Abstract
Congo Red is a complex aromatic azo dye whose metabolites can be toxic due to their carcinogenicity, mutagenicity, and various associated toxic effects on flora, fauna, and humans. Different technologies have been employed to degrade this dye, including biodegradation, radiation-based degradation, and chemical degradation with catalysts and photocatalysis. Among these, the use of TiO2-based materials combined with photocatalysis has proven to be an effective technology for its degradation. However, the wide bandgap of TiO2 limits its efficiency under visible light, prompting the need for modifications such as doping with metals, metalloids, and organic compounds. These modifications enhance its photocatalytic performance under visible light, achieving degradation efficiencies of up to 100% under optimal conditions. This article explores recent advances (from 2020 to the present) in the degradation of Congo Red using TiO2-based photocatalysts under visible light, focusing on their characteristics, synthesis methods, and degradation efficiencies. Additionally, it compares the TiO2-based photocatalysis with visible light to other available technologies, emphasizing its potential as a sustainable and efficient approach while addressing the importance of monitoring degradation byproducts to prevent the generation of equally or more toxic compounds.
1. Introduction
Congo Red is a synthetic azo dye widely recognized for its distinctive color change depending on pH levels; it appears blue-violet at a pH of 3.0 and red at pH 5.0. Chemically, its structure corresponds to 3,3′-(biphenyl-4,4′-diyldidiazene-2,1-diyl) bis (4-aminonaphthalene-1-sulfonate) and is functionally related to a 3,3′-(biphenyl-4,4′-diyldidiazene-2,1-diyl) bis (4-aminonaphthalene-1-sulfonic acid) as described by the NIH (2024). The chemical structure is shown in Figure 1 [1]. This compound, derived from benzidine, is a complex aromatic molecule whose metabolites can be highly toxic [2]. Although Congo Red is not directly toxic [2], it poses significant environmental concerns due to its carcinogenicity, mutagenicity, and harmful effects on flora and fauna [3]. Moreover, the dye is known to cause infertility, increase chemical oxygen demand in water bodies, and trigger allergic reactions through its toxic metabolites. It also negatively impacts the aesthetic and ecological quality of aquatic ecosystems [2].
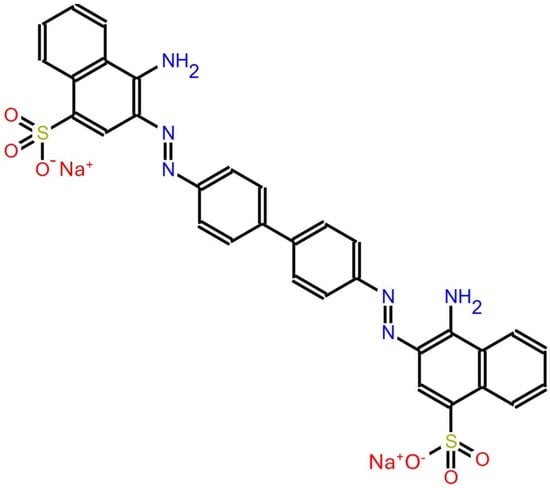
Figure 1.
Chemical structure of Congo Red [1].
This dye is extensively used as a colorant in the textile, printing, dyeing, and rubber industries, which often leads to its discharge into aquatic systems [3]. Once released into the environment, Congo Red persists due to its stable aromatic structure and high resistance to natural degradation. With a maximum absorption wavelength of 497 nm at a pH of 5, its detection in environmental monitoring efforts is relatively straightforward [4].
Regarding its toxicity, evaluations have been conducted using animal models. For instance, a study on New Zealand albino rabbits observed 100% mortality at doses of 6.0 and 8.0 g kg−1 of body weight, highlighting its high toxicity potential at elevated concentrations [5]. This underscores the need for strict monitoring and mitigation strategies.
To address the environmental challenges associated with Congo Red, various degradation technologies have been developed. These include photocatalysis, chemical, physical, and biological treatments, and irradiation techniques. Relevant information regarding these degradation methods is presented in Table 1.

Table 1.
Common methods and technologies for the degradation of Congo Red.
As shown in Table 1, photocatalysis is the most employed method for degrading Congo Red, with decolorization ranging from 81.9% to 98% [10,12,13,14,15,16]. This technique uses advanced materials such as titanium dioxide (TiO2) and zinc oxide (ZnO), often enhanced with dopants or co-catalysts, to decompose the dye under ultraviolet (UV) or visible light. However, while there are high degradation rates, these processes often lack direct toxicity assessments. Conversely, the combination of physical–chemical degradation with irradiation using visible light, UV light, gamma radiation, or microwaves necessitates shorter treatment times, often less than an hour, making it faster than other technologies (see Table 1).
Irradiation techniques, such as gamma radiation from sources like Co-60 [18,19], or microwave-assisted degradation [20], have also shown high efficiency, with degradation rates ranging from 86% to 100%. These technologies often monitor intermediate products through advanced analytical methods such as liquid chromatography-mass spectrometry (LC-MS), Fourier-transform infrared spectroscopy (FTIR), or gas chromatography-mass spectrometry (GC-MS). For example, Muneer et al. identified radiolytic products of Congo Red using FTIR and GC-MS, while Sales Solano et al. employed LC-MS to analyze metabolites formed during the Fenton’s process [18].
