Abstract
Increasing energy consumption and environmental pollution problems have forced people to turn their attention to the development and utilization of hydrogen energy, which requires that hydrogen energy can be efficiently prepared. However, the sluggish kinetics of hydrogen evolution reaction (HER) requires higher overpotential. It is urgent to design and fabricate catalysts to drive the procedure and decrease the overpotential of HER. It is well known that platinum catalysts are the best for HER, but their high cost limits their wide application. Transition metals such as Fe, Co, Mo and Ni are abundant, and transition metal phosphides are considered as promising HER catalysts. Nevertheless, catalysts in powder form are very easily soluble in the electrolyte, which leads to inferior cycling stability. In this work, Ni5P4 anchored on Ni foam was doped with Se powder. After SEM characterization, the Ni5P4-Se was anchored on Ni foam, which circumvents the use of the conductive additives and binder. The Ni5P4-Se formed a porous nanosheet structure with enhanced electron transfer capability. The prepared Ni5P4-Se exhibited high electrochemical performances. At 10 mA cm−2, the overpotential was only 128 mV and the Tafel slope is 163.14 mV dec−1. Additionally, the overpotential was stabilized at 128 mV for 30 h, suggesting its excellent cycling stability. The results show that Se doping can make the two phases achieve a good synergistic effect, which makes the Ni5P4-Se catalyst display excellent HER catalytic activity and stability.
1. Introduction
As an energy carrier with high specific energy density and zero greenhouse gas emissions, hydrogen can be used as a clean renewable energy on a large scale [1,2,3,4,5]. However, there is a high overpotential in the process of electrolysis of water, resulting in a large amount of energy consumption. It is necessary to use efficient catalysts to reduce this overpotential [6,7,8,9]. At present, the catalyst with the best hydrogen evolution performance is platinum group metals, but the scarcity and high price make large-scale application difficult [10,11,12,13,14,15,16].
Nickel is widely known for its excellent HER performance. Therefore, the enthusiasm of researchers to improve the performance of nickel-based catalysts in alkaline solution has not subsided [17,18]. The surface adsorption properties of nickel-based alloys and heterostructure catalysts can optimize their electrocatalytic activity and stability by fine-tuning the synergistic effect generated by adjacent elements [19,20]. Extensive work has found that phosphides have stable behavior in acidic and basic solutions and high current densities at low overpotentials, which make them show great potential as HER catalysts [21,22,23]. The phosphorus in the metal phosphide structure is moderately bonded to the reaction intermediate to participate in the reaction, resulting in the formation of surfaces with proton acceptor and hydride acceptor sites. These reasons make metal phosphides highly active [24,25,26]. Ni and P elements are abundant in the crust. Nickel phosphide compounds have exhibited good electrochemical hydrogen evolution performance in the previous reports [27]. Among them, Ni5P4 particles reveal superior HER performance in both acidic and alkaline solutions [27,28,29]. The preparation of these catalysts is mostly based on nanoparticles. Despite the large surface area of nanoparticles, issues such as uncontrolled agglomeration, high series resistance and susceptibility to oxidation are also detrimental to the overall performance. NiSe2/Ni5P4 nanosheets grown in situ on nitrogen-doped carbon nanofibers were prepared for water splitting [30]. NiSe2/Ni5P4, which has a unique porous nanostructure, was obtained by low-temperature phosphating/selenization of Ni(OH)2. In H2SO4 solution, the HER performance of NiSe2/Ni5P4 is excellent, with an overpotential of only 112 mV at 10 mA cm−2. The heterostructure of NiSe2/Ni5P4 optimizes the adsorption of hydrogen-containing intermediates, which enables the excellent HER activity of NiSe2/Ni5P4 on nitrogen-doped carbon nanofibers. Zhuo et al. obtained a HER catalyst grown on carbon paper by doping pyrite-phase nickel phosphide with Se [31]. Compared with undoped NiP2, the overpotential and Tafel slope of Se-doped NiP2 (NiP1.93Se0.07) have obvious advantages. The above results show that Se doping can improve the HER performance of NiP2. The two phases of biphasic catalysts are promisingly synergistic to optimize the adsorption–desorption behavior of intermediates at active sites [30,32,33]. Nevertheless, the catalyst in powder form is very easily soluble in the electrolyte, which leads to the inferior cycling stability. Moreover, the addition of the conductive additives and binder increases the whole cost.
Here, we used Ni foam as the substrate and sodium hypophosphite as the phosphorus source to prepare a Ni5P4 anchored on Ni foam for water electrolysis through a gas-phase reaction with a low-process cost. The structure, morphology and hydrogen evolution properties were characterized. After that, we tried to use selenium powder as the selenium source to dope the catalyst to form the Ni5P4-Se composite, which further reduced the overpotential. Among the four catalysts, the Ni5P4-Se anchored on Ni foam displayed the best HER performances. At 10 mA cm−2, the Ni5P4-Se composite displayed an overpotential of only 128 mV and possessed a Tafel slope of 163.14 mV dec−1. The Ni5P4-Se electrode also exhibited excellent cycling durability, with an overpotential stabilized at 128 mV when tested at 10 mA cm−2 for 10 h.
