Continuous Production of Fumaric Acid with Immobilised Rhizopus oryzae: The Role of pH and Urea Addition
Abstract
1. Introduction
2. Results and Discussion
2.1. Experimental Window Determination and Repeatability
2.2. The Role of pH and Urea Feed Rate on the Production of Fumaric Acid
3. Materials and Methods
3.1. Microorganism and Culture Conditions
3.2. Medium
3.3. Fermenter Design and Operation
3.4. Analytical Methods
3.5. Production Rate Calculations
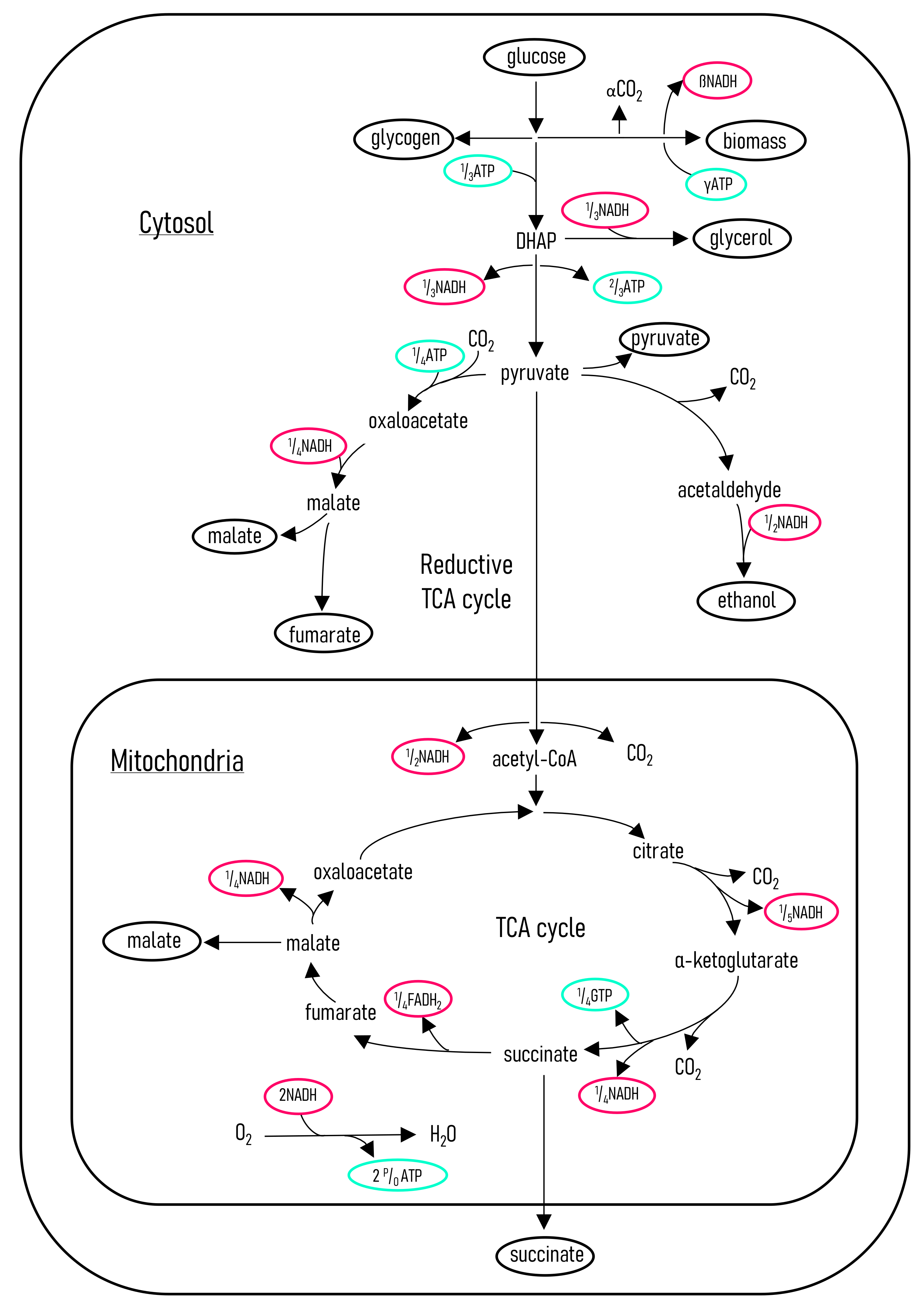
3.6. Metabolic Flux Model and Energy Calculations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| A | Acid concentration (mol L) |
| ATP | Adenosine triphosphate |
| C | Carbon mole concentration of specie(Cmol L) |
| e | Effluent |
| Equilibrium | |
| F | Faradays constant (96.5 kJ Volt e-mol) |
| f | Feed into reactor |
| FADH | Flavin adenine dinucleotide |
| GTP | Guanosine triphosphate |
| i | Intracellular |
| j | Designation of a specie |
| N | Moles of species (Cmol) |
| n | Number of protons |
| NADH | Nicotinamide adenine dinucleotide |
| o | Extracelluler |
| Dissociation equlibrium constant | |
| Proton motive force (Volt) | |
| P/O | Oxidative phosphorylation value () |
| Q | Volumetric (L h) |
| R | Gas constant (8.314 × 10 kJ mol K) |
| r | Rate of production (Cmol L h) |
| T | Temperature (K) |
| t | Time increment (s) |
| TCA | Tricarboxylic acid |
| V | Volume of the fermenter (L) |
| X | Biomass |
| Yield of biomass on glucose (g g) |
References
