Supported Metal Catalysts for the Synthesis of N-Heterocycles
Abstract
1. Introduction
2. Metal Catalysts Supported on Inorganic Matrices for the Synthesis of N-Heterocycles
2.1. Metal Catalysts Supported on Carbon
2.2. Metal Catalysts Supported on Silica
2.3. Metal Catalysts Supported on Metal Oxides
2.4. Metal Catalysts Supported on Minerals
2.5. Metal Catalysts Supported on Magnetic Core/Inorganic Shell Composites
3. Metal Catalysts Supported on Organic Matrices for the Synthesis of N-Heterocycles
3.1. Metal Catalysts Supported on Organic Polymers
3.2. Metal Catalysts Supported on Organic Polymer-Anchored Ligands
3.3. Metal Catalysts Supported on Biopolymers
4. Metal Catalysts Supported on Hybrid Inorganic-Organic Matrices for the Synthesis of N-Heterocycles
4.1. Metal Catalysts Supported on Carbon-Anchored Ligands
4.2. Metal Catalysts Supported on Silica-Anchored Ligands
4.3. Metal Catalysts Supported on Metal Oxides or Minerals-Anchored Ligands
4.4. Metal Catalysts Supported on Magnetic Core/Inorganic Shell Composite-Anchored Ligands
4.5. Metal Catalysts Supported on Magnetic Core/Organic Shell Composites
4.6. Metal Catalysts Supported on Metal Organic Frameworks
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Joule, J.A. Chapter Four—Natural Products Containing Nitrogen Heterocycles—Some Highlights 1990–2015. In Advances in Heterocyclic Chemistry; Scriven, E.F.V., Ramsden, C.A., Eds.; Academic Press: Cambridge, UK, 2016; pp. 81–106. [Google Scholar]
- Prandi, C.; Occhiato, E.G. From synthetic control to natural products: A focus on N-heterocycles. Pest Manag. Sci. 2019, 75, 2385–2402. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar Jain, S. A Comprehensive Review of N-Heterocycles as Cytotoxic Agents. Curr. Med. Chem. 2016, 23, 4338–4394. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Zadsirjan, V. Prescribed drugs containing nitrogen heterocycles: An overview. RSC Adv. 2020, 10, 44247–44311. [Google Scholar] [CrossRef]
- Elgemeie, G.H.; Helal, M.H.; El-Sayed, H.M. Recent trends in synthesis and application of nitrogen heterocyclic azo dyes. Pigm. Resin Technol. 2001, 30, 210–228. [Google Scholar] [CrossRef]
- Lipunova, G.N.; Nosova, E.V.; Charushin, V.N.; Chupakhin, O.N. Functionalized Quinazolines and Pyrimidines for Optoelectronic Materials. Curr. Org. Synth. 2018, 15, 793–814. [Google Scholar] [CrossRef]
- Albano, G.; Aronica, L.A.; Minotto, A.; Cacialli, F.; Di Bari, L. Chiral Oligothiophenes with Remarkable Circularly Polarized Luminescence and Electroluminescence in Thin Films. Chem. Eur. J. 2020, 26, 16622–16627. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, B.; Zhao, J.; Zhang, Q.; Lin, Z.; Weng, J.; Huang, W. Recent progress in 1,4-diazafluorene-cored optoelectronic materials: A review. Dyes Pigm. 2021, 191, 109365. [Google Scholar] [CrossRef]
- Albano, G.; Aronica, L.A. Potentiality and Synthesis of O- and N-Heterocycles: Pd-Catalyzed Cyclocarbonylative Sonogashira Coupling as a Valuable Route to Phthalans, Isochromans, and Isoindolines. Eur. J. Org. Chem. 2017, 2017, 7204–7221. [Google Scholar] [CrossRef]
