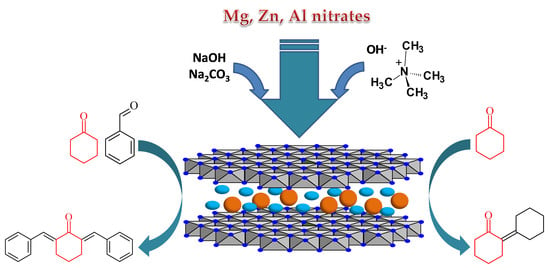
An Advanced Approach for MgZnAl-LDH Catalysts Synthesis Used in Claisen-Schmidt Condensation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Catalysts
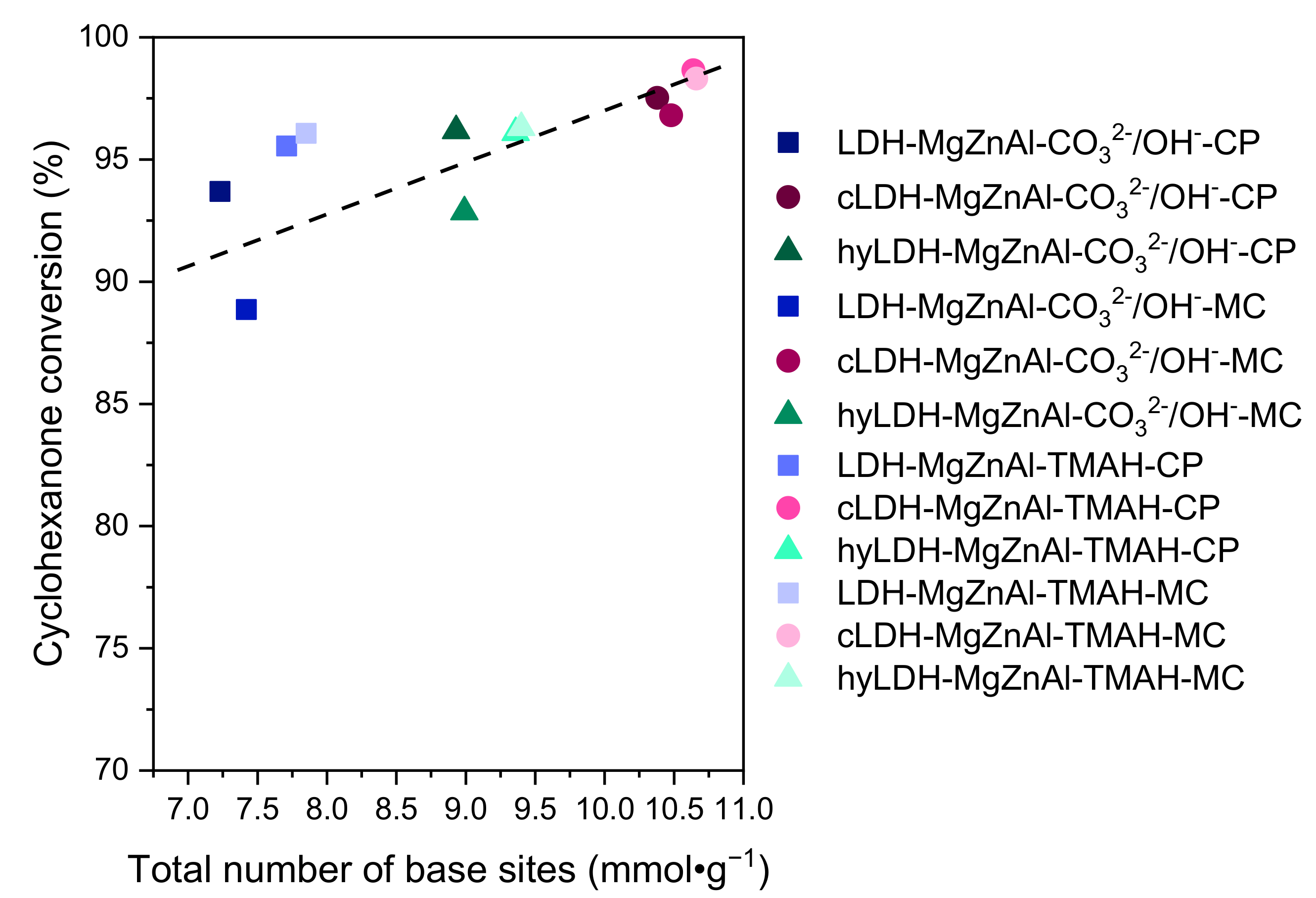
2.2. Catalytic Activity
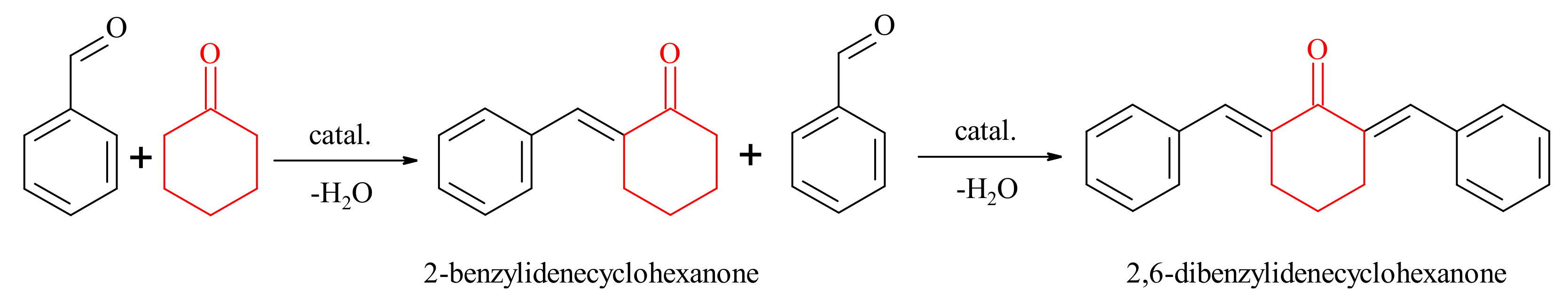
2.2.1. Claisen-Schmidt Condensation
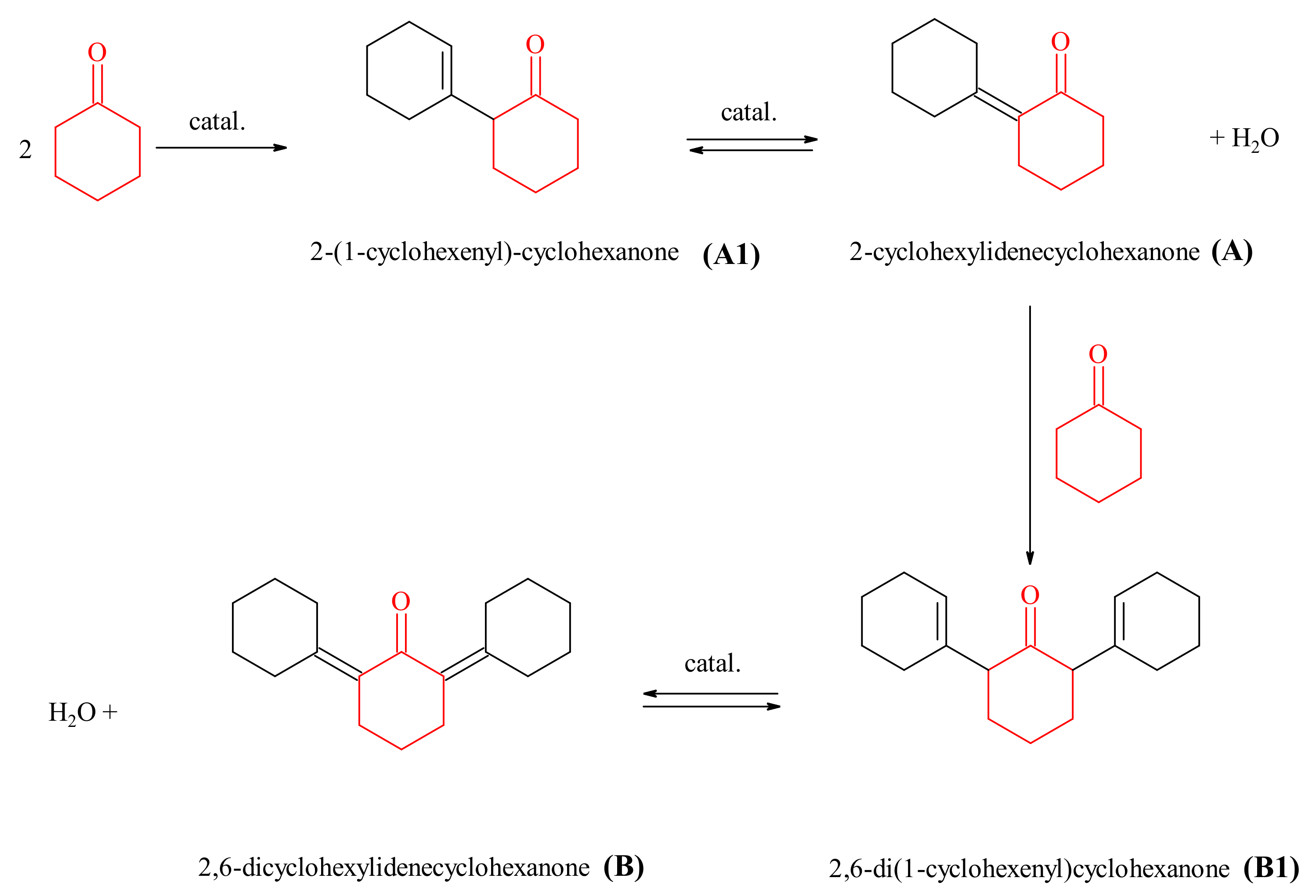
2.2.2. Cyclohexanone Self-Condensation
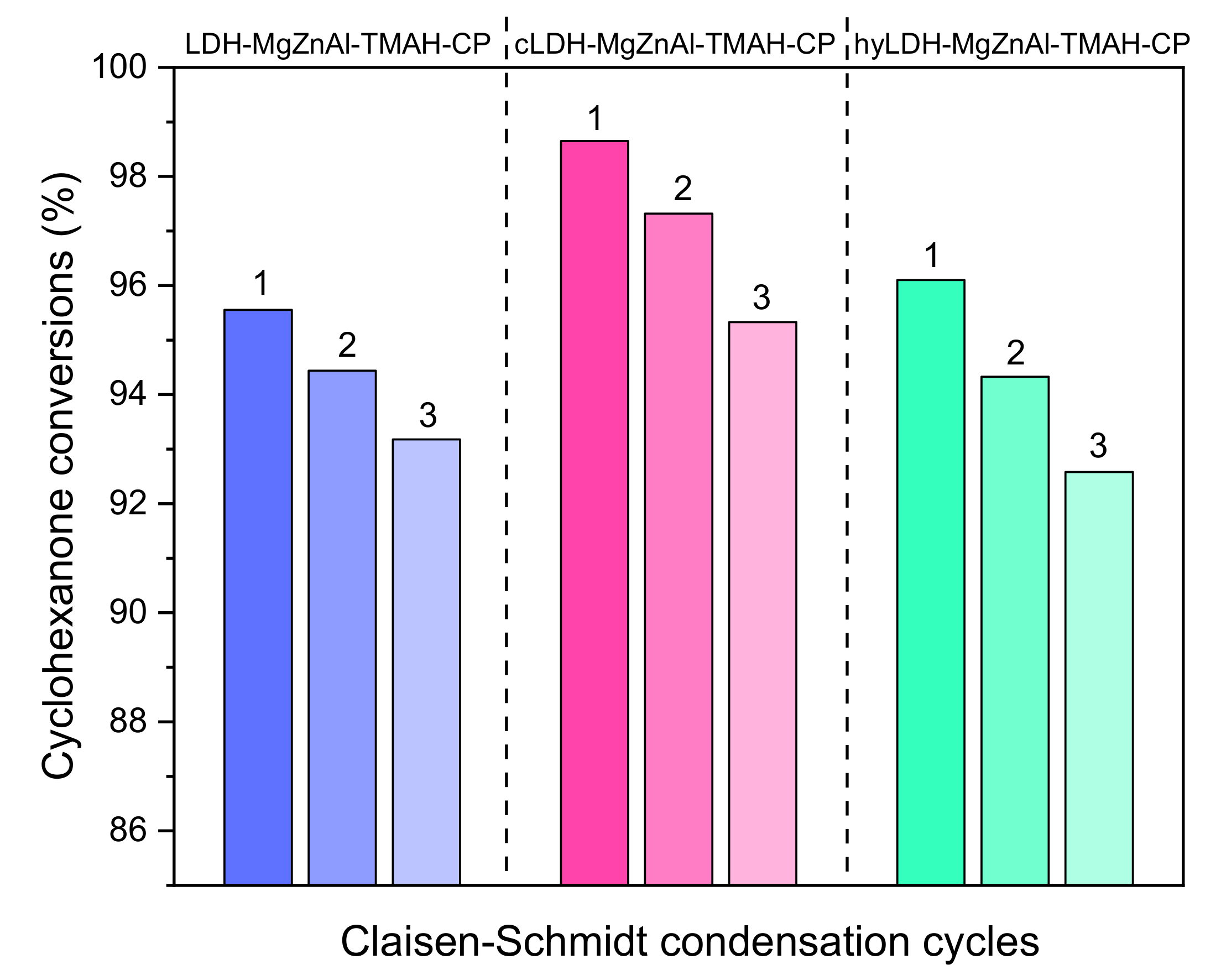
2.3. Catalyst Reusability
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Tests
3.3.1. Claisen-Schmidt Condensation
3.3.2. The Aldol Cyclohexanone Self-Condensation
3.4. Catalyst Recycling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Basahel, S.N.; Al-Thabaiti, S.A.; Narasimharao, K.; Ahmed, N.S.; Mokhtar, M. Nanostructured Mg–Al Hydrotalcite as Catalyst for Fine Chemical Synthesis. J. Nanosci. Nanotechnol. 2014, 14, 1931–1946. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, T.; Islam, Q.A.; Wu, Y. Layered double hydroxide photocatalysts for solar fuel production. Chin. J. Catal. 2021, 42, 1944–1975. [Google Scholar] [CrossRef]
- Yadav, G.D.; Wagh, D. Claisen-Schmidt Condensation using Green Catalytic Processes: A Critical Review. ChemistrySelect 2020, 5, 9059–9085. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, C.; Rad, S.; He, H.; Qin, L. A Comprehensive Review of Layered Double Hydroxide-Based Carbon Composites as an Environmental Multifunctional Material for Wastewater Treatment. Processes 2022, 10, 617. [Google Scholar] [CrossRef]
- Johnston, A.-L.; Lester, E.; Williams, O.; Gomes, R.L. Understanding Layered Double Hydroxide properties as sorbent materials for removing organic pollutants from environmental waters. J. Environ. Chem. Eng. 2021, 9, 105197. [Google Scholar] [CrossRef]
- Dias, A.C.; Fontes, M.P.F. Arsenic (V) removal from water using hydrotalcites as adsorbents: A critical review. Appl. Clay Sci. 2020, 191, 105615. [Google Scholar] [CrossRef]
- Yang, H.; Xiong, C.; Liu, X.; Liu, A.; Li, T.; Ding, R.; Shah, S.P.; Li, W. Application of layered double hydroxides (LDHs) in corrosion resistance of reinforced concrete-state of the art. Constr. Build. Mater. 2021, 307, 124991. [Google Scholar] [CrossRef]
