Abstract
An innovative strategy has been developed to activate the basal planes in molybdenum disulfide (MoS2) to improve their electrocatalytic activity by controlling surface electron accumulation (SEA) through aging, annealing, and nitrogen-plasma treatments. The optimal hydrogen evolution reaction (HER) performance was obtained on the surface treated with nitrogen-plasma for 120 s. An overpotential of 0.20 V and a Tafel slope of 120 mV dec−1 were achieved for the optimized condition. The angle-resolved photoemission spectroscopy measurement confirmed the HER efficiency enhanced by the SEA conjugated with the sulfur vacancy active sites in the MoS2 basal planes. This study provides new insight into optimizing MoS2 catalysts for energy applications.
1. Introduction
Hydrogen (H2) holds great attention as a green energy carrier for future technologies due to its high energy density and being environment friendly [1,2]. Electrochemical water-splitting is a promising route for sustainable H2 production due to its advantages of abundant sources and non-pollutants [2,3]. Hydrogen evolution reaction (HER) is one of the most efficient pathways to produce H2 from water splitting using suitable catalysts. Currently, platinum (Pt) and other noble metals or alloys are the most popular electrocatalysts for HER, while their scarcity and high cost limit their widespread utilization [4,5]. The new challenge for researchers is to develop earth-abundant, low-cost, and high-performance catalysts as an alternative to Pt and other noble metals for electrochemical H2 production.
Two dimensional (2D) transition metal dichalcogenides (TMDCs) have been extensively investigated as promising alternative catalysts for HERs due to their exceptional physicochemical properties including high mechanical properties, good semiconducting ability, large surface area, and high catalytic activity [6,7,8,9,10,11,12]. Among TMDCs, molybdenum disulfide (MoS2) has been found to be one of the most economical alternatives to noble metal catalysts for HER due to its high electron mobility, stability, non-toxic nature, low cost, and excellent electrocatalytic properties [13,14,15]. Due to its inert basal plane, the overall activity of the two-layered hexagonal phase MoS2 (2H-phase MoS2) catalyzes mainly from the active edge sites, which limits the practical application of MoS2 for HER [9,16,17,18]. Various techniques have been developed to overcome the limited catalytic activity of the MoS2 basal plane, such as interface electronic coupling, phase engineering, and introducing active unsaturated defects and strain [19,20,21,22,23,24]. The pioneer strategy to activate the basal plane of MoS2 that enhances HER activity was the introduction of sulfur (S)-vacancies into the basal plane [22,25,26].
In HER, the optimal H2 adsorption free energy (ΔGH) is 0 eV, where H2 is bound to the catalyst neither too strongly nor too weakly. For the basal plane of pristine 2H-MoS2, the ΔGH value is approximately 2 eV, making the basal plane inert to HER performance. The ΔGH values can be decreased with an increase in S-vacancies on the MoS2 basal plane. A ΔGH value between ±0.08 eV for S-vacancies in the range of 9–19% is the most favorable value for HER [22,27,28,29]. To date, a limited number of techniques have been used to create S-vacancies on 2D TMDC materials such as electrochemical reduction, H2 annealing, and plasma bombardment [29,30,31]. According to our previous work, long term air exposure (aging) spontaneously creates S-vacancies, which cause surface electron accumulation (SEA) on MoS2 surfaces [32].
In this report, we utilize and control SEA to activate the basal planes of 2H-MoS2. The SEA was performed through various techniques such as aging, annealing, and nitrogen (N2)-plasma treatments to create S-vacancies on the basal plane of 2H-MoS2. The effect of SEA on HER efficiency was investigated. Angle-resolved photoemission spectroscopy (ARPES) measurements were performed to confirm the electronic structures of different MoS2 surfaces. The results revealed that 2H-MoS2 was converted from intrinsic (fresh) to degenerate semiconductor (using N2-plasma treatments) by creating S-vacancies which provided more active sites and charge carriers for HER.
2. Results and Discussion
2.1. Structural Characterization
Figure 1a illustrates the crystal structure of the MoS2 monolayer formed by a hexagonal plane of Mo and S atoms. These triple planes are stacked together by weak van der Waals forces of attraction to form bulk MoS2. The van der Waals force allows the preparation of mono and a few layers of MoS2 flakes through a simple mechanical exfoliation technique using dicing tape (see Figure 1b). The bulk fresh surface was created by the exfoliation of the top layers of pristine MoS2 and the other pristine MoS2 crystal was subjected to N2-plasma treatments. The X-ray diffraction (XRD) pattern of pristine, fresh, and N2-plasma-treated bulk samples is shown in Figure 2. The diffraction peaks, located at 14.3°, 29.0°, 44.1°, 60.1°, and 77.9° were assigned to (002), (004), (006), (008), and (0010) planes of standard 2H-MoS2, respectively (JCPDS #872416) [32]. The diffraction peak along the (002) plane was the most prominent compared with the other peaks. Moreover, a broad reflection around 21° was observed for the fresh and pristine MoS2 samples which may have been due to the glass substrate [33]. A reduction in the intensity of diffraction peaks of fresh and N2-plasma MoS2 was observed when compared with pristine MoS2, and this may have been due to the absence of constructive interference from the bulk crystal planes [34]. The position of diffraction peaks did not show any shift, which indicated that there was no inner structural change in the MoS2 samples after N2-plasma treatment.
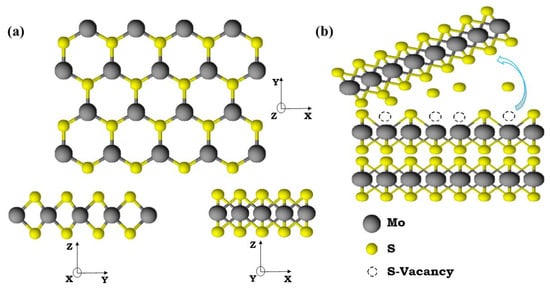
Figure 1.
(a) The crystal structure of the MoS2 mono layer. (b) The exfoliation process for obtaining a fresh MoS2 surface and the S-vacancies were created by aging and annealing processes on a fresh surface.