- Albano, G.; Aronica, L.A. Cyclization Reactions for the Synthesis of Phthalans and Isoindolines. Synthesis 2018, 50, 1209–1227. [Google Scholar]
- Albano, G.; Aronica, L.A. From Alkynes to Heterocycles through Metal-Promoted Silylformylation and Silylcarbocyclization Reactions. Catalysts 2020, 10, 1012. [Google Scholar] [CrossRef]
- Albano, G.; Aronica, L.A. Acyl Sonogashira Cross-Coupling: State of the Art and Application to the Synthesis of Heterocyclic Compounds. Catalysts 2020, 10, 25. [Google Scholar] [CrossRef]
- Albano, G.; Morelli, M.; Aronica, L.A. Synthesis of Functionalised 3-Isochromanones by Silylcarbocyclisation/Desilylation Reactions. Eur. J. Org. Chem. 2017, 2017, 3473–3480. [Google Scholar] [CrossRef]
- Aronica, L.A.; Albano, G.; Giannotti, L.; Meucci, E. Synthesis of N-Heteroaromatic Compounds through Cyclocarbonylative Sonogashira Reactions. Eur. J. Org. Chem. 2017, 2017, 955–963. [Google Scholar] [CrossRef]
- Albano, G.; Morelli, M.; Lissia, M.; Aronica, L.A. Synthesis of Functionalised Indoline and Isoquinoline Derivatives through a Silylcarbocyclisation/Desilylation Sequence. ChemistrySelect 2019, 4, 2505–2511. [Google Scholar] [CrossRef]
- Albano, G.; Giuntini, S.; Aronica, L.A. Synthesis of 3-Alkylideneisoindolin-1-ones via Sonogashira Cyclocarbonylative Reactions of 2-Ethynylbenzamides. J. Org. Chem. 2020, 85, 10022–10034. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Schlögl, R. Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2015, 54, 3465–3520. [Google Scholar] [CrossRef]
- Friend, C.M.; Xu, B. Heterogeneous Catalysis: A Central Science for a Sustainable Future. Acc. Chem. Res. 2017, 50, 517–521. [Google Scholar] [CrossRef]
- Astruc, D.; Lu, F.; Aranzaes, J.R. Nanoparticles as Recyclable Catalysts: The Frontier between Homogeneous and Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2005, 44, 7852–7872. [Google Scholar] [CrossRef]
- Heravi, M.M.; Alishiri, T. Application of nanomaterials in heterocyclic chemistry. Heterocycles 2012, 85, 545–586. [Google Scholar] [CrossRef]
- Elwahy, A.H.M.; Shaaban, M.R. Synthesis of heterocycles and fused heterocycles catalyzed by nanomaterials. RSC Adv. 2015, 5, 75659–75710. [Google Scholar] [CrossRef]
- Banerjee, B. Recent developments on nano-ZnO catalyzed synthesis of bioactive heterocycles. J. Nanostruct. Chem. 2017, 7, 389–413. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M. Advances in Magnetic Nanoparticles-Supported Palladium Complexes for Coupling Reactions. Molecules 2018, 23, 2532. [Google Scholar] [CrossRef]
- Kaur, G.; Devi, P.; Thakur, S.; Kumar, A.; Chandel, R.; Banerjee, B. Magnetically Separable Transition Metal Ferrites: Versatile Heterogeneous Nano-Catalysts for the Synthesis of Diverse Bioactive Heterocycles. ChemistrySelect 2019, 4, 2181–2199. [Google Scholar] [CrossRef]
- Aqeel Ashraf, M.; Liu, Z.; Yang, Y.; Zhang, D. Magnetic nanoparticles supported copper catalysts: Synthesis of heterocyclic scaffolds. Synth. Commun. 2020, 50, 2885–2905. [Google Scholar] [CrossRef]
