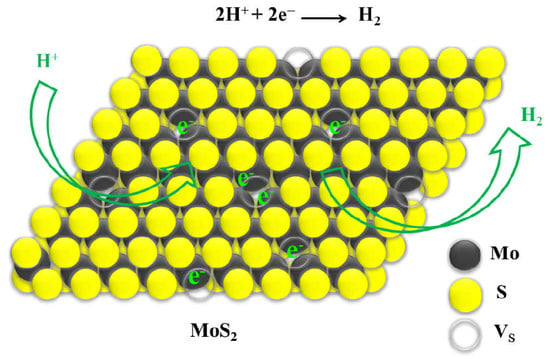
Efficient Hydrogen Evolution Reaction in 2H-MoS2 Basal Planes Enhanced by Surface Electron Accumulation
Abstract
1. Introduction
2. Results and Discussion
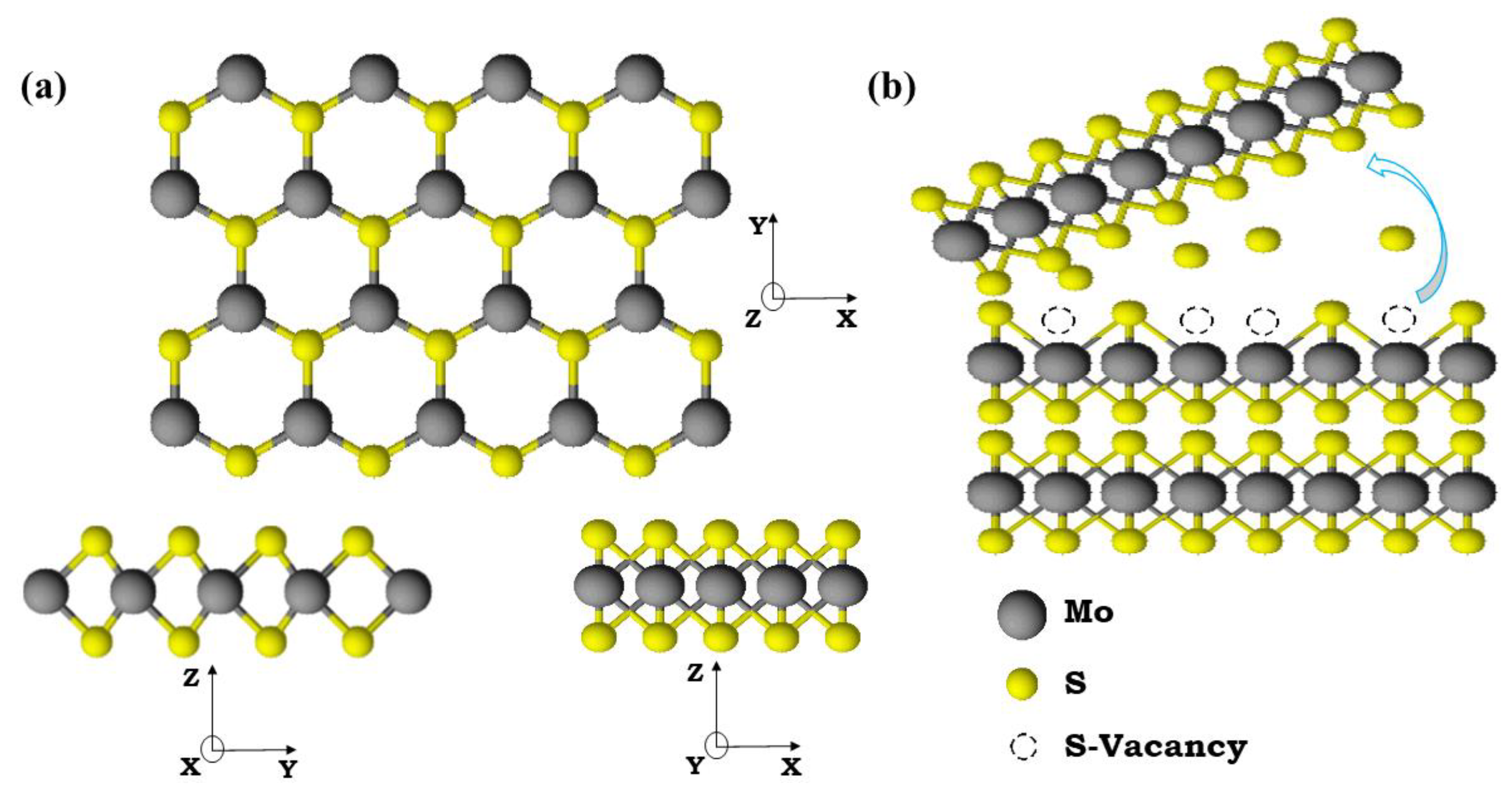
2.1. Structural Characterization
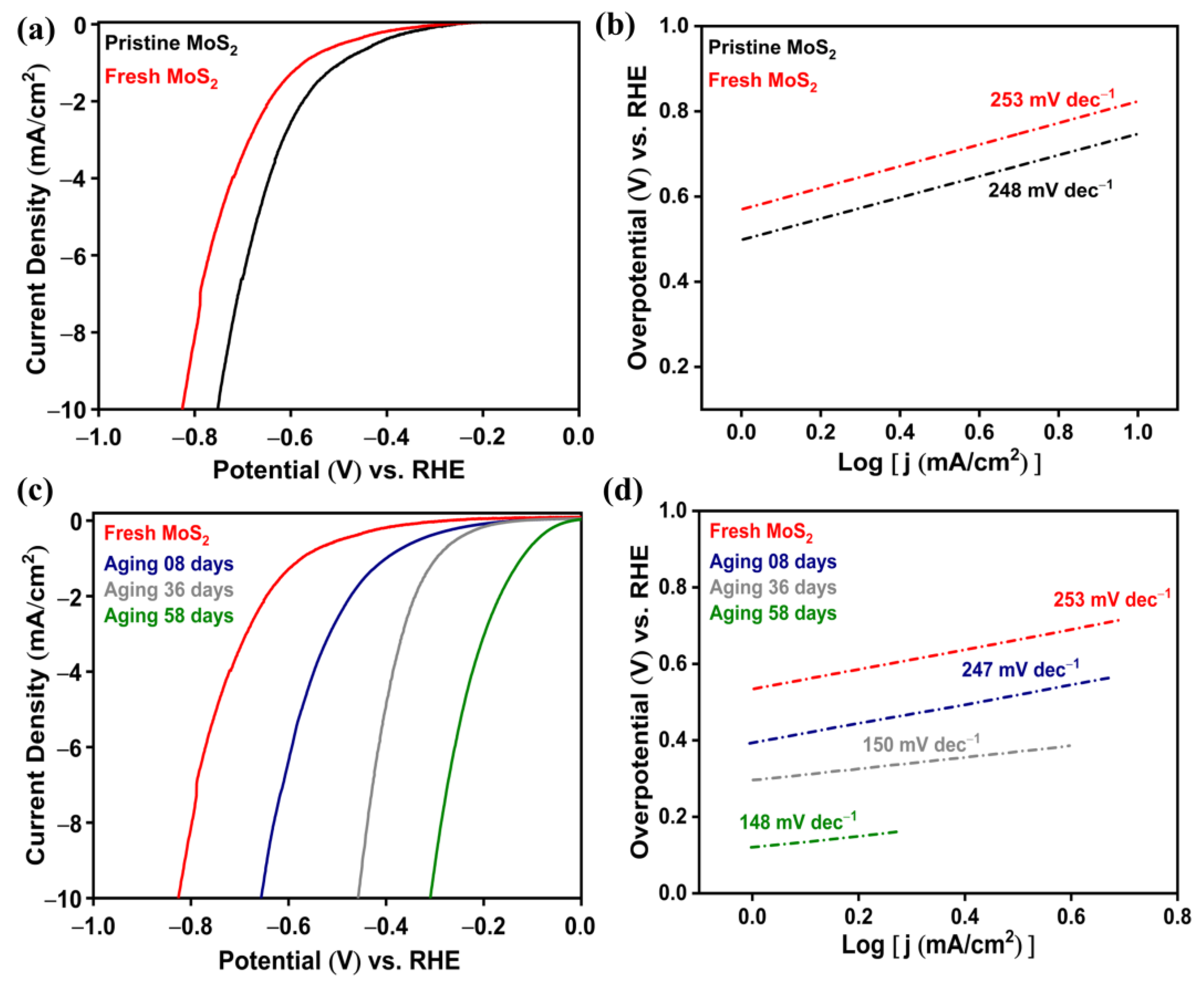
2.2. Electrochemical HER Efficiency Enhanced by SEA
2.2.1. SEA through Aging
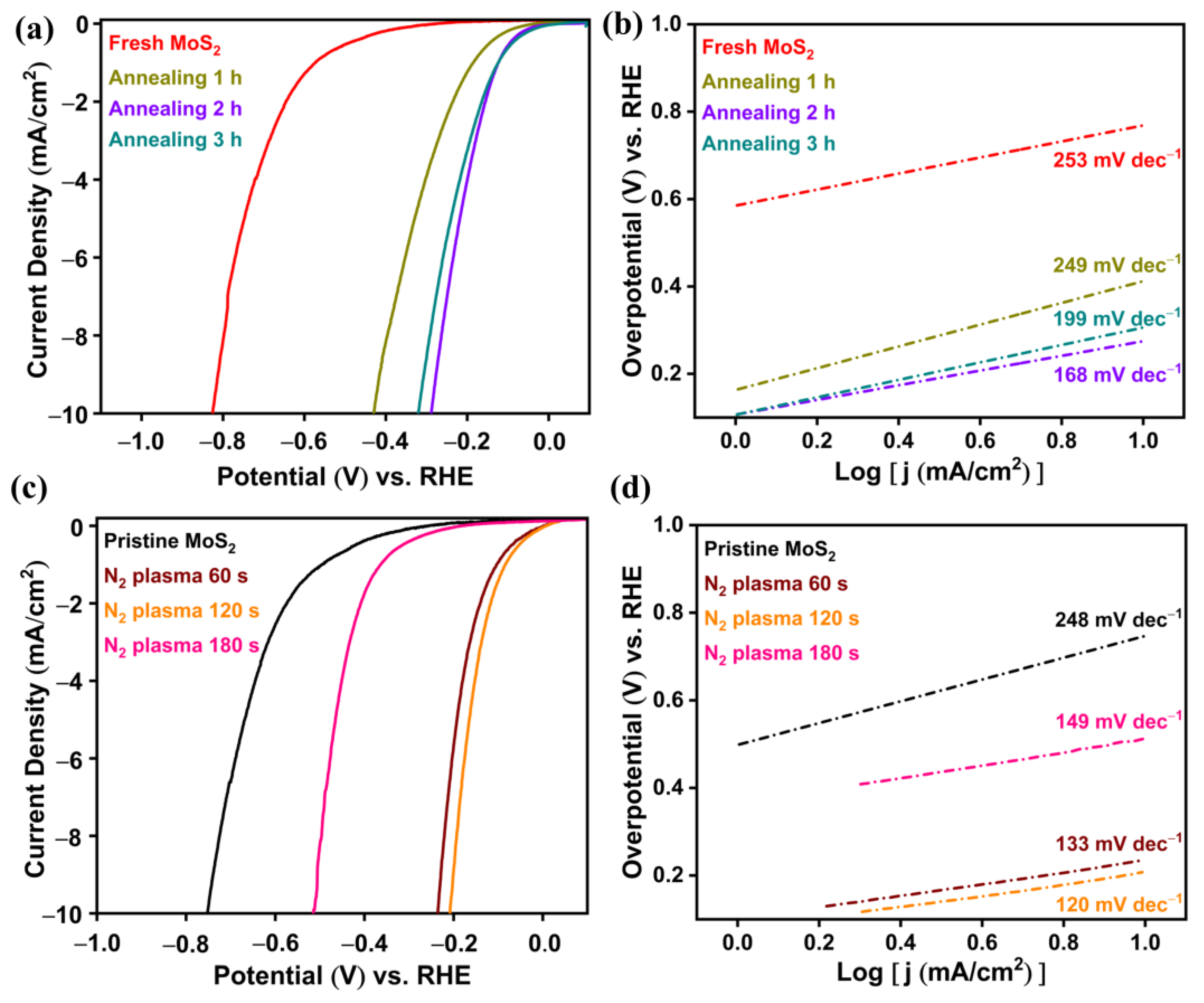
2.2.2. SEA through Annealing
2.2.3. SEA through N2-Plasma Treatment
2.3. Evidence of Enhanced SEA Observed by ARPES
2.4. Discussion
3. Experimental Section
3.1. Preparation of MoS2 Layer Crystals
3.2. Characterization of MoS2 Bulks
3.3. N2-Plasma Treatment of Pristine MoS2
3.4. Electrochemical Measurements
3.5. Angle-Resolved Photoelectron Spectroscopy (ARPES) Measurement
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, Y.; Zhou, Y.; Yang, D.R.; Xu, W.X.; Wang, C.; Wang, F.B.; Xu, J.J.; Xia, X.H.; Chen, H.Y. Energy Level Engineering of MoS2 by Transition-Metal Doping for Accelerating Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2017, 139, 15479–15485. [Google Scholar] [CrossRef]
- Wang, J.; Yan, M.; Zhao, K.; Liao, X.; Wang, P.; Pan, X.; Yang, W.; Mai, L. Field Effect Enhanced Hydrogen Evolution Reaction of MoS2 Nanosheets. Adv. Mater. 2017, 29, 1604464. [Google Scholar] [CrossRef]
- Tachibana, Y.; Vayssieres, L.; Durrant, J.R. Artificial Photosynthesis for Solar Water-Splitting. Nat. Photonics 2012, 6, 511–518. [Google Scholar] [CrossRef]
- Ouyang, Y.; Ling, C.; Chen, Q.; Wang, Z.; Shi, L.; Wang, J. Activating Inert Basal Planes of MoS2 for Hydrogen Evolution Reaction through the Formation of Different Intrinsic Defects. Chem. Mater. 2016, 28, 4390–4396. [Google Scholar] [CrossRef]
- Ding, Q.; Song, B.; Xu, P.; Jin, S. Efficient Electrocatalytic and Photoelectrochemical Hydrogen Generation Using MoS2 and Related Compounds. Chem 2016, 1, 699–726. [Google Scholar] [CrossRef]
- Prabhu, P.; Jose, V.; Lee, J.M. Design Strategies for Development of TMD-Based Heterostructures in Electrochemical Energy Systems. Matter 2020, 2, 526–553. [Google Scholar] [CrossRef]