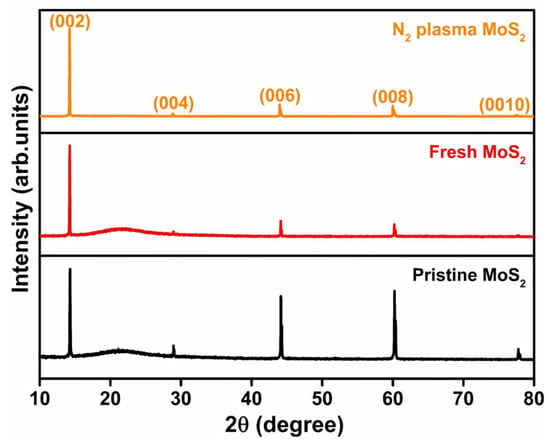
Figure 2.
XRD patterns of pristine, fresh, and N2-plasma-treated MoS2 bulk.
Figure 3 shows the Raman spectra of pristine, fresh, and N2-plasma-treated MoS2. The observed Raman modes at 385 and 411 cm−1 belong to the (in-plane) and (out-of-plane) modes of bulk MoS2, respectively [35,36]. The 2H-MoS2 phase contains four Raman active vibrations, namely, , , and , and the absence of the other two modes may have been due to the selection rules of the scattering geometry and limited rejection of Rayleigh scattered radiation [35,37,38]. After the exfoliation and plasma treatments, the positions of the observed two modes did not show any shift. However, the peak intensity of the N2-plasma-treated sample showed considerable decrement when compared with the pristine MoS2 sample. This indicated that the lattice distortion on the MoS2 basal plane was due to the gradual increase in S-vacancies and cracks caused by the N2-plasma treatment [31].
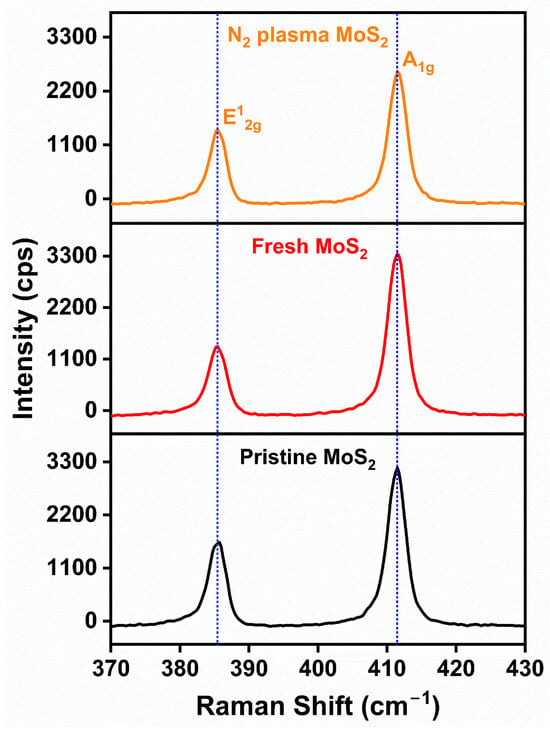
Figure 3.
Raman spectra of pristine, fresh, and N2-plasma-treated MoS2 bulk.
2.2. Electrochemical HER Efficiency Enhanced by SEA
2.2.1. SEA through Aging
To examine the effects of SEA on a MoS2 electrocatalyst for a HER, the fresh surface was exposed to air for a different number of days to create SEA on the MoS2 surface. Figure 4a shows the polarization curves of pristine and fresh MoS2 surfaces with an overpotential of 0.75 and 0.82 V, respectively. The fresh MoS2 used a high overpotential of 0.82 V (vs. RHE) to generate a 10 mA/cm2 current density. The higher overpotential of fresh MoS2 was due to the intrinsic (insulating) nature of the cleaved fresh surface, which was confirmed by ARPES and scanning tunneling spectroscopy (STS) measurements in our previous study [32]. The overpotential of the fresh surface was reduced by the aging effect, which created the S-vacancies. The aging effect of the fresh surface was examined over a different number of days, and their polarization curves are shown in Figure 4c. The overpotential reduced gradually with the increase in aging time and reached a minimum value of 0.31 V for a surface aging of 58 days. This reduction in the overpotential was evidence of the HER efficiency enhanced by SEA on the MoS2 basal plane due to the aging effect. Generally, the exposure of the sulfide surface to air causes desulfurization (escape of sulfur atoms) and adsorption of foreign molecules such as oxygen and water molecules. The ARPES results of an in situ-cleaved surface and the same surface kept for 11 h excluded the possibility of the SEA being induced by the adsorption of foreign molecules, and it was concluded that the SEA was induced by long-term air exposure originating from the S-vacancy surface defects [32]. The S-defects created a donor-like surface state close to the conduction band edge which increased the electron concentration at the MoS2 surface.
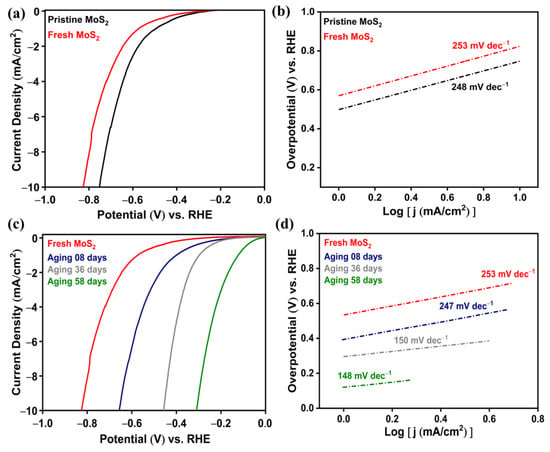
Figure 4.
(a) Polarization curves and (b) corresponding Tafel plots for pristine and fresh surfaces of MoS2. (c) Polarization curves and (d) corresponding Tafel plots of fresh and aging surfaces for 8, 36, and 58 days of MoS2.
Tafel slope is the linear fitting region in the Tafel plot according to the Tafel equation of η = b log |j| + a, where η is the overpotential, b is the Tafel slope, and a is the current density [39,40]. The Tafel plots of pristine and fresh MoS2 surfaces are presented in Figure 4b, with corresponding Tafel slopes of 248 and 253 mV dec−1, respectively. The Tafel plots of aging surfaces are illustrated in Figure 4d, and it was noted that the Tafel slope reduced with an increase in aging time. Finally, the lowest Tafel slope of 148 mV dec−1 was obtained for the MoS2 surface aged for 58 days. Generally, a lower Tafel slope requires less voltage to increase the current density in the HER mechanism by one order of magnitude. Hence, among these, MoS2 aged for 58 days was the most favorable catalyst for HER. Normally, HER activity depends on the number of active sites on, and electron density of, the catalyst. The major active sites for the electrocatalytic reaction were formed mainly by S-vacancies which were created on the MoS2 surface due to the spontaneous desulfurization in air [32].
