Pomegranate Peel Derived-Carbon for Highly Efficient Palladium-Based Catalysts for Acetylene Hydrochlorination
Abstract
1. Introduction
2. Results and Discussion
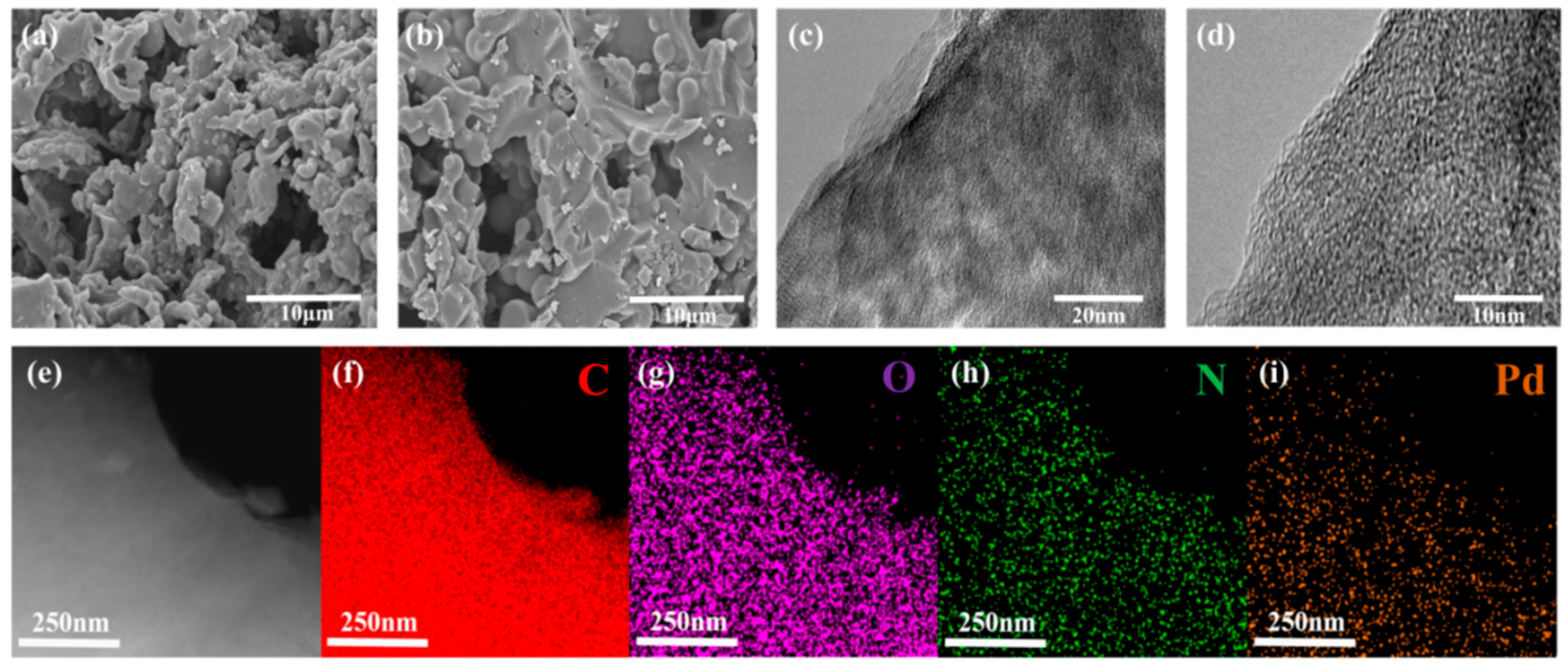
2.1. Characterization of PPC
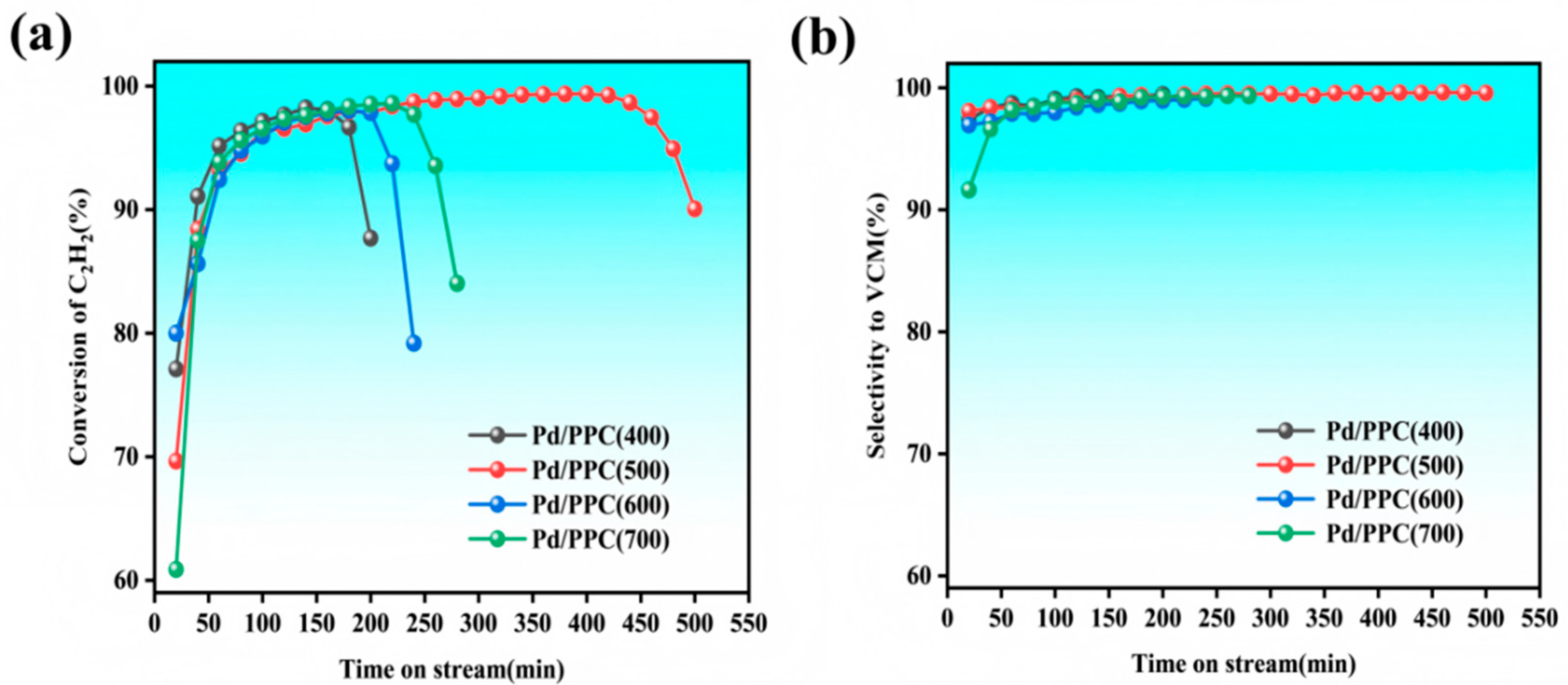
2.2. Catalytic Performance of the Pd/PPC Catalysts
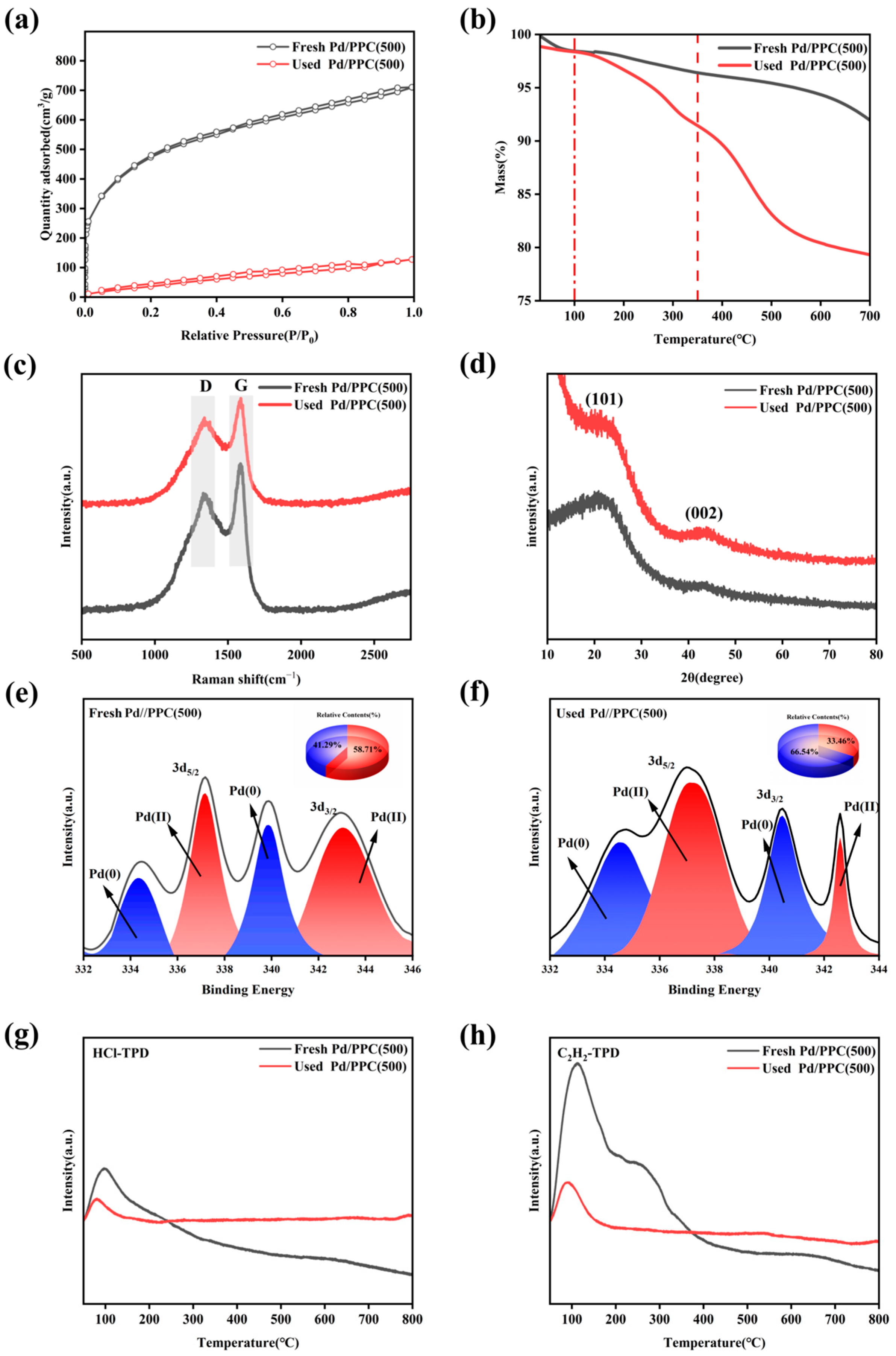
2.3. Characterization of the Pd/PPC Catalysts
3. Experimental Section
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Performance Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Zhao, L.; Zhang, Y.; Zhang, L.; Zan, X. Progress and challenges of mercury-free Catalysis for acetylene hydrochlorination. Catalysts 2020, 10, 1218. [Google Scholar] [CrossRef]
- Zhong, J.; Xu, Y.; Liu, Z. Heterogeneous non-mercury catalysts for acetylene hydrochlorination: Progress, challenges, and opportunities. Green Chem. 2018, 20, 2412–2427. [Google Scholar] [CrossRef]
- Shang, S.; Zhao, W.; Wang, Y.; Li, X.; Zhang, J.; Han, Y.; Li, W. Highly efficient Ru@ IL/AC to substitute mercuric catalyst for acetylene hydrochlorination. ACS Catal. 2017, 7, 3510–3520. [Google Scholar] [CrossRef]
- Man, B.; Zhang, H.; Zhang, C.; Li, X.; Zhu, M.; Dai, B.; Zhang, J. Effect of Ru/Cl ratio on the reaction of acetylene hydrochlorination. New J. Chem. 2017, 41, 14675–14682. [Google Scholar] [CrossRef]
- Krasnyakova, T.V.; Nikitenko, D.V.; Kobets, K.D.; Krasniakova, I.O.; Gogilchin, A.S.; Bugaev, A.L.; Mitchenkoet, S.A. Stereoselectivity of acetylene hydrochlorination over supported PdCl2/C catalysts. Kinet. Catal. 2023, 64, 294–302. [Google Scholar] [CrossRef]
- Saadi, W.; Rodríguez-Sánchez, S.; Ruiz, B.; Najar-Souissi, S.; Ouederni, A.; Fuente, E. From pomegranate peels waste to one-step alkaline carbonate activated carbons. Prospect as sustainable adsorbent for the renewable energy production. J. Environ. Chem. Eng. 2022, 10, 2213–3437. [Google Scholar] [CrossRef]
- Kaiser, S.K.; Surin, I.; Amorós-Pérez, A.; Büchele, S.; Krumeich, F.; Adam, H.C.; Román-Martínez, M.C.; Lillo-Ródenas, M.A.; Pérez-Ramírez, J. Design of carbon supports for metal-catalyzed acetylene hydrochlorination. Nat. Commun. 2021, 12, 4016. [Google Scholar] [CrossRef]
