The Nature of Active Sites in the Pd/C-Catalyzed Hydrogenation/Hydrodeoxygenation of Benzaldehyde
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterisation
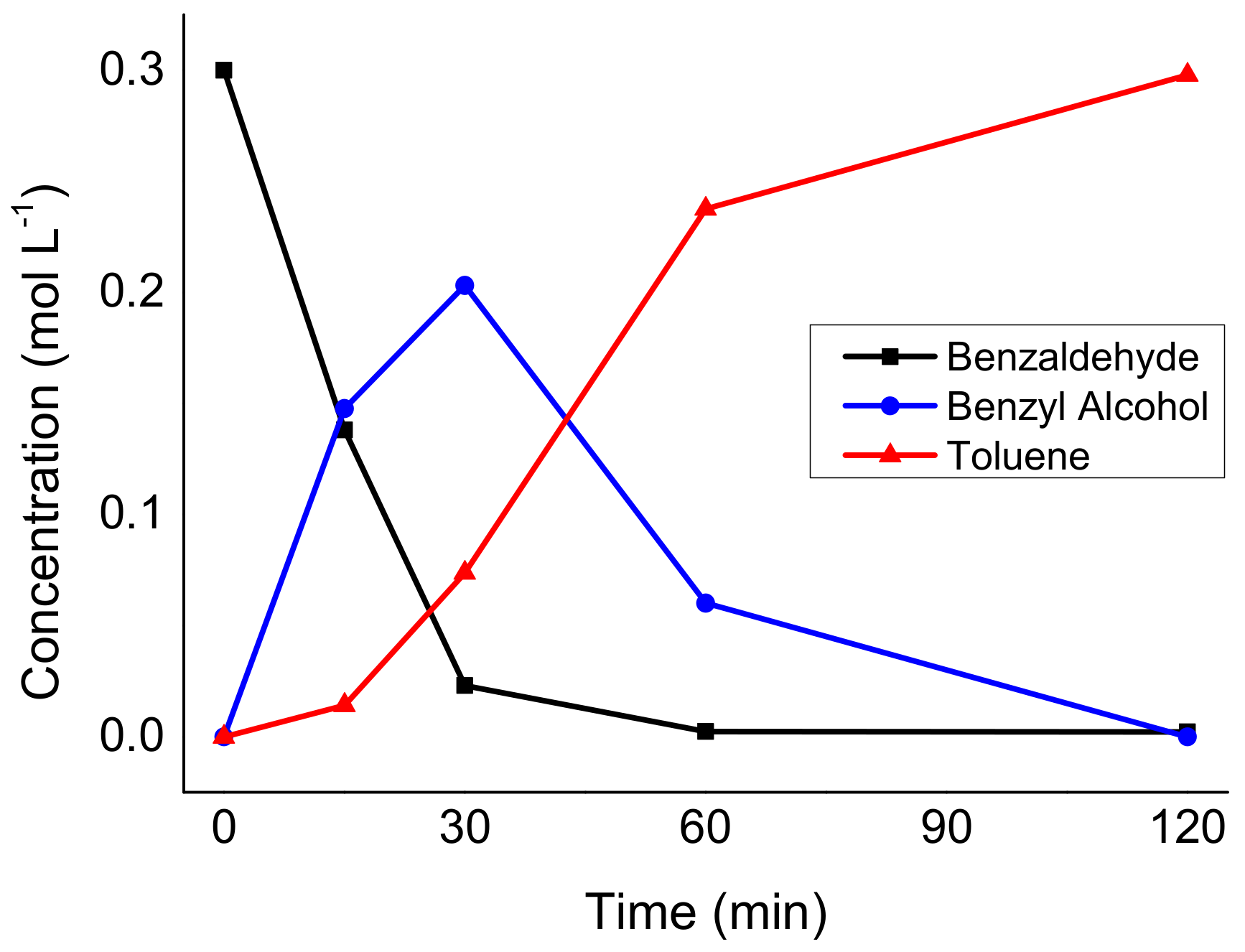
2.2. Catalytic Activity
2.3. Activity/Catalyst Properties Correlation
3. Materials and Methods
3.1. Catalyst Syntheses
3.2. Catalyst Washing
3.3. Catalysts Characterization
3.4. Catalytic Reactions
3.5. Recycling Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Personick, M.L.; Montemore, M.M.; Kaxiras, E.; Madix, R.J.; Biener, J.; Friend, C.M. Catalyst Design for Enhanced Sustainability through Fundamental Surface Chemistry. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150077. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, G.; Ozkan, U.S. Hydrogenation of Hexanal over Sulfided Ni-Mo/γ-Al2O3 Catalysts. J. Mol. Catal. A Chem. 2004, 217, 219–229. [Google Scholar] [CrossRef]
- Fu, J.; Lu, X.; Savage, P.E. Hydrothermal Decarboxylation and Hydrogenation of Fatty Acids over Pt/C. ChemSusChem 2011, 4, 481–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa, A.; Wang, D.; Veith, G.M.; Vindigni, F.; Prati, L. Sol Immobilization Technique: A Delicate Balance between Activity, Selectivity and Stability of Gold Catalysts. Catal. Sci. Technol. 2013, 3, 3036–3041. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, F.; Motta, D.; Bocelli, L.; Albonetti, S.; Roldan, A.; Hammond, C.; Villa, A.; Dimitratos, N. Investigation of the Catalytic Performance of Pd/CNFs for Hydrogen Evolution from Additive-Free Formic Acid Decomposition. C 2018, 4, 26. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, F.; Motta, D.; Roldan, A.; Hammond, C.; Villa, A.; Dimitratos, N. Hydrogen Generation from Additive-Free Formic Acid Decomposition Under Mild Conditions by Pd/C: Experimental and DFT Studies. Top. Catal. 2018, 61, 254–266. [Google Scholar] [CrossRef] [Green Version]
