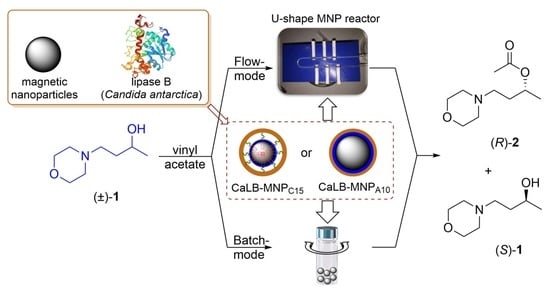
A Convenient U-Shape Microreactor for Continuous Flow Biocatalysis with Enzyme-Coated Magnetic Nanoparticles-Lipase-Catalyzed Enantiomer Selective Acylation of 4-(Morpholin-4-yl)butan-2-ol
Abstract
1. Introduction
2. Results and Discussion
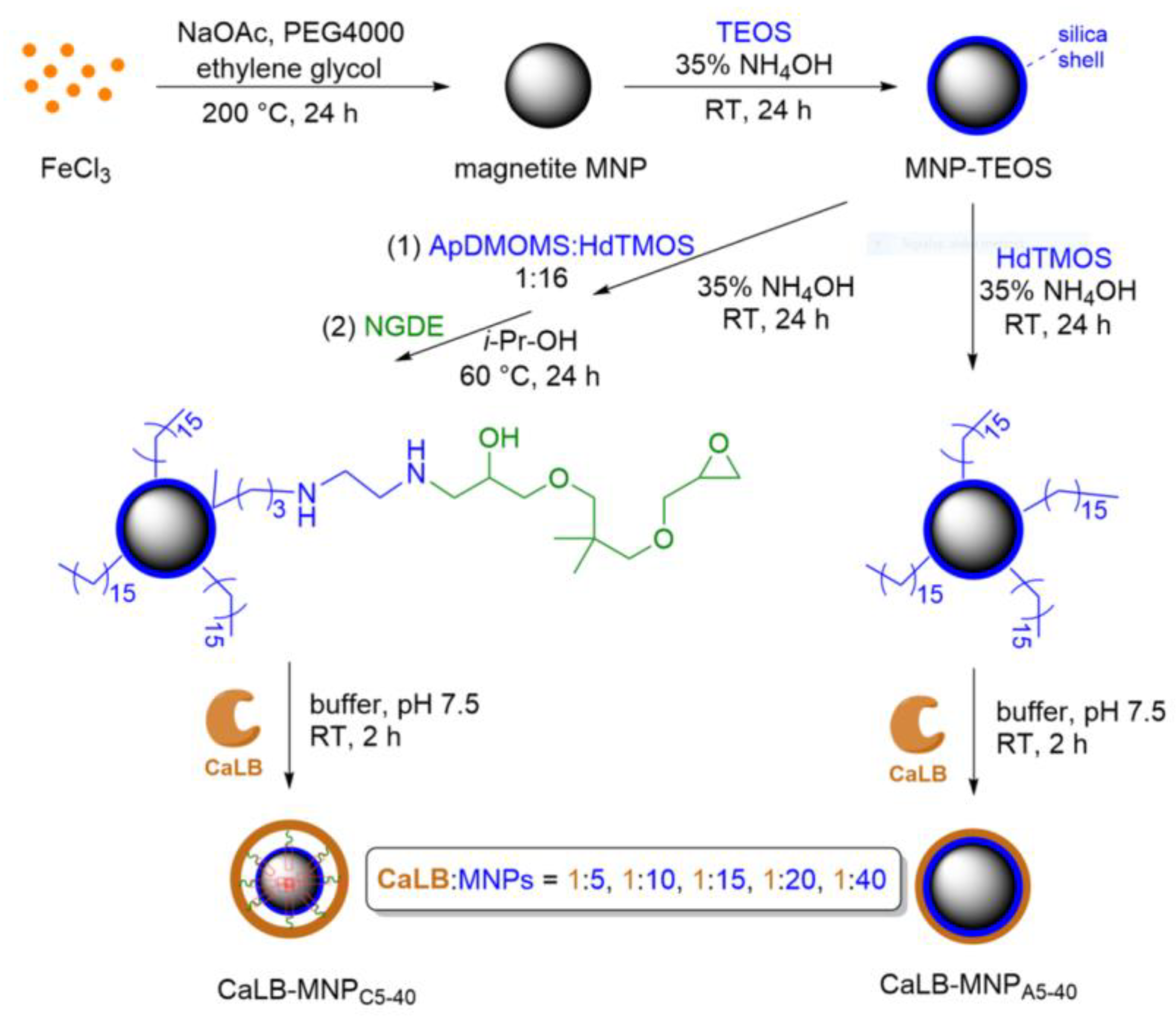
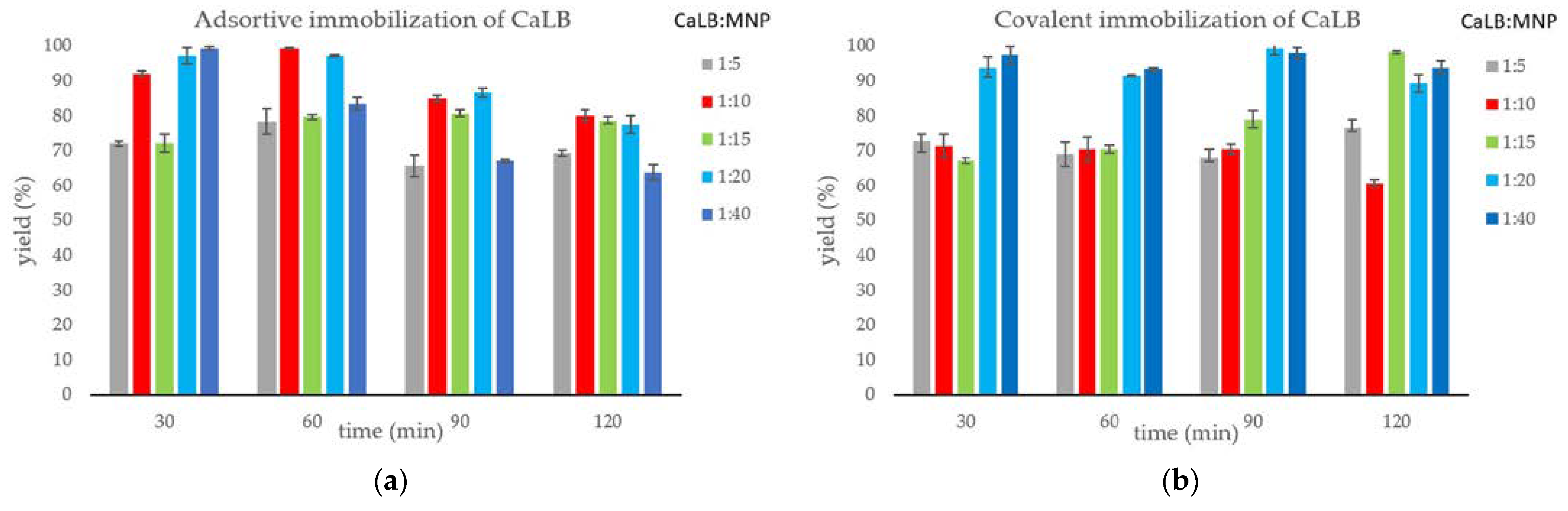
2.1. Immobilization of Lipase B from Candida antarctica onto Magnetic Nanoparticles
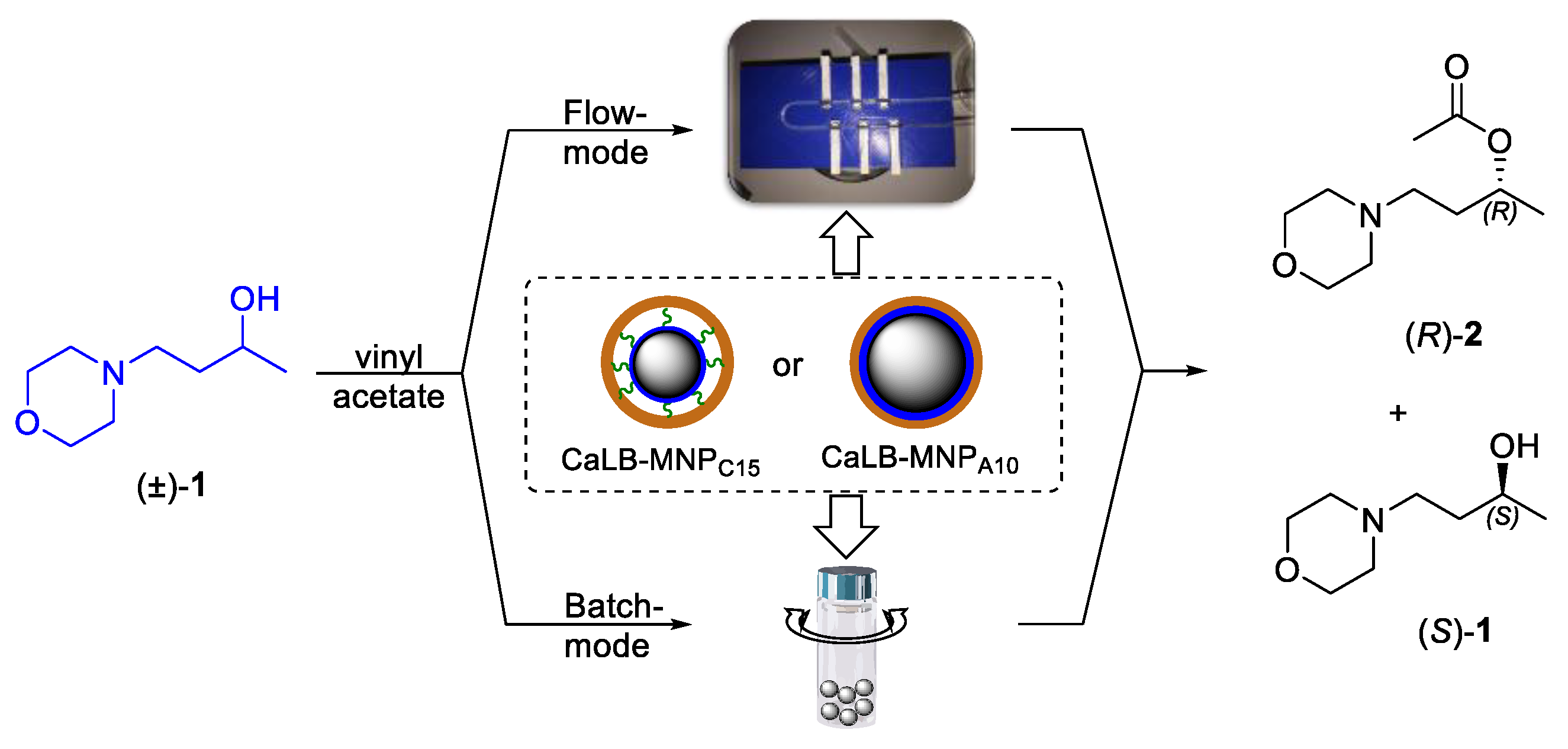
2.2. Kinetic Resolution of Racemic 4-(Morpholin-4-yl)butan-2-ol (±)-1 with CaLB-MNP Biocatalysts in Batch Mode
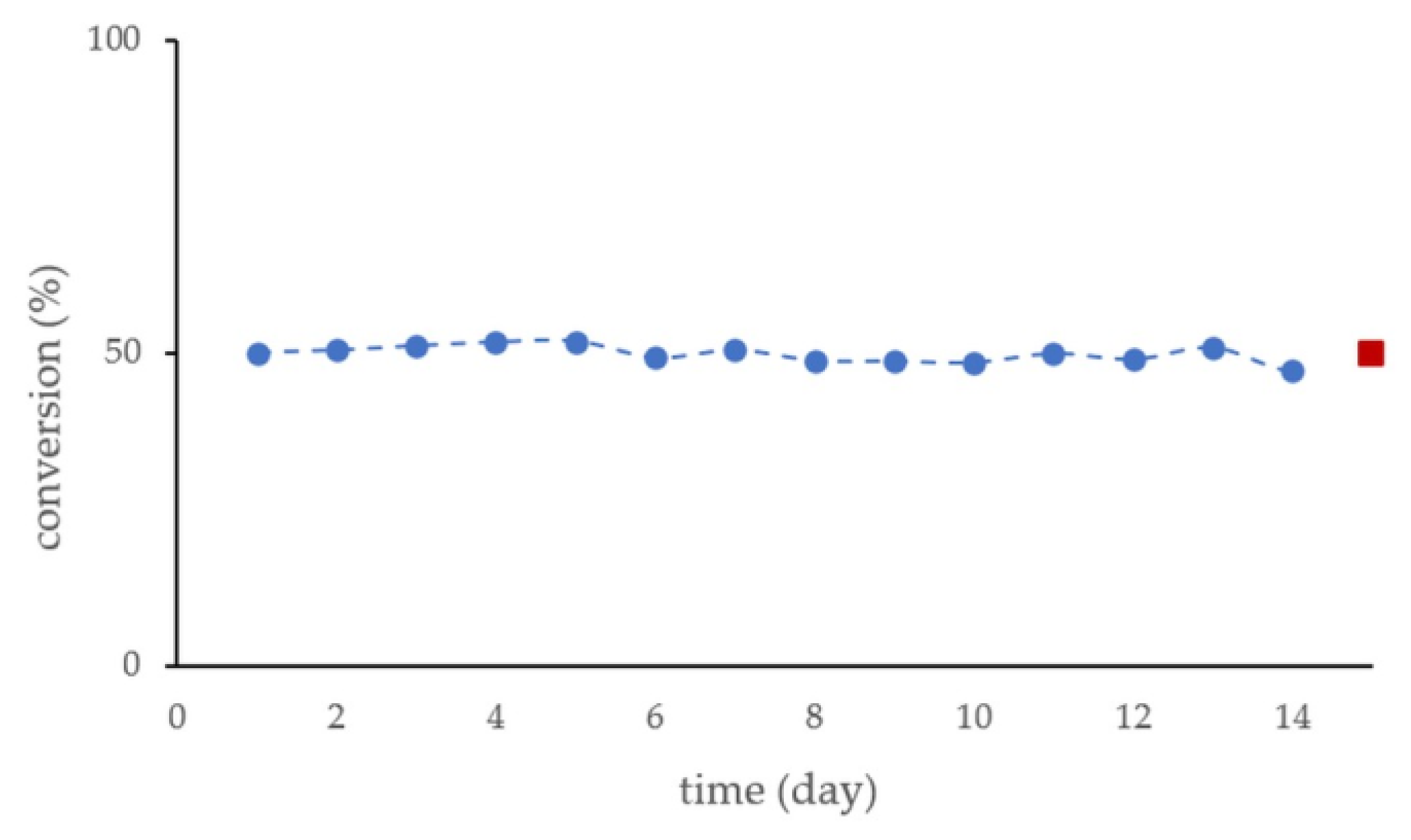
2.3. Kinetic Resolution of Racemic 4-(Morpholin-4-yl)butan-2-ol (±)-1 with CaLB-MNP Biocatalysts in the U-Shape Reactor in Continuous Flow Mode
3. Materials and Methods
3.1. Materials
3.2. Analytical and Separation Methods
3.3. Comparative Activity Tests of the CaLB-MNP Biocatalysts by Acetylation of (±)-1
3.4. Kinetic Resolution of (±)-1 by the CaLB-MNPC15 Biocatalysts in Batch Mode
3.4.1. (R)-4-(Morpholin-4-yl)butan-2-yl acetate (R)-2
3.4.2. (S)-4-(Morpholin-4-yl)butan-2-ol (S)-1
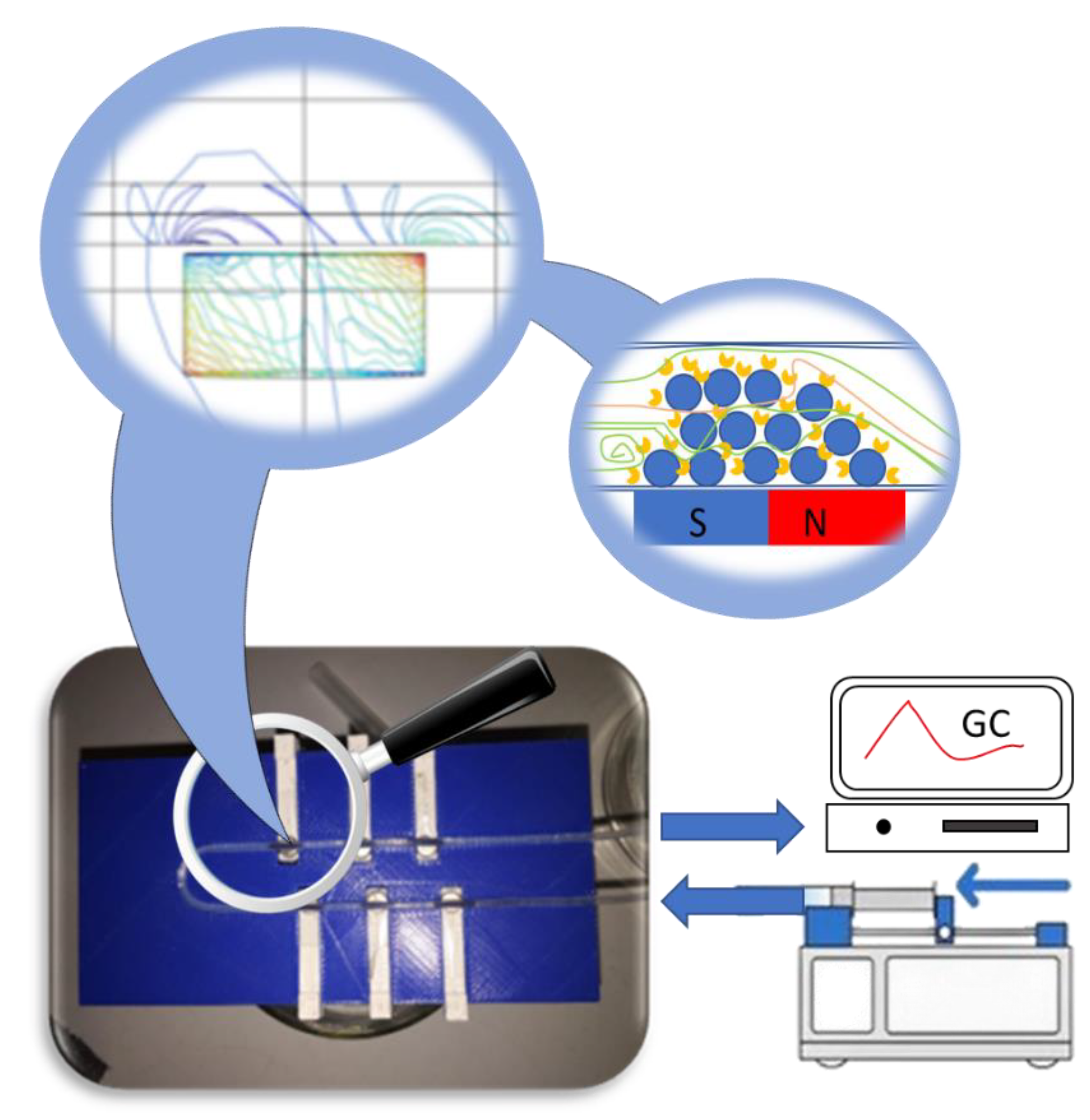
3.5. Design and Assembly of the U-Shape MNP Reactor
3.6. Kinetic Resolution of (±)-1 by the CaLB-MNPC15 Biocatalysts in the U-Shape Reactor in Continuous Flow Mode
3.6.1. (R)-4-(Morpholin-4-yl)butan-2-yl acetate (R)-2
3.6.2. (S)-4-(Morpholin-4-yl)butan-2-ol (S)-1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fasim, A.; More, V.S.; More, S.S. Large-scale production of enzymes for biotechnology uses. Curr. Opin. Biotechnol. 2021, 69, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.L.; Finnigan, W.; France, S.P.; Green, A.P.; Hayes, M.A.; Hepworth, L.J.; Lovelock, S.L.; Niikura, H.; Osuna, S.; Romero, E.; et al. Biocatalysis. Nat. Rev. Method. Prim. 2021, 1, 46. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Brady, D. Green chemistry, biocatalysis, and the chemical industry of the future. ChemSusChem 2022, 15, e202102628. [Google Scholar] [CrossRef]
- Fehér, A.; Bedő, S.; Fehér, C. Comparison of Enzymatic and Acidic Fractionation of Corn Fiber for Glucose-rich Hydrolysate and Bioethanol Production by Candida boidinii. Period. Polytech. Chem. Engin. 2021, 65, 320–330. [Google Scholar] [CrossRef]
- Bilal, M.; Fernandes, C.D.; Mehmood, T.; Nadeem, F.; Tabassam, Q.; Ferreira, L.F.R. Immobilized lipases-based nano-biocatalytic systems—A versatile platform with incredible biotechnological potential. Int. J. Biol. Macromol. 2021, 175, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Enespa, E.; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Fact. 2020, 19, 169. [Google Scholar] [CrossRef]
- Ismail, A.R.; Kashtoh, H.; Baek, K.H. Temperature-resistant and solvent-tolerant lipases as industrial biocatalysts: Biotechnological approaches and applications. Int. J. Biol. Macromol. 2021, 187, 127–142. [Google Scholar] [CrossRef]