- Mohapi, M.; Sefadi, J.S.; Mochane, M.J.; Magagula, S.I.; Lebelo, K. Effect of LDHs and Other Clays on Polymer Composite in Adsorptive Removal of Contaminants: A Review. Crystals 2020, 10, 957. [Google Scholar] [CrossRef]
- Shirin, V.A.; Sankar, R.; Johnson, A.P.; Gangadharappa, H.; Pramod, K. Advanced drug delivery applications of layered double hydroxide. J. Control Release 2020, 330, 398–426. [Google Scholar] [CrossRef]
- Lauermannová, A.-M.; Paterová, I.; Patera, J.; Skrbek, K.; Jankovský, O.; Bartůněk, V. Hydrotalcites in Construction Materials. Appl. Sci. 2020, 10, 7989. [Google Scholar] [CrossRef]
- Ho, P.H.; Ambrosetti, M.; Groppi, G.; Tronconi, E.; Palkovits, R.; Fornasari, G.; Vaccari, A.; Benito, P. Structured Catalysts-Based on Open-Cell Metallic Foams for Energy and Environmental Applications. Stud. Surf. Sci. Catal. 2019, 178, 303–327. [Google Scholar] [CrossRef]
- Sikander, U.; Sufian, S.; Salam, M. A review of hydrotalcite based catalysts for hydrogen production systems. Int. J. Hydrog. Energy 2017, 42, 19851–19868. [Google Scholar] [CrossRef]
- Bravo-Suárez, J.J.; Páez-Mozo, E.A.; Oyama, S.T. Review of the synthesis of layered double hydroxides: A thermodynamic approach. Quim. Nova 2004, 27, 601–614. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Martins, P.R.; Angnes, L.; Araki, K. Recent advances in ternary layered double hydroxide electrocatalysts for the oxygen evolution reaction. New J. Chem. 2020, 44, 9981–9997. [Google Scholar] [CrossRef]
- Kühl, S.; Schumann, J.; Kasatkin, I.; Hävecker, M.; Schlögl, R.; Behrens, M. Ternary and quaternary Cr or Ga-containing ex-LDH catalysts—Influence of the additional oxides onto the microstructure and activity of Cu/ZnAl2O4 catalysts. Catal. Today 2015, 246, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Ulibarri, M.A.; Pavlovic, I.; Barriga, C.; Hermosın, M.C.; Cornejo, J. Adsorption of anionic species on hydrotalcite-like compounds: Effect of interlayer anion and crystallinity. J. Appl. Clay Sci. 2001, 18, 17–27. [Google Scholar] [CrossRef]