- Matyjasik, W.; Matus, K.; Długosz, O.; Pulit-Prociak, J.; Banach, M. Preparation of biomass waste-derived carbon dots by the thermal degradation process. ACS Omega 2025, 10, 22529–22548. [Google Scholar] [CrossRef]
- Visser, E.D.; Seroka, N.S.; Khotseng, L. Catalytic properties of biochar as support material potential for direct methanol fuel cell: A review. ACS Omega 2023, 8, 40972–40981. [Google Scholar] [CrossRef]
- Portilla-Amaguaña, A.; Barraza-Burgos, J.; Guerrero-Perez, J.; Borugadda, V.B.; Dalai, A.K. Hydrothermal carbonization of green harvesting residues (GHRs) from sugar cane: Effect of temperature and water/GHR ratio on mass and energy yield. ACS Omega 2024, 9, 26325–26335. [Google Scholar] [CrossRef]
- Melgarejo-Sánchez, P.; Núñez-Gómez, D.; Martínez-Nicolás, J.J.; Hernández, F.; Legua, P.; Melgarejo, P. Pomegranate variety and pomegranate plant part, relevance from bioactive point of view: A review. Bioresour. Bioprocess. 2021, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; HolcroftAdel, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Al-Zoreky, N. Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. Int. J. Food Microbiol. 2009, 134, 244–248. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Vadhanam, M.V.; Kausar, H.; Jeyabalan, J.; Schultz, D.J.; Gupta, R.C. Anti-proliferative activity and protection against oxidative DNA damage by punicalagin isolated from pomegranate husk. Food Res. Int. 2012, 49, 345–353. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Hosseinzadeh, H. Protective effects of pomegranate (Punica granatum) and its main components against natural and chemical toxic agents: A comprehensive review. Phytomedicine 2023, 109, 154581. [Google Scholar] [CrossRef]
- Chen, C.; Han, B.; Zhu, X.; Jiang, C.; Wang, Y. A novel pomegranate peel-derived biochar for highly efficient removal of sulfamethoxazole by activation of peroxymonosulfate through a non-radical pathway. J. Environ. Chem. Eng. 2022, 10, 108184. [Google Scholar] [CrossRef]
- Khairy, G.M.; Hesham, A.M.; Jahin, E.S.; El-Korashy, S.A.; Awad, Y.M. Green synthesis of a novel eco-friendly hydro char from pomegranate peels loaded with iron nanoparticles for the removal of copper ions and methylene blue from aqueous solutions. J. Mol. Liq. 2022, 368, 120722. [Google Scholar] [CrossRef]