- Alijani, S.; Capelli, S.; Cattaneo, S.; Schiavoni, M.; Evangelisti, C.; Mohammed, K.M.H.; Wells, P.P.; Tessore, F.; Villa, A. Capping Agent Effect on Pd-Supported Nanoparticles in the Hydrogenation of Furfural. Catalysts 2019, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Baygazieva, E.K.; Yesmurzayeva, N.N.; Tatykhanova, G.S.; Mun, G.A.; Khutoryanskiy, V.V.; Kudaibergenov, S.E. Polymer Protected Gold Nanoparticles: Synthesis, Characterization and Application in Catalysis. J. Biol. Chem. 2014, 7, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Raveendran, P.; Fu, J.; Wallen, S.L. Completely “Green” Synthesis and Stabilization of Metal Nanoparticles. J. Am. Chem. Soc. 2003, 125, 13940–13941. [Google Scholar] [CrossRef]
- Crooks, R.M.; Zhao, M.; Sun, L.; Chechik, V.; Yeung, L.K. Dendrimer-Encapsulated Metal Nanoparticles: Synthesis, Characterization, and Applications to Catalysis. Acc. Chem. Res. 2001, 34, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.K.; Korgel, B.A. The Importance of the CTAB Surfactant on the Colloidal Seed-Mediated Synthesis of Gold Nanorods. Langmuir 2008, 24, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, C.; Tripkovic, D.; Sun, S.; Markovic, N.M.; Stamenkovic, V.R. Surfactant Removal for Colloidal Nanoparticles from Solution Synthesis: The Effect on Catalytic Performance. ACS Catal. 2012, 2, 1358–1362. [Google Scholar] [CrossRef]
- Niu, Z.; Li, Y. Removal and Utilization of Capping Agents in Nanocatalysis. Chem. Mater. 2014, 26, 72–83. [Google Scholar] [CrossRef]
- Ajitha, B.; Kumar Reddy, Y.A.; Reddy, P.S.; Jeon, H.-J.; Ahn, C.W. Role of Capping Agents in Controlling Silver Nanoparticles Size, Antibacterial Activity and Potential Application as Optical Hydrogen Peroxide Sensor. RSC Adv. 2016, 6, 36171–36179. [Google Scholar] [CrossRef]
