Hydrogen Peroxide Activation with Sulfidated Zero-Valent Iron for Synchronous Removal of Cr(VI) and BPA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation nZVI and S-nZVI
2.2. Experimental Procedures
2.3. Analysis
3. Results and Discussion
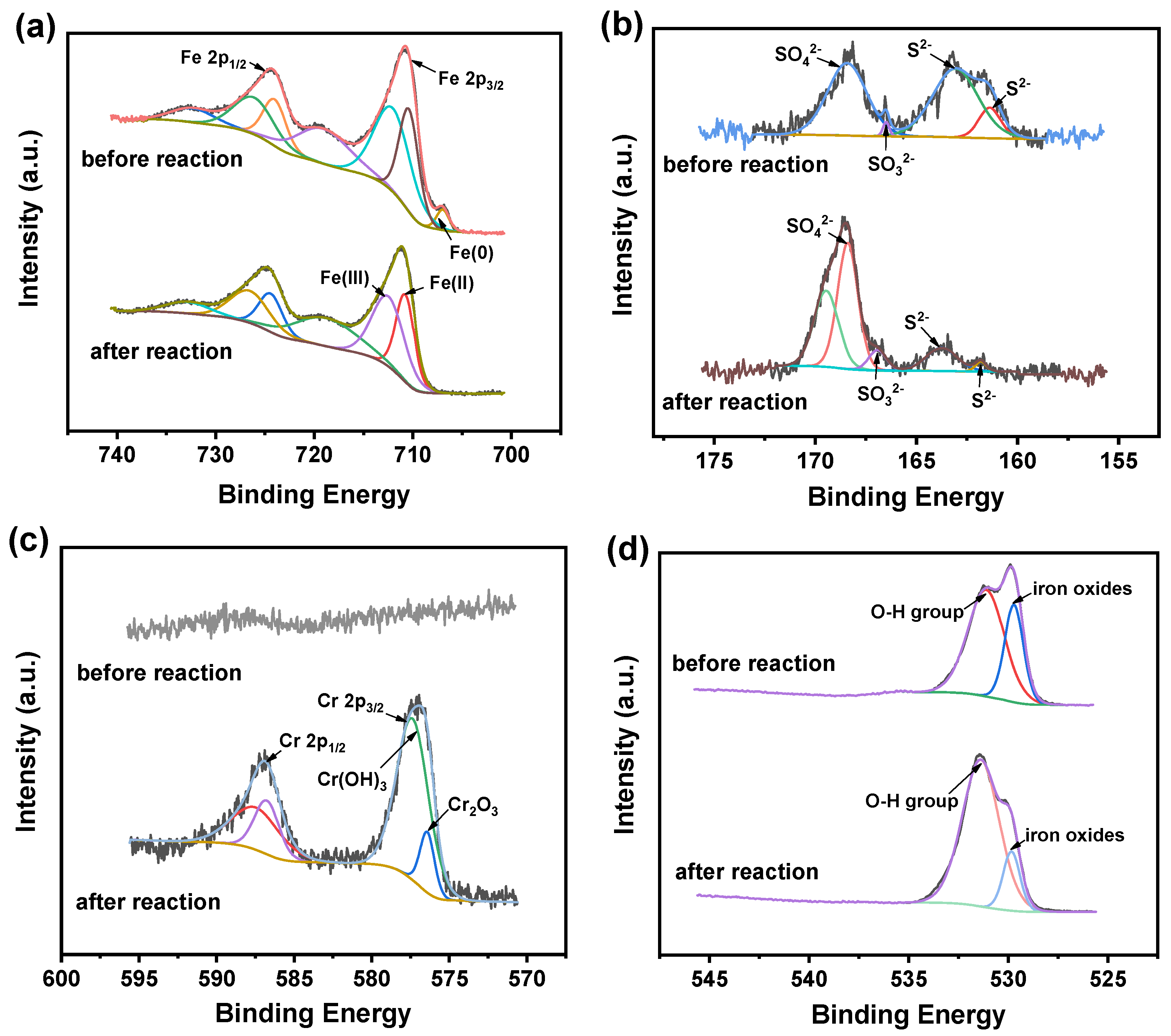
3.1. Characterization of S-nZVI
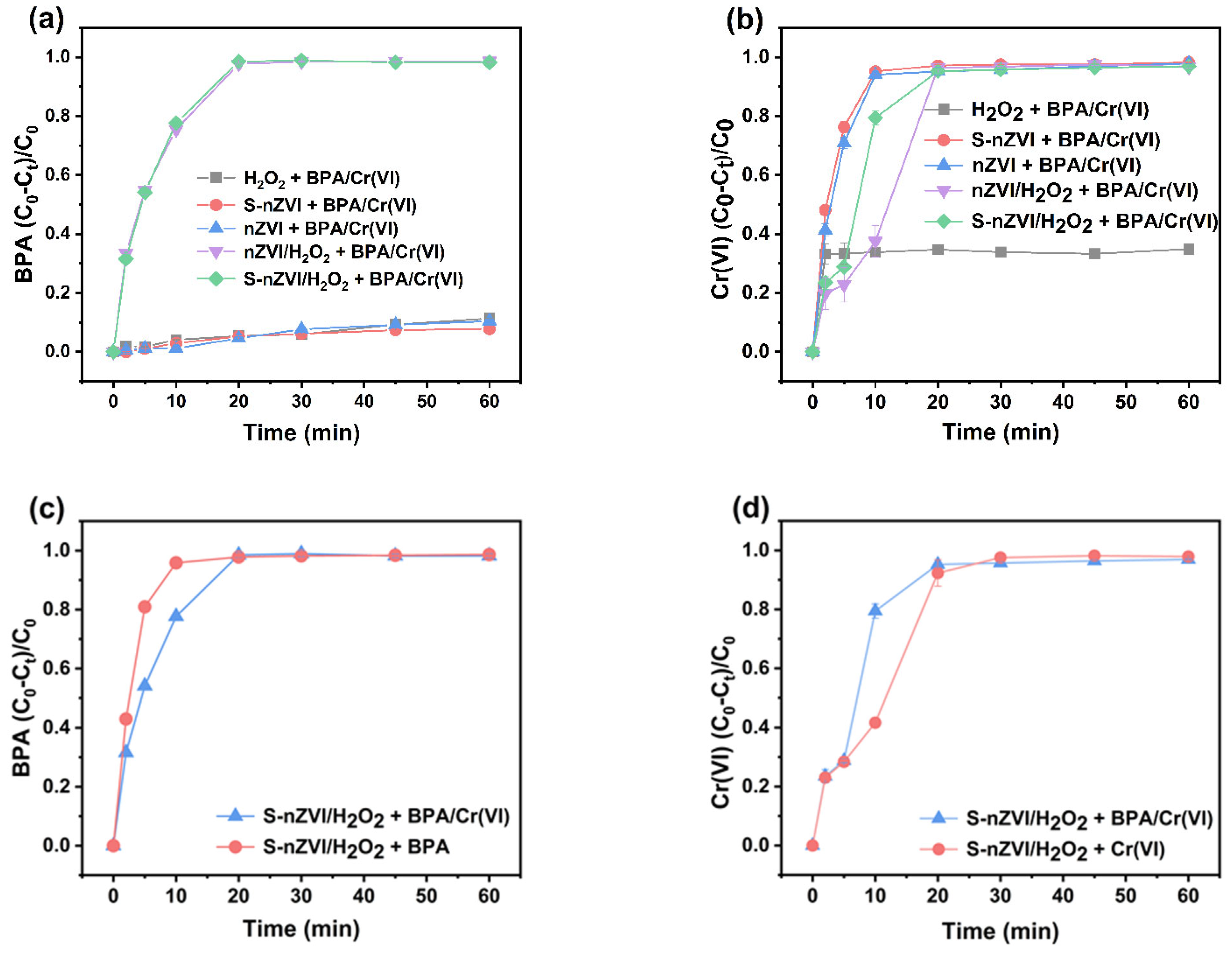
3.2. Cr(VI) and BPA Removal in Different Systems
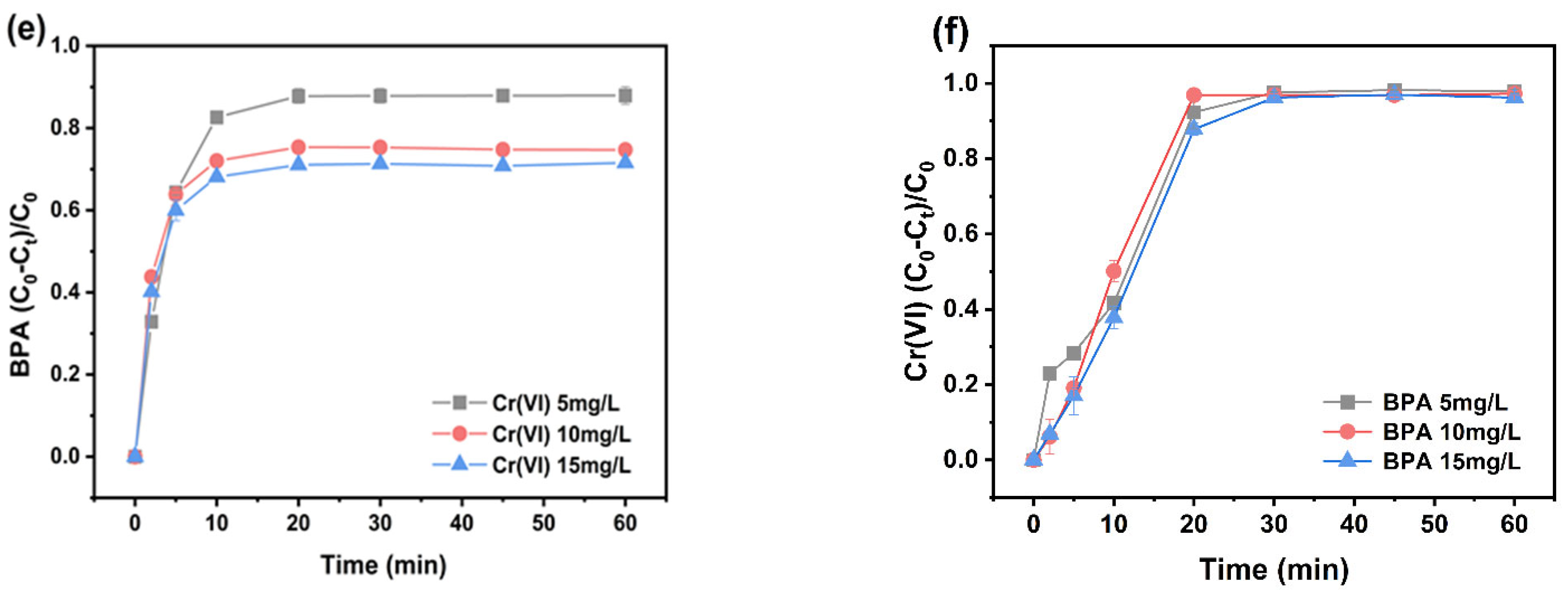
3.3. Effect of pH, Oxidant Dosage, Catalyst Dosage
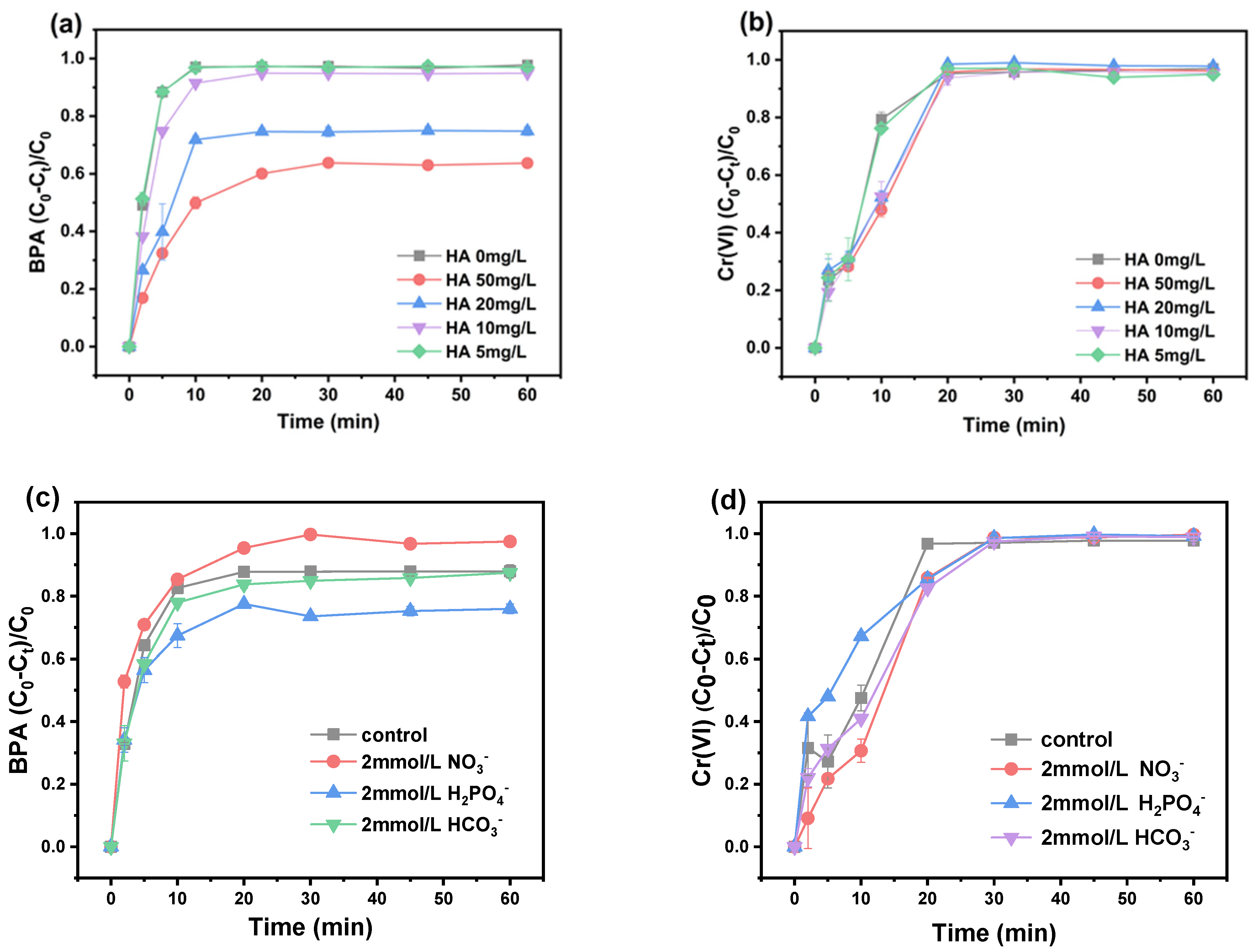
3.4. Effect of Water Matrices
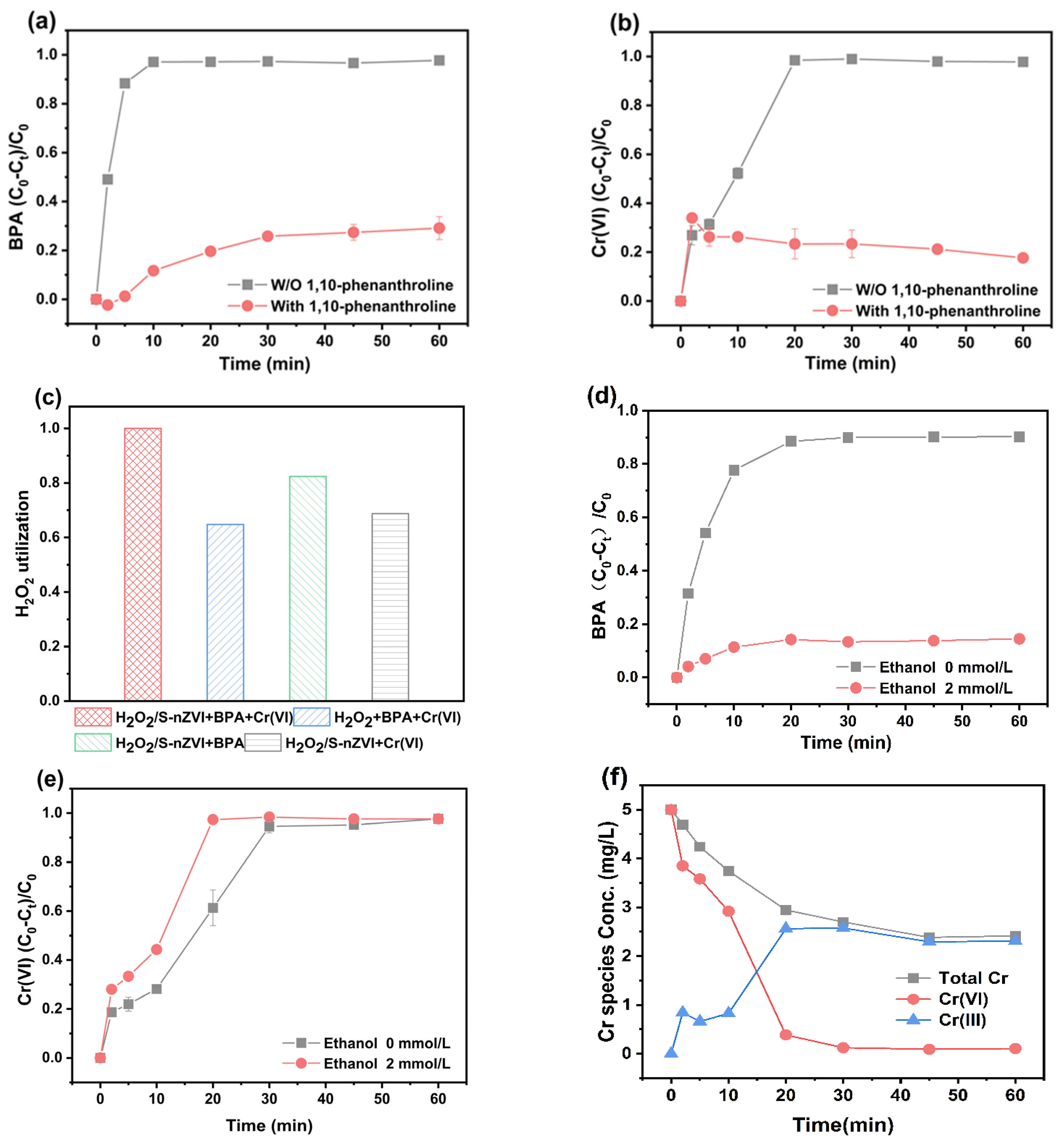
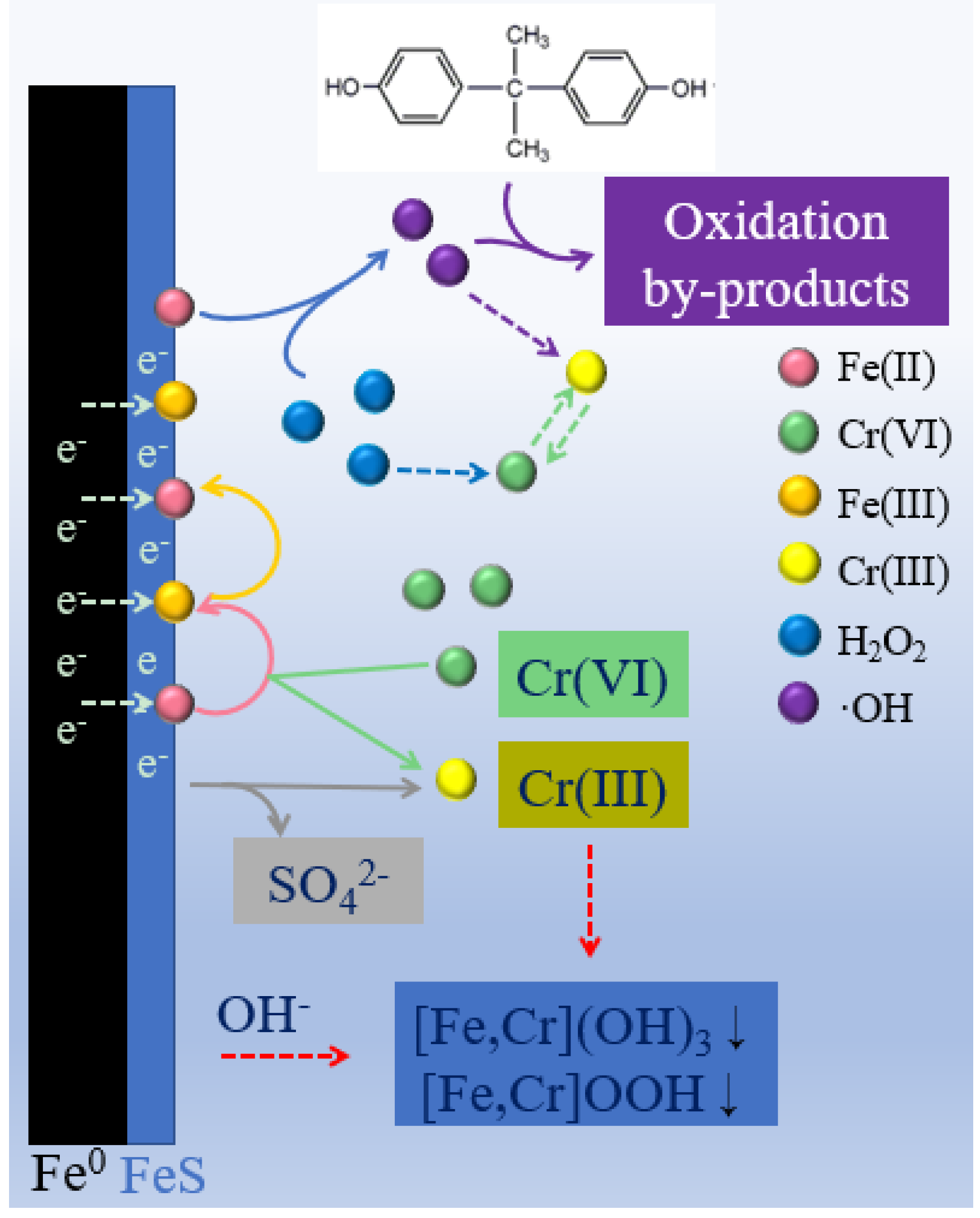
3.5. Proposed Reaction Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barrera-Díaz, C.E.; Lugo-Lugo, V.; Bilyeu, B. A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J. Hazard. Mater. 2012, 223–224, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Sun, Y.; Zhao, Y.; Wen, T.; Wang, X.; Chen, Z.; Sheng, G.; Chen, C.; Wang, X. Enhanced Photocatalytic Simultaneous Removals of Cr(VI) and Bisphenol A over Co(II)-Modified TiO2. Langmuir 2019, 35, 276–283. [Google Scholar] [CrossRef]
