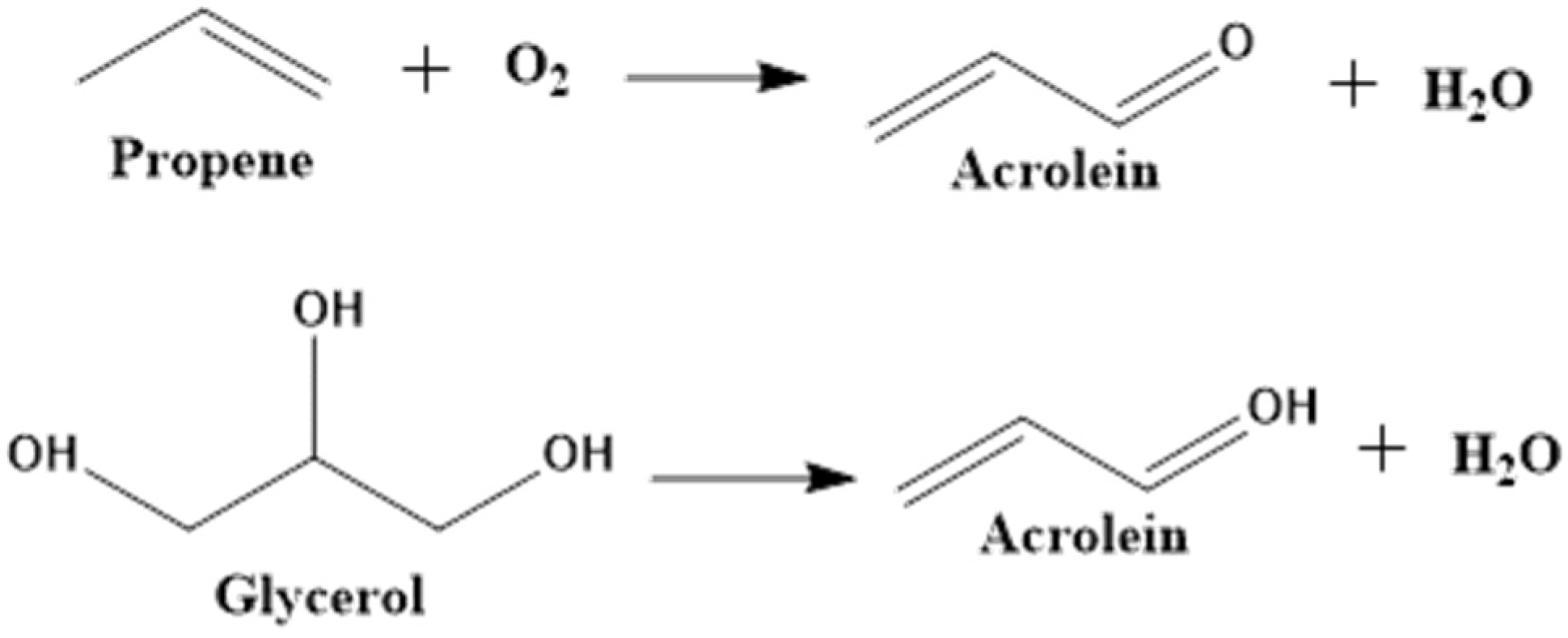
3.1. Dehydration of Glycerol to Acrolein
Acrolein (also called propenal), the simplest unsaturated aldehyde, is primarily used either as a biocide in drilling water or irrigation canals to control weed and algae or as a precursor to synthesize other chemicals such as acrylic acid, glutaraldehyde, and methionine [
86]. In industry, acrolein is prepared by the gas phase partial oxidation of propene over a Bi/Mo-mixed oxide catalyst. Alternatively, glycerol can be used as the feedstock to prepare acrolein via catalytic dehydration in either the gas or liquid phase. The catalysts used in the catalytic dehydration of glycerol for the production of acrolein include supported zeolites [
88], heteropoly acids (HPAs) [
89], mixed metal oxides [
90], phosphates [
91], and pyrophosphates [
91]. The reaction pathways for industrial acrolein preparation and glycerol to acrolein are depicted in
Figure 7. For comparison, the dehydration of glycerol in gas phase offers advantages over dehydration of glycerol in the liquid phase in terms of the ease of products’ separation and a higher acrolein yield [
92]. The recent studies on catalytic dehydration of glycerol for producing acrolein are summarized in
Table 4.