- Ambrosi, A.; Sofer, Z.; Pumera, M. 2H → 1T Phase Transition and Hydrogen Evolution Activity of MoS2, MoSe2, WS2 and WSe2 Strongly Depends on the MX2 Composition. Chem. Commun. 2015, 51, 8450–8453. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Gao, D.; Ding, J.; Chao, D.; Wang, J. TMD-Based Highly Efficient Electrocatalysts Developed by Combined Computational and Experimental Approaches. Chem. Soc. Rev. 2018, 47, 4332–4356. [Google Scholar] [CrossRef] [PubMed]
- Voiry, D.; Salehi, M.; Silva, R.; Fujita, T.; Chen, M.; Asefa, T.; Shenoy, V.B.; Eda, G.; Chhowalla, M. Conducting MoS2 Nanosheets as Catalysts for Hydrogen Evolution Reaction. Nano Lett. 2013, 13, 6222–6227. [Google Scholar] [CrossRef]
- Lukowski, M.A.; Daniel, A.S.; English, C.R.; Meng, F.; Forticaux, A.; Hamers, R.J.; Jin, S. Highly Active Hydrogen Evolution Catalysis from Metallic WS2 Nanosheets. Energy Environ. Sci. 2014, 7, 2608–2613. [Google Scholar] [CrossRef]
- Mahler, B.; Hoepfner, V.; Liao, K.; Ozin, G.A. Colloidal Synthesis of 1T-WS2 and 2H-WS2 Nanosheets: Applications for Photocatalytic Hydrogen Evolution. J. Am. Chem. Soc. 2014, 136, 14121–14127. [Google Scholar] [CrossRef] [PubMed]
- Lazar, P.; Otyepka, M. Role of the Edge Properties in the Hydrogen Evolution Reaction on MoS2. Chem. Eur. J. 2017, 23, 4863–4869. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y. Roadmap and Direction toward High-Performance MoS2 Hydrogen Evolution Catalysts. ACS Nano 2021, 15, 11014–11039. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Fang, B.; Wang, Z.; Wang, C.; Liu, Z.; Liu, F.; Wang, W.; Alfantazi, A.; Wang, D.; Wilkinson, D.P. MoS2 Nanosheets: A Designed Structure with High Active Site Density for the Hydrogen Evolution Reaction. ACS Catal. 2013, 3, 2101–2107. [Google Scholar] [CrossRef]
- Wu, L.; Longo, A.; Dzade, N.Y.; Sharma, A.; Hendrix, M.M.R.M.; Bol, A.A.; de Leeuw, N.H.; Hensen, E.J.M.; Hofmann, J.P. The Origin of High Activity of Amorphous MoS2 in the Hydrogen Evolution Reaction. ChemSusChem 2019, 12, 4383–4389. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Carey, B.J.; Zhang, W.; Chrimes, A.F.; Chen, L.; Kalantar-Zadeh, K.; Ou, J.Z.; Daeneke, T. Intercalated 2D MoS2 Utilizing a Simulated Sun Assisted Process: Reducing the HER Overpotential. J. Phys. Chem. C 2016, 120, 2447–2455. [Google Scholar] [CrossRef]
