Acetalization of Glycerol with Citral over Heteropolyacids Immobilized on KIT-6
Abstract
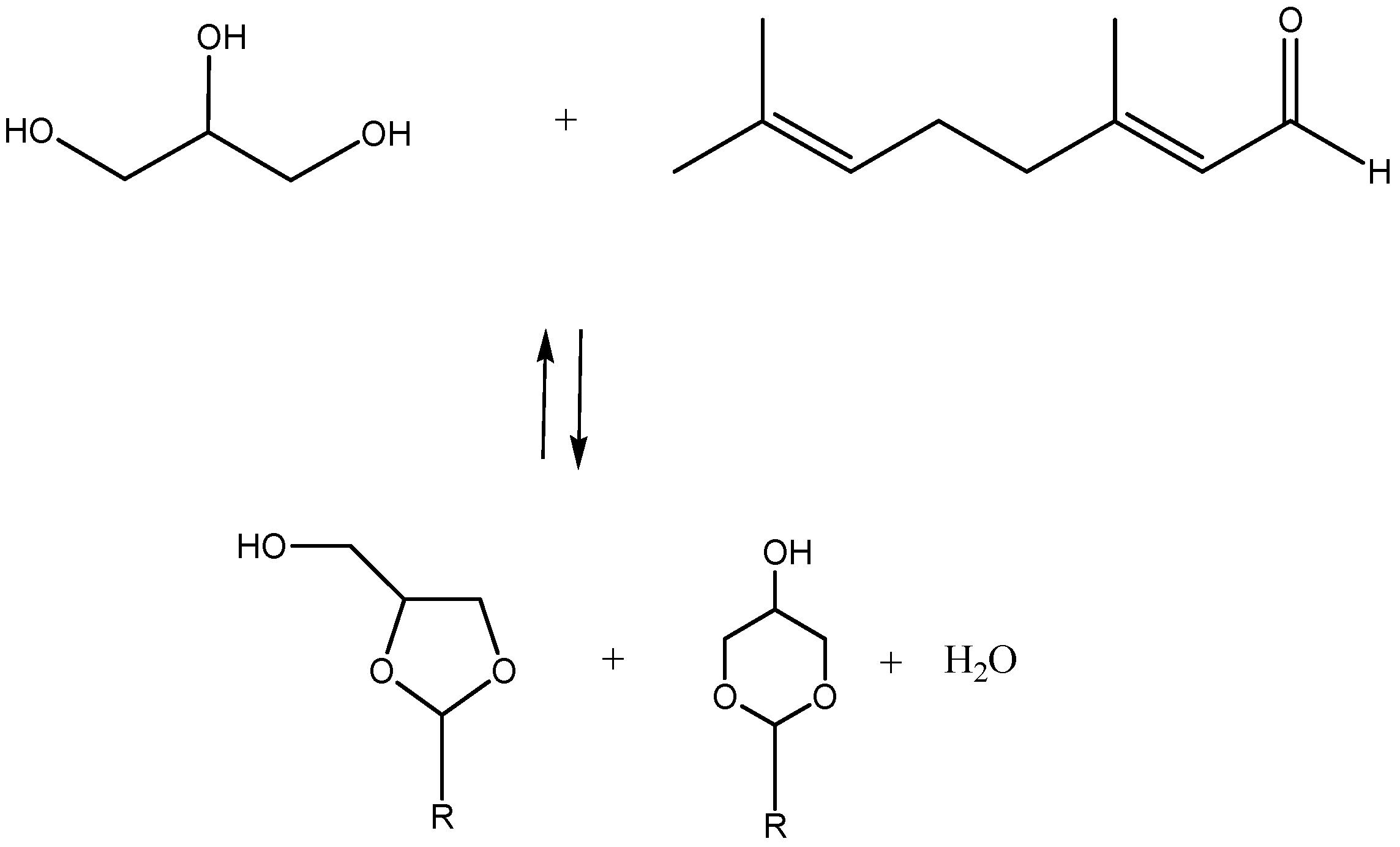
1. Introduction
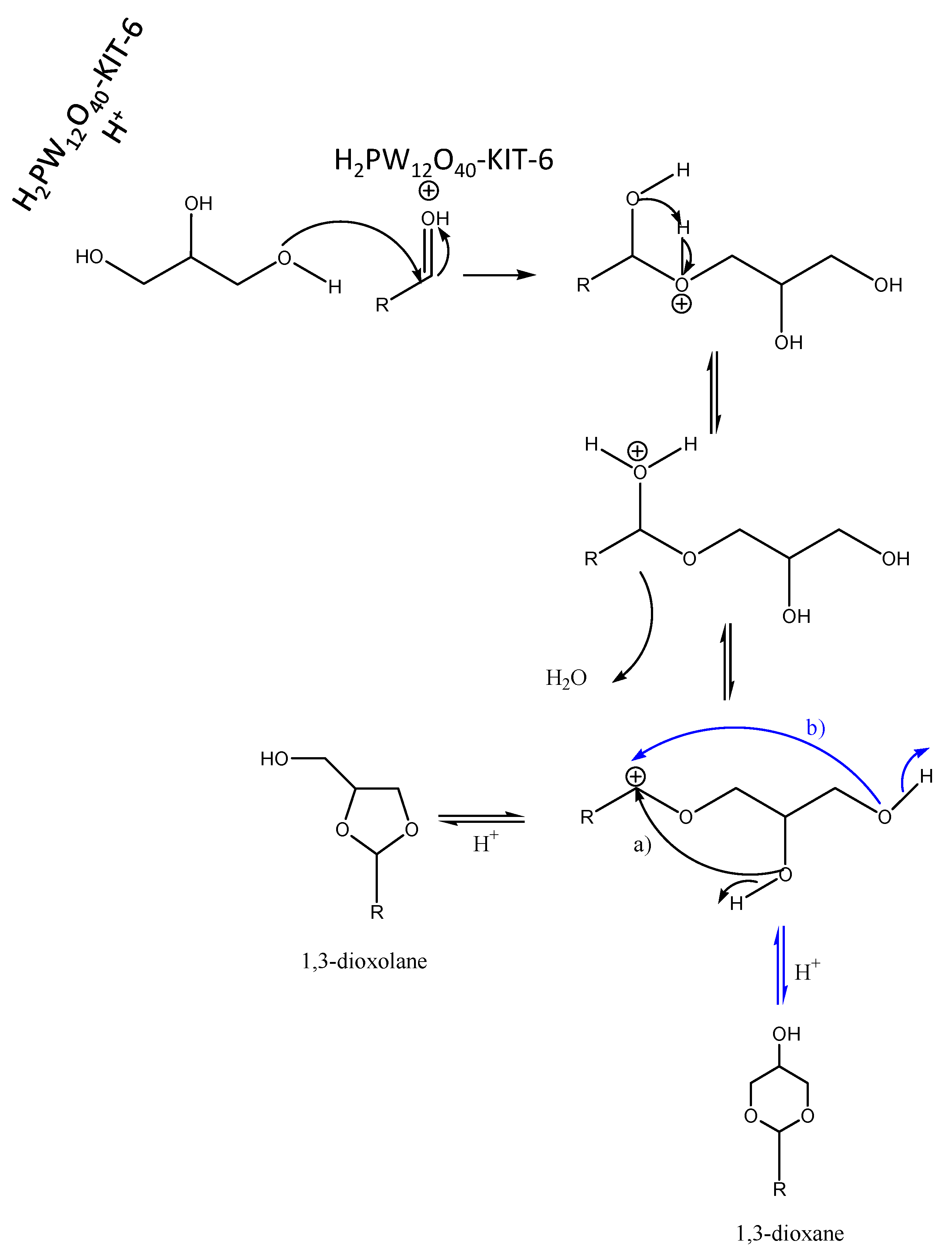
2. Results and Discussion
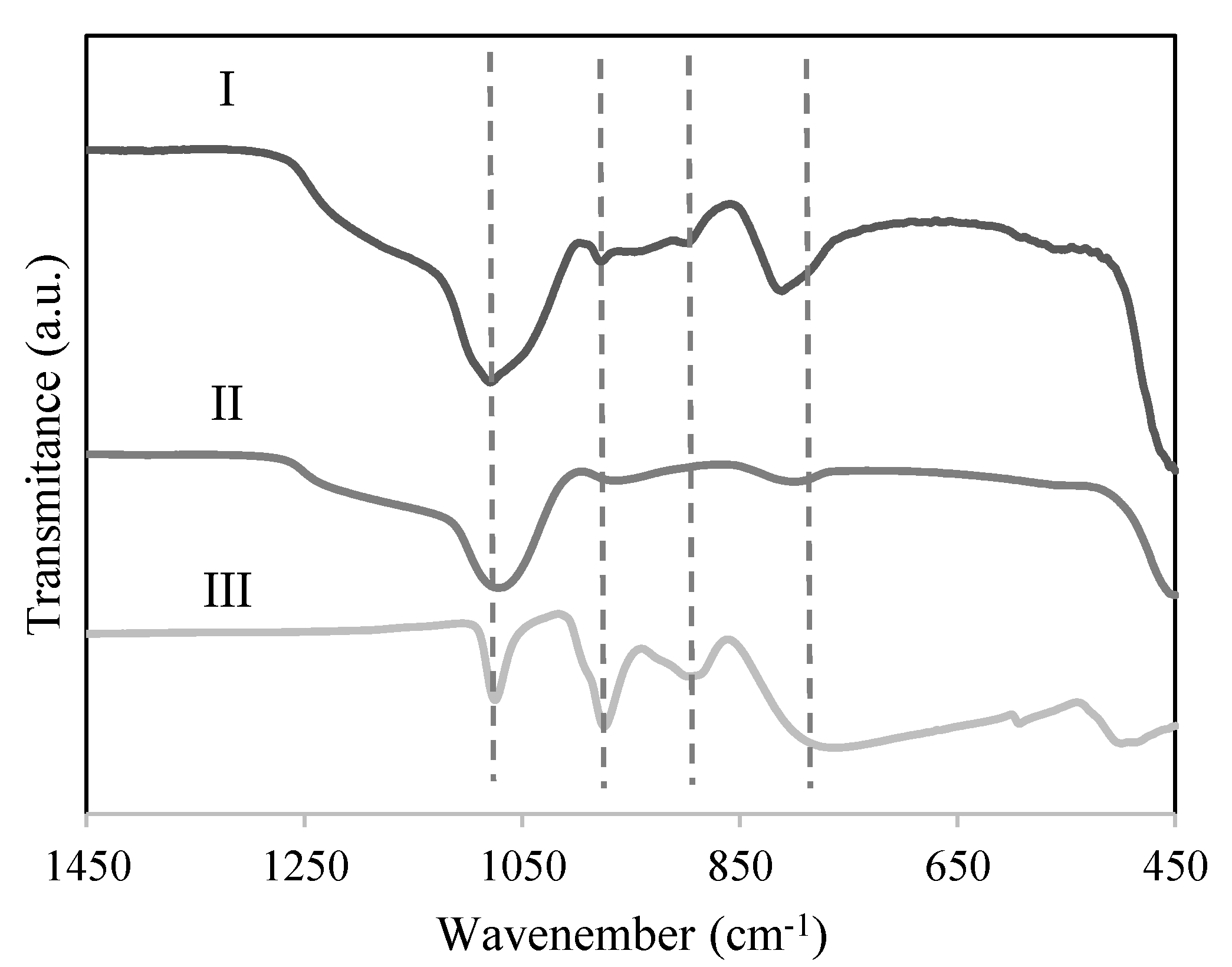
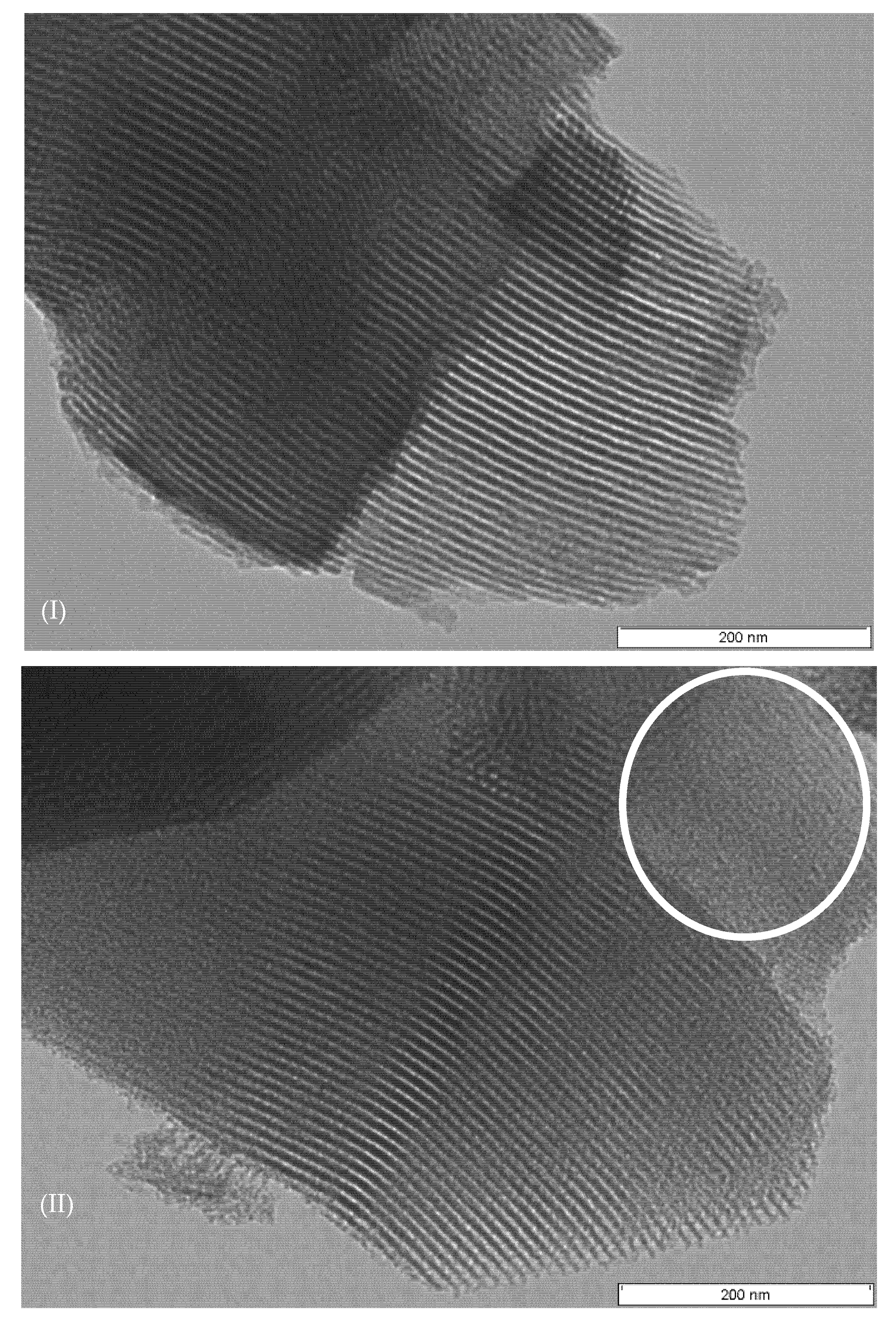
2.1. Catalyst Characterization
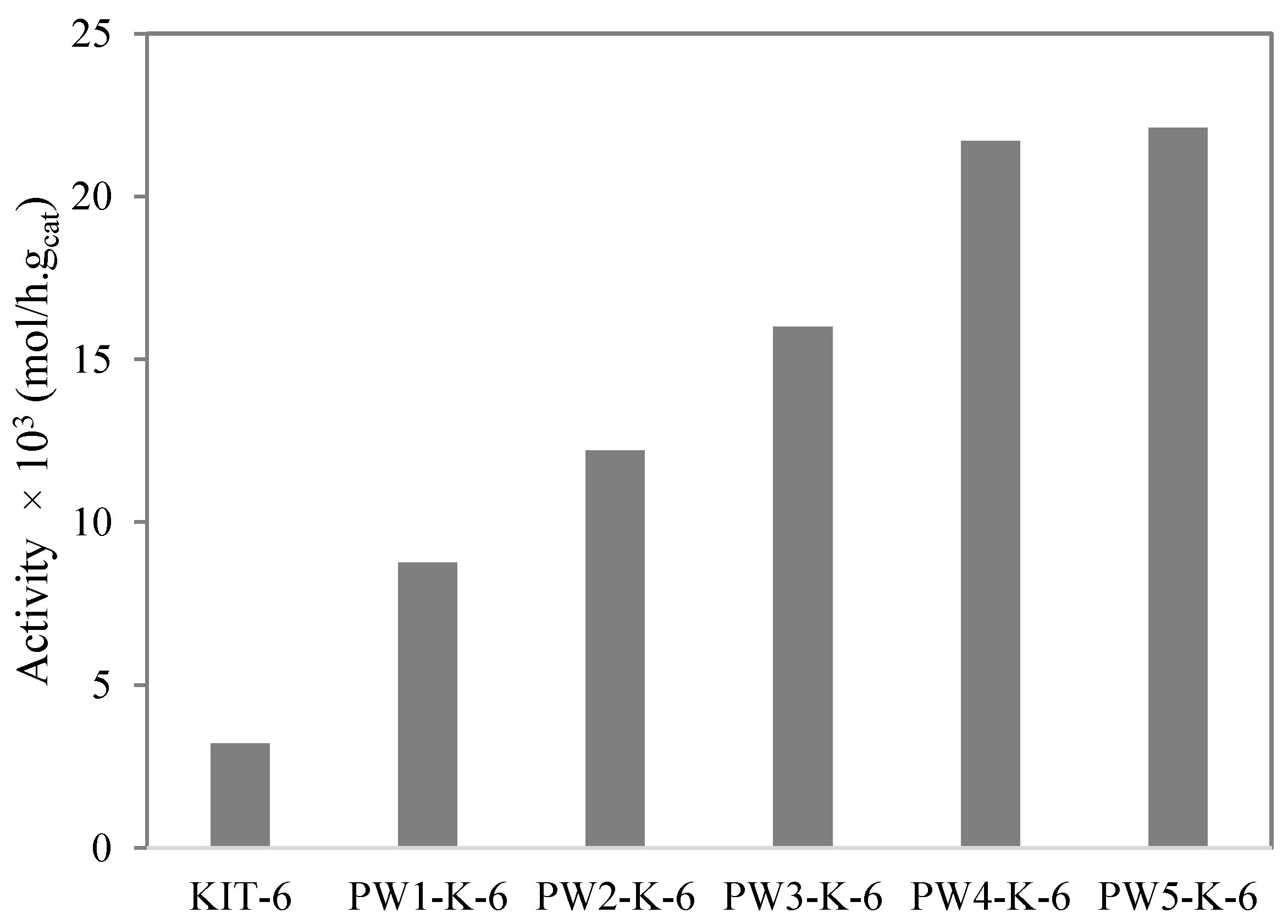
2.2. Catalytic Experiments
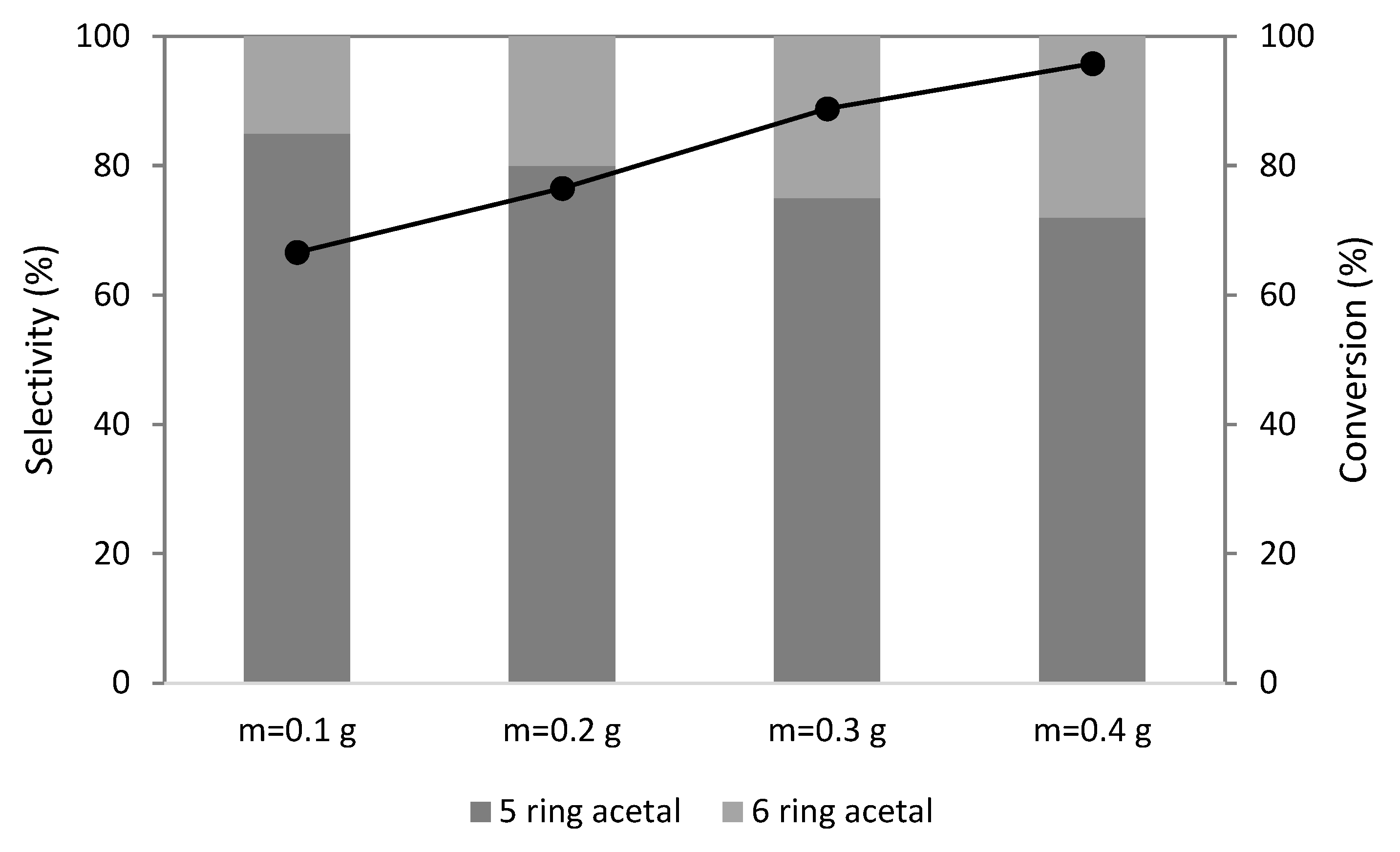
2.3. Catalyst Amount
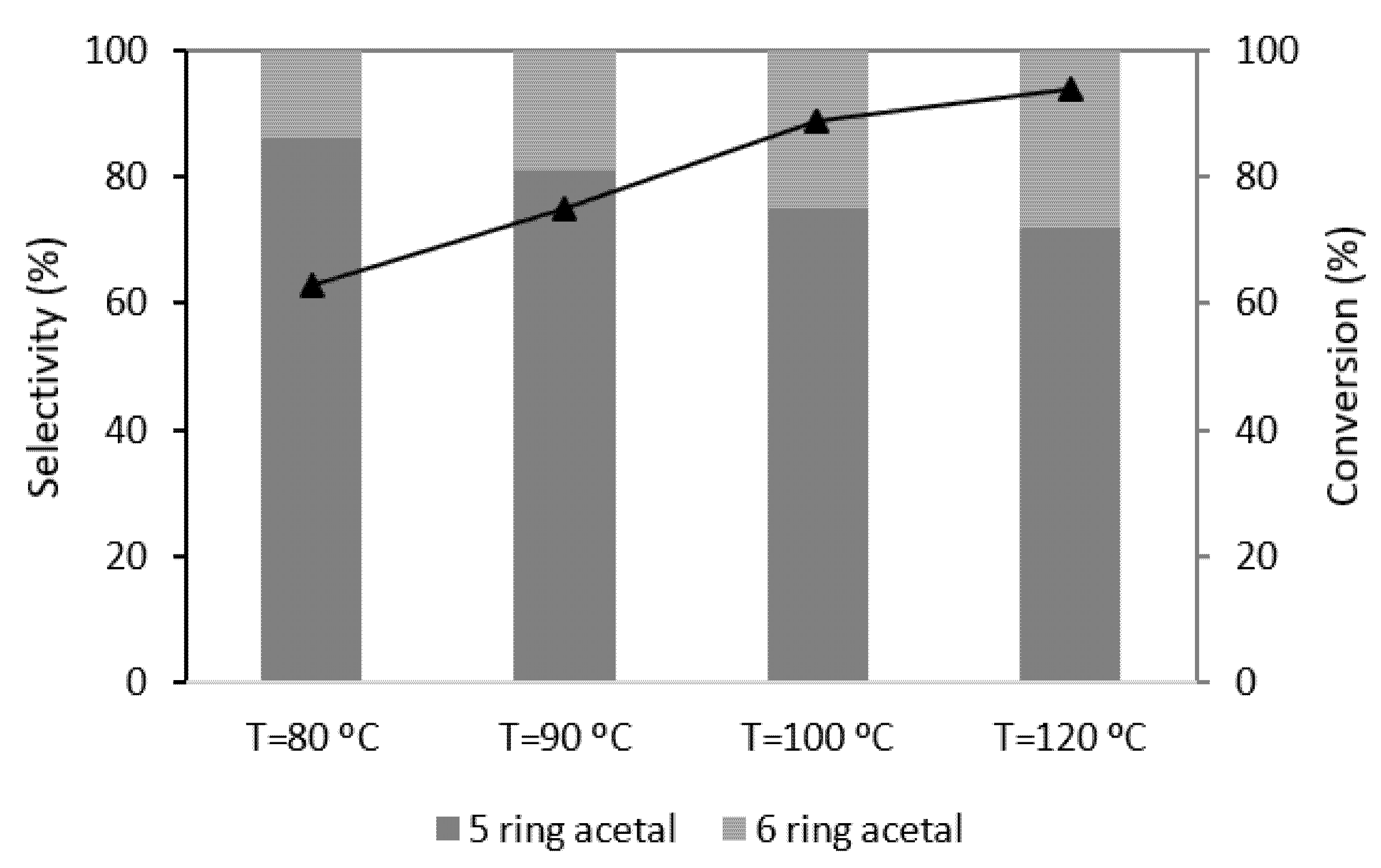
2.4. Temperature
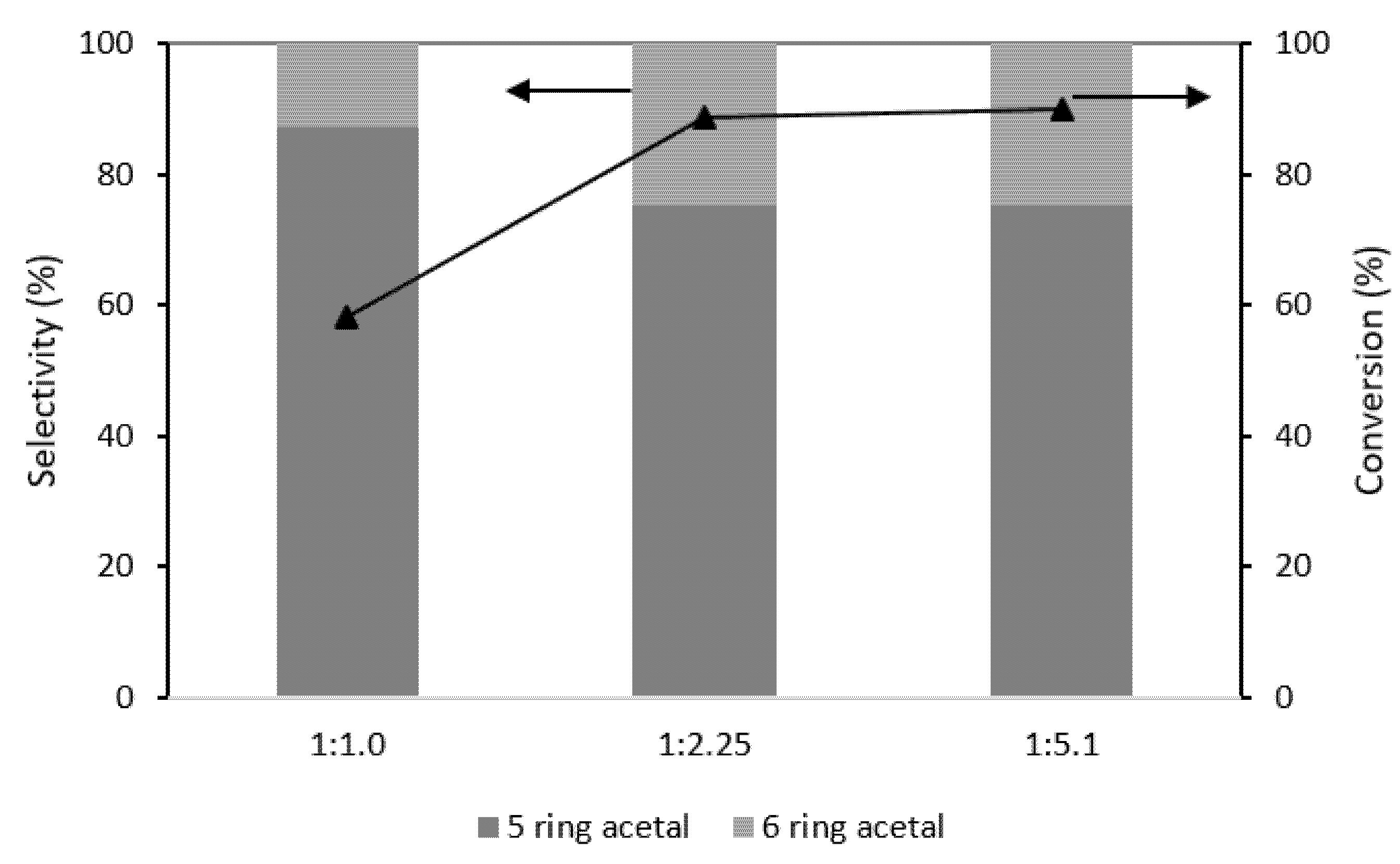
2.5. Glycerol: Citral Molar Ratio
3. Materials and Methods
3.1. Materials
3.2. Preparation of Catalysts
3.3. Materials Characterization
3.4. Catalytic Experiments
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alsultan, A.G.; Asikin-Mijan, N.; Ibrahim, Z.; Yunus, R.; Razali, S.Z.; Mansir, N.; Islam, A.; Seenivasagam, S.; Taufiq-Yap, Y.H. A Short Review on Catalyst, Feedstock, Modernised Process, Current State and Challenges on Biodiesel Production. Catalysts 2021, 11, 1261. [Google Scholar] [CrossRef]
- Avhad, M.R.; Marchetti, J.M. Innovation in solid heterogeneous catalysis for the generation of economically viable and ecofriendly biodiesel: A review. Catal. Rev. 2016, 58, 157–208. [Google Scholar] [CrossRef]
- Lotero, E.; Liu, Y.; Lopez, D.E.; Suwannakarn, K.; Bruce, D.A.; Goodwin, J.G. Synthesis of biodiesel via acid catalysis. Ind. Eng. Chem. Res. 2005, 44, 5353–5363. [Google Scholar] [CrossRef]
- Avhad, M.R.; Marchettin, J.M. A review on recent advancement in catalytic materials for biodiesel production. Renew. Sust. Energ. Rev. 2015, 50, 696–718. [Google Scholar] [CrossRef]