2.2.2. SEA through Annealing
To control the SEA and optimize HER efficiency, the fresh surface was annealed at 80 °C for different times (1, 2, and 3 h). The polarization curves of fresh and annealed MoS2 surfaces for different times are shown in Figure 5a and corresponding Tafel plots can be seen in Figure 5b. The overpotential of MoS2 decreased from 0.82 to 0.28 V when the fresh MoS2 surface was exposed to annealing at 80 °C for 2 h, and the Tafel slope attained the minimum value of 168 mV dec−1. Further, on increasing the heat treatment to 3 h, the overpotential and Tafel slope increased from 0.28 to 0.32 V and 168 to 199 mV dec−1, respectively. This could have been due to the increase in S-vacancies over the optimum when the fresh MoS2 surface was exposed to heat for more than 2 h. The catalytic activity depends on the density of S-vacancies. Li et al. observed maximal catalytic activity for MoS2 films when the density of S-vacancies was in the range of 7−10%. Further, an increase in the density of S-vacancies reduced the catalytic activity [41]. Density functional theory studies have revealed that the optimal HER activity of MoS2 occurs below the S-vacancy concentration of 12.5% [42]. The S-vacancies will disperse homogeneously like point defects up to a vacancy concentration of 12.5%, and with further increases in vacancy concentration (up to 18.75%) some of the vacancies will form agglomerate clusters. At higher vacancy concentrations, the combination of isolated point defects and clustered defects may induce structural defects in MoS2. The relatively strong hydrogen adsorption (ΔGH = −0.278 and −0.290 eV) at higher vacancy concentrations (15.63 and 18.75%) shows very high activity for proton adsorption, but desorption of *H to form H2 would be kinetically more difficult. Hence, the optimal hydrogen adsorption (ΔGH = ±0.15 eV) occurs below the S-vacancy concentration of 12.5%, which corresponds to the highly active sites for hydrogen evolution.
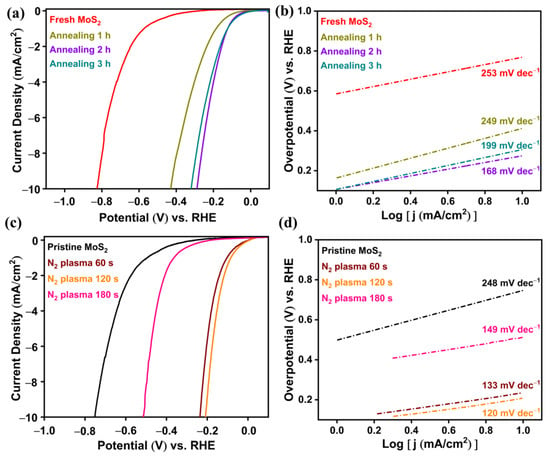
Figure 5.
(a) Polarization curves and (b) corresponding Tafel plots for fresh and annealed MoS2 at 80 °C for 1, 2, and 3 h. (c) Polarization curves and (d) corresponding Tafel plots of pristine and N2-plasma-treated MoS2 for 60, 120, and 180 s.
2.2.3. SEA through N2-Plasma Treatment
Further, N2-plasma treatment was conducted on pristine MoS2 surfaces over different times to enhance HER activity. The polarization curves and corresponding Tafel plots of pristine and N2-plasma-treated MoS2 surfaces over different times are shown in Figure 5c and Figure 5d, respectively. During plasma treatment, the pristine surface was kept 1 cm away from the ICP N2 plasma generator to avoid rapid S-vacancies and surface damage. The overpotential of the MoS2 surface reduced from 0.75 to 0.20 V on N2-plasma exposure for 120 s. Further increases in N2-plasma exposure time resulted in increases in overpotential. Thus, it was concluded that the optimal N2-plasma treatment time for better HER activity was 120 s. Generally, the Tafel slopes of 120, 40, and 30 mVdec−1 are an indication of the Volmer, Heyrovsky, and Tafel reaction steps in the HER mechanism, respectively [43,44]. The optimal N2-plasma treatment for 120 s exhibited the Tafel slope value of 120 mV dec−1 (see Figure 5d) and hence, it followed the Volmer mechanism of HER.
2.3. Evidence of Enhanced SEA Observed by ARPES
ARPES measurements were performed for the in situ-cleaved, annealed for 2 h, and N2-plasma treatment for 120 s -MoS2 surfaces to confirm the existence of SEA as shown in Figure 6a. The magnified normal spectra at Γ point can be seen in Figure 6b. The valence band edge (EV) of the in situ-cleaved surface was at a binding energy of −0.80 eV which is the indication of intrinsic Fermi level (EF) according to the bulk MoS2 band gap of 1.3 eV at 85 K [32]. The difference between EF and EV (EF − EV0.80 eV) of the in situ-cleaved surface was in good agreement with the reported value of 0.80 eV for in situ exfoliated MoS2 under UHV conditions [45]. The SEA of aging surfaces was confirmed by ARPES in our previous work [32]. The EV of the MoS2 surface annealed for 2 h resulted in red shift and moved to a binding energy of −1.16 eV. This moved EF to near the conduction band edge (EC) (EC − EF0.19 eV). The carrier concentration (n) of the annealing surface can be calculated using the following formula [46]:
where is the effective density of states function in the conduction band and is given by . Here, is the effective mass of an electron, k is Boltzmann’s constant, T is the room temperature, and h is Planck’s constant. The values of for bulk MoS2 are in the range of 0.45−0.73 m0; here m0 is the free electron rest mass [47]. The calculated n of annealing surfaces is 3.4 1016–7.0 1016 cm−3.