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; Technical Report; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2004. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- IMARC. Fumaric Acid Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2021–2026; Technical Report; IMARC: Noida, India, 2020. [Google Scholar]
- Grand View Research. Maleic Anhydride Market Size, Share & Trends Analysis Report By Application (1,4-BDO, UPR, Additives, Copolymers), by Region (Asia Pacific, North America, Europe, Central & South America, MEA), and Segment Forecasts, 2019–2025; Technical Report; Grand View Research: San Francisco, CA, USA, 2019. [Google Scholar]
- Nystrom, R.F.; Loo, Y.H.; Leak, J.C. Synthesis of Fumaric Acid-2-C14 and Maleic Anhydride-2-C14 1. J. Am. Chem. Soc. 1952, 74, 3434–3435. [Google Scholar] [CrossRef]
- Sebastian, J.; Hegde, K.; Kumar, P.; Rouissi, T.; Brar, S.K. Bioproduction of fumaric acid: An insight into microbial strain improvement strategies. Crit. Rev. Biotechnol. 2019, 39, 817–834. [Google Scholar] [CrossRef]
- Martin-Dominguez, V.; Estevez, J.; Ojembarrena, F.; Santos, V.; Ladero, M. Fumaric Acid Production: A Biorefinery Perspective. Fermentation 2018, 4, 33. [Google Scholar] [CrossRef]
- Felthouse, T.R.; Burnett, J.C.; Horrell, B.; Mummey, M.J.; Kuo, Y.J. Maleic Anhydride, Maleic Acid, and Fumaric Acid. Kirk-Othmer Encycl. Chem. Technol. 2001, 15, 1–48. [Google Scholar] [CrossRef]
- Li, Z.; Liu, N.; Cao, Y.; Jin, C.; Li, F.; Cai, C.; Yao, J. Effects of fumaric acid supplementation on methane production and rumen fermentation in goats fed diets varying in forage and concentrate particle size. J. Anim. Sci. Biotechnol. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Warren, R.; Barker, J.; Van de Kerkhof, P.; Reich, K.; Mrowietz, U. Switching from a fumaric acid ester mixture to dimethylfumarate monotherapy in psoriasis. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e352. [Google Scholar] [CrossRef] [PubMed]