- Huang, W.; Cheng, Q.; Ma, D. Recent reports on magnetic nanoparticles supported metallic catalysts: Synthesis of heterocycles. Synth. Commun. 2021, 51, 1321–1339. [Google Scholar] [CrossRef]
- Elwahy, A.H.M.; Shaaban, M.R. Synthesis of heterocycles catalyzed by iron oxide nanoparticles. Heterocycles 2017, 94, 595–655. [Google Scholar] [CrossRef]
- Bhaskaruni, S.V.H.S.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A review on multi-component green synthesis of N-containing heterocycles using mixed oxides as heterogeneous catalysts. Arab. J. Chem. 2020, 13, 1142–1178. [Google Scholar] [CrossRef]
- Sharma, A.; Gudala, S.; Ambati, S.R.; Penta, S.; Mahapatra, S.P.; Vedula, R.R.; Pola, S.; Acharya, B. Synthesis of Heterocyclic Compounds Catalyzed by Metal/Metal Oxide-Multiwall Carbon Nanotube Nanocomposites. J. Chin. Chem. Soc. 2017, 64, 589–606. [Google Scholar] [CrossRef]
- Rai, A.; Ranganath, K.V.S. Recyclable catalysts for the synthesis of heterocyclic compounds using carbon materials. J. Heterocycl. Chem. 2021, 58, 1039–1057. [Google Scholar] [CrossRef]
- Daştan, A.; Kulkarni, A.; Török, B. Environmentally benign synthesis of heterocyclic compounds by combined microwave-assisted heterogeneous catalytic approaches. Green Chem. 2012, 14, 17–37. [Google Scholar] [CrossRef]
- Mandoli, A. Recent Advances in Recoverable Systems for the Copper-Catalyzed Azide-Alkyne Cycloaddition Reaction (CuAAC). Molecules 2016, 21, 1174. [Google Scholar] [CrossRef]
- McAteer, C.H.; Murugan, R.; Subba Rao, Y.V. Chapter Six—Heterogeneously Catalyzed Synthesis of Heterocyclic Compounds. In Advances in Heterocyclic Chemistry; Scriven, E.F.V., Ramsden, C.A., Eds.; Academic Press: Cambridge, UK, 2017; pp. 173–205. [Google Scholar]
- Truong, C.C.; Ngo, H.L. Sustainable synthesis of nitrogen heterocycles from carbon dioxide and aromatic amines over heterogeneous catalysts. J. CO2 Util. 2020, 42, 101325. [Google Scholar] [CrossRef]
- Fraile, J.M.; García, J.I.; Mayoral, J.A.; Pires, E. Heterogenization on Inorganic Supports: Methods and Applications. In Heterogenized Homogeneous Catalysts for Fine Chemicals Production: Materials and Processes; Barbaro, P., Liguori, F., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 65–121. [Google Scholar]
- Gruber, M.; Chouzier, S.; Koehler, K.; Djakovitch, L. Palladium on activated carbon: A valuable heterogeneous catalyst for one-pot multi-step synthesis. Appl. Catal. A 2004, 265, 161–169. [Google Scholar] [CrossRef]
- Landge, S.M.; Schmidt, A.; Outerbridge, V.; Török, B. Synthesis of Pyrazoles by a One-Pot Tandem Cyclization-Dehydrogenation Approach on Pd/C/K-10 Catalyst. Synlett 2007, 2007, 1600–1604. [Google Scholar] [CrossRef]
- Weires, N.A.; Boster, J.; Magolan, J. Combined Pd/C and Montmorillonite Catalysis for One-Pot Synthesis of Benzimidazoles. Eur. J. Org. Chem. 2012, 2012, 6508–6512. [Google Scholar] [CrossRef] [PubMed]