- Mehta, A.; Grover, C.; Bhardwaj, K.K.; Gupta, R. Application of lipase purified from Aspergillus fumigatus in the syntheses of ethyl acetate and ethyl lactate. J. Oleo Sci. 2020, 69, 23–29. [Google Scholar] [CrossRef]
- Gkountela, C.; Rigopoulou, M.; Barampouti, E.M.; Vouyiouka, S. Enzymatic prepolymerization combined with bulk post-polymerization towards the production of bio-based polyesters: The case of poly(butylene succinate). Eur. Polym. J. 2021, 143, 110197. [Google Scholar] [CrossRef]
- Carvalho, W.C.A.; Luiz, J.H.H.; Fernandez-Lafuente, R.; Hirata, D.B.; Mendes, A.A. Eco-friendly production of trimethylolpropane triesters from refined and used soybean cooking oils using an immobilized low-cost lipase (Eversa>® Transform 2.0) as heterogeneous catalyst. Biomass Bioenergy 2021, 155, 106302. [Google Scholar] [CrossRef]
- Kumari, M.; Padhi, S.; Sharma, S.; Phukon, L.C.; Singh, S.P.; Rai, A.K. Biotechnological potential of psychrophilic microorganisms as the source of cold-active enzymes in food processing applications. 3 Biotech 2021, 11, 479. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, S.H.; Maiangwa, J.; Ali, M.S.M.; Normi, Y.M.; Sabri, S.; Leow, T.C. Thermostable lipases and their dynamics of improved enzymatic properties. Appl. Microbiol. Biotechnol. 2021, 105, 7069–7094. [Google Scholar] [CrossRef] [PubMed]
- Patkar, S.A.; Bjorking, F.; Zundel, M.; Schulein, M.; Svendsen, A.; Heldt Hansen, H.P.; Gormsen, E. Purification of two lipases from Candida antarctica and their inhibition by various inhibitors. Indian J. Chem. 1993, 32B, 76–80. [Google Scholar]
- Kirk, O.; Christensen, M.W. Lipases from Candida antarctica: Unique biocatalysts from a unique origin. Org. Process Res. Dev. 2002, 6, 446–451. [Google Scholar] [CrossRef]
- María, P.D.; Carboni-Oerlemans, C.; Tuin, B.; Bargeman, G.; Van Der Meer, A.; Van Gemert, R. Biotechnological applications of Candida antarctica lipase A: State-of-the-art. J. Mol. Catal. B Enzym. 2005, 37, 36–46. [Google Scholar] [CrossRef]
- Lima, R.N.; dos Anjos, C.S.; Orozco, E.V.M.; Porto, A.L.M. Versatility of Candida antarctica lipase in the amide bond formation applied in organic synthesis and biotechnological processes. Mol. Catal. 2019, 466, 75–105. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; Dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Basso, A.; Brady, D. New frontiers in enzyme immobilisation: Robust biocatalysts for a circular bio-based economy. Chem. Soc. Rev. 2021, 50, 5850–5862. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is enzyme immobilization a mature discipline? Some critical considerations to capitalize on the benefits of immobilization. Chem. Soc. Rev. 2022, 51, 6251–6290. [Google Scholar] [CrossRef]
