CO2 Methanation of Biogas over Ni-Mg-Al: The Effects of Ni Content, Reduction Temperature, and Biogas Composition
Abstract
1. Introduction
2. Results and Discussion
2.1. Effects of Temperature Reduction and Catalyst Loading on CO2 Conversion
2.2. Effect of Temperature Reaction on CO2 Conversion
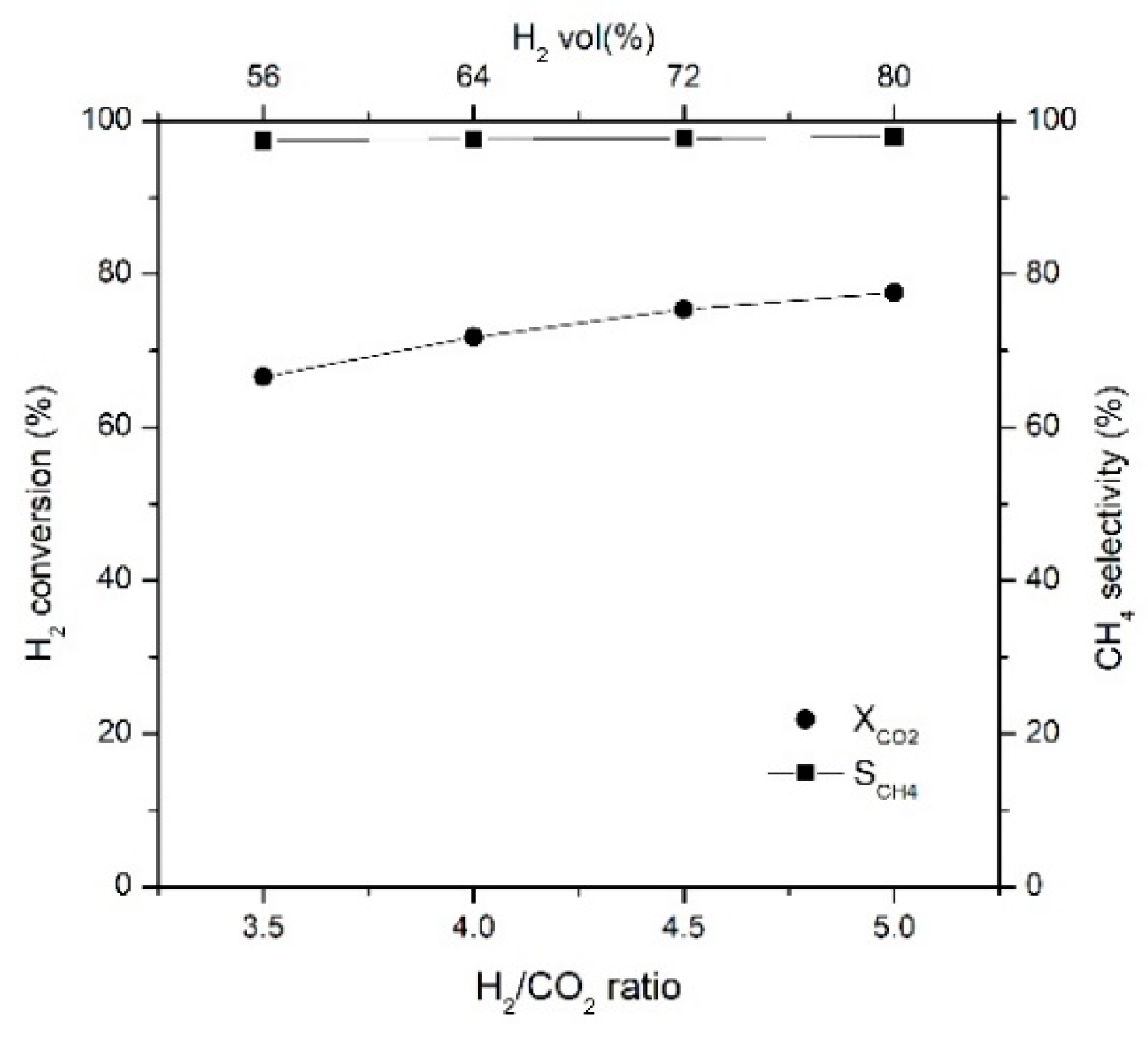
2.3. Effects of CO2 and H2 Concentrations on CO2 Conversion
2.4. Effect of CH4 Concentration on CO2 Conversion
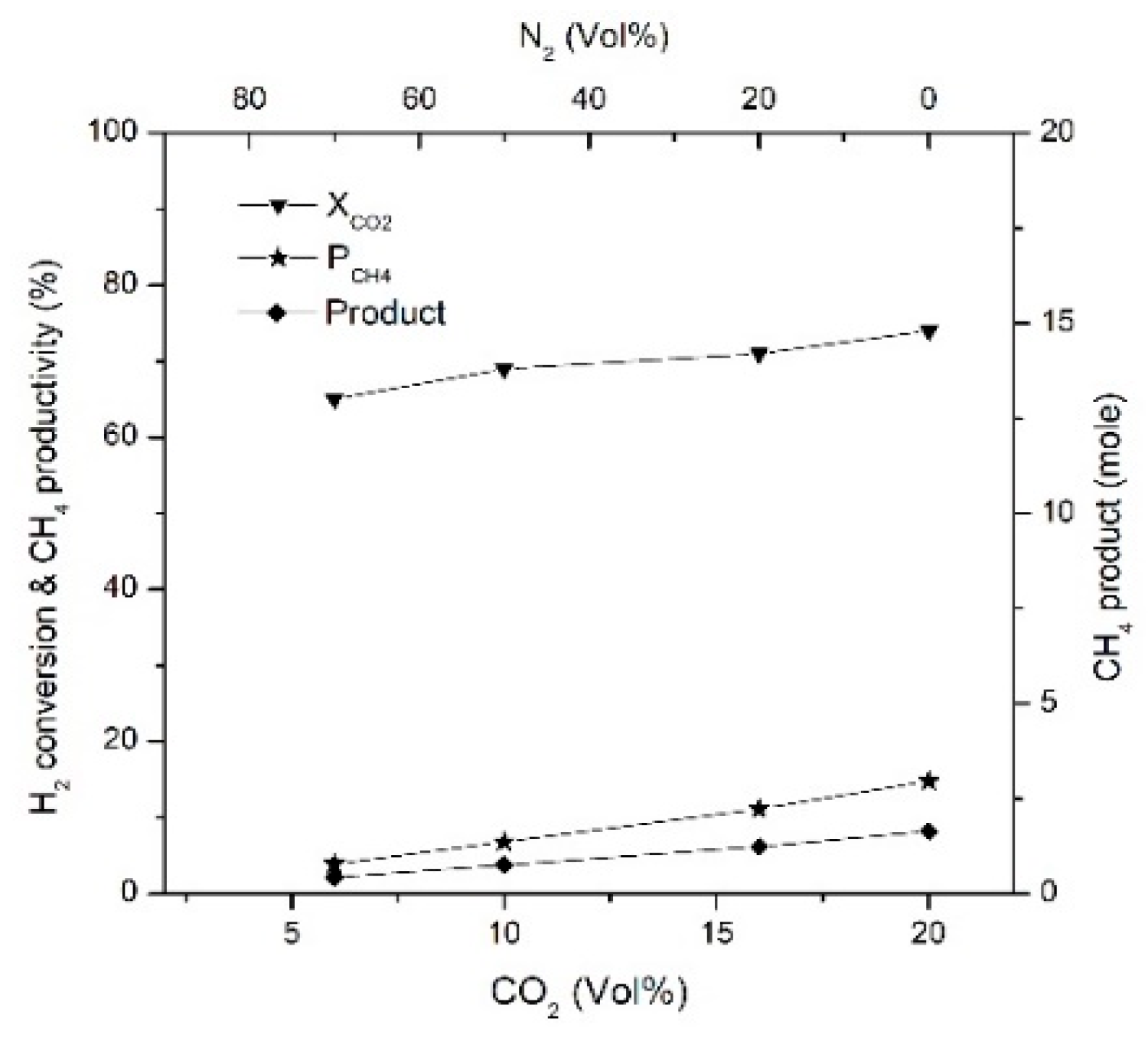
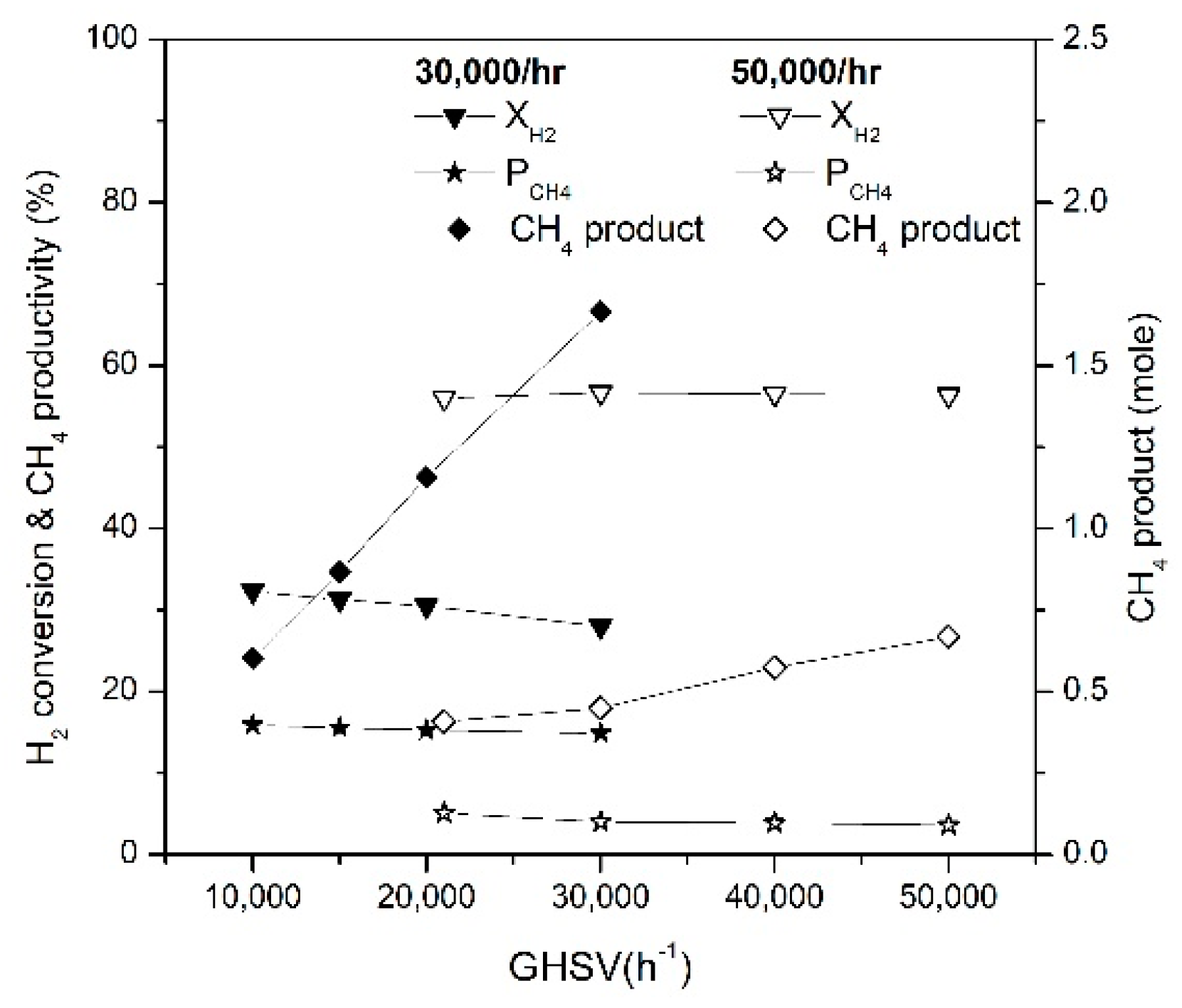
2.5. Effect of CO2 Concentration, H2/CO2 Ratio and GHSV on CH4 Productivity and Product
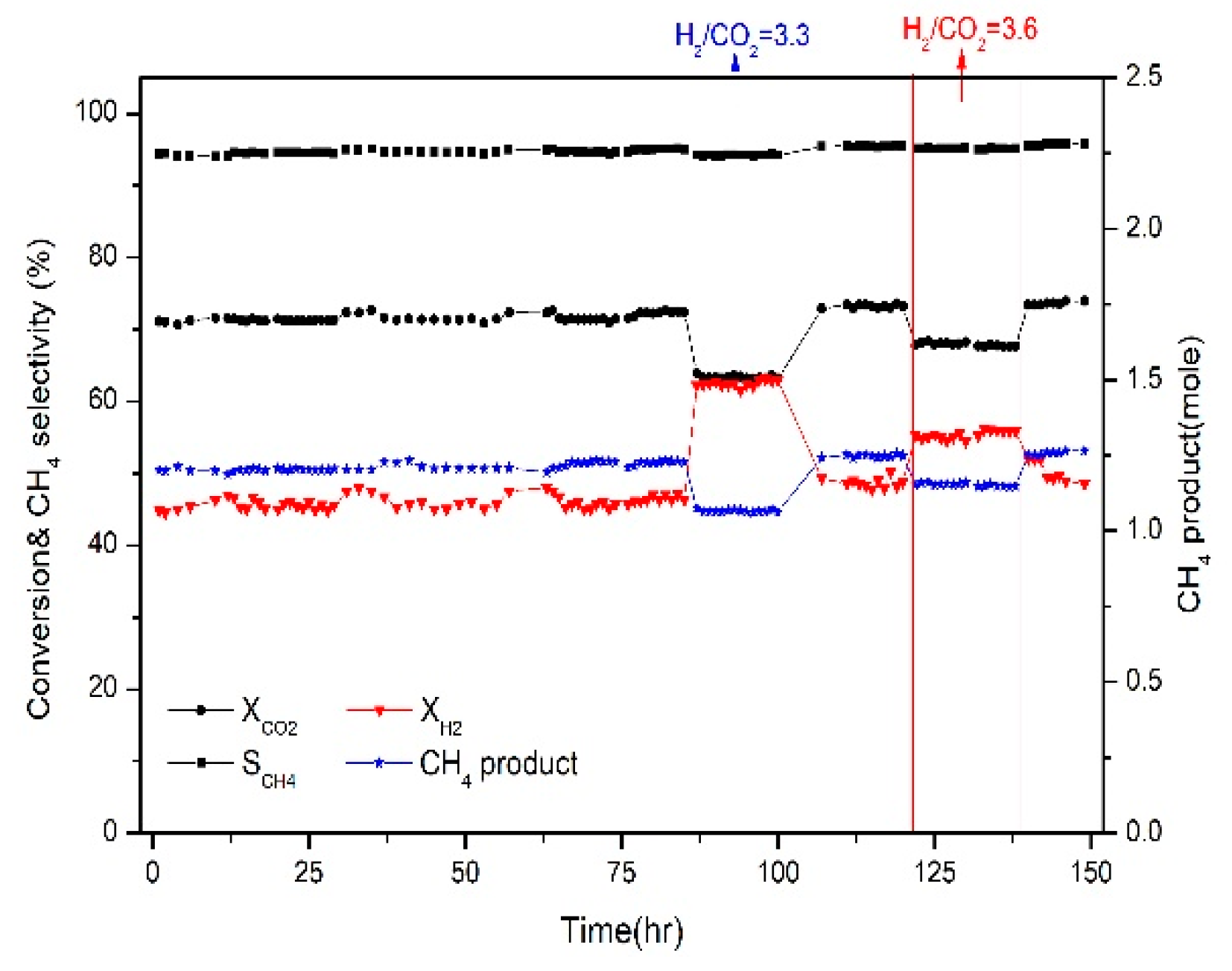
2.6. Catalytic Activity and Stability Tests
3. Characterization of the Catalysts
3.1. Surface Area Analysis
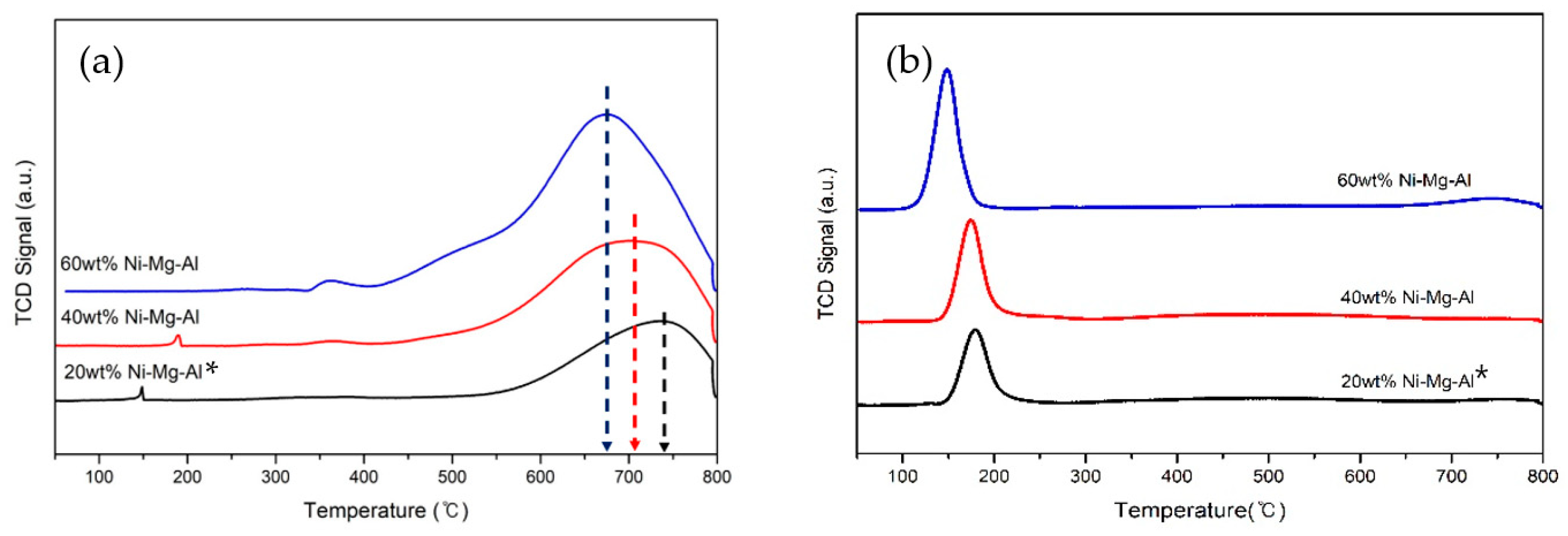
3.2. H2-Temperature Programmed Reduction (TPR) and H2-Chemisorption Analyses
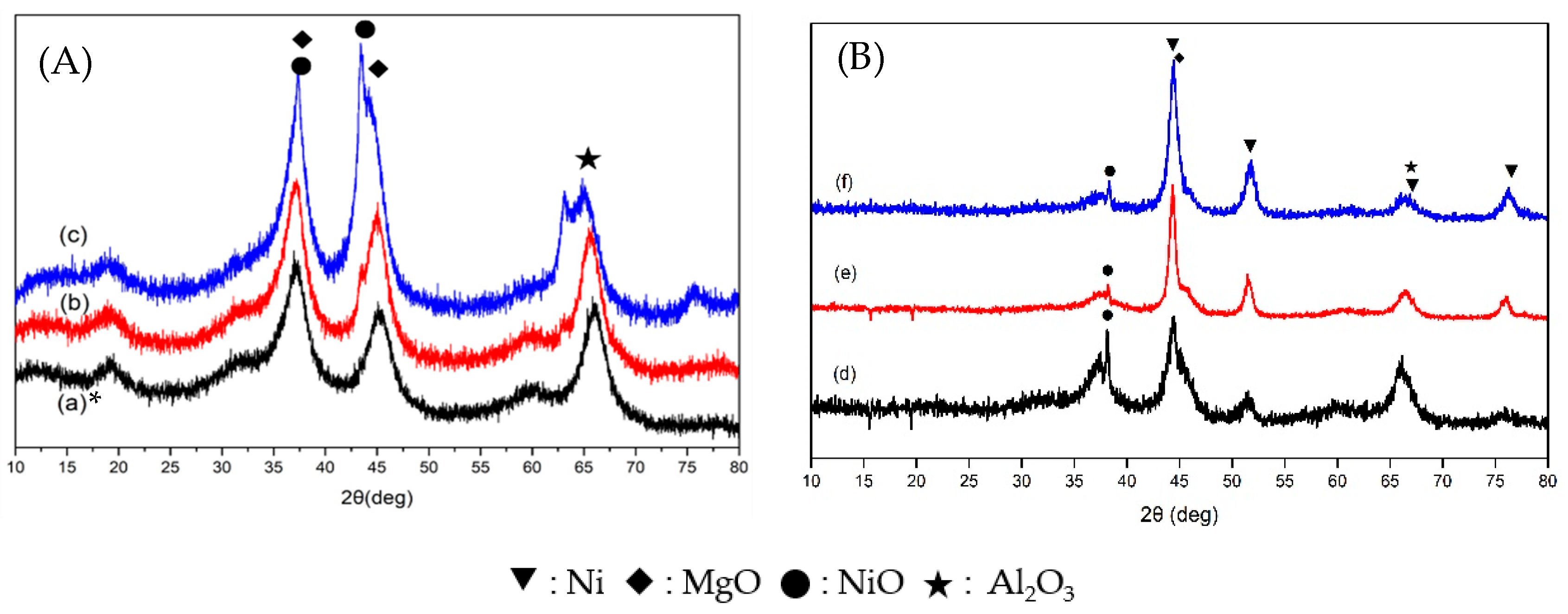
3.3. XRD Analysis
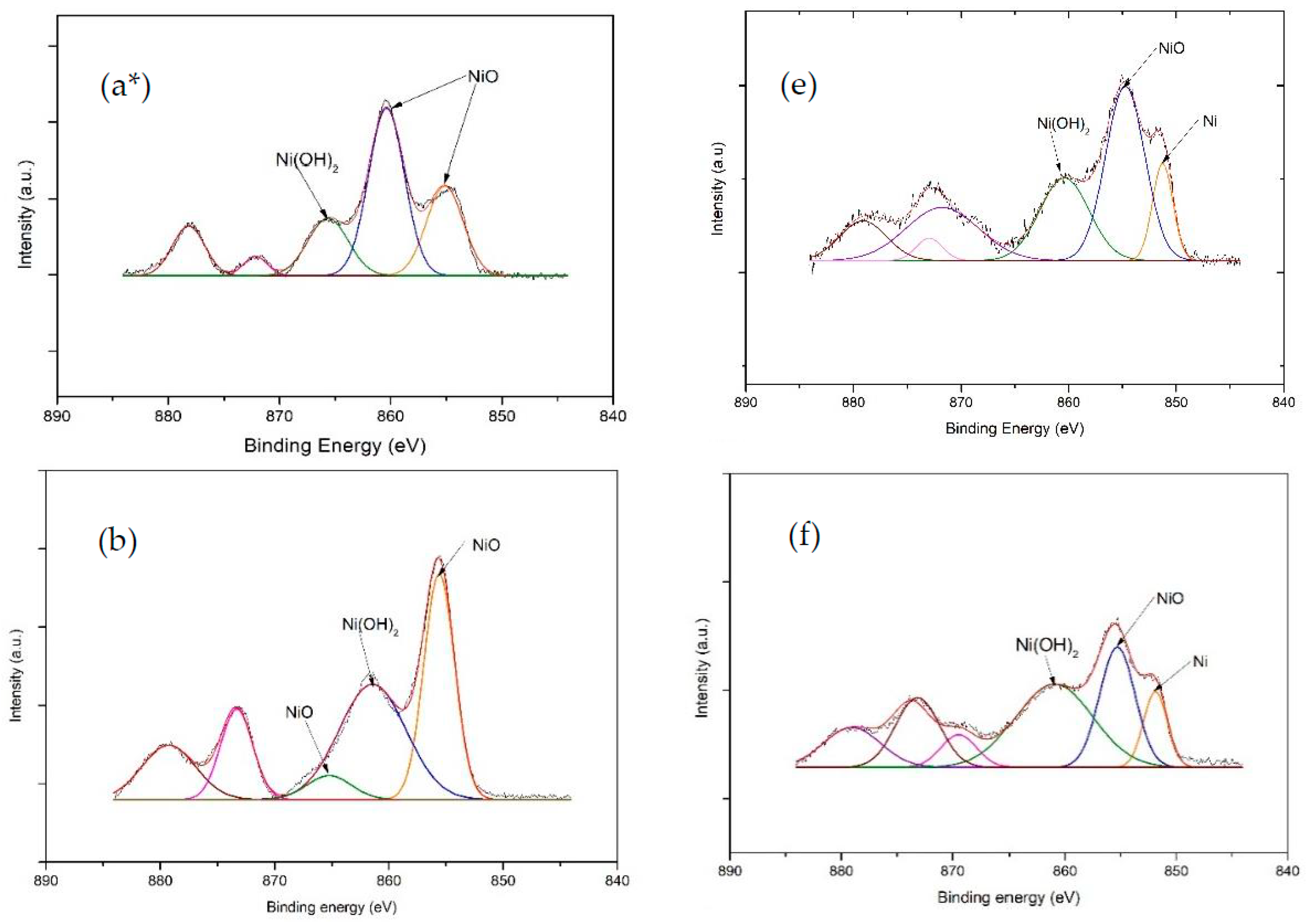
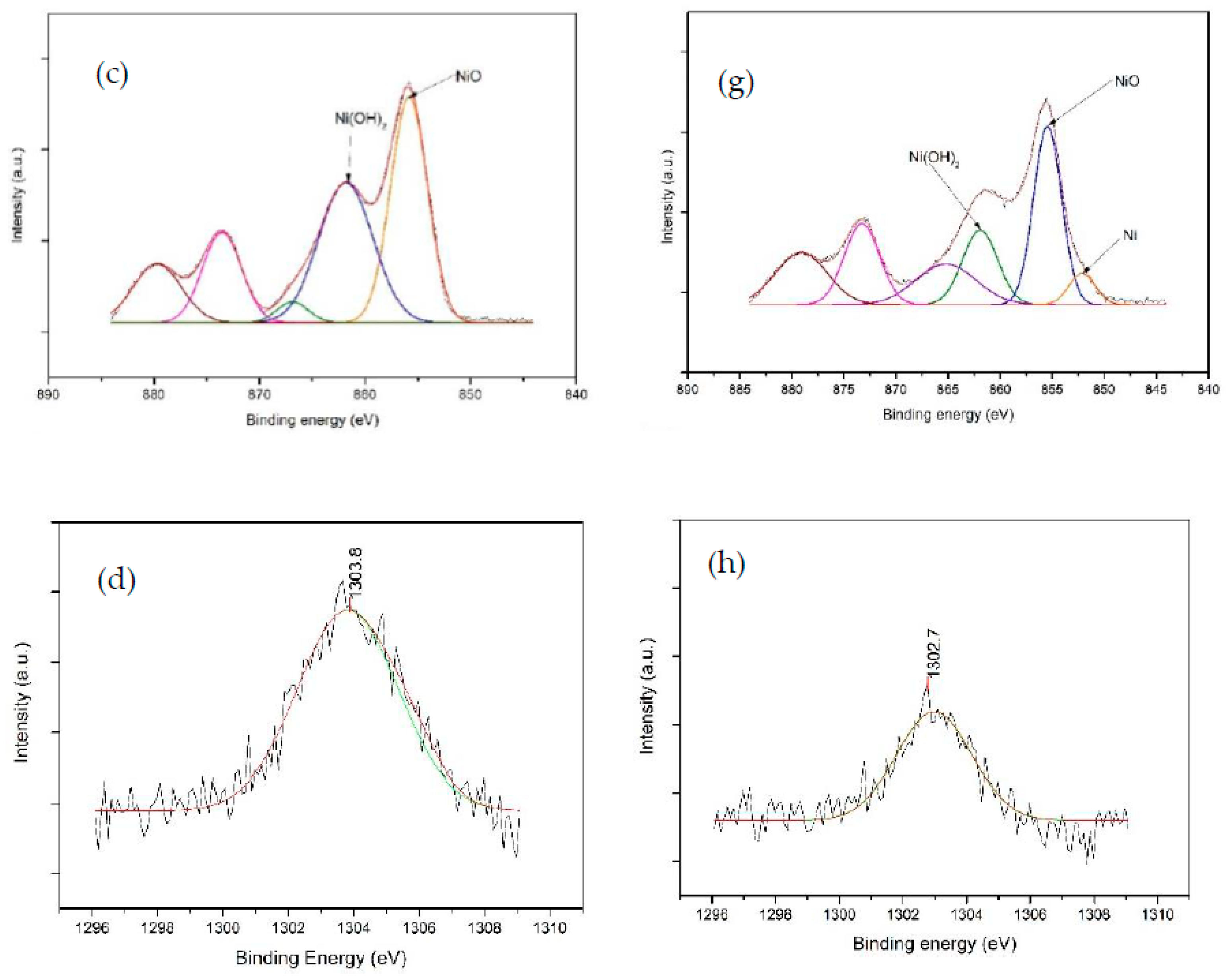
3.4. XPS Analysis
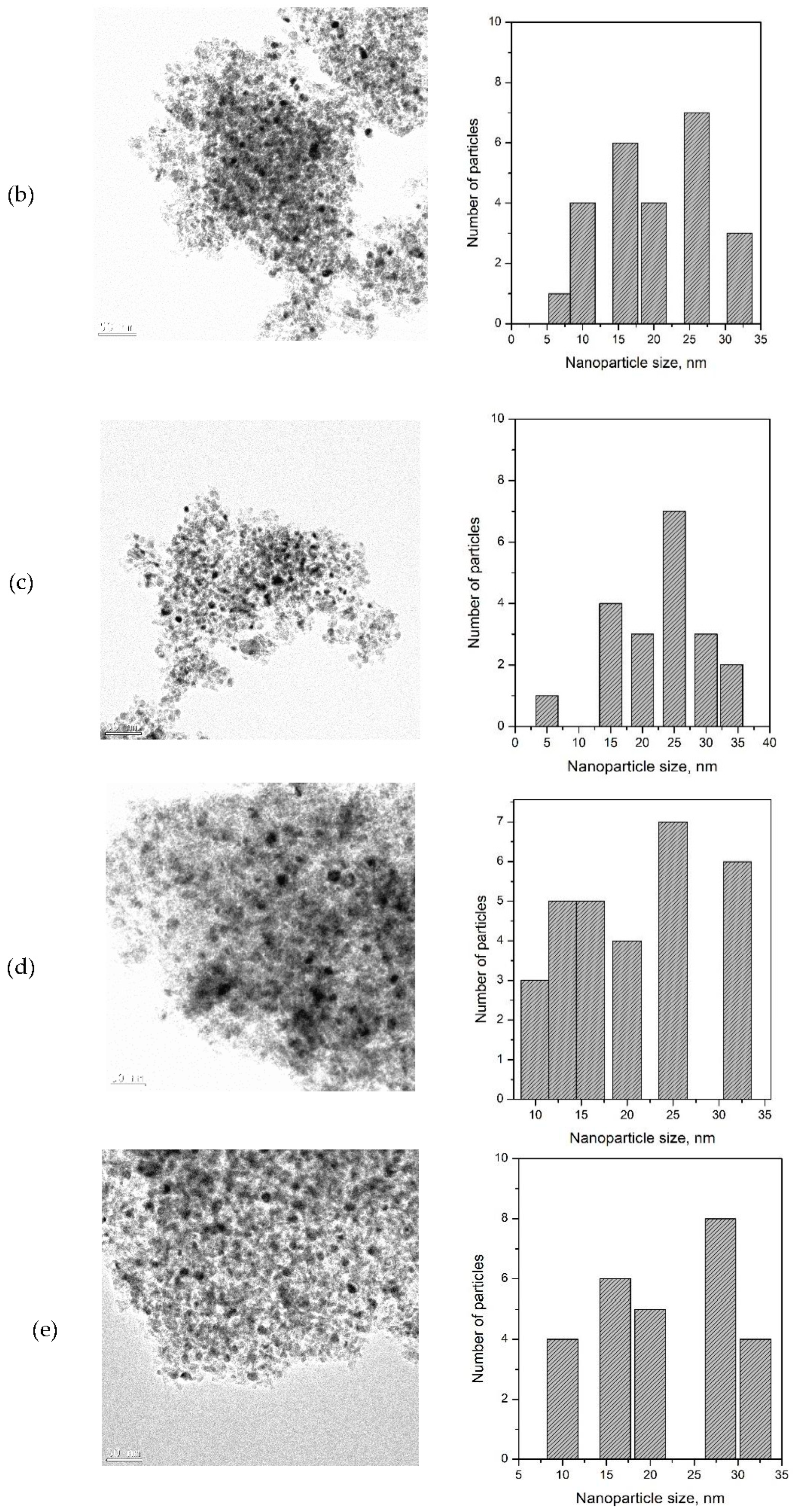
3.5. Transmission Electron Microscopy (TEM) Characterization
4. Experimental Methods
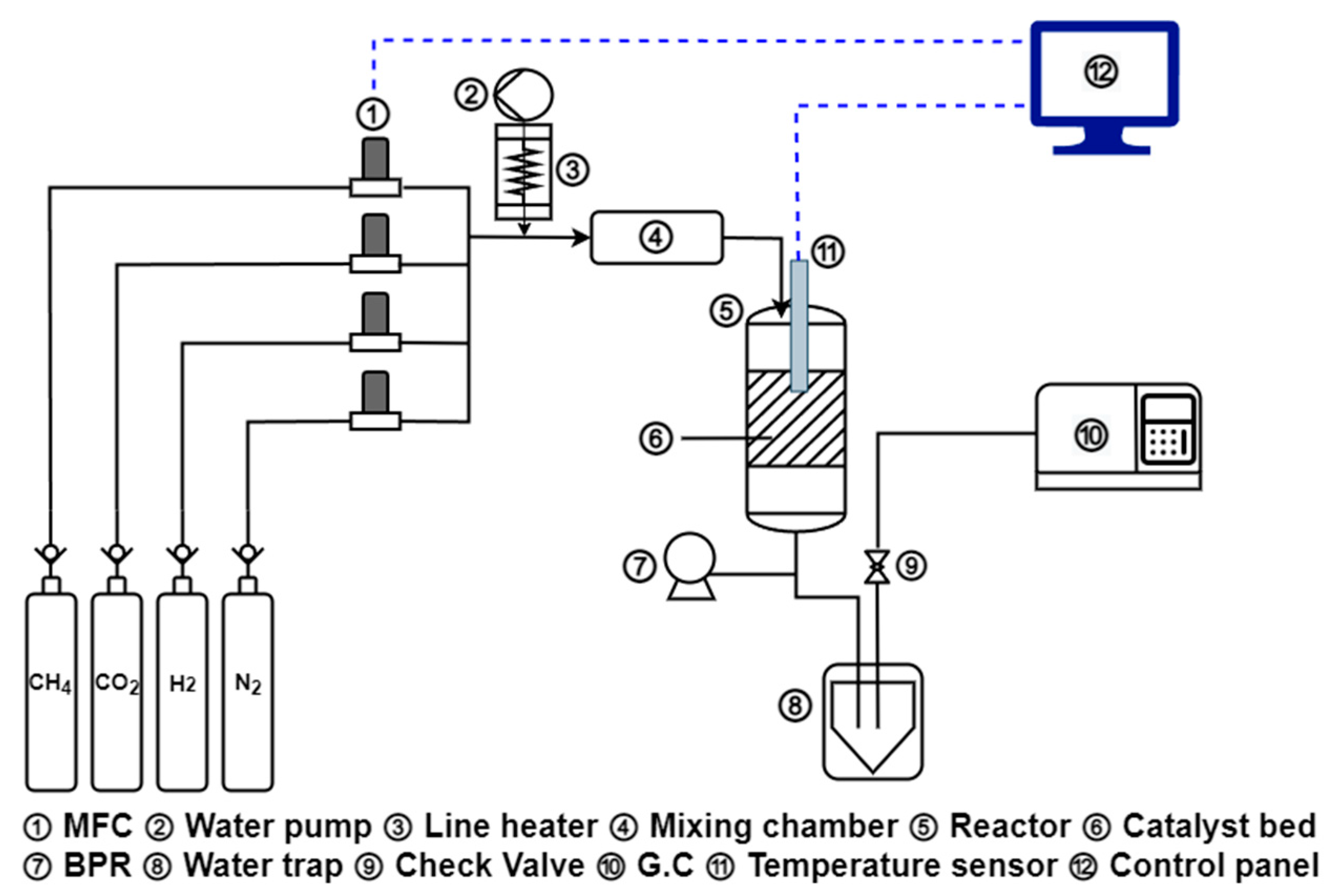
4.1. Methanation Reaction
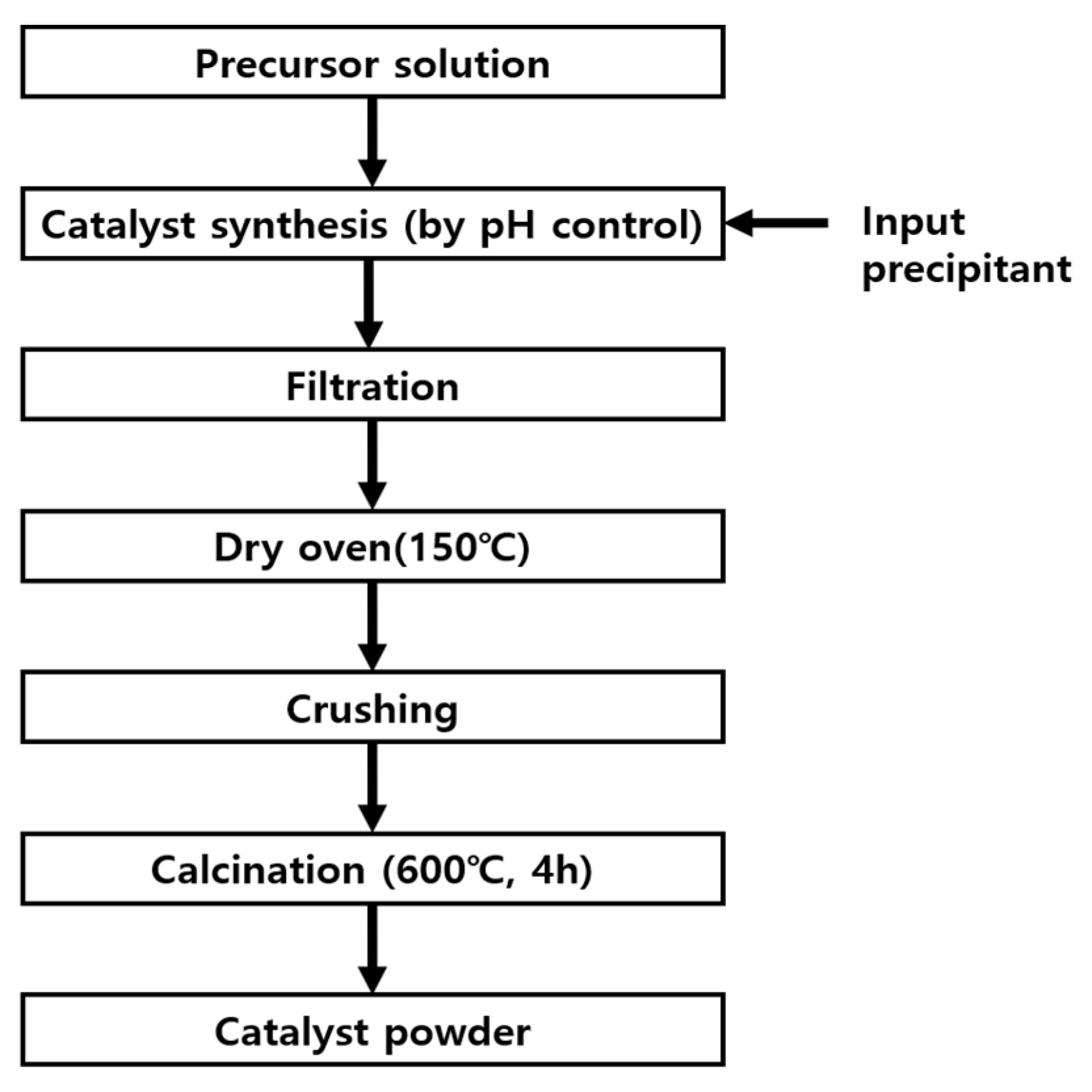
4.2. Catalyst Synthesis and Experimentation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gac, W.; Zawadzki, W.; Słowik, G.; Sienkiewicz, A.; Kierys, A. Nickel catalysts supported on silica microspheres for CO2 methanation. Microporous Mesoporous Mater. 2018, 272, 79–91. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, J.; Xu, Y.; Sun, Y. A review of CH4CO2 reforming to synthesis gas over Ni-based catalysts in recent years (2010–2017). Int. J. Hydrogen Energy 2018, 43, 15030–15054. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Mu, M.; Ding, F.; Liu, Z.; Guo, X.; Song, C. Organic acid-assisted preparation of highly dispersed Co/ZrO2 catalysts with superior activity for CO2 methanation. Appl. Catal. B Environ. 2019, 254, 531–540. [Google Scholar] [CrossRef]
- Bian, Z.; Chan, Y.M.; Yu, Y.; Kawi, S. Morphology dependence of catalytic properties of Ni/CeO2 for CO2 methanation: A kinetic and mechanism study. Catal. Today 2020, 347, 31–38. [Google Scholar] [CrossRef]