- Zarroug, M.; Najar, S.; Souissi, J.A.; Menéndez, E.G.; Calvo, A. Ouederni. Fast production of activated carbon from pomegranate peels by combining microwave heating and phosphoric acid activation for paracetamol adsorption. Environ. Eng. Sci. 2022, 39, 441–452. [Google Scholar] [CrossRef]
- Chavan, M.S.; Suryawanshi, S.; Kumbhar, A. bio-waste originated, heterogeneous catalysts based on pomegranate peel for knoevenagel condensation: A green approach. React. Kinet. Mech. Catal. 2023, 136, 2167–2180. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, Y.; Han, Y.; Liu, Y.; Cao, S. Nitrogen-doped porous carbon from biomass with superior catalytic performance for acetylene hydrochlorination. RSC Adv. 2020, 10, 14556–14569. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Jiang, A.; Zhou, X.; Guo, Z.; Wang, K. Environmentally friendly high-efficient metal-free catalyst for acetylene hydrochlorination derived from walnut shell-based N-doped biochar. Mol. Catal. 2022, 532, 112719. [Google Scholar] [CrossRef]
- Lan, G.; Wang, Y.; Qiu, Y.; Wang, X.; Liang, J.; Han, W.; Tang, H.; Liu, H.; Liu, J.; Li, Y. Wheat flour-derived N-doped mesoporous carbon extrudate as superior metal-free catalysts for acetylene hydrochlorination. Chem. Commun. 2018, 54, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Wang, F.; Wang, J. Bi/AC modified with phosphoric acid as catalyst in the hydrochlorination of acetylene. RSC Adv. 2017, 7, 7567–7575. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.; Wang, J. Enhanced stability of hydrochlorination of acetylene using polyaniline-modified Pd/HY catalysts. Catal. Commun. 2016, 74, 55–59. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.; Wang, J. Non-mercury catalytic acetylene hydrochlorination over a NH4F-urea-modified Pd/HY catalyst for vinyl chloride monomer production. New J. Chem. 2016, 40, 3019–3023. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; Yan, H.; Lian, L.; Si, J.; Long, Z.; Cui, X.; Wang, J.; Zhao, L.; Yang, C.; et al. Palladium-halloysite nanocomposites as an efficient heterogeneous catalyst for acetylene hydrochlorination. J. Mater. Res. Technol. 2021, 13, 2055–2065. [Google Scholar] [CrossRef]