- Dong, F.-X.; Yan, L.; Zhou, X.-H.; Huang, S.-T.; Liang, J.-Y.; Zhang, W.-X.; Guo, Z.-W.; Guo, P.-R.; Qian, W.; Kong, L.-J.; et al. Simultaneous adsorption of Cr(VI) and phenol by biochar-based iron oxide composites in water: Performance, kinetics and mechanism. J. Hazard. Mater. 2021, 416, 125930. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Hu, E.; Yang, S.; Gong, L.; He, F. Chromium(VI) removal by mechanochemically sulfidated zero valent iron and its effect on dechlorination of trichloroethene as a co-contaminant. Sci. Total Environ. 2019, 650, 419–426. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Fang, Y.; Yin, W.; Jiang, Y.; Ge, H.; Li, P.; Wu, J. Depth treatment of coal-chemical engineering wastewater by a cost-effective sequential heterogeneous Fenton and biodegradation process. Environ. Sci. Pollut. Res. 2018, 25, 13118–13126. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Tang, W.Z.; Chen, R.Z. Decolorization kinetics and mechanisms of commercial dyes by H2O2/iron powder system. Chemosphere 1996, 32, 947–958. [Google Scholar] [CrossRef]
- Martins, R.C.; Lopes, D.V.; Quina, M.J.; Quinta-Ferreira, R.M. Treatment improvement of urban landfill leachates by Fenton-like process using ZVI. Chem. Eng. J. 2012, 192, 219–225. [Google Scholar] [CrossRef]
- Minella, M.; Sappa, E.; Hanna, K.; Barsotti, F.; Maurino, V.; Minero, C.; Vione, D. Considerable Fenton and photo-Fenton reactivity of passivated zero-valent iron. RSC Adv. 2016, 6, 86752–86761. [Google Scholar] [CrossRef] [Green Version]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef]
