-C) through a one-step pyrolysis process. The prepared catalyst shows effective oxidation of 17α-ethinylestradiol (EE2). Similar work [93] has also been performed for PET MPs through controlled carbonization, resulting in a zero-valent iron-loaded porous carbon catalyst that can degrade tetracycline with high efficiency. To increase the thermal catalytic oxidation process efficiency and lower the reaction temperature, a Hopcalite (CuMnOx) catalyst is used and combined with dielectric barrier discharge (DBD) plasma [19], forming a two-stage reaction system for PS MPs’ complete degradation. In the first step, the DBD plasma generates ROS for PS-MP degradation, while in the second stage, the Hopcalite catalyst selectively converts the intermediates to CO2 with a high efficiency of 98.4% in 60 min. By using NiCl2 with HDPE, different structured carbons (core–shell carbon composites, nanosheets, and their hybrids) that show promising catalytic effects for peroxymonosulfate activation can be made for phenol degradation in water [49]. The morphology and proportions of defective carbon structures can be easily controlled by tuning the NiCl2-to-HDPE ratio. In the process, NiCl2 serves as both a catalyst and template for HDPE conversion. The working mechanism is that HDPE is firstly pyrolyzed into hydrogen and light hydrocarbons, which can reduce Ni2+ to zero-valent Ni0, which is the active site for light hydrocarbons conversion to carbon-rich compounds.
A summary of catalysts, reaction conditions, and the performance of thermocatalytic MP degradation is shown in Table 6. Despite the high reaction rate and efficiency of treating MPs using thermochemical methods (catalytic pyrolysis, hydrocracking, hydrogenolysis, oxidative degradation, and tandem upcycling), catalyst deactivation due to coking, active site leaching, and sintering is still a big issue that needs to be resolved [94]. Furthermore, there is also a product selectivity control challenge, as varied hydrocarbon chain lengths with O and halogen atoms tend to be formed, impairing the value of the products. Therefore, future thermal catalyst development should focus on tackling stability, product selectivity, and catalyst scaling issues.

Table 6.
Summary of catalysts, reaction conditions, and the performance of thermal catalytic MP degradation.
4.4. Bio and Bio-Inspired Catalysts
Biodegradation of MPs is considered the most environmentally friendly and cost-effective degradation technology to handle environmentally related MPs due to the widespread occurrence of microorganisms and their ecosystem compatibility [99]. MP biodegradation is primarily driven by microorganisms like bacteria, fungi, and algae that secrete enzymes to break down plastic polymers into simpler compounds. The process involves biofragmentation, where enzymes cleave polymer chains, followed by assimilation and mineralization, where microorganisms further break down these fragments, eventually incorporating them into their cells or converting them into harmless byproducts like CO2.
Mechanism: Four-stage biodegradation paradigm is usually applied for MPs—conditioning/colonization → fragmentation (oxidative/abiotic assist) → enzymatic depolymerization to oligomers/monomers → assimilation and mineralization (CO2/H2O, or CH4 anaerobically)—using key enzymes (e.g., PETase/MHETase, cutinases, laccases, peroxidases) and microbial taxa. MP degradability could be affected by polymer chemistry, crystallinity, hydrophobicity, particle size, additives, aging, etc. [100]. More information in terms of the general methods and mechanisms for microplastic degradation can be learned from the review works [101,102].
Immobilization of Candida rugosa-CrL enzyme in metal–organic frameworks (CrL-MOFs) is formulated for Bis-(hydroxyethyl) terephthalate (BHET) degradation [103] with increased stability and recyclability of enzyme-type catalysts. Due to the MOF’s adsorption capability to concentrate MP-related products, a 37% removal efficiency in 24 h can be achieved through the enzymatic degradation. Using a pre-concentration approach, hydrophilic bare Fe3O4 nanoaggregates are used to absorb major MPs via hydrogen bonding [104]. Besides adsorption, the hydrophilic Fe3O4 surfaces also show a peroxidase-like nanozyme activity to degrade organic pollutants. In total, 100 % of PP, PE, PVC, PS, and PET can be degraded through an autoclave-based heating approach. Modification of bacteria has also been conducted to improve MPs’ degradation performance. A Shewanella putrefaciens 200 Fe-reducing bacteria is prepared, taking advantage of the microbially driven Fenton reaction for PS-MP degradation [105]. ·OH produced by the microbially driven Fenton reaction shows a PS-MP degradation of 6.1 ± 0.6% weight loss in 14 days. The authors found that ·OH-induced oxidative stress could damage microorganisms, affecting continuous ·OH production. Therefore, timely replenishment of organic carbon sources is the key to sustaining the reproduction of microorganisms. In another work, an engineered bacterium made through the rational design of biological manganese oxides (BMO mediated by SDE-PsLAC) was used for PE MP degradation [106]. SDE-BMO shows significantly enhanced PE MP catalytic degradation due to weakened MPE inertia. The improvement of microbial MP degradation efficiency through metal ions is also observed by Xionge Li et al. [107]. During the study, they found that PE MP biodegradation by marine sediment bacteria tends to be enhanced under iron-enhanced conditions. The enhancement is due to the generation of multiple oxygen-containing functional groups resulting from the addition of Fe3+. Besides the bacterial modification approach, Mn-doped iron phosphate (LFMP) bio-inspired nanozymes have also been synthesized for PE and PP degradation [108]. Through Mn2+ doping, an expanded lithium iron phosphate (LFP) lattice structure with a narrowed bandgap can be realized, enhancing Li+ migration rates, which increases the peroxidase-like MP degradation activity.
The bio-Fenton approach is an emerging method for microplastic degradation, which combines Fenton oxidation and microorganisms. By combining a MnO2/g-C3N4/fly ash (MCNF) micromotor-induced Fenton reaction and B. subtilis microbial degradation, 60% PS degradation within 24 days and 66% PE degradation within 50 days are realized [109]. A clear elevated degradation process can be observed, as shown in Figure 7. The bio-Fenton method shows much better performance compared to the sole use of the Fenton reaction or microbial degradation. The authors claimed that the improved degradation performance is ascribed to the self-propelled motion of MCNF micromotors, which improves the mass transfer efficiency of the Fenton reaction.
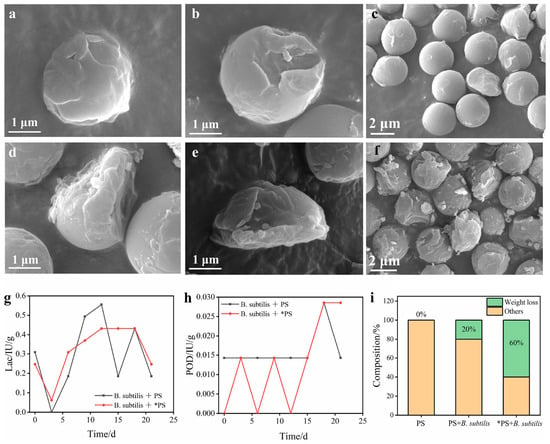
Figure 7.
(a–c) SEM images of PS degradation using B. subtilis soil for 24 days. (d–f) SEM images of PS degradation through Fenton reaction pretreatment and B. subtilis for 24 days. Enzyme activity of (g) laccase and (h) peroxidase concentrations using different catalyst combinations and (i) weight loss of PS using different catalyst combinations (*PS indicates Fenton reaction-pretreated PS) [109].
A summary of catalysts, reaction conditions, and the performance of biological catalytic MP degradation is shown in Table 7. Biodegradation offers significant advantages in terms of sustainability and circular economies under mild conditions; however, it faces challenges such as slow reaction rate, susceptibility to environmental changes, and complicated reaction mechanisms. Research is ongoing to enhance these processes through techniques like protein engineering and strain breeding, aiming to improve the efficiency and environmental safety of MP biodegradation. MP biodegradation is a slow process that can take days or months to proceed [110], lacking the capability to effectively remove the much-accumulated environmental MPs. Therefore, formulating eco-friendly solutions to accelerate MP degradation is the key.

Table 7.
Summary of catalysts, reaction conditions, and the performance of biological catalytic MP degradation.
4.5. Electrocatalysts
Electrochemical catalyst systems are increasingly important for MP treatment because they generate oxidants and reductants in situ, eliminating bulk chemical dosing and minimizing secondary pollution. Their activity is readily tuned by potential, electrode composition, and electrolytes, enabling modular reactors that scale from lab cells to flow systems and that operate in diverse matrices (including salted and fresh waters). Beyond simply degrading stubborn polymers (PE, PET, PP, PS) and PVC, advanced paired electrochemical systems can upcycle plastic-derived intermediates into value-added products (e.g., formate, carboxylic acid, etc.) while simultaneously producing H2, offering an efficient strategy to integrate renewable electricity and realize a green pathway.