2. Experimental
2.1. Synthesis
The synthesis of the Ni(OH)2 precursor: First, the Ni foam was divided into small pieces (1 × 1.5 cm), soaked in 1 M HCl solution for 15 min, and ultrasonically cleaned with ultrapure water and anhydrous ethanol for three times. Then, 6 mmol Ni(NO3)2·6H2O and 10 mmol urea were dissolved in 30 mL of deionized water, and a clear and transparent green solution was obtained after magnetic stirring for 30 min. The treated Ni foam was immersed in the green solution. The above solution was transferred to an autoclave, which was placed in an oven at 180 °C for 12 h. After cooling down to the room temperature, the sample was taken out and washed with ultrapure water for several times. Lastly, the Ni(OH)2 precursors in situ grown on the Ni foam were obtained.
The synthesis of the Ni5P4-Se nanocatalysts: The Ni(OH)2 precursor was placed on the downstream side of the tube furnace. In all, 1 g NaH2PO2·H2O and 50 mg Se powder were placed on the upstream side. Then, the tube furnace was heated to 300 °C at a heating rate of 2 °C min−1 under an argon atmosphere, which was kept for 120 min. After naturally cooling to room temperature, in situ Ni5P4-Se nanocatalysts grown on nickel foam were obtained. Single-phase Ni5P4 and NiSe2 were prepared by the above-mentioned method from equimolar amounts of NaH2PO2·H2O and Se powder as phosphorus and selenium sources, respectively.
2.2. Characterization
The physicochemical properties of the catalysts were mainly characterized using the following means. The microstructure, morphology and elemental constitution of samples were characterized on field emission scanning electron microscope (SEM, Quanta FEG 250), super-resolution SEM (Verios 460L, Hillsboro, OR, USA), transmission electron microscope (TEM, LaB6) and high-resolution TEM (Talos F200X, Hillsboro, OR, USA). The X-ray diffraction (XRD) patterns of the materials were obtained on a MiniFlex600 X-ray diffractometer (Rigaku, Japan). The scan rate is 5° min−1. The range is from 10° to 90°. An X-ray photoelectron spectrometer (XPS, ESCALAB 250Xi, Waltham, MA, USA) was used to determine the elemental composition and chemical valence of the samples. The samples for SEM, XRD and XPS characterizations are powder. For TEM characterization, the powder sample is dispersed in ethanol to form a homogeneous solution, which is dropwise added into Cu mesh.
2.3. Electrochemical Measurements
The electrochemical measurements were conducted on the CHI660E electrochemical workstation. In the three-electrode system, the prepared material was used as the working electrode, graphite as the counter electrode and saturated calomel electrode (SCE) as the reference electrode. The measured raw data potentials were relative to the SCE. They needed to be converted into a standard reversible hydrogen electrode (RHE) potential. The specific conversion formula is: E (vs. RHE) =E (vs. SCE) + (0.059 × pH) +0.241 V. Electrochemical measurements include: linear sweep voltammetry (LSV), cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS) and galvanostatic stability curve (V-t).
3. Results and Discussion
Ni(OH)2 anchored on Ni foam was prepared by hydrothermal method. As illustrated in Figure S1, the diffraction peaks of the Ni(OH)2 catalyst are very obvious and match well with JCPDS No. 74-2075. It is proved that the pure phase Ni(OH)2 was successfully obtained with good crystallinity. The Ni(OH)2 nanoarrays are relatively uniformly grown on the ligaments surface of the Ni foam (Figure S2a). The grown Ni(OH)2 precursor is a flower-like cluster structure composed of many nanosheets, which can be clearly observed in the local enlarged view of the Ni foam ligament (Figure S2b). Moreover, the content of Ni(OH)2 in the composites is about 24 wt%. The Ni(OH)2 precursor was subjected to phosphorization, selenization or simultaneous phosphorization and selenization reactions. The XRD patterns of the obtained products are shown in Figure 1a. The diffraction peaks of single-phase Ni5P4 and NiSe2 are matched with JCPDS No. 28-0883 and JCPDS No. 41-1495, respectively, indicating that the Ni(OH)2 precursor was completely converted into Ni5P4 and NiSe2. The XRD pattern of the Ni5P4-Se is almost close to that of the single-phase Ni5P4 (Figure 1a). No obvious selenium diffraction peaks were presented in the XRD pattern of the Ni5P4-Se due to the relatively low content of the Se element source. The doping of Se induces the formation of a two-phase heterojunction and reduces the crystallinity of the single-phase Ni5P4. Therefore, by observing the characteristic peaks of the Ni5P4 phase, it can be found that Se doping significantly weakens the peak intensity of the Ni5P4 phase. The corresponding microstructures were also characterized. It can be clearly observed in Figure S3a,b that a single Ni5P4 nanoarray grows uniformly on the Ni foam. Many active sites cannot be exposed in Figure S3c because of the accumulation of a large number of large sheets to form clusters or particles. The morphology of the layered NiSe2 array is displayed in Figure S4. The NiSe2 nanosheets grow more densely, which possess an approximate microstructure with the Ni(OH)2 precursor.