Biological treatments focus on using microorganisms, such as bacteria and fungi, or plant systems to degrade Congo Red. Previous studies have demonstrated that these methods achieve degradation rates between 80% and 97% [6,7,8,9], while also incorporating toxicity bioassays to ensure the environmental safety of the resulting products. For example, Fowsiya et al. demonstrated the ability of certain microorganisms to degrade the dye while reducing its toxicity [9]. Unlike physical–chemical methods that use irradiation, biological enzymes typically require longer treatment times, ranging from several hours to 6 days. This can be a significant drawback (see Table 1). Additionally, some enzymes, particularly purified ones, can be quite expensive [21] and may denature under extreme conditions such as high pressure, temperature [22], pH [23], and salt concentrations [24]. In contrast, materials like TiO2 are more affordable [25] and stable [26].
Assessing the toxicity of degradation byproducts is crucial to ensuring the environmental safety of treatments. Depending on the degradation method used, toxic intermediates can form. For example, during the ozonation of diclofenac, antiestrogenic compounds with dichloroaniline structures were detected, which were not present during photodegradation using UVA and UVC light [27]. Findings like these highlight the importance of thorough monitoring to ensure that degradation processes do not produce byproducts more hazardous than the original pollutant.
2. Degradation of Congo Red with TiO2-Based Photocatalysts: Advantages and Limitations
When selecting a material for degradation processes, several crucial factors must be taken into account to ensure efficiency and feasibility. These include the material’s availability, affordability, and suitability for large-scale applications. Additionally, a high specific surface area and the capability for surface functionalization are highly desirable characteristics. In photocatalysis applications, the material’s ability to enhance the absorption of UV radiation is particularly advantageous. TiO2 exemplifies many of these characteristics [28]. It is chemically inert, non-toxic, and possesses functional groups that can facilitate further surface modifications, making it well-suited for environmentally friendly processes [29].
Despite its advantages, TiO2 has a notable limitation: its wide band gap of approximately 3.2 eV (in the anatase crystalline form) restricts its photocatalytic activity to UV light. This dependence on UV radiation is problematic because UV constitutes only about 5% of the solar spectrum, whereas visible light represents a significantly larger portion at approximately 45%. Consequently, TiO2 demonstrates low energy efficiency when exposed to natural sunlight. Addressing this limitation by shifting its optical response from the UV to the visible spectrum could greatly enhance its practical applications, particularly in solar-driven photocatalysis [30].
Numerous studies have demonstrated the effectiveness of TiO2 in degrading Congo Red when combined with UV light. For instance, Nurdin et al. explored using TiO2 and UV radiation in degrading a 5 mg L−1 solution of Congo Red. Over 60 min, they observed a degradation rate (k’) of 0.018 ppm min−1 in a 4 mL solution [29]. Similarly, Harun et al. achieved a 66.99% degradation of 250 mL of 4 mg L−1 of Congo Red within 30 min using TiO2 and a UV lamp [31]. Another significant study by Turcu et al. used TiO2 and UV light, a pH of 5.5, and 0.05 g of powdered TiO2 in a 50 mL solution with 50 mg L−1 of Congo Red, achieving a remarkable 98.28% degradation in 90 min [32].
These studies underscore the potential of TiO2 as an effective photocatalyst for the degradation of Congo Red under UV light. However, they also highlight the limitations imposed by its dependence on UV radiation, emphasizing the importance of further research into strategies to improve its optical response. Possible approaches include doping with other elements, coupling TiO2 with other semiconductors, or developing composite materials to extend its absorption range into the visible spectrum. Enhancing the solar utilization efficiency of TiO2 could significantly advance its application in sustainable, large-scale environmental remediation processes.
While other materials such as Fe2O3, WO3, ZnSe, CdSe, CdS, and MoS2 have lower band gaps [33], TiO2 stands out due to its numerous advantages. These include low cost, easy accessibility, the ability to modify its surface with different molecules, physical and chemical stability, non-toxicity, photosensitivity, and biocompatibility. Additionally, TiO2 nanoparticles can be surface-modified to create stable, non-aggregating formulations [34]. These characteristics make TiO2 particularly suitable for the photocatalytic degradation of organic compounds, such as Congo Red, which will be explained further.
3. Degradation of Congo Red Using TiO2-Based Photocatalysts Under Visible Light
TiO2 has proven to be an efficient photocatalyst with a wide range of applications. However, its effectiveness and suitability for large-scale use can be significantly enhanced by modifying its properties to function under visible light. This is primarily achieved by reducing its band gap, allowing for a broader absorption spectrum. Over the years, extensive research has been conducted to optimize TiO2 for visible light photocatalysis, yielding promising results, as summarized in Table 2.

Table 2.
TiO2-based materials used in the degradation of Congo Red with visible light.