Mechanism: The electrochemical degradation of MPs can be divided into indirect oxidation and direct oxidation. For indirect oxidation, ROS is generated in the bulk solution or at the electrode surface. Then, these highly unstable and non-selective radicals attack and break the strong bonds within the MPs, while for direct oxidation, MPs can directly interact with the anode surface, leading to a direct electron transfer reaction. Regardless of the initiation pathway (indirect or direct oxidation), the degradation of MPs follows a general process of polymer attack and fragmentation.
Mao et al. developed a sustainable electrocatalytic upcycling method to convert PET microplastics into value-added products (H2, TPA, and formate) through a two-stage process using Mn0.1Ni0.9Co2O4-δ rod-shaped fiber spinel catalyst [112]. In the first stage, PET MPs (particle size <500 μm) are hydrolyzed with potassium hydroxide (KOH) to release terephthalic acid (TPA) and ethylene glycol (EG). In the second stage, the extracted EG is used as the anolyte and undergoes anodic oxidation on the Mn0.1Ni0.9Co2O4-δ rod-shaped fiber spinel catalyst, achieving formate production with over 95% Faradaic efficiency at 1.42 V vs. RHE, while the cathode simultaneously facilitates hydrogen evolution, thereby producing both clean energy and valuable chemicals from plastic waste. The superior performance is due to Mn doping, which changes the electronic structure, reducing the lattice oxygen oxidation in the NiCo2O4 spinel oxide as an OER electrocatalyst. Doping Mn, Co, or Ru as an intermediate layer between the titanium (Ti) substrate and La-Sb-SnO2 active layer has also been conducted to improve the lifespan of the electrode for PS MP electrochemical oxidation [113]. With the Co dopant as the interlayer, PS degradation performance (28% mass loss in 3h) is 4.54 × vs. no interlayer, 2.38 × vs. Ru, and 1.19 × vs. Mn dopant counterparts. What is more, a 5-time-prolonged lifespan can be achieved using Ti/La/Co/-Sb-SnO2 anode. Similar work has been conducted by the same group using La as a dopant for the Ti/Sb-SnO2 electrode [114]. The results show that La promoted the formation of ·OH radicals and increased the PS MP degradation efficiency by 77% compared to Ti/Sb-SnO2 only. Metal oxide has also been used as a dopant to improve the performance of electrochemical oxidation. CeO2-doped PbO2 anode has been prepared for PVC MP degradation [115]. Compared with undoped PbO2, the CeO2–PbO2 electrode shows a mass loss in PVC MPs and clear surface damage (cracks, reduced particle size) during treatment. The system achieves about 39% PVC weight loss in 6 h, attributed to persulfate/·OH generated from the sulfate electrolyte and high anode potential. Through CeO2 doping, β-PbO2 grains are refined, resulting in improved grain arrangement and an increased amount of active sites.
Ma et al. designed a novel Ni3N/W5N4 Janus nanostructure with a seamless heterointerface, synthesized via a transition metal nitride-induced growth method [116]. The seamless interface can enable synergistic charge redistribution, lower the reaction energy barrier, and facilitate efficient electron transfer. Within this heterointerface, Ni3N supplies abundant active sites for the hydrogen evolution reaction (HER), while W5N4 enhances electrical conductivity and provides strong stability under harsh seawater conditions. Moreover, the junction between Ni and W nitrides effectively modulates the d-band center, thereby optimizing the adsorption and desorption of reaction intermediates and contributing to the overall catalytic efficiency. Furthermore, the Janus architecture exhibits superb hydrophilicity and an interface synergy that grants Pt-like performance to the HER, with remarkable stability (~300 h) even under industrial current densities. Simultaneously, this catalyst drives the electroreforming of MPs in seawater, enabling the production of formic acid with ~85% Faradaic efficiency at an ultralow overpotential of 1.33 V.
Defect engineering, including heteroatom doping, vacancies, edge/planar defects, and nanoarchitecture control, has been extensively used to tune 2D graphitic carbon nitride (g-C3N4) for enhanced light absorption, charge separation, redox kinetics, and surface adsorption of MP waste remediation [117]. Through the approach, Yuan Wang and colleagues [118] report the development of a cost-effective, self-supporting electrodes composed of vacancy-rich NiFe-layered double-hydroxide (NiFe_V-LDH) nanoarrays grown in situ on carbon paper designed for electro-Fenton degradation of PVC microplastics. This electrode exhibits high selectivity (~76%) for generating H2O2. Density functional theory (DFT) calculations demonstrate that oxygen vacancies significantly lower the energy barrier for H2O2 desorption, enhancing catalytic performance. The optimized system leverages both direct cathodic reduction of PVC and ·OH-mediated oxidation driven by the electro-Fenton process. A summary of the catalysts, reaction conditions, and the performance of electro-catalytic MP degradation is shown in Table 8. Despite showing advantages for value-added chemical production for MP degradation, electrochemical approaches still suffer from many challenges, including incomplete MP degradation, high energy consumption, low energy efficiency, and a reaction system associated with high costs.

Table 8.
Summary of catalysts, reaction conditions, and the performance of electro-catalytic MP degradation.
4.6. Hybrid Catalysts Coupling Different Reaction Pathways
Hybrid catalyst systems have emerged as a promising strategy for the elimination of microplastics, as they integrate two or more catalytic mechanisms to enhance degradation efficiency. For example, the combination of photocatalysts and enzymatic catalysts can accelerate the breakdown of polymers while simultaneously improving conversion rates into environmentally benign byproducts. Compared with conventional single-catalyst approaches, hybrid systems offer distinct advantages, including higher degradation efficiency, faster reaction kinetics, and greater overall effectiveness, thereby positioning them as a sustainable and advanced solution for addressing MP pollution [119].
An MP-to-CH4 process with nearly 100% CH4 selectivity is demonstrated in the work of Ye et al. [120]. M. b-CDPCN biohybrid catalysts are constructed by assembling Methanosarcina barkeri (M. b) and carbon dot-functionalized polymeric carbon nitrides (CDPCN). A maximum CH4 yield of 7.24 ± 0.40 mmol g−1 can be achieved with the M. b-CDPCN biohybrids, which is 34.5-fold and 16.8-fold higher than M. b-PCN and M. b-CDPCN with cysteine, respectively. The methanogen–semiconductor biohybrids in the work use MPs as an electron and energy source for CH4 generation. Both the photooxidation methanogenesis (i.e., pyruvate/acetate-to-CH4 conversion by reactive species) and photoreduction methanogenesis (i.e., CO2-to-CH4 conversion by photoexcited electrons) work together to contribute to the high CH4 yield and selectivity.
Through the integration of carbocatalytic oxidation and hydrothermal (HT) hydrolysis with sulfate radicals, a magnetic N-doped nanocarbon spring is formulated [121] to degrade MPs. Results show that the magnetic nanohybrids can effectively activate peroxymonosulfate (PMS) to generate highly oxidizing radicals for MP degradation under hydrothermal conditions. In total, 40 wt% of MP removal can be achieved in 8 h through the hydrothermal-assisted catalytic PMS activation approach (using the N-doped nanocarbon springs) compared to 5% and 17% MP degradation without a catalyst and PMS-only conditions. What is more, through toxicity tests, the organic intermediates generated from the formulated MP degradation route have been proven to be environmentally benign and can be used as a carbon source for algae growth, indicating a green way for water environment MP handling. A hydrothermal-assisted Fenton-like reaction is also applied to convert ultrahigh-molecular-weight polyethylene (UHMWPE) MPs into carboxylic acids and H2 [22]. To achieve the goal, hierarchical porous carbon nitride-supported single-atom iron (FeSA-hCN) catalysts have been prepared by formulating a tandem MP degradation–hydrogen evolution reaction. Near-total degradation of ultrahigh-molecular-weight polyethylene into C3–C20 organics, with 64% selectivity toward carboxylic acids under neutral pH and hydrogen production at ~42 µmol/h under illumination, can be achieved. By coupling Fenton-like oxidative degradation of MPs into electron-donating intermediates with photocatalytic H2 evolution, both pollutant breakdown and clean energy production in a single FeSA-hCN catalyst can be realized.
