Effect of Modified Alumina Support on the Performance of Ni-Based Catalysts for CO2 Reforming of Methane
Abstract
1. Introduction
2. Results and Discussion
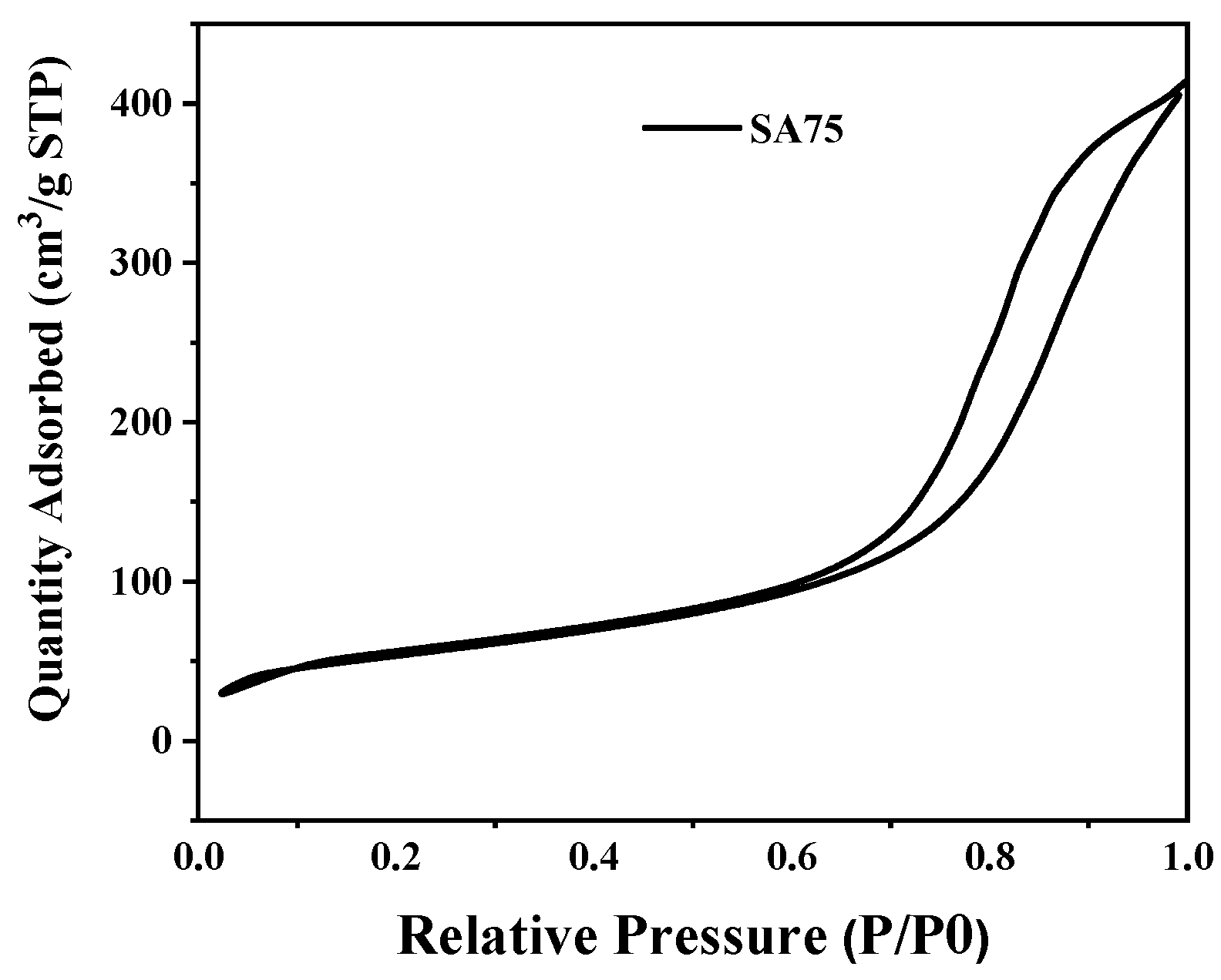
2.1. BET Analysis
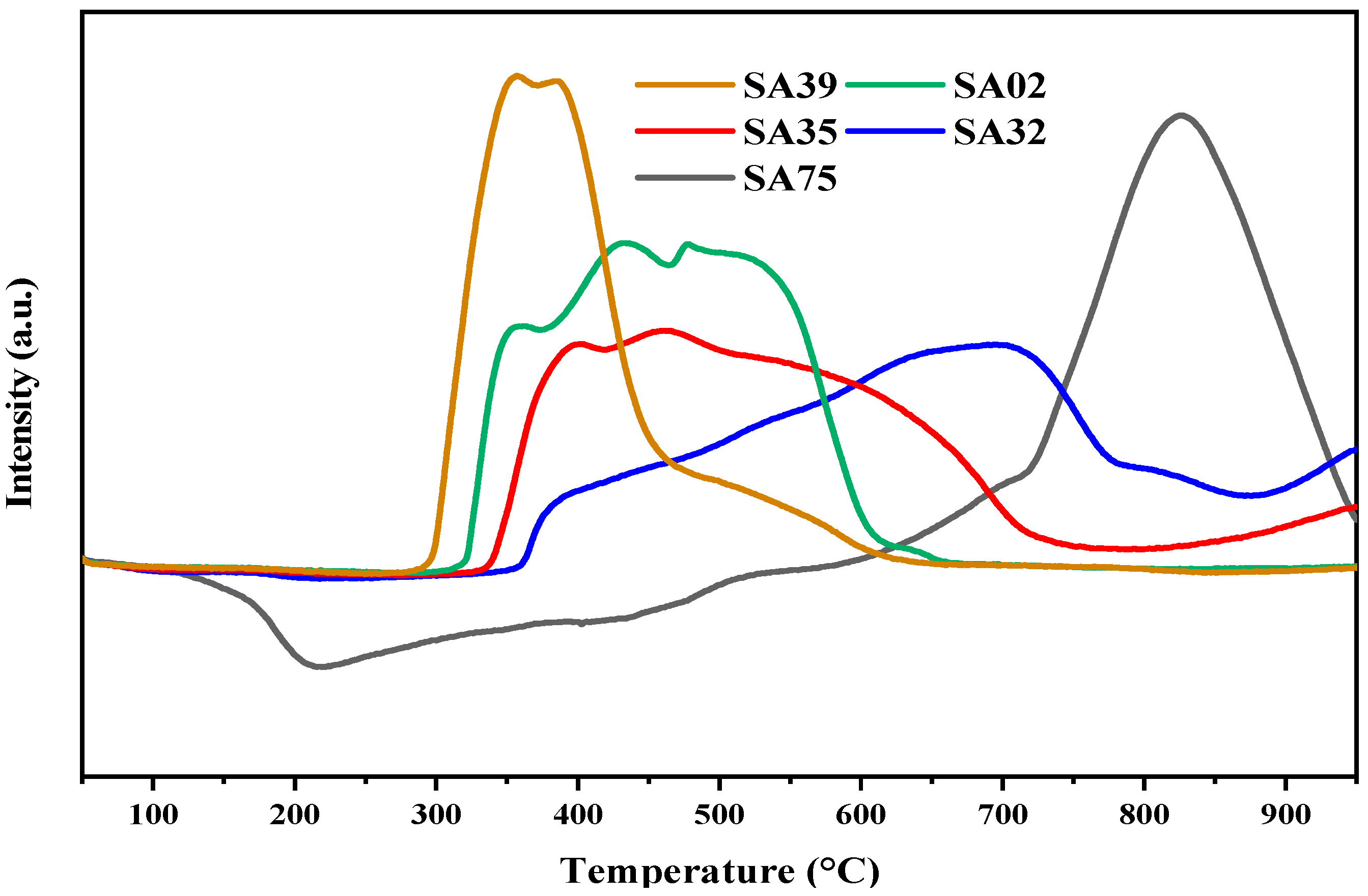
2.2. TPR
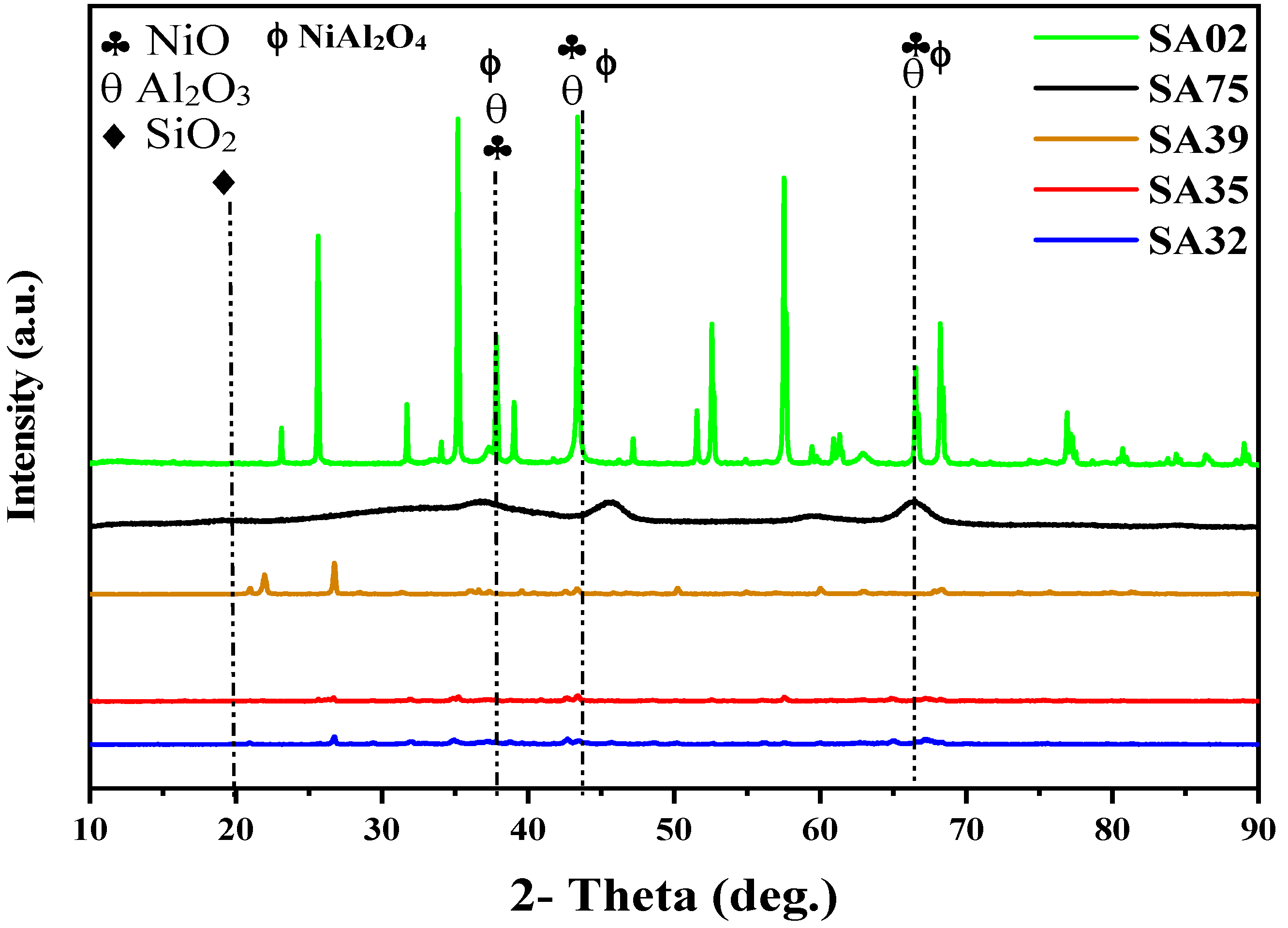
2.3. XRD Analysis
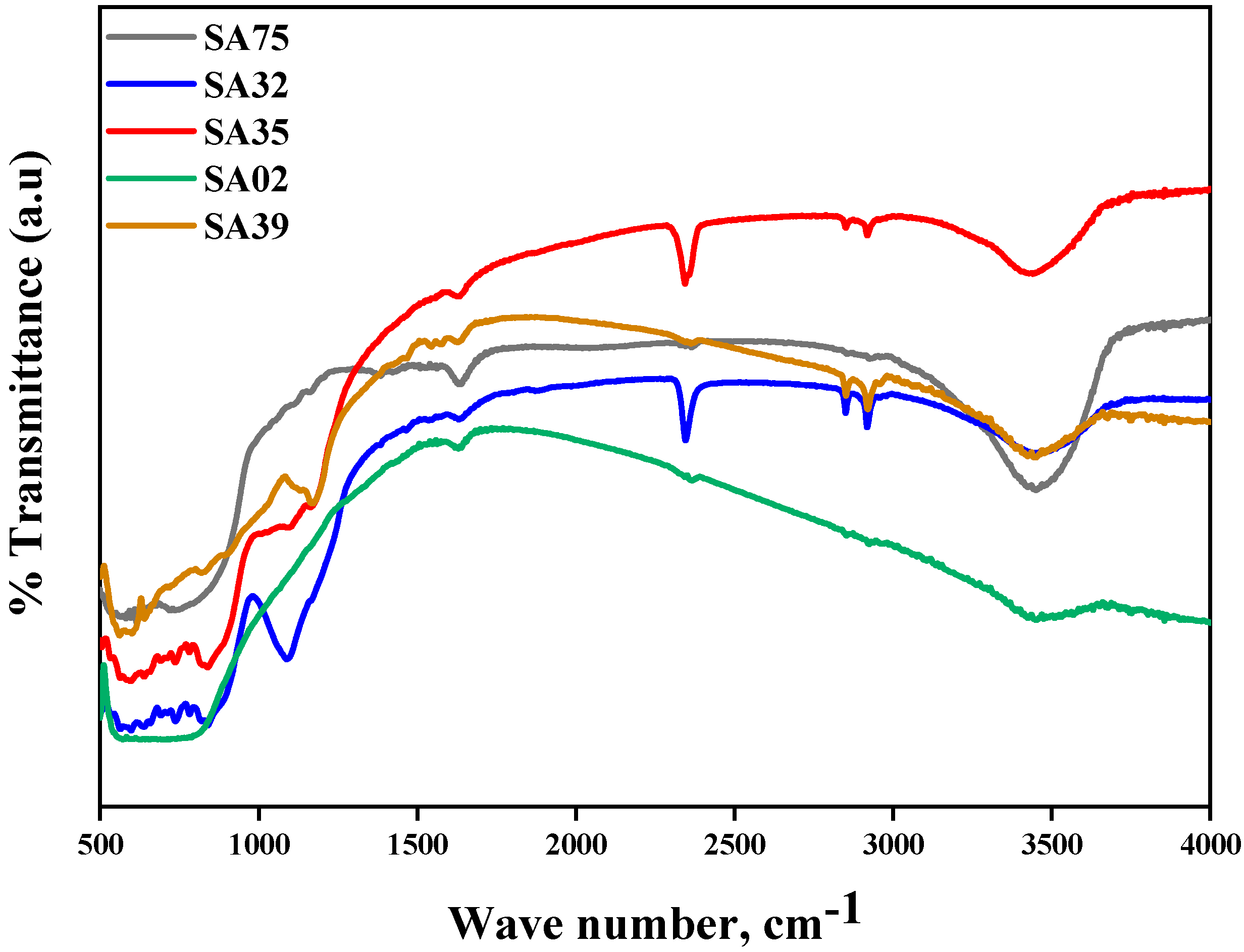
2.4. FTIR Analysis
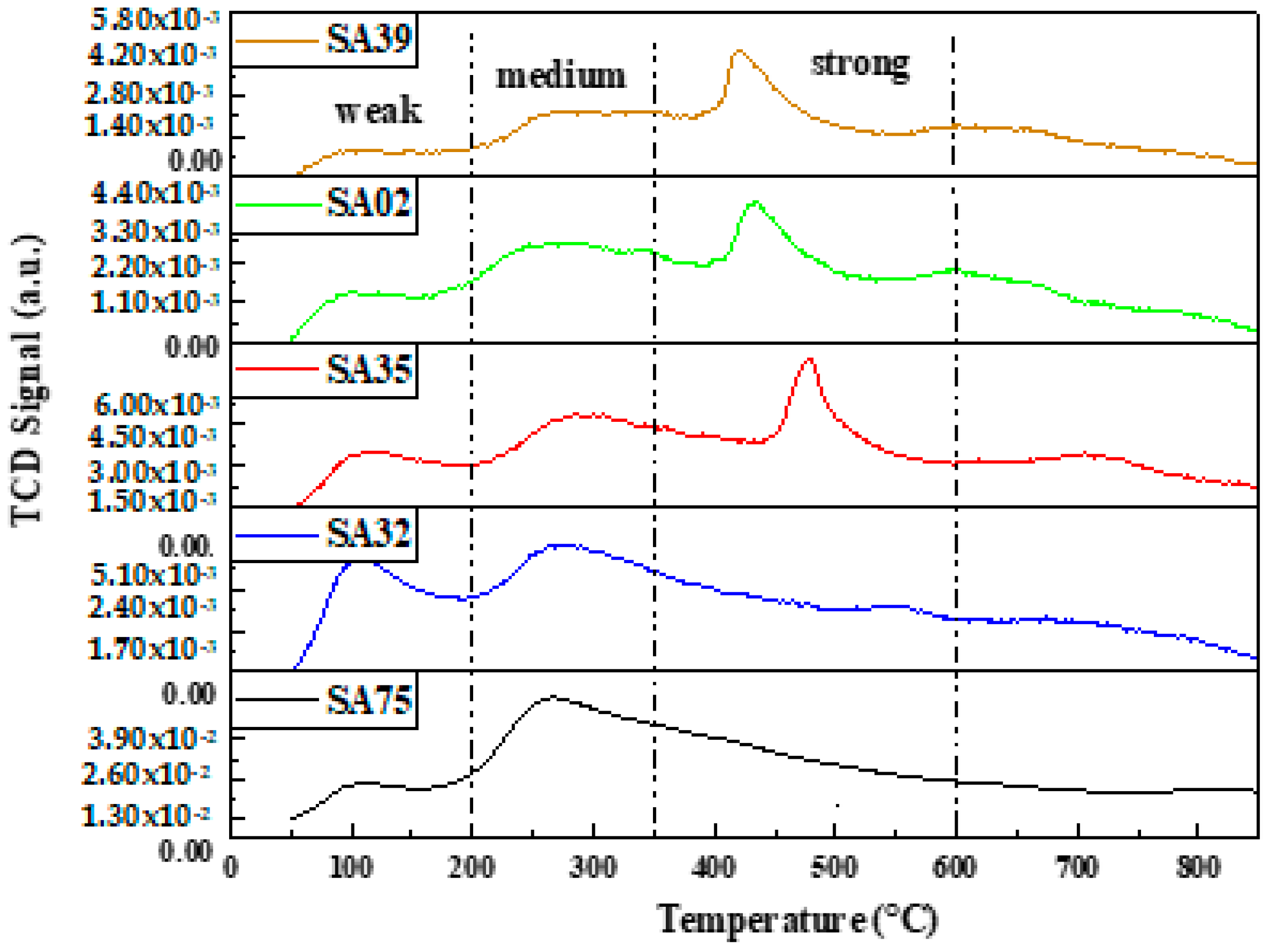
2.5. CO2-TPD
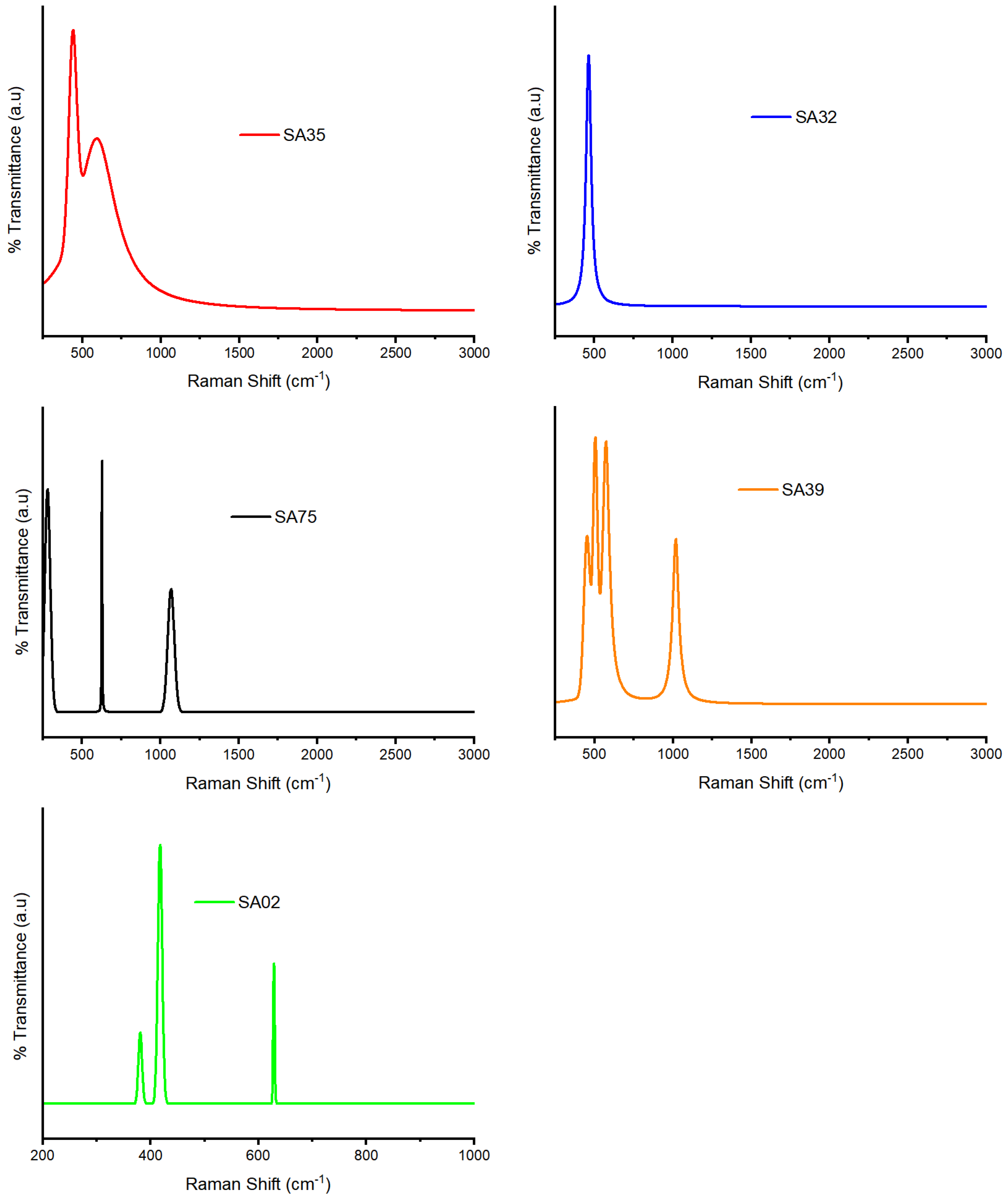
2.6. Raman Analysis
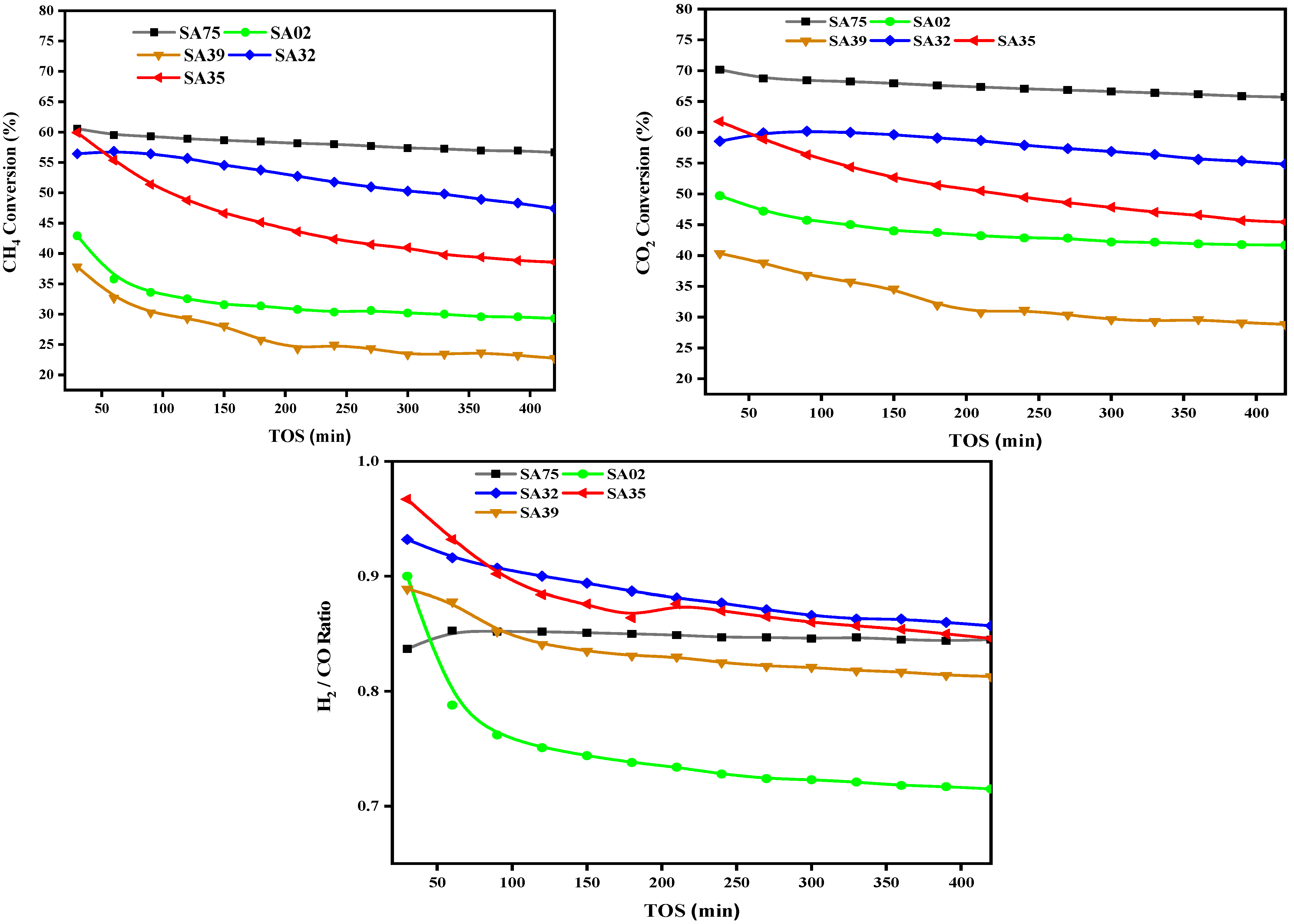
2.7. Catalyst Activity
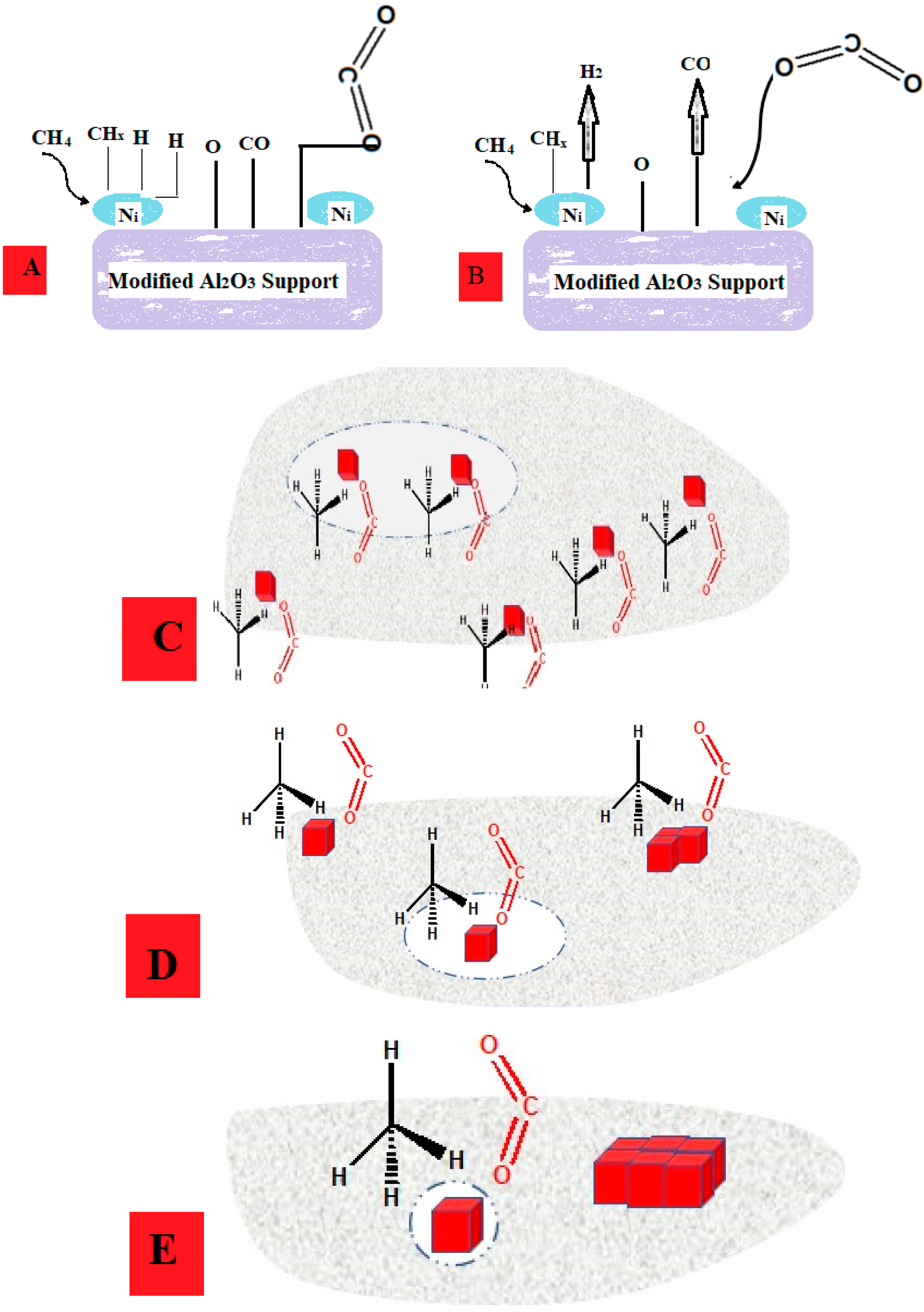
2.8. Mechanism of CRM
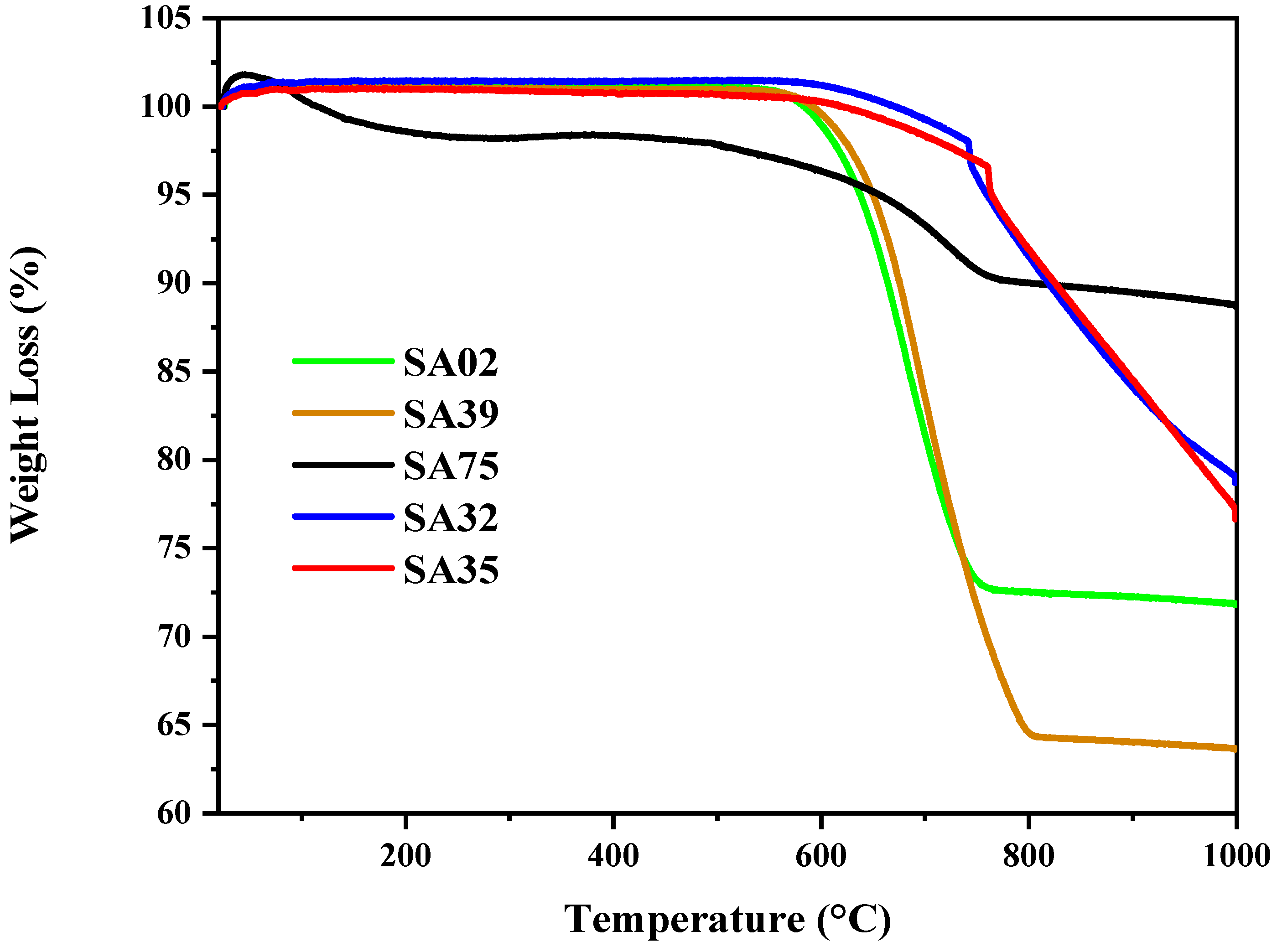
2.9. TGA
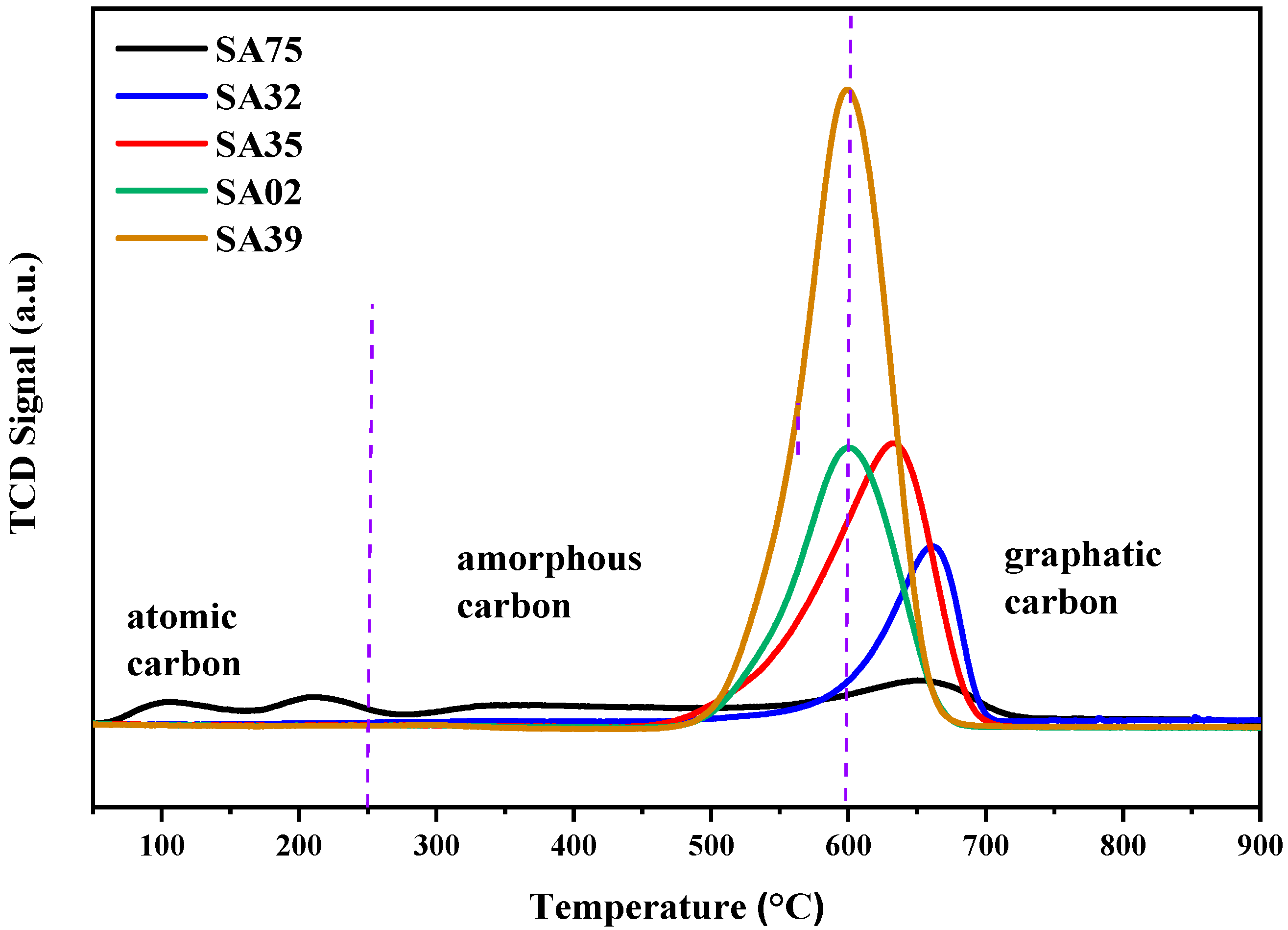
2.10. O2-TPO
3. Experimental Procedure
3.1. Catalysts’ Preparation
3.2. Synthesis of Catalysts
3.3. Catalyst Characterization
3.4. Catalytic Activity Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akri, M.; Zhao, S.; Li, X.; Zang, K.; Lee, A.F.; Isaacs, M.A.; Xi, W.; Gangarajula, Y.; Luo, J.; Ren, Y.; et al. Atomically dispersed nickel as coke-resistant active sites for methane dry reforming. Nat. Commun. 2019, 10, 5181–5191. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, I.R.; da Silva, A.A.A.; Xing, Y.T.; Rabelo-Neto, R.C.; Luchters, N.T.J.; Fletcher, J.C.Q.; Noronha, F.B.; Mattos, L.V. Long-term stability of Pt/Ce0.8Me0.2O2-γ/Al2O3 (Me=Gd, Nb, Pr, and Zr) catalysts for steam reforming of methane. Int. J. Hydrogen Energy 2022, 47, 15624–15640. [Google Scholar] [CrossRef]
- Araújo, J.C.S.; Oton, L.F.; Bessa, B.; Neto, A.B.S.; Oliveira, A.C.; Lang, R.; Otubo, L.; Bueno, J.M.C. The role of Pt loading on La2O3-Al2O3 support for methane conversion reactions via partial oxidation and steam reforming. Fuel 2019, 254, 115681–115696. [Google Scholar] [CrossRef]
- Ruan, G.; Zhang, Z.; Metals, M.Y.-R. Undefined Effect of SiO2 micro powder on properties of corundum-mullite composites. Rare Met. 2011, 30, 506–510. [Google Scholar] [CrossRef]
