Heterogeneous Metal-Activated Persulfate and Electrochemically Activated Persulfate: A Review
Abstract
:1. Introduction
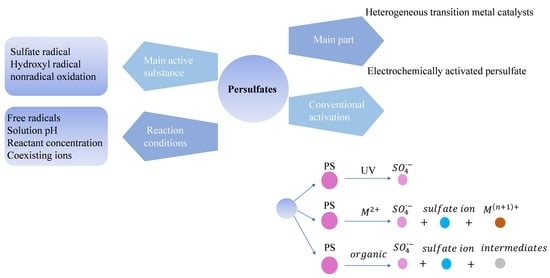
2. The Activation of Persulfates
2.1. The Properties of Persulfates
2.2. Activation Methods
2.3. The Effect of Reaction Conditions
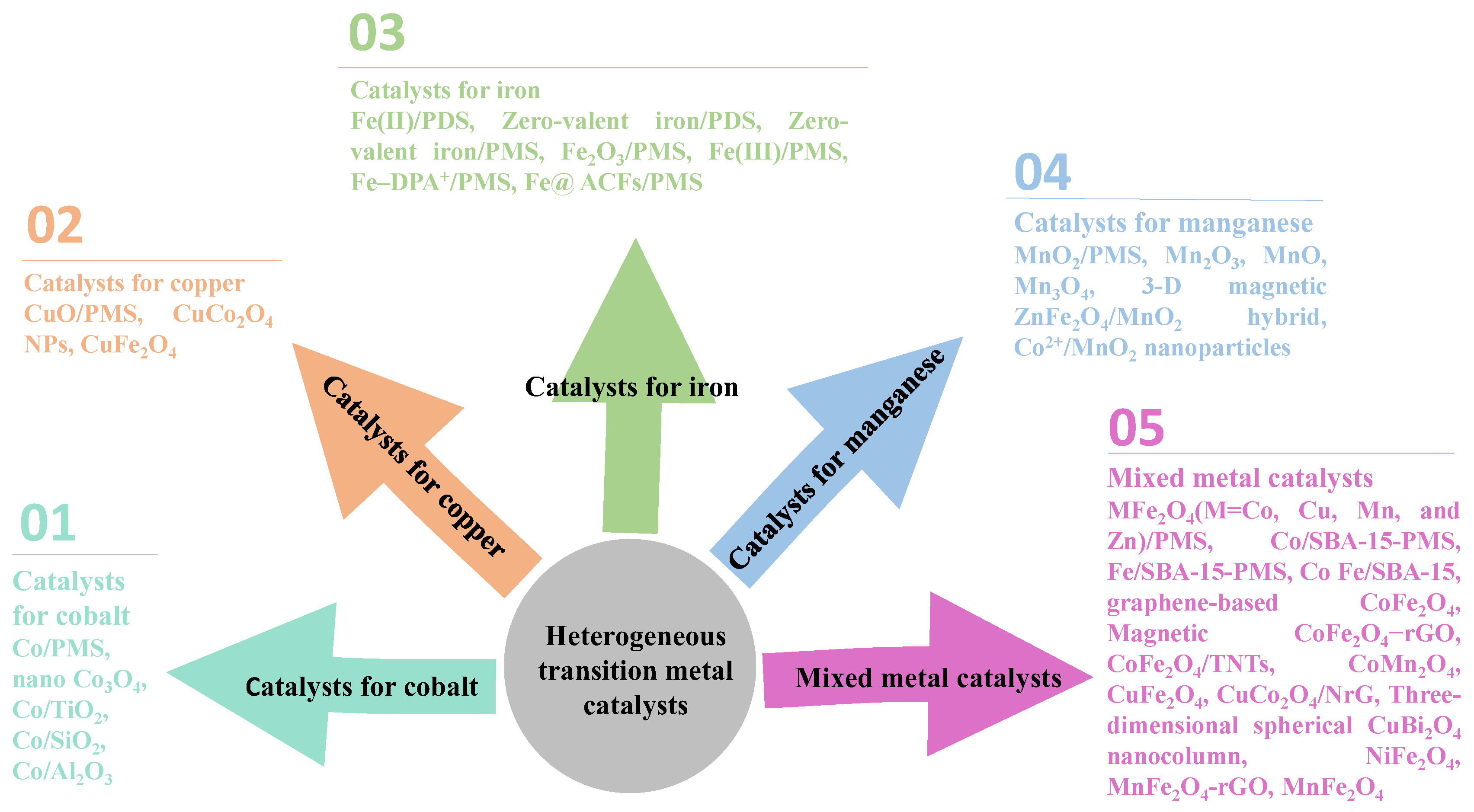
3. Heterogeneous Transition Metal Catalysts
3.1. Catalysts for Cobalt
3.2. Catalysts for Copper
3.3. Catalysts for Iron
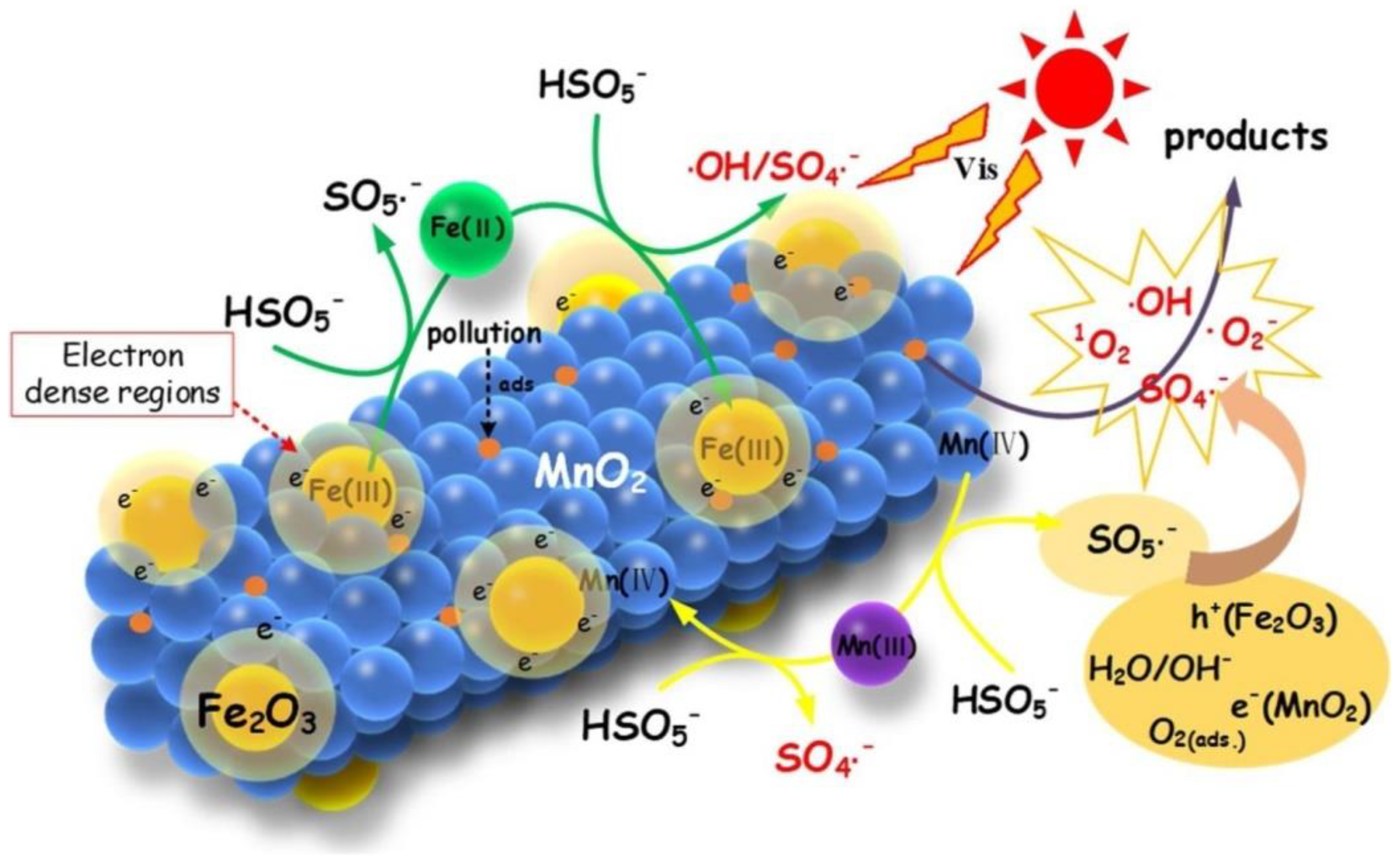
3.4. Catalysts for Manganese
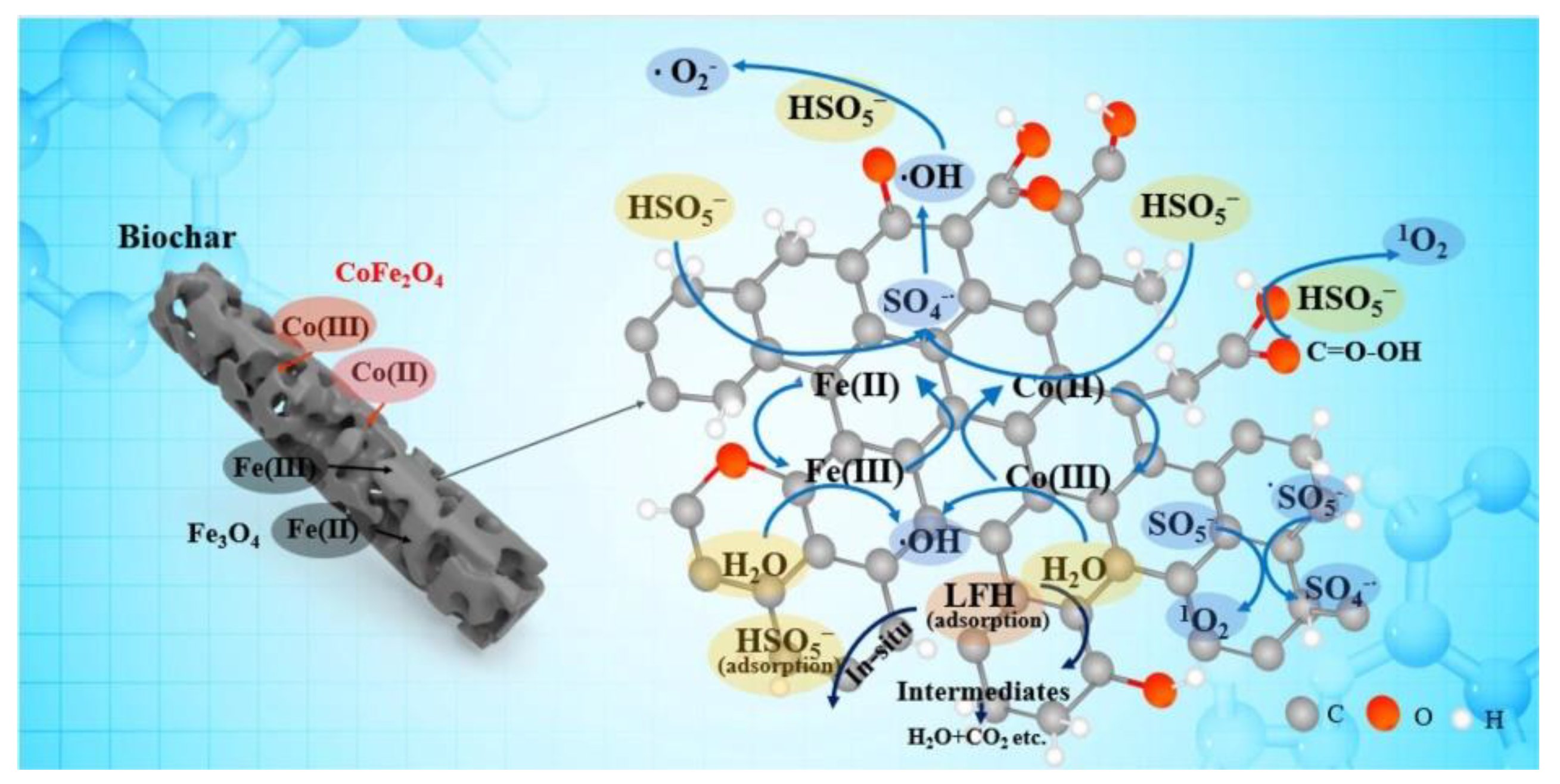
3.5. Mixed Metal Catalysts
4. Electrochemically Activated Persulfate
4.1. Electrochemically Activated Peroxydisulfate
4.2. Electrochemically Activated Peroxymonosulfate
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.J.; Wang, H.; Qi, Z.Y.; Ma, C.; Zhang, Z.H.; Zhao, B.; Wang, L.; Zhang, H.W.; Chong, Y.T.; Chen, X.; et al. Kinetics and mechanisms of electrocatalytic hydrodechlorination of diclofenac on Pd-Ni/PPy-rGO/Ni electrodes. Appl. Catal. B 2020, 268, 118696. [Google Scholar] [CrossRef]
- Arthur, R.B.; Ahern, J.C.; Patterson, H.H. Application of BiOX photocatalysts in remediation of persistent organic pollutants. Catalysts 2018, 8, 604. [Google Scholar] [CrossRef]
- Han, S.; Xiao, P.F. Catalytic degradation of tetracycline using peroxymonosulfate activated by cobalt and iron Co-loaded pomelo peel biochar nanocomposite: Characterization, performance and reaction mechanism. Sep. Purif. Technol. 2022, 287, 120533. [Google Scholar] [CrossRef]
- Yang, X.Y.; Cheng, X.W.; Elzatahry, A.A.; Chen, J.Y.; Alghamdi, A.; Deng, Y.H. Recyclable Fenton-like catalyst based on zeolite Y supported ultrafine, highly-dispersed Fe2O3 nanoparticles for removal of organics under mild conditions. Chin. Chem. Lett. 2019, 30, 324–330. [Google Scholar] [CrossRef]
- Chen, K.; Wang, G.H.; Li, W.B.; Wan, D.; Hu, Q.; Lu, L.L. Application of response surface methodology for optimization of Orange II removal by heterogeneous Fenton-like process using Fe3O4 nanoparticles. Chin. Chem. Lett. 2014, 25, 1455–1460. [Google Scholar] [CrossRef]
- Ji, Q.Q.; Li, J.; Xiong, Z.K.; Lai, B. Enhanced reactivity of microscale Fe/Cu bimetallic particles (mFe/Cu) with persulfate (PS) for p-nitrophenol (PNP) removal in aqueous solution. Chemosphere 2017, 172, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Mishra, I.M.; Dionysiou, D.D.; Kumar, V. Oxidative removal of Bisphenol A by UV-C/peroxymonosulfate (PMS): Kinetics, influence of co-existing chemicals and degradation pathway. Chem. Eng. J. 2015, 276, 193–204. [Google Scholar] [CrossRef]
- Nfodzo, P.; Choi, H. Sulfate radicals destroy pharmaceuticals and personal care products. Environ. Eng. Sci. 2011, 28, 605–609. [Google Scholar] [CrossRef]
- Zhang, R.C.; Sun, P.Z.; Boyer, T.H.; Zhao, L.; Huang, C.H. Degradation of pharmaceuticals and metabolite in synthetic human urine by UV, UV/H2O2, and UV/PDS. Environ. Sci. Technol. 2015, 49, 3056–3066. [Google Scholar] [CrossRef]
- Antoniou, M.G.; de la Cruz, A.A.; Dionysiou, D.D. Intermediates and reaction pathways from the degradation of microcystin-LR with sulfate radicals. Environ. Sci. Technol. 2010, 44, 7238–7244. [Google Scholar] [CrossRef]
- Antoniou, M.G.; de la Cruz, A.A.; Dionysiou, D.D. Degradation of microcystin-LR using sulfate radicals generated through photolysis, thermolysis and e(-) transfer mechanisms. Appl. Catal. B 2010, 96, 290–298. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lo, S.L.; Kuo, J.; Huang, C.P. Promoted degradation of perfluorooctanic acid by persulfate when adding activated carbon. J. Hazard. Mater. 2013, 261, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.F.; Dong, C.X.; Kong, D.Y.; Lu, J.H.; Zhou, Q.S. Heat-activated persulfate oxidation of atrazine: Implications for remediation of groundwater contaminated by herbicides. Chem. Eng. J. 2015, 263, 45–54. [Google Scholar] [CrossRef]