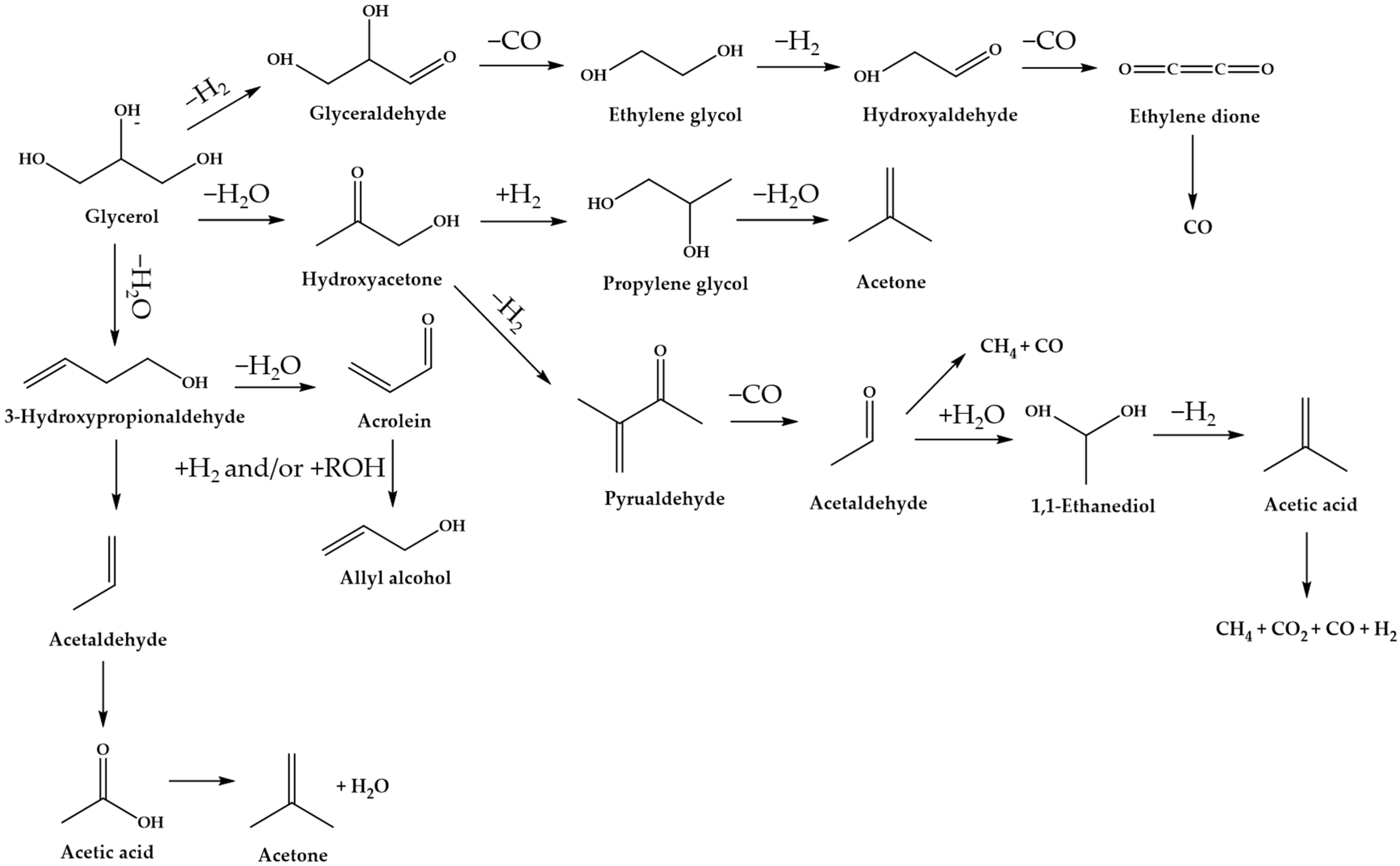
Typically, the reactions of solid acid catalysts involved in the dehydration of glycerol include (i) the formation of acetol on the Lewis acid sites, and (ii) the formation of acrolein on Brønsted acid sites (
Figure 8) [
9].
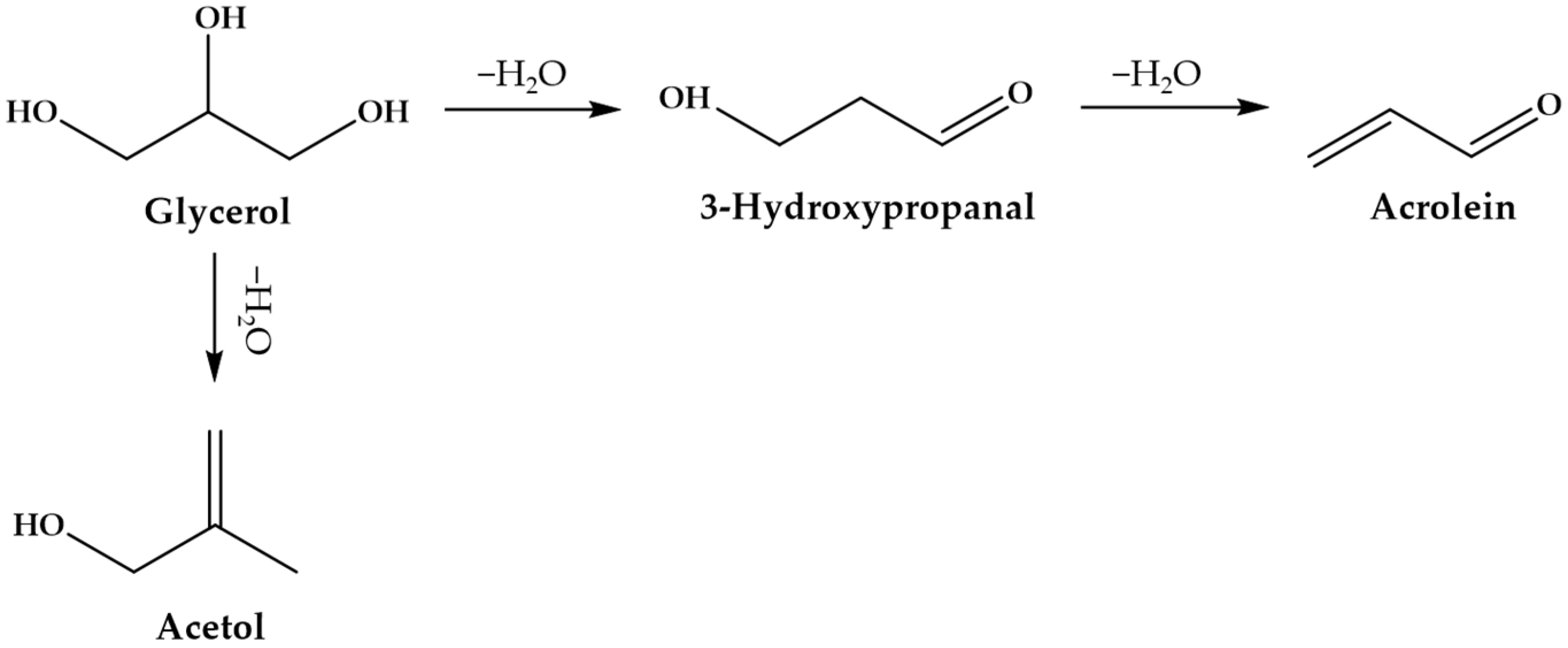
Figure 3 shows that glycerol dehydration over a solid acid catalyst often results in the formation of 3-hydroxypropanal, which is accompanied by acetol formation as the by-product. The obtained 3-hydroxypropanal further proceeds with the dehydration reaction to form acrolein. Particularly, as suggested by Chai et al. [
108], Brønsted acid sites are favorable for producing acrolein, and the formation of acetol is promoted by Lewis acid sites. In the presence of strong Brønsted acid sites, coke deposition might occur on the catalyst surface, thus leading to catalyst deactivation [
109]. To reuse the spent catalyst, catalyst regeneration by burning off the coke can be carried out either continuously in situ or periodically ex situ [
110]. Thus, the amount and strength of the acidic sites are essential to the catalytic dehydration of glycerol to produce acrolein with respect to unwanted side reactions and coke formation. For example, Viswanadham et al. [
96] applied Keggin-type vanadium-containing phosphomolybdic acid (H
4PMo
11VO
40) supported on a mesoporous molecular sieve (i.e., MCM-
41) in glycerol dehydration, and the results showed that acrolein selectivity was proportional to the loading of H
4PMo
11VO
40 up to 40 wt % and then decreased with further increases in H
4PMo
11VO
40 loading. Based on the results from temperature-programmed desorption of ammonia (TPD-NH
3), it was found that the concentration of H
4PMo
11VO
40 is related to the acidity of the catalyst. Similar results were reported by Ginjupalli et al. [
95], where the influence of the acidic strength of a range of tungsten oxide supported on metal phosphate catalysts on the catalytic performance, reaction variables, and reactant functionalities during the gas phase glycerol dehydration for acrolein production was evaluated. In addition to the type, amount, and strength of the acidic sites, the pore size of the catalyst is another important operational variable affecting the catalytic dehydration of glycerol. The porosity and distance of internal channels of the catalyst play a significant role in the diffusion and the adsorption–desorption of the molecules, thereby affecting coke formation on the catalyst surface. Ali et al. [
100] evaluated the influence of
b-axis channel length (i.e., 60–250 nm) of HZSM-5 zeolites on the acrolein selectivity and coke formation obtained from glycerol dehydration, and the results showed that the HZSM-5 catalyst with a
b-axis channel length of 60 nm led to the highest glycerol conversion (100%) and acrolein selectivity (88%). The shortest channel length could lead to a high availability of active sites and improved diffusion, which, in turn, drastically limit coke formation. In terms of the effect of pore size, Zhang et al. [