- Therdthianwong, S.; Siangchin, C.; Therdthianwong, A. Improvement of coke resistance of Ni/Al2O3 catalyst in CH4/CO2 reforming by ZrO2 addition. Fuel Process. Technol. 2008, 89, 160–168. [Google Scholar] [CrossRef]
- Pompeo, F.; Nichio, N.N.; Souza, M.M.V.M.; Cesar, D.V.; Ferretti, O.A.; Schmal, M. Study of Ni and Pt catalysts supported on α-Al2O3 and ZrO2 applied in methane reforming with CO2. Appl. Catal. A Gen. 2007, 316, 175–183. [Google Scholar] [CrossRef]
- Moogi, S.; Hyun Ko, C.; Hoon Rhee, G.; Jeon, B.H.; Ali Khan, M.; Park, Y.K. Influence of catalyst synthesis methods on anti-coking strength of perovskites derived catalysts in biogas dry reforming for syngas production. Chem. Eng. J. 2022, 437, 135348. [Google Scholar] [CrossRef]
- Da Costa-Serra, J.F.; Chica, A. Bioethanol steam reforming on Co/ITQ-18 catalyst: Effect of the crystalline structure of the delaminated zeolite ITQ-18. Int. J. Hydrogen Energy 2011, 36, 3862–3869. [Google Scholar] [CrossRef]
- Da Costa-Serra, J.F.; Guil-López, R.; Chica, A. Co/ZnO and Ni/ZnO catalysts for hydrogen production by bioethanol steam reforming. Influence of ZnO support morphology on the catalytic properties of Co and Ni active phases. Int. J. Hydrogen Energy 2010, 35, 6709–6716. [Google Scholar] [CrossRef]
- Zhou, L.; Li, L.; Wei, N.; Li, J.; Basset, J.M. Effect of NiAl2O4 Formation on Ni/Al2O3 Stability during Dry Reforming of Methane. ChemCatChem 2015, 7, 2508–2516. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Liu, Y.; Zhang, Y. Dry reforming of methane over Ni/MgO-Al2O3 catalysts prepared by two-step hydrothermal method. Appl. Surf. Sci. 2016, 389, 25–33. [Google Scholar] [CrossRef]
- Fuertes, A.; Da Costa-Serra, J.F.; Chica, A. New Catalysts based on Ni-Birnessite and Ni-Todorokite for the Efficient Production of Hydrogen by Bioethanol Steam Reforming. Energy Procedia 2012, 29, 181–191. [Google Scholar] [CrossRef][Green Version]