The efficiency of TiO2-based photocatalysis in degrading pollutants like Congo Red depends on several factors, including the concentration of the catalyst, pH levels, the initial concentration of the dye, and reaction time. Studies have extensively characterized these variables, presenting key findings in the Supplementary Data. For example, Asaad Mahdi et al. proposed a detailed mechanism for the degradation of Congo Red using TiO2 under visible light. According to their findings, the degradation process involves the cleavage of amine groups and oxygenation of the dye, leading to the formation of new compounds. The results from LC-MS revealed two intensity peaks corresponding to hydroxy-napthalene-1-sulfonic acid, which resulted from the cleavage of the C6H6 ring, along with the bond cleavages of N‚N, and C-N. Additionally, the sustained activity of hydroxy free radicals contributed to the formation of 4-carboxy-butanoate and naphthalene, with respective molecular weights of 138 and 122. Reactive oxygen species (ROS) can cause the cleavage or reduction of sulfonic, amine, nitro, and hydroxyl groups bonded to the C6H6 ring, leading to the production of 3-carboxy propanoate, malonic acid, and malonate [35]. The authors also proposed a mechanism of action for the decolorization of Congo Red (see Figure 2).
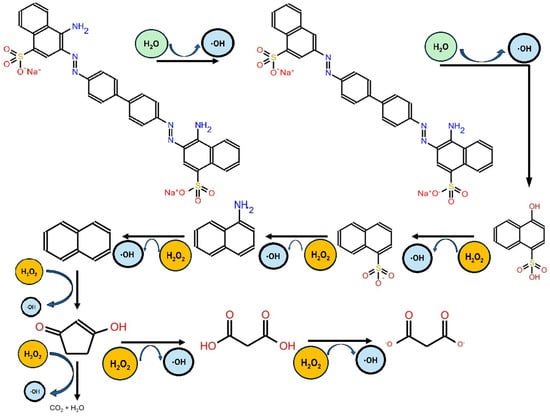
Figure 2.
Mechanism of decolorization of Congo Red via reactive oxygen species (ROS), according to Asaad Mahdi et al. [35].
3.1. Modification of TiO2 Photocatalyst Using Metals
A critical development in this field has been the doping of TiO2 with various materials to decrease its band gap and enhance its performance under visible light. Numerous studies have demonstrated that doping TiO2 with metals can significantly improve its photocatalytic efficiency, including Fe [36,37,38,46,47,55], Co [38,41], Cu [39,45,50,54], Zn [39,50,51], Cr [44], Ag [47], Au [48], Pd [48,51], and Sn [56], as well as metalloids like Sb [51]. Degradation rates as high as 99% [35,38,46,54] and even 100% [37,50] have been reported in studies using these doped catalysts (see Figure 3).
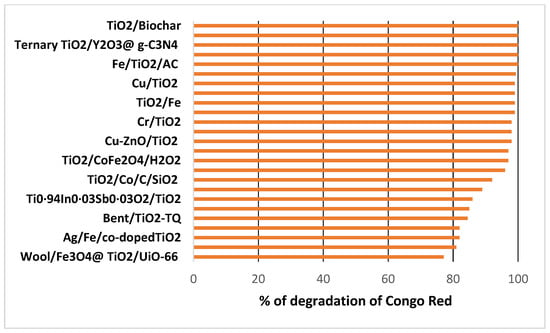
Figure 3.
Percentage of Congo Red degradation using TiO2 along with various dopants and co-dopants.
According to Zaleska [57], co-doping with metallic species can create a new energy level within the band gap of TiO2. In a typical photocatalytic reaction, the process begins with the absorption of a photon (hv1) that has energy equal to or greater than the band gap of TiO2 (approximately 3.3 eV for the anatase phase). This absorption generates an electron-hole pair on the surface of the TiO2 nanoparticle. The absorbed energy promotes an electron to the conduction band, resulting in the formation of a positive hole in the valence band. The excited electrons and holes can recombine, dissipating the input energy as heat, or they may become trapped in metastable surface states. Alternatively, they can react with electron donors and acceptors that are adsorbed on the semiconductor surface or within the surrounding electrical double layer of the charged particles. When these holes react with water, they can produce hydroxyl radicals, which possess a high redox oxidizing potential. Depending on the reaction conditions, the holes, hydroxyl radicals, superoxide anions (O2−), H2O2, and O2 can play vital roles in the photocatalytic reaction mechanism. Furthermore, when TiO2 is metal-doped, a new energy level is generated in the band gap due to the dispersion of metal nanoparticles within the TiO2 matrix. In this scenario, an electron can be excited from the defect state to the TiO2 conduction band by a photon with energy equal to hv2. An added benefit of transition metal doping is the improved trapping of electrons, which helps to inhibit electron–hole recombination during irradiation. Additionally, the reduction in charge carrier recombination leads to enhanced photoactivity [57].