- Diao, Z.-H.; Xu, X.-R.; Jiang, D.; Liu, J.-J.; Kong, L.-J.; Li, G.; Zuo, L.-Z.; Wu, Q.-H. Simultaneous photocatalytic Cr(VI) reduction and ciprofloxacin oxidation over TiO2/Fe0 composite under aerobic conditions: Performance, durability, pathway and mechanism. Chem. Eng. J. 2017, 315, 167–176. [Google Scholar] [CrossRef]
- Yin, X.; Liu, W.; Ni, J. Removal of coexisting Cr(VI) and 4-chlorophenol through reduction and Fenton reaction in a single system. Chem. Eng. J. 2014, 248, 89–97. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, P.; Cheng, X.; Li, H.; Huo, X.; Zhang, Y. Simultaneous removal of methylene blue and total dissolved copper in zero-valent iron/H2O2 Fenton system: Kinetics, mechanism and degradation pathway. J. Colloid Interface Sci. 2019, 555, 383–393. [Google Scholar] [CrossRef]
- Diao, Z.-H.; Xu, X.-R.; Chen, H.; Jiang, D.; Yang, Y.-X.; Kong, L.-J.; Sun, Y.-X.; Hu, Y.-X.; Hao, Q.-W.; Liu, L. Simultaneous removal of Cr(VI) and phenol by persulfate activated with bentonite-supported nanoscale zero-valent iron: Reactivity and mechanism. J. Hazard. Mater. 2016, 316, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Diao, Z.-H.; Xu, X.-R.; Jiang, D.; Kong, L.-J.; Sun, Y.-X.; Hu, Y.-X.; Hao, Q.-W.; Chen, H. Bentonite-supported nanoscale zero-valent iron/persulfate system for the simultaneous removal of Cr(VI) and phenol from aqueous solutions. Chem. Eng. J. 2016, 302, 213–222. [Google Scholar] [CrossRef]