111] observed that hierarchical-structured zeolites with diverse meso-porosity demonstrated better stability and acrolein selectivity compared with conventional zeolites with sole microporous structure. Additionally, the presence of mesopores in the zeolites is helpful for making the catalyst more tolerant to coke formation, particularly in the case of an open and interconnected mesopore architecture. As suggested by the Kelvin equation (Equation (4)), catalyst deactivation might be caused by pore condensation, and a greater degree of pore condensation can be observed in the smaller mesoporous structures [
112].
where
Pc and
P0 are the pressure at which pore condensation occurs and the saturated vapor pressure, respectively;
T and
R represent the absolute temperature and gas constant, respectively;
σVL is the vapor–liquid surface tension;
θ represents the contact angle;
nL and
nV are the molar density of the bulk liquid and vapor phase, respectively; and
H is the pore width.
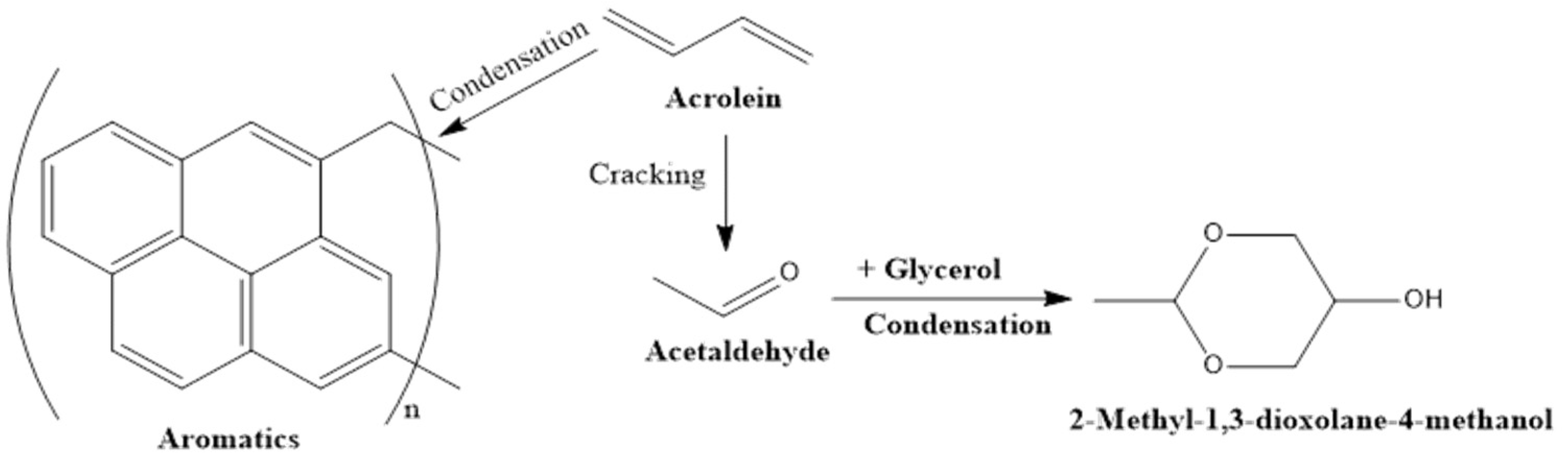
Undeniably, coke formation and the associated catalyst deactivation are the major challenges faced by the catalytic dehydration of glycerol for acrolein production. The possible reaction pathways for coke formation during glycerol conversion to acrolein are shown in
Figure 9. In addition to the modification of the pore size of the catalyst, as earlier discussed in this section, doping the catalyst with noble metals (e.g., Ru, Pt, or Pd) is another solution [
113]. Doping Ru, Pd, or Pt into the catalyst together with H
2 addition are effective in preventing coke formation through the hydrogenation of the coke precursors, consequently extending the catalyst’s lifetime [
113]. Trakarnpruk [
114] reported that Pt doping in H
3PW
12O
40/Zr-MCM-
41 catalyst was capable of suppressing coke formation and dramatically enhancing catalyst stability. In another study, Ma et al. [
115] synthesized and tested H
3PW
12O
40/MCM-
41, H
3PW
12O
40/Zr-MCM-
41, and Pd-H
3PW
12O
40/Zr-MCM-
41 in the catalytic dehydration of glycerol for acrolein production, and the results indicated that Pd doping did not alter the mesoporous structure of the catalyst but decreased the specific surface area, pore volume, and pore size. Although no significant change was observed in the total acidity of the catalyst, the amount of Brønsted acid sites increased with a decrease in the amount of Lewis acid sites. Additionally, the use of Pd-H
3PW
12O
40/Zr-MCM-
41 led to the highest glycerol conversion of 94% and acrolein selectivity of 85%, which were accompanied by higher catalyst stability over 50 h of reaction compared to H
3PW
12O
40/Zr-MCM-
41. The third approach to mitigate coke formation is co-feeding oxygen or air through the oxidation of coke precursors to form CO and CO
2. Nadji et al. [
90], for example, studied the effect of O
2 on acrolein production from glycerol. When co-feeding O
2, the glycerol conversion and acrolein selectivity were 100% and 85%, respectively, during 8 h of reaction. Conversely, a significant decrease in the glycerol conversion from 99% to 55% was found after 8 h of reaction, along with a relatively lower acrolein selectivity ranging from 70% to 85%. Based on the results, the authors speculated that co-feeding O
2 in the glycerol dehydration is not only helpful for preventing the formation of coke but also suppressing acetol formation. Similar results were observed by Dalil et al. [
116], where the dehydration of glycerol was performed over WO
3/TiO
2 in 10 mol % O
2/Ar at 280 °C. However, the presence of O
2 promotes the formation of carboxylic acids (e.g., formic acid, acetic acid, and acrylic acid), which could be attributed to the oxidation of aldehydes [