- Guan, Y.H.; Ma, J.; Li, X.C.; Fang, J.Y.; Chen, L.W. Influence of pH on the formation of sulfate and hydroxyl radicals in the UV/peroxymonosulfate system. Environ. Sci. Technol. 2011, 45, 9308–9314. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.S.; Cheng, X.S.; Dai, H.X.; Yan, B.B.; Chen, G.Y.; Hou, L.A.; Wang, S.B. Influences and mechanisms of phosphate ions onto persulfate activation and organic degradation in water treatment: A review. Water Res. 2022, 222, 118896. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, H.; Zhong, X.; Hou, L.W. Ultrasound enhanced heterogeneous activation of peroxymonosulfate by a bimetallic Fe-Co/SBA-15 catalyst for the degradation of Orange II in water. J. Hazard. Mater. 2015, 283, 70–79. [Google Scholar] [CrossRef]
- Oyekunle, D.T.; Gendy, E.A.; Ifthikar, J.; Chen, Z.Q. Heterogeneous activation of persulfate by metal and non-metal catalyst for the degradation of sulfamethoxazole: A review. Chem. Eng. J. 2022, 437, 135277. [Google Scholar] [CrossRef]
- Guo, R.N.; Zhu, Y.L.; Cheng, X.W.; Li, J.J.; Crittenden, J.C. Efficient degradation of lomefloxacin by Co-Cu-LDH activating peroxymonosulfate process: Optimization, dynamics, degradation pathway and mechanism. J. Hazard. Mater. 2020, 399, 122966. [Google Scholar] [CrossRef]
- Guo, R.N.; Wang, Y.Y.; Li, J.J.; Cheng, X.W.; Dionysiou, D.D. Sulfamethoxazole degradation by visible light assisted peroxymonosulfate process based on nanohybrid manganese dioxide incorporating ferric oxide. Appl. Catal. B 2020, 278, 119297. [Google Scholar] [CrossRef]
- Xu, C.Y.; Yang, G.R.; Li, J.; Zhang, S.Q.; Fang, Y.P.; Peng, F.; Zhang, S.S.; Qiu, R.L. Efficient purification of tetracycline wastewater by activated persulfate with heterogeneous Co-V bimetallic oxides. J. Colloid Interface Sci. 2022, 619, 188–197. [Google Scholar] [CrossRef]
- Zheng, X.X.; Niu, X.J.; Zhang, D.Q.; Lv, M.Y.; Ye, X.Y.; Ma, J.L.; Lin, Z.; Fu, M.L. Metal-based catalysts for persulfate and peroxymonosulfate activation in heterogeneous ways: A review. Chem. Eng. J. 2022, 429, 132323. [Google Scholar] [CrossRef]
- Li, J.J.; Guo, R.N.; Ma, Q.L.; Nengzi, L.C.; Cheng, X.W. Efficient removal of organic contaminant via activation of potassium persulfate by γ-Fe2O3/α-MnO2 nanocomposite. Sep. Purif. Technol. 2019, 227, 115669. [Google Scholar] [CrossRef]
- Qin, Y.H.; Sun, M.; Liu, H.J.; Qu, J.H. AuPd/Fe3O4-based three-dimensional electrochemical system for efficiently catalytic degradation of 1-butyl-3-methylimidazolium hexafluorophosphate. Electrochim. Acta 2015, 186, 328–336. [Google Scholar] [CrossRef]
- Sun, X.P.; Liu, Z.B.; Sun, Z.R. Electro-enhanced degradation of atrazine via Co-Fe oxide modified graphite felt composite cathode for persulfate activation. Chem. Eng. J. 2022, 433, 133789. [Google Scholar] [CrossRef]
- Matzek, L.W.; Tipton, M.J.; Farmer, A.T.; Steen, A.D.; Carter, K.E. Understanding electrochemically activated persulfate and its application to ciprofloxacin abatement. Environ. Sci. Technol. 2018, 52, 5875–5883. [Google Scholar] [CrossRef]
- Cai, J.J.; Zhou, M.H.; Zhang, Q.Z.; Tian, Y.S.; Song, G. The radical and non-radical oxidation mechanism of electrochemically activated persulfate process on different cathodes in divided and undivided cell. J. Hazard. Mater. 2021, 416, 125804. [Google Scholar] [CrossRef]
- Liang, C.J.; Bruell, C.J.; Marley, M.C.; Sperry, K.L. Thermally activated persulfate oxidation of trichloroethylene (TCE) and 1,1,1-trichloroethane (TCA) in aqueous systems and soil slurries. Soil Sediment Contam. 2003, 12, 207–228. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Degradation of organic contaminants in water with sulfate radicals generated by the conjunction of peroxymonosulfate with cobalt. Environ. Sci. Technol. 2003, 37, 4790–4797. [Google Scholar] [CrossRef]
- Bandala, E.R.; Pelaez, M.A.; Dionysiou, D.D.; Gelover, S.; Garcia, J.; Macias, D. Degradation of 2,4-dichlorophenoxyacetic acid (2,4-D) using cobalt-peroxymonosulfate in Fenton-like process. J. Photoch. Photobio. A 2007, 186, 357–363. [Google Scholar] [CrossRef]
- Tsitonaki, A.; Petri, B.; Crimi, M.; Mosbaek, H.; Siegrist, R.L.; Bjerg, P.L. In situ chemical oxidation of contaminated soil and groundwater using persulfate: A review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 55–91. [Google Scholar] [CrossRef]
