Photocatalytic Oxidation of Pesticides with TiO2-CeO2 Thin Films Using Sunlight
Abstract
1. Introduction
2. Results and Discussion
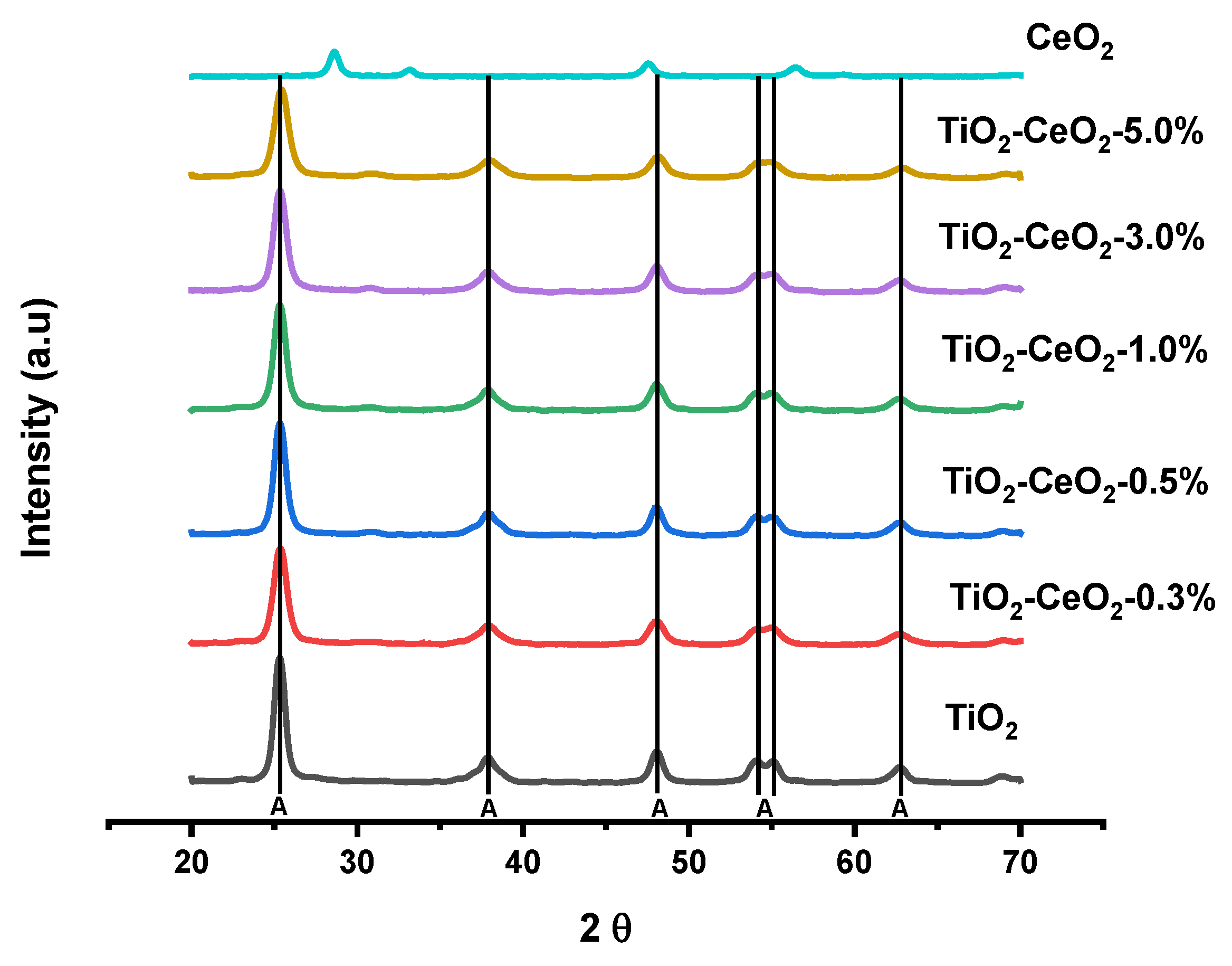
2.1. XRD
2.2. XPS
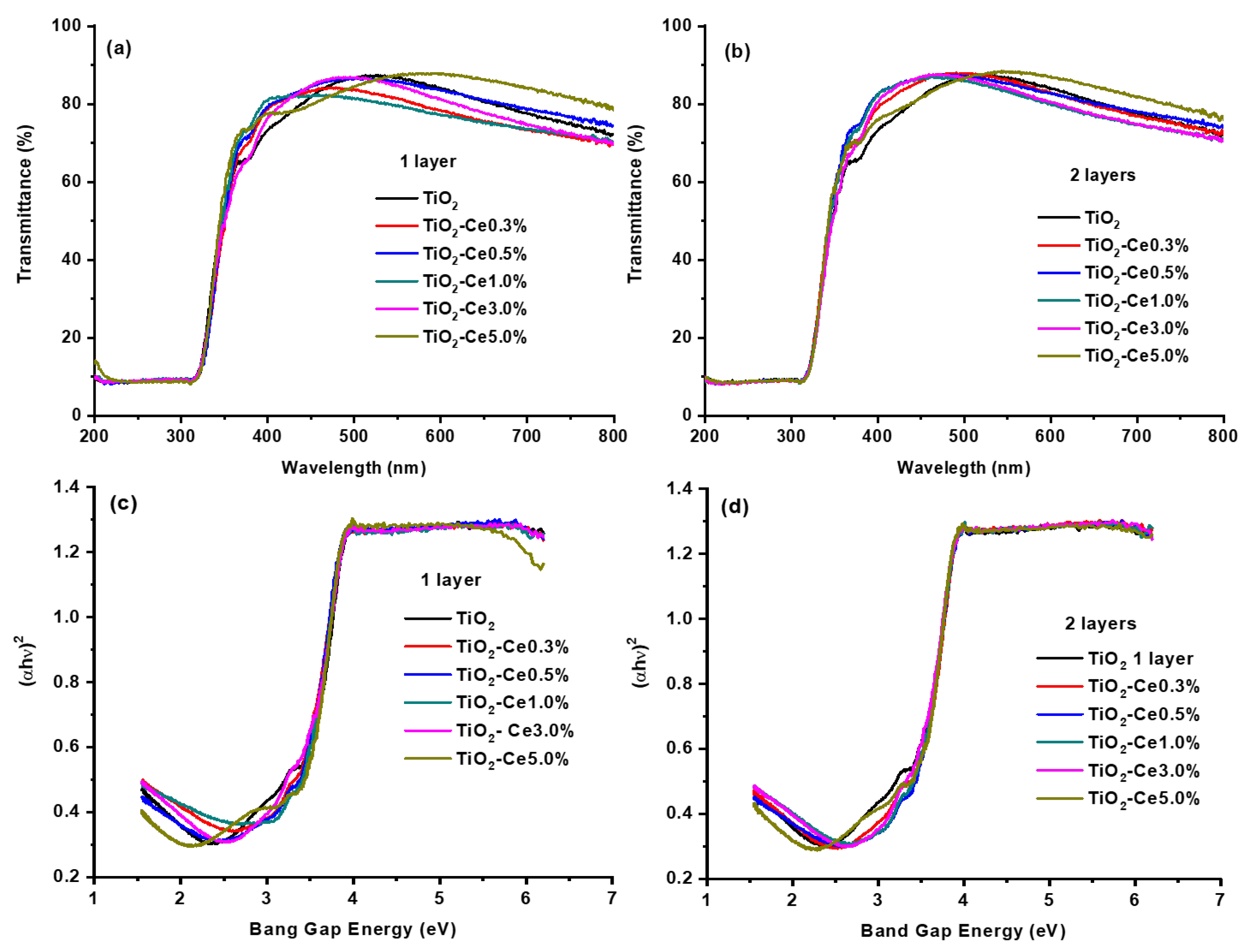
2.3. Transmittance UV-Vis Spectroscopy
2.4. UV-Vis Diffuse Reflectance Spectroscopy
| Sample | Thickness, t (nm) | Band Gap Energy, Eg (eV) [λ (nm)] * | ||
|---|---|---|---|---|
| One layer | Two Layers | One Layer | Two Layers | |
| TiO2 | 159.48 | 167.35 | 3.38 (366.86) | 3.40 (364.70) |
| TiO2-CeO2-0.3% | 136.97 | 134.39 | 3.36 (369.04) | 3.41 (363.63) |
| TiO2-CeO2-0.5% | 154.01 | 133.23 | 3.38 (366.86) | 3.43 (361.51) |
| TiO2-CeO2-1.0% | 125.66 | 125.42 | 3.39 (365.78) | 3.42 (362.57) |
| TiO2-CeO2-3.0% | 147.48 | 133.14 | 3.40 (364.71) | 3.37 (367.95) |
| TiO2-CeO2-5.0% | 230.03 | 182.18 | 3.37 (367.95) | 3.35 (370.15) |
2.5. Profilometry and AFM
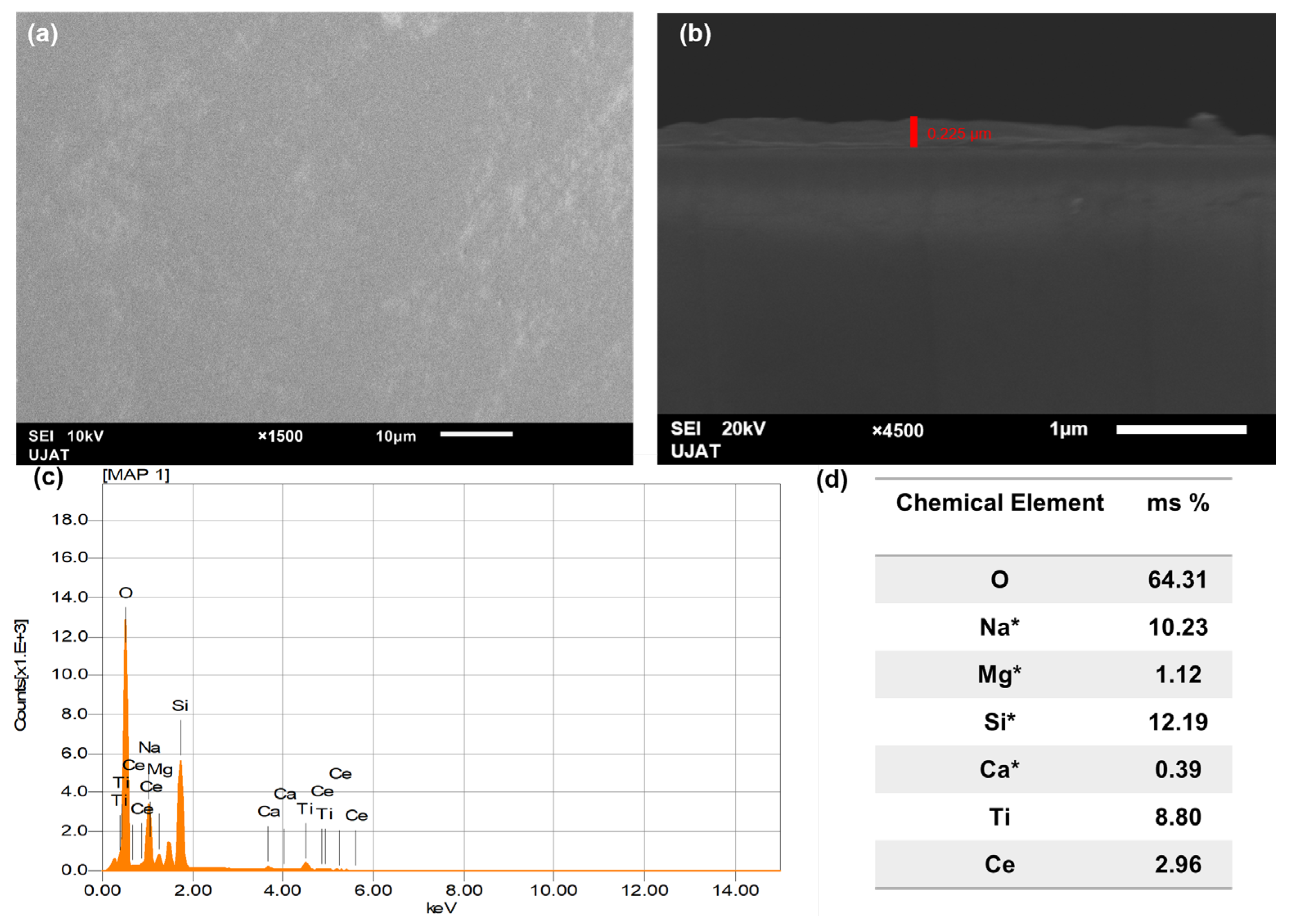
2.6. SEM
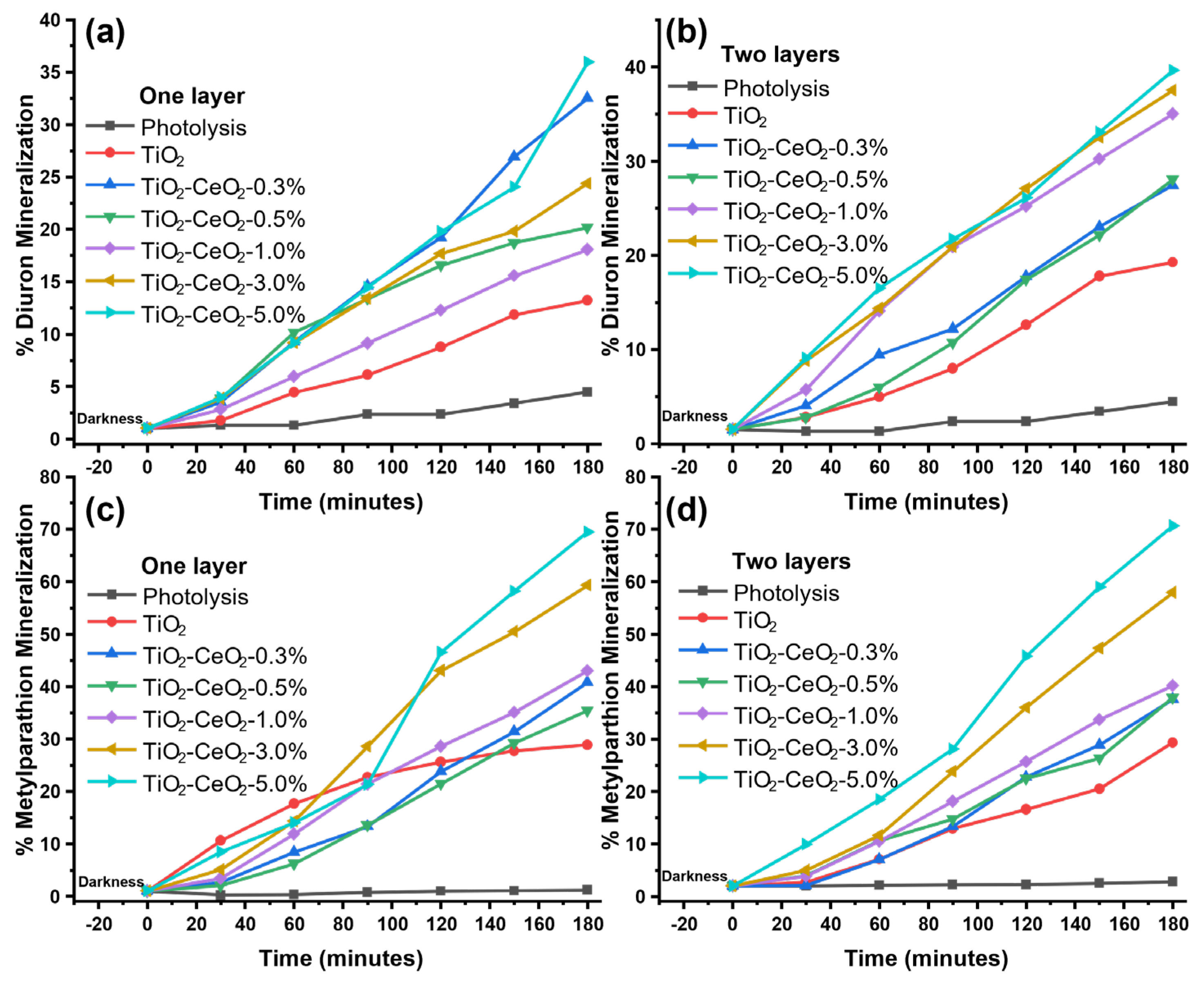
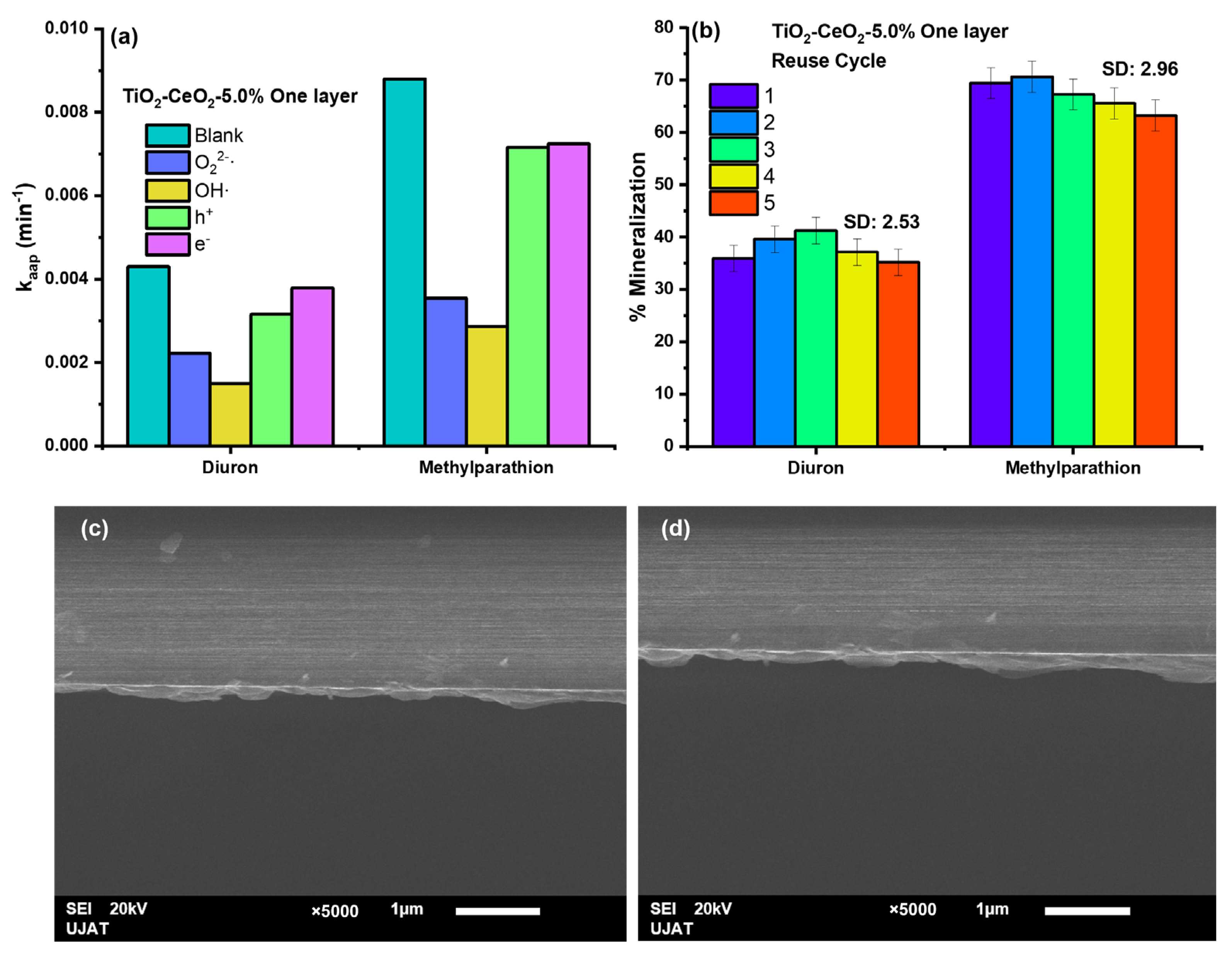
2.7. Photocatalytic Test
3. Materials and Methods
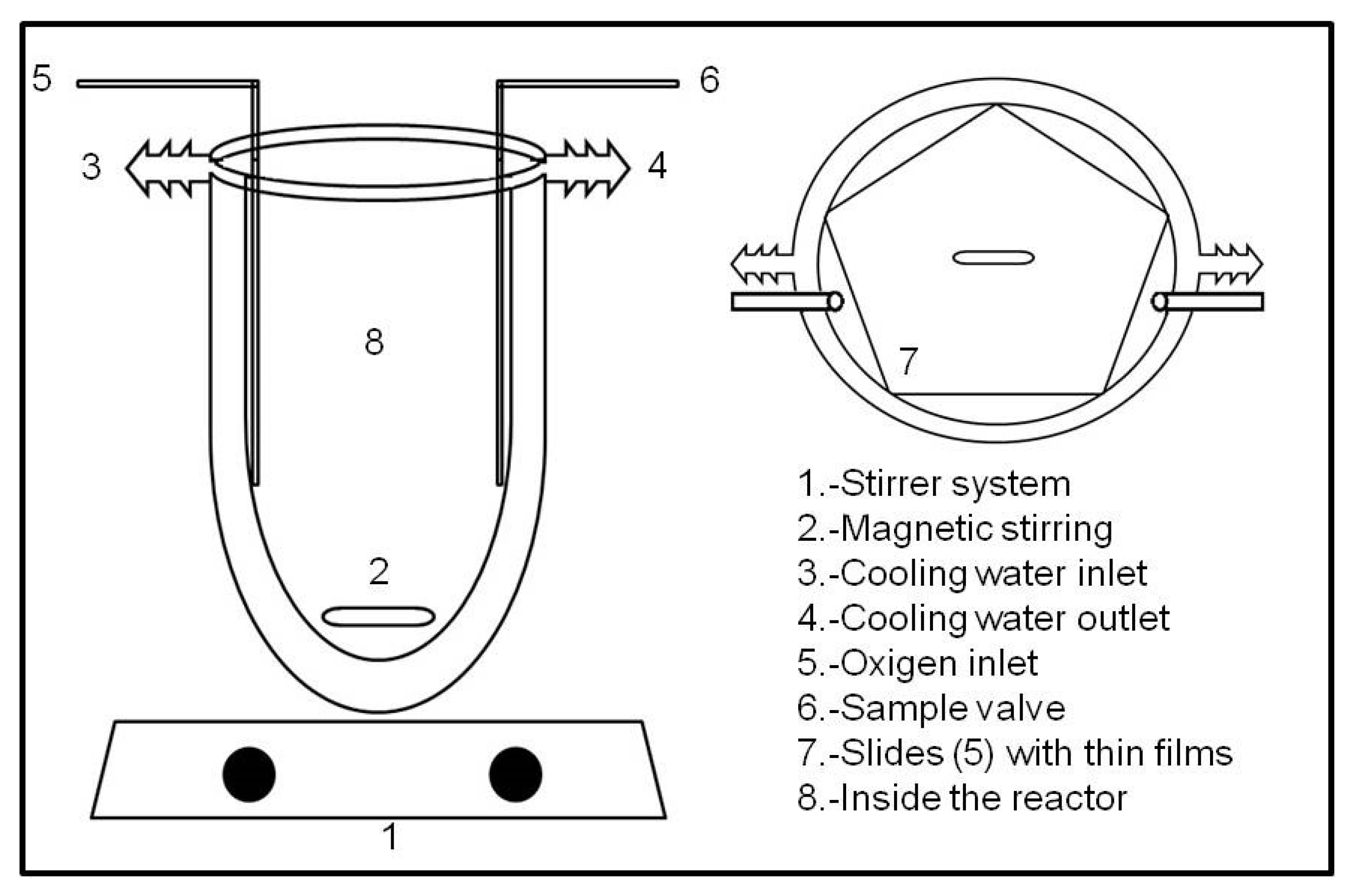
3.1. Thin Films Preparations
3.2. Sample Characterization
3.3. Photocatalytic Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced Reactivity of Titanium Dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X. Titanium Dioxide Photocatalysis: Present Situation and Future Approaches. Comptes Rendus Chim. 2006, 9, 750–760. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’shea, K.E.; Byrne, J.A.; Dionysiou, D.D. New Insights into the Mechanism of Visible Light Photocatalysis. J. Phys. Chem. Lett. 2014, 5, 2543–2554. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P.; Soutar, A.; Cherrie, J.W.; Granath, F.; Andersen, A.; Anttila, A.; Blettner, M.; Gaborieau, V.; Klug, S.J.; Langard, S.; et al. Mortality among Workers Employed in the Titanium Dioxide Production Industry in Europe. Cancer Causes Control 2004, 15, 697–706. [Google Scholar] [CrossRef]