- Benson, E.E.; Kubiak, C.P.; Sathrum, A.J.; Smieja, J.M. Electrocatalytic and homogeneous approaches to conversion of CO2 to liquid fuels. Chem. Soc. Rev. 2009, 38, 89–99. [Google Scholar] [CrossRef]
- Solis-Garcia, A.; Louvier-Hernandez, J.F.; Almendarez-Camarillo, A.; Fierro-Gonzalez, J. Participation of surface bicarbonate, formate and methoxy species in the carbon dioxide methanation catalyzed by ZrO2-supported Ni. Appl. Catal. B Environ. 2017, 218, 611–620. [Google Scholar] [CrossRef]
- Gnanakumar, E.S.; Chandran, N.; Kozhevnikov, I.V.; Grau-Atienza, A.; Fernández, E.V.R.; Sepulveda-Escribano, A.; Shiju, N.R. Highly efficient nickel-niobia composite catalysts for hydrogenation of CO2 to methane. Chem. Eng. Sci. 2019, 194, 2–9. [Google Scholar] [CrossRef]
- Panagiotopoulou, P. Hydrogenation of CO2 over supported noble metal catalysts. Appl. Catal. A Gen. 2017, 542, 63–70. [Google Scholar] [CrossRef]
- Chai, S.; Men, Y.; Wang, J.; Liu, S.; Song, Q.; An, W.; Kolb, G. Boosting CO2 methanation activity on Ru/TiO2 catalysts by exposing (001) facets of anatase TiO2. J. CO2 Util. 2019, 33, 242–252. [Google Scholar] [CrossRef]
- Li, W.; Nie, X.; Jiang, X.; Zhang, A.; Ding, F.; Liu, M.; Liu, Z.; Guo, X.; Song, C. ZrO2 support imparts superior activity and stability of Co catalysts for CO2 methanation. Appl. Catal. B Environ. 2018, 220, 397–408. [Google Scholar] [CrossRef]
- Liu, H.; Xu, S.; Zhou, G.; Xiong, K.; Jiao, Z.; Wang, S. CO2 hydrogenation to methane over Co/KIT-6 catalysts: Effect of Co content. Fuel 2018, 217, 570–576. [Google Scholar] [CrossRef]
- Veselovskaya, J.V.; Parunin, P.D.; Netskina, O.V.; Kibis, L.S.; Lysikov, A.I.; Okunev, A.G. Catalytic methanation of carbon dioxide captured from ambient air. Energy 2018, 159, 766–773. [Google Scholar] [CrossRef]
- Siakavelas, G.I.; Charisiou, N.D.; AlKhoori, A.; AlKhoori, S.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Yentekakis, I.V.; Polychronopoulou, K.; Goula, M.A. Highly selective and stable Ni/La-M (M=Sm, Pr, and Mg)-CeO2 catalysts for CO2 methanation. J. CO2 Util. 2021, 51, 101618. [Google Scholar] [CrossRef]
- Ahmad, W.; Younis, M.N.; Shawabkeh, R.; Ahmed, S. Synthesis of lanthanide series (La, Ce, Pr, Eu & Gd) promoted Ni/γ-Al2O3 catalysts for methanation of CO2 at low temperature under atmospheric pressure. Catal. Commun. 2017, 100, 121–126. [Google Scholar]