- Yang, S.Y.; Wang, P.; Yang, X.; Shan, L.; Zhang, W.Y.; Shao, X.T.; Niu, R. Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: Persulfate, peroxymonosulfate and hydrogen peroxide. J. Hazard. Mater. 2010, 179, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Waldemer, R.H.; Tratnyek, P.G.; Johnson, R.L.; Nurmi, J.T. Oxidation of chlorinated ethenes by heat-activated persulfate: Kinetics and products. Environ. Sci. Technol. 2007, 41, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
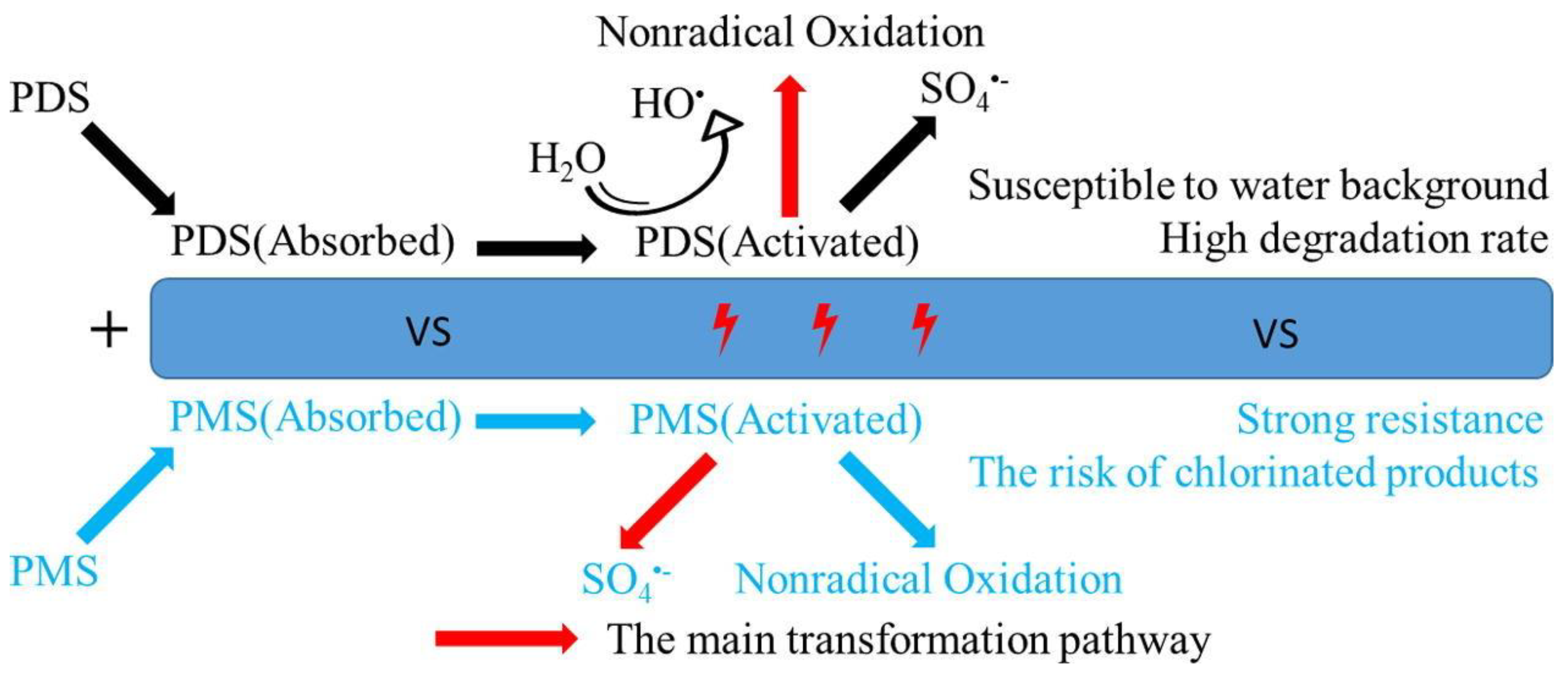
- Song, H.R.; Yan, L.X.; Wang, Y.W.; Jiang, J.; Ma, J.; Li, C.P.; Wang, G.; Gu, J.; Liu, P. Electrochemically activated PMS and PDS: Radical oxidation versus nonradical oxidation. Chem. Eng. J. 2020, 391, 123560. [Google Scholar] [CrossRef]
- Liang, C.J.; Bruell, C.J.; Marley, M.C.; Sperry, K.L. Persulfate oxidation for in situ remediation of TCE. I. Activated by ferrous ion with and without a persulfate-thiosulfate redox couple. Chemosphere 2004, 55, 1213–1223. [Google Scholar] [CrossRef]
- Yuan, S.H.; Liao, P.; Alshawabkeh, A.N. Electrolytic manipulation of persulfate reactivity by iron electrodes for trichloroethylene degradation in groundwater. Environ. Sci. Technol. 2014, 48, 656–663. [Google Scholar] [CrossRef]
- Wang, S.L.; Zhou, N. Removal of carbamazepine from aqueous solution using sono-activated persulfate process. Ultrason. Sonochem. 2016, 29, 156–162. [Google Scholar] [CrossRef]
- He, L.Y.; Chen, H.; Wu, L.; Zhang, Z.L.; Ma, Y.F.; Zhu, J.; Liu, J.X.; Yan, X.K.; Li, H.; Yang, L. Synergistic heat/UV activated persulfate for the treatment of nanofiltration concentrated leachate. Ecotoxicol. Environ. Saf. 2021, 208, 111522. [Google Scholar] [CrossRef]
- Fang, G.D.; Gao, J.; Dionysiou, D.D.; Liu, C.; Zhou, D.M. Activation of persulfate by quinones: Free radical reactions and implication for the degradation of PCBs. Environ. Sci. Technol. 2013, 47, 4605–4611. [Google Scholar] [CrossRef]
- Wang, S.L.; Ning, Z.; Si, W.; Qi, Z.; Zhi, Y. Modeling the oxidation kinetics of sono-activated persulfate’s process on the degradation of humic acid. Ultrason. Sonochem. 2015, 23, 128–134. [Google Scholar] [CrossRef]
- Bennedsen, L.R.; Muff, J.; Sogaard, E.G. Influence of chloride and carbonates on the reactivity of activated persulfate. Chemosphere 2012, 86, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.D.; Long, M.C. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Stathatos, E.; Dionysiou, D.D. Heterogeneous activation of oxone using Co3O4. J. Phys. Chem. B 2005, 109, 13052–13055. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Q.; Liang, H.W.; Zhou, G.L.; Wang, S.B. Supported cobalt catalysts by one-pot aqueous combustion synthesis for catalytic phenol degradation. J. Colloid Interface Sci. 2013, 394, 394–400. [Google Scholar] [CrossRef]
- Ji, F.; Li, C.; Liu, Y.; Liu, P. Heterogeneous activation of peroxymonosulfate by Cu/ZSM5 for decolorization of Rhodamine B. Sep. Purif. Technol. 2014, 135, 1–6. [Google Scholar] [CrossRef]
- Ji, F.; Li, C.L.; Deng, L. Performance of CuO/Oxone system: Heterogeneous catalytic oxidation of phenol at ambient conditions. Chem. Eng. J. 2011, 178, 239–243. [Google Scholar] [CrossRef]
- Fu, C.; Yi, X.L.; Liu, Y.; Zhou, H. Cu2+ activated persulfate for sulfamethazine degradation. Chemosphere 2020, 257, 127294. [Google Scholar] [CrossRef]
- Ye, Y.X.; Wan, J.; Li, Q.; Huang, Y.B.; Pan, F.; Xia, D.S. Catalytic oxidation of dyeing wastewater by copper oxide activating persulfate: Performance, mechanism and application. Int. J. Environ. Res. 2021, 15, 1–10. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Zhang, Y.B.; Quan, X.; Chen, S. Enhanced oxidation of 4-chlorophenol using sulfate radicals generated from zero-valent iron and peroxydisulfate at ambient temperature. Sep. Purif. Technol. 2010, 71, 302–307. [Google Scholar] [CrossRef]
- Liang, C.J.; Guo, Y.Y. Mass transfer and chemical oxidation of naphthalene particles with zerovalent iron activated persulfate. Environ. Sci. Technol. 2010, 44, 8203–8208. [Google Scholar] [CrossRef]
- Liang, C.J.; Lai, M.C. Trichloroethylene degradation by zero valent iron activated persulfate oxidation. Environ. Eng. Sci. 2008, 25, 1071–1077. [Google Scholar] [CrossRef]