5]. Recently, Xie et al. [
99] used microwave heating in the catalytic glycerol dehydration to prevent coke formation. Their newly designed microwave system consists of feedstock storage, peristaltic pump, preheater, quartz reactor, catalyst bed, microwave oven, infrared irradiation thermometer, temperature controller, liquid product storage, and water seal. The authors reported that the glycerol conversion (83.8%) and acrolein selectivity (53.5%) obtained from electric heating were lower than those obtained from microwave heating at 250 °C (glycerol conversion: 100% and acrolein selectivity: 71.1%), which might be due to the differences in the heating mechanism between microwave heating and conventional heating. In conventional heating, heat is transferred from the reactor wall to the interior of the catalyst bed by conduction, resulting in a lower temperature at the interior of the catalyst bed. In microwave heating, the electromagnetic energy absorbed by the material is directly converted into heat at the molecular level. The major challenges for applying microwave irradiation in glycerol dehydration include safety issues, the lack of an accurate temperature measuring device, and the difficulty of selecting construction materials.
To date, many efforts have been aimed at suppressing coke formation and prolonging catalyst lifetime for glycerol dehydration to produce acrolein through (i) doping noble metals, (ii) modifying the porosity and channel length, and (iii) co-feeding O
2; however, the catalysts will eventually be deactivated. Thus, catalyst regeneration by burning off the coke in air or O
2 has been carried out through in situ regeneration [
117] or periodic regeneration [
118], but explosion might occur under high O
2 concentration (i.e., 7%) [
113]. It is essential for future studies to design and develop innovative reactor configurations for the catalyst regeneration used in the catalytic dehydration of glycerol for producing acrolein.
3.2. Oxidation of Glycerol to Lactic Acid
Lactic acid is an essential ingredient in the food industry as an acidulant or inhibitor for bacterial spoilage, in the textile industry as a mordant to improve color durability, in the cosmetic industry as a moisturizer, and in the dairy industry as a pH regulator, as well as an important monomer for manufacturing biopolymer polylactic acid (PLA) [
119]. Traditionally, lactic acid is synthesized through sugar fermentation using carbohydrates as the carbon substrate; this method suffers from poor productivity and expensive operating costs due to the high cost of the enzyme, complex post-treatment by purification, and low scalability. As such, recent studies have employed glycerol as the feedstock to produce lactic acid via oxidation, as summarized in
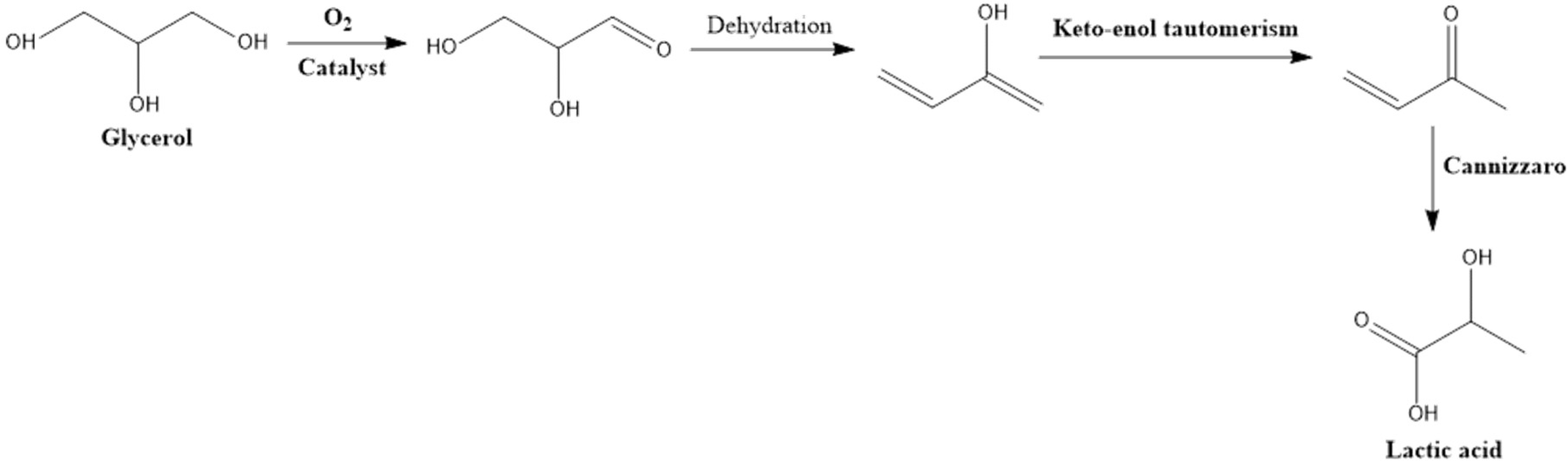
Table 5. The proposed reaction mechanism for converting glycerol to lactic acid via oxidation is depicted in
Figure 10. As illustrated in
Figure 10, glycerol conversion to lactic acid follows these steps:
Glycerol is initially dehydrogenated to glyceraldehyde in the presence of homogenous base and metal sites;
Dehydration of glyceraldehyde is followed by keto-enol tautomerism to form pyrualdehyde;
The formed pyrualdehyde is converted to lactic acid by an intra-molecular Cannizzaro reaction.