- Karthikeyan Thirunavukkarasu, G.; Bacova, J.; Monfort, O.; Dworniczek, E.; Paluch, E.; Bilal Hanif, M.; Rauf, S.; Motlochova, M.; Capek, J.; Hensel, K.; et al. Critical Comparison of Aerogel TiO2 and P25 Nanopowders: Cytotoxic Properties, Photocatalytic Activity and Photoinduced Antimicrobial/Antibiofilm Performance. Appl. Surf. Sci. 2022, 579, 152145. [Google Scholar] [CrossRef]
- Dell’Edera, M.; Lo Porto, C.; De Pasquale, I.; Petronella, F.; Curri, M.L.; Agostiano, A.; Comparelli, R. Photocatalytic TiO2-Based Coatings for Environmental Applications. Catalisys Today 2021, 380, 62–83. [Google Scholar] [CrossRef]
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Gas Sensors Based on TiO2 Nanostructured Materials for the Detection of Hazardous Gases: A Review. Nano Mater. Sci. 2021, 3, 390–403. [Google Scholar] [CrossRef]
- Mahmoudi-Qashqay, S.; Zamani-Meymian, M.R.; Sadati, S.J. Improving Antibacterial Ability of Ti-Cu Thin Films with Co-Sputtering Method. Sci. Rep. 2023, 13, 16593. [Google Scholar] [CrossRef]
- Mechiakh, R.; Sedrine, N.B.; Chtourou, R.; Bensaha, R. Correlation between Microstructure and Optical Properties of Nano-Crystalline TiO2 Thin Films Prepared by Sol-Gel Dip Coating. Appl. Surf. Sci. 2010, 257, 670–676. [Google Scholar] [CrossRef]
- Lukong, V.T.; Ukoba, K.; Jen, T.C. Review of Self-Cleaning TiO2 Thin Films Deposited with Spin Coating. Int. J. Adv. Manuf. Technol. 2022, 122, 3525–3546. [Google Scholar] [CrossRef]
- Kumar Patnaik, R.; Divya, N. A Brief Review on the Synthesis of TiO2 Thin Films and Its Application in Dye Degradation. Mater. Today Proc. 2023, 72, 2749–2756. [Google Scholar] [CrossRef]
- Pérez, J.A.B.; Courel, M.; Pal, M.; Delgado, F.P.; Mathews, N.R. Effect of Ytterbium Doping Concentration on Structural, Optical and Photocatalytic Properties of TiO2 Thin Films. Ceram. Int. 2017, 43, 15777–15784. [Google Scholar] [CrossRef]