- Singh, S.; Patel, A. 12-Tungstophosphoric acid supported on mesoporous molecular material: Synthesis, characterization and performance in biodiesel production. J. Clean. Prod. 2014, 72, 46–56. [Google Scholar] [CrossRef]
- Castanheiro, J.E.; Fonseca, I.M.; Ramos, A.M.; Vital, J. Tungstophosphoric acid immobilised in SBA-15 as an efficient heterogeneous acid catalyst for the conversion of terpenes and free fatty acids. Micropor. Mesopor. Mat. 2017, 249, 16–24. [Google Scholar] [CrossRef]
- Smirnov, A.A.; Selishcheva, S.A.; Yakovlev, V.A. Acetalization catalysts for synthesis of valuable oxygenated fuel additives from glycerol. Catalysts 2018, 8, 595. [Google Scholar] [CrossRef]
- Natalia, B.; Thomas, R.; Martin, K.; Stephan, A.S. Valorisation of Glycerol as Renewable Feedstock: Comparison of the Exploration of Chemical Transformation Methods Aided by High Throughput Experimentation. Comb. Chem. High. Throughput Screen. 2012, 15, 123–135. [Google Scholar]
- Rodrigues, A.; Bordado, J.C.; dos Santos, R.G. Upgrading the Glycerol from Biodiesel Production as a Source of Energy Carriers and Chemicals—A Technological Review for Three Chemical Pathways. Energies 2017, 10, 1817. [Google Scholar] [CrossRef]
- Rahmat, N.; Abdullah, A.Z.; Mohamed, A.R. Recent progress on innovative and potential technologies for glycerol transformation into fuel additives: A critical review. Renew. Sustain. Energy Rev. 2010, 14, 987–1000. [Google Scholar] [CrossRef]
- Checa, M.; Nogales-Delgado, S.; Montes, V.; Encinar, J.M. Recent Advances in Glycerol Catalytic Valorization: A Review. Catalysts 2020, 10, 1279. [Google Scholar] [CrossRef]