- Remonatto, D.; Miotti, R.H., Jr.; Monti, R.; Bassan, J.C.; de Paula, A.V. Applications of immobilized lipases in enzymatic reactors: A review. Proc. Biochem. 2022, 114, 1–20. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Rios, N.S.; Carballares, D.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Immobilization of lipases via interfacial activation on hydrophobic supports: Production of biocatalysts libraries by altering the immobilization conditions. Catal. Today 2021, 362, 130–140. [Google Scholar] [CrossRef]
- Nandini, S.; Anurag, V.; Ishman, K.; Soham, C. A comprehensive review on the potential use of immobilized lipase as biosensor–present scenario and prospects. Res. J. Biotechnol. 2022, 17, 145–150. [Google Scholar] [CrossRef]
- Abaházi, E.; Lestál, D.; Boros, Z.; Poppe, L. Tailoring the spacer arm for covalent immobilization of Candida antarctica lipase B-thermal stabilization by bisepoxide-activated aminoalkyl resins in continuous-flow reactors. Molecules 2016, 21, 767. [Google Scholar] [CrossRef] [PubMed]
- Weiser, D.; Nagy, F.; Bánóczi, G.; Oláh, M.; Farkas, A.; Szilágyi, A.; László, K.; Gellért, Á.; Marosi, G.; Kemény, S.; et al. Immobilization engineering-How to design advanced sol-gel systems for biocatalysis? Green Chem. 2017, 19, 3927–3937. [Google Scholar] [CrossRef]
- Dudu, A.I.; Lacatus, M.A.; Bencze, L.C.; Paizs, C.; Tosa, M.I. Green Process for the Enzymatic Synthesis of Aroma Compounds Mediated by Lipases Entrapped in Tailored Sol-Gel Matrices. ACS Sustain. Chem. Eng. 2021, 9, 5461–5469. [Google Scholar] [CrossRef]
- Razzaghi, M.; Homaei, A.; Vianello, F.; Azad, T.; Sharma, T.; Nadda, A.K.; Stevanto, R.; Bilal, M.; Iqbal, H.M.N. Industrial applications of immobilized nano-biocatalysts. Bioproc. Biosys. Engin. 2022, 45, 237–256. [Google Scholar] [CrossRef]
- Shuai, W.; Das, R.K.; Naghdi, M.; Brar, S.K.; Verma, M. A review on the important aspects of lipase immobilization on nanomaterials. Biotechnol. Appl. Biochem. 2017, 64, 496–508. [Google Scholar] [CrossRef]
- Kumar, N.; Chauhan, N.S. Nano-biocatalysts: Potential biotechnological applications. Indian J. Microbiol. 2021, 61, 441–448. [Google Scholar] [CrossRef]
- Weiser, D.; Sóti, P.L.; Bánóczi, G.; Bódai, V.; Kiss, B.; Gellért, Á.; Nagy, Z.K.; Koczka, B.; Szilágyi, A.; Marosi, G.; et al. Bioimprinted lipases in PVA nanofibers as efficient immobilized biocatalysts. Tetrahedron 2016, 72, 7335–7342. [Google Scholar] [CrossRef]
- Wang, C.; Wang, N.; Liu, X.; Wan, P.; He, X.; Shang, Y. Expanding application of immobilized Candida antarctica lipase B: A green enzyme catalyst for Knoevenagel condensation reaction. Fibers Polym. 2018, 19, 1611–1617. [Google Scholar] [CrossRef]
- Nagy, F.; Sánta-Bell, E.; Jipa, M.; Hornyánszky, G.; Szilágyi, A.; László, K.; Katona, G.; Paizs, C.; Poppe, L.; Balogh-Weiser, D. Cross-linked enzyme-adhered nanoparticles (CLEANs) for continuous-flow bioproduction. ChemSusChem 2022, 15, e202102284. [Google Scholar] [CrossRef] [PubMed]