- Oh, S.Y.; Kim, H.W.; Park, J.M.; Park, H.S.; Yoon, C. Oxidation of polyvinyl alcohol by persulfate activated with heat, Fe2+, and zero-valent iron. J. Hazard. Mater. 2009, 168, 346–351. [Google Scholar] [CrossRef]
- Oh, S.Y.; Kang, S.G.; Chiu, P.C. Degradation of 2,4-dinitrotoluene by persulfate activated with zero-valent iron. Sci. Total Environ. 2010, 408, 3464–3468. [Google Scholar] [CrossRef] [PubMed]
- Volpe, A.; Pagano, M.; Mascolo, G.; Lopez, A.; Ciannarella, R.; Locaputo, V. Simultaneous Cr(VI) reduction and non-ionic surfactant oxidation by peroxymonosulphate and iron powder. Chemosphere 2013, 91, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Zhang, Y.Q.; Huang, S.B.; Du, X.Z. Degradation of p-chloroaniline by persulfate activated with zero-valent iron. Chem. Eng. J. 2012, 203, 269–276. [Google Scholar] [CrossRef]
- Rastogi, A.; Ai-Abed, S.R.; Dionysiou, D.D. Sulfate radical-based ferrous-peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems. Appl. Catal. B 2009, 85, 171–179. [Google Scholar] [CrossRef]
- Ji, F.; Li, C.L.; Wei, X.Y.; Yu, J. Efficient performance of porous Fe2O3 in heterogeneous activation of peroxymonosulfate for decolorization of Rhodamine B. Chem. Eng. J. 2013, 231, 434–440. [Google Scholar] [CrossRef]
- Oh, W.D.; Lua, S.K.; Dong, Z.L.; Lim, T.T. High surface area DPA-hematite for efficient detoxification of bisphenol A via peroxymonosulfate activation. J. Mater. Chem. A 2014, 2, 15836–15845. [Google Scholar] [CrossRef]
- Gong, F.; Wang, L.; Li, D.W.; Zhou, F.Y.; Yao, Y.Y.; Lu, W.T.; Huang, S.Q.; Chen, W.X. An effective heterogeneous iron-based catalyst to activate peroxymonosulfate for organic contaminants removal. Chem. Eng. J. 2015, 267, 102–110. [Google Scholar] [CrossRef]
- Tan, C.Q.; Gao, N.Y.; Deng, Y.; Deng, J.; Zhou, S.Q.; Li, J.; Xin, X.Y. Radical induced degradation of acetaminophen with Fe3O4 magnetic nanoparticles as heterogeneous activator of peroxymonosulfate. J. Hazard. Mater. 2014, 276, 452–460. [Google Scholar] [CrossRef]
- Rao, L.J.; Yang, Y.F.; Liu, X.D.; Huang, Y.F.; Chen, M.X.; Yao, Y.Y.; Wang, W.T. Heterogeneous activation of persulfate by supporting ferric oxalate onto activated carbon fibers for organic contaminants removal. Mater. Res. Bull. 2020, 130, 110919. [Google Scholar] [CrossRef]
- Zhao, J.J.; Sun, Y.J.; Zhang, Y.; Zhang, B.T.; Yin, M.; Chen, L. Heterogeneous activation of persulfate by activated carbon supported iron for efficient amoxicillin degradation. Environ. Technol. Innov. 2021, 21, 101259. [Google Scholar] [CrossRef]
- Shao, F.L.; Wang, Y.J.; Mao, Y.R.; Shao, T.; Shang, J.G. Degradation of tetracycline in water by biochar supported nanosized iron activated persulfate. Chemosphere 2020, 261, 127844. [Google Scholar] [CrossRef] [PubMed]
- Pervez, M.N.; He, W.; Zarra, T.; Naddeo, V.; Zhao, Y.P. New sustainable approach for the production of Fe3O4/Graphene oxide-activated persulfate system for dye removal in real wastewater. Water 2020, 12, 733. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, G.; Yan, J.W.; Zhao, L.B.; Zhou, X.; Wang, H.L.; Feng, Y.K.; Guo, Y.F.; Wu, J.F.; Chen, W.T.; et al. The decolorization of methyl orange by persulfate activated with natural vanadium-titanium magnetite. Appl. Surf. Sci. 2020, 509, 144886. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, Y.Z.; Zhang, W.T.; Zhang, Y.L.; Yuan, G.E. Oxidative degradation of Orange G in aqueous solution by persulfate activated with pyrite. Water Sci. Technol. 2020, 82, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Ning, H.; Zhai, Y.B.; Li, S.H.; Liu, X.M.; Wang, T.F.; Wang, B.; Liu, Y.L.; Qiu, Z.Z.; Li, C.T.; Zhu, Y. Fe(II) activated persulfate assisted hydrothermal conversion of sewage sludge: Focusing on nitrogen transformation mechanism and removal effectiveness. Chemosphere 2020, 244, 125473. [Google Scholar] [CrossRef] [PubMed]
- Saputra, E.; Muhammad, S.; Sun, H.Q.; Ang, H.M.; Tade, M.O.; Wang, S.B. Manganese oxides at different oxidation states for heterogeneous activation of peroxymonosulfate for phenol degradation in aqueous solutions. Appl. Catal. B 2013, 142, 729–735. [Google Scholar] [CrossRef]