- Er, D.; Ye, H.; Frey, N.C.; Kumar, H.; Lou, J.; Shenoy, V.B. Prediction of Enhanced Catalytic Activity for Hydrogen Evolution Reaction in Janus Transition Metal Dichalcogenides. Nano Lett. 2018, 18, 3943–3949. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Gong, Y.; Lin, J.; Li, B.; He, Y.; Pantelides, S.T.; Zhou, W.; Vajtai, R.; Ajayan, P.M. Defects Engineered Monolayer MoS2 for Improved Hydrogen Evolution Reaction. Nano Lett. 2016, 16, 1097–1103. [Google Scholar] [CrossRef]
- Voiry, D.; Fullon, R.; Yang, J.; DeCarvalho Castro E Silva, C.; Kappera, R.; Bozkurt, I.; Kaplan, D.; Lagos, M.J.; Batson, P.E.; Gupta, G.; et al. The Role of Electronic Coupling between Substrate and 2D MoS2 Nanosheets in Electrocatalytic Production of Hydrogen. Nat. Mater. 2016, 15, 1003–1009. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Z.C.; Dai, H.; Wang, Q.; Yang, R.; Yu, H.; Liao, M.; Zhang, J.; Chen, W.; Wei, Z.; et al. Boundary Activated Hydrogen Evolution Reaction on Monolayer MoS2. Nat. Commun. 2019, 10, 1348. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Han, J.; Zhang, Y.; Zhang, X.; Xu, P.; Yuan, Q.; Samad, L.; Wang, X.; Wang, Y.; Zhang, Z.; et al. Contributions of Phase, Sulfur Vacancies, and Edges to the Hydrogen Evolution Reaction Catalytic Activity of Porous Molybdenum Disulfide Nanosheets. J. Am. Chem. Soc. 2016, 138, 7965–7972. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tsai, C.; Koh, A.L.; Cai, L.; Contryman, A.W.; Fragapane, A.H.; Zhao, J.; Han, H.S.; Manoharan, H.C.; Abild-Pedersen, F.; et al. Erratum: Activating and Optimizing MoS2 Basal Planes for Hydrogen Evolution through the Formation of Strained Sulphur Vacancies. Nat. Mater. 2016, 15, 364. [Google Scholar] [CrossRef] [PubMed]
- Lukowski, M.A.; Daniel, A.S.; Meng, F.; Forticaux, A.; Li, L.; Jin, S. Enhanced Hydrogen Evolution Catalysis from Chemically Exfoliated Metallic MoS2 Nanosheets. J. Am. Chem. Soc. 2013, 135, 10274–10277. [Google Scholar] [CrossRef] [PubMed]