- Phan, C.M.; Nguyen, H.M. Role of Capping Agent in Wet Synthesis of Nanoparticles. J. Phys. Chem. A 2017, 121, 3213–3219. [Google Scholar] [CrossRef]
- Neri, G.; Musolino, M.G.; Milone, C.; Pietropaolo, D.; Galvagno, S. Particle Size Effect in the Catalytic Hydrogenation of 2,4-Dinitrotoluene over Pd/C Catalysts. Appl. Catal. A Gen. 2001, 208, 307–316. [Google Scholar] [CrossRef]
- Johnson, J.A.; Makis, J.J.; Marvin, K.A.; Rodenbusch, S.E.; Stevenson, K.J. Size-Dependent Hydrogenation of p-Nitrophenol with Pd Nanoparticles Synthesized with Poly(Amido)Amine Dendrimer Templates. J. Phys. Chem. C 2013, 117, 22644–22651. [Google Scholar] [CrossRef]
- Gao, D.; Zhou, H.; Wang, J.; Miao, S.; Yang, F.; Wang, G.; Wang, J.; Bao, X. Size-Dependent Electrocatalytic Reduction of CO2 over Pd Nanoparticles. J. Am. Chem. Soc. 2015, 137, 4288–4291. [Google Scholar] [CrossRef]
- Wilson, O.M.; Knecht, M.R.; Garcia-Martinez, J.C.; Crooks, R.M. Effect of Pd Nanoparticle Size on the Catalytic Hydrogenation of Allyl Alcohol. J. Am. Chem. Soc. 2006, 128, 4510–4511. [Google Scholar] [CrossRef] [Green Version]
- Zorn, K.; Giorgio, S.; Halwax, E.; Henry, C.R.; Grönbeck, H.; Rupprechter, G. CO Oxidation on Technological Pd-Al2O3 Catalysts: Oxidation State and Activity. J. Phys. Chem. C 2011, 115, 1103–1111. [Google Scholar] [CrossRef]
- Xiong, H.; Wiebenga, M.H.; Carrillo, C.; Gaudet, J.R.; Pham, H.N.; Kunwar, D.; Oh, S.H.; Qi, G.; Kim, C.H.; Datye, A.K. Design Considerations for Low-Temperature Hydrocarbon Oxidation Reactions on Pd Based Catalysts. Appl. Catal. B Environ. 2018, 236, 436–444. [Google Scholar] [CrossRef]