- Mallesham, B.; Sudarsanam, P.; Reddy, B.M. Eco-friendly synthesis of bio-additive fuels from renewable glycerol using nanocrystalline SnO2-based solid acids. Catal. Sci. Technol. 2014, 4, 803–881. [Google Scholar] [CrossRef]
- Kundu, S.K.; Singuru, R.; Hayashi, T.; Hijikata, Y.; Irle, S.; Mondal, J. Constructing sulfonic acid functionalized anthracene derived conjugated porous organic polymer for efficient metal-free catalytic acetalization of bio-Glycerol. ChemistrySelect 2017, 2, 4705–4716. [Google Scholar] [CrossRef]
- Konwar, L.J.; Samikannu, A.; Mäki-Arvela, P.; Boström, D.; Mikkola, J.-P. Lignosulfonate-based macro/mesoporous solid protonic acids for acetalization of glycerol to bio-additives. Appl. Catal. B Environ. 2018, 220, 314–323. [Google Scholar] [CrossRef]
- Gonzalez-Arellano, C.; Arancon, R.A.D.; Luque, R. Al-SBA-15 catalysed cross-esterification and acetalisation of biomass-derived platform chemicals. Green Chem. 2014, 16, 4985–4993. [Google Scholar] [CrossRef]
- Serafim, H.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Valorization of glycerol into fuel additives over zeolites as catalysts. Chem. Eng. J. 2011, 178, 291–296. [Google Scholar] [CrossRef]
- Silva, P.H.R.; Gonçalves, V.L.C.; Mota, C.J.A. Glycerol acetals as anti-freezing additives for biodiesel. Bioresour. Technol. 2010, 101, 6225–6229. [Google Scholar] [CrossRef]
- Antunes, M.M.; Mendes, R.F.; Paz, F.A.A.; Valente, A.A. Versatile Coordination Polymer Catalyst for Acid Reactions Involving Biobased Heterocyclic Chemicals. Catalysts 2021, 11, 190. [Google Scholar] [CrossRef]