- Rossy, C.; Majimel, J.; Delapierre, M.T.; Fouquet, E.; Felpin, F.-X. Palladium and copper-supported on charcoal: A heterogeneous multi-task catalyst for sequential Sonogashira-Click and Click-Heck reactions. J. Organomet. Chem. 2014, 755, 78–85. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Taft, B.R. Heterogeneous Copper-in-Charcoal-Catalyzed Click Chemistry. Angew. Chem. Int. Ed. 2006, 45, 8235–8238. [Google Scholar] [CrossRef]
- Lee, C.-T.; Huang, S.; Lipshutz, B.H. Copper-in-Charcoal-Catalyzed, Tandem One-Pot Diazo Transfer-Click Reactions. Adv. Synth. Catal. 2009, 351, 3139–3142. [Google Scholar] [CrossRef]
- Alonso, F.; Moglie, Y.; Radivoy, G.; Yus, M. Multicomponent Synthesis of 1,2,3-Triazoles in Water Catalyzed by Copper Nanoparticles on Activated Carbon. Adv. Synth. Catal. 2010, 352, 3208–3214. [Google Scholar] [CrossRef]
- Alonso, F.; Moglie, Y.; Radivoy, G.; Yus, M. Click chemistry from organic halides, diazonium salts and anilines in water catalysed by copper nanoparticles on activated carbon. Org. Biomol. Chem. 2011, 9, 6385–6395. [Google Scholar] [CrossRef] [PubMed]
- Albaladejo, M.J.; Alonso, F.; Yus, M. Synthesis of Indolizines and Heterocyclic Chalcones Catalyzed by Supported Copper Nanoparticles. Chem. Eur. J. 2013, 19, 5242–5245. [Google Scholar] [CrossRef] [PubMed]
- Siddiki, S.M.A.H.; Touchy, A.S.; Chaudhari, C.; Kon, K.; Toyao, T.; Shimizu, K.-i. Synthesis of 2,5-disubstituted pyrroles via dehydrogenative condensation of secondary alcohols and 1,2-amino alcohols by supported platinum catalysts. Org. Chem. Front. 2016, 3, 846–851. [Google Scholar] [CrossRef]
- Godino-Ojer, M.; Milla-Diez, L.; Matos, I.; Durán-Valle, C.J.; Bernardo, M.; Fonseca, I.M.; Pérez Mayoral, E. Enhanced Catalytic Properties of Carbon supported Zirconia and Sulfated Zirconia for the Green Synthesis of Benzodiazepines. ChemCatChem 2018, 10, 5215–5223. [Google Scholar] [CrossRef]
- Shaygan Nia, A.; Rana, S.; Döhler, D.; Noirfalise, X.; Belfiore, A.; Binder, W.H. Click chemistry promoted by graphene supported copper nanomaterials. Chem. Commun. 2014, 50, 15374–15377. [Google Scholar] [CrossRef]
- Hussain, N.; Gogoi, P.; Das, M.R.; Sengupta, P.; Fedorov, V.E.; Asanov, I.P.; Kozlova, M.N.; Artemkina, S.B. Development of novel efficient 2D nanocomposite catalyst towards the three-component coupling reaction for the synthesis of imidazo [1,2-a] pyridines. Appl. Catal. A 2017, 542, 368–379. [Google Scholar] [CrossRef]
- Demirci, T.; Çelik, B.; Yıldız, Y.; Eriş, S.; Arslan, M.; Sen, F.; Kilbas, B. One-pot synthesis of Hantzsch dihydropyridines using a highly efficient and stable PdRuNi@GO catalyst. RSC Adv. 2016, 6, 76948–76956. [Google Scholar] [CrossRef]
- Santra, S.; Ranjan, P.; Bera, P.; Ghosh, P.; Mandal, S.K. Anchored palladium nanoparticles onto single walled carbon nanotubes: Efficient recyclable catalyst for N-containing heterocycles. RSC Adv. 2012, 2, 7523–7533. [Google Scholar] [CrossRef]
- Godino-Ojer, M.; López-Peinado, A.J.; Maldonado-Hódar, F.J.; Bailón-García, E.; Pérez-Mayoral, E. Cobalt oxide-carbon nanocatalysts with highly enhanced catalytic performance for the green synthesis of nitrogen heterocycles through the Friedländer condensation. Dalton Trans. 2019, 48, 5637–5648. [Google Scholar] [CrossRef]