- Lv, Q.; Meng, Q.; Liu, W.; Sun, N.; Jiang, K.; Ma, L.; Peng, Z.; Cai, W.; Liu, C.; Ge, J.; et al. Pd–PdO Interface as Active Site for HCOOH Selective Dehydrogenation at Ambient Condition. J. Phys. Chem. C 2018, 122, 2081–2088. [Google Scholar] [CrossRef]
- Capelli, S.; Motta, D.; Evangelisti, C.; Dimitratos, N.; Prati, L.; Pirola, C.; Villa, A. Effect of Carbon Support, Capping Agent Amount, and Pd NPs Size for Bio-Adipic Acid Production from Muconic Acid and Sodium Muconate. Nanomaterials 2020, 10, 505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.-F.; Jang, D.-Y.; Jang, H.-G.; Kim, G.-J. Hydrogenation and Dehydrogenation Reactions Catalyzed by CNTs Supported Palladium Catalysts. Catal. Today 2012, 186, 109–114. [Google Scholar] [CrossRef]
- Menegazzo, F.; Canton, P.; Pinna, F.; Pernicone, N. Bimetallic Pd–Au Catalysts for Benzaldehyde Hydrogenation: Effects of Preparation and of Sulfur Poisoning. Catal. Commun. 2008, 9, 2353–2356. [Google Scholar] [CrossRef]
- Divakar, D.; Manikandan, D.; Kalidoss, G.; Sivakumar, T. Hydrogenation of Benzaldehyde over Palladium Intercalated Bentonite Catalysts: Kinetic Studies. Catal. Lett. 2008, 125, 277. [Google Scholar] [CrossRef]
- Simone, D.O.; Kennelly, T.; Brungard, N.L.; Farrauto, R.J. Reversible Poisoning of Palladium Catalysts for Methane Oxidation. Appl. Catal. 1991, 70, 87–100. [Google Scholar] [CrossRef]
- Fernandes, V.R.; Pinto, A.M.F.R.; Rangel, C.M. Hydrogen Production from Sodium Borohydride in Methanol–Water Mixtures. Int. J. Hydrogen Energy 2010, 35, 9862–9868. [Google Scholar] [CrossRef]
- López-Corral, I.; Germán, E.; Juan, A.; Volpe, M.A.; Brizuela, G.P. DFT Study of Hydrogen Adsorption on Palladium Decorated Graphene. J. Phys. Chem. C 2011, 115, 4315–4323. [Google Scholar] [CrossRef]
- Cabria, I.; López, M.J.; Fraile, S.; Alonso, J.A. Adsorption and Dissociation of Molecular Hydrogen on Palladium Clusters Supported on Graphene. J. Phys. Chem. C 2012, 116, 21179–21189. [Google Scholar] [CrossRef]