- Widyastuti, E.; Chiu, C.T.; Hsu, J.L.; Chieh Lee, Y. Photocatalytic Antimicrobial and Photostability Studies of TiO2/ZnO Thin Films. Arab. J. Chem. 2023, 16, 105010. [Google Scholar] [CrossRef]
- Momeni, M.; Saghafian, H.; Golestani-Fard, F.; Barati, N.; Khanahmadi, A. Effect of SiO2 Addition on Photocatalytic Activity, Water Contact Angle and Mechanical Stability of Visible Light Activated TiO2 Thin Films Applied on Stainless Steel by a Sol Gel Method. Appl. Surf. Sci. 2017, 392, 80–87. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, X.; Zhang, N.; Zhao, Q.; He, X.; Feng, J. Photocatalytic Mechanism of TiO2-CeO2 Films Prepared by Magnetron Sputtering under UV and Visible Light. Surf. Sci. 2005, 595, 203–211. [Google Scholar] [CrossRef]
- Guergova, D.; Stoyanova, E.; Stoychev, D.; Avramova, I.; Stefanov, P. Self-Healing Effect of Ceria Electrodeposited Thin Films on Stainless Steel in Aggressive 0.5 Mol/L NaCl Aqueous Solution. J. Rare Earths 2015, 33, 1212–1227. [Google Scholar] [CrossRef]
- Kumar, V.; Chen, W.F.; Zhang, X.; Jiang, Y.; Koshy, P.; Sorrell, C.C. Properties and Performance of Photocatalytic CeO2, TiO2, and CeO2–TiO2 Layered Thin Films. Ceram. Int. 2019, 45, 22085–22094. [Google Scholar] [CrossRef]
- Kumari, V.; Sharma, A.; Kumar, N.; Sillanpää, M.; Makgwane, P.R.; Ahmaruzzaman, M.; Hosseini-Bandegharaei, A.; Rani, M.; Chinnamuthu, P. TiO2-CeO2 Assisted Heterostructures for Photocatalytic Mitigation of Environmental Pollutants: A Compre-hensive Study on Band Gap Engineering and Mechanistic Aspects. Inorg. Chem. Commun. 2023, 151, 110564. [Google Scholar] [CrossRef]
- Huong, H.T.; Nhu, T.T.Q.; Nang, H.X.; Tuan, P.A.; Huy, P.T. Super-Hydrophilic Ce3+-Doped TiO2-SiO2 Nanocomposite Thin Films with High Optical Transmittance by a Simple Spin-Coating Method. Thin Solid Film. 2023, 768, 139730. [Google Scholar] [CrossRef]