In Miao et al.’s work [122], an innovative electro-Fenton-like (EF-like) technology is designed to degrade PVC MPs in an aqueous environment by employing a TiO2/graphite (TiO2/C) cathode. The system can simultaneously promote cathodic reductive dechlorination and ·OH oxidation, enabling effective degradation. Using the idea of process intensification and fabricating heterogeneous materials to modulate the interfacial electronic structure, ternary heterogeneous interpenetrating catalysts, Fe1−xS/FeMoO4/MoS2 (Mo5Fe5), are also prepared to enhance MP degradation through a piezo-photocatalytic approach [123]. Under sonication and simulated light conditions, 58.46% of PS-MPs can be degraded in 30 h. The superior performance is due to the ternary heterogeneous structure, where piezoelectric response and illumination both favor the generation of H2O2, leading to the rapid activation of ROS for MP degradation.
An innovative photo-electrocatalytic–biocatalytic hybrid system that transforms real-world PET MPs into value-added chemicals using solar energy has also been formulated [124]. By improving charge transport, suppressing electron–hole recombination, and lowering reaction barriers through Zr doping, the process begins with a Zr-doped hematite (α-Fe2O3) photoanode, which extracts electrons from hydrolyzed PET solutions derived from post-consumer plastic waste. These electrons are transferred to a carbon-based cathode, where they activate various redox enzymes to catalyze organic transformations such as C–H oxyfunctionalization, C=O amination, and asymmetric C=C hydrogenation.
Feng and co-workers [125] designed a CoNi alloy catalyst anchored on carbon nanocages and interconnected by CNTs, then surface fluorinated using a solvent-free CF4 plasma treatment. The F-functionalized heterostructure shows high activity and durability for seawater electrolysis, enabling a paired system where the anode electro-oxidizes PET MPs into value-added products (e.g., formate), while the cathode produces H2 with Pt-like HER performance. The hollow 1D/3D architecture, Co–Ni synergy, and reconstructable metal–F bonds in alkaline media enhance charge transfer and reaction kinetics. What is more, the proposed coupling strategy can have a potential saving of 165 mV at a current density of 10 mA cm−2. To better illustrate, Figure 8 showcases some of the hybrid catalyst design strategies.
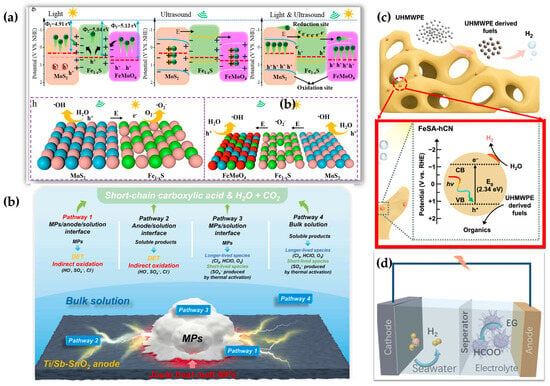
Figure 8.
Hybrid catalyst design strategy illustration coupling different reaction mechanisms: (a) piezo-photocatalytic enhanced microplastic degradation [123]; (b) joule heat-assisting electrochemical degradation [110]; (c) tandem MP degradation and H2 production [22]; (d) H2 evolution coupled with microplastic upcycling [125].
Besides the aforementioned methods, a novel joule heat-assisted electrochemical strategy is also developed by using a hybrid membrane consisting of a Sb-SnO2-coated titanium mesh (Ti/Sb-SnO2) and a carbon felt (as an assistant) as an anode [110]. In the specially designed reactor, PE MPs can be captured by the anode, being softened or even melted by the interface Joule heat, which enables a direct electron transfer from anode to MPs and makes the most use of short-lived active species. Above 99 % PE MP removal efficiency can be achieved within 6 h without nanoplastic generation and chlorinated compounds. A summary of the novel catalysts developed for the hybrid approach to MP degradation is shown in Table 9. Hybrid MP degradation approaches show great promise in terms of realizing a fast reaction rate and efficient material utilization. However, there is still more to be conducted, especially for accomplishing a robust and complicated system design. In this regard, a stable and active catalyst is the key.

Table 9.
Summary of catalysts, reaction conditions, and performance of hybrid catalytic MP degradation.
For a more detailed literature summary for catalysts used for microplastics degradation, the following review papers [102,119,126,127] can be referred to, within which [126] focuses on chemical catalytic processes. Work from Hettiarachchi et al. [127] stresses biocatalytic approaches. While Miranda et al. [119] review the catalytic removal of MPs in water bodies. while more detailed information in terms of the general methods and mechanisms for MP degradation can be found in the review work [102].
In summary, depending on the targeted products, MP degradation can proceed either through complete mineralization into harmless end products (CO2 and H2O) or through upcycling into value-added chemicals and fuels (H2, formate, methanol, hydrocarbons, etc.). Compared to mineralization to degrade the MPs, upcycling offers greater advantages by enabling resource recovery, promoting greener production pathways, and contributing to the development of a circular economy. Nevertheless, it also encompasses significant technical and logistical challenges across collection, processing, and end-use applications. For instance, the initial obstacle involves efficient collection and removal, as MPs are dispersed across aquatic, terrestrial, and atmospheric environments, with diverse polymer compositions, particle sizes, and the inclusion of other organic components that make it extremely challenging to achieve a high separation efficiency [128]. Conventional filtration and sedimentation techniques often struggle with high energy demand and limited scalability, while advanced approaches such as membrane technologies, magnetic separation, or coagulation-flocculation require further optimization for large-scale deployment. Following recovery, heterogeneity in polymer type, surface oxidation, and the presence of additives or adsorbed pollutants complicate standardized upcycling pathways. Thermal, catalytic, and biological conversion strategies offer potential for transforming MPs into fuels, carbonaceous materials, or chemical precursors; however, these methods face barriers related to energy intensity, catalyst deactivation, and process integration. Besides the advanced catalyst development, logistically, the establishment of reliable supply chains for MP feedstocks remains underdeveloped, as most recovery efforts are fragmented and localized. To realize commercial-scale upcycling, consistent feedstock quality that can align with regulatory frameworks is a must. Therefore, addressing these challenges requires coordinated strategies that link recovery technologies with scalable conversion processes and harmonized policy frameworks to facilitate sustainable MP valorization.
5. Challenges and Future Opportunities for Catalytic Microplastic Degradation and Upcycling
To mitigate MP pollution in the current ecosystem, strategies can be developed from two ends: cut the input or increase the output. Cutting the input can include end-of-pipe (EOP) treatment and cleaner production (CP). The EOP treatment, which is also called “pollute first, treat later”, refers to technologies and practices used to remove pollutants from waste streams after they have been generated, typically before discharge into the environment [129], while CP requires the development of degradable and environmentally friendly plastics alternatives, limiting the generation of pollution at the source. Considering the early stage of degradable plastics development and the maturity of fossil fuel-based plastics, complete blocking and controlling plastic pollution from the source will not be seen soon. Therefore, it is vital to develop clean and green technologies to collect and degrade MPs existing in the environment.
As summarized in this work, MP degradation could be achieved through photocatalytic, advanced oxidation, electrochemical, thermal, biological, and hybrid processes, as shown in Figure 9. Photocatalytic and biological degradation routes provide a green and sustainable way to mitigate MP pollution. However, they suffer from low process efficiency, low carbon recyclability, long degradation time, and are susceptible to environmental conditions. AOPs provide an effective and fast elimination way of breaking down MP molecular chains into benign substances through strong, non-selective free radicals. However, the process has limited effectiveness and low efficiency at the laboratory scale. Furthermore, nanoplastics or other toxic byproducts tend to be generated. In terms of efficiency, the thermal catalytic route is promising with a high reaction rate and products with added value. In exchange, it is also an energy-intensive process. Based on current research, hybrid approaches integrating the advantages of different catalytic pathways seem to be a promising way out for improved energy and material utilization efficiency.
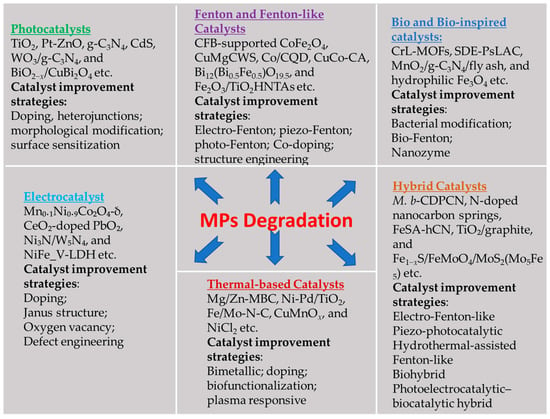
Figure 9.
Summary of different types of catalysts and the catalyst activity enhancement methods used for MP degradation.