- Ezhilmathi, P.; Thirumalai, K.; Swaminathan, M.; Krishnasamy, K. One-Pot Synthesis of Tetra Substituted Imidazoles Catalyzed by Fly Ash Supported Bi2O3-ZnO. J. Nanosci. Nanotechnol. 2019, 19, 8163–8171. [Google Scholar] [CrossRef]
- Kazemi Movahed, S.; Salari, P.; Kasmaei, M.; Armaghan, M.; Dabiri, M.; Amini, M.M. Copper nanoparticles incorporated on a mesoporous carbon nitride, an excellent catalyst in the Huisgen 1,3-dipolar cycloaddition and N-arylation of N-heterocycles. Appl. Organomet. Chem. 2018, 32, e3914. [Google Scholar] [CrossRef]
- Phatake, V.V.; Bhanage, B.M. Cu@U-g-C3N4 Catalyzed Cyclization of o-Phenylenediamines for the Synthesis of Benzimidazoles by Using CO2 and Dimethylamine Borane as a Hydrogen Source. Catal. Lett. 2019, 149, 347–359. [Google Scholar] [CrossRef]
- Gong, Z.; Lei, Y.; Zhou, P.; Zhang, Z. One-pot synthesis of N-substituted pyrroles from nitro compounds and 2,5-hexadione over a heterogeneous cobalt catalyst. New J. Chem. 2017, 41, 10613–10618. [Google Scholar] [CrossRef]
- Ma, Z.; Song, T.; Yuan, Y.; Yang, Y. Synergistic catalysis on Fe-Nx sites and Fe nanoparticles for efficient synthesis of quinolines and quinazolinones via oxidative coupling of amines and aldehydes. Chem. Sci. 2019, 10, 10283–10289. [Google Scholar] [CrossRef]
- Song, T.; Ren, P.; Ma, Z.; Xiao, J.; Yang, Y. Highly Dispersed Single-Phase Ni2P Nanoparticles on N,P-Codoped Porous Carbon for Efficient Synthesis of N-Heterocycles. ACS Sustain. Chem. Eng. 2020, 8, 267–277. [Google Scholar] [CrossRef]
- Díaz-Ortiz, A.; de la Hoz, A.; Moreno, A.; Sánchez-Migallón, A.; Valiente, G. Synthesis of 1,3,5-triazines in solvent-free conditions catalysed by silica-supported lewis acids. Green Chem. 2002, 4, 339–343. [Google Scholar] [CrossRef]
- Soltani Rad, M.N.; Behrouz, S.; Doroodmand, M.M.; Movahediyan, A. Copper-doped silica cuprous sulfate (CDSCS) as a highly efficient and new heterogeneous nano catalyst for [3+2] Huisgen cycloaddition. Tetrahedron 2012, 68, 7812–7821. [Google Scholar] [CrossRef]
- Inamdar, S.M.; More, V.K.; Mandal, S.K. CuO nano-particles supported on silica, a new catalyst for facile synthesis of benzimidazoles, benzothiazoles and benzoxazoles. Tetrahedron Lett. 2013, 54, 579–583. [Google Scholar] [CrossRef]
- Maleki, A. One-pot multicomponent synthesis of diazepine derivatives using terminal alkynes in the presence of silica-supported superparamagnetic iron oxide nanoparticles. Tetrahedron Lett. 2013, 54, 2055–2059. [Google Scholar] [CrossRef]
- Arabpourian, K.; Behbahani, F.K. Synthesis of Pyrrole Derivatives Promoted by Fe(ClO4)3/SiO2 as an Environmentally Friendly Catalyst. Russ. J. Org. Chem. 2019, 55, 682–685. [Google Scholar] [CrossRef]
- Safari, J.; Gandomi-Ravandi, S.; Naseh, S. Efficient, green and solvent-free synthesis of tetrasubstituted imidazoles using SbCl3/SiO2 as heterogeneous catalyst. J. Chem. Sci. 2013, 125, 827–833. [Google Scholar] [CrossRef]