- Bolar, S.; Shit, S.; Murmu, N.C.; Samanta, P.; Kuila, T. Activation Strategy of MoS2 as HER Electrocatalyst through Doping-Induced Lattice Strain, Band Gap Engineering, and Active Crystal Plane Design. ACS Appl. Mater. Interfaces 2021, 13, 765–780. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.Y.; Yang, X.; Tseng, C.C.; Min, S.; Lin, S.H.; Hsu, C.L.; Li, H.; Idriss, H.; Kuo, J.L.; Huang, K.W.; et al. High-Sulfur-Vacancy Amorphous Molybdenum Sulfide as a High Current Electrocatalyst in Hydrogen Evolution. Small 2016, 12, 5530–5537. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Du, M.; Mleczko, M.J.; Koh, A.L.; Nishi, Y.; Pop, E.; Bard, A.J.; Zheng, X. Kinetic Study of Hydrogen Evolution Reaction over Strained MoS2 with Sulfur Vacancies Using Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2016, 138, 5123–5129. [Google Scholar] [CrossRef] [PubMed]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Nørskov, J.K. Biomimetic Hydrogen Evolution: MoS2 Nanoparticles as Catalyst for Hydrogen Evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef]
- Tsai, C.; Chan, K.; Nørskov, J.K.; Abild-Pedersen, F. Theoretical Insights into the Hydrogen Evolution Activity of Layered Transition Metal Dichalcogenides. Surf. Sci. 2015, 640, 133–140. [Google Scholar] [CrossRef]
- Tsai, C.; Abild-Pedersen, F.; Nørskov, J.K. Tuning the MoS2 Edge-Site Activity for Hydrogen Evolution via Support Interactions. Nano Lett. 2014, 14, 1381–1387. [Google Scholar] [CrossRef]
- Kiriya, D.; Lobaccaro, P.; Nyein, H.Y.Y.; Taheri, P.; Hettick, M.; Shiraki, H.; Sutter-Fella, C.M.; Zhao, P.; Gao, W.; Maboudian, R.; et al. General Thermal Texturization Process of MoS2 for Efficient Electrocatalytic Hydrogen Evolution Reaction. Nano Lett. 2016, 16, 4047–4053. [Google Scholar] [CrossRef]
- Nguyen, A.D.; Nguyen, T.K.; Le, C.T.; Kim, S.; Ullah, F.; Lee, Y.; Lee, S.; Kim, K.; Lee, D.; Park, S.; et al. Nitrogen-Plasma-Treated Continuous Monolayer MoS2 for Improving Hydrogen Evolution Reaction. ACS Omega 2019, 4, 21509–21515. [Google Scholar] [CrossRef] [PubMed]
- Siao, M.D.; Shen, W.C.; Chen, R.S.; Chang, Z.W.; Shih, M.C.; Chiu, Y.P.; Cheng, C.M. Two-Dimensional Electronic Transport and Surface Electron Accumulation in MoS2. Nat. Commun. 2018, 9, 1442. [Google Scholar] [CrossRef] [PubMed]