- Saputra, E.; Muhammad, S.; Sun, H.Q.; Ang, H.M.; Tade, M.O.; Wang, S.B. Shape-controlled activation of peroxymonosulfate by single crystal alpha-Mn2O3 for catalytic phenol degradation in aqueous solution. Appl. Catal. B 2014, 154, 246–251. [Google Scholar] [CrossRef]
- Saputra, E.; Muhammad, S.; Sun, H.Q.; Patel, A.; Shukla, P.; Zhu, Z.H.; Wang, S.B. Alpha-MnO2 activation of peroxymonosulfate for catalytic phenol degradation in aqueous solutions. Catal. Commun. 2012, 26, 144–148. [Google Scholar] [CrossRef]
- Wang, Y.X.; Indrawirawan, S.; Duan, X.G.; Sun, H.Q.; Ang, H.M.; Tade, M.O.; Wang, S.B. New insights into heterogeneous generation and evolution processes of sulfate radicals for phenol degradation over one-dimensional alpha-MnO2 nanostructures. Chem. Eng. J. 2015, 266, 12–20. [Google Scholar] [CrossRef]
- Wang, Y.X.; Sun, H.Q.; Ang, H.M.; Tade, M.O.; Wang, S.B. Synthesis of magnetic core/shell carbon nanosphere supported manganese catalysts for oxidation of organics in water by peroxymonosulfate. J. Colloid Interface Sci. 2014, 433, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Sun, H.Q.; Ang, H.M.; Tade, M.O.; Wang, S.B. Facile synthesis of hierarchically structured magnetic MnO2/ZnFe2O4 hybrid materials and their performance in heterogeneous activation of peroxymonosulfate. ACS Appl. Mater. Interfaces 2014, 6, 19914–19923. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.D.; Zhang, G.K.; Guo, S. Efficient activation of peroxymonosulfate by manganese oxide for the degradation of azo dye at ambient condition. J. Colloid Interface Sci. 2015, 454, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.W.; Sun, H.Q.; Patel, A.; Shukla, P.; Zhu, Z.H.; Wang, S.B. Excellent performance of mesoporous Co3O4/MnO2 nanoparticles in heterogeneous activation of peroxymonosulfate for phenol degradation in aqueous solutions. Appl. Catal. B 2012, 127, 330–335. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.W.; Shao, P.H.; Cui, F.Y. Activation of peroxymonosulfate with magnetic Fe3O4-MnO2 core-shell nanocomposites for 4-chlorophenol degradation. Chem. Eng. J. 2015, 262, 854–861. [Google Scholar] [CrossRef]
- Luo, S.L.; Duan, L.; Sun, B.Z.; Wei, M.Y.; Li, X.X.; Xu, A.H. Manganese oxide octahedral molecular sieve (OMS-2) as an effective catalyst for degradation of organic dyes in aqueous solutions in the presence of peroxymonosulfate. Appl. Catal. B 2015, 164, 92–99. [Google Scholar] [CrossRef]
- Ren, Y.M.; Lin, L.Q.; Ma, J.; Yang, J.; Feng, J.; Fan, Z.J. Sulfate radicals induced from peroxymonosulfate by magnetic ferrospinel MFe2O4 (M = Co, Cu, Mn, and Zn) as heterogeneous catalysts in the water. Appl. Catal. B 2015, 165, 572–578. [Google Scholar] [CrossRef]
- Stoyanova, M.; Slavova, I.; Christoskova, S.; Ivanova, V. Catalytic performance of supported nanosized cobalt and iron-cobalt mixed oxides on MgO in oxidative degradation of Acid Orange 7 azo dye with peroxymonosulfate. Appl. Catal. A 2014, 476, 121–132. [Google Scholar] [CrossRef]
- You, Y.; Shi, Z.K.; Li, Y.H.; Zhao, Z.J.; He, B.; Cheng, X.W. Magnetic cobalt ferrite biochar composite as peroxymonosulfate activator for removal of lomefloxacin hydrochloride. Sep. Purif. Technol. 2021, 272, 118889. [Google Scholar] [CrossRef]
- Hu, L.X.; Yang, F.; Zou, L.P.; Yuan, H.; Hu, X. Fe/SBA-15 catalyst coupled with peroxymonosulfate for heterogeneous catalytic degradation of rhodamine B in water. Chin. J. Catal. 2015, 36, 1785–1797. [Google Scholar] [CrossRef]
- Xu, L.J.; Chu, W.; Gan, L. Environmental application of graphene-based CoFe2O4 as an activator of peroxymonosulfate for the degradation of a plasticizer. Chem. Eng. J. 2015, 263, 435–443. [Google Scholar] [CrossRef]
- Yao, Y.J.; Yang, Z.H.; Zhang, D.W.; Peng, W.C.; Sun, H.Q.; Wang, S.B. Magnetic CoFe2O4-Graphene hybrids: Facile synthesis, characterization, and catalytic properties. Ind. Eng. Chem. Res. 2012, 51, 6044–6051. [Google Scholar] [CrossRef]
- Du, Y.C.; Ma, W.J.; Liu, P.X.; Zou, B.H.; Ma, J. Magnetic CoFe2O4 nanoparticles supported on titanate nanotubes (CoFe2O4/TNTs) as a novel heterogeneous catalyst for peroxymonosulfate activation and degradation of organic pollutants. J. Hazard. Mater. 2016, 308, 58–66. [Google Scholar] [CrossRef]