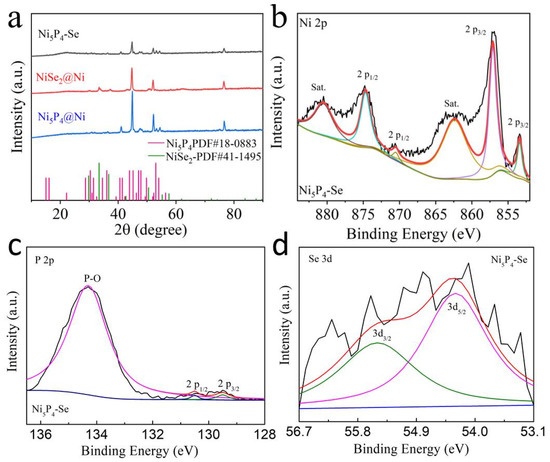
Figure 1.
(a) XRD patterns of Ni5P4, NiSe2 and Ni5P4-Se. XPS spectra of Ni5P4-Se: (b) Ni 2p, (c) P 2p, (d) Se 3d.
The high-resolution XPS spectras of Ni5P4 and NiSe2 are included in Figure S5. The Ni 2p spectrum of Ni5P4 in Figure S5a illustrates that the peaks located at 853.4 eV and 870.5 eV correspond to Ni 2p3/2 and Ni 2p1/2, respectively. The peaks centered at 857.1 eV (2p3/2) and 874.7 Ev (2p1/2) can be ascribed to the oxidation state of Ni. Two doublets are located at 129.6 eV and 130.4 eV in the P 2p spectrum, which belong to P 2p3/2 and P 2p1/2, respectively. The characteristic peak at 134.2 eV suggests the existence of the P–O bond. There are two peaks at 855.8 eV and 873.1 eV in the Ni 2p spectrum of NiSe2, which are ascribed to Ni 2p3/2 and Ni 2p1/2, respectively (Figure S5c). The peak at 854 eV (2p3/2) corresponds to nickel oxide. In addition, two satellite peaks were observed at 855.8 eV and 879.5 eV. There are two peaks here at 55.1 eV and 54.5 eV in the Se 3d spectrum corresponding to Se 3d3/2 and Se 3d5/2, respectively. (Figure S5d).
The XPS measurement of the Ni5P4-Se was conducted to investigate the chemical composition and valences (Figure 1b–d). Similarly, several deconvoluted peaks (853.4 eV for Ni 2p3/2 and 870.5 eV for Ni 2p1/2) were obtained in C 1s spectra (Figure 1b). The peaks at 857.1 eV (2p3/2) and 874.7 eV (2p1/2) can be owing to the oxidation state of Ni. The two doublets at 129.6 eV and 130.4 eV in P 2p spectra are attributed to P 2p3/2 and P 2p1/2, respectively (Figure 1c). The characteristic peak at 134.2 eV is ascribed to the P–O bond. In the Se 3d spectrum shown in Figure 1d, the peaks located at 54.9 eV and 54.2 eV belong to Se 3d3/2 and Se 3d5/2, respectively. Compared with the single-phase Ni5P4, the P 2p peak exhibits a small positive shift (0.4 eV) after Se doping. In contrast, the two peaks of Se 3d5/2 and 3d3/2 are negatively shifted (0.2 eV) compared to the single-phase NiSe2. This phenomenon demonstrates that electrons are transferred from P to Se at the locally formed heterostructure interface, suggesting the formation of a strong internal electronic effect heterointerface. These will lead to the transfer and redistribution of electrons. Because of this electronic effect, the heterointerface provides more reactive sites, reduces the catalytic reaction overpotential and improves the catalytic efficiency.
The nanosheet layer uniformly grown on the ligament surface of the Ni foam was obtained through the steam reaction jointly driven by sodium hypophosphite and selenium powder (Figure 2a,b). The partial enlarged view of the nanosheet layer is displayed in Figure 2c. A regular nanosheet array with a size of about 500–1000 nm can be observed in the local area. Since these regular nanosheet arrays are not similar to other large-scale curved nanosheets, we infer that the Ni5P4/NiSe2 heterojunction structure is locally grown on the ligaments of the Ni foam. Energy Dispersive Spectrometer (EDS) and elemental analysis were performed on this region to further analyze the doping situation of Se. In the regular nanosheet region in Figure 2d–f, we can clearly observe that the content of the Se element is higher than that of the P element, while the P element is dominant in the large-sized nanosheet region. The morphological structure of the Ni5P4-Se composites were verified by the TEM images (Figure S6). From the above results, it can be roughly concluded that the Se-doped Ni5P4 phase is mainly concentrated in this region. The synergistic effect of the regular two-phase structure nanosheets provides more active sites for HER. The Energy Dispersive Spectrometer (EDS) spectrum is shown in Figure S7. The ratio of the Se and P atoms in this region is about 1:1, which further verifies the successful doping of Se into Ni5P4.