- Du, J.; Bao, J.; Lu, C.; Werner, D. Reductive sequestration of chromate by hierarchical FeS@Fe0 particles. Water Res. 2016, 102, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Fontmorin, J.M.; Castillo, R.C.B.; Tang, W.Z.; Sillanpaa, M. Stability of 5,5-dimethyl-1-pyrroline-N-oxide as a spin-trap for quantification of hydroxyl radicals in processes based on Fenton reaction. Water Res. 2016, 99, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Bao, J.; Fu, X.; Lu, C.; Kim, S.H. Mesoporous sulfur-modified iron oxide as an effective Fenton-like catalyst for degradation of bisphenol A. Appl. Catal. B Environ. 2016, 184, 132–141. [Google Scholar] [CrossRef]
- Liu, C.-M.; Diao, Z.-H.; Huo, W.-Y.; Kong, L.-J.; Du, J.-J. Simultaneous removal of Cu2+ and bisphenol A by a novel biochar-supported zero valent iron from aqueous solution: Synthesis, reactivity and mechanism. Environ. Pollut. 2018, 239, 698–705. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Heibati, B.; Asadi, A.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Reduction of noxious Cr(VI) ion to Cr(III) ion in aqueous solutions using H2O2 and UV/H2O2 systems. J. Ind. Eng. Chem. 2016, 33, 197–200. [Google Scholar] [CrossRef]