- Yao, Y.J.; Cai, Y.M.; Wu, G.D.; Wei, F.Y.; Li, X.Y.; Chen, H.; Wang, S.B. Sulfate radicals induced from peroxymonosulfate by cobalt manganese oxides (CoxMn3-xO4) for Fenton-Like reaction in water. J. Hazard. Mater. 2015, 296, 128–137. [Google Scholar] [CrossRef]
- Ding, Y.B.; Zhu, L.H.; Wang, N.; Tang, H.Q. Sulfate radicals induced degradation of tetrabromobisphenol A with nanoscaled magnetic CuFe2O4 as a heterogeneous catalyst of peroxymonosulfate. Appl. Catal. B 2013, 129, 153–162. [Google Scholar] [CrossRef]
- Oh, W.D.; Lua, S.K.; Dong, Z.L.; Lim, T.T. Performance of magnetic activated carbon composite as peroxymonosulfate activator and regenerable adsorbent via sulfate radical-mediated oxidation processes. J. Hazard. Mater. 2015, 284, 1–9. [Google Scholar] [CrossRef]
- Xu, Y.; Ai, J.; Zhang, H. The mechanism of degradation of bisphenol A using the magnetically separable CuFe2O4/peroxymonosulfate heterogeneous oxidation process. J. Hazard. Mater. 2016, 309, 87–96. [Google Scholar] [CrossRef]
- Bikkarolla, S.K.; Papakonstantinou, P. CuCo2O4 nanoparticles on nitrogenated graphene as highly efficient oxygen evolution catalyst. J. Power Sources 2015, 281, 243–251. [Google Scholar] [CrossRef]
- Oh, W.D.; Lua, S.K.; Dong, Z.L.; Lim, T.T. A novel three-dimensional spherical CuBi2O4 consisting of nanocolumn arrays with persulfate and peroxymonosulfate activation functionalities for 1H-benzotriazole removal. Nanoscale 2015, 7, 8149–8158. [Google Scholar] [CrossRef]
- Wang, Z.L.; Du, Y.C.; Liu, Y.L.; Zou, B.H.; Xiao, J.Y.; Ma, J. Degradation of organic pollutants by NiFe2O4/peroxymonosulfate: Efficiency, influential factors and catalytic mechanism. RSC Adv. 2016, 6, 11040–11048. [Google Scholar] [CrossRef]
- Yao, Y.J.; Cai, Y.M.; Lu, F.; Wei, F.Y.; Wang, X.Y.; Wang, S.B. Magnetic recoverable MnFe2O4 and MnFe2O4-graphene hybrid as heterogeneous catalysts of peroxymonosulfate activation for efficient degradation of aqueous organic pollutants. J. Hazard. Mater. 2014, 270, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Mirzaei, A.A.; Atashi, H. Hydrothermal synthesis of Fe-Ni-Ce nano-structure catalyst for Fischer-Tropsch synthesis: Characterization and catalytic performance. J. Alloys Compd. 2019, 799, 546–555. [Google Scholar] [CrossRef]
- Lv, C.N.; Shi, J.D.; Tang, Q.J.; Hu, Q. Tetracycline removal by activating persulfate with diatomite loading of Fe and Ce. Molecules 2020, 25, 5531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhou, T.T.; He, L.; Xu, D.Q.; Bai, L. Oxidative degradation of Rhodamine B by Ag@CuO nanocomposite activated persulfate. Synth. Met. 2020, 267, 116479. [Google Scholar] [CrossRef]
- Niu, L.J.; Xian, G.; Long, Z.Q.; Zhang, G.M.; Zhu, J.; Li, J.W. MnCeOx with high efficiency and stability for activating persulfate to degrade AO7 and ofloxacin. Ecotoxicol. Environ. Saf. 2020, 191, 110228. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.T.; Teng, Y.G.; Zhao, J.J. Activated carbon supported nanoscale zero valent iron for cooperative adsorption and persulfate-driven oxidation of ampicillin. Environ. Technol. Innov. 2020, 19, 100956. [Google Scholar] [CrossRef]
- Yang, J.Y.; Huang, M.Y.; Wang, S.S.; Mao, X.Y.; Hu, Y.M.; Chen, X. Efficient removal of levofloxacin by activated persulfate with magnetic CuFe2O4/MMT-k10 nanocomposite: Characterization, response surface methodology, and degradation mechanism. Water 2020, 12, 3583. [Google Scholar] [CrossRef]
- Chen, W.S.; Jhou, Y.C.; Huang, C.P. Mineralization of dinitrotoluenes in industrial wastewater by electro-activated persulfate oxidation. Chem. Eng. J. 2014, 252, 166–172. [Google Scholar] [CrossRef]
- Wang, Y.R.; Chu, W. Degradation of 2,4,5-trichlorophenoxyacetic acid by a novel Electro-Fe(II)/Oxone process using iron sheet as the sacrificial anode. Water Res. 2011, 45, 3883–3889. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Liu, C.C.; Guo, Y.Z.; Shan, N.; Meng, C.X.; Sun, L.Y. Removal of COD from landfill leachate by an electro/Fe2+/peroxydisulfate process. Chem. Eng. J. 2014, 250, 76–82. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Qiu, J.J. Degradation of Acid Orange 7 in aqueous solution by a novel electro/Fe2+/peroxydisulfate process. J. Hazard. Mater. 2012, 215, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, Y.K.; Zhao, Q.; Ji, M. Treatment of secondary effluent using a three-dimensional electrode system: COD removal, biotoxicity assessment, and disinfection effects. Chem. Eng. J. 2014, 243, 1–6. [Google Scholar]