The main challenges of the current MP degradation process include many aspects from MP collection to quantification, identification, processing, and degradation products. For instance, it is easy to tackle industrially produced MPs with high abundance and purity, while it is extremely challenging to handle and process environmentally relevant microplastics, which refer to the plastic particles found in nature, including in the human diet. These differ substantially from the virgin plastic particles from the shelves that are used in many laboratory experiments [130]. Not only is it challenging to remove and collect those MPs, but it is also challenging for catalytic process development and performance evaluation. In addition, unsatisfying degradation performance, including reaction rate, product selectivity, catalyst activity, and recyclability, represent another big challenge. Furthermore, there is a lack of standard characterization metrics (mass loss, CO2 evolution, TOC, FTIR/Raman, GPC/SEC, SEM/AFM) and caution about inconsistent protocols that complicate cross-study comparisons, resulting in scattered and chaotic research results. For instance, in most of the studies reviewed on MP degradation and upcycling, conversion efficiency is assessed using a mass-based metric, typically by filtering residual MPs through submicron-sized filters. However, this method overlooks the presence of nanoplastics that may not be fully degraded, potentially leading to overestimated degradation performance and the unintended release of more harmful nanoplastic particles. Currently, there is a significant gap in understanding the toxicological effects and physicochemical properties of nanoplastics, particularly in relation to their size. Therefore, we propose the establishment of a lower size limit for MPs to enable fair comparisons across studies and promote a unified framework for evaluating degradation performance. This lower boundary should be selected with consideration of its environmental and health impact, which warrants further research, especially on the size-dependent toxicity of MPs. Finally, secondary pollution caused by MP degradation products can be a huge issue. Despite gaining increased attention, most of the studies available lack systematic and long-term studies for the effects of products from MP degradation on human and living organisms in the ecosystem, leaving some claimed green and sustainable processes questionable.
Therefore, future directions are multifold for the catalytic MP degradation and upcycling: (1) catalyst development toward improved reaction rate, product selectivity, and energy and material utilization efficiency based on green and sustainable materials; (2) formulated intensified catalytic processes coupling advantages from thermal, electrical, biological, and photocatalytic approaches; (3) building modeling tools to predict and identify possible methods and materials for MPs treatment; (4) collecting experimental and inventory data, such as MP biodistribution, toxicity studies, and life-cycle assessments [131]; (5) making modeling and mathematical simulations to guide MP treatment [2]; (6) and finally, developing catalytic strategies through scaling up for practical, real-world applications, addressing issues like cost, energy consumption, and long-term performance under various environmental conditions. In a nutshell, catalytic degradation and upcycling of microplastics are still in their infancy, with no established standards to guide the process. Strong regulations and economic incentives are needed to drive research and development in this field.
Author Contributions
Paper structure formulation, topic pick, literature search, data analysis and summary, and draft manuscript preparation, C.Z.; literature collection, analysis, and draft manuscript preparation, G.Z.; manuscript editing, review, and finalization, P.-X.G. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to express our thanks for the financial support from the National Oceanic and Atmospheric Administration (NOAA) through the University of Alabama (Award No.: NA24OARX417C0421-T1-01) and the support from the National Institute of Standards and Technology (NIST) through 3D Array Technology, LLC (3DAT, co-founded by P.-X.G.) with Award No.: 70NANB22H118.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Mohamed Nor, N.H.; Kooi, M.; Diepens, N.J.; Koelmans, A.A. Lifetime Accumulation of Microplastic in Children and Adults. Environ. Sci. Technol. 2021, 55, 5084–5096. [Google Scholar] [CrossRef]
- Thompson, R.C.; Courtene-Jones, W.; Boucher, J.; Pahl, S.; Raubenheimer, K.; Koelmans, A.A. Twenty Years of Microplastic Pollution Research—What Have We Learned? Science 2024, 386, eadl2746. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, U.; Sun, J.; Zhu, C.; Wu, M.; Zhao, B.; Gao, P. Plastic Recycling: A Review on Life Cycle, Methods, Misconceptions, and Techno—Economic Analysis. Adv. Sustain. Syst. 2023, 7, 2200471. [Google Scholar] [CrossRef]
- Sutkar, P.R.; Gadewar, R.D.; Dhulap, V.P. Recent Trends in Degradation of Microplastics in the Environment: A State-of-the-Art Review. J. Hazard. Mater. Adv. 2023, 11, 100343. [Google Scholar] [CrossRef]
- Puteri, M.N.; Gew, L.T.; Ong, H.C.; Ming, L.C. Technologies to Eliminate Microplastic from Water: Current Approaches and Future Prospects. Environ. Int. 2025, 199, 109397. [Google Scholar] [CrossRef]
- Welden, N.A.; Lusher, A. Microplastics. In Plastic Waste and Recycling; Elsevier: Amsterdam, The Netherlands, 2020; pp. 223–249. ISBN 9780128178805. [Google Scholar]
- Bermúdez, J.R.; Swarzenski, P.W. A Microplastic Size Classification Scheme Aligned with Universal Plankton Survey Methods. MethodsX 2021, 8, 101516. [Google Scholar] [CrossRef]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a Consensus on the Definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Efimova, I.; Bagaeva, M.; Bagaev, A.; Kileso, A.; Chubarenko, I.P. Secondary Microplastics Generation in the Sea Swash Zone With Coarse Bottom Sediments: Laboratory Experiments. Front. Mar. Sci. 2018, 5, 313. [Google Scholar] [CrossRef]
- Gopalakrishnan, K.K.; Sivakumar, R.; Kashian, D. The Microplastics Cycle: An In-Depth Look at a Complex Topic. Appl. Sci. 2023, 13, 10999. [Google Scholar] [CrossRef]
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A Global Perspective on Microplastics. J. Geophys. Res. Ocean. 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Mattsson, K.; Johnson, E.V.; Malmendal, A.; Linse, S.; Hansson, L.-A.; Cedervall, T. Brain Damage and Behavioural Disorders in Fish Induced by Plastic Nanoparticles Delivered through the Food Chain. Sci. Rep. 2017, 7, 11452. [Google Scholar] [CrossRef]
- Tiwari, M.; Rathod, T.D.; Ajmal, P.Y.; Bhangare, R.C.; Sahu, S.K. Distribution and Characterization of Microplastics in Beach Sand from Three Different Indian Coastal Environments. Mar. Pollut. Bull. 2019, 140, 262–273. [Google Scholar] [CrossRef]