- Gal, C.A.; Barabás, L.E.; Vári, J.H.B.; Moisa, M.E.; Weiser-Balogh, D.; Bencze, L.C.; Poppe, L.; Paizs, C. Lipase on carbon nanotubes-an active, selective, stable and easy-to-optimize nanobiocatalyst for kinetic resolutions. React. Chem. Eng. 2021, 6, 2391–2399. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Rasheed, T.; Iqbal, H.M.N. Magnetic nanoparticles as versatile carriers for enzymes immobilization: A review. Int. J. Biol. Macromol. 2018, 120, 2530–2544. [Google Scholar] [CrossRef] [PubMed]
- Matveeva, V.G.; Bronstein, L.M. Magnetic nanoparticle-containing supports as carriers of immobilized enzymes: Key factors influencing the biocatalyst performance. Nanomaterials 2021, 11, 2257. [Google Scholar] [CrossRef]
- Netto, C.G.C.M.; Toma, H.E.; Andrade, L.H. Superparamagnetic nanoparticles as versatile carriers and supporting materials for enzymes. J. Mol. Catal. B Enzym. 2013, 85–86, 71–92. [Google Scholar] [CrossRef]
- Arsalan, A.; Younus, H. Enzymes and nanoparticles: Modulation of enzymatic activity via nanoparticles. Int. J. Biol. Macromol. 2018, 118, 1833–1847. [Google Scholar] [CrossRef]
- Tan, Z.; Bilal, M.; Li, X.; Ju, F.; Teng, Y.; Iqbal, H.M.N. Nanomaterial-immobilized lipases for sustainable recovery of biodiesel–A review. Fuel 2022, 316, 123429. [Google Scholar] [CrossRef]
- Okura, N.S.; Sabi, G.J.; Crivellenti, M.C.; Gomes, R.A.B.; Fernandez-Lafuente, R.; Mendes, A.A. Improved immobilization of lipase from Thermomyces lanuginosus on a new chitosan-based heterofunctional support: Mixed ion exchange plus hydrophobic interactions. Int. J. Biol. Macromol. 2020, 163, 550–561. [Google Scholar] [CrossRef]
- Netto, C.G.C.M.; Andrade, L.H.; Toma, H.E. Enantioselective transesterification catalysis by Candida antarctica lipase immobilized on superparamagnetic nanoparticles. Tetrahedron Asymmetry 2009, 20, 2299–2304. [Google Scholar] [CrossRef]
- Nicolás, P.; Lassalle, V.; Ferreira, M.L. Immobilization of CALB on lysine-modified magnetic nanoparticles: Influence of the immobilization protocol. Bioproc. Biosyst. Engin. 2018, 41, 171–184. [Google Scholar] [CrossRef]
- SreeHarsha, N.; Ghorpade, R.V.; Alzahrani, A.M.; Al-Dhubiab, B.E.; Venugopala, K.N. Immobilization studies of Candida antarctica lipase B on gallic acid resin-grafted magnetic iron oxide nanoparticles. Int. J. Nanomed. 2019, 14, 3235–3244. [Google Scholar] [CrossRef]
- Vahidi, A.K.; Yang, Y.; Ngo, T.P.N.; Li, Z. Simple and efficient immobilization of extracellular his-tagged enzyme directly from cell culture supernatant as active and recyclable nanobiocatalyst: High-performance production of biodiesel from waste grease. ACS Catal. 2015, 5, 3157–3161. [Google Scholar] [CrossRef]