- Safari, J.; Naseh, S.; Zarnegar, Z.; Akbari, Z. Applications of microwave technology to rapid synthesis of substituted imidazoles on silica-supported SbCl3 as an efficient heterogeneous catalyst. J. Taibah Univ. Sci. 2014, 8, 323–330. [Google Scholar] [CrossRef]
- Atar, A.B.; Kim, J.S.; Lim, K.T.; Jeong, Y.T. Bridging homogeneous and heterogeneous catalysis with CAN·SiO2 as a solid catalyst for four-component reactions for the synthesis of tetrasubstituted pyrroles. New J. Chem. 2015, 39, 396–402. [Google Scholar] [CrossRef]
- Maddila, S.; Valand, J.; Bandaru, H.; Yalagala, K.; Lavanya, P. Ag Loaded on SiO2 as an Efficient and Recyclable Heterogeneous Catalyst for the Synthesis of Chloro-8-substituted-9H-purines. J. Heterocycl. Chem. 2016, 53, 319–324. [Google Scholar] [CrossRef]
- So, M.-H.; Liu, Y.; Ho, C.-M.; Lam, K.-Y.; Che, C.-M. Silica-Supported Gold Nanoparticles Catalyzed One-Pot, Tandem Aerobic Oxidative Cyclization Reaction for Nitrogen-Containing Polyheterocyclic Compounds. ChemCatChem 2011, 3, 386–393. [Google Scholar] [CrossRef]
- Abdollahi-Alibeik, M.; Rezaeipoor-Anari, A. BF3/MCM-41 as nano structured solid acid catalyst for the synthesis of 3-iminoaryl-imidazo [1,2-a] pyridines. Catal. Sci. Technol. 2014, 4, 1151–1159. [Google Scholar] [CrossRef]
- Samanta, P.K.; Banerjee, R.; Richards, R.M.; Biswas, P. Mesoporous silica supported ytterbium as catalyst for synthesis of 1,2-disubstituted benzimidazoles and 2-substituted benzimidazoles. Appl. Organomet. Chem. 2018, 32, e4507. [Google Scholar] [CrossRef]
- Samanta, P.K.; Biswas, R.; Das, T.; Nandi, M.; Adhikary, B.; Richards, R.M.; Biswas, P. Mesoporous silica supported samarium as recyclable heterogeneous catalyst for synthesis of 5-substituted tetrazole and 2-substituted benzothiazole. J. Porous Mater. 2019, 26, 145–155. [Google Scholar] [CrossRef]
- Naeimi, H.; Nejadshafiee, V. Efficient one-pot click synthesis of β-hydroxy-1,2,3-triazoles catalyzed by copper(i)@phosphorated SiO2via multicomponent reaction in aqueous media. New J. Chem. 2014, 38, 5429–5435. [Google Scholar] [CrossRef]
- Kumar, A.; Koul, S.; Razdan, T.K.; Kapoor, K.K. A new and convenient one-pot solid supported synthesis of 2,4,6-triarylpyridines. Tetrahedron Lett. 2006, 47, 837–842. [Google Scholar] [CrossRef]
- Mukherjee, N.; Ahammed, S.; Bhadra, S.; Ranu, B.C. Solvent-free one-pot synthesis of 1,2,3-triazole derivatives by the ‘Click’ reaction of alkyl halides or aryl boronic acids, sodium azide and terminal alkynes over a Cu/Al2O3 surface under ball-milling. Green Chem. 2013, 15, 389–397. [Google Scholar] [CrossRef]
- Agalave, S.G.; Pharande, S.G.; Gade, S.M.; Pore, V.S. Alumina-Supported Copper Iodide: An Efficient and Recyclable Catalyst for Microwave-Assisted Synthesis of 1,4-Disubstituted 1,2,3-Triazoles via Three-Component Reaction in Water. Asian J. Org. Chem. 2015, 4, 943–951. [Google Scholar] [CrossRef]