- Nobre, C.R.; Santana, M.F.M.; Maluf, A.; Cortez, F.S.; Cesar, A.; Pereira, C.D.S.; Turra, A. Assessment of Microplastic Toxicity to Embryonic Development of the Sea Urchin Lytechinus Variegatus (Echinodermata: Echinoidea). Mar. Pollut. Bull. 2015, 92, 99–104. [Google Scholar] [CrossRef]
- Ali, S.S.; Alsharbaty, M.H.M.; Al-Tohamy, R.; Khalil, M.A.; Schagerl, M.; Al-Zahrani, M.; Sun, J. Microplastics as an Emerging Potential Threat: Toxicity, Life Cycle Assessment, and Management. Toxics 2024, 12, 909. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Sima, J.; Song, J.; Du, X.; Lou, F.; Zhu, Y.; Lei, J.; Huang, Q. Complete Degradation of Polystyrene Microplastics through Non-Thermal Plasma-Assisted Catalytic Oxidation. J. Hazard. Mater. 2024, 480, 136313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Hou, Y.; Wang, Y.; Wang, Q.; Shan, X.; Liu, J. Highly Efficient Low-Temperature Biodegradation of Polyethylene Microplastics by Using Cold-Active Laccase Cell-Surface Display System. Bioresour. Technol. 2023, 382, 129164. [Google Scholar] [CrossRef]
- Uheida, A.; Mejía, H.G.; Abdel-Rehim, M.; Hamd, W.; Dutta, J. Visible Light Photocatalytic Degradation of Polypropylene Microplastics in a Continuous Water Flow System. J. Hazard. Mater. 2021, 406, 124299. [Google Scholar] [CrossRef]
- Lin, J.; Hu, K.; Wang, Y.; Tian, W.; Hall, T.; Duan, X.; Sun, H.; Zhang, H.; Cortés, E.; Wang, S. Tandem Microplastic Degradation and Hydrogen Production by Hierarchical Carbon Nitride-Supported Single-Atom Iron Catalysts. Nat. Commun. 2024, 15, 8769. [Google Scholar] [CrossRef]
- Martín, A.J.; Mondelli, C.; Jaydev, S.D.; Pérez-Ramírez, J. Catalytic Processing of Plastic Waste on the Rise. Chem 2021, 7, 1487–1533. [Google Scholar] [CrossRef]
- Li, H.; Aguirre-Villegas, H.A.; Allen, R.D.; Bai, X.; Benson, C.H.; Beckham, G.T.; Bradshaw, S.L.; Brown, J.L.; Brown, R.C.; Cecon, V.S.; et al. Expanding Plastics Recycling Technologies: Chemical Aspects, Technology Status and Challenges. Green Chem. 2022, 24, 8899–9002. [Google Scholar] [CrossRef]
- Jehanno, C.; Alty, J.W.; Roosen, M.; De Meester, S.; Dove, A.P.; Chen, E.Y.-X.; Leibfarth, F.A.; Sardon, H. Critical Advances and Future Opportunities in Upcycling Commodity Polymers. Nature 2022, 603, 803–814. [Google Scholar] [CrossRef]
- Zhao, X.; Korey, M.; Li, K.; Copenhaver, K.; Tekinalp, H.; Celik, S.; Kalaitzidou, K.; Ruan, R.; Ragauskas, A.J.; Ozcan, S. Plastic Waste Upcycling toward a Circular Economy. Chem. Eng. J. 2022, 428, 131928. [Google Scholar] [CrossRef]
- Napper, I.E.; Davies, B.F.R.; Clifford, H.; Elvin, S.; Koldewey, H.J.; Mayewski, P.A.; Miner, K.R.; Potocki, M.; Elmore, A.C.; Gajurel, A.P.; et al. Reaching New Heights in Plastic Pollution—Preliminary Findings of Microplastics on Mount Everest. One Earth 2020, 3, 621–630. [Google Scholar] [CrossRef]
- Zimmermann, L.; Göttlich, S.; Oehlmann, J.; Wagner, M.; Völker, C. What Are the Drivers of Microplastic Toxicity? Comparing the Toxicity of Plastic Chemicals and Particles to Daphnia Magna. Environ. Pollut. 2020, 267, 115392. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, J. The Chemical Behaviors of Microplastics in Marine Environment: A Review. Mar. Pollut. Bull. 2019, 142, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rafa, N.; Ahmed, B.; Zohora, F.; Bakya, J.; Ahmed, S.; Ahmed, S.F.; Mofijur, M.; Chowdhury, A.A.; Almomani, F. Microplastics as Carriers of Toxic Pollutants: Source, Transport, and Toxicological Effects. Environ. Pollut. 2024, 343, 123190. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Hu, B.; Wang, H. Analytical Methods for Microplastics in the Environment: A Review. Environ. Chem. Lett. 2023, 21, 383–401. [Google Scholar] [CrossRef]
- Wang, Z.-M.; Wagner, J.; Ghosal, S.; Bedi, G.; Wall, S. SEM/EDS and Optical Microscopy Analyses of Microplastics in Ocean Trawl and Fish Guts. Sci. Total Environ. 2017, 603–604, 616–626. [Google Scholar] [CrossRef]
- Ivleva, N.P.; Wiesheu, A.C.; Niessner, R. Microplastic in Aquatic Ecosystems. Angew. Chem. Int. Ed. 2017, 56, 1720–1739. [Google Scholar] [CrossRef]
- Primpke, S.; Lorenz, C.; Rascher-Friesenhausen, R.; Gerdts, G. An Automated Approach for Microplastics Analysis Using Focal Plane Array (FPA) FTIR Microscopy and Image Analysis. Anal. Methods 2017, 9, 1499–1511. [Google Scholar] [CrossRef]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.P.; Ribeiro-Claro, P.J.A. Identification of Microplastics Using Raman Spectroscopy: Latest Developments and Future Prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef]
- Batel, A.; Borchert, F.; Reinwald, H.; Erdinger, L.; Braunbeck, T. Microplastic Accumulation Patterns and Transfer of Benzo[a]Pyrene to Adult Zebrafish (Danio Rerio) Gills and Zebrafish Embryos. Environ. Pollut. 2018, 235, 918–930. [Google Scholar] [CrossRef]
- Matsuguma, Y.; Takada, H.; Kumata, H.; Kanke, H.; Sakurai, S.; Suzuki, T.; Itoh, M.; Okazaki, Y.; Boonyatumanond, R.; Zakaria, M.P.; et al. Microplastics in Sediment Cores from Asia and Africa as Indicators of Temporal Trends in Plastic Pollution. Arch. Environ. Contam. Toxicol. 2017, 73, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Peez, N.; Janiska, M.-C.; Imhof, W. The First Application of Quantitative 1H NMR Spectroscopy as a Simple and Fast Method of Identification and Quantification of Microplastic Particles (PE, PET, and PS). Anal. Bioanal. Chem. 2019, 411, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Richter, F.; Rein, G. The Role of Heat Transfer Limitations in Polymer Pyrolysis at the Microscale. Front. Mech. Eng. 2018, 4, 18. [Google Scholar] [CrossRef]
- Zeng, G.; Zhao, B.; Deng, C.; Zhu, C.; Cheng, M.M.-C.; Gao, P.-X. Microplastic Detection and Monitoring in Biological and Environmental Systems: A Mini Review of Techniques and Strategies. Int. J. High Speed Electron. Syst. 2026, 35, 2640002. [Google Scholar] [CrossRef]
- Cristoni, S.; Dusi, G.; Brambilla, P.; Albini, A.; Conti, M.; Brambilla, M.; Bruno, A.; Di Gaudio, F.; Ferlin, L.; Tazzari, V.; et al. SANIST: Optimization of a Technology for Compound Identification Based on the European Union Directive with Applications in Forensic, Pharmaceutical and Food Analyses. J. Mass Spectrom. 2017, 52, 16–21. [Google Scholar] [CrossRef]
- Elumalai, P.V.; Dhinesh, B.; Jayakar, J.; Nambiraj, M.; Hariharan, V. Effects of Antioxidants to Reduce the Harmful Pollutants from Diesel Engine Using Preheated Palm Oil–Diesel Blend. J. Therm. Anal. Calorim. 2022, 147, 2439–2453. [Google Scholar] [CrossRef]
- Maddison, C.; Sathish, C.I.; Lakshmi, D.; Wayne, O.; Palanisami, T. An Advanced Analytical Approach to Assess the Long-Term Degradation of Microplastics in the Marine Environment. npj Mater. Degrad. 2023, 7, 59. [Google Scholar] [CrossRef]
- Pfohl, P.; Wagner, M.; Meyer, L.; Domercq, P.; Praetorius, A.; Hüffer, T.; Hofmann, T.; Wohlleben, W. Environmental Degradation of Microplastics: How to Measure Fragmentation Rates to Secondary Micro- and Nanoplastic Fragments and Dissociation into Dissolved Organics. Environ. Sci. Technol. 2022, 56, 11323–11334. [Google Scholar] [CrossRef] [PubMed]