- Ali, S.; Wang, L.; Yan, H.; Yang, C.; Wang, J.; Sun, H.; Li, X.; Wu, R.; Liang, C. Enhanced catalytic performance of palladium-based catalysts modified with the dibenzofuran ligand for acetylene hydrochlorination. Appl. Organomet. Chem. 2024, 38, 119382–119391. [Google Scholar] [CrossRef]
- Lian, L.; Wang, L.; Yan, H.; Lian, L.; Wang, L.; Yan, H.; Ali, S.; Wang, J.; Zhao, L.; Yang, C.; et al. Non-mercury catalytic acetylene hydrochlorination over Bi/CNTs catalysts for vinyl chloride monomer production. J. Mater. Res. Technol-JMRT. 2020, 9, 14961–14968. [Google Scholar] [CrossRef]
- Dang, L.; Wang, L.; Yan, H.; Long, Z.; Yang, C.; Wang, J.; Guan, Q.; Sun, H.; Li, X.; Wu, R.; et al. Metal immobilized in a USY zeolite-supported (4-CB) TPPB: A new strategy of enhanced stability for acetylene hydrochlorination. Microporous Mesoporous Mat. 2024, 377, 113204. [Google Scholar] [CrossRef]
- Yang, H.; Wang, L.; Yan, H.; Dang, L.; Wang, J.; Sun, H. Hierarchical porous carbon from PVC plastic wastes with efficient catalytic performance for acetylene hydrochlorination. New J. Chem. 2025, 49, 36125–36142. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, H.; Gao, G.; Wang, M.; Huang, W.; Ma, L.; Yao, Y.; Qu, Z.; Xie, P.; Dai, B.; et al. Asymmetric Ru-In atomic pairs promote highly active and stable acetylene hydrochlorination. Nat. Commun. 2024, 15, 6035. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, C.; Qiao, X.; Guan, Q.; Li, W. Regulating the coordination environment of Ru single-atom catalysts and unravelling the reaction path of acetylene hydrochlorination. Green Energy Environ. 2023, 8, 1141–1153. [Google Scholar] [CrossRef]
- Yang, L.Q.; Zhang, C.; Li, W.L.; Li, W.; Liu, G.; Wu, M.; Liu, J.; Zhang, J. Global optimization of process parameters for low-temperature SiNx based on orthogonal experiments. Adv. Manuf. 2023, 11, 181–190. [Google Scholar] [CrossRef]
- Liu, D.; Xia, S.; Tang, H.; Zhong, D.; Wang, B.; Cai, X.; Lin, R. Parameter optimization of PEMFC stack under steady working condition using orthogonal experimental design. Int. J. Energy Res. 2019, 43, 2571–2582. [Google Scholar] [CrossRef]