- Procópio, A.M.S.; do Carmo Lima Carvalho, J.; Silveira, T.H.R.; Valério, A.L.; Vaz, I.C.F.; Cabral, A.C.T.; de Freitas, M.R.; da Silva, M.C.; Filho, F.M. CeO2 Thin Film Supported on TiO2 Porous Ceramics. Mater. Lett. 2020, 276, 128224. [Google Scholar] [CrossRef]
- Contreras-García, M.E.; García-Benjume, M.L.; Macías-Andrés, V.I.; Barajas-Ledesma, E.; Medina-Flores, A.; Espitia-Cabrera, M.I. Synergic Effect of the TiO2-CeO2 Nanoconjugate System on the Band-Gap for Visible Light Photocatalysis. Mater. Sci. Eng. B 2014, 183, 78–85. [Google Scholar] [CrossRef]
- Galindo, F.; Gómez, R.; Aguilar, M. Photodegradation of the Herbicide 2,4-Dichlorophenoxyacetic Acid on Nanocrystalline TiO2-CeO2 Sol-Gel Catalysts. J. Mol. Catal. A Chem. 2008, 281, 119–125. [Google Scholar] [CrossRef]
- Jiang, Y.; Jin, Z.; Chen, C.; Duan, W.; Liu, B.; Chen, X.; Yang, F.; Guo, J. Cerium-Doped Mesoporous-Assembled SiO2/P25 Nanocomposites with Innovative Visible-Light Sensitivity for the Photocatalytic Degradation of Organic Dyes. RSC Adv. 2017, 7, 12856–12870. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Mansingh, S.; Babu, P.; Parida, K. Black Titania an Emerging Photocatalyst: Review Highlighting the Synthesis Techniques and Photocatalytic Activity for Hydrogen Generation. Nanoscale Adv. 2021, 3, 5487–5524. [Google Scholar] [CrossRef]
- Moongraksathum, B.; Chen, Y.W. CeO2–TiO2 Mixed Oxide Thin Films with Enhanced Photocatalytic Degradation of Organic Pollutants. J. Sol-Gel Sci. Technol. 2017, 82, 772–782. [Google Scholar] [CrossRef]
- Reguero-Márquez, G.A.; Lunagómez-Rocha, M.A.; Cervantes-Uribe, A.; del Angel, G.; Rangel, I.; Torres-Torres, J.G.; González, F.; Godavarthi, S.; Arevalo-Perez, J.C.; Espinosa de los Monteros, A.E.; et al. Photodegradation of 2,4-D (Dichlorophenoxyacetic Acid) with Rh/TiO2; Comparative Study with Other Noble Metals (Ru, Pt, and Au). RSC Adv. 2022, 12, 25711–25721. [Google Scholar] [CrossRef]
- Liu, G.; Mamat, M.; Baikeli, Y.; Dong, X. Photocatalytic Degradation of Methylene Blue by TiO2/Nd2O3 Composite Thin Films. Heliyon 2024, 10, e29894. [Google Scholar] [CrossRef]