3.2. Modification of TiO2 Photocatalyst Using Non-Metals
Additionally, the efficiency of TiO2 under visible light can be enhanced using other materials. For instance, graphene oxide has achieved a degradation rate of up to 99% [35], while bentonite combined with quantum dots has also shown high effectiveness [43]. Organic compounds like cellulose with biochar have reached a complete degradation of 100% [33]. In contrast, recyclable wool achieved only a 77.1% degradation rate [55] (see Figure 3). Furthermore, activated carbon as a dopant has resulted in a 100% degradation rate [37] and 98% when used as a co-dopant [40]. Notably, 100% degradation has also been attained with nano Y2O3@g-C3N4 hybrids [52]. The enhancement of efficiency in TiO2 co-doped with non-metal can be explained by three distinct mechanisms [57]: (a) band gap narrowing, (b) impurity energy level, and (c) oxygen vacancies. In the band gap narrowing mechanism, the nitrogen 2p states hybridize with oxygen 2p states in anatase TiO2 doped with nitrogen, as their energy levels are very close. This interaction narrows the band gap of N-TiO2, enabling it to absorb visible light. The impurity energy level mechanism involves the substitution of oxygen sites in TiO2 with nitrogen atoms, which create isolated impurity energy levels above the valence band. Under UV light irradiation, electrons are excited from both the valence band and the impurity energy levels; however, when illuminated with visible light, only electrons in the impurity energy level are excited. Lastly, in the oxygen vacancies mechanism, oxygen-deficient sites form at the grain boundaries, which are critical for enhancing visible light activity. The presence of nitrogen dopants in these oxygen-deficient sites helps to prevent reoxidation, further improving performance [57].
3.3. Impact of the Synthesis Process on Degradation Ability
The synthesis process also plays a crucial role in determining the efficiency of these photocatalysts. Many catalysts prepared via the hydrothermal method [3,35,37,40,52] achieved the highest degradation percentages [35,37,40,54], often comparable to or exceeding other methodologies and technologies described in Table 2. In contrast, sol–gel methods were the most frequently employed [33,38,44,46,47,49,50,54,55], yielding degradation percentages above 90%. Only one study utilized a solvothermal method and reported a high degradation percentage (98%) [39]. Most photocatalysts with lower degradation percentages were produced using alternate methods, except for a study by Tian et al., which achieved a 77.1% degradation using both solvothermal and hydrothermal techniques [55]. Rani also reported an 82% degradation rate with the same synthesis method. Additionally, Magdalane et al. achieved an 85% degradation using a microwave-assisted method [36], Sayqal et al. [43] reported an 84.5% degradation from a co-precipitation method, Musmade et al. [45] obtained a rate of 89% with a precipitation method, and Moulahi et al. achieved 86% using a precipitation method [51]. Pouthika et al. reported 81% degradation with a microwave ultrasonic-assisted method [53], while Ali et al. obtained 80% degradation with a co-precipitation method for synthesizing the photocatalyst [56]. In some instances, the photocatalytic process using TiO2 was enhanced with chemical reagents like H2O2. For example, Magdalane et al. increased the degradation percentage from 85% (using only TiO2-doped cobalt ferrite nanoparticles) to 97% when utilizing TiO2-doped cobalt ferrite nanoparticles combined with H2O2 [36].
The synthesis process significantly influences the characteristics of the materials. Factors such as catalyst concentration, pH, initial concentration of the colorant, and reaction time were primarily considered by different authors (see Table 2). The degradation methods using TiO2-based photocatalysts and visible light that achieved 100% degradation typically operated in an acidic pH range (1–5), with catalyst concentrations from 0.03–0.06% w/v, dye concentrations from 10 to 100 mg L−1, and reaction times spanning 10 to 140 min [3,33,37,50,52]. Moreover, in TiO2-doped catalysts, the anatase phase predominated, and smaller particle sizes were associated with increased degradation efficiency. The doping materials were vital in decreasing the band gap, making the photocatalysts suitable for use under visible light (see Supplementary Data, Table S1).
4. Perspectives
As summarized in Table 2, TiO2-based photocatalysts are often co-doped with other materials to enhance various features such as surface area, band gap, absorptivity, particle size, reusability, affordability, and the ability to degrade different pollutants. The sustainability and development of green processes are becoming increasingly important, leading to the production of more composites from eco-friendly and recyclable sources. In terms of synthesis methods, hydrothermal, solvothermal, and sol–gel techniques are expected to remain the predominant approaches, as they yield photocatalysts with greater degradation capabilities and are simpler to implement compared to other methods. However, while some TiO2-based photocatalysts can achieve 100% degradation or mineralization of Congo Red, further studies are needed to assess the toxicity of intermediate and final products in cases where complete degradation is not attained. Analytic techniques such as HPLC, MS, TOC, FTIR, LC, and bioassays can be effective in identifying degradation products, as indicated in Table 1.