- Wang, F.; Xu, L.; Zhang, J.; Zhao, Y.; Li, H.; Li, H.X.; Wu, K.; Xu, G.Q.; Chen, W. Tuning the metal-support interaction in catalysts for highly efficient methane dry reforming reaction. Appl. Catal. B Environ. 2016, 180, 511–520. [Google Scholar] [CrossRef]
- Elharati, M.A.; Lee, K.M.; Hwang, S.; Mohammed Hussain, A.; Miura, Y.; Dong, S.; Fukuyama, Y.; Dale, N.; Saunders, S.; Kim, T.; et al. The effect of silica oxide support on the catalytic activity of nickel-molybdenum bimetallic catalyst toward ethanol steam reforming for hydrogen production. Chem. Eng. J. 2022, 441, 135916. [Google Scholar] [CrossRef]
- Xu, Y.; Du, X.H.; Li, J.; Wang, P.; Zhu, J.; Ge, F.J.; Zhou, J.; Song, M.; Zhu, W.Y. A comparison of Al2O3 and SiO2 supported Ni-based catalysts in their performance for the dry reforming of methane. J. Fuel Chem. Technol. 2019, 47, 199–208. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, R.; Zu, Y.; Zhu, L.; Mei, Y.; Luo, Y.; He, D. Low-temperature dry reforming of methane tuned by chemical speciations of active sites on the SiO2 and γ-Al2O3 supported Ni and Ni-Ce catalysts. Chin. J. Chem. Eng. 2022, 48, 76–90. [Google Scholar] [CrossRef]
- Rabelo-Neto, R.C.; Sales, H.B.E.; Inocêncio, C.V.M.; Varga, E.; Oszko, A.; Erdohelyi, A.; Noronha, F.B.; Mattos, L.V. CO2 reforming of methane over supported LaNiO3 perovskite-type oxides. Appl. Catal. B Environ. 2018, 221, 349–361. [Google Scholar] [CrossRef]
- Cao, P.; Zhao, H.; Adegbite, S.; Yang, B.; Lester, E.; Wu, T. Stabilized CO2 reforming of CH4 on modified Ni/Al2O3 catalysts via in-situ K2CO3-enabled dynamic coke elimination reaction. Fuel 2021, 298, 120599. [Google Scholar] [CrossRef]