- Li, Z.; Sheng, J.; Zhang, Y.; Li, X.; Xu, Y. Role of CeO2 as Oxygen Promoter in the Accelerated Photocatalytic Degradation of Phenol over Rutile TiO2. Appl. Catal. B 2015, 166–167, 313–319. [Google Scholar] [CrossRef]
- Moongraksathum, B.; Chen, Y.W. Anatase TiO2 Co-Doped with Silver and Ceria for Antibacterial Application. Catal. Today 2018, 310, 68–74. [Google Scholar] [CrossRef]
- Li, F.B.; Li, X.Z.; Hou, M.F.; Cheah, K.W.; Choy, W.C.H. Enhanced Photocatalytic Activity of Ce3+-TiO2 for 2-Mercaptobenzothiazole Degradation in Aqueous Suspension for Odour Control. Appl. Catal. A Gen. 2005, 285, 181–189. [Google Scholar] [CrossRef]
- Xiu, Z.; Xing, Z.; Li, Z.; Wu, X.; Yan, X.; Hu, M.; Cao, Y.; Yang, S.; Zhou, W. Ti3+-TiO2/Ce3+-CeO2 Nanosheet Heterojunctions as Efficient Visible-Light-Driven Photocatalysts. Mater. Res. Bull. 2018, 100, 191–197. [Google Scholar] [CrossRef]
- Manifacier, J.C.; Gasiot, J.; Fillard, J.P. A Simple Method for the determination of the optical Constants n, k and the Thickness of a Weakly Thin Film. J. Phys. E Sci. Instrum. 1976, 9, 1002–1004. [Google Scholar] [CrossRef]
- Štengl, V.; Bakardjieva, S.; Murafa, N. Preparation and Photocatalytic Activity of Rare Earth Doped TiO2 Nanoparticles. Mater. Chem. Phys. 2009, 114, 217–226. [Google Scholar] [CrossRef]
- Kayani, Z.N.; Maria; Riaz, S.; Naseem, S. Magnetic and Antibacterial Studies of Sol-Gel Dip Coated Ce Doped TiO2 Thin Films: Influence of Ce Contents. Ceram. Int. 2020, 46, 381–390. [Google Scholar] [CrossRef]
- Sta, I.; Jlassi, M.; Hajji, M.; Boujmil, M.F.; Jerbi, R.; Kandyla, M.; Kompitsas, M.; Ezzaouia, H. Structural and Optical Properties of TiO2 Thin Films Prepared by Spin Coating. J. Solgel Sci. Technol. 2014, 72, 421–427. [Google Scholar] [CrossRef]