- Zlateski, V.; Fuhrer, R.; Koehler, F.M.; Wharry, S.; Zeltner, M.; Stark, W.J.; Moody, T.S.; Grass, R.N. Efficient magnetic recycling of covalently attached enzymes on carbon-coated metallic nanomagnets. Bioconjugate Chem. 2014, 25, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Afzal, H.A.; Ghorpade, R.V.; Thorve, A.K.; Nagaraja, S.; Al-Dhubiab, B.E.; Meravanige, G.; Rasool, S.T.; Roopashree, T.S. Epoxy functionalized polymer grafted magnetic nanoparticles by facile surface initiated polymerization for immobilization studies of Candida antarctica lipase B. React. Function. Polym. 2020, 147, 104454. [Google Scholar] [CrossRef]
- Nicolás, P.; Lassalle, V.; Ferreira, M.L. Development of a magnetic biocatalyst useful for the synthesis of ethyloleate. Bioproc. Biosyst. Engin. 2014, 37, 585–591. [Google Scholar] [CrossRef]
- Spelmezan, C.G.; Bencze, L.C.; Katona, G.; Irimie, F.D.; Paizs, C.; Tosa, M.I. Effcient and stable magnetic chitosan-lipase B from Candida antarctica bioconjugates in the enzymatic kinetic resolution of racemic heteroarylethanols. Molecules 2020, 25, 350. [Google Scholar] [CrossRef]
- Nicolás, P.; Lassalle, V.L.; Ferreira, M.L. About the role of typical spacer/crosslinker on the design of efficient magnetic biocatalysts based on nanosized magnetite. J. Mol. Catal. B Enzym. 2015, 122, 296–304. [Google Scholar] [CrossRef]
- Costa, V.M.; De Souza, M.C.M.; Fechine, P.B.A.; Macedo, A.C.; Gonçalves, L.R.B. Nanobiocatalytic systems based on lipase-Fe3O4 and conventional systems for isoniazid synthesis: A comparative study. Braz. J. Chem. Engin. 2016, 33, 661–673. [Google Scholar] [CrossRef]
- Modenez, I.A.; Sastre, D.E.; Moares, F.C.; Marques Netto, C.G.C. Influence of dlutaraldehyde cross-linking modes on the recyclability of immobilized lipase B from Candida antarctica for transesterification of soy bean oil. Molecules 2018, 23, 2230. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Neto, D.M.A.; Fechine, P.B.A.; Lopes, A.A.S.; Gonçalves, L.R.B.; Dos Santos, J.C.S.; de Souza, M.C.M.; Fernandez-Lafuente, R. Ethyl butyrate synthesis catalyzed by lipases A and B from Candida antarctica immobilized onto magnetic nanoparticles. Improvement of biocatalysts’ performance under ultrasonic irradiation. Int. J. Mol. Sci. 2019, 20, 5807. [Google Scholar] [CrossRef]
- Vasilescu, C.; Todea, A.; Nan, A.; Circu, M.; Turcu, R.; Benea, I.; Peter, F. Enzymatic synthesis of short-chain flavor esters from natural sources using tailored magnetic biocatalysts. Food Chem. 2019, 296, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Brandão Junior, J.; Ferreira, D.C.L.; Maia, F.S.; Mota, G.F.; Nascimento, J.G.A.; Monteiro, R.R.C.; Fonseca, A.M.; Maria Souza, C.M. Immobilization of CalB Lipase by adsorption on magnetic nanoparticles: A heterogeneous biocatalysis/Imobilização da lipase CalB por adsorção em nanopartículas magnéticas: Um biocatalisador heterogêneo. Braz. J. Dev. 2021, 7, 76015–76024. [Google Scholar] [CrossRef]