5. Conclusions
Congo Red is an organic dye known to have detrimental effects on both the environment and human health. Various methods have been proposed for its degradation, with photocatalysis utilizing different materials being a promising approach. Titanium dioxide (TiO2) stands out as a potential photocatalyst for the degradation of this dye. Despite its many advantages—such as availability, affordability, suitability for large-scale use, high specific surface area, enhanced absorption of ultraviolet (UV) radiation, and the ability for further surface functionalization—TiO2 has some limitations. Notably, it is inert, non-toxic, and possesses a wide bandgap, which restricts its effectiveness under visible light. However, this limitation can be addressed by employing various dopants and synthesis methods to enhance its performance in visible light. Several factors, including the preparation method, irradiation time, initial concentration of both the photocatalyst and the dye, pH, and temperature can influence the degradation efficiency of TiO2. Among the synthesis methods, sol–gel is frequently used, though the hydrothermal method often achieves the highest degradation rates, sometimes approaching 100%. Doping TiO2 with different elements or compounds can provide advantages such as predominance of the anatase phase, reduced particle size (which increases efficiency), and a decrease in the band gap. It is also crucial to conduct monitoring tests for degradation products and bioassays to ensure that the degradation products do not possess equal or greater toxicity than the original compound.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15010084/s1, Table S1: Characterization of photocatalysts employed in the photodegradation of Congo Red.
Author Contributions
Conceptualization, G.E.Q.-V. and J.F.V.-C.; methodology, G.E.Q.-V. and J.F.V.-C.; formal analysis, G.E.Q.-V., J.F.V.-C., R.E.N.-S., M.M.R.-D., A.S.-S. and D.L.-M.; investigation, G.E.Q.-V. and J.F.V.-C.; data curation, J.F.V.-C.; writing—original draft preparation, G.E.Q.-V.; writing—review and editing G.E.Q.-V., J.F.V.-C., R.E.N.-S., M.M.R.-D., A.S.-S. and D.L.-M.; visualization, G.E.Q.-V., J.F.V.-C. and R.E.N.-S.; supervision, J.F.V.-C.; project administration, J.F.V.-C.; funding acquisition, G.E.Q.-V. and J.F.V.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council of Humanities Science, and Technology. (CONAHCYT), C.V.U. number: 740156.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Oyekanmi, A.A.; Ahmad, A.; Mohd Setapar, S.H.; Alshammari, M.B.; Jawaid, M.; Hanafiah, M.M.; Abdul Khalil, H.P.S.; Vaseashta, A. Sustainable Durio zibethinus-derived biosorbents for congo red removal from aqueous solution: Statistical optimization, isotherms and mechanism studies. Sustainability 2021, 13, 13264. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Allehyani, E.S.; Al-Harbi, S.A.; Hasan, Z.; Abomuti, M.A.; Rajor, H.K.; Oh, S. Investigation of Congo Red Toxicity towards Different Living Organisms: A Review. Processes 2023, 11, 807. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, K.; Sun, F.; Liu, Y.; Chen, J. Enhanced Performance of Photocatalytic Treatment of Congo Red Wastewater by CNTs-Ag Modified TiO2 Under Visible Light. Environ. Sci. Pollut. Res. 2022, 29, 15516–15525. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, W.A.A.; El-Gawad, H.H.A.; Mousa, H.A.; Handal, H.T.; Galal, H.R.; Ibrahem, I.A.; El-Beih, A.A.; Fawzy, M.M.; Ahmed, M.A.M.; Mekkey, S.D.; et al. Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater. Nanotechnol. Rev. 2024, 13, 20240001. [Google Scholar] [CrossRef]
- NIH. Congo Red. Compound Summary. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Congo-red#section=Information-Sources (accessed on 25 November 2024).
- Asses, N.; Ayed, L.; Hkiri, N.; Hamdi, M. Congo Red Decolorization and Detoxification by Aspergillus niger: Removal Mechanisms and Dye Degradation Pathway. BioMed Res. Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Mota, T.R.; Kato, C.G.; Peralta, R.A.; Bracht, A.; de Morais, G.R.; Baesso, M.L.; de Souza, C.G.M. Decolourization of Congo Red by Ganoderma lucidum Laccase: Evaluation of Degradation Products and Toxicity. Water Air Soil Pollut. 2015, 226, 1–11. [Google Scholar] [CrossRef]