- Arévalo-Pérez, J.C.; de la Cruz-Romero, D.; Cordero-García, A.; Lobato-García, C.E.; Aguilar-Elguezabal, A.; Torres-Torres, J.G. Photodegradation of 17 α-Methyltestosterone Using TiO2 -Gd3+ and TiO2-Sm3+ Photocatalysts and Simulated Solar Radiation as an Activation Source. Chemosphere 2020, 249, 126497. [Google Scholar] [CrossRef]
- Vo, T.L.N.; Dao, T.T.; Duong, A.T.; Bui, V.H.; Nguyen, V.H.; Nguyen, D.L.; Nguyen, D.C.; Nguyen, T.H.; Nguyen, H.T. Enhanced Photocatalytic Degradation of Organic Dyes Using Ce–Doped TiO2 Thin Films. J. Sol-Gel Sci. Technol. 2023, 108, 423–434. [Google Scholar] [CrossRef]
- Chen, N.; Xia, J.; Li, L.; Lv, Q.; Zhao, K.; Ahmad, M.; Xiao, Z.; Wang, S.; Ye, F.; Zhang, Q. Comprehensive Enhancement of Photocatalytic H2O2 Generation and Antibacterial Efficacy on Carbon Nitride through a Straightforward Polydopamine Coating Strategy. Surf. Interfaces 2025, 56, 105566. [Google Scholar] [CrossRef]
- Haider, A.J.; Al-Anbari, R.H.; Kadhim, G.R.; Salame, C.T. Exploring Potential Environmental Applications of TiO2 Nanoparticles. Energy Procedia 2017, 119, 332–345. [Google Scholar] [CrossRef]
- Chung, L.; Chen, W.F.; Koshy, P.; Sorrell, C.C. Effect of Ce-Doping on the Photocatalytic Performance of TiO2 Thin Films. Mater. Chem. Phys. 2017, 197, 236–239. [Google Scholar] [CrossRef]
- Sani, S.F.A.; Othman, M.H.U.; Alqahtani, A.; Almugren, K.S.; Alkallas, F.H.; Bradley, D.A. Low-Cost Commercial Borosilicate Glass Slides for Passive Radiation Dosimetry. PLoS ONE 2020, 15, e0241550. [Google Scholar] [CrossRef]
- Ameen, S.; Shaheer Akhtar, M.; Seo, H.K.; Shin, H.S. Solution-Processed CeO2/TiO2 Nanocomposite as Potent Visible Light Photocatalyst for the Degradation of Bromophenol Dye. Chem. Eng. J. 2014, 247, 193–198. [Google Scholar] [CrossRef]
- Dundar, I.; Mere, A.; Mikli, V.; Krunks, M.; Acik, I.O. Thickness Effect on Photocatalytic Activity of TiO2 Thin Films Fabricated by Ultrasonic Spray Pyrolysis. Catalysts 2020, 10, 1058. [Google Scholar] [CrossRef]