- Nene, A.; Sadeghzade, S.; Viaroli, S.; Yang, W.; Uchenna, U.P.; Kandwal, A.; Liu, X.; Somani, P.; Galluzzi, M. Recent Advances and Future Technologies in Nano-Microplastics Detection. Environ. Sci. Eur. 2025, 37, 7. [Google Scholar] [CrossRef]
- He, J.; Han, L.; Wang, F.; Ma, C.; Cai, Y.; Ma, W.; Xu, E.G.; Xing, B.; Yang, Z. Photocatalytic Strategy to Mitigate Microplastic Pollution in Aquatic Environments: Promising Catalysts, Efficiencies, Mechanisms, and Ecological Risks. Crit. Rev. Environ. Sci. Technol. 2022, 53, 504–526. [Google Scholar] [CrossRef]
- Wang, C.; Sun, R.; Huang, R.; Wang, H. Superior Fenton-like Degradation of Tetracycline by Iron Loaded Graphitic Carbon Derived from Microplastics: Synthesis, Catalytic Performance, and Mechanism. Sep. Purif. Technol. 2021, 270, 118773. [Google Scholar] [CrossRef]
- Ren, S.; Xu, X.; Zhu, Z.-S.; Yang, Y.; Tian, W.; Hu, K.; Zhong, S.; Yi, J.; Duan, X.; Wang, S. Catalytic Transformation of Microplastics to Functional Carbon for Catalytic Peroxymonosulfate Activation: Conversion Mechanism and Defect of Scavenging. Appl. Catal. B Environ. 2024, 342, 123410. [Google Scholar] [CrossRef]
- Hou, Q.; Zhen, M.; Qian, H.; Nie, Y.; Bai, X.; Xia, T.; Laiq Ur Rehman, M.; Li, Q.; Ju, M. Upcycling and Catalytic Degradation of Plastic Wastes. Cell Rep. Phys. Sci. 2021, 2, 100514. [Google Scholar] [CrossRef]
- Jeyaraj, J.; Baskaralingam, V.; Stalin, T.; Muthuvel, I. Mechanistic Vision on Polypropylene Microplastics Degradation by Solar Radiation Using TiO2 Nanoparticle as Photocatalyst. Environ. Res. 2023, 233, 116366. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, H.; Huo, P. Plastic Degradation and Conversion by Photocatalysis. In Plastic Degradation and Conversion by Photocatalysis (Volume 2): From Waste to Wealth; American Chemical Society: Washington, DC, USA, 2024; pp. 1–22. ISBN 9780841296091. [Google Scholar]
- Wang, X.; Zhu, Z.; Jiang, J.; Li, R.; Xiong, J. Preparation of Heterojunction C3N4/WO3 Photocatalyst for Degradation of Microplastics in Water. Chemosphere 2023, 337, 139206. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Z.; Liu, H.; Li, Y.; Zhang, G. Efficient Photocatalytic Degradation of Microplastics by Constructing a Novel Z-Scheme Fe-Doped BiO2−x/BiOI Heterojunction with Full-Spectrum Response: Mechanistic Insights and Theory Calculations. J. Hazard. Mater. 2024, 480, 136080. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Jin, M.; Zhu, J.; Zhou, Q.; Fu, L.; Wu, W. Photocatalytic Technologies for Transformation and Degradation of Microplastics in the Environment: Current Achievements and Future Prospects. Catalysts 2023, 13, 846. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, X. Nanomaterial ZnO Synthesis and Its Photocatalytic Applications: A Review. Nanomaterials 2025, 15, 682. [Google Scholar] [CrossRef]
- Surana, M.; Pattanayak, D.S.; Yadav, V.; Singh, V.K.; Pal, D. An Insight Decipher on Photocatalytic Degradation of Microplastics: Mechanism, Limitations, and Future Outlook. Environ. Res. 2024, 247, 118268. [Google Scholar] [CrossRef]
- Kang, S.; Sun, T.; Ma, Y.; Du, M.; Gong, M.; Zhou, C.; Chai, Y.; Qiu, B. Artificial Photosynthesis Bringing New Vigor into Plastic Wastes. SmartMat 2023, 4, e1202. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, L.; Nekliudov, A. Construction of Loading g-C3N4/TiO2 on Waste Cotton-Based Activated Carbon S-Scheme Heterojunction for Enhanced Photocatalytic Degradation of Microplastics: Performance, DFT Calculation and Mechanism Study. Opt. Mater. 2024, 154, 115786. [Google Scholar] [CrossRef]
- Cao, B.; Wan, S.; Wang, Y.; Guo, H.; Ou, M.; Zhong, Q. Highly-Efficient Visible-Light-Driven Photocatalytic H2 Evolution Integrated with Microplastic Degradation over MXene/ZnxCd1-XS Photocatalyst. J. Colloid Interface Sci. 2022, 605, 311–319. [Google Scholar] [CrossRef]
- Maulana, D.A.; Ibadurrohman, M. Slamet Synthesis of Nano-Composite Ag/TiO2 for Polyethylene Microplastic Degradation Applications. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1011, 012054. [Google Scholar] [CrossRef]
- Tofa, T.S.; Ye, F.; Kunjali, K.L.; Dutta, J. Enhanced Visible Light Photodegradation of Microplastic Fragments with Plasmonic Platinum/Zinc Oxide Nanorod Photocatalysts. Catalysts 2019, 9, 819. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, H.; Li, Y.; Zhang, G. Efficient Photocatalytic Degradation of Polystyrene Microplastics in Water over Core–Shell BiO2−x/CuBi2O4 Heterojunction with Full Spectrum Light Response. J. Colloid Interface Sci. 2025, 686, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, D.; Wu, X.; Wu, D.; Su, N.; Wang, Y.; Chen, F.; Fu, C.; Wang, J.; Zhang, Q. Synergistic Dual-Defect Band Engineering for Highly Efficient Photocatalytic Degradation of Microplastics via Nb-Induced Oxygen Vacancies in SnO2 Quantum Dots. J. Mater. Chem. A 2025, 13, 4429–4443. [Google Scholar] [CrossRef]
- Quilumbaquin, W.; Castillo-Cabrera, G.X.; Borrero-González, L.J.; Mora, J.R.; Valle, V.; Debut, A.; Loor-Urgilés, L.D.; Espinoza-Montero, P.J. Photoelectrocatalytic Degradation of High-Density Polyethylene Microplastics on TiO2-Modified Boron-Doped Diamond Photoanode. iScience 2024, 27, 109192. [Google Scholar] [CrossRef]
- Lin, L.; Yi, J.; Wang, J.; Qian, Q.; Chen, Q.; Cao, C.; Zhou, W. Enhancing Microplastic Degradation through Synergistic Photocatalytic and Pretreatment Approaches. Langmuir 2024, 40, 22582–22590. [Google Scholar] [CrossRef]
- He, Y.; Rehman, A.U.; Xu, M.; Not, C.A.; Ng, A.M.C.; Djurišić, A.B. Photocatalytic Degradation of Different Types of Microplastics by TiOx/ZnO Tetrapod Photocatalysts. Heliyon 2023, 9, e22562. [Google Scholar] [CrossRef]
- Jiang, R.; Lu, G.; Dang, T.; Wang, M.; Liu, J.; Yan, Z.; Xie, H. Insight into the Degradation Process of Functional Groups Modified Polystyrene Microplastics with Dissolvable BiOBr-OH Semiconductor-Organic Framework. Chem. Eng. J. 2023, 470, 144401. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Hidalgo-Jiménez, J.; Sauvage, X.; Saito, K.; Guo, Q.; Edalati, K. Phase and Sulfur Vacancy Engineering in Cadmium Sulfide for Boosting Hydrogen Production from Catalytic Plastic Waste Photoconversion. Chem. Eng. J. 2025, 504, 158730. [Google Scholar] [CrossRef]
- Xiao, Y.; Tian, Y.; Xu, W.; Zhu, J. Photodegradation of Microplastics through Nanomaterials: Insights into Photocatalysts Modification and Detailed Mechanisms. Materials 2024, 17, 2755. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, H.; Song, H.; Yi, J.; Wang, X.; Chen, Z.; Zhu, X. Photocatalysis toward Microplastics Conversion: A Critical Review. ACS Catal. 2024, 14, 8694–8719. [Google Scholar] [CrossRef]
- Gu, X.; Li, L.; Wu, Y.; Dong, W. Enhancement of Microplastics Degradation with MIL-101 Modified BiOI Photocatalyst under Light and Dark Alternated System. J. Environ. Chem. Eng. 2024, 12, 112958. [Google Scholar] [CrossRef]