- Garbarino, G.; Phung, T.K.; Pampararo, G.; Riani, P.; Busca, G. Modification of the properties of γ-alumina as a support for nickel and molybdate catalysts by addition of silica. Catal. Today 2021, 378, 57–64. [Google Scholar] [CrossRef]
- Maryam, K.M.; Huang, B.; Calvin, H.B.; Todd, M.A.; Brian, F.W. Synthesis and characterization of silica doped alumina catalyst support with superior thermal stability and unique pore properties. J. Porous Mater. 2016, 23, 475–487. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Hao, M.; Bai, J.; Fang, B.; Liang, J.; Gao, P.; Ding, Y.; Li, H.; Wang, F. A simple fabrication of mineral supported Ni-NiAl2O4 nanocomposites with a novel transition layer. Mater. Charact. 2022, 192, 112194. [Google Scholar] [CrossRef]
- Pettit, F.S.; Randklev, E.H.; Felten, E.J. Formation of NiAl2O4 by Solid State Reaction. J. Am. Ceram. Soc. 1966, 49, 199–203. [Google Scholar] [CrossRef]
- Yang, R.; Qi, Z.; Gao, Y.; Yang, J.; Zhou, Y.; Liu, H.; Peng, L.; Jiao, J. Effects of alumina sols on the sintering of α-alumina ceramics. Ceram. Int. 2020, 46, 20865–20870. [Google Scholar] [CrossRef]
- Vlaev, L.; Damyanov, D.; Mohamed, M.M. Infrared spectroscopy study of the nature and reactivity of a hydrate coverage on the surface of γ-Al2O3. Colloids Surf. 1989, 36, 427–437. [Google Scholar] [CrossRef]
- Calatayud, M.; Collins, S.E.; Baltanás, M.A.; Bonivardi, A.L. Stability of formate species on β-Ga2O3. Phys. Chem. Chem. Phys. 2009, 11, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Reshetenko, T.V.; Avdeeva, L.B.; Khassin, A.A.; Kustova, G.N.; Ushakov, V.A.; Moroz, E.M.; Shmakov, A.N.; Kriventsov, V.V.; Kochubey, D.I.; Pavlyukhin, Y.T.; et al. Coprecipitated iron-containing catalysts (Fe-Al2O3, Fe-Co-Al2O3, Fe-Ni-Al2O3) for methane decomposition at moderate temperatures I. Genesis of calcined and reduced catalysts. Appl. Catal. A Gen. 2004, 268, 127–138. [Google Scholar] [CrossRef]