- Iark, D.; dos Reis Buzzo, A.J.; Garcia, J.A.A.; Côrrea, V.G.; Helm, C.V.; Corrêa, R.C.G.; Peralta, R.A.; Moreira, R.D.F.P.M.; Bracht, A.; Peralta, R.M. Enzymatic degradation and detoxification of azo dye Congo red by a new laccase from Oudemansiella canarii. Bioresour. Technol. 2019, 289, 121655. [Google Scholar] [CrossRef] [PubMed]
- Fowsiya, J.; Madhumitha, G.; Al-Dhabi, N.A.; Arasu, M.V. Photocatalytic degradation of Congo red using Carissa edulis extract capped zinc oxide nanoparticles. J. Photochem. Photobiol. B Biol. 2016, 162, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Said, A.; Ali, F.; Raziq, F.; Ali, Z.; Bilal, M.; Reinert, L.; Begum, T.; Iqbal, H.M.N. Photocatalytic Degradation of Congo Red Dye from Aqueous Environment Using Cobalt Ferrite Nanostructures: Development, Characterization, and Photocatalytic Performance. Water Air Soil Pollut. 2020, 231, 50. [Google Scholar] [CrossRef]
- Umamaheswari, C.; Lakshmanan, A.; Nagarajan, N. Green synthesis, characterization and catalytic degradation studies of gold nanoparticles against congo red and methyl orange. J. Photochem. Photobiol. B Biol. 2018, 178, 33–39. [Google Scholar] [CrossRef]
- Laouedj, N.; Ahmed, B. ZnO-Assisted Photocatalytic Degradation of Congo Red and Benzopurpurine 4B in Aqueous Solution. J. Chem. Eng. Process Technol. 2011, 2, 1–9. [Google Scholar] [CrossRef]
- Hitkari, G.; Chowdhary, P.; Kumar, V.; Singh, S.; Motghare, A. Potential of Copper-Zinc Oxide nanocomposite for photocatalytic degradation of congo red dye. Clean. Chem. Eng. 2022, 1, 100003. [Google Scholar] [CrossRef]
- Fardood, S.T.; Moradnia, F.; Ramazani, A. Green synthesis and characterisation of ZnMn2O4 nanoparticles for photocatalytic degradation of Congo red dye and kinetic study. Micro Nano Lett. 2019, 14, 986–991. [Google Scholar] [CrossRef]
- Wang, S.; Luo, C.; Tan, F.; Cheng, X.; Ma, Q.; Wu, D.; Li, P.; Zhang, F.; Ma, J. Degradation of Congo red by UV photolysis of nitrate: Kinetics and degradation mechanism. Sep. Purif. Technol. 2021, 262, 118276. [Google Scholar] [CrossRef]
- Güy, N.; Özacar, M. The influence of noble metals on photocatalytic activity of ZnO for Congo red degradation. Int. J. Hydrogen Energy 2016, 41, 20100–20112. [Google Scholar] [CrossRef]
- Solano, A.M.S.; Garcia-Segura, S.; Martínez-Huitle, C.A.; Brillas, E. Degradation of acidic aqueous solutions of the diazo dye Congo Red by photo-assisted electrochemical processes based on Fenton’s reaction chemistry. Appl. Catal. B Environ. 2015, 168–169, 559–571. [Google Scholar] [CrossRef]
- Muneer, M.; Saeed, M.; Bhatti, I.A.; Haq, A.-U.; Khosa, M.K.; Jamal, M.A.; Ali, S. Radiation induced degradation of Congo red dye: A mechanistic study. Nukleonika 2019, 64, 49–53. [Google Scholar] [CrossRef]
- Ma, H.; Wang, M.; Yang, R.; Wang, W.; Zhao, J.; Shen, Z.; Yao, S. Radiation degradation of Congo Red in aqueous solution. Chemosphere 2007, 68, 1098–1104. [Google Scholar] [CrossRef]
- Zhang, Z.; Shan, Y.; Wang, J.; Ling, H.; Zang, S.; Gao, W.; Zhao, Z.; Zhang, H. Investigation on the rapid degradation of congo red catalyzed by activated carbon powder under microwave irradiation. J. Hazard. Mater. 2007, 147, 325–333. [Google Scholar] [CrossRef]
- Feng, S.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Cheng, D.; Varjani, S.; Lei, Z.; Liu, Y. Roles and applications of enzymes for resistant pollutants removal in wastewater treatment. Bioresour. Technol. 2021, 335, 125278. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kermanshahi-Pour, A.; Brar, S.K.; He, Q.S.; Rainey, J.K. Influence of elevated pressure and pressurized fluids on microenvironment and activity of enzymes. Biotechnol. Adv. 2023, 68, 108219. [Google Scholar] [CrossRef]
- Zin, Z.M.; Yahaya, N.; Bashah, N.; Ibrahim, K.; Rusli, N.; Smedley, K.; Mohd, K.; Zainol, M. Effect of pH extraction buffer on antioxidant enzymes activities in water lily’s leaves and petioles. Food Res. 2022, 6, 34–44. [Google Scholar] [CrossRef]
- Braham, S.A.; Siar, E.-H.; Arana-Peña, S.; Carballares, D.; Morellon-Sterling, R.; Bavandi, H.; de Andrades, D.; Kornecki, J.F.; Fernandez-Lafuente, R. Effect of concentrated salts solutions on the stability of immobilized enzymes: Influence of inactivation conditions and immobilization protocol. Molecules 2021, 26, 968. [Google Scholar] [CrossRef]