- Moharregh-Khiabani, D.; Linker, R.; Gold, R.; Stangel, M. Fumaric Acid and its Esters: An Emerging Treatment for Multiple Sclerosis. Curr. Neuropharmacol. 2009, 7, 60–64. [Google Scholar] [CrossRef]
- Abe, A.; Oda, Y.; Asano, K.; Sone, T. Rhizopus delemar is the proper name for Rhizopus oryzae fumaric-malic acid producers. Mycologia 2007, 99, 714–722. [Google Scholar] [CrossRef]
- Papadaki, A.; Androutsopoulos, N.; Patsalou, M.; Koutinas, M.; Kopsahelis, N.; Castro, A.; Papanikolaou, S.; Koutinas, A. Biotechnological Production of Fumaric Acid: The Effect of Morphology of Rhizopus arrhizus NRRL 2582. Fermentation 2017, 3, 33. [Google Scholar] [CrossRef]
- Roa Engel, C.A.; van Gulik, W.M.; Marang, L.; van der Wielen, L.A.; Straathof, A.J. Development of a low pH fermentation strategy for fumaric acid production by Rhizopus oryzae. Enzym. Microb. Technol. 2011, 48, 39–47. [Google Scholar] [CrossRef]
- Odoni, D.I.; Tamayo-Ramos, J.A.; Sloothaak, J.; van Heck, R.G.; Martins dos Santos, V.A.; de Graaff, L.H.; Suarez-Diez, M.; Schaap, P.J. Comparative proteomics of Rhizopus delemar ATCC 20344 unravels the role of amino acid catabolism in fumarate accumulation. PeerJ 2017, 5, e3133. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, Y.; Du, J.; Tsao, G.T. Mycelial Pellet Formation by Rhizopus oryzae ATCC 20344. Appl. Biochem. Biotechnol. 2000, 84–86, 779–790. [Google Scholar] [CrossRef]