- Castanheiro, J.E.; Vital, J.; Fonseca, I.M.; Ramos, A.M. Acetalization of glycerol with hexanal in the presence of SBA-15 with sulfonic acid groups. Catal. Today 2022, 484–386, 2–11. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Velty, A. Synthesis of hyacinth, vanilla, and blossom orange fragrances: The benefit of using zeolites and delaminated zeolites as catalysts. Appl. Catal. A Gen. 2004, 263, 155–161. [Google Scholar] [CrossRef]
- Castanheiro, J.E.; Vital, J.; Fonseca, I.M.; Ramos, A.M. Glycerol conversion into biofuel additives by acetalization with pentanal over heteropolyacids immobilized on zeolites. Catal. Today 2020, 346, 76–80. [Google Scholar] [CrossRef]
- Deutsch, J.; Martin, A.; Lieske, H. Investigations on heterogeneously catalysed condensations of glycerol to cyclic acetals. J. Catal. 2007, 245, 428–435. [Google Scholar] [CrossRef]
- Lopes, N.F.; Caiado, M.; Canhao, P.; Castanheiro, J.E. Synthesis of bio-fuel additives from glycerol over poly(vinyl Alcohol) with sulfonic acid groups. Energy Sources A: Recovery Util. Environ. Eff. 2015, 37, 1928–1936. [Google Scholar] [CrossRef][Green Version]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Moteki, T.; Gokhale, A.A.; Flaherty, D.W.; Toste, F.D. Production of fuels and chemicals from biomass: Condensation reactions and beyond. Chem 2016, 1, 32–58. [Google Scholar] [CrossRef]
- Bruneau, C.; Fischmeister, C.; Mandelli, D.; Carvalho, W.A.; dos Santos, E.N.; Dixneuf, P.H.; Fernandes, L.S. Transformations of terpenes and terpenoids via carbon-carbon double bond metathesis. Catal. Sci. Technol. 2018, 8, 3989–4004. [Google Scholar] [CrossRef]