- Mikhalitsyna, E.A.; Kataev, V.A.; Larrañaga, A.; Lepalovskij, V.N.; Kurlyandskaya, G.V. Nanocrystallization in FINEMET-Type Fe73.5Nb3Cu1Si13.5B9 and Fe72.5Nb1.5Mo2Cu1.1Si14.2B8.7 Thin Films. Materials 2020, 13, 348. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.; Kumar, B.; Sinha, J.; Ghosh, S.; Roy, S.S.; Kaviraj, B. Cost Effective Liquid Phase Exfoliation of MoS2 Nanosheets and Photocatalytic Activity for Wastewater Treatment Enforced by Visible Light. Sci. Rep. 2020, 10, 10759. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From Bulk to Monolayer MoS2: Evolution of Raman Scattering. Adv. Funct. Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Molina-Sánchez, A.; Hummer, K.; Wirtz, L. Vibrational and Optical Properties of MoS2: From Monolayer to Bulk. Surf. Sci. Rep. 2015, 70, 554–586. [Google Scholar] [CrossRef]
- Verble, J.L.; Wieting, T.J. Lattice Mode Degeneracy in MoS2 and Other Layer Compounds. Phys. Rev. Lett. 1970, 25, 362–365. [Google Scholar] [CrossRef]
- Kong, D.; Wang, H.; Cha, J.J.; Pasta, M.; Koski, K.J.; Yao, J.; Cui, Y. Synthesis of MoS2 and MoSe2 Films with Vertically Aligned Layers. Nano Lett. 2013, 13, 1341. [Google Scholar] [CrossRef]
- Sarker, S.; Peters, J.; Chen, X.; Li, B.; Chen, G.; Yan, L.; Richins, S.K.; Das, S.; Zhou, M.; Luo, H. Engineering Molybdenum Diselenide and Its Reduced Graphene Oxide Hybrids for Efficient Electrocatalytic Hydrogen Evolution. ACS Appl. Nano Mater. 2018, 1, 2143–2152. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, T.; Sun, L.; Sun, Y.; Hu, T.; Xu, K.; Ma, F. Hydrothermal Synthesis of 3D Hierarchical MoSe2/NiSe2 Composite Nanowires on Carbon Fiber Paper and Their Enhanced Electrocatalytic Activity for the Hydrogen Evolution Reaction. J. Mater. Chem. A 2017, 5, 19752–19759. [Google Scholar] [CrossRef]