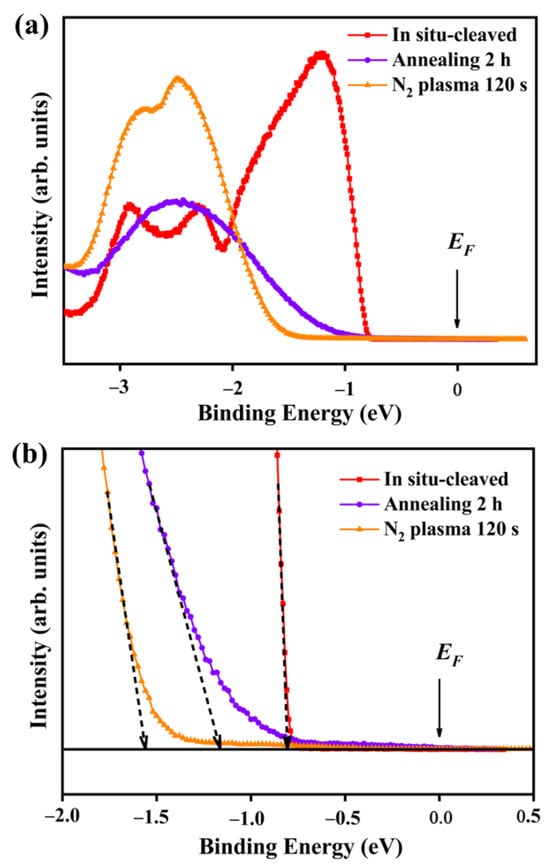
Figure 6.
ARPES characterization of MoS2 surfaces at different conditions. (a) The normal emission spectra and (b) magnified normal emission spectra of in situ-cleaved, annealed for 2 h, and N2-plasma-treated for 120 s MoS2 surfaces. The black dotted lines represent the extrapolation of the linear region of the respective data points to find the EV values. The experimental data of the in situ-cleaved surface were taken from our previously published work [32].
The EV showed a remarkable red shift and moved to −1.56 eV for the N2-plasma-treated MoS2 surface. This moved the EF to beyond EC (EF − EC 0.26 eV), which showed the n-type degenerate semiconductor nature. n can be calculated using the following formula [48]:
The calculated n value was in the range of 1.50 1020–3.10 1020 cm−3. This value was around five orders of magnitude higher than the MoS2 bulk value (n 2 1015 cm−3) [49]. The higher n on the N2-plasma-treated MoS2 surface denotes the highly conductive nature of the basal plane. This result was consistent with the electrochemical HER results in which the lowest overpotential was obtained for the N2-plasma treatment for 120 s on the MoS2 surface. Hence, it was confirmed that HER activity can be enhanced by producing conjugated active sites and SEA in the 2H-MoS2 basal plane.
The best HER activity of N2-plasma-treated MoS2 with an overpotential of 0.20 V was compared with other MoS2-based catalysts including nanoflakes [50], thin films [51,52,53], hierarchical hollow architectures [54], homostructures [55], and hybrid nanostructures [56,57], and is tabulated in Table 1. It was noticed that MoS2 bulks performed better than MoS2-based nanostructures and, hence, control of SEA can be a promising strategy to enhance HER activity in 2D TMDCs. In addition, the obtained overpotential of 0.20 V was higher than the overpotential of Ru doped 2H-MoS2 (0.16 V at 10 mAcm−2 in 0.5 M H2SO4) [58], and the MoS2 nanosheet (0.07 V 10 mAcm−2 in 0.5 M H2SO4 under back-gate voltage of 3V) [59]. This suggests that HER activity in MoS2 basal planes can be improved further by a combination of SEA with doping or Fermi level modulation.

Table 1.
The comparison of MoS2 bulk overpotential with reported MoS2 nanostructures.
2.4. Discussion
The schematic of SEA and its enhancement on HER in 2H-MoS2 is shown in Figure 7. The SEA was produced by the S-vacancies which act as donor-like surface states. The accumulated free electrons were injected from the donor-like surface states and leave the positively charged state on the surface which produced the downward band bending. This moved the EC below EF according to the ARPES measurement of the N2-plasma-treated MoS2 surface due to its n-type degenerate nature. S-vacancies are the origin of high surface electron concentration and, meanwhile, provide abundant active sites. The conjugated SEA and HER active sites provide the required conditions to enhance the electrochemical activity of HER.
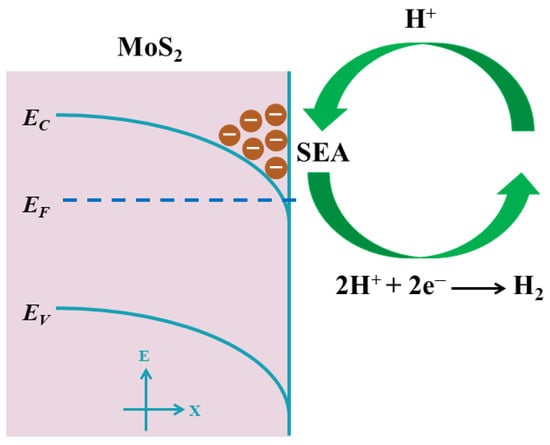
Figure 7.
Schematic diagram of SEA and its effect on HER in MoS2. Surface band bending and SEA are produced by the donor-like surface states.
Usually, in MoS2, the metallic 1T-phase basal plane is considered for the HER due to the possible source of active sites [9,23]. The SEA in 2H-MoS2 enhances HER activity and this result may be comparable with the excellent HER activity of the metallic 1T-phase MoS2 [7,23,60]. In our previous reports, we observed SEA formation in MoS2 and MoSe2 due to the S- and Se-vacancies, respectively [32,48]. The MoS2 catalyst has been widely investigated for HER but, unexpectedly, the effect of SEA on HER efficiency has been ignored in previous reports. This investigation gives a new insight into the tuning of basal plane active sites through S-vacancies by keeping the edge defects that can enhance HER activity. Moreover, the catalytic vacancy sites and abundant free electrons at the surface are the major areas responsible for efficient HER activity.
3. Experimental Section
3.1. Preparation of MoS2 Layer Crystals
The chemical vapor transport method was used to synthesize MoS2 single crystals. Fine powders of sulfur (99.99%) and molybdenum (99.99%) were used as source materials. Bromine (Br2) was used as the transport agent and enabled an effective and faster vapor transport to produce MoS2 single crystals. The source materials including sulfur and molybdenum powders together with Br2 were sealed in a quartz ampoule of a length of 30 cm at a vacuum of 10−5 Torr. The inner and outer diameters of the quartz ampoule were 1.3 and 1.6 cm, respectively. The preheated material temperatures of growth MoS2 single crystals were kept at 950 and 1000 °C, respectively. The controlling temperatures of the source and crystallization ends were maintained at 1050 and 960 °C, respectively. After ten days of processing, the as-synthesized MoS2 layer crystals area was in the range of a few square millimeters to centimeters. The thickness of the crystals ranged from a few nanometers to hundreds of nanometers. Typical mechanical exfoliation was used for obtaining fresh surface MoS2 from the pristine layer crystals using dicing tape.