- Vannucci, J.A.; Nichio, N.N.; Pompeo, F. Solketal synthesis from ketalization of glycerol with acetone: A kinetic study over a sulfated zirconia catalyst. Catal. Today 2021, 372, 238–245. [Google Scholar] [CrossRef]
- Melero, J.A.; van Grieken, R.; Morales, G.; Paniagua, M. Acidic Mesoporous Silica for the Acetylation of Glycerol: Synthesis of Bioadditives to Petrol Fuel. Energy Fuel 2007, 21, 1782–1791. [Google Scholar] [CrossRef]
- Narkhede, N.; Patel, A. Room temperature acetalization of glycerol to cyclic acetals over anchored silicotungstates under solvent free conditions. RSC Adv. 2014, 4, 19294–19301. [Google Scholar] [CrossRef]
- Akinnawo, C.A.; Mosia, L.; Alimi, O.A.; Oseghale, C.O.; Fapojuwo, D.P.; Bingwa, N.; Meijboom, R. Eco-friendly synthesis of valuable fuel bio-additives from glycerol. Catal. Commun. 2021, 152, 106287. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V. Catalysis by Heteropoly Acids and Multicomponent Polyoxometalates in Liquid-Phase Reactions. Chem. Rev. 1998, 98, 171–198. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Thomas, H.J.; Climent, M.J.; Romanelli, G.P.; Iborra, S. Heteropolycompounds as catalysts for biomass product transformations. Catal. Rev. 2016, 58, 497–586. [Google Scholar] [CrossRef]
- Patel, A.; Narkhede, N.; Singh, S.; Pathan, S. Keggin-type lacunary and transition metal substituted polyoxometalates as heterogeneous catalysts: A recent progress. Catal. Rev. 2016, 58, 337–370. [Google Scholar] [CrossRef]