- Gheju, M.; Iovi, A. Kinetics of hexavalent chromium reduction by scrap iron. J. Hazard. Mater. 2006, 135, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.S.; Rafatullah, M.; Al-Karkhi, A.F.M.; Tow, T.T. Removal of Terasil Red R dye by using Fenton oxidation: A statistical analysis. Desalin. Water Treat. 2014, 52, 4583–4591. [Google Scholar] [CrossRef]
- Guo, S.; Yang, W.; You, L.; Li, J.; Chen, J.; Zhou, K. Simultaneous reduction of Cr(VI) and degradation of tetracycline hydrochloride by a novel iron-modified rectorite composite through heterogeneous photo-Fenton processes. Chem. Eng. J. 2020, 393, 124758. [Google Scholar] [CrossRef]
- Abdul, N.A.; Abdul-Talib, S.; Amir, A. Nano-pyrite as a Reductant to Remove Chromium in Groundwater. KSCE J. Civ. Eng. 2019, 23, 992–999. [Google Scholar] [CrossRef]
- Rehman, F.; Sayed, M.; Khan, J.A.; Shah, N.S.; Khan, H.M.; Dionysiou, D.D. Oxidative removal of brilliant green by UV/S2O82−, UV/HSO5− and UV/H2O2 processes in aqueous media: A comparative study. J. Hazard. Mater. 2018, 357, 506–514. [Google Scholar] [CrossRef]
- Mak, M.S.H.; Rao, P.; Lo, I.M.C. Effects of hardness and alkalinity on the removal of arsenic(V) from humic acid-deficient and humic acid-rich groundwater by zero-valent iron. Water Res. 2009, 43, 4296–4304. [Google Scholar] [CrossRef]