3.2. Characterization of MoS2 Bulks
An X-ray diffraction (XRD-Bruker D2 phaser diffractometer) system with Cu Kα1 radiation (λ = 1.54056 Å) and Raman spectroscopy (Jobin-Yvon LabRAM HR800) with a 633 nm He-Ne laser as the excitation source were used to examine the structural characterization of the MoS2 bulks. The pristine surface (non-fresh) meant that the surface of the as-synthesized crystal was exposed to air for a prolonged period. The fresh surface was obtained by stripping the top layers of the bulk crystal with dicing tape. The N2-plasma treatment was performed on a different pristine MoS2 surface.
3.3. N2-Plasma Treatment of Pristine MoS2
An inductively coupled plasma (ICP) system with a commercial 13.56 MHz RF source was used to perform the N2-plasma treatment on the MoS2/glassy carbon electrode. In the quartz tube, the distance between the samples and the center of the coil was maintained at 1 cm and the samples were placed on the downstream side. After that, the vacuum in the chamber was maintained at 10 mTorr. High purity N2 (99.999%) was allowed into the quartz tube at a flow rate of 400 sccm. The N2-plasma was generated under a pressure of 130 mTorr due to excited N2 ions when powered by 100 W at ambient temperature. Finally, the pristine MoS2 surfaces were exposed to a N2-plasma atmosphere for fixed different time intervals.
3.4. Electrochemical Measurements
An electrochemical test system (Biologic Bi-stat) was used to perform the HER activity measurements using a standard three electrode cell at ambient temperature. The working electrode had a rotating disk electrode (RDE, PINE AFE5T050GC) with a glassy carbon (GC) disk (diameter 5 mm) and a PTFE shroud (diameter 15 mm). The platinum foil and Ag/AgCl were utilized as counter and reference electrodes, respectively. The measured potential values were transferred to a reversible hydrogen electrode (RHE), where ERHE = EAg/AgCl + 0.059 pH + E°Ag/AgCl. The HER polarization curves were recorded in a 0.5 M H2SO4 (pH ≈ 0.3) electrolyte at a scan rate of 5 mVs−1. The high purity N2 gas was passed through the electrolyte to remove other gases. As-prepared MoS2 catalyst was loaded onto the surface of the GC electrode and thread sealant (Loctite®) was used to cover the uncovered GC area beside the MoS2 catalyst. For the pristine surface, the bulk MoS2 crystal was placed on the surface of the GC electrode and fixed. For the fresh surface, first we fixed the pristine bulk on the GC electrode and removed the top surface using dicing tape; then for the annealing process, the electrode with the fresh surface was exposed to heating. For the N2-plasma-treated samples, first we fixed the pristine bulk on the GC electrode and then exposed it to plasma. Cyclic voltammetry (CV) was performed in the potential range of −1.0 to +0.2 V (vs. RHE) and a scan rate of 50 mVs−1 until the curves reached a stable state. Linear sweep voltammetry (LSV) was executed in the same potential range as CV with a scan rate of 5 mVs−1. The electrochemical measurements were repeated three times to obtain concordant data.
3.5. Angle-Resolved Photoelectron Spectroscopy (ARPES) Measurement
The ARPES experiment was performed at the National Synchrotron Radiation Research Center (NSRRC) in Hsinchu, Taiwan, at a BL21B1 U9-CGM beamline. The photoemission spectra of MoS2 annealed and N2-plasma-treated surfaces were measured in a UHV chamber equipped with a hemispherical analyzer (Scienta R4000) with a collecting angle of 15 at a base pressure of 5.6 10−11 Torr. The polarization was invariably in the angular dispersive plane. The spectra of all MoS2 surfaces were recorded with a photon energy of 42 eV at 85 K. The energy resolution was better than 23 meV and the angular resolution was 0.2. After loading samples into a load–lock vacuum chamber, the samples were left in a vacuum until the pressure and temperature reached the required steady state.
4. Conclusions
We improved the electrocatalytic activity of 2H-MoS2 by activating its basal planes through SEA using aging, annealing, and nitrogen-plasma treatments. The SEA caused by the S-vacancies was catalytically active, and a high electron concentration in the order of ~1020 cm−3 was obtained for the N2-plasma-treated MoS2 surface. The optimal HER performance was obtained for the surface which underwent N2-plasma treatment for 120 s, with an overpotential of 0.20 V vs RHE at 10 mA cm−2. The ARPES measurements confirmed that the HER efficiency enhanced by the SEA conjugated with the S-vacancy active sites in the 2H-MoS2 basal planes. This work provides an efficient and low cost MoS2-based HER catalyst and also opens up comprehensive insights into basal planes of 2D TMDCs through SEA for energy related applications.