- Guo, Y.; Li, K.; Yu, X.; Clark, J.H. Mesoporous H3PW12O40-silica composite: Efficient and reusable solid acid catalyst for the synthesis of diphenolic acid from levulinic acid. Appl. Catal. B Env. 2008, 81, 182–191. [Google Scholar] [CrossRef]
- Gagea, B.C.; Lorgouilloux, Y.; Altintas, Y.; Jacobs, P.A.; Martens, J.A. Bifunctional conversion of n-decane over HPW heteropoly acid incorporated into SBA-15 during synthesis. J. Catal. 2009, 265, 99–108. [Google Scholar] [CrossRef]
- Chai, S.-H.; Wang, H.-P.; Liang, Y.; Xu, B.-Q. Sustainable production of acrolein: Gas-phase dehydration of glycerol over 12-tungstophosphoric acid supported on ZrO2 and SiO2. Green Chem. 2008, 10, 1087–1093. [Google Scholar] [CrossRef]
- Chen, L.; Nohair, B.; Zhao, D.; Kaliaguine, S. Glycerol acetalization with formaldehyde using heteropolyacid salts supported on mesostructured silica. Appl. Catal. A Gen. 2018, 549, 207–215. [Google Scholar] [CrossRef]
- Pizzio, L.R.; Vásquez, P.G.; Cáceres, C.V.; Blanco, M.N. Supported Keggin type heteropolycompounds for ecofriendly reactions. Appl. Catal. A Gen. 2003, 256, 125–139. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, J.; Liao, M.; Li, J.; Zhang, L.; Guo, J.; Wu, H. Deep oxidative desulfurization of dibenzothiophene by novel POM-based IL immobilized on well-ordered KIT-6. Chem. Eng. J. 2021, 418, 129470. [Google Scholar] [CrossRef]
- Sheng, X.; Kong, J.; Zhou, Y.; Zhang, Y.; Zhang, Z.; Zhou, S. Direct synthesis, characterization and catalytic application of SBA-15 mesoporous silica with heteropolyacid incorporated into their framework. Micropor. Mesopor. Mater. 2014, 187, 7–13. [Google Scholar] [CrossRef]