- Takehira, K.; Shishido, T.; Shouro, D.; Murakami, K.; Honda, M.; Kawabata, T.; Takaki, K. Novel and effective surface enrichment of active species in Ni-loaded catalyst prepared from Mg–Al hydrotalcite-type anionic clay. Appl. Catal. A: Gen. 2005, 279, 41–51. [Google Scholar] [CrossRef]
- Marchi, A.; Apesteguía, C. Impregnation-induced memory effect of thermally activated layered double hydroxides. Appl. Clay Sci. 1998, 13, 35–48. [Google Scholar] [CrossRef]
- Teodorescu, F.; Pălăduţă, A.-M.; Pavel, O. Memory effect of hydrotalcites and its impact on cyanoethylation reaction. Mater. Res. Bull. 2013, 48, 2055–2059. [Google Scholar] [CrossRef]
- Barbosa, C.A.; Ferreira, A.M.D.; Constantino, V.; Coelho, A.C.V. Preparation and Characterization of Cu(II) Phthalocyanine Tetrasulfonate Intercalated and Supported on Layered Double Hydroxides. J. Incl. Phenom. Macrocycl. Chem. 2002, 42, 15–23. [Google Scholar] [CrossRef]
- He, J.; Wei, M.; Li, B.; Kang, Y.; Evans, D.G.; Duan, X. Preparation of Layered Double Hydroxides. Struct. Bond. 2005, 119, 89–119. [Google Scholar] [CrossRef]
- Pavel, O.D.; Stamate, A.-E.; Zăvoianu, R.; Bucur, I.C.; Bîrjega, R.; Angelescu, E.; Pârvulescu, V.I. Mechano-chemical versus co-precipitation for the preparation of Y-modified LDHs for cyclohexene oxidation and Claisen-Schmidt condensations. Appl. Catal. A: Gen. 2020, 605, 117797. [Google Scholar] [CrossRef]
- Chen, L.; Sun, B.; Wang, X.; Qiao, F.; Ai, S. 2D ultrathin nanosheets of Co–Al layered double hydroxides prepared in l-asparagine solution: Enhanced peroxidase-like activity and colorimetric detection of glucose. J. Mater. Chem. B 2013, 1, 2268–2274. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Liu, Z.; Xie, C.; Feng, S.; Liu, D.; Shao, M.; Wang, S. Layered Double Hydroxide Nanosheets with Multiple Vacancies Obtained by Dry Exfoliation as Highly Efficient Oxygen Evolution Electrocatalysts. Angew. Chem. Int. Ed. 2017, 56, 5867–5871. [Google Scholar] [CrossRef]
- Musella, E.; Gualandi, I.; Scavetta, E.; Rivalta, A.; Venuti, E.; Christian, M.; Morandi, V.; Mullaliu, A.; Giorgetti, M.; Tonelli, D. Newly developed electrochemical synthesis of Co-based layered double hydroxides: Toward noble metal-free electro-catalysis. J. Mater. Chem. A 2019, 7, 11241–11249. [Google Scholar] [CrossRef]