- Diasanayake, M.A.K.L.; Senadeera, G.K.R.; Sarangika, H.N.M.; Ekanayake, P.M.P.C.; Thotawattage, C.A.; Divarathne, H.K.D.W.M.N.R.; Kumari, J.M.K.W. TiO2 as a Low Cost, Multi Functional Material. Mater. Today Proc. 2016, 3 (Suppl. S1), S40–S47. [Google Scholar] [CrossRef]
- Rahim, S.; Ghamsari, M.S.; Radiman, S.; Hamzah, A. Highly stable TiO2 Sol with High Photocatalytic Properties. In The Sol-Gel Process: Uniformity, Polymers and Applications; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; pp. 773–785. [Google Scholar]
- Pan, D.; Zhang, C.; Wang, C.-S.; Zhang, P.; Jiao, X.-Y.; Ma, Q.-R.; Wang, L.-T.; Li, D.-J.; Li, L.-P. Unravelling hidden threats of water disinfection: Toxicity evaluation and toxic products identification during diclofenac degradation. Environ. Pollut. 2024, 345, 123424. [Google Scholar] [CrossRef]
- Petronella, F.; Truppi, A.; Dell’edera, M.; Agostiano, A.; Curri, M.L.; Comparelli, R. Scalable synthesis of mesoporous TiO2 for environmental photocatalytic applications. Materials 2019, 12, 1853. [Google Scholar] [CrossRef] [PubMed]
- Nurdin, M.; Widianingsih, E.; Azis, T.; Wibowo, D. Preparation of Visible Photocatalyst N-TiO2 and Its Activity on Congo Red Degradation. ARPN J. Eng. Appl. Sci. 2015, 10, 6250–6256. [Google Scholar]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Harun, N.H.; Rahman, M.A.; Kamarudin, W.W.; Irwan, Z.; Muhammud, A.; Akhir, N.E.F.M.; Yaafar, M.R. Photocatalytic Degradation of Congo Red Dye Based on Titanium Dioxide Using Solar and UV Lamp. J. Fundam. Appl. Sci. 2018, 10, 832–846. [Google Scholar] [CrossRef]
- Turcu, E.; Coromelci, C.G.; Harabagiu, V.; Ignat, M. Enhancing the Photocatalytic Activity of TiO2 for the Degradation of Congo Red Dye by Adjusting the Ultrasonication Regime Applied in Its Synthesis Procedure. Catalysts 2023, 13, 345. [Google Scholar] [CrossRef]
- Kong, L.; Tunku, U.; Rahman, A. Synthesis and Characterisations of Titanium Dioxide (TiO2)/Cellulose Biochar Composites for Photocatalytic Degradation of Congo Red. Doctoral Dissertation, Universiti Tunku Abdul Rahman, Jaya, Malaysia, 2020. [Google Scholar]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium dioxide nanoparticles: Prospects and applications in medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef]
- Mahdi, M.A.; Farhan, M.A.; Mahmoud, Z.H.; Rheima, A.M.; Abbas, Z.S.; Kadhim, M.M.; Al-Bayati, A.D.J.; Jaber, A.S.; Hachim, S.K.; Ismail, A.H. Direct sunlight photodegradation of congo red in aqueous solution by TiO2/rGO binary system: Experimental and DFT study. Arab. J. Chem. 2023, 16, 104992. [Google Scholar] [CrossRef]
- Magdalane, C.M.; Priyadharsini, G.M.A.; Kaviyarasu, K.; Jothi, A.I.; Simiyon, G.G. Synthesis and characterization of TiO2 doped cobalt ferrite nanoparticles via microwave method: Investigation of photocatalytic performance of congo red degradation dye. Surf. Interfaces 2021, 25, 101296. [Google Scholar] [CrossRef]
- Baruah, M.; Ezung, S.L.; Supong, A.; Bhomick, P.C.; Kumar, S.; Sinha, D. Synthesis, characterization of novel Fe-doped TiO2 activated carbon nanocomposite towards photocatalytic degradation of Congo red, E. coli, and S. aureus. Korean J. Chem. Eng. 2021, 38, 1277–1290. [Google Scholar] [CrossRef]
- Imran, M.; Saeed, Z.; Pervaiz, M.; Mehmood, K.; Ejaz, R.; Younas, U.; Nadeem, H.A.; Hussain, S. Enhanced visible light photocatalytic activity of TiO2 co-doped with Fe, Co, and S for degradation of Cango red. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 255, 119644. [Google Scholar] [CrossRef] [PubMed]
- Landge, V.K.; Huang, C.M.; Hakke, V.S.; Sonawane, S.H.; Manickam, S.; Hsieh, M.C. Solar-Energy-Driven Cu-ZnO/TiO2 Nanocomposite Photocatalyst for the Rapid Degradation of Congo Red Azo Dye. Catalysts 2022, 12, 605. [Google Scholar] [CrossRef]
- Aprilita, N.H.; Amalia, D.; Wahyuni, E.T. Removal of the Hazardous Congo Red Dye through Degradation under Visible Light Photocatalyzed by C,N Co-Doped TiO2 Prepared from Chicken Egg White. Sci. World J. 2022, 2022, 1–8. [Google Scholar] [CrossRef]