Alkali is capable of catalyzing dehydrogenation and dehydration reactions when subjected to hydrothermal conditions; thus, it has been extensively applied in lactic acid production from glycerol. For example, Zhang et al. [
130] performed selective oxidation of glycerol over Pt/AC to produce lactic acid in different basic solutions including LiOH, NaOH, KOH, and Ba(OH)
2, and the results showed that the order for lactic acid selectivity was as follows: LiOH > NaOH > KOH > Ba(OH)
2. The highest selectivity of lactic acid (69.3%) was achieved at 90 °C for 6 h and a LiOH-to-glycerol molar ratio of 1.5, which achieved a glycerol conversion of 100%. Despite the use of Pt/AC demonstrating excellent catalytic stability in glycerol oxidation, it had a detrimental influence on the conversion of the intermediate toward lactic acid formation by shifting the reaction to form glyceric acid. Yang et al. [
131] observed that 100% glycerol conversion and 94.6% lactic acid selectivity were achieved when conducting oxidation in NaOH solution, at 180 °C, for 8 h, and at 1.4 MPa of N
2. Similarly, Yin et al. [
132] tested different Cu-based catalysts (i.e., Cu/hydroxyapatite, Cu/MgO, and Cu/ZrO
2) for glycerol conversion to lactic acid in NaOH solution. They observed that both Cu/hydroxyapatite and Cu/MgO exhibited better catalytic performance than Cu/ZrO
2, which was mainly due to the differences in the basicity among Cu-based catalysts. A maximum selectivity of lactic acid of 90% was obtained at 230 °C for 2 h and at a NaOH concentration of 1.1 mol/L, along with a 91% of glycerol conversion. Notably, glycerol conversion in alkaline solution is usually operated at severe conditions (i.e., temperature of 280–290 °C) as dehydrogenation is an energy-demanding process. Nevertheless, alkali-assisted C-C bond cleavage might be simulated in harsh conditions, thereby leading to the formation of a series of undesirable by-products such as acetic acid, acrylic acid, formic acid, and oxalic acid. Consequently, to limit C-C bond cleavage forming undesired by-products, dehydrogenation must be performed at moderate conditions, which can be achieved using noble-metals-based catalysts (e.g., Pt and Pd). Feng et al. [
133] synthesized a Pt-based catalyst (i.e., Pt/L-Nb
2O
5) and used it in the glycerol conversion for producing lactic acid under base-free conditions. The results showed that the presence of Lewis acid sites of Pt/L-Nb
2O
5 was helpful for the transformation of pyruvic aldehyde to lactic acid. The authors also reported that the selectivity of lactic acid and glycerol conversion were 91% and 81%, respectively, when using Pt/L-Nb
2O
5 as the catalyst. Marques et al. [
134] investigated glycerol conversion to lactic acid over Pd/C, and a 99% glycerol conversion and a 46% lactic acid selectivity were obtained. In addition to Pd and Pt, the efficiency of using Au-Pt or Au-Pd alloy in lactic acid production from glycerol oxidation was also assessed because: (i) Au is an effective catalyst for alcohol oxidation by molecular oxygen in the liquid phase; (ii) Au commonly shows excellent catalytic performance and is highly resistant to catalyst deactivation. Thus, several previous studies were carried out to apply Au-Pt/C [
135], Au-Pt/TiO
2 [
136], or Au-Pt/CeO
2 [
137] in glycerol oxidation to enhance lactic acid production. In general, the use of bimetallic catalysts demonstrated improved catalytic performance in terms of glycerol conversion and lactic acid selectivity compared with monometallic catalysts [
137]. However, noble-metals-based catalysts tend to be deactivated and show poor recyclability, which might result from over-oxidation, metal leaching and sintering, and poisoning by molecular oxygen. Catalyst deactivation could be limited, to some extent, by purging N
2 into the catalyst support [
138]. To enhance catalytic reducibility, several strategies have been developed [
119]:
Pt and Pd supported by activated carbon showed great stability during glycerol oxidation to produce lactic acid;
The development of bimetallic catalysts supported on CeO2 where insignificant loss in the catalytic activity was observed upon recycling five times;
Adding non-noble metal promoters.
Overall, to date, a large gap remains in the development of excellent heterogeneous catalysts for the oxidation of glycerol for producing lactic acid; thus, more work is required.