- Li, M.; Zhao, F.P.; Sillanpaa, M.; Meng, Y.; Yin, D.L. Electrochemical degradation of 2-diethylamino-6-methyl-4-hydroxypyrimidine using three-dimensional electrodes reactor with ceramic particle electrodes. Sep. Purif. Technol. 2015, 156, 588–595. [Google Scholar] [CrossRef]
- Long, Y.Y.; Feng, Y.; Li, X.; Suo, N.; Chen, H.; Wang, Z.W.; Yu, Y.Z. Removal of diclofenac by three-dimensional electro-Fenton-persulfate (3D electro-Fenton-PS). Chemosphere 2019, 219, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Long, A.H.; Zhang, H. Selective oxidative degradation of toluene for the recovery of surfactant by an electro/Fe2+/persulfate process. Environ. Sci. Pollut. Res. 2015, 22, 11606–11616. [Google Scholar] [CrossRef] [PubMed]
- Long, A.H.; Lei, Y.; Zhang, H. In situ chemical oxidation of organic contaminated soil and groundwater using activated persulfate process. Prog. Chem. 2014, 26, 898–908. [Google Scholar]
- Anipsitakis, G.P.; Dionysiou, D.D.; Gonzalez, M.A. Cobalt-mediated activation of peroxymonosulfate and sulfate radical attack on phenolic compounds. Implications of chloride ions. Environ. Sci. Technol. 2006, 40, 1000–1007. [Google Scholar] [CrossRef]
- Ledjeri, A.; Yahiaoui, I.; Kadji, H.; Aissani-Benissad, F.; Amrane, A.; Fourcade, F. Combination of the Electro/Fe3+/peroxydisulfate (PDS) process with activated sludge culture for the degradation of sulfamethazine. Environ. Toxicol. Phar. 2017, 53, 34–39. [Google Scholar] [CrossRef]
- Hu, C.Y.; Hou, Y.Z.; Lin, Y.L.; Deng, Y.G.; Hua, S.J.; Du, Y.F.; Chen, C.W.; Wu, C.H. Investigation of iohexol degradation kinetics by using heat-activated persulfate. Chem. Eng. J. 2020, 379, 122403. [Google Scholar] [CrossRef]
- Lin, H.; Wu, J.; Zhang, H. Degradation of bisphenol A in aqueous solution by a novel electro/Fe3+/peroxydisulfate process. Sep. Purif. Technol. 2013, 117, 18–23. [Google Scholar] [CrossRef]
- Yan, S.D.; Xiong, W.H.; Xing, S.Y.; Shao, Y.Q.; Guo, R.; Zhang, H. Oxidation of organic contaminant in a self-driven electro/natural maghemite/peroxydisulfate system: Efficiency and mechanism. Sci. Total Environ. 2017, 599, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhong, X.; Ciotonea, C.; Fan, X.H.; Mao, X.Y.; Li, Y.T.; Deng, B.; Zhang, H.; Royer, S. Efficient degradation of clofibric acid by electro-enhanced peroxydisulfate activation with Fe-Cu/SBA-15 catalyst. Appl. Catal. B 2018, 230, 1–10. [Google Scholar] [CrossRef]
- Deng, B.; Li, Y.T.; Tan, W.H.; Wang, Z.X.; Yu, Z.W.; Xing, S.Y.; Lin, H.; Zhang, H. Degradation of bisphenol A by electro-enhanced heterogeneous activation of peroxydisulfate using Mn-Zn ferrite from spent alkaline Zn-Mn batteries. Chemosphere 2018, 204, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, H.; Hou, L.W. Degradation of C. I. Acid Orange 7 in aqueous solution by a novel electro/Fe3O4/PDS process. J. Hazard. Mater. 2014, 276, 182–191. [Google Scholar] [CrossRef]
- Lin, H.; Li, Y.T.; Mao, X.Y.; Zhang, H. Electro-enhanced goethite activation of peroxydisulfate for the decolorization of Orange II at neutral pH: Efficiency, stability and mechanism. J. Taiwan Inst. Chem. Eng. 2016, 65, 390–398. [Google Scholar] [CrossRef]
- Huang, R.H.; Yan, H.H.; Li, L.S.; Deng, D.Y.; Shu, Y.H.; Zhang, Q.Y. Catalytic activity of Fe/SBA-15 for ozonation of dimethyl phthalate in aqueous solution. Appl. Catal. B 2011, 106, 264–271. [Google Scholar] [CrossRef]
- Hu, L.X.; Yang, F.; Lu, W.C.; Hao, Y.; Yuan, H. Heterogeneous activation of oxone with CoMg/SBA-15 for the degradation of dye Rhodamine B in aqueous solution. Appl. Catal. B 2013, 134, 7–18. [Google Scholar] [CrossRef]
- Hu, L.X.; Yang, X.P.; Dang, S.T. An easily recyclable Co/SBA-15 catalyst: Heterogeneous activation of peroxymonosulfate for the degradation of phenol in water. Appl. Catal. B 2011, 102, 19–26. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, Z.Y.; Zhang, H. Electro-assisted heterogeneous activation of persulfate by Fe/SBA-15 for the degradation of Orange II. J. Hazard. Mater. 2016, 313, 209–218. [Google Scholar] [CrossRef]