- Patel, A.; Pithadia, D. Low temperature synthesis of bio-fuel additives via valorization of glycerol with benzaldehyde as well as furfural over a novel sustainable catalyst, 12-tungstosilicic acid anchored to ordered cubic nano-porous MCM-48. Appl. Catal. A Gen. 2020, 602, 117729. [Google Scholar] [CrossRef]
- Wang, B.; Shen, Y.; Sun, J.; Xu, F.; Sun, R. Conversion of platform chemical glycerol to cyclic acetals promoted by acidic ionic liquids. RSC Adv. 2014, 36, 18917–18923. [Google Scholar] [CrossRef]
- Aksnes, G.; Albriktsen, P.; Juvvik, P.; Thelin, H.; Sjoberg, B.; Larsen, E. Studies of Cyclic Acetal and Ketal Isomers of Glycerol. Acta Chem. Scand. 1965, 19, 920–930. [Google Scholar] [CrossRef]
- Kulkarni, R.M.; Arvind, N. Acetalization of glycerol and benzaldehyde to synthesize biofuel additives using SO42−/CeO2–ZrO2 catalyst. Heliyon 2021, 7, e06018. [Google Scholar] [CrossRef]
- Li, R.; Song, H.; Chen, J. Propylsulfonic acid functionalized SBA-15 mesoporous silica as efficient catalysts for the acetalization of glycerol. Catalysts 2018, 8, 297. [Google Scholar] [CrossRef]
- Pirez, C.; Caderon, J.-M.; Dacquin, J.-P.; Lee, A.F.; Wilson, K. Tunable KIT-6 Mesoporous Sulfonic Acid Catalysts for Fatty Acid Esterification. ACS Catal. 2012, 2, 1607–1614. [Google Scholar] [CrossRef]
 ) PW2-K-6; (×) PW3-K-6; (☐) PW4-K-6; (+) PW5-K-6.
) PW2-K-6; (×) PW3-K-6; (☐) PW4-K-6; (+) PW5-K-6.
 ) PW2-K-6; (×) PW3-K-6; (☐) PW4-K-6; (+) PW5-K-6.
) PW2-K-6; (×) PW3-K-6; (☐) PW4-K-6; (+) PW5-K-6.

 ) PW4-K-6; (●) PW5-K-6.
) PW4-K-6; (●) PW5-K-6.
 ) PW4-K-6; (●) PW5-K-6.
) PW4-K-6; (●) PW5-K-6.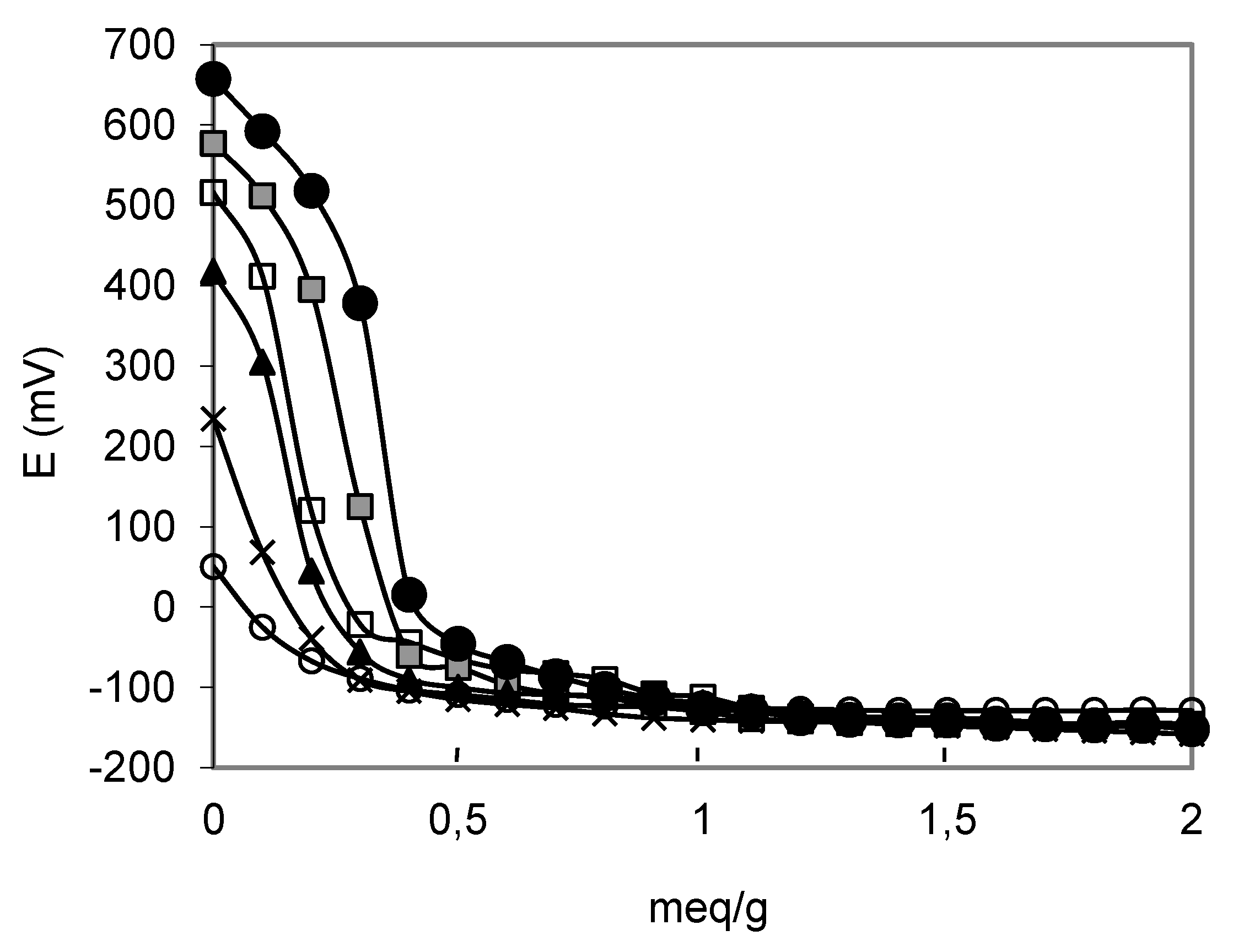
 ) PW3-K-6; (▲) PW4-K-6; (☐) PW5-K-6.