- Capelli, S.; Motta, D.; Evangelisti, C.; Dimitratos, N.; Prati, L.; Pirola, C.; Villa, A. Bio Adipic Acid Production from Sodium Muconate and Muconic Acid: A Comparison of Two Systems. ChemCatChem 2019, 11, 3075–3084. [Google Scholar] [CrossRef]
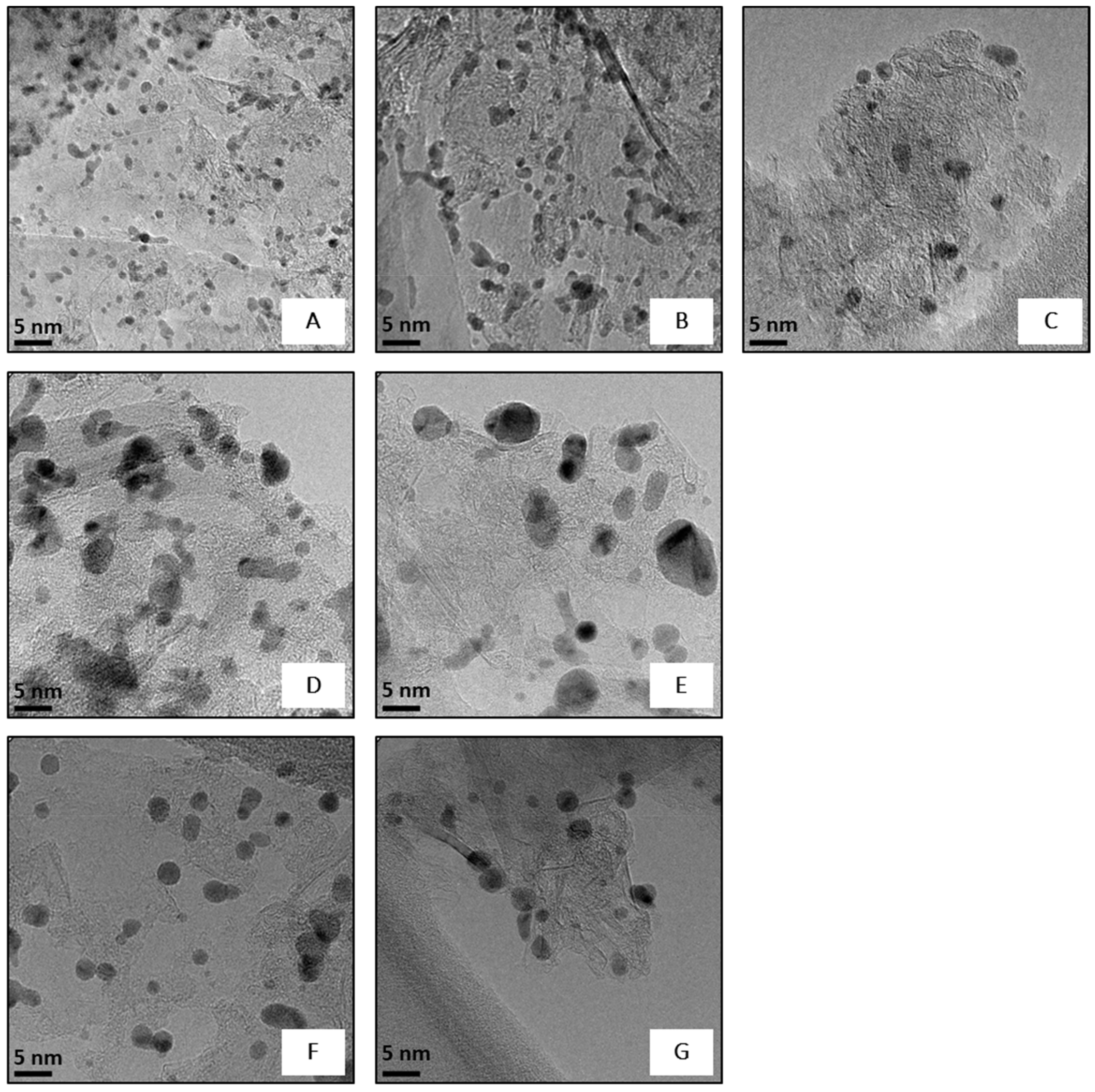



| Catalyst | Metal Loading * (wt%) | Mean NPs Size § (nm) | Pdexposure ç (atomic %) | Pd(0) ç | Pd(0)exposure ° (%) | |
|---|---|---|---|---|---|---|
| w-Pd/GNP0.5-H2O-fast | 1.0 ± 0.1 | 3.6 ± 1.1 | 0.55 | B.E. (eV) | 335.5 | 0.37 |
| Atomic (%) | 68 | |||||
| w-Pd/GNP0.2-H2O-fast | 1.0 ± 0.1 | 4.6 ± 1.4 | 0.55 | B.E. (eV) | 336.0 | 0.44 |
| Atomic (%) | 79 | |||||
| w-Pd/GNP1.0-H2O-fast | 1.0 ± 0.1 | 4.4 ± 2.0 | 0.27 | B.E. (eV) | 336.0 | 0.16 |
| Atomic (%) | 63 | |||||
| w-Pd/GNP0.5-EtOH-fast | 1.0 ± 0.1 | 5.7 ± 2.1 | 0.54 | B.E. (eV) | 335.7 | 0.47 |
| Atomic (%) | 88 | |||||
| w-Pd/GNP0.5-EtOH/H2O-fast | 1.0 ± 0.1 | 8.3 ± 3.9 | 0.37 | B.E. (eV) | 335.9 | 0.34 |
| Atomic (%) | 93 | |||||
| w-Pd/GNP0.5-H2O-medium | 1.1 ± 0.1 | 5.5 ± 2.1 | 0.37 | B.E. (eV) | 335.7 | 0.36 |
| Atomic (%) | 99 | |||||
| w-Pd/GNP0.5-H2O-slow | 1.0 ± 0.1 | 4.7 ± 1.5 | 0.45 | B.E. (eV) | 336.1 | 0.45 |
| Atomic (%) | 99 | |||||
| Sample | Initial Activity * (s−1) | Conversion * (%) | Selectivitybenzyl alcohol * (%) | Selectivitytoluene * (%) |
|---|---|---|---|---|
| Pd/GNP0.5-H2O-fast | 0.48 | 40 | 88 | 12 |
| w-Pd/GNP0.5-H2O-fast | 0.59 | 54 | 91 | 9 |
| w-Pd/GNP0.2-H2O-fast | 0.66 | 60 | 94 | 6 |
| w-Pd/GNP1.0-H2O-fast | 0.34 | 31 | 91 | 9 |
| w-Pd/GNP0.5-EtOH-fast | 0.79 | 72 | 91 | 9 |
| w-Pd/GNP0.5-EtOH/H2O-fast | 0.54 | 49 | 95 | 5 |
| w-Pd/GNP0.5-H2O-medium | 0.60 | 54 | 94 | 6 |
| w-Pd/GNP0.5-H2O-slow | 0.76 | 70 | 94 | 6 |