- Naude, A.; Nicol, W. Improved continuous fumaric acid production with immobilised Rhizopus oryzae by implementation of a revised nitrogen control strategy. New Biotechnol. 2018, 44, 13–22. [Google Scholar] [CrossRef]
- Swart, R.M.; Le Roux, F.; Naude, A.; De Jongh, N.W.; Nicol, W. Fumarate production with Rhizopus oryzae: Utilising the Crabtree effect to minimise ethanol by-product formation. Biotechnol. Biofuels 2020, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lv, C.; Xu, Q.; Li, S.; Huang, H.; Ouyang, P. Enhanced acid tolerance of Rhizopus oryzae during fumaric acid production. Bioprocess Biosyst. Eng. 2015, 38, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Aita, G.M. Fumaric Acid Production by Rhizopus oryzae ATCC® 20344™ from Lignocellulosic Syrup. Bioenergy Res. 2018, 11, 330–340. [Google Scholar] [CrossRef]
- Taymaz-Nikerel, H.; Jamalzadeh, E.; Borujeni, A.E.; Verheijen, P.J.; Van Gulik, W.M.; Heijnen, S.J. A thermodynamic analysis of dicarboxylic acid production in microorganisms. In Biothermodynamics: The Role of Thermodynamics in Biochemical Engineering; EPFL Press: Lausanne, Switzerland, 2013; pp. 547–579. [Google Scholar]
- Kenealy, W.; Zaady, E.; Du Preez, J.C. Biochemical aspects of fumaric acid accumulation by Rhizopus arrhizus. Appl. Environ. Microbiol. 1986, 52, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Naude, A.; Nicol, W. Fumaric acid fermentation with immobilised Rhizopus oryzae: Quantifying time-dependent variations in catabolic flux. Process. Biochem. 2017, 56, 8–20. [Google Scholar] [CrossRef]