- Ferreira, A.C.; Branco, J.B. Methanation of CO2 over nanostructured nickel-4f block element bimetallic oxides. Int. J. Hydrogen Energy 2019, 44, 6505–6513. [Google Scholar] [CrossRef]
- Li, S.; Liu, G.; Zhang, S.; An, K.; Ma, Z.; Wang, L.; Liu, Y. Cerium-modified Ni-La2O3/ZrO2 for CO2 methanation. J. Energy Chem. 2020, 43, 155–164. [Google Scholar] [CrossRef]
- Ye, R.-P.; Gong, W.; Sun, Z.; Sheng, Q.; Shi, X.; Wang, T.; Yao, Y.; Razink, J.J.; Lin, L.; Zhou, Z.; et al. Enhanced stability of Ni/SiO2 catalyst for CO2 methanation: Derived from nickel phyllosilicate with strong metal-support interactions. Energy 2019, 188, 116059. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Graça, I.; Bebiano, S.S.; Lopes, J.M.; Henriques, C. Micro- and mesoporous supports for CO2 methanation catalysts: A comparison between SBA-15, MCM-41 and USY zeolite. Chem. Eng. Sci. 2018, 175, 72–83. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Zhang, X.; Wang, Z.; Wang, X.; Peng, H. Design of Ni-ZrO2@SiO2 catalyst with ultra-high sintering and coking resistance for dry reforming of methane to prepare syngas. J. CO2 Util. 2018, 27, 297–307. [Google Scholar] [CrossRef]
- Romero-Sáez, M.; Dongil, A.; Benito, N.; Espinoza-González, R.; Escalona, N.; Gracia, F. CO2 methanation over nickel-ZrO2 catalyst supported on carbon nanotubes: A comparison between two impregnation strategies. Appl. Catal. B Environ. 2018, 237, 817–825. [Google Scholar] [CrossRef]
- Ocampo, F.; Louis, B.; Kiwi-Minsker, L.; Roger, A.-C. Effect of Ce/Zr composition and noble metal promotion on nickel based CexZr1−xO2 catalysts for carbon dioxide methanation. Appl. Catal. A Gen. 2011, 392, 36–44. [Google Scholar] [CrossRef]
- Garbarino, G.; Wang, C.; Cavattoni, T.; Finocchio, E.; Riani, P.; Flytzani-Stephanopoulos, M.; Busca, G. A study of Ni/La-Al2O3 catalysts: A competitive system for CO2 methanation. Appl. Catal. B Environ. 2019, 248, 286–297. [Google Scholar] [CrossRef]
- Tada, S.; Shimizu, T.; Kameyama, H.; Haneda, T.; Kikuchi, R. Ni/CeO2 catalysts with high CO2 methanation activity and high CH4 selectivity at low temperatures. Int. J. Hydrogen Energy 2012, 37, 5527–5531. [Google Scholar] [CrossRef]