- Rong, F.; Zhao, J.; Yang, Q.; Li, C. Nanostructured hybrid NiFeOOH/CNT electrocatalysts for oxygen evolution reaction with low overpotential. RSC Adv. 2016, 6, 74536–74544. [Google Scholar] [CrossRef]
- Ciotta, E.; Pizzoferrato, R.; Di Vona, M.L.; Ferrari, I.V.; Richetta, M.; Varone, A. Increasing the Electrical Conductivity of Layered Double Hydroxides by Intercalation of Ionic Liquids. Mater. Sci. Forum 2018, 941, 2209–2213. [Google Scholar] [CrossRef]
- Pavel, O.D.; Stamate, A.-E.; Bacalum, E.; Cojocaru, B.; Zăvoianu, R.; Pârvulescu, V.I. Catalytic behavior of Li-Al-LDH prepared via mechanochemical and co-precipitation routes for cyanoethylation reaction. Catal. Today 2021, 366, 227–234. [Google Scholar] [CrossRef]
- Iyi, N.; Matsumoto, T.; Kaneko, Y.; Kitamura, K. A Novel Synthetic Route to Layered Double Hydroxides Using Hexamethylenetetramine. Chem. Lett. 2004, 33, 1122–1123. [Google Scholar] [CrossRef]
- Schmidt, J.G. Ueber die Einwirkung von Aldehyd auf Furfurol. Ber. Dtsch. Chem. Ges. 1880, 13, 2342–2345. [Google Scholar] [CrossRef] [Green Version]
- Claisen, L. Condensationen der Aldehyde mit Acetessig- und Malonsäureäther. Ber. Dtsch. Chem. Ges. 1881, 14, 345–349. [Google Scholar] [CrossRef] [Green Version]
- Sallum, L.O.; Duarte, V.S.; Custodio, J.M.F.; Faria, E.C.M.; da Silva, A.M.; Lima, R.S.; Camargo, A.J.; Napolitano, H.B. Cyclohexanone-Based Chalcones as Alternatives for Fuel Additives. ACS Omega 2022, 7, 11871–11886. [Google Scholar] [CrossRef] [PubMed]
- Eagon, S.; Hammill, J.T.; Fitzsimmons, K.; Sienko, N.; Nguyen, B.; Law, J.; Manjunath, A.; Wilkinson, S.P.; Thompson, K.; Glidden, J.E.; et al. Antimalarial activity of 2,6-dibenzylidenecyclohexanone derivatives. Bioorganic Med. Chem. Lett. 2021, 47, 128216. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, J.; Gu, X. Modified calcium oxide as stable solid base catalyst for Aldol condensation reaction. J. Chem. Sci. 2013, 125, 313–320. [Google Scholar] [CrossRef] [Green Version]
- Sluban, M.; Cojocaru, B.; Parvulescu, V.I.; Iskra, J.; Korošec, R.C.; Umek, P. Protonated titanate nanotubes as solid acid catalyst for aldol condensation. J. Catal. 2017, 346, 161–169. [Google Scholar] [CrossRef]