- Ghule, A.V.; Ghule, K.; Punde, T.; Liu, J.Y.; Tzing, S.H.; Chang, J.Y.; Chang, H.; Ling, Y.C. In situ monitoring of NiO– Al2O3 nanoparticles synthesis by thermo-Raman spectroscopy. Mater. Chem. Phys. 2010, 119, 86–92. [Google Scholar] [CrossRef]
- Shi, Y.; Tian, S.; Shi, Q.; Zhang, Y.; Waheed, A.; Cao, Y.; Li, G. Cascade Aldol Condensation of an Aldehyde via the Aerobic Oxidation of Ethanol over an Au/NiO Composite. Nanoscale Adv. 2019, 1, 3654–3659. [Google Scholar] [CrossRef]
- Cao, Y.; Su, Y.; Xu, L.; Yang, X.; Han, Z.; Cao, R.; Li, G. Ionic liquids modified oxygen vacancy-rich amorphous FeNi hydroxide nanoclusters on carbon-based materials as an efficient electrocatalyst for electrochemical water oxidation. J. Energy Chem. 2022, 71, 167–173. [Google Scholar] [CrossRef]
- Gunduz-Meric, G.; Kaytakoglu, S.; Degirmenci, L. Ni,Co/SiO2 and Ni/SiO2,Co bimetallic microsphere catalysts indicating high activity and stability in the dry reforming of methane. React. Kinet. Mech. Catal. 2020, 129, 403–419. [Google Scholar] [CrossRef]
- Deb, M.; Banerjee, R.; Majumder, A.; Sastry, G.R.K. Multi objective optimization of performance parameters of a single cylinder diesel engine with hydrogen as a dual fuel using pareto-based genetic algorithm. Int. J. Hydrogen Energy 2014, 39, 8063–8077. [Google Scholar] [CrossRef]
- Ha, Q.L.M.; Armbruster, U.; Atia, H.; Schneider, M.; Lund, H.; Agostini, G.; Radnik, J.; Vuong, H.T.; Martin, A. Development of Active and Stable Low Nickel Content Catalysts for Dry Reforming of Methane. Catalysts 2017, 7, 157. [Google Scholar] [CrossRef]
- Fouskas, A.; Kollia, M.; Kambolis, A.; Papadopoulou, C.; Matralis, H. Boron-modified Ni/Al2O3 catalysts for reduced carbon deposition during dry reforming of methane. Appl. Catal. A Gen. 2014, 474, 125–134. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Kumar, R.; Kasim, S.O.; Ibrahim, A.A.; Fakeeha, A.H.; Abasaeed, A.E.; Alrasheed, R.; Bagabas, A.; Chaudhary, M.L.; Frusteri, F.; et al. The effect of modifier identity on the performance of Ni-based catalyst supported on γ-Al2O3 in dry reforming of methane. Catal. Today 2020, 348, 236–242. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Naeem, M.A.; Fakeeha, A.H.; Abasaeed, A.E. CO2 Reforming of Methane to Produce Syngas over γ-Al2O3-Supported Ni–Sr Catalysts. Bull. Chem. Soc. Jpn. 2013, 86, 742–748. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Bagabas, A.A.; Lanre, M.S.; Osman, A.I.; Kasim, S.O.; Ibrahim, A.A.; Arasheed, R.; Alkhalifa, A.; Elnour, A.Y.; Abasaeed, A.E.; et al. Catalytic Performance of Metal Oxides Promoted Nickel Catalysts Supported on Mesoporous γ-Alumina in Dry Reforming of Methane. Process 2020, 8, 522. [Google Scholar] [CrossRef]
- Li, K.; Pei, C.; Li, X.; Chen, S.; Zhang, X.; Liu, R.; Gong, J. Dry reforming of methane over La2O2CO3-modified Ni/Al2O3 catalysts with moderate metal support interaction. Appl. Catal. B Environ. 2020, 264, 118448. [Google Scholar] [CrossRef]
- Amin, M.H.; Mantri, K.; Newnham, J.; Tardio, J.; Bhargava, S.K. Highly stable ytterbium promoted Ni/γ-Al2O3 catalysts for carbon dioxide reforming of methane. Appl. Catal. B Environ. 2012, 119, 217–226. [Google Scholar] [CrossRef]
- Rahemi, N.; Haghighi, M.; Babaluo, A.A.; Jafari, M.F.; Khorram, S. Non-thermal plasma assisted synthesis and physicochemical characterizations of Co and Cu doped Ni/Al2O3 nanocatalysts used for dry reforming of methane. Int. J. Hydrogen Energy 2013, 38, 16048–16061. [Google Scholar] [CrossRef]
- Chein, R.Y.; Fung, W.Y. Syngas production via dry reforming of methane over CeO2 modified Ni/Al2O3 catalysts. Int. J. Hydrogen Energy 2019, 44, 14303–14315. [Google Scholar] [CrossRef]