- Hammud, H.H.; Traboulsi, H.; Karnati, R.K.; Bakir, E.M. Photodegradation of Congo Red by Modified P25-Titanium Dioxide with Cobalt-Carbon Supported on SiO2 Matrix, DFT Studies of Chemical Reactivity. Catalysts 2022, 12, 248. [Google Scholar] [CrossRef]
- Prashanth, V.; Remya, N. Synthesis of TiO2 using Calotropis gigantea for visible light excitation and degradation of congo red dye. J. Hazard. Toxic Radioact. Waste 2021, 25, 04021026. [Google Scholar] [CrossRef]
- Sayqal, A.; Alfi, A.A.; Alatawi, N.M.; Al-Ghamdi, S.A.; Alatawi, I.S.; Alsharari, A.M.; Alessa, H.; El-Metwaly, N.M. Breakdown cost and recycling processes of Bentonite/TiO2 quantum dots of photo and solar degradation of Congo Red dye and industrial dyes wastes. Opt. Mater. 2024, 157, 116408. [Google Scholar] [CrossRef]
- Wahyuni, E.T.; Wahyuni, S.; Lestari, N.D.; Suherman, S. Utilization of tannery wastewater as a source of Cr doped into TiO2 for improving its activity under visible light in the Congo red degradation. React. Kinet. Catal. Lett. 2023, 136, 1067–1084. [Google Scholar] [CrossRef]
- Musmade, S.; Hase, D.P.; Waghmare, A.S.; Kadam, K.R.; Khedkar, J.; Gadhave, A.G.; Bhavsar, K.S.; Murade, V.D. Synthesis of shape controlled Cu2O and Cu2O/TiO2-QD composite for degradation of Congo red dye under visible-light irradiation. J. Water Environ. Nanotechnol. 2023, 8, 406–416. [Google Scholar] [CrossRef]
- Wahyuni, E.T.; Lestari, N.D.; Cinjana, I.R.; Annur, S.; Natsir, T.A.; Mudasir, M. Doping TiO2 with Fe from iron rusty waste for enhancing its activity under visible light in the Congo red dye photodegradation. J. Eng. Appl. Sci. 2023, 70, 9. [Google Scholar] [CrossRef]
- Rani, A.; Kumar, S.; Dhiman, L.; Singh, V. Photocatalytic Degradation of Congo Red and Methyl Orange Dye Under Visible Light Using Silver and Iron co-doped TiO2 Nanoparticles. Indian J. Pure Appl. Phys. 2022, 60, 325–334. [Google Scholar]
- Mousa, S.A.; Tareq, S.; Muhammed, E.A. Studying the Photodegradation of Congo Red Dye from Aqueous Solutions Using Bimetallic Au–Pd/TiO2 Photocatalyst. Baghdad Sci. J. 2021, 18, 1261–1268. [Google Scholar] [CrossRef]
- Nafis, N.; Sartika, H.; Rahmi, D.M. Photocatalytic Degradation of Congo Red with Sol-Gel Immobilized TiO2 Thin Layers on the Inner Surface of a Glass Tube Column. Chem Trendz J. 2024, 1, 1–23. [Google Scholar]
- Mohammed, A.M.; Mohtar, S.S.; Aziz, F.; Aziz, M.; Ul-Hamid, A.; Salleh, W.N.W.; Yusof, N.; Jaafar, J.; Ismail, A.F. Ultrafast degradation of Congo Red dye using a facile one-pot solvothermal synthesis of cuprous oxide/titanium dioxide and cuprous oxide/zinc oxide p-n heterojunction photocatalyst. Mater. Sci. Semicond. Process. 2021, 122, 105481. [Google Scholar] [CrossRef]
- Moulahi, A. New and effective photocatalysts for removal of mordant black 17 and Congo red pollutants: In3+ and Sb5+ doped and codoped TiO2 semiconductor. Opt. Mater. 2023, 137, 113573. [Google Scholar] [CrossRef]
- Abumousa, R.A.; Bououdina, M.; Aissa, M.A.B.; Khezami, L.; Modwi, A. Efficient photocatalytic degradation of Congo red and other dyes by ternary TiO2/Y2O3@ g-C3N4 nanohybrid. J. Mater. Sci. Mater. Electron. 2024, 35, 486. [Google Scholar] [CrossRef]
- Pouthika, K.; Madhumitha, G. Tailoring interfacial charge separation in Z-Scheme CuO@TiO2@ halloysite heterostructure for efficient photocatalytic removal of Congo red. J. Taiwan Inst. Chem. Eng. 2024, 166, 105752. [Google Scholar] [CrossRef]
- Bibi, S.; Shah, S.S.; Muhammad, F.; Siddiq, M.; Kiran, L.; Aldossari, S.A.; Mushab, M.S.S.; Sarwar, S. Cu-doped mesoporous TiO2 photocatalyst for efficient degradation of organic dye via visible light photocatalysis. Chemosphere 2023, 339, 139583. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, H.; Chen, P.; Song, Y.; Zhang, J. Magnetically Recyclable Wool/Fe3O4@TiO2/UiO-66 Core-Shell Structured Composite for Photocatalytic Removal of Methylene Blue, Congo Red, Tetracycline Hydrochloride and Cr (VI) Ions. Fibers Polym. 2022, 23, 2780–2797. [Google Scholar] [CrossRef]
- Ali, N.; Khan, A.; Riaz, A.; Asiri, A.M.; Kamal, T. Photocatalytic Performance Evaluation of Bismuth Doped Tin-Dioxide under UV and Direct Sunlight Irradiation for Congo Red Dye Degradation. J. Chem. Soc. Pak. 2020, 42, 687. [Google Scholar]
- Zaleska, A. Doped-TiO2: A Review. Recent Pat. Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).