- Jain, D.; Khatri, C.; Rani, A. Synthesis and characterization of novel solid base catalyst from fly ash. Fuel 2011, 90, 2083–2088. [Google Scholar] [CrossRef]
- Motiur Rahman, A.F.M.; Ali, R.; Jahng, Y.; Kadi, A.A. A Facile Solvent Free Claisen-Schmidt Reaction: Synthesis of α,α′-bis-(Substituted-benzylidene)cycloalkanones and α,α′-bis-(Substituted-alkylidene)cycloalkanones. Molecules 2012, 17, 571–583. [Google Scholar] [CrossRef] [Green Version]
- Mortezaei, Z.; Zendehdel, M.; Bodaghifard, M.A. Synthesis and characterization of functionalized NaP Zeolite@CoFe2O4 hybrid materials: A micro–meso-structure catalyst for aldol condensation. Res. Chem. Intermed. 2020, 46, 2169–2193. [Google Scholar] [CrossRef]
- Vashishtha, M.; Mishra, M.; Undre, S.; Singh, M.; Shah, D.O. Molecular mechanism of micellar catalysis of cross aldol reaction: Effect of surfactant chain length and surfactant concentration. J. Mol. Catal. A: Chem. 2015, 396, 143–154. [Google Scholar] [CrossRef]
- Hu, Z.G.; Liu, J.; Zeng, P.L.; Dong, Z.B. Synthesis of α, α′-bis(Substituted Benzylidene)Ketones Catalysed by a SOCl2/EtOH reagent. J. Chem. Res. 2004, 2004, 55–56. [Google Scholar] [CrossRef]
- Miyata, S. The Syntheses of Hydrotalcite-Like Compounds and Their Structures and Physico-Chemical Properties I: The Systems Mg2+-Al3+-NO3−, Mg2+-Al3+-Cl−, Mg2+-Al3+-ClO4−, Ni2+-Al3+-Cl− and Zn2+-Al3+-Cl−. Clays Clay Miner. 1975, 23, 369–375. [Google Scholar] [CrossRef]
- Musker, W.K. A Reinvestigation of the Pyrolysis of Tetramethylammonium Hydroxide. J. Am. Chem. Soc. 1964, 86, 960–961. [Google Scholar] [CrossRef]
- Pavel, O.; Zăvoianu, R.; Bîrjega, R.; Angelescu, E.; Pârvulescu, V. Mechanochemical versus co-precipitated synthesized lanthanum-doped layered materials for olefin oxidation. Appl. Catal. A: Gen. 2017, 542, 10–20. [Google Scholar] [CrossRef]
- Frost, R.L.; Cash, G.A.; Kloprogge, J. ‘Rocky Mountain leather’, sepiolite and attapulgite—An infrared emission spectroscopic study. Vib. Spectrosc. 1998, 16, 173–184. [Google Scholar] [CrossRef]
- Singh, D.K.; Pandey, D.K.; Yadav, R.R.; Singh, D. A study of nanosized zinc oxide and its nanofluid. Pramana 2012, 78, 759–766. [Google Scholar] [CrossRef]