- Zhou, L. Preparation of calcium fluoride using phosphogypsum by orthogonal experiment. Open Chem. 2018, 16, 864–868. [Google Scholar] [CrossRef]
- Qiu, Y.; Ali, S.; Lan, G.; Tong, H.; Fan, J.; Liu, H.; Li, B.; Han, W.; Tang, H.; Liu, H.; et al. Defect-rich activated carbons as active and stable metal-free catalyst for acetylene hydrochlorination. Carbon 2019, 146, 406–412. [Google Scholar] [CrossRef]
- Ma, X.; Bai, Y.; Chen, S.; He, Z.; Wu, P.; Qi, Y.; Zhang, S. A composite of pineapple leaf-derived porous carbon integrated with ZnCo-MOF for high-performance supercapacitors. Phys. Chem. Chem. Phys. 2024, 26, 28746–28756. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fan, D.; Lan, G.; Cheng, Z.; Sun, X.; Qiu, Y.; Han, W.; Tang, H.; Liu, H.; Zhu, Y.; et al. The reaction mechanism of acetylene hydrochlorination on defective carbon supported ruthenium catalysts identified by DFT calculations and experimental approaches. Inorg. Chem. Front. 2022, 9, 458–467. [Google Scholar] [CrossRef]
- Dai, Z.B. Effect of carbon defects on the nitrogen-doped carbon catalytic performance for acetylene hydrochlorination. Appl. Catal. A-Gen. 2018, 564, 72–78. [Google Scholar]
- Lu, F.; Wei, C.; Yin, X.; Zhang, L.; Zan, X. The effect of sp2 content in carbon on its catalytic activity for acetylene hydrochlorination. Nanomaterials 2022, 12, 2619. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, K.; Yuan, X. Study on quantitative structure of coke deposition in UDH catalyst for acetylene hydrochlorination. Energy Source Part A 2023, 45, 5458–5464. [Google Scholar] [CrossRef]
- Lu, F.; Wang, Q.; Zhu, M.; Dai, B. Deactivation and regeneration of nitrogen doped carbon catalyst for acetylene hydrochlorination. Molecules 2023, 28, 956. [Google Scholar] [CrossRef]
- Cen, Y.; Yue, Y.; Wang, S.; Lu, J.; Wang, B.; Jin, C.; Guo, L.; Hu, Z.; Zhao, J. Adsorption behavior and electron structure engineering of Pd-Based catalysts for acetylene hydrochlorination. Catalysts 2019, 10, 24–40. [Google Scholar] [CrossRef]