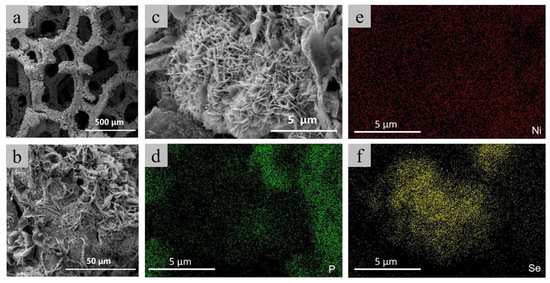
Figure 2.
Low magnification SEM images (a–c) and corresponding elemental distributions of Ni5P4-Se composites (d–f).
The electrochemical HER performances of the Ni(OH)2 precursor, the single-phase Ni5P4 and NiSe2 and the Ni5P4-Se were measured using a three-electrode system. The LSV curves of the four catalysts at 10 mVs−1 are depicted in Figure 3a. The overpotential of the the Ni5P4-Se is only 128 mV at 10 mA cm−2, which is significantly better than that of the single-phase Ni5P4 (150 mV), NiSe2 (211 mV) and Ni(OH)2 (278 mV). To explore the reaction kinetics in depth, we fitted the Tafel slope according to the Tafel equation. The Tafel slope of the Ni5P4-Se electrocatalyst is 163.14 mV dec−1 in Figure 3b, which has obvious advantages compared with Ni5P4 (177.94 mV dec−1), NiSe2 (183.94 mV dec−1) and Ni(OH)2 (208.77 mV dec−1). The Ni5P4-Se catalysts exhibited good reaction kinetics and a higher electron transfer efficiency, which makes the catalytic reaction easier. Meanwhile, in order to explore the charge transfer kinetics, the electrochemical impedance spectra (Figure 3c) show the charge transfer resistance of the four catalysts. The semicircle of the Nyquist plot refers to the charge transfer resistance Rct during the HER process. Obviously, the electrocatalyst of the Ni5P4-Se exhibits the smallest semicircular diameter, which further proves that the doping of Se accelerates the electron transfer of the single-phase Ni5P4 and the HER catalytic kinetics are stronger. The long-cycle stability of the Ni5P4-Se electrode was measured at 10 mA cm−2 for 30 h (Figure 3d). The overpotential was stabilized at 128 mV, demonstrating its excellent cycling durability. Moreover, the SEM image of the Ni5P4-Se catalyst is displayed in Figure S8. As we can see, the Ni5P4-Se catalyst exhibits a nanosheet structure, which maintains its original morphological structure, further demonstrating its structural stability. The comparison of the catalytic activity of catalysts is presented in Table S1 [34,35,36,37,38,39,40,41,42,43].
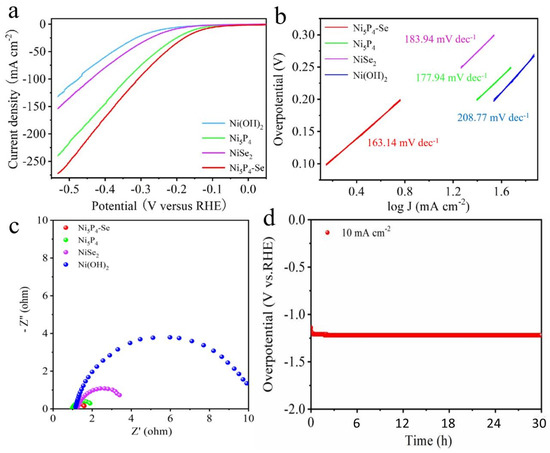
Figure 3.
(a) Polarization curve, (b) Tafel slope, (c) Nyquist plots of Ni(OH)2, Ni5P4, NiSe2, Ni5P4−Se samples measured in 1 M KOH electrolyte., (d) V-t curves of Ni5P4−Se at 10 mA cm−2.
An electrochemically active surface area (ECSA), which was evaluated by measuring electrochemical double layer capacitance (Cdl), was used to further study the electrocatalytic kinetic performance of the catalyst. The Faradaic potential region was selected as shown in Figure 4. The CV curves were tested at 10, 20, 50, 80 and 100 mV s−1, respectively. The ECSAs of the catalysts were obtained by linear fitting. The Cdl values of the obtained Ni(OH)2, Ni5P4, NiSe2, and Ni5P4-Se electrocatalysts are 2.32, 53.69, 28.32, 17.19, and 61.74 mF cm−2, respectively (Figure 5). Compared with the single-phase Ni5P4 and NiSe2, the Se-doped electrocatalyst can expose more active sites to drive the catalysis, which is more conducive to the hydrogen evolution reaction.