- Li, G.; Zhang, D.; Qiao, Q.; Yu, Y.; Peterson, D.; Zafar, A.; Kumar, R.; Curtarolo, S.; Hunte, F.; Shannon, S.; et al. All The Catalytic Active Sites of MoS2 for Hydrogen Evolution. J. Am. Chem. Soc. 2016, 138, 16632–16638. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Dong, L.; Tan, L.; Tang, Q. First-Principles Study of Sulfur Vacancy Concentration Effect on the Electronic Structures and Hydrogen Evolution Reaction of MoS2. Nanotechnology 2021, 32, 145718. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, S.; Salamone, M.M.; Robertson, A.W.; Nayak, S.; Kim, H.; Tsang, S.C.E.; Pasta, M.; Warner, J.H. Edge-Enriched 2D MoS2 Thin Films Grown by Chemical Vapor Deposition for Enhanced Catalytic Performance. ACS Catal. 2017, 7, 877–886. [Google Scholar] [CrossRef]
- Sun, Y.; Alimohammadi, F.; Zhang, D.; Guo, G. Enabling Colloidal Synthesis of Edge-Oriented MoS2 with Expanded Interlayer Spacing for Enhanced HER Catalysis. Nano Lett. 2017, 17, 1963–1969. [Google Scholar] [CrossRef]
- Wang, X.; Cormier, C.R.; Khosravi, A.; Smyth, C.M.; Shallenberger, J.R.; Addou, R.; Wallace, R.M. In Situ Exfoliated 2D Molybdenum Disulfide Analyzed by XPS. Surf. Sci. Spectra 2020, 27, 014019. [Google Scholar] [CrossRef]
- Neamen, D.A. Semiconductor Physics and Devices: Basic Principles, 4th ed.; McGraw Hill: New York, NY, USA, 2012. [Google Scholar]
- Peelaers, H.; Van DeWalle, C.G. Effects of Strain on Band Structure and Effective Masses in MoS2. Phys. Rev. B 2012, 86, 241401. [Google Scholar] [CrossRef]
- Chang, Y.S.; Chen, C.Y.; Ho, C.J.; Cheng, C.M.; Chen, H.R.; Fu, T.Y.; Huang, Y.T.; Ke, S.W.; Du, H.Y.; Lee, K.Y.; et al. Surface Electron Accumulation and Enhanced Hydrogen Evolution Reaction in MoSe2 Basal Planes. Nano Energy 2021, 84, 105922. [Google Scholar] [CrossRef]
- Tiong, K.K.; Liao, P.C.; Ho, C.H.; Huang, Y.S. Growth and Characterization of Rhenium-Doped MoS Single Crystals. J. Cryst. Growth 1999, 205, 543–547. [Google Scholar] [CrossRef]
- Yang, F.; Cao, Z.-F.; Wang, J.; Wang, S.; Zhong, H. Novel Preparation of High Activity 1T-Phase MoS2 Ultra-Thin Flakes by Layered Double Hydroxide for Enhanced Hydrogen Evolution Performance. Int. J. Hydrog. Energy 2019, 44, 21229–21237. [Google Scholar] [CrossRef]
- Kim, J.; Takahashi, K.; Takaetsu, T.; Funatsu, T. Characterization of Vertically Aligned MoS2 Thin Film on Mo Electrode for Hydrogen Evolution Catalyst. J. Jpn. Inst. Energy 2021, 100, 283–287. [Google Scholar] [CrossRef]