- Löfberg, A.; Guerrero-Caballero, J.; Kane, T.; Rubbens, A.; Jalowiecki-Duhamel, L. Ni/CeO2 based catalysts as oxygen vectors for the chemical looping dry reforming of methane for syngas production. Appl. Catal. B Environ. 2017, 212, 159–174. [Google Scholar] [CrossRef]
- Ratchahat, S.; Sudoh, M.; Suzuki, Y.; Kawasaki, W.; Watanabe, R.; Fukuhara, C. Development of a powerful CO2 methanation process using a structured Ni/CeO2 catalyst. J. CO2 Util. 2018, 24, 210–219. [Google Scholar] [CrossRef]
- Yan, X.; Hu, T.; Liu, P.; Li, S.; Zhao, B.; Zhang, Q.; Jiao, W.; Chen, S.; Wang, P.; Lu, J.; et al. Highly efficient and stable Ni/CeO2-SiO2 catalyst for dry reforming of methane: Effect of interfacial structure of Ni/CeO2 on SiO2. Appl. Catal. B Environ. 2019, 246, 221–231. [Google Scholar] [CrossRef]
- Akbari, E.; Alavi, S.M.; Rezaei, M. CeO2 Promoted Ni-MgO-Al2O3 nanocatalysts for carbon dioxide reforming of methane. J. CO2 Util. 2018, 24, 128–138. [Google Scholar] [CrossRef]
- Ray, K.; Bhardwaj, R.; Singh, B.; Deo, G. Developing descriptors for CO2 methanation and CO2 reforming of CH4 over Al2O3 supported Ni and low-cost Ni based alloy catalysts. Phys. Chem. Chem. Phys. 2018, 20, 15939–15950. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Chang, S.W.; Lee, S.M.; Kim, S.S.; Chung, W.J.; Lee, J.C.; Cho, Y.J.; Shin, K.S.; Moon, D.H.; Nguyen, D.D. Developing Ni-based honeycomb-type catalysts using different binary oxide-supported species for synergistically enhanced CO2 methanation activity. Fuel 2019, 250, 277–284. [Google Scholar] [CrossRef]
- Moghaddam, S.V.; Rezaei, M.; Meshkani, F.; Daroughegi, R. Carbon dioxide methanation over Ni-M/Al2O3 (M: Fe, CO, Zr, La and Cu) catalysts synthesized using the one-pot sol-gel synthesis method. Int. J. Hydrogen Energy 2018, 43, 16522–16533. [Google Scholar] [CrossRef]
- Stangeland, K.; Kalai, D.; Li, H.; Yu, Z. CO2 Methanation: The Effect of Catalysts and Reaction Conditions. Energy Procedia 2017, 105, 2022–2027. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Ahmad, A. CO2 methanation over heterogeneous catalysts: Recent progress and future prospects. Green Chem. 2015, 17, 2647–2663. [Google Scholar] [CrossRef]
- Guo, M.; Lu, G. The effect of impregnation strategy on structural characters and CO2 methanation properties over MgO modified Ni/SiO2 catalysts. Catal. Commun. 2014, 54, 55–60. [Google Scholar] [CrossRef]