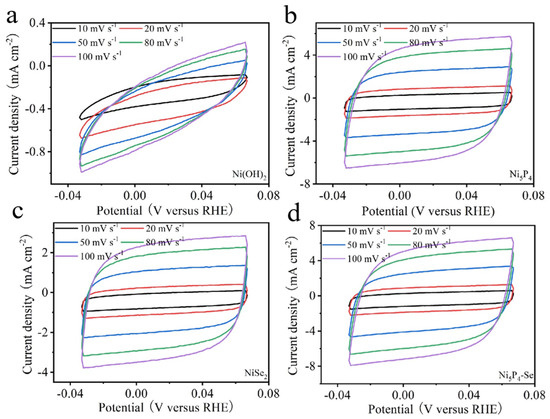
Figure 4.
CV curves of Ni(OH)2 (a), Ni5P4 (b), NiSe2 (c), Ni5P4−Se (d).
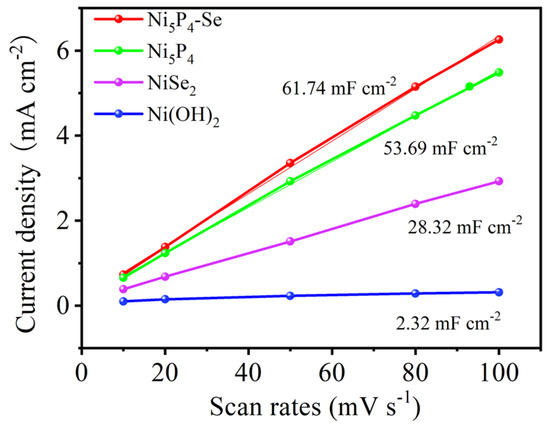
Figure 5.
The Cdl values of Ni(OH)2, Ni5P4, NiSe2, Ni5P4−Se.
4. Conclusions
In this work, we successfully prepared a Ni5P4 catalyst on Ni foam and studied the effect of Se doping on the catalytic performances and structure of single-phase Ni5P4 catalysts. After characterization, the Ni5P4-Se displayed a unique porous nanosheet structure, which is beneficial to expose a large quantity of active sites and greatly improve the catalytic performances. The Ni5P4-Se composite catalyst exhibited amazing catalytic performances, with an overpotential of only 128 mV and a Tafel slope of 163.14 mV dec−1 at 10 mA cm−2. The overpotential was stabilized at 128 mV for 30 h, demonstrating its superior cycling stability. The direct synthesis of the Ni5P4-Se on the Ni foam ensured good electrical contact between the catalyst and the conductive support, fast electron transfer, mass transfer and bubble release, thereby endowing more active sites and stability. The strategy of directly constructing composite catalysts on porous Ni foam provides a new idea for designing efficient water electrolysis catalysts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal12091055/s1, Figure S1: XRD patterns of Ni(OH)2 precursors; Figure S2: (a–b) SEM images of Ni(OH)2 precursor; Figure S3: (a–c) SEM images of Ni5P4; Figure S4: (a–c) SEM images of NiSe2; Figure S5: High-resolution XPS of Ni5P4 and NiSe2, (a) XPS of Ni 2p in pure Ni5P4, (b) XPS of P 2p in pure Ni5P4, (c) XPS of Ni 2p in NiSe2, (d) XPS of Se 3d in NiSe2; Figure S6: TEM images (a-b) of Ni5P4-Se composites; Figure S7: Energy spectrum for Ni5P4-Se composites; Figure S8: SEM image of Ni5P4-Se catalyst after long cycles; Table S1: Comparison of the catalytic activity of catalysts [34,35,36,37,38,39,40,41,42,43].