- Lv, X.; Hu, Y.; Tang, J.; Sheng, T.; Jiang, G.; Xu, X. Effects of co-existing ions and natural organic matter on removal of chromium (VI) from aqueous solution by nanoscale zero valent iron (nZVI)-Fe3O4 nanocomposites. Chem. Eng. J. 2013, 218, 55–64. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, H.; Yin, J. Arsenic and chromate removal from water by iron chips—Effects of anions. Front. Environ. Sci. Eng. China 2008, 2, 203–208. [Google Scholar] [CrossRef]
- Ma, J.; Yang, Y.; Jiang, X.; Xie, Z.; Li, X.; Chen, C.; Chen, H. Impacts of inorganic anions and natural organic matter on thermally activated persulfate oxidation of BTEX in water. Chemosphere 2018, 190, 296–306. [Google Scholar] [CrossRef]
- Døssing, L.N.; Dideriksen, K.; Stipp, S.L.S.; Frei, R. Reduction of hexavalent chromium by ferrous iron: A process of chromium isotope fractionation and its relevance to natural environments. Chem. Geol. 2011, 285, 157–166. [Google Scholar] [CrossRef]
- Funahashi, S.; Uchida, F.; Tanaka, M. Reactions of hydrogen peroxide with metal complexes. 3. Thermodynamic and kinetic studies on the formation, dissociation and decomposition of peroxochromium(VI) complexes in acid media. Inorg. Chem. 1978, 17, 2784–2789. [Google Scholar] [CrossRef]