- Tan, J.; Wang, J.; Zhang, Z.; Ma, Z.; Wang, L.; Liu, Y. Highly dispersed and stable Ni nanoparticles confined by MgO on ZrO2 for CO2 methanation. Appl. Surf. Sci. 2019, 481, 1538–1548. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Abu-Dahrieh, J.K.; Atia, H.; Armbruster, U.; Ibrahim, A.A.; Khan, W.U.; Abasaeed, A.E.; Fakeeha, A.H. Effect of pre-treatment and calcination temperature on Al2O3-ZrO2 supported Ni-Co catalysts for dry reforming of methane. Int. J. Hydrogen Energy 2019, 44, 21546–21558. [Google Scholar] [CrossRef]
- Feng, X.; Feng, J.; Li, W. Insight into MgO promoter with low concentration for the carbon-deposition resistance of Ni-based catalysts in the CO2 reforming of CH4. Chin. J. Catal. 2018, 39, 88–98. [Google Scholar] [CrossRef]
- Vázquez, F.V.; Kihlman, J.; Mylvaganam, A.; Simell, P.; Koskinen-Soivi, M.-L.; Alopaeus, V. Modeling of nickel-based hydrotalcite catalyst coated on heat exchanger reactors for CO2 methanation. Chem. Eng. J. 2018, 349, 694–707. [Google Scholar] [CrossRef]
- Su, X.; Xu, J.; Liang, B.; Duan, H.; Hou, B.; Huang, Y. Catalytic carbon dioxide hydrogenation to methane: A review of recent studies. J. Energy Chem. 2016, 25, 553–565. [Google Scholar] [CrossRef]
- Champon, I.; Bengaouer, A.; Chaise, A.; Thomas, S.; Roger, A.-C. Carbon dioxide methanation kinetic model on a commercial Ni/Al2O3 catalyst. J. CO2 Util. 2019, 34, 256–265. [Google Scholar] [CrossRef]
- Hu, F.; Tong, S.; Lu, K.; Chen, C.-M.; Su, F.-Y.; Zhou, J.; Lu, Z.-H.; Wang, X.; Feng, G.; Zhang, R. Reduced graphene oxide supported Ni-Ce catalysts for CO2 methanation: The support and ceria promotion effects. J. CO2 Util. 2019, 34, 676–687. [Google Scholar] [CrossRef]
- Han, D.; Kim, Y.; Byun, H.; Cho, W.; Baek, Y. CO2 Methanation of Biogas over 20 wt% Ni-Mg-Al Catalyst: On the Effect of N2, CH4, and O2 on CO2 Conversion Rate. Catalysts 2020, 10, 1201. [Google Scholar] [CrossRef]
- Wierzbicki, D.; Baran, R.; Dębek, R.; Motak, M.; Grzybek, T.; Gálvez, M.E.; Da Costa, P. The influence of nickel content on the performance of hydrotalcite-derived catalysts in CO2 methanation reaction. Int. J. Hydrogen Energy 2017, 42, 23548–23555. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, Y.; Zhang, L.; Hu, S.; Xiang, J.; Wang, Y.; Xu, L.; Liu, Q.; Zhang, S.; Hu, X. Impacts of nickel loading on properties, catalytic behaviors of Ni/γ–Al2O3 catalysts and the reaction intermediates formed in methanation of CO2. Int. J. Hydrogen Energy 2019, 44, 9291–9306. [Google Scholar] [CrossRef]