Author Contributions
Conceptualization and writing—review and editing, R.-S.C.; methodology, V.K., C.-Y.C., Y.-T.H. and C.-M.C.; formal analysis and writing—original draft preparation, V.K. and H.K.B.; investigation, C.-M.C., R.K.U., R.S., K.-Y.L., H.-Y.D. and L.-C.C.; supervision, H.-Y.D., L.-C.C., K.-H.C. and R.-S.C.; resources and validation, L.-C.C., K.-H.C. and R.-S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Science and Technology (MOST) of Taiwan grant numbers MOST 111-2112-M-011-004-MY3, MOST 108-2628-M-011-001-MY3, MOST 109-2622-E-011-034, MOST 110-2622-E-011-017, MOST 112-2112-M-131-003, MOST 111-2112-M-131-003, NSTC 112-2124-M-001-007 and NSTC 112-2811-M-001-065, Chang Gung University, Taiwan grant number URRPD2N0021, and the Academia Sinica, Taiwan grant number AS-iMATE-112-12. And the APC was funded by Ministry of Science and Technology (MOST) of Taiwan.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Corresponding author R.-S.C. thanks the support of the Ministry of Science and Technology (MOST) of Taiwan. Corresponding author H.-Y.D. expresses gratitude for the financial support provided by Ministry of Science and Technology (MOST) of Taiwan and Chang Gung University, Taiwan. Author R.S. acknowledges the financial support provided by the Ministry of Science and Technology (MOST) of Taiwan and Academia Sinica, Taiwan. Author V.K. thanks the Ministry of Education (MOE) Elite Scholarship Program and the National Taiwan University of Science and Technology (NTUST) Scholarship Program for additional financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shi, Y.; Zhou, Y.; Yang, D.R.; Xu, W.X.; Wang, C.; Wang, F.B.; Xu, J.J.; Xia, X.H.; Chen, H.Y. Energy Level Engineering of MoS2 by Transition-Metal Doping for Accelerating Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2017, 139, 15479–15485. [Google Scholar] [CrossRef]
- Wang, J.; Yan, M.; Zhao, K.; Liao, X.; Wang, P.; Pan, X.; Yang, W.; Mai, L. Field Effect Enhanced Hydrogen Evolution Reaction of MoS2 Nanosheets. Adv. Mater. 2017, 29, 1604464. [Google Scholar] [CrossRef]
- Tachibana, Y.; Vayssieres, L.; Durrant, J.R. Artificial Photosynthesis for Solar Water-Splitting. Nat. Photonics 2012, 6, 511–518. [Google Scholar] [CrossRef]
- Ouyang, Y.; Ling, C.; Chen, Q.; Wang, Z.; Shi, L.; Wang, J. Activating Inert Basal Planes of MoS2 for Hydrogen Evolution Reaction through the Formation of Different Intrinsic Defects. Chem. Mater. 2016, 28, 4390–4396. [Google Scholar] [CrossRef]
- Ding, Q.; Song, B.; Xu, P.; Jin, S. Efficient Electrocatalytic and Photoelectrochemical Hydrogen Generation Using MoS2 and Related Compounds. Chem 2016, 1, 699–726. [Google Scholar] [CrossRef]
- Prabhu, P.; Jose, V.; Lee, J.M. Design Strategies for Development of TMD-Based Heterostructures in Electrochemical Energy Systems. Matter 2020, 2, 526–553. [Google Scholar] [CrossRef]
- Ambrosi, A.; Sofer, Z.; Pumera, M. 2H → 1T Phase Transition and Hydrogen Evolution Activity of MoS2, MoSe2, WS2 and WSe2 Strongly Depends on the MX2 Composition. Chem. Commun. 2015, 51, 8450–8453. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Gao, D.; Ding, J.; Chao, D.; Wang, J. TMD-Based Highly Efficient Electrocatalysts Developed by Combined Computational and Experimental Approaches. Chem. Soc. Rev. 2018, 47, 4332–4356. [Google Scholar] [CrossRef] [PubMed]
- Voiry, D.; Salehi, M.; Silva, R.; Fujita, T.; Chen, M.; Asefa, T.; Shenoy, V.B.; Eda, G.; Chhowalla, M. Conducting MoS2 Nanosheets as Catalysts for Hydrogen Evolution Reaction. Nano Lett. 2013, 13, 6222–6227. [Google Scholar] [CrossRef]
- Lukowski, M.A.; Daniel, A.S.; English, C.R.; Meng, F.; Forticaux, A.; Hamers, R.J.; Jin, S. Highly Active Hydrogen Evolution Catalysis from Metallic WS2 Nanosheets. Energy Environ. Sci. 2014, 7, 2608–2613. [Google Scholar] [CrossRef]
- Mahler, B.; Hoepfner, V.; Liao, K.; Ozin, G.A. Colloidal Synthesis of 1T-WS2 and 2H-WS2 Nanosheets: Applications for Photocatalytic Hydrogen Evolution. J. Am. Chem. Soc. 2014, 136, 14121–14127. [Google Scholar] [CrossRef] [PubMed]
- Lazar, P.; Otyepka, M. Role of the Edge Properties in the Hydrogen Evolution Reaction on MoS2. Chem. Eur. J. 2017, 23, 4863–4869. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y. Roadmap and Direction toward High-Performance MoS2 Hydrogen Evolution Catalysts. ACS Nano 2021, 15, 11014–11039. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Fang, B.; Wang, Z.; Wang, C.; Liu, Z.; Liu, F.; Wang, W.; Alfantazi, A.; Wang, D.; Wilkinson, D.P. MoS2 Nanosheets: A Designed Structure with High Active Site Density for the Hydrogen Evolution Reaction. ACS Catal. 2013, 3, 2101–2107. [Google Scholar] [CrossRef]
- Wu, L.; Longo, A.; Dzade, N.Y.; Sharma, A.; Hendrix, M.M.R.M.; Bol, A.A.; de Leeuw, N.H.; Hensen, E.J.M.; Hofmann, J.P. The Origin of High Activity of Amorphous MoS2 in the Hydrogen Evolution Reaction. ChemSusChem 2019, 12, 4383–4389. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Carey, B.J.; Zhang, W.; Chrimes, A.F.; Chen, L.; Kalantar-Zadeh, K.; Ou, J.Z.; Daeneke, T. Intercalated 2D MoS2 Utilizing a Simulated Sun Assisted Process: Reducing the HER Overpotential. J. Phys. Chem. C 2016, 120, 2447–2455. [Google Scholar] [CrossRef]