- de Jongh, N.W.; Swart, R.M.; Nicol, W. Fed-batch growth of Rhizopus oryzae: Eliminating ethanol formation by controlling glucose addition. Biochem. Eng. J. 2021, 169, 107961. [Google Scholar] [CrossRef]
- Feillet, F.; Leonard, J.V. Alternative pathway therapy for urea cycle disorders. J. Inherit. Metab. Dis. 1998, 21, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Abbott, D.A.; Suir, E.; Van Maris, A.J.; Pronk, J.T. Physiological and transcriptional responses to high concentrations of lactic acid in anaerobic chemostat cultures of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2008, 74, 5759–5768. [Google Scholar] [CrossRef] [PubMed]
- Villadsen, J.; Nielsen, J.; Lidén, G. Bioreaction Engineering Principles; Springer: Boston, MA, USA, 2011; Volume 53, pp. 1689–1699. [Google Scholar] [CrossRef]
- Di Caprio, F. A fattening factor to quantify the accumulation ability of microorganisms under N-starvation. New Biotechnol. 2021, 66, 70–78. [Google Scholar] [CrossRef]
- Osmani, S.A.; Scrutton, M.C. The sub-cellular localisation and regulatory properties of pyruvate carboxylase from Rhizopus arrhizus. Eur. J. Biochem. 1985, 147, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Maris, A.J.; Konings, W.N.; Dijken, J.P.; Pronk, J.T. Microbial export of lactic and 3-hydroxypropanoic acid: Implications for industrial fermentation processes. Metab. Eng. 2004, 6, 245–255. [Google Scholar] [CrossRef] [PubMed]

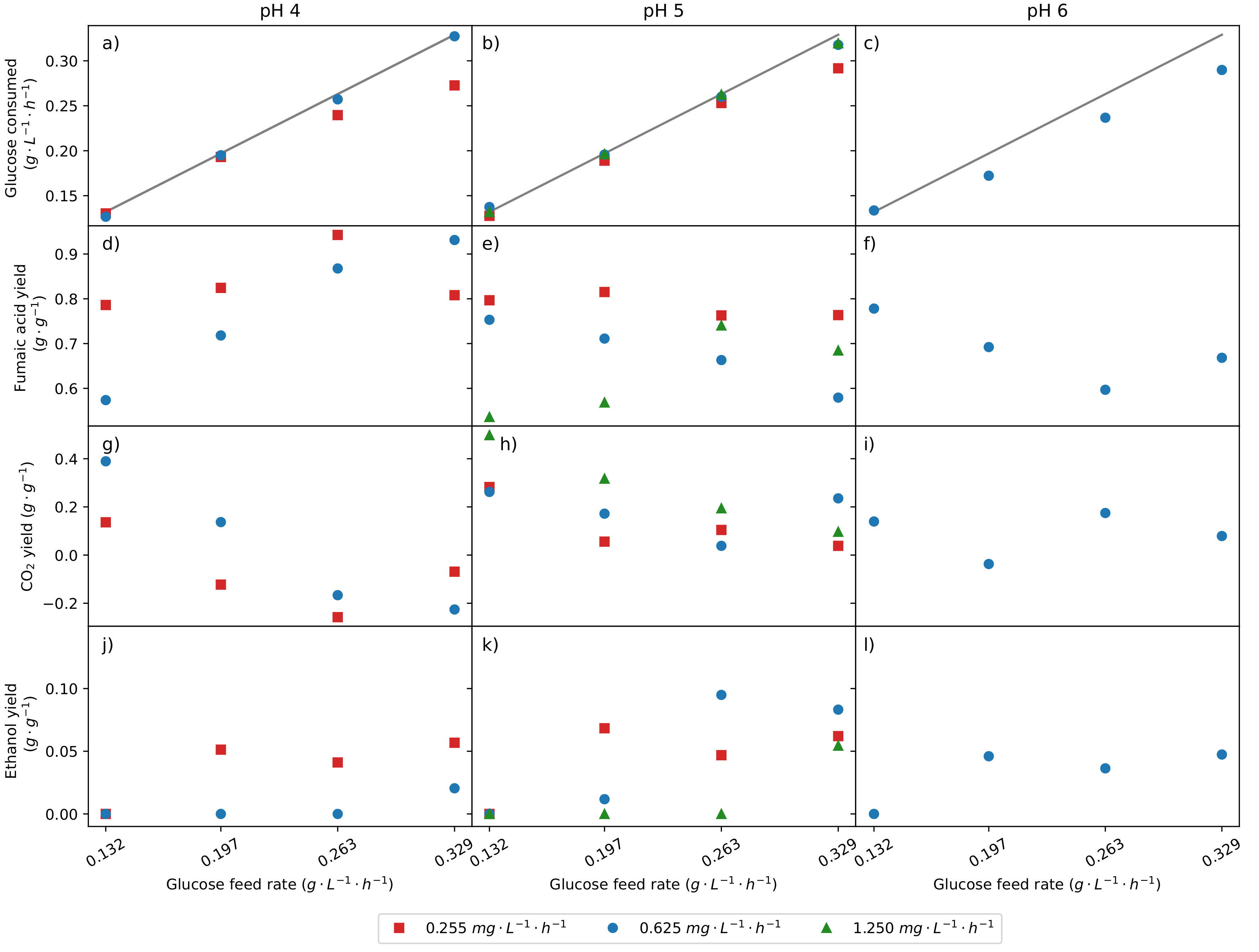

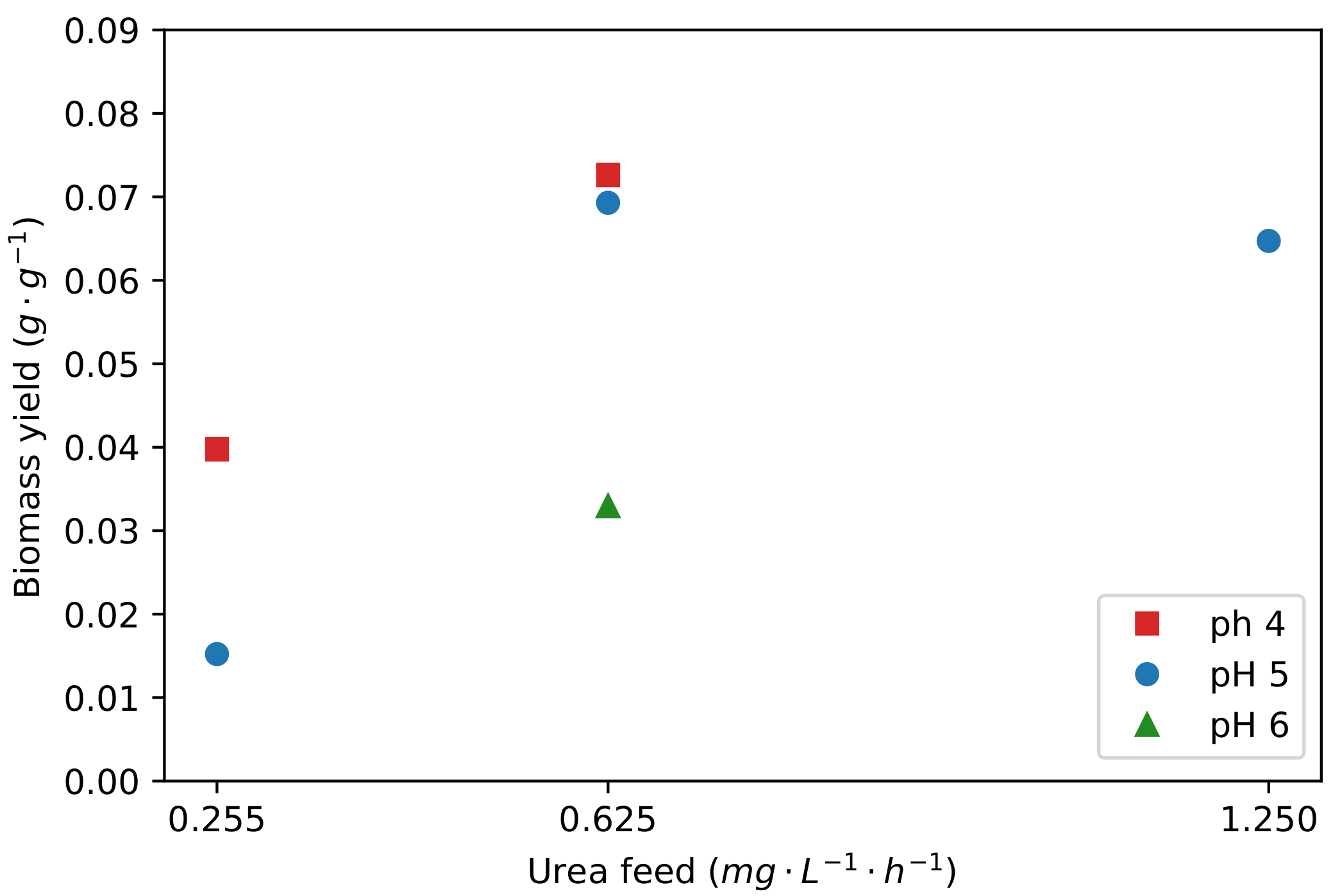
| Separate Run Repeats | Repeats within a Run | |||||||
|---|---|---|---|---|---|---|---|---|