- Quindimil, A.; De-La-Torre, U.; Pereda-Ayo, B.; Davó-Quiñonero, A.; Bailón-García, E.; Lozano-Castelló, D.; González-Marcos, J.A.; Bueno-López, A.; González-Velasco, J.R. Effect of metal loading on the CO2 methanation: A comparison between alumina supported Ni and Ru catalysts. Catal. Today 2019, 356, 419–432. [Google Scholar] [CrossRef]
- Mutz, B.; Carvalho, H.W.; Mangold, S.; Kleist, W.; Grunwaldt, J.-D. Methanation of CO2: Structural response of a Ni-based catalyst under fluctuating reaction conditions unraveled by operando spectroscopy. J. Catal. 2015, 327, 48–53. [Google Scholar] [CrossRef]
- Peymani, M.; Alavi, S.M.; Rezaei, M. Synthesis Gas Production by Catalytic Partial Oxidation of Propane on Mesoporous Nanocrystalline Ni/Al2O3Catalysts. Appl. Catal. A Gen. 2017, 529, 1–9. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, X.; Rui, N.; Hu, X.; Liu, C. Structural effect of Ni/ZrO2 catalyst on CO2 methanation with enhanced activity. Appl. Catal. B Environ. 2019, 244, 159–169. [Google Scholar] [CrossRef]
- Dannesboe, C.; Hansen, J.B.; Johannsen, I. Catalytic methanation of CO2 in biogas: Experimental results from a reactor at full scale. React. Chem. Eng. 2020, 5, 183–189. [Google Scholar] [CrossRef]
- Jürgensen, L.; Ehimen, E.A.; Born, J.; BoHolm-Nielsena, J. Dynamic biogas upgrading based on the Sabatier process: Thermodynamic and dynamic process simulation. Bioresour. Technol. 2015, 178, 323–329. [Google Scholar] [CrossRef]
- Jaffar, M.M.; Nahil, M.A.; Williams, P.T. Parametric study of CO2 methanation for synthetic natural gas production. Energy Technol. 2019, 7, 1900795. [Google Scholar] [CrossRef]
- Erdöhelyi, A.; Pásztor, M.; Solymosi, F. Catalytic hydrogenation of CO2 over supported palladium. J. Catal. 1986, 98, 166–177. [Google Scholar] [CrossRef]
- Agnelli, M.; Kolb, M.; Mirodatos, C. Co Hydrogenation on a Nickel Catalyst: 1. Kinetics and Modeling of a Low-Temperature Sintering Process. J. Catal. 1994, 148, 9–21. [Google Scholar] [CrossRef]