- Anandan, K.; Siva, D.; Rajesh, K. Structural and optical properties of (ZnO/MgO) nanocomposites. Int. J. Eng. Res. Technol. 2018, 7, 493–499. [Google Scholar] [CrossRef]
- Yousefi, S.; Ghasemi, B.; Tajally, M.; Asghari, A. Optical properties of MgO and Mg(OH) 2 nanostructures synthesized by a chemical precipitation method using impure brine. J. Alloys Compd. 2017, 711, 521–529. [Google Scholar] [CrossRef]
- Winter, F.; Xia, X.; Hereijgers, B.P.C.; Bitter, J.H.; van Dillen, A.J.; Muhler, A.M.; de Jong, K.P. On the Nature and Accessibility of the Brønsted-Base Sites in Activated Hydrotalcite Catalysts. J. Phys. Chem. B 2006, 110, 9211–9218. [Google Scholar] [CrossRef] [Green Version]
- Pavel, O.; Bîrjega, R.; Che, M.; Costentin, G.; Angelescu, E.; Şerban, S. The activity of Mg/Al reconstructed hydrotalcites by “memory effect” in the cyanoethylation reaction. Catal. Commun. 2008, 9, 1974–1978. [Google Scholar] [CrossRef]
- Debecker, D.P.; Gaigneaux, E.M.; Busca, G. Exploring, Tuning, and Exploiting the Basicity of Hydrotalcites for Applications in Heterogeneous Catalysis. Chem. Eur. J. 2009, 15, 3920–3935. [Google Scholar] [CrossRef]
- Parida, K.; Das, J. Mg/Al hydrotalcites: Preparation, characterisation and ketonisation of acetic acid. J. Mol. Catal. A Chem. 2000, 151, 185–192. [Google Scholar] [CrossRef]
- Pavel, O.; Zăvoianu, R.; Bîrjega, R.; Angelescu, E. The effect of ageing step elimination on the memory effect presented by Mg0.75Al0.25 hydrotalcites (HT) and their catalytic activity for cyanoethylation reaction. Catal. Commun. 2011, 12, 845–850. [Google Scholar] [CrossRef]
- Angelescu, E.; Bîrjega, R.; Pavel, O.; Che, M.; Costentin, G.; Popoiu, S. Hydrotalcites (HTs) and mesoporous mixed oxides obtained from HTs, basic solid catalysts for cyclohexanone condensation. Stud. Surf. Sci. Catal. 2005, 156, 257–264. [Google Scholar] [CrossRef]
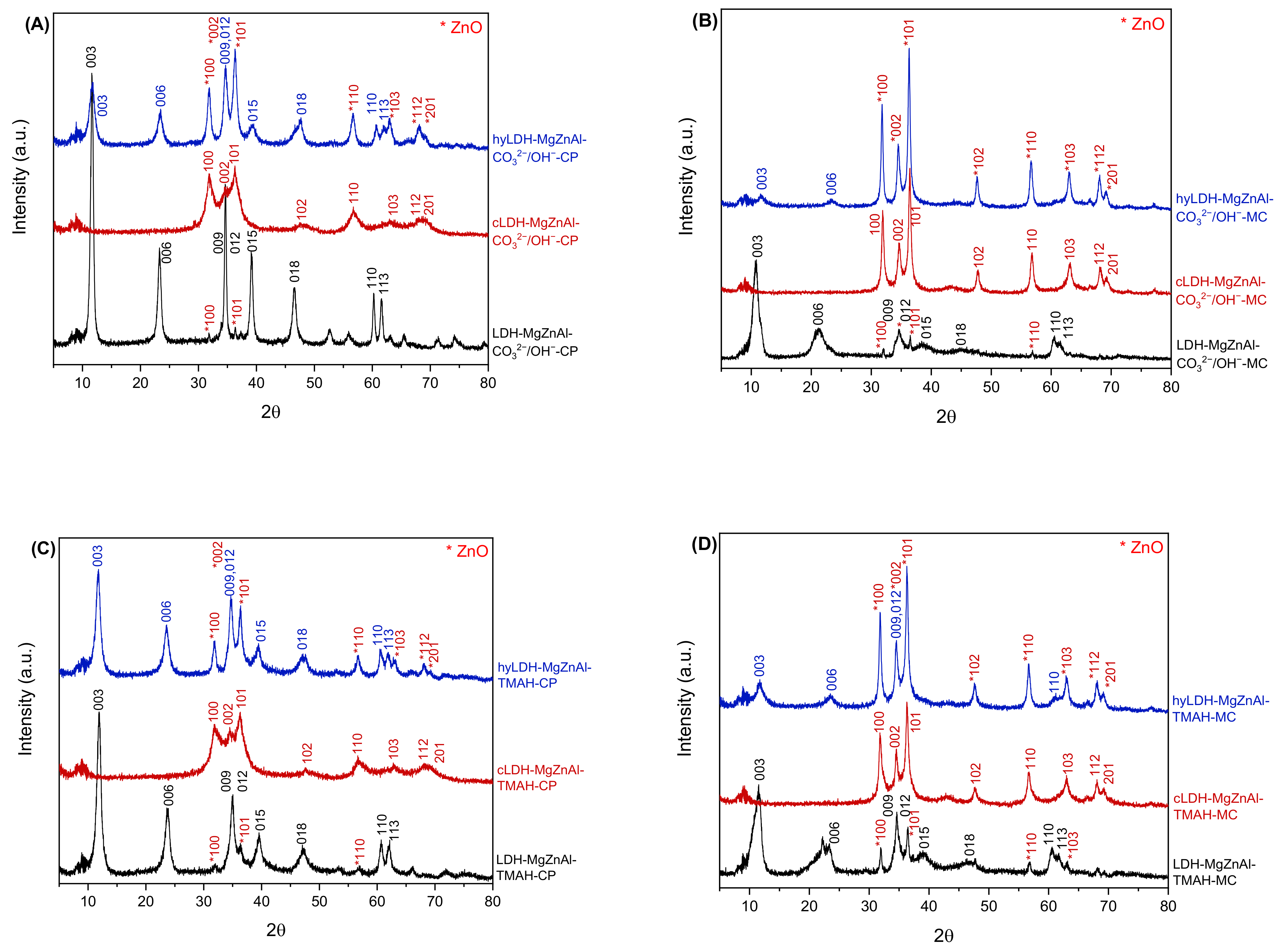
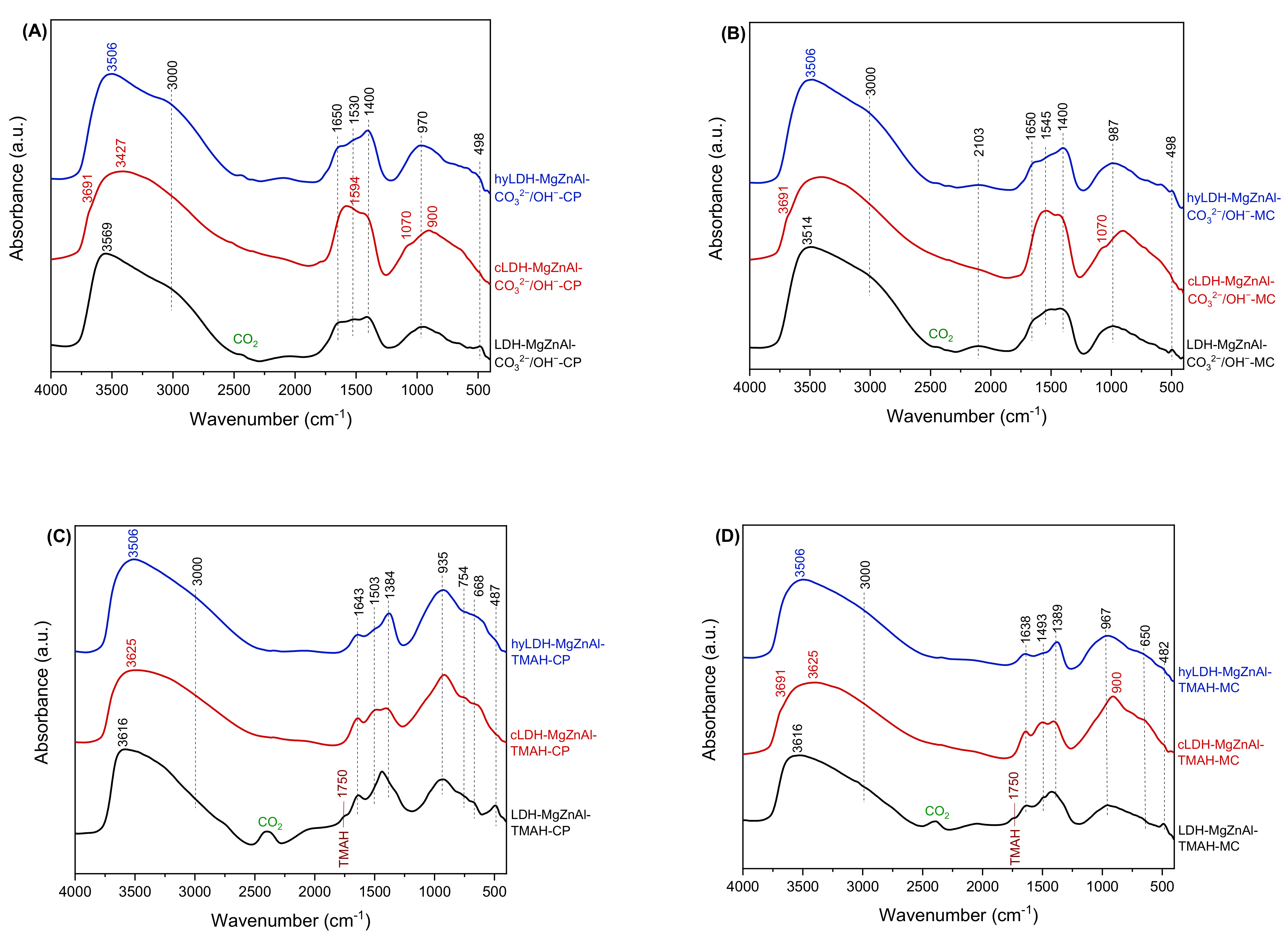
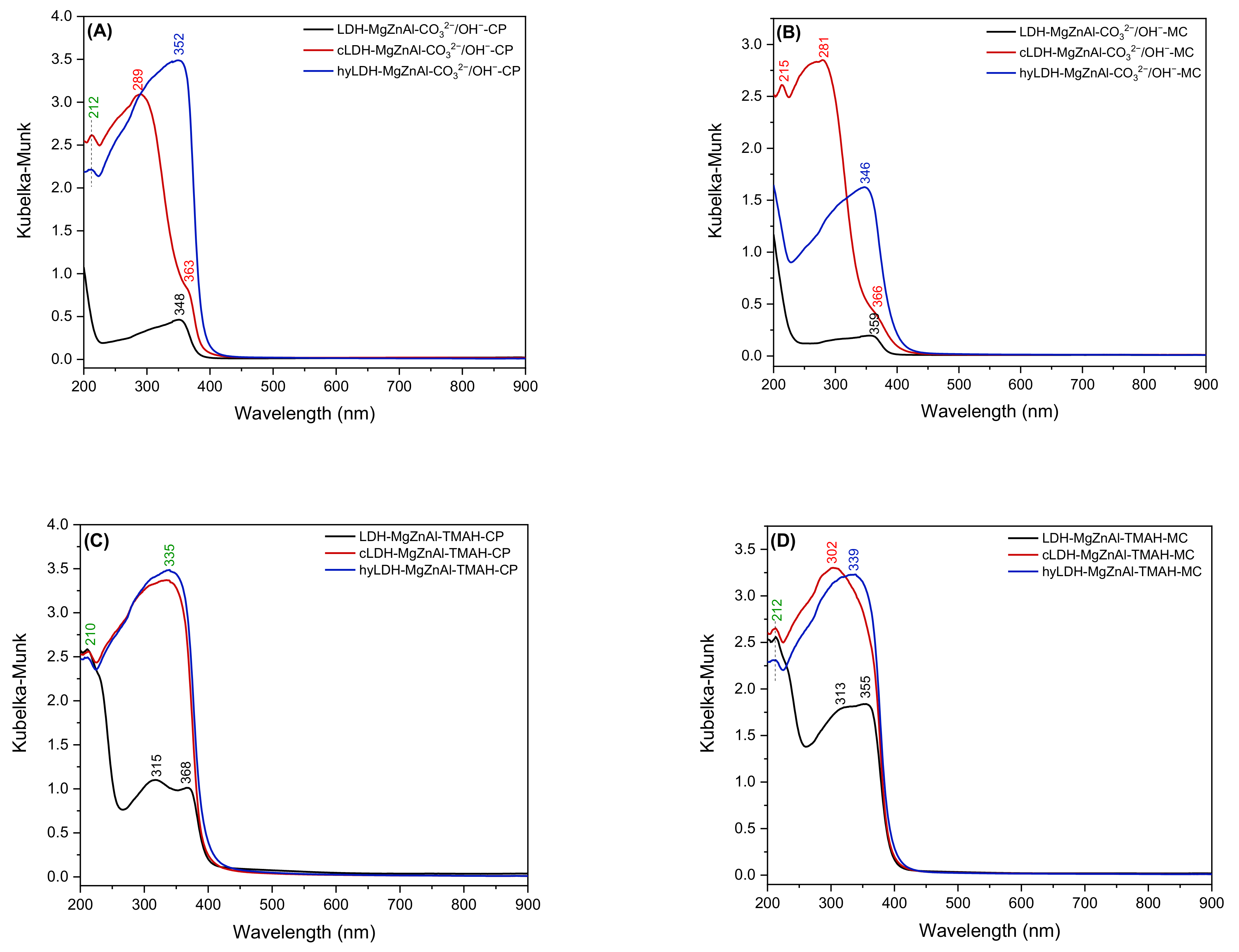
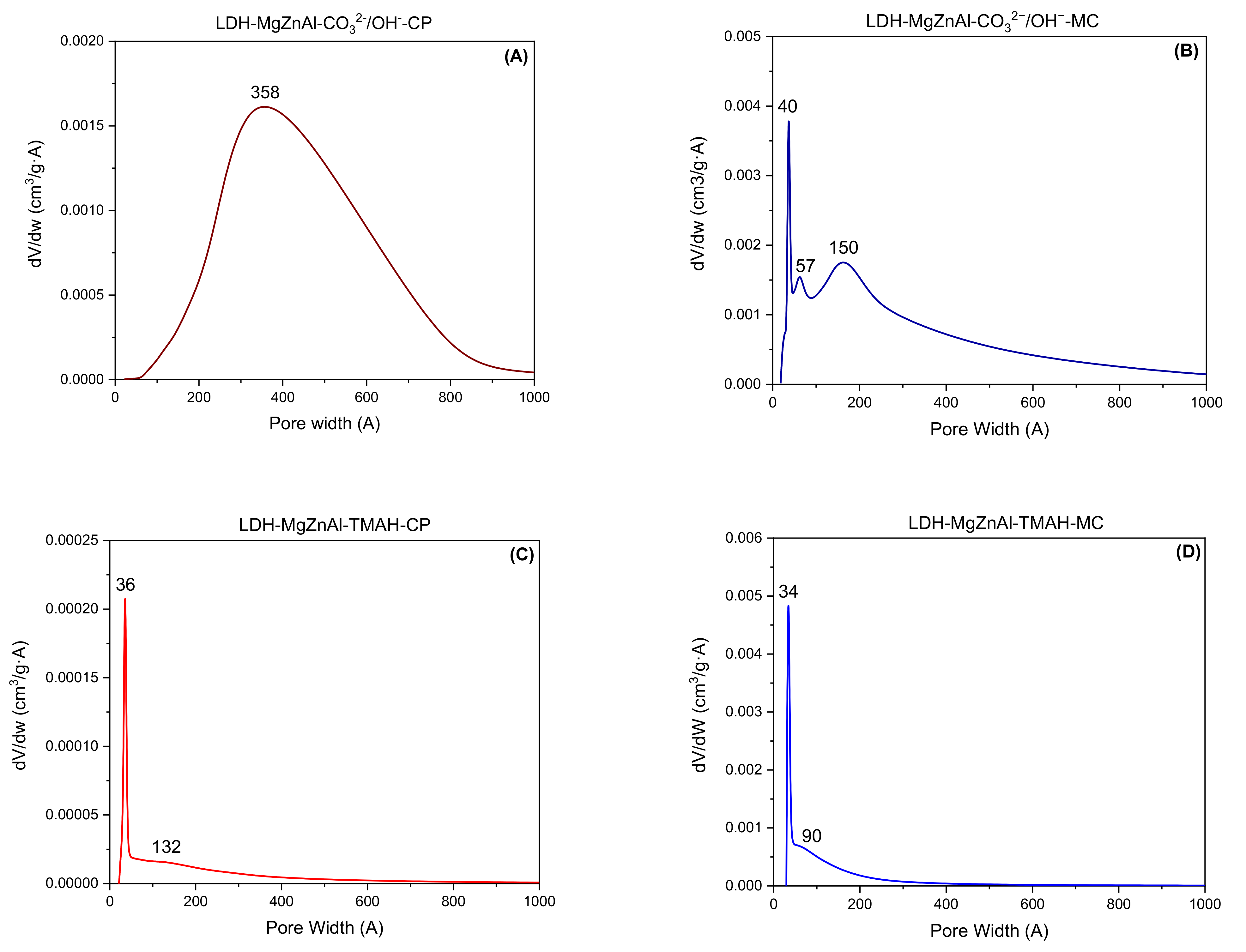

| Hydrotalcite Samples | Lattice Parameters | IFS * (Å) | 2θ003 (°) | I003/I006 | I003/I110 | FWHM003 | D ** (Å) | |
| a (Å) | c (Å) | |||||||