Author Contributions
Conceptualization, C.A. and J.L.; methodology, Y.W.; validation, C.A., Y.W. and S.W.; formal analysis, P.J.; investigation, C.A. and P.J.; resources, S.W. and J.L.; data curation, C.A., L.G. and C.Z.; writing—original draft preparation, C.A.; writing—review and editing, S.W. and C.Z.; supervision, N.H.; project administration, S.W. and J.L.; funding acquisition, S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Fund of China (Grant No.: 11632004, 52005151, U1864208), the Research Program of Local Science and Technology Development under the Guidance of Central (216Z4402G), the National Science and Technology Major Project (2017-VII-0011-0106), the Science and Technology Planning Project of Tianjin (20ZYJDJC00030), the Key Program of Research and Development of Hebei Province (202030507040009), the Natural Science Foundation of Hebei Province (E2021202008), the Opening Foundation of State Key Laboratory of Tribology in Tsinghua University (SKLTKF20B03), the Fund for Innovative Research Groups of Natural Science Foundation of Hebei Province (A2020202002) and the Key Project of Natural Science Foundation of Tianjin (S20ZDF077). We also acknowledge support from the ‘‘Yuanguang” Scholar Program of Hebei University of Technology.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, Z.; Qing, H.; Zhou, K.; Sun, D.; Wu, R. Metal-organic framework-derived nanocomposites for electrocatalytic hydrogen evolution reaction. Prog. Mater. Sci. 2020, 108, 100618. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.-Y. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef] [PubMed]
- El-Hakam, S.A.; ALShrifi, F.T.; Salama, R.S.; Gamal, S.; El-Yazeed, W.S.A.; Ibrahim, A.A.; Ahmed, A.I. Application of nanostructured mesoporous silica/bismuth vanadate composite catalysts for the degradation of methylene blue and brilliant green. J. Mater. Res. Technol. 2022, 18, 1963–1976. [Google Scholar] [CrossRef]
- Alshorifi, F.T.; Alswat, A.A.; Mannaa, M.A.; Alotaibi, M.T.; El-Bahy, S.M.; Salama, R.S. Facile and green synthesis of silver quantum dots immobilized onto a polymeric CTS-PEO blend for the photocatalytic degradation of p-nitrophenol. ACS Omega 2021, 6, 30432–30441. [Google Scholar] [CrossRef] [PubMed]
- Alshorifi, F.T.; Ali, S.L.; Salama, R.S. Promotional synergistic effect of Cs-Au NPs on the performance of Cs-Au/MgFe2O4 catalysts in catalyisi 3,4-dihydropyrimidin-2(1H)-ones and degradation of RhB dye. J. Inorg. Organomet. Polym. 2022. [Google Scholar] [CrossRef]
- Chhetri, K.; Muthurasu, A.; Dahal, B.; Kim, T.; Mukhiya, T.; Chae, S.-H.; Ko, T.H.; Choi, Y.C.; Kim, H.Y. Engineering the abundant heterointerfaces of integrated bimetallic sulfide-coupled 2D MOF-derived mesoporous CoS2 nanoarray hybrids for electrocatalytic water splitting. Mater. Today Nano 2022, 17, 100146. [Google Scholar] [CrossRef]
- Zhou, Z.; Pei, Z.; Wei, L.; Zhao, S.; Jian, X.; Chen, Y. Electrocatalytic hydrogen evolution under neutral pH conditions: Current understandings, recent advances, and future prospects. Energy Environ. Sci. 2020, 13, 3185–3206. [Google Scholar] [CrossRef]
- Kandel, M.R.; Pan, U.N.; Paudel, D.R.; Dhakal, P.P.; Kim, N.H.; Lee, J.H. Hybridized bimetallic phosphides of Ni-Mo, Co-Mo, and Co-Ni in a single ultrathin-3D-nanosheets for efficient HER and OER in alkaline media. Compos. Part B Eng. 2022, 239, 109992. [Google Scholar] [CrossRef]
- Wu, H.M.; Feng, C.Q.; Zhang, L.; Zhang, J.J.; Wilkinson, D.P. Non-noble metal electrocatalysts for the hydrogen evolution reaction in water electrolysis. Electrochem. Energy Rev. 2021, 4, 473–507. [Google Scholar] [CrossRef]
- Jiao, P.; Wu, S.; Zhu, C.; Ye, D.; Qin, C.; An, C.; Hu, N.; Deng, Q. Non-precious transition metal single-atom catalysts for oxygen reduction reaction: Progress and prospects. Nanoscale 2022. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xiao, B.; Lin, Z.; Xu, Y.; Lin, Y.; Meng, F.; Zhang, Q.; Gu, L.; Fang, B.; Guo, S.; et al. PtSe2/Pt Heterointerface with reduced coordination for boosted hydrogen evolution reaction. Angew. Chem. 2021, 133, 23576–23581. [Google Scholar] [CrossRef]