- Meng, Z.; Sun, C.; Wang, C.; Wang, Z.; Deng, S.; Yang, H. Boosting Photocatalytic Capability by Dispersing TiO2 into Chitin Matrix for Polystyrene Microplastics Degradation. Appl. Surf. Sci. 2025, 711, 164104. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Multivalent Metal Catalysts in Fenton/Fenton-like Oxidation System: A Critical Review. Chem. Eng. J. 2023, 466, 143147. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Wang, J. Fenton/Fenton-like Processes with in-Situ Production of Hydrogen Peroxide/Hydroxyl Radical for Degradation of Emerging Contaminants: Advances and Prospects. J. Hazard. Mater. 2021, 404, 124191. [Google Scholar] [CrossRef]
- Meyerstein, D. Re-Examining Fenton and Fenton-like Reactions. Nat. Rev. Chem. 2021, 5, 595–597. [Google Scholar] [CrossRef]
- Xiao, J.; Guo, S.; Wang, D.; An, Q. Fenton—Like Reaction: Recent Advances and New Trends. Chem.—A Eur. J. 2024, 30, e202304337. [Google Scholar] [CrossRef] [PubMed]
- Zarghami Qaretapeh, M.; Kouchakipour, S.; Hosseinzadeh, M.; Dashtian, K. Cuttlefish Bone-Supported CoFe2O4 Nanoparticles Enhance Persulfate Fenton-like Process for the Degradation of Polystyrene Nanoplastics. Chem. Eng. J. 2024, 490, 151833. [Google Scholar] [CrossRef]
- Ye, J.; Li, N.; Gao, W.; Peng, W.; Peng, X.; Cheng, Z.; Yan, B.; Fu, Y.; Zhan, S.; Chen, G.; et al. Bimetal-Carbon Engineering for Polypropylene Conversion into Hydrocarbons and Ketones in a Fenton-like System. Appl. Catal. B Environ. Energy 2024, 358, 124411. [Google Scholar] [CrossRef]
- Camilli, E.; Pighin, A.F.; Copello, G.J.; Villanueva, M.E. Cobalt/Carbon Quantum Dots Core-Shell Nanoparticles as an Improved Catalyst for Fenton-like Reaction. Nano-Struct. Nano-Objects 2024, 37, 101097. [Google Scholar] [CrossRef]
- Ye, Q.; Xu, H.; Hunter, T.N.; Harbottle, D.; Kale, G.M.; Tillotson, M.R. Advanced Polystyrene Nanoplastic Remediation through Electro-Fenton Process: Degradation Mechanisms and Pathways. J. Environ. Chem. Eng. 2025, 13, 118907. [Google Scholar] [CrossRef]
- Wu, M.; Wang, R.; Miao, L.; Sun, P.; Zhou, B.; Xiong, Y.; Dong, X. Synergistically Piezocatalytic and Fenton-like Activation of H2O2 by a Ferroelectric Bi12(Bi0.5Fe0.5)O19.5 Catalyst to Boost Degradation of Polyethylene Terephthalate Microplastic (PET-MPs). J. Colloid Interface Sci. 2025, 682, 738–750. [Google Scholar] [CrossRef]
- Xue, Z.; Yu, X.; Ke, X.; Zhao, J. A Novel Route for Microplastic Mineralization: Visible-Light-Driven Heterogeneous Photocatalysis and Photothermal Fenton-like Reaction. Environ. Sci. Nano 2024, 11, 113–122. [Google Scholar] [CrossRef]
- Hu, K.; Zhou, P.; Yang, Y.; Hall, T.; Nie, G.; Yao, Y.; Duan, X.; Wang, S. Degradation of Microplastics by a Thermal Fenton Reaction. Acs Es&T Eng. 2022, 2, 110–120. [Google Scholar] [CrossRef]
- Zanaty, M.; Zaki, A.H.; El-Dek, S.I.; Abdelhamid, H.N. Zeolitic Imidazolate Framework@hydrogen Titanate Nanotubes for Efficient Adsorption and Catalytic Oxidation of Organic Dyes and Microplastics. J. Environ. Chem. Eng. 2024, 12, 112547. [Google Scholar] [CrossRef]
- Bule Možar, K.; Miloloža, M.; Martinjak, V.; Radovanović-Perić, F.; Bafti, A.; Ujević Bošnjak, M.; Markić, M.; Bolanča, T.; Cvetnić, M.; Kučić Grgić, D.; et al. Evaluation of Fenton, Photo-Fenton and Fenton-like Processes in Degradation of PE, PP, and PVC Microplastics. Water 2024, 16, 673. [Google Scholar] [CrossRef]
- Shen, M.; Song, B.; Zhou, C.; Hu, T.; Zeng, G.; Zhang, Y. Advanced Oxidation Processes for the Elimination of Microplastics from Aqueous Systems: Assessment of Efficiency, Perspectives and Limitations. Sci. Total Environ. 2022, 842, 156723. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Liu, Y.; Lou, X.; Zhang, Q.; Chen, J. Rational Design of Chemical Catalysis for Plastic Recycling. ACS Catal. 2022, 12, 4659–4679. [Google Scholar] [CrossRef]
- Wang, H.; Chen, H.; Wan, Q.; Zheng, Y.; Wan, Y.; Liu, X.; Song, X.; Ma, W.; Huo, P. Catalytic Degradation of Polyethylene Terephthalate Microplastics by Co-N/C@CeO2 Composite in Thermal-Assisted Activation PMS System: Process Mechanism and Toxicological Analysis. Chem. Eng. J. 2025, 514, 163192. [Google Scholar] [CrossRef]
- Wang, J.; Sun, C.; Huang, Q.-X.; Chi, Y.; Yan, J.-H. Adsorption and Thermal Degradation of Microplastics from Aqueous Solutions by Mg/Zn Modified Magnetic Biochars. J. Hazard. Mater. 2021, 419, 126486. [Google Scholar] [CrossRef]
- Nabgan, W.; Nabgan, B.; Tuan Abdullah, T.A.; Ikram, M.; Jadhav, A.H.; Jalil, A.A.; Ali, M.W. Highly Active Biphasic Anatase-Rutile Ni-Pd/TNPs Nanocatalyst for the Reforming and Cracking Reactions of Microplastic Waste Dissolved in Phenol. ACS Omega 2022, 7, 3324–3340. [Google Scholar] [CrossRef]
- Ma, S.; Tang, S.; Zhao, Y. Upcycling Polystyrene Microplastics to Fe/Mo-Doped Sponge-Carbon: Mo5+ Enhanced Electron Transfer for Boosting Fenton-like Performance. Chem. Eng. J. 2024, 498, 155460. [Google Scholar] [CrossRef]
- Sun, R.; Yang, J.; Huang, R.; Wang, C. Controlled Carbonization of Microplastics Loaded Nano Zero-Valent Iron for Catalytic Degradation of Tetracycline. Chemosphere 2022, 303, 135123. [Google Scholar] [CrossRef]
- Hao, Y.; Che, X.; Hou, X.; Shi, L.; Huang, L. Recent Advances in the Thermo-Catalytic Upcycling of Polyethylene Waste. ACS Appl. Polym. Mater. 2025, 7, 6597–6612. [Google Scholar] [CrossRef]
- Vo, T.-P.; Rintala, J.; Dai, L.; Oh, W.-D.; He, C. The Role of Ubiquitous Metal Ions in Degradation of Microplastics in Hot-Compressed Water. Water Res. 2023, 245, 120672. [Google Scholar] [CrossRef]
- Daligaux, V.; Richard, R.; Manero, M.-H. Deactivation and Regeneration of Zeolite Catalysts Used in Pyrolysis of Plastic Wastes—A Process and Analytical Review. Catalysts 2021, 11, 770. [Google Scholar] [CrossRef]
- Razali, N.A.; Wan Abdullah, W.R.; Mohd Zikir, N. Effect of Thermo-Photocatalytic Process Using Zinc Oxide on Degradation of Macro/Micro-Plastic in Aqueous Environment. J. Sustain. Sci. Manag. 2020, 15, 1–14. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, S.; Sun, B.; Han, Y.; Zhang, Z.; Shen, X.; Li, Z. Adsorption Efficiency and In-Situ Catalytic Thermal Degradation Behaviour of Microplastics from Water over Fe-Modified Lignin-Based Magnetic Biochar. Sep. Purif. Technol. 2025, 353, 128468. [Google Scholar] [CrossRef]
- Li, S.; Yang, Y.; Yang, S.; Zheng, H.; Zheng, Y.; M, J.; Nagarajan, D.; Varjani, S.; Chang, J.-S. Recent Advances in Biodegradation of Emerging Contaminants—Microplastics (MPs): Feasibility, Mechanism, and Future Prospects. Chemosphere 2023, 331, 138776. [Google Scholar] [CrossRef]
- Gao, W.; Xu, M.; Zhao, W.; Yang, X.; Xin, F.; Dong, W.; Jia, H.; Wu, X. Microbial Degradation of (Micro)Plastics: Mechanisms, Enhancements, and Future Directions. Fermentation 2024, 10, 441. [Google Scholar] [CrossRef]
- Thakur, B.; Singh, J.; Singh, J.; Angmo, D.; Vig, A.P. Biodegradation of Different Types of Microplastics: Molecular Mechanism and Degradation Efficiency. Sci. Total Environ. 2023, 877, 162912. [Google Scholar] [CrossRef]
- Du, H.; Xie, Y.; Wang, J. Microplastic Degradation Methods and Corresponding Degradation Mechanism: Research Status and Future Perspectives. J. Hazard. Mater. 2021, 418, 126377. [Google Scholar] [CrossRef]