- Melero, J.A.; Calleja, G.; Martinez, F.; Molina, R. Nanocomposite of crystalline Fe2O3 and CuO particles and mesostructured SBA-15 silica as an active catalyst for wet peroxide oxidation processes. Catal. Commun. 2006, 7, 478–483. [Google Scholar] [CrossRef]
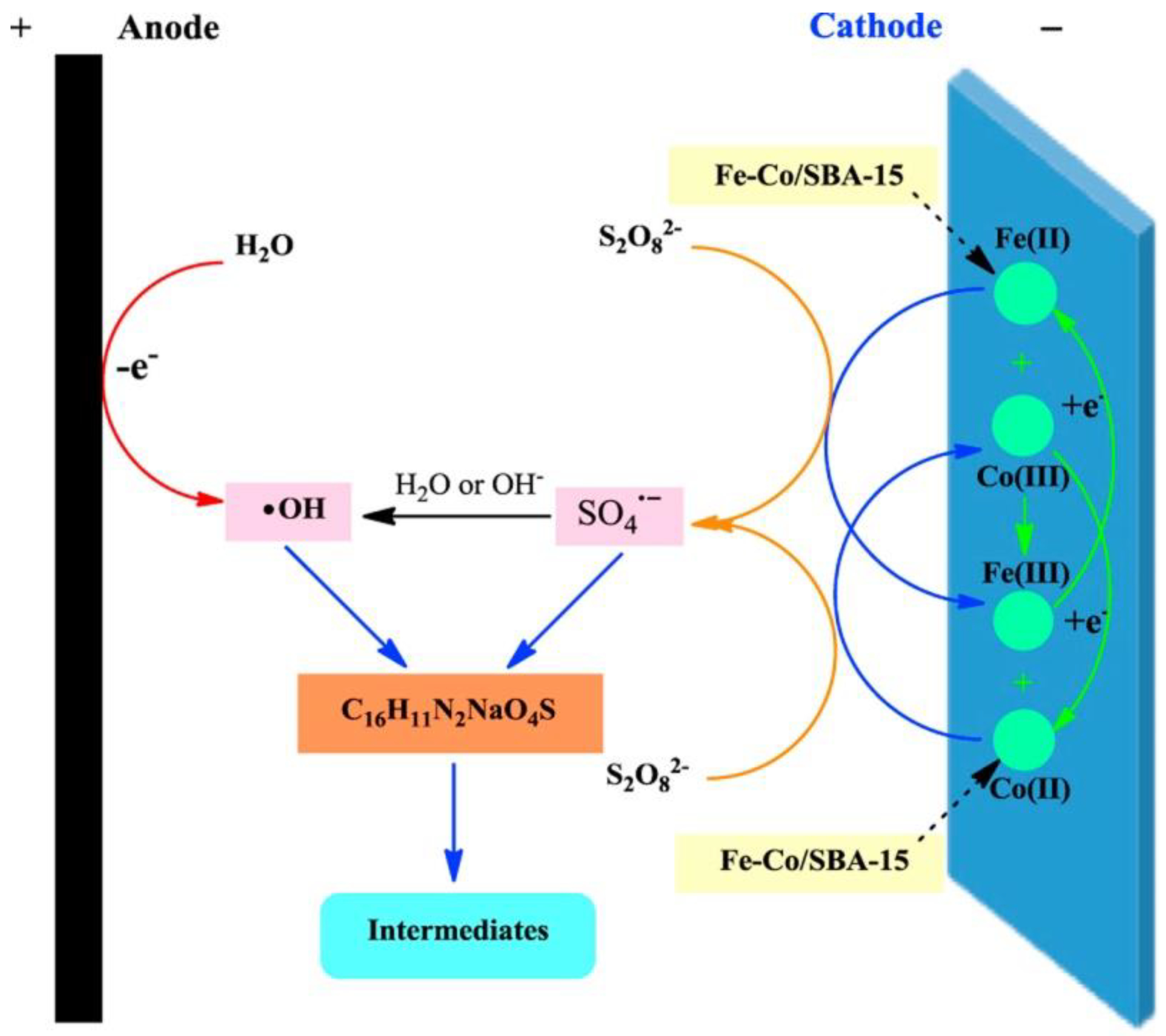
- Cai, C.; Zhang, H.; Zhong, X.; Hou, L.W. Electrochemical enhanced heterogeneous activation of peroxydisulfate by Fe-Co/SBA-15 catalyst for the degradation of Orange II in water. Water Res. 2014, 66, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wu, J.; Zhang, H. Degradation of clofibric acid in aqueous solution by an EC/Fe3+/PMS process. Chem. Eng. J. 2014, 244, 514–521. [Google Scholar] [CrossRef]
- Yan, S.D.; Geng, J.Y.; Guo, R.; Du, Y.; Zhang, H. Hydronium jarosite activation of peroxymonosulfate for the oxidation of organic contaminant in an electrochemical reactor driven by microbial fuel cell. J. Hazard. Mater. 2017, 333, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.P.; Chen, X.; Liu, S.S.; Xing, Q.J.; Dong, W.H.; Luo, X.B.; Dai, W.L.; Xiao, X.; Luo, J.M.; Crittenden, J. Electrochemical oxidation and advanced oxidation processes using a 3D hexagonal Co3O4 array anode for 4-nitrophenol decomposition coupled with simultaneous CO2 conversion to liquid fuels via a flower-like CuO cathode. Water Res. 2019, 150, 330–339. [Google Scholar] [CrossRef]
- Li, J.; Lin, H.; Zhu, K.M.; Zhang, H. Degradation of Acid Orange 7 using peroxymonosulfate catalyzed by granulated activated carbon and enhanced by electrolysis. Chemosphere 2017, 188, 139–147. [Google Scholar] [CrossRef]
- Song, H.R.; Yan, L.X.; Jiang, J.; Ma, J.; Zhang, Z.X.; Zhang, J.M.; Liu, P.X.; Yang, T. Electrochemical activation of persulfates at BDD anode: Radical or nonradical oxidation? Water Res. 2018, 128, 393–401. [Google Scholar] [CrossRef]
- Liu, Z.; Ding, H.J.; Zhao, C.; Wang, T.; Wang, P.; Dionysiou, D.D. Electrochemical activation of peroxymonosulfate with ACF cathode: Kinetics, influencing factors, mechanism, and application potential. Water Res. 2019, 159, 111–121. [Google Scholar] [CrossRef]
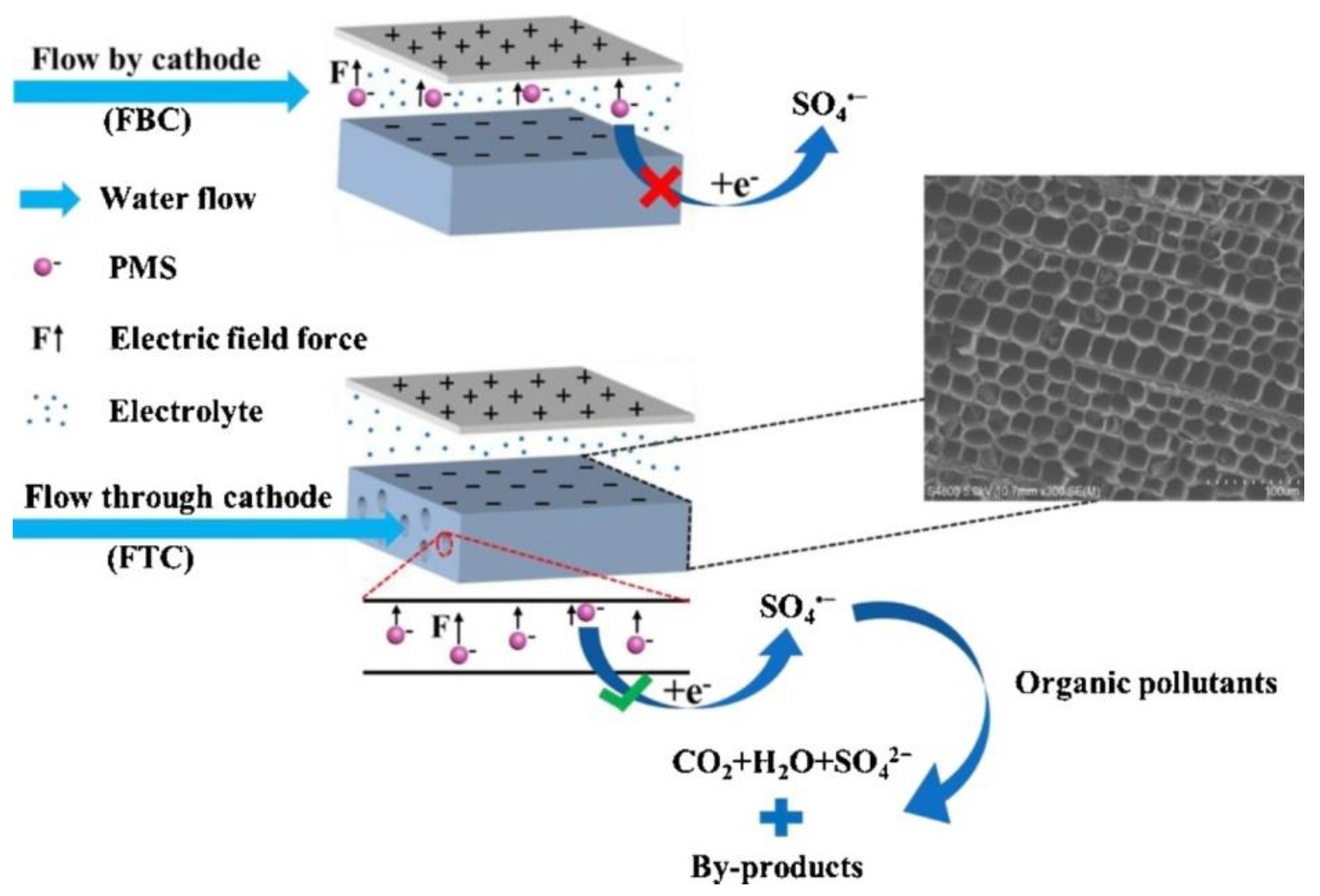
- Zhang, Y.; Kang, W.D.; Yu, H.T.; Chen, S.; Quan, X. Electrochemical activation of peroxymonosulfate in cathodic micro-channels for effective degradation of organic pollutants in wastewater. J. Hazard. Mater. 2020, 398, 122879. [Google Scholar] [CrossRef]
- Lei, J.M.; Cai, Q.; Yang, Q.; Wang, Y.Y. Oxidative removal of dichloromethane by electro-activated persulfate in a dual-chamber reactor. Environ. Eng. Sci. 2020, 37, 596–605. [Google Scholar] [CrossRef]
- Miao, D.T.; Liu, G.S.; Wei, Q.P.; Hu, N.X.; Zheng, K.Z.; Zhu, C.W.; Liu, T.; Zhou, K.C.; Yu, Z.M.; Ma, L. Electro-activated persulfate oxidation of malachite green by boron-doped diamond (BDD) anode: Effect of degradation process parameters. Water Sci. Technol. 2020, 81, 925–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Liang, Y.; Jin, P.; Zhao, B.; Zhang, Z.; He, X.; Tan, Z.; Wang, L.; Cheng, X. Heterogeneous Metal-Activated Persulfate and Electrochemically Activated Persulfate: A Review. Catalysts 2022, 12, 1024. https://doi.org/10.3390/catal12091024
Li J, Liang Y, Jin P, Zhao B, Zhang Z, He X, Tan Z, Wang L, Cheng X. Heterogeneous Metal-Activated Persulfate and Electrochemically Activated Persulfate: A Review. Catalysts. 2022; 12(9):1024. https://doi.org/10.3390/catal12091024
Chicago/Turabian StyleLi, Junjing, Yiqi Liang, Pengliang Jin, Bin Zhao, Zhaohui Zhang, Xiaojia He, Zilin Tan, Liang Wang, and Xiuwen Cheng. 2022. "Heterogeneous Metal-Activated Persulfate and Electrochemically Activated Persulfate: A Review" Catalysts 12, no. 9: 1024. https://doi.org/10.3390/catal12091024
APA StyleLi, J., Liang, Y., Jin, P., Zhao, B., Zhang, Z., He, X., Tan, Z., Wang, L., & Cheng, X. (2022). Heterogeneous Metal-Activated Persulfate and Electrochemically Activated Persulfate: A Review. Catalysts, 12(9), 1024. https://doi.org/10.3390/catal12091024