- Watwe, V.S.; Kulkarni, S.D.; Kulkarni, P.S. Cr(VI)-Mediated Homogeneous Fenton Oxidation for Decolorization of Methylene Blue Dye: Sludge Free and Pertinent to a Wide pH Range. ACS Omega 2021, 6, 27288–27296. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Gai, L.; Tang, J.; Fu, J.; Wang, Q.; Zeng, E.Y. Reduction of Cr(VI) in simulated groundwater by FeS-coated iron magnetic nanoparticles. Sci. Total Environ. 2017, 595, 743–751. [Google Scholar] [CrossRef]
- Song, X.; Ren, C.; Zhao, Q.; Su, B. Simultaneous removal of Cr(VI) and triclosan from aqueous solutions through Fe3O4 magnetic nanoscale-activated persulfate oxidation. Chem. Eng. J. 2020, 381, 122586. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Li, X.-Q.; Cao, J.; Zhang, W.-X.; Wang, H.P. Characterization of zero-valent iron nanoparticles. Adv. Colloid Interface Sci. 2006, 120, 47–56. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Han, Y.-S.; Park, S.W.; Hayes, K.F. Aerobic oxidation of mackinawite (FeS) and its environmental implication for arsenic mobilization. Geochim. Cosmochim. Acta 2010, 74, 3182–3198. [Google Scholar] [CrossRef]
- Rajajayavel, S.R.C.; Ghoshal, S. Enhanced reductive dechlorination of trichloroethylene by sulfidated nanoscale zerovalent iron. Water Res. 2015, 78, 144–153. [Google Scholar] [CrossRef]