- Li, M.; Zhang, S.; Lv, L.; Wang, M.; Zhang, W.; Pan, B. A Thermally Stable Mesoporous ZrO2-CeO2-TiO2 Visible Light Photocatalyst. Chem. Eng. J. 2013, 229, 118–125. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, W.S.; Jo, D.H.; Kim, J.S.; Park, J.M. Effect of Ce Conversion Underlayer Coating on the Photo-Catalytic Activity of TiO2 Sol-Gel Film Deposited on Hot-Dip GI. J. Ind. Eng. Chem. 2014, 20, 1965–1972. [Google Scholar] [CrossRef]
- Monteros, A.E.D.L.; Lafaye, G.; Cervantes, A.; Del Angel, G.; Barbier, J.; Torres, G. Catalytic Wet Air Oxidation of Phenol over Metal Catalyst (Ru,Pt) Supported on TiO2-CeO2 Oxides. Catal. Today 2015, 258, 564–569. [Google Scholar] [CrossRef]
- Lalliansanga; Tiwari, D.; Lee, S.M.; Kim, D.J. Photocatalytic Degradation of Amoxicillin and Tetracycline by Template Synthesized Nano-Structured Ce3+@TiO2 Thin Film Catalyst. Environ. Res. 2022, 210, 112914. [Google Scholar] [CrossRef]



| Sample | Crystallite Size, D (nm) | Strain, Ɛ |
|---|---|---|
| TiO2 | 10.66 | 0.01549 |
| TiO2-CeO2-0.3% | 9.70 | 0.01702 |
| TiO2-CeO2-0.5% | 9.64 | 0.01713 |
| TiO2-CeO2-1.0% | 9.55 | 0.01728 |
| TiO2-CeO2-3.0% | 9.08 | 0.01818 |
| TiO2-CeO2-5.0% | 8.14 | 0.02027 |
| Sample | Ti 2p | O 1s | Ce 3d | ||||
|---|---|---|---|---|---|---|---|
| Ti4+ | Ti3+ | O2− | OH | H2O | Ce4+ | Ce3+ | |
| TiO2 | 458.12 | 456.49 | 529.37 | 531.60 | 533.49 | - | - |
| 97.1 | 2.9 | 67.3 | 26 | 6.7 | - | - | |
| TiO2-CeO2-5.0% | 458.20 | 456.49 | 529.45 | 531.50 | 533.39 | 882, 888 | 880, 884 |
| 95.8 | 4.2 | 73.1 | 23.7 | 3.3 | 49.20, 51.60 | 50.90, 48.40 | |
| One Layer | kaap (min−1) | T1/2 (min) | TOC (%) | Two Layers | kaap (min−1) | T1/2 (min) | TOC (%) |
|---|---|---|---|---|---|---|---|
| Photolysis D MP | 0.0004 | 1732 | 4.45 | Photolysis D MP | 0.0004 | 1732 | 4.45 |
| 0.0005 | 1386 | 1.22 | 0.0005 | 1386 | 1.22 | ||
| TiO2 D MP | 0.0024 | 289 | 13.20 | TiO2 D MP | 0.0023 | 301 | 19.25 |
| 0.0021 | 330 | 28.88 | 0.0051 | 136 | 29.32 | ||
| TiO2-CeO2 0.3% D MP | 0.0043 | 161 | 32.52 | TiO2-CeO2 0.3% D MP | 0.0012 | 578 | 27.41 |