| LDH-MgZnAl-CO32−/OH−-CP | 3.0717 | 22.8689 | 2.82 | 11.6259 | 2.96 | 5.54 | 0.6064 | 131.8 |
| hyLDH-MgZnAl-CO32−/OH−-CP | 3.0497 | 22.8017 | 2.80 | 11.6421 | 1.97 | 3.42 | 1.1043 | 72.4 |
| LDH-MgZnAl-CO32−/OH−-MC | 3.0504 | 22.4314 | 2.68 | 11.8652 | 2.54 | 5.33 | 0.8885 | 90.0 |
| hyLDH-MgZnAl-CO32−/OH−-MC | 3.0497 | 22.6427 | 2.75 | 11.7234 | 2.20 | 4.48 | 0.9709 | 82.3 |
| LDH-MgZnAl-TMAH-CP | 3.0617 | 24.7662 | 3.46 | 10.8279 | 3.57 | 5.08 | 1.1644 | 68.6 |
| hyLDH-MgZnAl-TMAH-CP | 2.9757 | 22.7965 | 2.80 | 11.6610 | 1.59 | 1.13 | 0.9020 | 88.6 |
| LDH-MgZnAl-TMAH-MC | 3.0580 | 23.6969 | 3.10 | 11.3150 | 2.83 | 3.45 | 1.6050 | 49.8 |
| hyLDH-MgZnAl-TMAH-MC | 3.0535 | 22.7515 | 2.78 | 11.6700 | 2.18 | 3.45 | 1.1800 | 67.7 |
| Mixed oxides samples | a (Å) | 2θ101 (°) | I003 | FWHM101 | D *** (Å) | |||
| cLDH-MgZnAl-CO32−/OH−-CP | 4.9536 | 36.2400 | 279 | 1.8266 | 45.8 | |||
| cLDH-MgZnAl-CO32−/OH−-MC | 4.9483 | 36.3628 | 257 | 1.5500 | 53.9 | |||
| cLDH-MgZnAl-TMAH-CP | 4.9332 | 36.3949 | 736 | 0.4925 | 169.9 | |||
| cLDH-MgZnAl-TMAH-MC | 4.9449 | 36.3055 | 175 | 0.6023 | 138.9 | |||
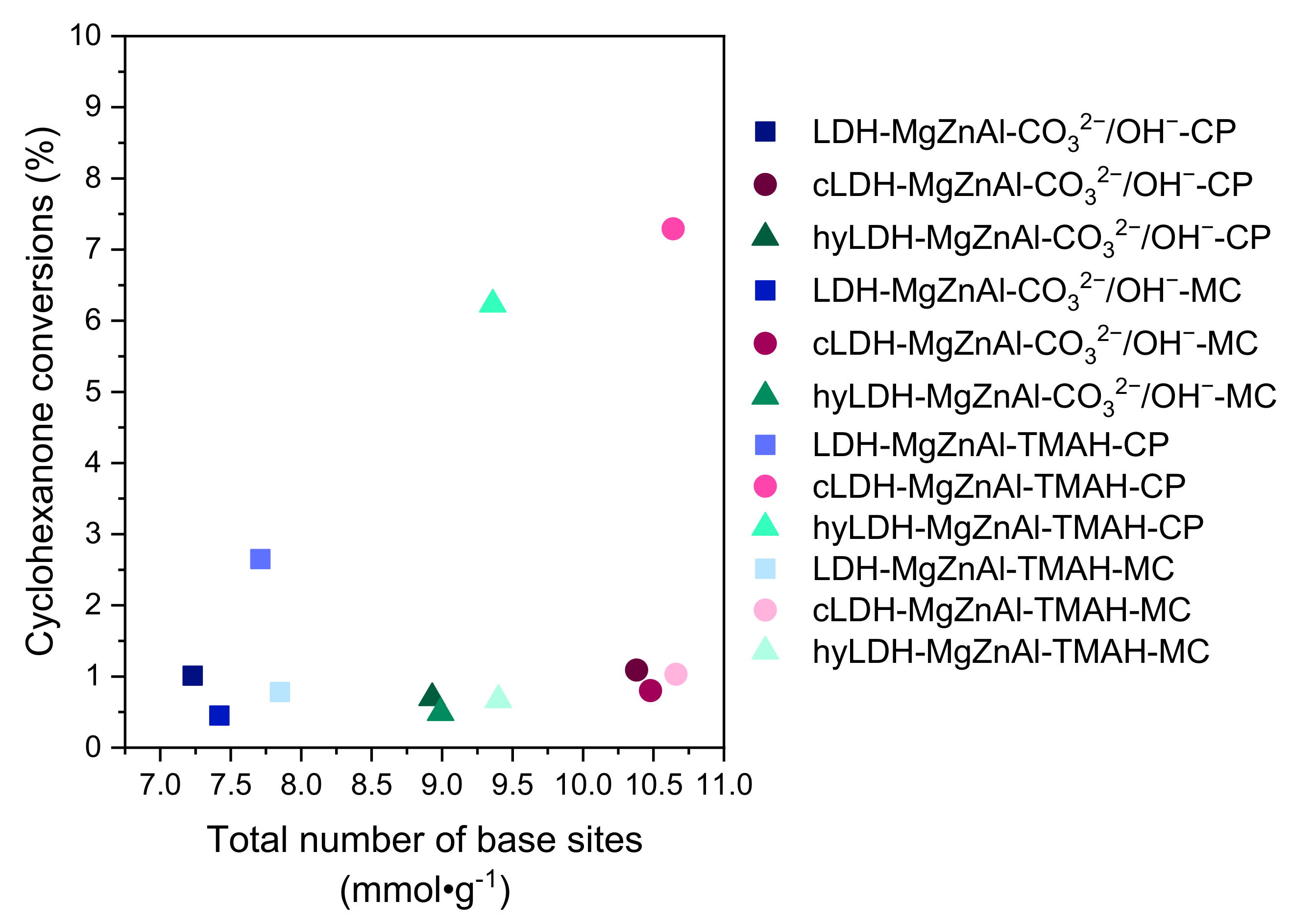
| Hydrotalcite Samples | Surface Area (m2∙g−1) | Pore Volume (cm3∙g−1) | Average Pore Width (Å) | Total Number of Base Sites (mmol∙g−1) * | Distribution of Base Sites | |
|---|---|---|---|---|---|---|
| Strong Base Sites (mmol∙g−1) ** | Weak and Medium Base Sites (mmol∙g−1) *** | |||||
| LDH-MgZnAl-CO32−/OH−-CP | 69 | 0.387 | 222 | 7.23 | 0.48 | 6.75 |
| cLDH-MgZnAl-CO32−/OH−-CP | 258 | 0.843 | 124 | 10.38 | 0.53 | 9.85 |
| hyLDH-MgZnAl-CO32−/OH−-CP | 25 | 0.186 | 238 | 8.93 | 0.57 | 8.36 |
| LDH-MgZnAl-CO32−/OH−-MC | 150 | 0.498 | 132 | 7.42 | 0.51 | 6.91 |
| cLDH-MgZnAl-CO32−/OH−-MC | 266 | 0.845 | 121 | 10.48 | 0.57 | 9.91 |
| hyLDH-MgZnAl-CO32−/OH−-MC | 28 | 0.203 | 208 | 8.99 | 0.61 | 8.38 |
| LDH-MgZnAl-TMAH-CP | 2 | 0.005 | 115 | 7.71 | 0.50 | 7.21 |
| cLDH-MgZnAl-TMAH-CP | 235 | 0.765 | 112 | 10.64 | 0.55 | 10.09 |
| hyLDH-MgZnAl-TMAH-CP | 1 | 0.004 | 118 | 9.36 | 0.59 | 8.77 |
| LDH-MgZnAl-TMAH-MC | 44 | 0.118 | 106 | 7.85 | 0.54 | 7.31 |
| cLDH-MgZnAl-TMAH-MC | 241 | 0.798 | 124 | 10.66 | 0.56 | 10.10 |