3.3. Selective Hydrogenolysis of Glycerol to 1,3-Propanediol
1,3-propanediol has been extensively applied in the synthesis of polymers such polyethers, polyurethanes, and polyesters; most importantly, polypropylene terephthalate (PPT) fibers can be manufactured based on 1,3-propanediol and terephthalic acid [
139]. During 1,3-propanediol formation, it is essential to selectively break down the secondary C-O bond of glycerol, which still remains a big challenge because of the similarities in the activation energies among the three C-O bonds of glycerol, thus complicating the discrimination. Additionally, the accessibility of the secondary C-O bond is restricted due to steric hindrance [
140]; it was reported that a range of by-products (e.g., 1-propanol and 2-propanol) can be generated in an excessive hydrogenolysis [
141]. As shown in
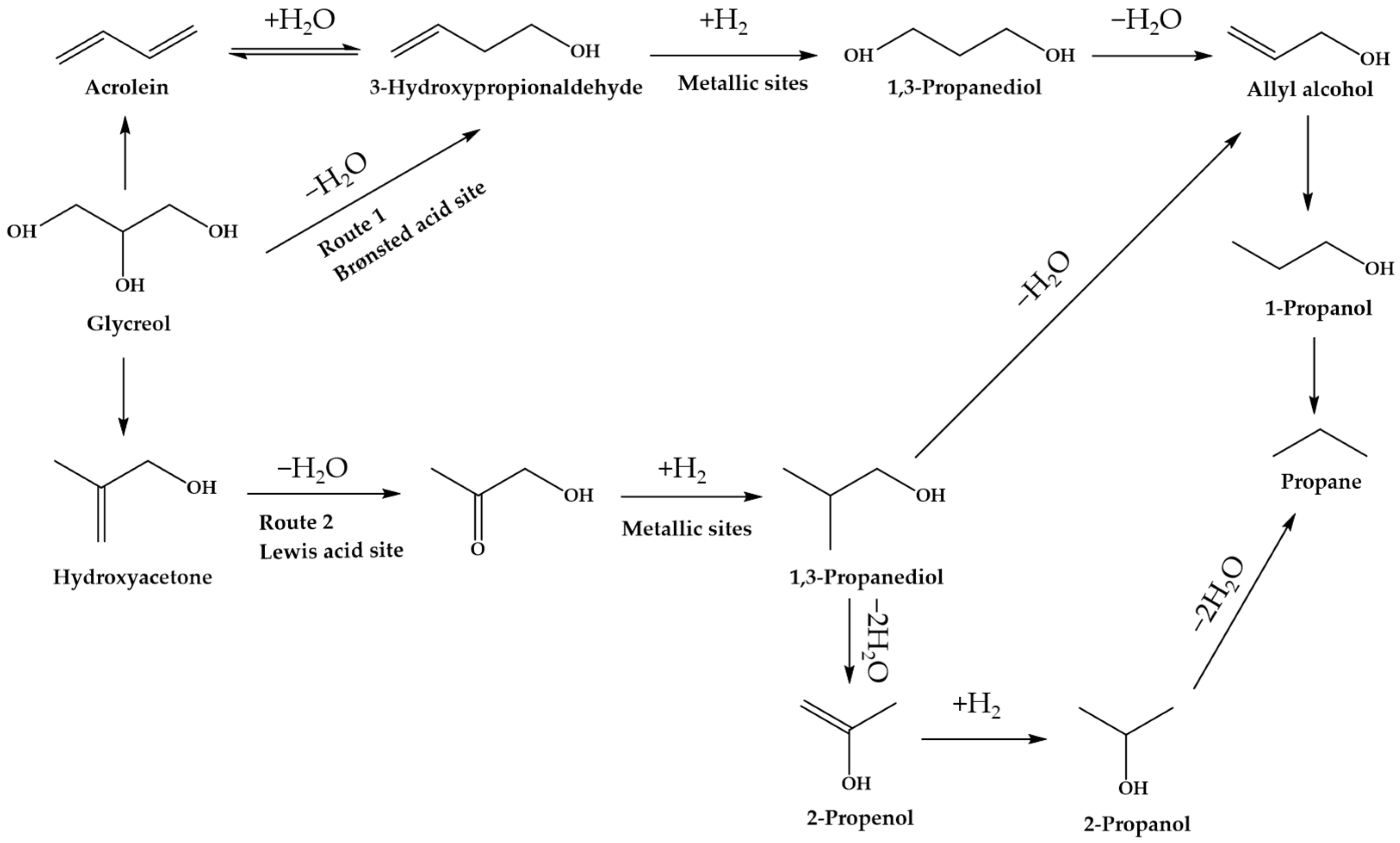
Figure 11, glycerol hydrogenolysis to produce 1,3-propanediol occurs on Brønsted acid sites at high a hydrogen pressure via Route 1. At higher temperatures, more glycerol can be converted into 3-hydroxypropionaldehyde (3-HPA) as an intermediate, while the formation of acrolein and monoalcohols can be stimulated. Thus, the operating temperature applied in the hydrogenolysis of glycerol for 1,3-propadeniol is usually below 200 °C. In addition to Brønsted acid sites, the hydrogenolysis reaction can also occur at Lewis acid sites where 1,2-propanediol formation is promoted by Route 2. Glycerol is initially dehydrated to form hydroxyacetone, which further undergoes hydrogenation into 1,2-propanediol [
142].