- Hansen, J.N.; Prats, H.; Toudahl, K.K.; Secher, N.M.; Chan, K.; Kibsgaard, J.; Chorkendorff, I. Is there anything better than Pt for HER? ACS Energy Lett. 2021, 6, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, H.; Gao, W.; Xue, W.; Liu, Z.; Huang, J.; Pan, X.; Huang, Y. Surface-engineered PtNi-O nanostructure with record-high performance for electrocatalytic hydrogen evolution reaction. J. Am. Chem. Soc. 2018, 140, 9046–9050. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Xu, T.; Lu, Z.; Wu, X.; Wan, P.; Sun, X.; Jiang, L. Under-water superaerophobic pine-shaped Pt nanoarray electrode for ultrahigh-performance hydrogen evolution. Adv. Funct. Mater. 2015, 25, 1737–1744. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, T.; Liu, D.; Wang, Z.; Deng, Q.; Qu, D.; Xie, Z.; Tang, H.; Li, J. Confining nano-sized platinum in nitrogen doped ordered mesoporous carbon: An effective approach toward efficient and robust hydrogen evolution electrocatalyst. J. Colloid Interface Sci. 2018, 530, 595–602. [Google Scholar] [CrossRef]
- An, C.-H.; Kang, W.; Deng, Q.-B.; Hu, N. Pt and Te codoped ultrathin MoS2 nanosheets for enhanced hydrogen evolution reaction with wide pH range. Rare Met. 2022, 41, 378–384. [Google Scholar] [CrossRef]
- Gong, M.; Wang, D.-Y.; Chen, C.-C.; Hwang, B.-J.; Dai, H. A mini review on nickel-based electrocatalysts for alkaline hydrogen evolution reaction. Nano Res. 2016, 9, 28–46. [Google Scholar] [CrossRef]
- Cao, Z.; Zhou, T.; Ma, X.; Shen, Y.; Deng, Q.; Zhang, W.; Zhao, Y. Hydrogen production from urea sewage on NiFe-based porous electrocatalysts. ACS Sustain. Chem. Eng. 2020, 8, 11007–11015. [Google Scholar] [CrossRef]
- Kou, T.; Smart, T.; Yao, B.; Chen, I.; Thota, D.; Ping, Y.; Li, Y. Theoretical and experimental insight into the effect of nitrogen doping on hydrogen evolution activity of Ni3S2 in alkaline medium. Adv. Energy Mater. 2018, 8, 1703538. [Google Scholar] [CrossRef]
- Jin, H.; Liu, X.; Chen, S.; Vasileff, A.; Li, L.; Jiao, Y.; Song, L.; Zheng, Y.; Qiao, S.-Z. Heteroatom-doped transition metal electrocatalysts for hydrogen evolution reaction. ACS Energy Lett. 2019, 4, 805–810. [Google Scholar] [CrossRef]
- El-Refaei, S.M.; Russo, P.A.; Pinna, N. Recent advances in multimetal and doped transition-metal phosphides for the hydrogen evolution reaction at different pH values. ACS Appl. Mater. Interfaces 2021, 13, 22077–22097. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhao, H.; Li, T.; Liang, J.; Lu, S.; Chen, G.; Gao, S.Y.; Asiri, A.M.; Wu, Q.; Sun, X. Iron-based phosphides as electrocatalysts for the hydrogen evolution reaction: Recent advances and future prospects. J. Mater. Chem. A 2020, 8, 19729–19745. [Google Scholar] [CrossRef]
- Weng, C.-C.; Ren, J.-T.; Yuan, Z.-Y. Transition metal phosphide-based materials for efficient electrochemical hydrogen evolution: A critical review. ChemSusChem 2020, 13, 3357–3375. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Yang, Z.; Chen, Y.; Yuan, Z. Nickel cobalt phosphide with three-dimensional nanostructure as a highly efficient electrocatalyst for hydrogen evolution reaction in both acidic and alkaline electrolytes. Nano Res. 2019, 12, 375–380. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, Z.; Chen, Z.; Lv, C.; Humphrey, M.G.; Zhang, C. Cobalt phosphide nanorods as an efficient electrocatalyst for the hydrogen evolution reaction. Nano Energy 2014, 9, 373–382. [Google Scholar] [CrossRef]
- Chhetri, K.; Dahal, B.; Tiwari, A.P.; Mukhiya, T.; Muthurasu, A.; Ojha, G.P.; Lee, M.; Kim, T.; Chae, S.-H.; Kim, H.Y. Controlled selenium infiltration of cobalt phosphide nanostructure arrays from a two-dimensional cobalt metal-organic framework: A self-supported electrode for flexible quasi-solid-state asymmetric supercapacitors. ACS Appl. Energy Mater. 2021, 4, 404–415. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Y.; Zhao, J.; Yang, K.; Liang, J.; Liu, D.; Hu, W.; Liu, D.; Liu, Y.; Liu, C. Monodispersed nickel phosphide nanocrystals with different phases: Synthesis, characterization and electrocatalytic properties for hydrogen evolution. J. Mater. Chem. A 2015, 3, 1656–1665. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, L.; Liu, R.; Hu, Y.; Xiong, T.; Qiu, W.; Balogun, M.S.; Pan, A.; Tong, Y. Nitrogen treatment generates tunable nanohybridization of Ni5P4 nanosheets with nickel hydr(oxy)oxides for efficient hydrogen production in alkaline, seawater and acidic media. Appl. Catal. B Environ. 2019, 251, 181–194. [Google Scholar] [CrossRef]