| hyLDH-MgZnAl-TMAH-MC | 9 | 0.104 | 118 | 9.40 | 0.64 | 8.76 |
| Catalysts | Conv. C6H10O (%) | Sel. A (%) | Sel. A1 (%) | Sel. B (%) | Sel. B1 (%) |
|---|---|---|---|---|---|
| LDH-MgZnAl-CO32−/OH−-CP | 1.01 | 69.00 | 27.31 | 1.93 | 1.76 |
| cLDH-MgZnAl-CO32−/OH−-CP | 1.09 | 75.31 | 24.31 | 0.22 | 0.16 |
| hyLDH-MgZnAl-CO32−/OH−-CP | 0.70 | 69.74 | 29.64 | 0.44 | 0.17 |
| LDH-MgZnAl-CO32−/OH−-MC | 0.45 | 66.39 | 33.61 | 0.00 | 0.00 |
| cLDH-MgZnAl-CO32−/OH−-MC | 0.80 | 73.74 | 26.26 | 0.00 | 0.00 |
| hyLDH-MgZnAl-CO32−/OH−-MC | 0.49 | 69.87 | 30.13 | 0.00 | 0.00 |
| LDH-MgZnAl-TMAH-CP | 2.65 | 73.35 | 11.36 | 8.32 | 6.97 |
| cLDH-MgZnAl-TMAH-CP | 7.29 | 82.48 | 15.08 | 1.70 | 0.74 |
| hyLDH-MgZnAl-TMAH-CP | 6.23 | 75.73 | 12.20 | 1.53 | 0.55 |
| LDH-MgZnAl-TMAH-MC | 0.78 | 76.82 | 19.75 | 1.98 | 1.45 |
| cLDH-MgZnAl-TMAH-MC | 1.03 | 80.30 | 19.70 | 0.00 | 0.00 |
| hyLDH-MgZnAl-TMAH-MC | 0.67 | 74.21 | 25.79 | 0.00 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zăvoianu, R.; Mihăilă, S.-D.; Cojocaru, B.; Tudorache, M.; Pârvulescu, V.I.; Pavel, O.D.; Oikonomopoulos, S.; Jacobsen, E.E. An Advanced Approach for MgZnAl-LDH Catalysts Synthesis Used in Claisen-Schmidt Condensation. Catalysts 2022, 12, 759. https://doi.org/10.3390/catal12070759
Zăvoianu R, Mihăilă S-D, Cojocaru B, Tudorache M, Pârvulescu VI, Pavel OD, Oikonomopoulos S, Jacobsen EE. An Advanced Approach for MgZnAl-LDH Catalysts Synthesis Used in Claisen-Schmidt Condensation. Catalysts. 2022; 12(7):759. https://doi.org/10.3390/catal12070759
Chicago/Turabian StyleZăvoianu, Rodica, Silvana-Denisa Mihăilă, Bogdan Cojocaru, Mădălina Tudorache, Vasile I. Pârvulescu, Octavian Dumitru Pavel, Solon Oikonomopoulos, and Elisabeth Egholm Jacobsen. 2022. "An Advanced Approach for MgZnAl-LDH Catalysts Synthesis Used in Claisen-Schmidt Condensation" Catalysts 12, no. 7: 759. https://doi.org/10.3390/catal12070759
APA StyleZăvoianu, R., Mihăilă, S.-D., Cojocaru, B., Tudorache, M., Pârvulescu, V. I., Pavel, O. D., Oikonomopoulos, S., & Jacobsen, E. E. (2022). An Advanced Approach for MgZnAl-LDH Catalysts Synthesis Used in Claisen-Schmidt Condensation. Catalysts, 12(7), 759. https://doi.org/10.3390/catal12070759