Until now, a great deal of effort has been directed to the design of an effective catalyst that can improve the selectivity toward 1,3-propanediol formation, among which Pt-W-based catalysts have recently been broadly investigated due to their suitable activity in the selective formation of 1,3-propanediol and potential industrial applications. Specifically, Pt-W-based catalysts offer dual roles including (i) active metal Pt species promoting the activation of H
2 to provide the hydrogen source and (ii) Brønsted acid sites forming when the hydrogen species reach WO
x species through hydrogen spillover, which is responsible for activating glycerol [
141,
142]. Edake et al. [
143] investigated the hydrogenolysis of glycerol over Pt-WO
3/Al
2O
3 at 240–300 °C and ambient pressure in a fluidized bed reactor, and the highest glycerol conversion and 1,3-propanediol selectivity of 99% and 14%, respectively, were attained at 260 °C, a H
2-to-glycerol ratio of 28, and a WHSV of 0.14 h
−1. Al
2O
3 is an efficient catalyst support that provides a higher surface area and serves as an anchor to fix glycerol on the surface, so has been regarded as one of the most effective supports used in glycerol hydrogenolysis to produce a high yield and selectivity of 1,3-propanediol, e.g., selectivity and yield of 66% and 42%, respectively, as reported by Zhu et al. [
144]. In addition to Al
2O
3, a variety of catalyst supports have been tested such as SiO
2 [
145], ZrO
2 [
146], WO
3 [
147], AlPO
4 [
148], SBA-15 [
149], and AlOOH [
150]. In a study, Priya et al. [
95] evaluated the influence of the catalyst support on glycerol conversion into 1,3-propanediol at 260 °C, 10 wt % of glycerol loading, and a 0.1 MPa of H
2 initial pressure, and the tested supports included ZrO
2, sulfated ZrO
2, Al
2O
3, AlPO
4, activated carbon, and Y-Zeolite. Among them, AlPO
4 was identified as the best catalyst support, resulting in a glycerol conversion of 100% and a 1,3-propanediol selectivity of 35.4%. The results suggested that the existence of weak acid sites play a positive role in the formation of 1,3-propanediol, and a high strength of weak acid sites was found in Pt/AlPO
4 based on the NH
3-TPD analysis, ensuring the highest catalytic performance during the hydrogenolysis of glycerol for 1,3-propanediol production. In another study, Zhu et al. [
151] modified TiO
2-supported Pt-WO
x catalyst by sulfate doping and then employed it in 1,3-propanediol production via hydrogenolysis since sulfate doping was reported to be helpful for increasing the surface area of the catalyst and improving metal dispersion [
152]. The results showed that sulfate-doped Pt-WO
x/S-TiO
2 achieved 100% glycerol conversion and 36% 1,3-propanediol selectivity at 120 °C and 4 MPa. Conversely hand, nonsulfate-doped Pt-WO
x/TiO
2 led to a significantly lower glycerol conversion of 57% but a higher selectivity of 66% toward 1,3-propanediol formation. This could be due to the over-strong hydrogenolysis activity of sulfate-doped catalyst. Subsequently, the addition of sulfate-doped Pt-WO
x/S-TiO
2 was found to achieve a glycerol conversion of 75% and a 1,3-propanediol selectivity of 47% when lowering the severity of the reaction, i.e., temperature of 100 °C, implying the beneficial impacts of sulfate on hydrogenolysis. This high hydrogenolysis efficiency offered by sulfate doping is related to the better dispersion of Pt and WO
x species and higher concentrations of Brønsted acid sites. Additionally, a better catalytic stability was detected when using the sulfate-doped Pt-WO
x/S-TiO
2 as the catalyst, i.e., the glycerol conversion reduced from 100% to 83% and 82% at the third and fourth run, respectively, resulting from stronger antileaching in the presence of sulfate doping. Nevertheless, several challenges remain [
141]:
A deep understanding of the synergistic influence between Pt species and WOx species is needed;
Characterization tools to elucidate the correlation between glycerol activation and the surface and structure properties of catalyst are lacing;
More effective catalysts must be developed for the selective hydrogenolysis of glycerol by different modification and catalyst preparation methods.
Apart from Pt-W-based catalysts, Ir-Re-based catalysts, as other widely investigated catalysts, have also been investigated to enhance 1,3-propanediol selectivity and yield from the hydrogenolysis of 1,3-propanediol [
153,
154,
155,
156,
157]. Chanklang et al. [
61], for instance, explored the effectiveness of using Ir-ReO
x/H-ZSM-5 in glycerol hydrogenolysis to produce 1,3-propanediol at 180–240 °C and 2–8 MPa of H
2 for 1–8 h, and the incorporation of Re led to better Ir dispersion. They also found that a maximum yield of 1,3-propanediol of 2.8% with a glycerol conversion of 14.9% at 220 °C and 4 MPa of H
2 for 8 h. Liu et al. [
154] synthesized an Ir-ReOx catalyst supported on SiO
2, and the results showed that the highest 1,3-propanediol yield and selectivity were 32% and 47%, respectively, at 120 °C and 8 MPa of H
2 for 24 h, which was accompanied by a glycerol conversion of 69%. In addition to H-ZSM-5 and SiO
2, KIT-6, as another ordered mesoporous silica with a cubic arrangement of interconnected pores, was also investigated in the preparation of an Ir-ReO
x-based catalyst to enhance catalytic performance in glycerol hydrogenolysis for 1,3-propanediol production [
157]. Re is a rare earth element, which significantly hinders its large-scale application. The most recent investigations into the hydrogenolysis of glycerol to produce 1,3-propanediol in the presence of either Pt-W-based catalysts or Ir-Re-based catalysts are summarized in
Table 6.