- De Souza, M.C.M.; Dos Santos, K.P.; Freire, R.M.; Barreto, A.C.H.; Fechine, P.B.A.; Gonçalves, L.R.B. Production of flavor esters catalyzed by Lipase B from Candida antarctica immobilized on magnetic nanoparticles. Braz. J. Chem. Engin. 2017, 34, 681–690. [Google Scholar] [CrossRef]
- Mehrasbi, M.R.; Mohammadi, J.; Peyda, M.; Mohammadi, M. Covalent immobilization of Candida antarctica lipase on core-shell magnetic nanoparticles for production of biodiesel from waste cooking oil. Renew. Energy 2017, 101, 593–602. [Google Scholar] [CrossRef]
- Gkantzou, E.; Patila, M.; Stamatis, H. Magnetic microreactors with immobilized enzymes-from assemblage to contemporary applications. Catalysts 2018, 8, 282. [Google Scholar] [CrossRef]
- Weiser, D.; Bencze, L.C.; Bánóczi, G.; Ender, F.; Kiss, R.; Kókai, E.; Szilágyi, A.; Vértessy, B.G.; Farkas, O.; Paizs, C.; et al. Phenylalanine Ammonia-Lyase-Catalyzed Deamination of an Acyclic Amino Acid: Enzyme Mechanistic Studies Aided by a Novel Microreactor Filled with Magnetic Nanoparticles. ChemBioChem 2015, 16, 2283–2288. [Google Scholar] [CrossRef] [PubMed]
- Ender, F.; Weiser, D.; Nagy, B.; Bencze, L.C.; Paizs, C.; Pálovics, P.; Poppe, L. Microfluidic multiple cell chip reactor filled with enzyme-coated magnetic nanoparticles-An efficient and flexible novel tool for enzyme catalyzed biotransformations. J. Flow Chem. 2016, 6, 43–52. [Google Scholar] [CrossRef]
- Imarah, A.O.; Csuka, P.; Bataa, N.; Decsi, B.; Sánta-Bell, E.; Molnár, Z.; Balogh-Weiser, D.; Poppe, L. Magnetically agitated nanoparticle-based batch reactors for biocatalysis with immobilized aspartate ammonia-lyase. Catalysts 2021, 11, 483. [Google Scholar] [CrossRef]
- Reichert, C.; Hoell, W.H.; Franzreb, M. Mass transfer enhancement in stirred suspensions of magnetic particles by the use of alternating magnetic fields. Powder Technol. 2004, 145, 131–138. [Google Scholar] [CrossRef]
- Žnidaršič-Plazl, P. Biocatalytic process intensification via efficient biocatalyst immobilization, miniaturization, and process integration. Curr. Opin. Green Sustain. Chem. 2021, 32, 100546. [Google Scholar] [CrossRef]
- Bras, E.J.S.; Chu, V.; Conde, J.P.; Fernandes, P. Recent developments in microreactor technology for biocatalysis applications. React. Chem. Engin. 2021, 6, 815–827. [Google Scholar] [CrossRef]
- Schätz, A.; Grass, R.N.; Kainz, Q.; Stark, W.J.; Reiser, O. Cu(II)−Azabis(oxazoline) Complexes Immobilized on Magnetic Co/C Nanoparticles: Kinetic Resolution of 1,2-Diphenylethane-1,2-diol under Batch and Continuous-Flow Conditions. Chem. Mater. 2010, 22, 305–310. [Google Scholar] [CrossRef]
- Román-Pizarro, V.; Ramírez-Gutiérrez, M.; Gómez-Hens, A.; Fernández-Romero, J.M. Usefulness of magnetically-controlled MNPs-enzymes microreactors for the fluorimetric determination of total cholesterol in serum. Talanta 2020, 208, 120426. [Google Scholar] [CrossRef] [PubMed]