) PW3-K-6; (▲) PW4-K-6; (☐) PW5-K-6.
 ) PW3-K-6; (▲) PW4-K-6; (☐) PW5-K-6.
) PW3-K-6; (▲) PW4-K-6; (☐) PW5-K-6.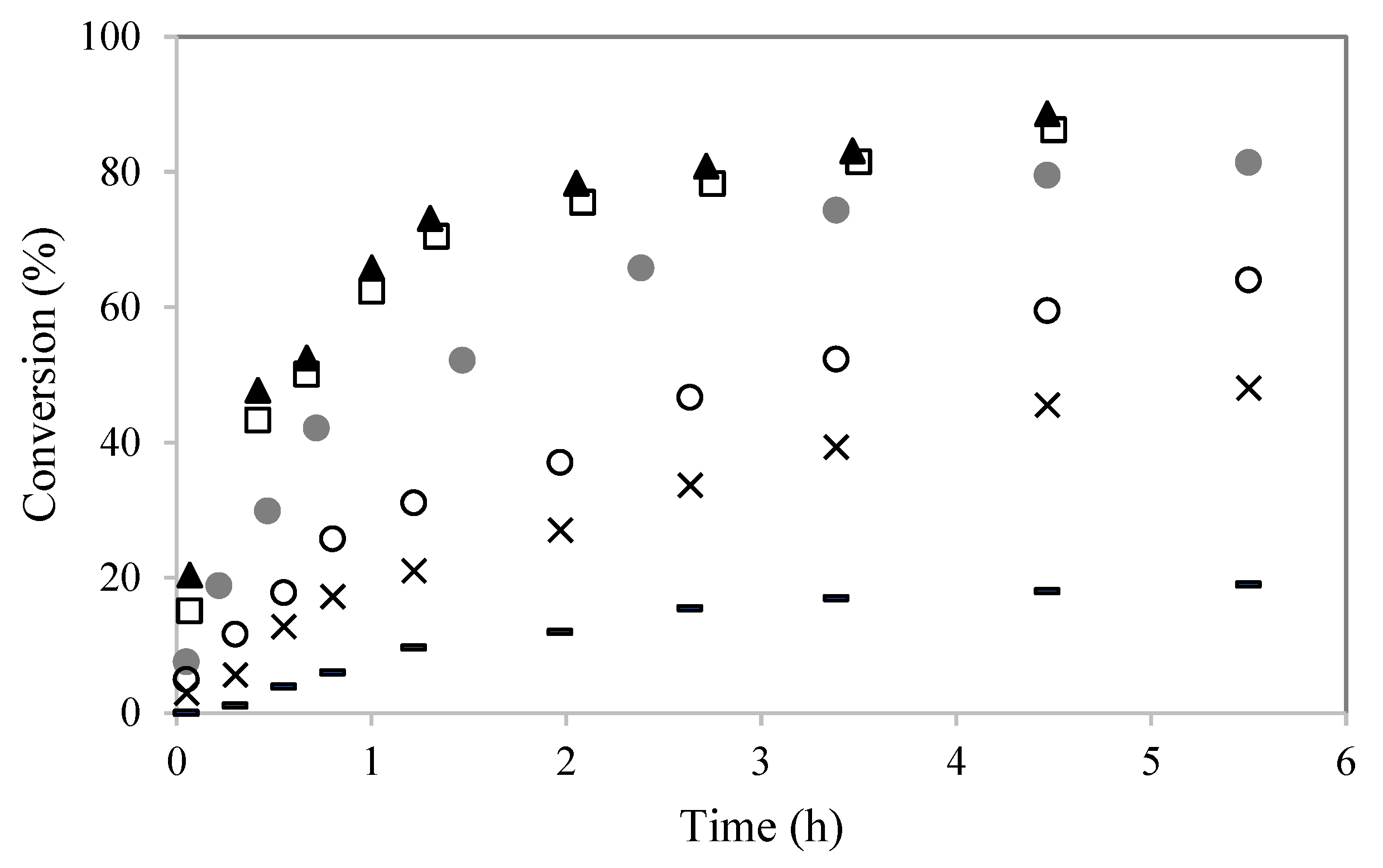

| Materials | HPW Amount (wt%) | ABET (m2/g) | VT a (cm3/g) |
|---|---|---|---|
| KIT-6 | - | 780 | 0.98 |
| PW1-K-6 | 1.5 | 765 | 0.92 |
| PW2-K-6 | 5.5 | 743 | 0.89 |
| PW3-K-6 | 8.1 | 724 | 0.86 |
| PW4-K-6 | 10.5 | 702 | 0.83 |
| PW5-K-6 | 15.6 | 692 | 0.81 |
| Sample | Conversion 1 (%) | Selectivity (%) | |
|---|---|---|---|
| 5R Acetal | 6R Acetal | ||
| KIT-6 | 18 | 85 | 15 |
| PW1-K-6 | 46 | 83 | 17 |
| PW2-K-6 | 60 | 82 | 16 |
| PW3-K-6 | 80 | 79 | 21 |
| PW4-K-6 | 89 | 75 | 25 |
| PW5-K-6 | 86 | 75 | 25 |
| Sample | Conversion 1 (%) | Selectivity (%) | |
|---|---|---|---|
| 5R Acetal | 6R Acetal | ||
| 1st utilization | 89 | 75 | 25 |
| 2nd utilization | 87 | 76 | 24 |
| 3rd utilization | 88 | 76 | 24 |
| 4th utilization | 87 | 76 | 24 |
| 5th utilization | 88 | 76 | 24 |
| Catalyst | 5R Acetal | 6R Acetal | Conversion (%) | Activity (h−1) | Reference |
|---|---|---|---|---|---|
| ZrO2-350 a | 29 | 46 | 64 | 5.2 | [30] |
| PW4-K-6 b | 75 | 25 | 89 | 596 | Present work |
| PW4-SBA-15 b,c | 76 | 24 | 86 | 489 | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castanheiro, J. Acetalization of Glycerol with Citral over Heteropolyacids Immobilized on KIT-6. Catalysts 2022, 12, 81. https://doi.org/10.3390/catal12010081
Castanheiro J. Acetalization of Glycerol with Citral over Heteropolyacids Immobilized on KIT-6. Catalysts. 2022; 12(1):81. https://doi.org/10.3390/catal12010081
Chicago/Turabian StyleCastanheiro, José. 2022. "Acetalization of Glycerol with Citral over Heteropolyacids Immobilized on KIT-6" Catalysts 12, no. 1: 81. https://doi.org/10.3390/catal12010081
APA StyleCastanheiro, J. (2022). Acetalization of Glycerol with Citral over Heteropolyacids Immobilized on KIT-6. Catalysts, 12(1), 81. https://doi.org/10.3390/catal12010081