- Bhimanapati, G.R.; Hankins, T.; Lei, Y.; Vilá, R.A.; Fuller, I.; Terrones, M.; Robinson, J.A. Growth and Tunable Surface Wettability of Vertical MoS2 Layers for Improved Hydrogen Evolution Reactions. ACS Appl. Mater. Interfaces 2016, 8, 22190–22195. [Google Scholar] [CrossRef]
- Liu, N.; Kim, J.; Oh, J.; Nguyen, Q.T.; Sahu, B.B.; Han, J.G.; Kim, S. Growth of Multiorientated Polycrystalline MoS2 Using Plasma-Enhanced Chemical Vapor Deposition for Efficient Hydrogen Evolution Reactions. Nanomaterials 2020, 10, 1465. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zheng, L.; Zhu, Z.; Chen, J.; Kang, J.; Huang, Z.; Yang, D. MoS2 Nanosheet Arrays Rooted on Hollow RGO Spheres as Bifunctional Hydrogen Evolution Catalyst and Supercapacitor Electrode. Nano-Micro Lett. 2018, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, J.; Feng, C.; Xu, G.; Xie, C.; Yuan, X.; Xiang, B. MoS2 Nanosheet/MoS2 Flake Homostructures for Efficient Electrocatalytic Hydrogen Evolution MoS2 Nanosheet/MoS2 Fl Ake Homostructures for Efficient Electrocatalytic Hydrogen Evolution. Mater. Res. Express 2019, 6, 085005. [Google Scholar] [CrossRef]
- Bojarska, Z.; Mazurkiewicz-Pawlicka, M.; Mierzwa, B.; Plocinski, T.; Makowski, L. Effect of the Carbon Support on MoS2 hybrid Nanostructures Prepared by an Impinging Jet Reactor for Hydrogen Evolution Reaction Catalysis. J. Environ. Chem. Eng. 2022, 10, 108038. [Google Scholar] [CrossRef]
- Singh, A.K.; Prasad, J.; Azad, U.P.; Singh, A.K.; Prakash, R.; Singh, K.; Srivastava, A.; Alaferdov, A.A.; Moshkalev, S.A. Vanadium Doped Few-Layer Ultrathin MoS2 Nanosheets on Reduced Graphene Oxide for High-Performance Hydrogen Evolution Reaction. RSC Adv. 2019, 9, 22232–22239. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, W.; Hu, Y.; Zhang, Y.; Dang, J.; Wu, Y.; Chen, B.; Zhao, H.; Li, Z. Single Atom Ru Doping 2H-MoS2 as Highly Efficient Hydrogen Evolution Reaction Electrocatalyst in a Wide pH Range. Appl. Catal. B Environ. 2021, 298, 120490. [Google Scholar] [CrossRef]