- Gebreyohannes, A.Y.; Giorno, L.; Vankelecom, I.F.J.; Verbiest, T.; Aimar, P. Effect of operational parameters on the performance of a magnetic responsive biocatalytic membrane reactor. Chem. Eng. J. 2017, 308, 853–862. [Google Scholar] [CrossRef]
- Hajar, M.; Vahabzadeh, F. Biolubricant production from castor oil in a magnetically stabilized fluidized bed reactor using lipase immobilized on Fe3O4 nanoparticles. Ind. Crops Prod. 2016, 94, 544–556. [Google Scholar] [CrossRef]
- Ou, Z.; Pan, J.; Tang, L.; Shi, H. Continuous enantiomer-selective acylation reaction of 1-phenylethanamine in a magnetic fluidized bed reactor system (MFBRS). J. Chem. Technol. Biotechnol. 2019, 94, 1951–1957. [Google Scholar] [CrossRef]
- Aldeghi, M.; Malhotra, S.; Selwood, D.L.; Chan, A.W.E. Two- and Three-dimensional Rings in Drugs. Chem. Biol. Drug Des. 2014, 83, 450–461. [Google Scholar] [CrossRef]
- Lovering, F.; Bikker, J.; Humblet, C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef]
- Kourounakis, A.P.; Xanthopoulos, D.; Tzara, A. Morpholine as a privileged structure: A review on the medicinal chemistry and pharmacological activity of morpholine containing bioactive molecules. Med. Res. Rev. 2020, 40, 709–752. [Google Scholar] [CrossRef]
- Tzara, A.; Xanthopoulos, D.; Kourounakis, A.P. Morpholine As a Scaffold in Medicinal Chemistry: An Update on Synthetic Strategies. ChemMedChem 2020, 15, 392–403. [Google Scholar] [CrossRef]
- Moran, H. Basische Ester von Derivaten der Tetrahydropyran-4-Carbonsäure Und Deren Pharmakologisch Nicht Giftige Säureadditionssalze. German Patent DE 1,518,403 B1, 16 July 1970. [Google Scholar]
- Moran, H. Mucolytic Salts, Compositions and Process for Treating Mucus. U.S. Patent US 3,567,835, 2 March 1971. [Google Scholar]
- Martinsson, J.; Faernegardh, K.; Joensson, M.; Ringom, R.; Gustafsson Sheppard, N.; Helleday, T.; Groth, P. Bisarylsulfonamide Derivatives Useful in the Treatment of Inflammation and Cancer. U.S. Patent US 10,000,449 B2, 19 July 2018. [Google Scholar]
- Csuka, P.; Molnár, Z.; Tóth, V.; Imarah, A.O.; Balogh-Weiser, D.; Vértessy, B.G.; Poppe, L. Immobilization of the Aspartate Ammonia-Lyase from Pseudomonas fluorescens R124 on Magnetic Nanoparticles: Characterization and Kinetics. ChemBioChem 2022, 23, e202100708. [Google Scholar] [CrossRef] [PubMed]
- Sánta-Bell, E.; Molnár, Z.; Varga, A.; Nagy, F.; Hornyánszky, G.; Paizs, C.; Balogh-Weiser, D.; Poppe, L. “Fishing and hunting”–Selective immobilization of a recombinant phenylalanine ammonia-lyase from fermentation media. Molecules 2019, 24, 4146. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Choi, Y.K.; Kim, Y.H.; Park, E.S.; Kim, E.J.; Kim, M.-J.; Park, J. Aminocyclopentadienyl Ruthenium Complexes as Racemization Catalysts for Dynamic Kinetic Resolution of Secondary Alcohols at Ambient Temperature. J. Org. Chem. 2004, 69, 1972–1977. [Google Scholar] [CrossRef] [PubMed]