| Catalyst | Pdexposure (Atomic %) | Pd(0) (Atomic %) | Pd(0)exposure (%) |
|---|---|---|---|
| w-Pd/GNP0.5-H2O-fast fresh | 0.55 | 68 | 0.37 |
| w-Pd/GNP0.5-H2O-fast used | 0.43 | 87 | 0.37 |
| Catalysts | PVA/Metal (wt%/wt%) | Solvent | NaBH4 Addition |
|---|---|---|---|
| w-Pd/GNP0.5-H2O-fast | 0.5 | H2O | Instantaneous |
| w-Pd/GNP0.2-H2O-fast | 0.2 | H2O | Instantaneous |
| w-Pd/GNP1.0-H2O-fast | 1.0 | H2O | Instantaneous |
| w-Pd/GNP0.5-EtOH-fast | 0.5 | EtOH | Instantaneous |
| w-Pd/GNP0.5-EtOH/H2O-fast | 0.5 | EtOH/H2O (1:1 vol/vol) | Instantaneous |
| w-Pd/GNP0.5-H2O-medium | 0.5 | H2O | Over 2 min |
| w-Pd/GNP0.5-H2O-slow | 0.5 | H2O | Over 4 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capelli, S.; Cattaneo, S.; Stucchi, M.; Vandegehuchte, B.D.; Chieregato, A.; Villa, A.; Prati, L. The Nature of Active Sites in the Pd/C-Catalyzed Hydrogenation/Hydrodeoxygenation of Benzaldehyde. Catalysts 2022, 12, 251. https://doi.org/10.3390/catal12030251
Capelli S, Cattaneo S, Stucchi M, Vandegehuchte BD, Chieregato A, Villa A, Prati L. The Nature of Active Sites in the Pd/C-Catalyzed Hydrogenation/Hydrodeoxygenation of Benzaldehyde. Catalysts. 2022; 12(3):251. https://doi.org/10.3390/catal12030251
Chicago/Turabian StyleCapelli, Sofia, Stefano Cattaneo, Marta Stucchi, Bart D. Vandegehuchte, Alessandro Chieregato, Alberto Villa, and Laura Prati. 2022. "The Nature of Active Sites in the Pd/C-Catalyzed Hydrogenation/Hydrodeoxygenation of Benzaldehyde" Catalysts 12, no. 3: 251. https://doi.org/10.3390/catal12030251
APA StyleCapelli, S., Cattaneo, S., Stucchi, M., Vandegehuchte, B. D., Chieregato, A., Villa, A., & Prati, L. (2022). The Nature of Active Sites in the Pd/C-Catalyzed Hydrogenation/Hydrodeoxygenation of Benzaldehyde. Catalysts, 12(3), 251. https://doi.org/10.3390/catal12030251