- Song, Z.; Wang, Q.; Guo, C.; Li, S.; Yan, W.; Jiao, W.; Qiu, L.; Yan, X.; Li, R. Improved effect of Fe on the stable NiFe/Al2O3 catalyst in low-temperature dry reforming of methane. Ind. Eng. Chem. Res. 2020, 59, 17250–17258. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, C.; Chen, B.; Zhang, Y.; Qiu, J. An active and coke-resistant dry reforming catalyst comprising nickel-tungsten alloy nanoparticles. Catal. Commun. 2015, 69, 123–128. [Google Scholar] [CrossRef]
- Shen, J.; Reule, A.A.C.; Semagina, N. Ni/MgAl2O4 catalyst for low-temperature oxidative dry methane reforming with CO2. Int. J. Hydrogen Energy 2019, 44, 4616–4629. [Google Scholar] [CrossRef]

| Catalysts | BET (m2/g) | Catalyst Weight (mg) | Feed Ratio | Activation Temp. (°C) | Reaction Temp. (°C) | TOS (h) | Conversion (%) | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CO2 | CH4 | Inert | CH4 | CO2 | |||||||
| 5%Ni/Al2O3 | 132 | 200 | 1 | 1 | 0 | 700 | 700 | 5 | 60 | 70 | [32] |
| Ni/Al.550 | 178 | - | 9 | 9 | 2 | 700 | 650 | 8 | 48 | 52 | [33] |
| Ni/Al | 191 | 100 | 1 | 1 | 0 | 800 | 700 | 24 | 55 | 65 | [34] |
| 5Ni3SiAl | 222.08 | 100 | 6 | 6 | 1 | 800 | 700 | 7 | 65 | 70 | [35] |
| Ni/Al | 189.5 | 300 | 5 | 5 | 1 | 600 | 700 | 6 | 79.9 | - | [36] |
| 5NiAl | 182 | 100 | 3 | 3 | 1 | 600 | 700 | 7 | 79 | 85 | [37] |
| 5Ni/Al2O3 | 160.8 | 250 | 3 | 3 | 14 | 650 | 650 | 50 | 57 | 61 | [38] |
| Ni/γ-Al2O3 | 123 | - | 1 | 1 | 2 | 700 | 700 | 20 | 55 | 64 | [39] |
| Ni/Al2O3 | 113 | - | 1 | 1 | 0 | 700 | 850 | 24 | 80 | 85 | [40] |
| 10Ni/Al2O3 | 120 | 100 | 1 | 1 | 0 | 700 | 750 | 4 | 60 | 70 | [41] |
| Ni/Al2O3 | 200 | 100 | 1 | 1 | 0 | 550 | 450 | 20 | 8 | 10 | [42] |
| SA75 | 260 | 100 | 3 | 3 | 1 | 700 | 700 | 7 | 58.2 | 67.7 | This work |
| SA32 | 30 | 100 | 3 | 3 | 1 | 700 | 700 | 7 | 52.8 | 58.7 | |
| SA35 | 12 | 100 | 3 | 3 | 1 | 700 | 700 | 7 | 43.5 | 50.5 | |
| SA02 | 3 | 100 | 3 | 3 | 1 | 700 | 700 | 7 | 31.2 | 43.3 | |
| SA39 | 0.3 | 100 | 3 | 3 | 1 | 700 | 700 | 7 | 24.7 | 31 | |
| Support Name | Surface Area (m2/g) | Pore Diameter | Pore Volume (cm3/g) | Purity (wt.%) |