| Glucose feed rate () | 0.132 * | 0.197 * | 0.227 * | 0.263 † | ||||
| Glucose consumed () | 0.133 | 0.142 | 0.197 | 0.195 | 0.222 | 0.226 | 0.240 | 0.236 |
| Fumaric yield () | 0.733 | 0.774 | 0.727 | 0.695 | 0.900 | 0.894 | 0.943 | 0.912 |
| By-product yield () | 0.076 | 0.073 | 0.074 | 0.040 | 0.093 | 0.116 | 0.139 | 0.108 |
| Phase | pH | Urea Feed () | Ygx () | H:C | N:C |
|---|---|---|---|---|---|
| Growth | 5 | Excess | 0.180 | 1.410 | 0.189 |
| Production | 5 | 0.255 | 0.013 | 0.807 | 0.128 |
| Production | 5 | 0.625 | 0.080 | 0.644 | 0.120 |
| Production | 5 | 1.250 | 0.066 | 1.041 | 0.148 |
| Production | 4 | 0.625 | 0.071 | 1.030 | 0.123 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swart, R.M.; Ronoh, D.K.; Brink, H.; Nicol, W. Continuous Production of Fumaric Acid with Immobilised Rhizopus oryzae: The Role of pH and Urea Addition. Catalysts 2022, 12, 82. https://doi.org/10.3390/catal12010082
Swart RM, Ronoh DK, Brink H, Nicol W. Continuous Production of Fumaric Acid with Immobilised Rhizopus oryzae: The Role of pH and Urea Addition. Catalysts. 2022; 12(1):82. https://doi.org/10.3390/catal12010082
Chicago/Turabian StyleSwart, Reuben Marc, Dominic Kibet Ronoh, Hendrik Brink, and Willie Nicol. 2022. "Continuous Production of Fumaric Acid with Immobilised Rhizopus oryzae: The Role of pH and Urea Addition" Catalysts 12, no. 1: 82. https://doi.org/10.3390/catal12010082
APA StyleSwart, R. M., Ronoh, D. K., Brink, H., & Nicol, W. (2022). Continuous Production of Fumaric Acid with Immobilised Rhizopus oryzae: The Role of pH and Urea Addition. Catalysts, 12(1), 82. https://doi.org/10.3390/catal12010082