3.4. Selective Hydrogenolysis of Glycerol to 1,2-Propanediol
Currently, the transformation of glycerol into 1,2-propanediol by selective hydrogenolysis has received much attention due to the ability of 1,2-propanediol as an industrial monomer to produce polyester resins, detergents, antifreeze agents, additives in paint, food, etc. Industrially, 1,2-propanediol is synthesized through the hydration of propylene [
164]. When using glycerol as the feed, selective hydrogenolysis of glycerol for producing 1,2-propanediol has been extensively carried out over noble metals (e.g., Pd, Pt, and Ru) because of their capability to active hydrogen molecules. Oberhauser et al. [
165] found that Pt nanoparticles supported on carbonaceous catalyst supports can catalyze glycerol hydrogenolysis at 160 and 180 °C. The used carbonaceous supports were Katjen Black EC-600JD, Vulcan XC-72, and fewer-layer graphene. Among them, Katjen Black EC-600JD exhibited the highest surface area and contributed to retarding the agglomeration of metal nanoparticles, along with showing the highest selectivity for 1,2-propanediol of 70%, achieved at 160 °C. Silveira et al. [
166] prepared a Ru catalyst supported on sugarcane-straw-derived active carbon and commercial activated carbon, which was tested by conducting glycerol hydrogenolysis at 200 °C, 5 MPa, 6 h, and 10 vol % of glycerol loading. The results showed that the formation of 1,2-propanediol was more favorable than 1,3-propanediol formation under the investigated conditions, which might be due to the dominance of Lewis sites. To further enhance the performance of Ru-based catalysts in glycerol hydrogenolysis, Sherbi et al. [
16] added another metal, Cu, into the preparation of Ru-based catalyst supported on carbon nanotubes (CNTs), and this developed catalyst reached 93.4% 1,2-propanediol selectivity at 200 °C, 5 MPa of H
2, 20 h, and 20 wt % of glycerol loading, along with glycerol conversion of 18%. Similar results were reported by Wu et al. [
167], where Ru-Cu/CNT catalysts were applied in glycerol hydrogenolysis to enhance 1,2-propanediol production, and the results showed that the bimetallic catalysts showed a higher selectivity toward 1,2-propanediol formation than single-metal catalysts due to the hydrogen spillover effect resulting from the presence of highly dispersed tiny Ru clusters on the surface of Cu particles. The main benefits of using Ru-based catalysts for glycerol hydrogenolysis were reviewed [
168], as indicated below:
The combination of active metal particles and acid support could lead to the formation of 1,2-propanediol as the main product throughout glycerol conversion, especially under mild conditions (i.e., temperature below 180 °C);
The occurrence of over-hydrogenolysis in the presence of Ru-based catalysts can be identified when conducting the reaction under severe conditions (i.e., temperature above 240 °C), which causes the product formation to shift from 1,2-propanediol to 1-propanol and propane.
Pd, as another noble metal, has also been used to synthesize catalysts in the 1,2-propanediol production from glycerol. Mauriello et al. [
169] studied the hydrogenolysis of glycerol at 180 °C and 5 bar without adding hydrogen gas; instead, an external hydrogen source, i.e., 2-propanol, was used as the reaction medium, and the investigated catalysts included Pd/Co, Pd/CoO, Pd/Co
3O
4, Pd/Fe, Pd/Fe
2O
3, Pd/Fe
2O
3, and Pd/SiO
2. The interaction between Pd and other metals (i.e., Co and Fe) can modify the electronic properties of Pd and results in the formation of bimetallic Pd-Co or Pd-Fe sites, thereby promoting catalytic performance in the hydrogenolysis of glycerol, creating a shift toward 1,2-propanediol production. In addition to noble metals, transition metals such as Ni [
170], Cu [
171], and Co [
172] have been broadly explored in the selective hydrogenolysis of glycerol to generate 1,2-propanediol due to their low price and high activity and selectivity [
173]. Among them, Cu-based catalysts are the most commonly utilized catalysts in glycerol hydrogenolysis, which is primarily related to their strong ability to cleave C-O bonds [
164]. To date, Cu-based catalysts, either monometallic or bimetallic, have been designed: Cu-Zn/Al
2O
3 by Mishra et al. [
171], Cu/SiO
2 by Shan et al. [
174], Cu-Ni/Y zeolite by de Andrade et al. [
175], Cu/ZnO by Wang et al. [
176], Cu/metal oxides (i.e., Al
2O
3, SiO
2, ZnO, and MgO) by Zhou et al. [
177], Cu/AlOOH by Wu et al. [
178], Cu-Ni/SiO
2 by Lee et al. [
179], Cu-Ru/TiO
2 by Salazar et al. [
180], Cu-Ni/Al
2O
3 by Poddar et al. [
181], and Co/dolomite by Azri et al. [
182]. The underlying mechanism involved in Cu-catalyzed glycerol hydrogenolysis was reviewed by Montassier et al. [
183]. Initially, glycerol is dehydrogenated on Cu to produce glyceraldehyde, and then the formation of 1,2-propanediol is achieved through a nucleophilic reaction of water or absorbed OH species, dihydroxylation, and hydrogenation of aldehyde (2-hydroxy acrolein) [
164]. The relevant studies on the selective hydrogenolysis of glycerol for 1,2-propanediol production over either noble-metals-based catalysts or transition-metals-based catalysts are summarized in
Table 7. Several studies have been performed to compare the catalytic performance amongst various noble-metals-based and transition-metals-based catalysts in 1,2-propanediol production by glycerol hydrogenolysis. For example, Kang et al. [
184] prepared and characterized various bimetallic catalysts including Pt-Cu/SiO
2, Pd-Cu/SiO
2, Ag-Cu/SiO
2, and Ni-Cu/SiO
2 using a series of analytical techniques, and found that Pt-Cu/SiO
2 demonstrated the largest metal particles dispersion and smallest particle size. In the following hydrogenolysis investigation at 200 °C, 4 MPa of H
2, and 12 h, the authors reported that using Pt-Cu/SiO
2 led to an almost 100% glycerol conversion and the highest selectivity to 1,2-propanediol of 96% of the considered bimetallic catalysts (i.e., Pd-Cu/SiO
2, Ag-Cu/SiO
2, and Ni-Cu/SiO
2) and monometallic catalysts (i.e., Cu/SiO
2 and Pt/SiO
2). This was accompanied by a relatively high catalytic stability offered by Pt-Cu/SiO
2, which could be due to the decreased metal agglomeration tendency in the presence of Pt. Von Held Soares et al. [
185] conducted glycerol hydrogenolysis over Pt/Fe
3O
4, Pd/Fe
3O
4, and Ni/Fe
3O
4, and the order of catalytic activity was as follows: Pt > Pd > Ni.
Overall, the transformation of glycerol to value-added C3 chemicals (i.e., acrolein, lactic acid, 1,3-propanediol, and 1,2-propanediol) over heterogeneous catalysts is an essential component of achieving the sustainable and economic production of biodiesel. To ensure a high yield of the target products, proper catalyst design including the selection of active metal species, support material, catalyst preparation method, and the type and strength of acid sites) and reaction conditions must be carefully determined.






_Xu.png)