- Er, D.; Ye, H.; Frey, N.C.; Kumar, H.; Lou, J.; Shenoy, V.B. Prediction of Enhanced Catalytic Activity for Hydrogen Evolution Reaction in Janus Transition Metal Dichalcogenides. Nano Lett. 2018, 18, 3943–3949. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Gong, Y.; Lin, J.; Li, B.; He, Y.; Pantelides, S.T.; Zhou, W.; Vajtai, R.; Ajayan, P.M. Defects Engineered Monolayer MoS2 for Improved Hydrogen Evolution Reaction. Nano Lett. 2016, 16, 1097–1103. [Google Scholar] [CrossRef]
- Voiry, D.; Fullon, R.; Yang, J.; DeCarvalho Castro E Silva, C.; Kappera, R.; Bozkurt, I.; Kaplan, D.; Lagos, M.J.; Batson, P.E.; Gupta, G.; et al. The Role of Electronic Coupling between Substrate and 2D MoS2 Nanosheets in Electrocatalytic Production of Hydrogen. Nat. Mater. 2016, 15, 1003–1009. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Z.C.; Dai, H.; Wang, Q.; Yang, R.; Yu, H.; Liao, M.; Zhang, J.; Chen, W.; Wei, Z.; et al. Boundary Activated Hydrogen Evolution Reaction on Monolayer MoS2. Nat. Commun. 2019, 10, 1348. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Han, J.; Zhang, Y.; Zhang, X.; Xu, P.; Yuan, Q.; Samad, L.; Wang, X.; Wang, Y.; Zhang, Z.; et al. Contributions of Phase, Sulfur Vacancies, and Edges to the Hydrogen Evolution Reaction Catalytic Activity of Porous Molybdenum Disulfide Nanosheets. J. Am. Chem. Soc. 2016, 138, 7965–7972. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tsai, C.; Koh, A.L.; Cai, L.; Contryman, A.W.; Fragapane, A.H.; Zhao, J.; Han, H.S.; Manoharan, H.C.; Abild-Pedersen, F.; et al. Erratum: Activating and Optimizing MoS2 Basal Planes for Hydrogen Evolution through the Formation of Strained Sulphur Vacancies. Nat. Mater. 2016, 15, 364. [Google Scholar] [CrossRef] [PubMed]
- Lukowski, M.A.; Daniel, A.S.; Meng, F.; Forticaux, A.; Li, L.; Jin, S. Enhanced Hydrogen Evolution Catalysis from Chemically Exfoliated Metallic MoS2 Nanosheets. J. Am. Chem. Soc. 2013, 135, 10274–10277. [Google Scholar] [CrossRef] [PubMed]
- Bolar, S.; Shit, S.; Murmu, N.C.; Samanta, P.; Kuila, T. Activation Strategy of MoS2 as HER Electrocatalyst through Doping-Induced Lattice Strain, Band Gap Engineering, and Active Crystal Plane Design. ACS Appl. Mater. Interfaces 2021, 13, 765–780. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.Y.; Yang, X.; Tseng, C.C.; Min, S.; Lin, S.H.; Hsu, C.L.; Li, H.; Idriss, H.; Kuo, J.L.; Huang, K.W.; et al. High-Sulfur-Vacancy Amorphous Molybdenum Sulfide as a High Current Electrocatalyst in Hydrogen Evolution. Small 2016, 12, 5530–5537. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Du, M.; Mleczko, M.J.; Koh, A.L.; Nishi, Y.; Pop, E.; Bard, A.J.; Zheng, X. Kinetic Study of Hydrogen Evolution Reaction over Strained MoS2 with Sulfur Vacancies Using Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2016, 138, 5123–5129. [Google Scholar] [CrossRef] [PubMed]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Nørskov, J.K. Biomimetic Hydrogen Evolution: MoS2 Nanoparticles as Catalyst for Hydrogen Evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef]
- Tsai, C.; Chan, K.; Nørskov, J.K.; Abild-Pedersen, F. Theoretical Insights into the Hydrogen Evolution Activity of Layered Transition Metal Dichalcogenides. Surf. Sci. 2015, 640, 133–140. [Google Scholar] [CrossRef]
- Tsai, C.; Abild-Pedersen, F.; Nørskov, J.K. Tuning the MoS2 Edge-Site Activity for Hydrogen Evolution via Support Interactions. Nano Lett. 2014, 14, 1381–1387. [Google Scholar] [CrossRef]
- Kiriya, D.; Lobaccaro, P.; Nyein, H.Y.Y.; Taheri, P.; Hettick, M.; Shiraki, H.; Sutter-Fella, C.M.; Zhao, P.; Gao, W.; Maboudian, R.; et al. General Thermal Texturization Process of MoS2 for Efficient Electrocatalytic Hydrogen Evolution Reaction. Nano Lett. 2016, 16, 4047–4053. [Google Scholar] [CrossRef]
- Nguyen, A.D.; Nguyen, T.K.; Le, C.T.; Kim, S.; Ullah, F.; Lee, Y.; Lee, S.; Kim, K.; Lee, D.; Park, S.; et al. Nitrogen-Plasma-Treated Continuous Monolayer MoS2 for Improving Hydrogen Evolution Reaction. ACS Omega 2019, 4, 21509–21515. [Google Scholar] [CrossRef] [PubMed]
- Siao, M.D.; Shen, W.C.; Chen, R.S.; Chang, Z.W.; Shih, M.C.; Chiu, Y.P.; Cheng, C.M. Two-Dimensional Electronic Transport and Surface Electron Accumulation in MoS2. Nat. Commun. 2018, 9, 1442. [Google Scholar] [CrossRef] [PubMed]
- Mikhalitsyna, E.A.; Kataev, V.A.; Larrañaga, A.; Lepalovskij, V.N.; Kurlyandskaya, G.V. Nanocrystallization in FINEMET-Type Fe73.5Nb3Cu1Si13.5B9 and Fe72.5Nb1.5Mo2Cu1.1Si14.2B8.7 Thin Films. Materials 2020, 13, 348. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.; Kumar, B.; Sinha, J.; Ghosh, S.; Roy, S.S.; Kaviraj, B. Cost Effective Liquid Phase Exfoliation of MoS2 Nanosheets and Photocatalytic Activity for Wastewater Treatment Enforced by Visible Light. Sci. Rep. 2020, 10, 10759. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From Bulk to Monolayer MoS2: Evolution of Raman Scattering. Adv. Funct. Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Molina-Sánchez, A.; Hummer, K.; Wirtz, L. Vibrational and Optical Properties of MoS2: From Monolayer to Bulk. Surf. Sci. Rep. 2015, 70, 554–586. [Google Scholar] [CrossRef]
- Verble, J.L.; Wieting, T.J. Lattice Mode Degeneracy in MoS2 and Other Layer Compounds. Phys. Rev. Lett. 1970, 25, 362–365. [Google Scholar] [CrossRef]
- Kong, D.; Wang, H.; Cha, J.J.; Pasta, M.; Koski, K.J.; Yao, J.; Cui, Y. Synthesis of MoS2 and MoSe2 Films with Vertically Aligned Layers. Nano Lett. 2013, 13, 1341. [Google Scholar] [CrossRef]