- Zhang, W.; Liao, X.; Pan, X.; Yan, M.; Li, Y.; Tian, X.; Zhao, Y.; Xu, L.; Mai, L. Superior Hydrogen Evolution Reaction Performance in 2H-MoS2 to That of 1T Phase. Small 2019, 15, 1900964. [Google Scholar] [CrossRef]
- Tang, Q.; Jiang, D.E. Mechanism of Hydrogen Evolution Reaction on 1T-MoS2 from First Principles. ACS Catal. 2016, 6, 4953–4961. [Google Scholar] [CrossRef]




| S. No | System | Overpotential at 10 mA/cm2 (V) | Reference |
|---|---|---|---|
| 1. | MoS2 bulk (N2-plasma-treated) | 0.20 | This work |
| 2. | 1T-MoS2 ultra-thin flakes | 0.25 | [50] |
| 3. | MoS2 thin films | 0.38 | [51] |
| 4. | MoS2 thin films (ozone treated for 10 min) | 0.36 | [52] |
| 5. | MoS2 thin films | 0.45 | [53] |
| 6. | h-rGO@MoS2 | 0.23 | [54] |
| 7. | MoS2 nanosheet/MoS2 nanoflake | 0.26 | [55] |
| 8. | MoS2/GO | 0.21 | [56] |
| 9. | MoS2/rGO | 0.30 | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishnamoorthy, V.; Bangolla, H.K.; Chen, C.-Y.; Huang, Y.-T.; Cheng, C.-M.; Ulaganathan, R.K.; Sankar, R.; Lee, K.-Y.; Du, H.-Y.; Chen, L.-C.; et al. Efficient Hydrogen Evolution Reaction in 2H-MoS2 Basal Planes Enhanced by Surface Electron Accumulation. Catalysts 2024, 14, 50. https://doi.org/10.3390/catal14010050
Krishnamoorthy V, Bangolla HK, Chen C-Y, Huang Y-T, Cheng C-M, Ulaganathan RK, Sankar R, Lee K-Y, Du H-Y, Chen L-C, et al. Efficient Hydrogen Evolution Reaction in 2H-MoS2 Basal Planes Enhanced by Surface Electron Accumulation. Catalysts. 2024; 14(1):50. https://doi.org/10.3390/catal14010050
Chicago/Turabian StyleKrishnamoorthy, Vimal, Hemanth Kumar Bangolla, Chi-Yang Chen, Yu-Ting Huang, Cheng-Maw Cheng, Rajesh Kumar Ulaganathan, Raman Sankar, Kuei-Yi Lee, He-Yun Du, Li-Chyong Chen, and et al. 2024. "Efficient Hydrogen Evolution Reaction in 2H-MoS2 Basal Planes Enhanced by Surface Electron Accumulation" Catalysts 14, no. 1: 50. https://doi.org/10.3390/catal14010050
APA StyleKrishnamoorthy, V., Bangolla, H. K., Chen, C.-Y., Huang, Y.-T., Cheng, C.-M., Ulaganathan, R. K., Sankar, R., Lee, K.-Y., Du, H.-Y., Chen, L.-C., Chen, K.-H., & Chen, R.-S. (2024). Efficient Hydrogen Evolution Reaction in 2H-MoS2 Basal Planes Enhanced by Surface Electron Accumulation. Catalysts, 14(1), 50. https://doi.org/10.3390/catal14010050