- Toşa, M.; Pilbák, S.; Moldovan, P.; Paizs, C.; Szatzker, G.; Szakács, G.; Novák, L.; Irimie, F.-D.; Poppe, L. Lipase-catalyzed kinetic resolution of racemic 1-heteroarylethanols−experimental and QM/MM study. Tetrahedron Asymmetry 2008, 19, 1844–1852. [Google Scholar] [CrossRef]
- Csajági, C.; Szatzker, G.; Tőke, E.R.; Ürge, L.; Darvas, F.; Poppe, L. Enantiomer selective acylation of racemic alcohols by lipases in continuous-flow bioreactors. Tetrahedron Asymmetry 2008, 19, 237–246. [Google Scholar] [CrossRef]
- Boudrant, J.; Woodley, J.M.; Fernandez-Lafuente, R. Parameters necessary to define an immobilized enzyme preparation. Proc. Biochem. 2020, 90, 66–80. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Rossi, D.; Pedrali, A.; Urbano, M.; Gaggeri, R.; Serra, M.; Fernandez, L.; Fernandez, M.; Caballero, J.; Ronsisvalle, S.; Prezzavento, O.; et al. Identification of a potent and selective σ1 receptor agonist potentiating NGF-induced neurite outgrowth in PC12 cells. Bioorg. Med. Chem. 2011, 19, 6210–6224. [Google Scholar] [CrossRef]
- Nakamura, Y.; Ohta, T.; Oe, Y. A formal anti-Markovnikov hydroamination of allylic alcohols via tandem oxidation/1,4-conjugate addition/1,2-reduction using a Ru catalyst. Chem. Commun. 2015, 51, 7459–7462. [Google Scholar] [CrossRef]



| Product | Yield 1 (%) | ee2 (%) | [α] 3 |
|---|---|---|---|
| (R)-2 | 47.5 | >99 | +1.2 |
| (S)-1 | 37.5 | >99 | +3.2 |
| (S)-2 4 | 90 4 | >99 | −1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imarah, A.O.; Silva, F.M.W.G.; Tuba, L.; Malta-Lakó, Á.; Szemes, J.; Sánta-Bell, E.; Poppe, L. A Convenient U-Shape Microreactor for Continuous Flow Biocatalysis with Enzyme-Coated Magnetic Nanoparticles-Lipase-Catalyzed Enantiomer Selective Acylation of 4-(Morpholin-4-yl)butan-2-ol. Catalysts 2022, 12, 1065. https://doi.org/10.3390/catal12091065
Imarah AO, Silva FMWG, Tuba L, Malta-Lakó Á, Szemes J, Sánta-Bell E, Poppe L. A Convenient U-Shape Microreactor for Continuous Flow Biocatalysis with Enzyme-Coated Magnetic Nanoparticles-Lipase-Catalyzed Enantiomer Selective Acylation of 4-(Morpholin-4-yl)butan-2-ol. Catalysts. 2022; 12(9):1065. https://doi.org/10.3390/catal12091065
Chicago/Turabian StyleImarah, Ali O., Fausto M. W. G. Silva, László Tuba, Ágnes Malta-Lakó, József Szemes, Evelin Sánta-Bell, and László Poppe. 2022. "A Convenient U-Shape Microreactor for Continuous Flow Biocatalysis with Enzyme-Coated Magnetic Nanoparticles-Lipase-Catalyzed Enantiomer Selective Acylation of 4-(Morpholin-4-yl)butan-2-ol" Catalysts 12, no. 9: 1065. https://doi.org/10.3390/catal12091065
APA StyleImarah, A. O., Silva, F. M. W. G., Tuba, L., Malta-Lakó, Á., Szemes, J., Sánta-Bell, E., & Poppe, L. (2022). A Convenient U-Shape Microreactor for Continuous Flow Biocatalysis with Enzyme-Coated Magnetic Nanoparticles-Lipase-Catalyzed Enantiomer Selective Acylation of 4-(Morpholin-4-yl)butan-2-ol. Catalysts, 12(9), 1065. https://doi.org/10.3390/catal12091065