| 0.0075 | 92 | 41.83 | 0.0059 | 117 | 37.57 | ||
| TiO2-CeO2 0.5%D MP | 0.0031 | 224 | 20.18 | TiO2-CeO2 0.5% D MP | 0.0011 | 630 | 28.06 |
| 0.0047 | 147 | 35.40 | 0.0050 | 139 | 37.91 | ||
| TiO2-CeO2 1.0%D MP | 0.0027 | 257 | 18.08 | TiO2-CeO2 1.0% D MP | 0.0036 | 193 | 34.99 |
| 0.0082 | 85 | 42.99 | 0.0049 | 141 | 40.17 | ||
| TiO2-CeO2 3.0%D MP | 0.0037 | 187 | 24.38 | TiO2-CeO2 3.0% D MP | 0.0032 | 217 | 37.57 |
| 0.0025 | 277 | 59.30 | 0.0083 | 84 | 57.95 | ||
| TiO2-CeO2 5.0% D MP | 0.0044 | 158 | 35.93 | TiO2-CeO2 5.0% D MP | 0.0050 | 138 | 38.55 |
| 0.0087 | 80 | 69.46 | 0.0088 | 79 | 70.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, T.A.T.; Gerónimo, E.S.; Torres, J.G.T.; del Angel Montes, G.A.; Vázquez, I.R.; García, A.C.; Uribe, A.C.; Pavon, A.A.S.; Pérez, J.C.A. Photocatalytic Oxidation of Pesticides with TiO2-CeO2 Thin Films Using Sunlight. Catalysts 2025, 15, 46. https://doi.org/10.3390/catal15010046
Pérez TAT, Gerónimo ES, Torres JGT, del Angel Montes GA, Vázquez IR, García AC, Uribe AC, Pavon AAS, Pérez JCA. Photocatalytic Oxidation of Pesticides with TiO2-CeO2 Thin Films Using Sunlight. Catalysts. 2025; 15(1):46. https://doi.org/10.3390/catal15010046
Chicago/Turabian StylePérez, Tania Arelly Tinoco, Evaristo Salaya Gerónimo, José Gilberto Torres Torres, Gloria Alicia del Angel Montes, Israel Rangel Vázquez, Adrian Cordero García, Adrian Cervantes Uribe, Adib Abiu Silahua Pavon, and Juan Carlos Arevalo Pérez. 2025. "Photocatalytic Oxidation of Pesticides with TiO2-CeO2 Thin Films Using Sunlight" Catalysts 15, no. 1: 46. https://doi.org/10.3390/catal15010046
APA StylePérez, T. A. T., Gerónimo, E. S., Torres, J. G. T., del Angel Montes, G. A., Vázquez, I. R., García, A. C., Uribe, A. C., Pavon, A. A. S., & Pérez, J. C. A. (2025). Photocatalytic Oxidation of Pesticides with TiO2-CeO2 Thin Films Using Sunlight. Catalysts, 15(1), 46. https://doi.org/10.3390/catal15010046