- Ledendecker, M.; Calderon, S.K.; Papp, C.; Steinruck, H.-P.; Antonietti, M.; Shalom, M. The synthesis of nanostructured Ni5P4 films and their use as a non-noble bifunctional electrocatalyst for full water splitting. Angew. Chem. Int. Edit. 2015, 54, 12361–12365. [Google Scholar] [CrossRef]
- Yu, J.; Tian, Y.; Lin, Z.; Liu, Q.; Liu, J.; Chen, R.; Zhang, H.; Wang, J. NiSe2/Ni5P4 nanosheets on nitrogen-doped carbon nano-fibred skeleton for efficient overall water splitting. Colloid Surf. A Physicochem. Eng. Asp. 2021, 614, 126189. [Google Scholar] [CrossRef]
- Zhuo, J.; Caban-Acevedo, M.; Liang, H.; Samad, L.; Ding, Q.; Fu, Y.; Li, M.; Jin, S. High-performance electrocatalysis for hydrogen evolution reaction using Se-doped pyrite-phase nickel diphosphide nanostructures. ACS Catal. 2015, 5, 6355–6361. [Google Scholar] [CrossRef]
- Li, K.; Xu, J.; Chen, C.; Xie, Z.; Liu, D.; Qu, D.; Tang, H.; Wei, Q.; Deng, Q.; Li, J.; et al. Activating the hydrogen evolution activity of Pt electrode via synergistic interaction with NiS2. J. Colloid Interface Sci. 2021, 582, 591–597. [Google Scholar] [CrossRef]
- Wang, C.; An, C.; Qin, C.; Gomaa, H.; Deng, Q.; Wu, S.; Hu, N. Noble metal-based catalysts with core-shell structure for oxygen reduction reaction: Progress and prospective. Nanomaterials 2022, 12, 2480. [Google Scholar] [CrossRef]
- Ding, Y.H.; Li, H.Y.; Hou, Y. Phosphorus-doped nickel sulfides/nickel foam as electrode materials for electrocatalytic water splitting. Int. J. Hydrogen Energy 2018, 43, 19002–19009. [Google Scholar] [CrossRef]
- Liu, H.H.; Zeng, S.; He, P.; Dong, F.Q.; He, M.Q.; Zhang, Y.; Wang, S.; Li, C.X.; Liu, M.Z.; Jia, L.P. Samarium oxide modified Ni-Co nanosheets based three-dimensional honeycomb film on nickel foam: A highly efficient electrocatalyst for hydrogen evolution reaction. Electrochim. Acta 2019, 299, 405–414. [Google Scholar] [CrossRef]
- Wang, Y.R.; Wang, Z.J.; Jin, C.; Li, C.; Li, X.W.; Li, Y.F.; Yang, R.Z.; Liu, M.L. Enhanced overall water electrolysis on a bifunctional perovskite oxide through interfacial engineering. Electrochim. Acta 2019, 318, 120–129. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.Z.; Gao, G.P.; Chen, H.; Wang, B.; Zhou, J.Z.; Soo, M.T.; Hong, M.; Yan, X.C.; Qian, G.R.; et al. A Heterostructure Coupling of Exfoliated Ni-Fe Hydroxide Nanosheet and Defective Graphene as a Bifunctional Electrocatalyst for Overall Water Splitting. Adv. Mater. 2017, 29, 8. [Google Scholar] [CrossRef]
- Li, Y.; Cai, P.W.; Ci, S.Q.; Wen, Z.H. Strongly Coupled 3D Nanohybrids with Ni2P/Carbon Nanosheets as pH-Universal Hydrogen Evolution Reaction Electrocatalysts. ChemElectroChem 2017, 4, 340–344. [Google Scholar] [CrossRef]
- Qian, H.X.; Li, K.Y.; Mu, X.B.; Zou, J.Z.; Xie, S.H.; Xiong, X.B.; Zeng, X.R. Nanoporous NiFeMoP alloy as a bifunctional catalyst for overall water splitting. Int. J. Hydrogen Energy 2020, 45, 16447–16457. [Google Scholar] [CrossRef]
- Lv, C.C.; Peng, Z.; Zhao, Y.X.; Huang, Z.P.; Zhang, C. The hierarchical nanowires array of iron phosphide integrated on a carbon fiber paper as an effective electrocatalyst for hydrogen generation. J. Mater. Chem. 2016, 4, 1454–1460. [Google Scholar] [CrossRef]
- Song, J.H.; Zhu, C.Z.; Xu, B.Z.; Fu, S.F.; Engelhard, M.H.; Ye, R.F.; Du, D.; Beckman, S.P.; Lin, Y.H. Bimetallic Cobalt-Based Phosphide Zeolitic Imidazolate Framework: CoPx Phase-Dependent Electrical Conductivity and Hydrogen Atom Adsorption Energy for Efficient Overall Water Splitting. Adv. Energy Mater. 2017, 7, 9. [Google Scholar] [CrossRef]
- Read, C.G.; Callejas, J.F.; Holder, C.F.; Schaak, R.E. General Strategy for the Synthesis of Transition Metal Phosphide Films for Electrocatalytic Hydrogen and Oxygen Evolution. ACS Appl. Mater. Interfaces 2016, 8, 12798–12803. [Google Scholar] [CrossRef] [PubMed]
- Amorim, I.; Xu, J.Y.; Zhang, N.; Xiong, D.H.; Thalluri, S.M.; Thomas, R.; Sousa, J.P.S.; Araujo, A.; Li, H.; Liu, L.F. Bi-metallic cobalt-nickel phosphide nanowires for electrocatalysis of the oxygen and hydrogen evolution reactions. Catal. Today 2020, 358, 196–202. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).