|---|---|---|---|---|
| SA-6175 | High (>120) 260.0 | 10 nm | 0.83 | <0.05% Na2O |
| SA-3132 | Intermediate (10–120) 30.0 | 1.2 µm | 0.55 | 17.9% SiO2 |
| SA-3135 | Intermediate (10–120) 12.0 | 1.2 µm | 0.53 | 17.9% SiO2 |
| SA-5202 | Low (0–10) 3.0 | 1.2 µm/ 16.1 nm | 0.3/0.01 | <0.05 SiO2 |
| SA-5239 | Low (0–10) 0.3 | 1.8/150 µm | 0.43 | 12% SiO2 |
| Catalyst Tested | Catalyst’s Abbreviated Name | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|---|
| 5% Ni/SA-6175 | SA75 | 189.0 | 0.66 | 12.8 |
| 5% Ni/SA-3132 | SA32 | 25.3 | 0.14 | 22.5 |
| 5% Ni/SA-3135 | SA35 | 15.6 | 0.11 | 30.0 |
| 5% Ni/SA-5202 | SA02 | 2.7 | 0.01 | 17.4 |
| 5% Ni/SA-5239 | SA39 | 2.3 | 0.01 | 17.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alreshaidan, S.B.; Ibrahim, A.A.; Fakeeha, A.H.; Almutlaq, A.M.; Ali, F.A.A.; Al-Fatesh, A.S. Effect of Modified Alumina Support on the Performance of Ni-Based Catalysts for CO2 Reforming of Methane. Catalysts 2022, 12, 1066. https://doi.org/10.3390/catal12091066
Alreshaidan SB, Ibrahim AA, Fakeeha AH, Almutlaq AM, Ali FAA, Al-Fatesh AS. Effect of Modified Alumina Support on the Performance of Ni-Based Catalysts for CO2 Reforming of Methane. Catalysts. 2022; 12(9):1066. https://doi.org/10.3390/catal12091066
Chicago/Turabian StyleAlreshaidan, Salwa Bader, Ahmed A. Ibrahim, Anis H. Fakeeha, Abdulaziz M. Almutlaq, Fekri Abdulraqeb Ahmed Ali, and Ahmed S. Al-Fatesh. 2022. "Effect of Modified Alumina Support on the Performance of Ni-Based Catalysts for CO2 Reforming of Methane" Catalysts 12, no. 9: 1066. https://doi.org/10.3390/catal12091066
APA StyleAlreshaidan, S. B., Ibrahim, A. A., Fakeeha, A. H., Almutlaq, A. M., Ali, F. A. A., & Al-Fatesh, A. S. (2022). Effect of Modified Alumina Support on the Performance of Ni-Based Catalysts for CO2 Reforming of Methane. Catalysts, 12(9), 1066. https://doi.org/10.3390/catal12091066