- Tan, M.; Wang, X.; Wang, X.; Zou, X.; Ding, W.; Lu, X. Influence of calcination temperature on textural and structural properties, reducibility, and catalytic behavior of mesoporous γ-alumina-supported Ni–Mg oxides by one-pot template-free route. J. Catal. 2015, 329, 151–166. [Google Scholar] [CrossRef]
- Nakayama, T.; Ichikuni, N.; Sato, S.; Nozaki, F. Ni/Mgo catalyst prepared using citric acid for hydrogenation of carbon dioxide. Appl. Catal. A Gen. 1997, 158, 185–199. [Google Scholar] [CrossRef]
- Carbarino, G.; Riani, P.; Magistri, L.; Busca, G. A study of the methanation of carbon dioxide on Ni/Al2O3 catalysts at atmospheric pressure. Int. J. Hydrogen Energy 2014, 39, 11557–11565. [Google Scholar] [CrossRef]
- Song, F.; Zhong, Q.; Yu, Y.; Shi, M.; Wu, Y.; Hu, J.; Song, Y. Obtaining well-dispersed Ni/Al2O3 catalyst for CO2 methanation with a microwave-assisted method. Int. J. Hydrogen Energy 2017, 42, 4174–4183. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; Smart, R.S.C. X-ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef]
- Jiang, H.; Gao, Q.; Wang, S.; Chen, Y.; Zhang, M. The synergistic effect of Pd NPs and UiO-66 for enhanced activity of carbon dioxide methanation. J. CO2 Util. 2019, 31, 167–172. [Google Scholar] [CrossRef]
- Ou, Z.; Qin, C.; Niu, J.; Zhang, L.; Ran, J. A comprehensive DFT study of CO2 catalytic conversion by H2 over Pt-doped Ni catalysts. Int. J. Hydrogen Energy 2018, 44, 819–834. [Google Scholar] [CrossRef]
- Lim, J.Y.; McGregor, J.; Sederman, A.; Dennis, J. Kinetic studies of CO2 methanation over a Ni/γ-Al2O3 catalyst using a batch reactor. Chem. Eng. Sci. 2016, 141, 28–45. [Google Scholar] [CrossRef]
- Westermann, A.; Azambre, B.; Bacariza, M.C.; Graça, I.; Ribeiro, M.F.; Lopes, J.M.; Henriques, C. Insight into CO2 methanation mechanism over NiUSY zeolites: An operando IR study. Appl. Catal. B Environ. 2015, 174–175, 120–125. [Google Scholar] [CrossRef]
- Miguel, C.; Mendes, A.; Madeira, L. Intrinsic kinetics of CO2 methanation over an industrial nickel-based catalyst. J. CO2 Util. 2018, 25, 128–136. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Zhang, L.; Zhang, H. Bimetallic Ni–Pd/SBA-15 alloy as an effective catalyst for selective hydrogenation of CO2 to methane. Int. J. Hydrogen Energy 2019, 44, 13354–13363. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Jiang, X.; Zhu, J.; Liu, Z.; Guo, X.; Song, C. A short review of recent advances in CO2 hydrogenation to hydrocarbons over heterogeneous catalysts. RSC Adv. 2018, 8, 7651–7669. [Google Scholar] [CrossRef]