- Pratt, A.R.; Muir, I.J.; Nesbitt, H.W. X-ray photoelectron and Auger electron spectroscopic studies of pyrrhotite and mechanism of air oxidation. Geochim. Cosmochim. Acta 1994, 58, 827–841. [Google Scholar] [CrossRef]
- Zhu, X.; Le, T.T.; Du, J.; Xu, T.; Cui, Y.; Ling, H.; Kim, S.H. Novel core-shell sulfidated nano-Fe(0) particles for chromate sequestration: Promoted electron transfer and Fe(II) production. Chemosphere 2021, 284, 131379. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, H.; Zhu, X.; Zhou, T.; Su, F.; Du, J.; Bao, J. Hydrogen Peroxide Activation with Sulfidated Zero-Valent Iron for Synchronous Removal of Cr(VI) and BPA. Catalysts 2022, 12, 252. https://doi.org/10.3390/catal12030252
Ling H, Zhu X, Zhou T, Su F, Du J, Bao J. Hydrogen Peroxide Activation with Sulfidated Zero-Valent Iron for Synchronous Removal of Cr(VI) and BPA. Catalysts. 2022; 12(3):252. https://doi.org/10.3390/catal12030252
Chicago/Turabian StyleLing, Haibo, Xiaowei Zhu, Ting Zhou, Fan Su, Jiangkun Du, and Jianguo Bao. 2022. "Hydrogen Peroxide Activation with Sulfidated Zero-Valent Iron for Synchronous Removal of Cr(VI) and BPA" Catalysts 12, no. 3: 252. https://doi.org/10.3390/catal12030252
APA StyleLing, H., Zhu, X., Zhou, T., Su, F., Du, J., & Bao, J. (2022). Hydrogen Peroxide Activation with Sulfidated Zero-Valent Iron for Synchronous Removal of Cr(VI) and BPA. Catalysts, 12(3), 252. https://doi.org/10.3390/catal12030252