- Sarker, S.; Peters, J.; Chen, X.; Li, B.; Chen, G.; Yan, L.; Richins, S.K.; Das, S.; Zhou, M.; Luo, H. Engineering Molybdenum Diselenide and Its Reduced Graphene Oxide Hybrids for Efficient Electrocatalytic Hydrogen Evolution. ACS Appl. Nano Mater. 2018, 1, 2143–2152. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, T.; Sun, L.; Sun, Y.; Hu, T.; Xu, K.; Ma, F. Hydrothermal Synthesis of 3D Hierarchical MoSe2/NiSe2 Composite Nanowires on Carbon Fiber Paper and Their Enhanced Electrocatalytic Activity for the Hydrogen Evolution Reaction. J. Mater. Chem. A 2017, 5, 19752–19759. [Google Scholar] [CrossRef]
- Li, G.; Zhang, D.; Qiao, Q.; Yu, Y.; Peterson, D.; Zafar, A.; Kumar, R.; Curtarolo, S.; Hunte, F.; Shannon, S.; et al. All The Catalytic Active Sites of MoS2 for Hydrogen Evolution. J. Am. Chem. Soc. 2016, 138, 16632–16638. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Dong, L.; Tan, L.; Tang, Q. First-Principles Study of Sulfur Vacancy Concentration Effect on the Electronic Structures and Hydrogen Evolution Reaction of MoS2. Nanotechnology 2021, 32, 145718. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, S.; Salamone, M.M.; Robertson, A.W.; Nayak, S.; Kim, H.; Tsang, S.C.E.; Pasta, M.; Warner, J.H. Edge-Enriched 2D MoS2 Thin Films Grown by Chemical Vapor Deposition for Enhanced Catalytic Performance. ACS Catal. 2017, 7, 877–886. [Google Scholar] [CrossRef]
- Sun, Y.; Alimohammadi, F.; Zhang, D.; Guo, G. Enabling Colloidal Synthesis of Edge-Oriented MoS2 with Expanded Interlayer Spacing for Enhanced HER Catalysis. Nano Lett. 2017, 17, 1963–1969. [Google Scholar] [CrossRef]
- Wang, X.; Cormier, C.R.; Khosravi, A.; Smyth, C.M.; Shallenberger, J.R.; Addou, R.; Wallace, R.M. In Situ Exfoliated 2D Molybdenum Disulfide Analyzed by XPS. Surf. Sci. Spectra 2020, 27, 014019. [Google Scholar] [CrossRef]
- Neamen, D.A. Semiconductor Physics and Devices: Basic Principles, 4th ed.; McGraw Hill: New York, NY, USA, 2012. [Google Scholar]
- Peelaers, H.; Van DeWalle, C.G. Effects of Strain on Band Structure and Effective Masses in MoS2. Phys. Rev. B 2012, 86, 241401. [Google Scholar] [CrossRef]
- Chang, Y.S.; Chen, C.Y.; Ho, C.J.; Cheng, C.M.; Chen, H.R.; Fu, T.Y.; Huang, Y.T.; Ke, S.W.; Du, H.Y.; Lee, K.Y.; et al. Surface Electron Accumulation and Enhanced Hydrogen Evolution Reaction in MoSe2 Basal Planes. Nano Energy 2021, 84, 105922. [Google Scholar] [CrossRef]
- Tiong, K.K.; Liao, P.C.; Ho, C.H.; Huang, Y.S. Growth and Characterization of Rhenium-Doped MoS Single Crystals. J. Cryst. Growth 1999, 205, 543–547. [Google Scholar] [CrossRef]
- Yang, F.; Cao, Z.-F.; Wang, J.; Wang, S.; Zhong, H. Novel Preparation of High Activity 1T-Phase MoS2 Ultra-Thin Flakes by Layered Double Hydroxide for Enhanced Hydrogen Evolution Performance. Int. J. Hydrog. Energy 2019, 44, 21229–21237. [Google Scholar] [CrossRef]
- Kim, J.; Takahashi, K.; Takaetsu, T.; Funatsu, T. Characterization of Vertically Aligned MoS2 Thin Film on Mo Electrode for Hydrogen Evolution Catalyst. J. Jpn. Inst. Energy 2021, 100, 283–287. [Google Scholar] [CrossRef]
- Bhimanapati, G.R.; Hankins, T.; Lei, Y.; Vilá, R.A.; Fuller, I.; Terrones, M.; Robinson, J.A. Growth and Tunable Surface Wettability of Vertical MoS2 Layers for Improved Hydrogen Evolution Reactions. ACS Appl. Mater. Interfaces 2016, 8, 22190–22195. [Google Scholar] [CrossRef]
- Liu, N.; Kim, J.; Oh, J.; Nguyen, Q.T.; Sahu, B.B.; Han, J.G.; Kim, S. Growth of Multiorientated Polycrystalline MoS2 Using Plasma-Enhanced Chemical Vapor Deposition for Efficient Hydrogen Evolution Reactions. Nanomaterials 2020, 10, 1465. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zheng, L.; Zhu, Z.; Chen, J.; Kang, J.; Huang, Z.; Yang, D. MoS2 Nanosheet Arrays Rooted on Hollow RGO Spheres as Bifunctional Hydrogen Evolution Catalyst and Supercapacitor Electrode. Nano-Micro Lett. 2018, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, J.; Feng, C.; Xu, G.; Xie, C.; Yuan, X.; Xiang, B. MoS2 Nanosheet/MoS2 Flake Homostructures for Efficient Electrocatalytic Hydrogen Evolution MoS2 Nanosheet/MoS2 Fl Ake Homostructures for Efficient Electrocatalytic Hydrogen Evolution. Mater. Res. Express 2019, 6, 085005. [Google Scholar] [CrossRef]
- Bojarska, Z.; Mazurkiewicz-Pawlicka, M.; Mierzwa, B.; Plocinski, T.; Makowski, L. Effect of the Carbon Support on MoS2 hybrid Nanostructures Prepared by an Impinging Jet Reactor for Hydrogen Evolution Reaction Catalysis. J. Environ. Chem. Eng. 2022, 10, 108038. [Google Scholar] [CrossRef]
- Singh, A.K.; Prasad, J.; Azad, U.P.; Singh, A.K.; Prakash, R.; Singh, K.; Srivastava, A.; Alaferdov, A.A.; Moshkalev, S.A. Vanadium Doped Few-Layer Ultrathin MoS2 Nanosheets on Reduced Graphene Oxide for High-Performance Hydrogen Evolution Reaction. RSC Adv. 2019, 9, 22232–22239. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, W.; Hu, Y.; Zhang, Y.; Dang, J.; Wu, Y.; Chen, B.; Zhao, H.; Li, Z. Single Atom Ru Doping 2H-MoS2 as Highly Efficient Hydrogen Evolution Reaction Electrocatalyst in a Wide pH Range. Appl. Catal. B Environ. 2021, 298, 120490. [Google Scholar] [CrossRef]
- Zhang, W.; Liao, X.; Pan, X.; Yan, M.; Li, Y.; Tian, X.; Zhao, Y.; Xu, L.; Mai, L. Superior Hydrogen Evolution Reaction Performance in 2H-MoS2 to That of 1T Phase. Small 2019, 15, 1900964. [Google Scholar] [CrossRef]
- Tang, Q.; Jiang, D.E. Mechanism of Hydrogen Evolution Reaction on 1T-MoS2 from First Principles. ACS Catal. 2016, 6, 4953–4961. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).