- Wang, H.; Tian, Y.; Xu, M.; Cheng, X.; Huang, J.; Li, H.; Li, Y.; Shi, Y.; Ye, L.; Liu, H.; et al. Development of ionic liquids promoted low content ruthenium catalysts for acetylene hydrochlorination. Catal. Today 2024, 434, 114696. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Dong, Y.; Li, W.; Zhao, S.; Zhang, J. Characteristics of activated carbons modulate the catalytic performance for acetylene hydrochlorination. Mol. Catal. 2020, 483, 110707. [Google Scholar] [CrossRef]
- Liu, X.; Conte, M.; Elias, D.; Lu, L.; Morgan, D.J.; Freakley, S.J.; Johnston, P.; Kiely, C.J.; Hutchings, G.J. Investigation of the active species in the carbon-supported gold catalyst for acetylene hydrochlorination. Catal. Sci. Technol. 2016, 6, 5144–5153. [Google Scholar] [CrossRef]
- Dong, X.; Liu, G.; Chen, Z.; Zhang, Q.; Xu, Y.; Liu, Z. Enhanced performance of Pd-[DBU][Cl]/AC mercury-free catalysts in acetylene hydrochlorination. Chin. J. Catal. 2023, 46, 137–147. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, Y.; Xu, X.; Di, S.; Wang, B.; Xu, H.; Ni, J.; Guo, L.; Pan, Z.; Li, X. Stabilizing Au (III) in supported-ionic-liquid-phase (SILP) catalyst using CuCl2 via a redox mechanism. Appl. Catal. B Environ. 2017, 206, 175–183. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wang, L.; Yan, H.; Liu, J.; Ali, S.; Yang, C.; Wu, R.; Wang, J.; Wei, Y.; Sun, H.; et al. Pomegranate Peel Derived-Carbon for Highly Efficient Palladium-Based Catalysts for Acetylene Hydrochlorination. Catalysts 2025, 15, 983. https://doi.org/10.3390/catal15100983
Li Z, Wang L, Yan H, Liu J, Ali S, Yang C, Wu R, Wang J, Wei Y, Sun H, et al. Pomegranate Peel Derived-Carbon for Highly Efficient Palladium-Based Catalysts for Acetylene Hydrochlorination. Catalysts. 2025; 15(10):983. https://doi.org/10.3390/catal15100983
Chicago/Turabian StyleLi, Zonglin, Lu Wang, Haijun Yan, Jindou Liu, Shahid Ali, Chao Yang, Ronglan Wu, Jide Wang, Yana Wei, Hui Sun, and et al. 2025. "Pomegranate Peel Derived-Carbon for Highly Efficient Palladium-Based Catalysts for Acetylene Hydrochlorination" Catalysts 15, no. 10: 983. https://doi.org/10.3390/catal15100983
APA StyleLi, Z., Wang, L., Yan, H., Liu, J., Ali, S., Yang, C., Wu, R., Wang, J., Wei, Y., Sun, H., & Liang, C. (2025). Pomegranate Peel Derived-Carbon for Highly Efficient Palladium-Based Catalysts for Acetylene Hydrochlorination. Catalysts, 15(10), 983. https://doi.org/10.3390/catal15100983