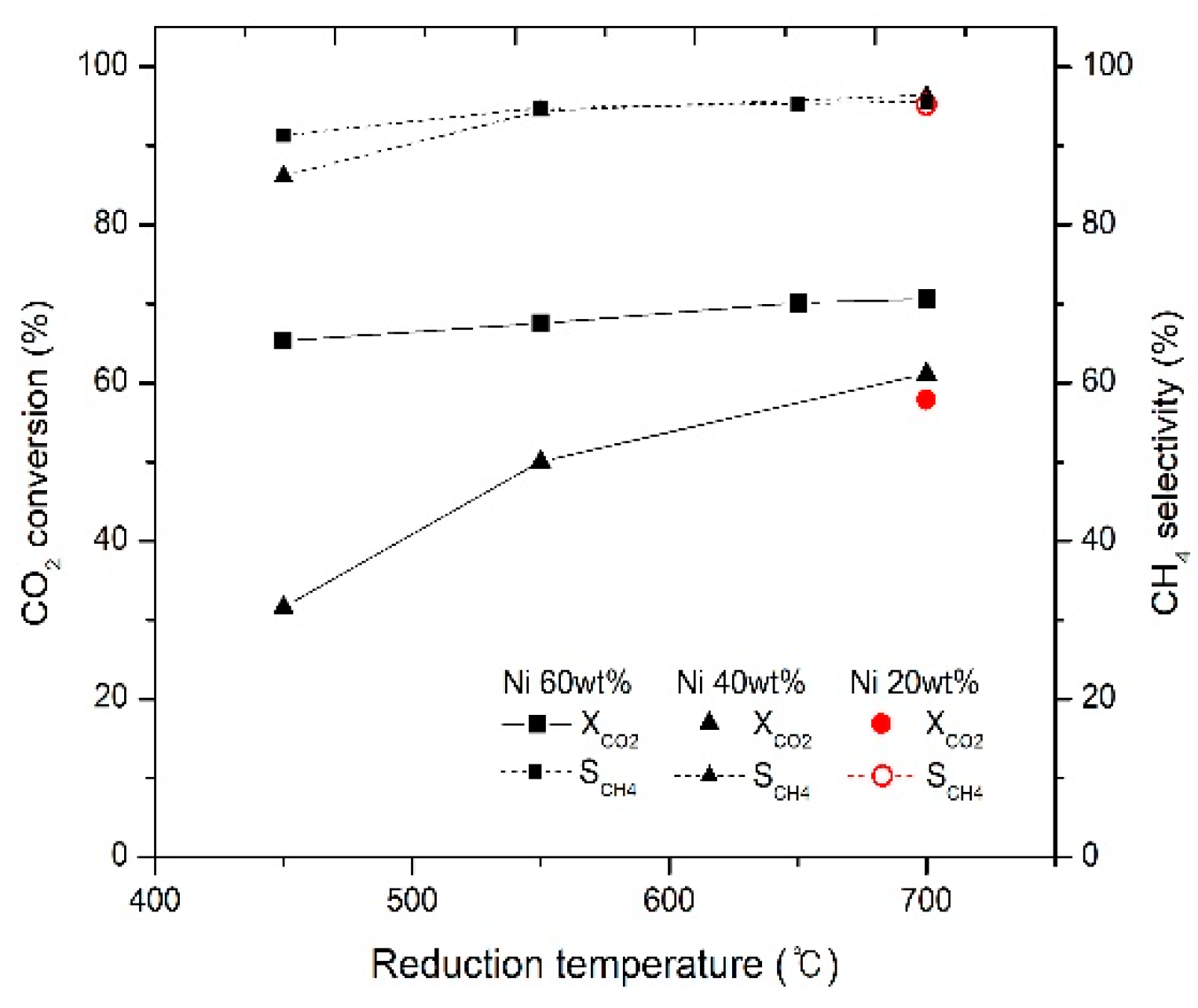

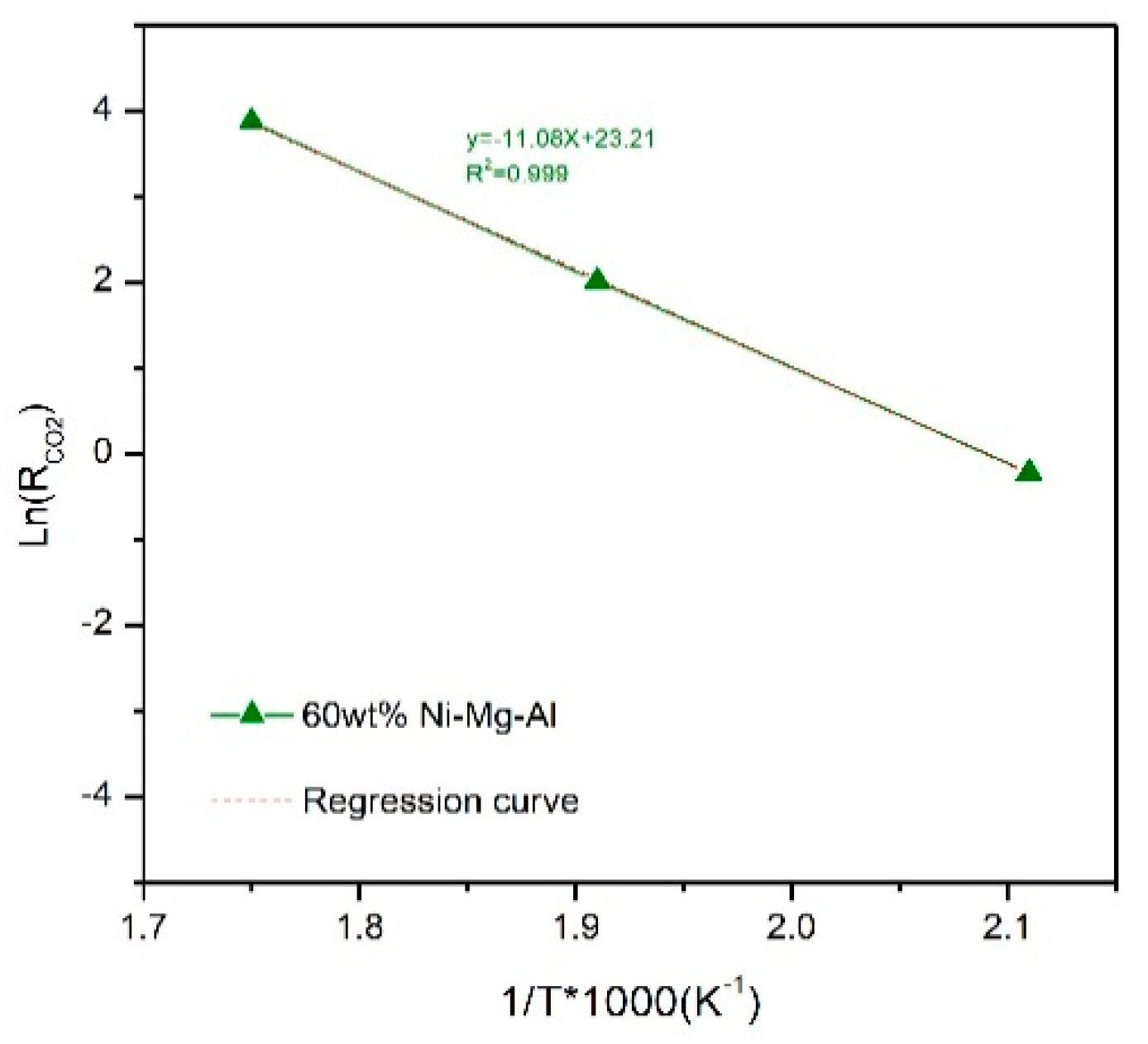
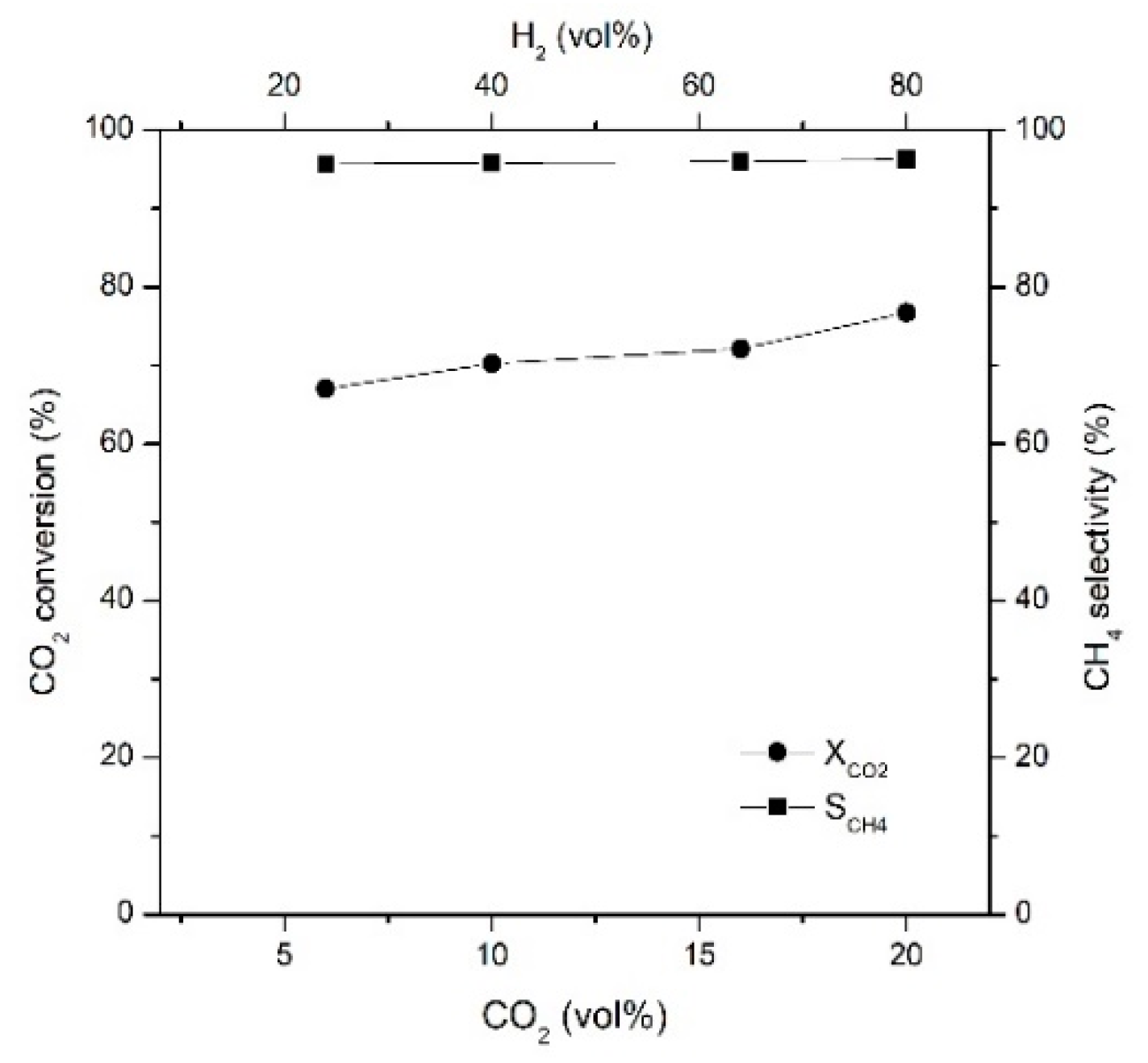




| Ni Catalyst | 20 wt% Ni-Mg-Al | 40 wt% Ni-Mg-Al | 60 wt% Ni-Mg-Al |
|---|---|---|---|
| Reaction temperature (°C) | 340 | 298 | 291 |
| Ni Catalyst | BET (m2/g) | Total Pore Volume (m3/g) | Pore Size (Å) | |
|---|---|---|---|---|
| * 20 (wt%) | fresh | 180.3 | 0.36 | 81.5 |
| spent | 148.9 | 0.30 | 81.1 | |
| 40 (wt%) | fresh | 155.8 | 0.31 | 77.4 |
| spent | 107.8 | 0.32 | 82.4 | |
| 60 (wt%) | fresh | 140.9 | 0.32 | 91.5 |
| spent | 110.8 | 0.23 | 85.9 | |
| Ni Catalyst | Ni Dispersion (%) | Ni Particle Size (nm) | TOF (s−1) |
|---|---|---|---|
| 20 wt% * | 3.57 | 28.3 | 0.01093 |
| 40 wt% * | 3.68 | 27.5 | 0.02366 |
| 60 wt% | 3.42 | 29.6 | 0.02593 |
| Items | Ni (wt%) | Mg (wt%) | Al2O3(wt%) |
|---|---|---|---|
| 20 wt% | 15.9 (20) | 3.2 (5) | 79.8 (75) |
| 40 wt% | 36.8 (40) | 2.0 (5) | 61.2 (55) |
| 60 wt% | 41.5 (60) | 1.0 (5) | 57.5 (35) |
| Parameter | Conditions |
|---|---|
| Temperature (°C) | 200~450 |
| Pressure(bar) | 1 |
| GHSV (/h) | 10,000~50,000 |
| N2 (vol%) | Balance gas |
| CO2 (vol%) | 6, 10, 16, 20 |
| H2/CO2 | 3.5, 4, 4.5, 5 |
| CH4 (vol%) | 0, 6.3, 10.8, 16.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.; Cho, W.; Baek, Y. CO2 Methanation of Biogas over Ni-Mg-Al: The Effects of Ni Content, Reduction Temperature, and Biogas Composition. Catalysts 2022, 12, 1054. https://doi.org/10.3390/catal12091054
Han D, Cho W, Baek Y. CO2 Methanation of Biogas over Ni-Mg-Al: The Effects of Ni Content, Reduction Temperature, and Biogas Composition. Catalysts. 2022; 12(9):1054. https://doi.org/10.3390/catal12091054
Chicago/Turabian StyleHan, Danbee, Wonjun Cho, and Youngsoon Baek. 2022. "CO2 Methanation of Biogas over Ni-Mg-Al: The Effects of Ni Content, Reduction Temperature, and Biogas Composition" Catalysts 12, no. 9: 1054. https://doi.org/10.3390/catal12091054
APA StyleHan, D., Cho, W., & Baek, Y. (2022). CO2 Methanation of Biogas over Ni-Mg-Al: The Effects of Ni Content, Reduction Temperature, and Biogas Composition. Catalysts, 12(9), 1054. https://doi.org/10.3390/catal12091054