- Rincon, I.; Hidalgo, T.; Armani, G.; Rojas, S.; Horcajada, P. Enzyme_Metal—Organic Framework Composites as Novel Approach for Microplastic Degradation. ChemSusChem 2024, 17, e202301350. [Google Scholar] [CrossRef]
- Zandieh, M.; Liu, J. Removal and Degradation of Microplastics Using the Magnetic and Nanozyme Activities of Bare Iron Oxide Nanoaggregates. Angew. Chem. 2022, 134, e202212013. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Chen, Z.; Yu, Z.; Xue, J.; Luan, T.; Chen, S.; Zhou, S. Mechanisms of Polystyrene Microplastic Degradation by the Microbially Driven Fenton Reaction. Water Res. 2022, 223, 118979. [Google Scholar] [CrossRef]
- Zhang, A.; Hou, Y.; Hou, S.; Wang, Y.; Wang, Q. Enhancement of Microplastics Degradation Efficiency: Microbial Laccase-Driven Radical Chemical Coupling Catalysis. Chem. Eng. J. 2025, 507, 160579. [Google Scholar] [CrossRef]
- Li, X.; Li, G.; Wang, J.; Li, X.; Yang, Y.; Song, D. Elucidating Polyethylene Microplastic Degradation Mechanisms and Metabolic Pathways via Iron-Enhanced Microbiota Dynamics in Marine Sediments. J. Hazard. Mater. 2024, 466, 133655. [Google Scholar] [CrossRef]
- Wan, P.; Chen, G.; Fan, J.; Tan, W.; Li, X.; Chen, L.; Li, K. Modulating Ion Migration Realizes Both Enhanced and Long-Term-Stable Nanozyme Activity for Efficient Microplastic Degradation. Chem. Sci. 2025, 16, 15955–15963. [Google Scholar] [CrossRef]
- Ren, J.; Meng, Y.; Wang, Z.; Xie, G. Degradation of Microplastics by Microbial in Combination with a Micromotor. ACS Sustain. Chem. Eng. 2025, 13, 4018–4027. [Google Scholar] [CrossRef]
- Huang, Q.-S.; Chen, S.-Q.; Zhao, X.-M.; Song, L.-J.; Deng, Y.-M.; Xu, K.-W.; Yan, Z.-F.; Wu, J. Enhanced Degradation of Polyethylene Terephthalate (PET) Microplastics by an Engineered Stenotrophomonas Pavanii in the Presence of Biofilm. Sci. Total Environ. 2024, 955, 177129. [Google Scholar] [CrossRef]
- Shao, D.; Zhao, W.; Ji, S.; Yang, C.; Zhang, J.; Guo, R.; Zhang, B.; Lyu, W.; Feng, J.; Xu, H.; et al. Joule Heat Assisting Electrochemical Degradation of Polyethylene Microplastics Melted on Anode. Appl. Catal. B Environ. Energy 2024, 357, 124281. [Google Scholar] [CrossRef]
- Mao, Y.; Fan, S.; Li, X.; Shi, J.; Wang, M.; Niu, Z.; Chen, G. Trash to Treasure: Electrocatalytic Upcycling of Polyethylene Terephthalate (PET) Microplastic to Value-Added Products by Mn0.1Ni0.9Co2O4-δ RSFs Spinel. J. Hazard. Mater. 2023, 457, 131743. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Liu, Z.; Wang, B.; Tao, M.; Ji, H.; Xiang, X.; Fu, Z.; Liao, L.; Liao, P.; Chen, R. Effective Degradation of Polystyrene Microplastics by Ti/La/Co-Sb-SnO2 Anodes: Enhanced Electrocatalytic Stability and Electrode Lifespan. Sci. Total Environ. 2024, 922, 171002. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, B.; Liu, Z.; Yang, H.; Chen, Z.; Sun, X. La-Doped Ti/Sb-SnO2 Electrode Enhanced Removal of Microplastics by Advanced Electrocatalysis Oxidation Process (AEOP) Strategy. Desalination Water Treat. 2024, 320, 100622. [Google Scholar] [CrossRef]
- Ning, Z.; Duan, X.; Li, Y.; Zhao, X.; Chang, L. Degradation of Polyvinyl Chloride Microplastics via Electrochemical Oxidation with a CeO2–PbO2 Anode. J. Clean. Prod. 2023, 432, 139668. [Google Scholar] [CrossRef]
- Ma, F.; Wang, S.; Gong, X.; Liu, X.; Wang, Z.; Wang, P.; Liu, Y.; Cheng, H.; Dai, Y.; Zheng, Z.; et al. Highly Efficient Electrocatalytic Hydrogen Evolution Coupled with Upcycling of Microplastics in Seawater Enabled via Ni3N/W5N4 Janus Nanostructures. Appl. Catal. B Environ. 2022, 307, 121198. [Google Scholar] [CrossRef]
- Kuila, S.K.; Dhanda, A.; Samal, B.; Ghangrekar, M.M.; Dubey, B.K.; Kundu, T.K. Defect Engineered 2D Graphitic Carbon Nitride for Photochemical, (Bio)Electrochemical, and Microplastic Remediation Advancements. ACS Es&T Water 2024, 4, 4283–4298. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, H.; Xu, Q.; Dong, M.; Yang, J.; Yang, W.; Feng, Y.; Su, Z.-M. Vacancy-Rich NiFe-LDH/Carbon Paper as a Novel Self-Supporting Electrode for the Electro-Fenton Degradation of Polyvinyl Chloride Microplastics. J. Hazard. Mater. 2025, 485, 136797. [Google Scholar] [CrossRef]
- Miranda Zoppas, F.; Sacco, N.; Soffietti, J.; Devard, A.; Akhter, F.; Marchesini, F.A. Catalytic Approaches for the Removal of Microplastics from Water: Recent Advances and Future Opportunities. Chem. Eng. J. Adv. 2023, 16, 100529. [Google Scholar] [CrossRef]
- Ye, J.; Chen, Y.; Gao, C.; Wang, C.; Hu, A.; Dong, G.; Chen, Z.; Zhou, S.; Xiong, Y. Sustainable Conversion of Microplastics to Methane with Ultrahigh Selectivity by a Biotic–Abiotic Hybrid Photocatalytic System. Angew. Chem. 2022, 134, e202213244. [Google Scholar] [CrossRef]
- Kang, J.; Zhou, L.; Duan, X.; Sun, H.; Ao, Z.; Wang, S. Degradation of Cosmetic Microplastics via Functionalized Carbon Nanosprings. Matter 2019, 1, 745–758. [Google Scholar] [CrossRef]
- Miao, F.; Liu, Y.; Gao, M.; Yu, X.; Xiao, P.; Wang, M.; Wang, S.; Wang, X. Degradation of Polyvinyl Chloride Microplastics via an Electro-Fenton-like System with a TiO2/Graphite Cathode. J. Hazard. Mater. 2020, 399, 123023. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Liu, W.; Jin, Q.; Lin, H. Piezo-Photocatalytic Enhanced Microplastic Degradation on Hetero-Interpenetrated Fe1−xS/FeMoO4/ MoS2 by Producing H2O2 and Self-Fenton Action. Chem. Eng. J. 2025, 508, 160935. [Google Scholar] [CrossRef]
- Kim, J.; Jang, J.; Hilberath, T.; Hollmann, F.; Park, C.B. Photoelectrocatalytic Biosynthesis Fuelled by Microplastics. Nat. Synth. 2022, 1, 776–786. [Google Scholar] [CrossRef]
- Feng, Z.-Y.; Jiang, J.-C.; Jin, B.; Meng, L.-Y. Hydrogen Evolution Coupled with Microplastic Upcycling in Seawater via Solvent-Free Plasma F-Functionalized CoNi Alloy Catalysts. J. Alloys Compd. 2024, 1008, 176593. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Lyagin, I.V.; Maslova, O.V.; Senko, O.V.; Stepanov, N.A.; Aslanli, A.G.G. Catalytic Degradation of Microplastics. Russ. Chem. Rev. 2023, 92, RCR5069. [Google Scholar] [CrossRef]
- Zandieh, M.; Griffiths, E.; Waldie, A.; Li, S.; Honek, J.; Rezanezhad, F.; Van Cappellen, P.; Liu, J. Catalytic and Biocatalytic Degradation of Microplastics. Exploration 2024, 4, 20230018. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Mo, A.; Jiang, J.; Liang, Y.; Cao, X.; He, D. Removal of Microplastics in Water: Technology Progress and Green Strategies. Green Anal. Chem. 2022, 3, 100042. [Google Scholar] [CrossRef]
- Hettiarachchi, H.; Meegoda, J.N. Microplastic Pollution Prevention: The Need for Robust Policy Interventions to Close the Loopholes in Current Waste Management Practices. Int. J. Environ. Res. Public Health 2023, 20, 6434. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Redondo-Hasselerharm, P.E.; Nor, N.H.M.; de Ruijter, V.N.; Mintenig, S.M.; Kooi, M. Risk Assessment of Microplastic Particles. Nat. Rev. Mater. 2022, 7, 138–152. [Google Scholar] [CrossRef]
- Xayachak, T.; Haque, N.; Lau, D.; Pramanik, B.K. The Missing Link: A Systematic Review of Microplastics and Its Neglected Role in Life-Cycle Assessment. Sci. Total Environ. 2024, 954, 176513. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).