- Maddila, S.; Maddila, S.N.; Jonnalagadda, S.B.; Lavanya, P. Reusable Ce-V Loaded Alumina Catalyst for Multicomponent Synthesis of Substituted Pyridines in Green Media. J. Heterocycl. Chem. 2016, 53, 658–664. [Google Scholar] [CrossRef]
- Park, J.W.; Song, Y.-H. Synthesis of thienopyrimidine-pyrazolo [3,4-b] pyridine hybrids. Heterocycl. Commun. 2017, 23, 281–285. [Google Scholar] [CrossRef]
- Zsolnai, D.; Mayer, P.; Szőri, K.; London, G. Pd/Al2O3-catalysed redox isomerisation of allyl alcohol: Application in aldol condensation and oxidative heterocyclization reactions. Catal. Sci. Technol. 2016, 6, 3814–3820. [Google Scholar] [CrossRef]
- Mazarío, J.; Raad, Z.; Concepción, P.; Cerdá-Moreno, C.; Domine, M.E. Pd supported on mixed metal oxide as an efficient catalyst for the reductive amination of bio-derived acetol to 2-methylpiperazine. Catal. Sci. Technol. 2020, 10, 8049–8063. [Google Scholar] [CrossRef]
- Bellezza, D.; Zaragozá, R.J.; José Aurell, M.; Ballesteros, R.; Ballesteros-Garrido, R. Acceptorless dehydrogenative condensation: Synthesis of indoles and quinolines from diols and anilines. Org. Biomol. Chem. 2021, 19, 677–683. [Google Scholar] [CrossRef]
- Hao, L.; Zhao, Y.; Yu, B.; Zhang, H.; Xu, H.; Liu, Z. Au catalyzed synthesis of benzimidazoles from 2-nitroanilines and CO2/H2. Green Chem. 2014, 16, 3039–3044. [Google Scholar] [CrossRef]
- Tang, L.; Guo, X.; Yang, Y.; Zha, Z.; Wang, Z. Gold nanoparticles supported on titanium dioxide: An efficient catalyst for highly selective synthesis of benzoxazoles and benzimidazoles. Chem. Commun. 2014, 50, 6145–6148. [Google Scholar] [CrossRef]
- Tang, L.; Yang, Y.; Wen, L.; Zhang, S.; Zha, Z.; Wang, Z. Supported gold-catalyzed and ammonia-promoted selective synthesis of quinazolines in aqueous media. Org. Chem. Front. 2015, 2, 114–118. [Google Scholar] [CrossRef]
- Tzani, M.A.; Gabriel, C.; Lykakis, I.N. Selective Synthesis of Benzimidazoles from o-Phenylenediamine and Aldehydes Promoted by Supported Gold Nanoparticles. Nanomaterials 2020, 10, 2405. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wang, Y.; Yu, C.; Zhao, P. Heterogeneously copper-catalyzed oxidative synthesis of imidazo [1,2-a] pyridines using 2-aminopyridines and ketones under ligand-and additive-free conditions. RSC Adv. 2014, 4, 27301–27307. [Google Scholar] [CrossRef]
- Geesi, M.H.; Ouerghi, O.; Elsanousi, A.; Kaiba, A.; Riadi, Y. Ultrasound-Assisted Preparation of Cu-Doped TiO2 Nanoparticles as a Nanocatalyst for Sonochemical Synthesis of Pyridopyrimidines. Polycycl. Aromat. Compd. 2020, 42, 80–90. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S.; Santos, L.L. Multisite Solid Catalyst for Cascade Reactions: The Direct Synthesis of Benzodiazepines from Nitro Compounds. Chem. Eur. J. 2009, 15, 8834–8841. [Google Scholar] [CrossRef]
- Ruiz, V.R.; Corma, A.; Sabater, M.J. New route for the synthesis of benzimidazoles by a one-pot multistep process with mono and bifunctional solid catalysts. Tetrahedron 2010, 66, 730–735. [Google Scholar] [CrossRef]
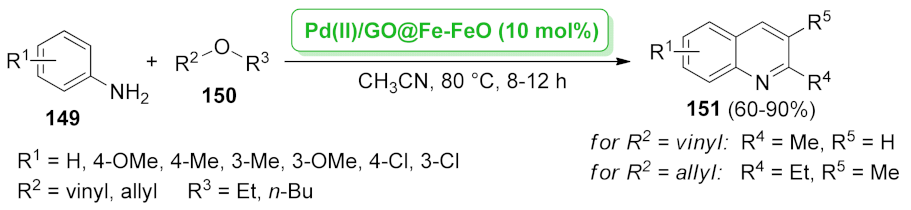
- Climent, M.J.; Corma, A.; Hernández, J.C.; Hungría, A.B.; Iborra, S.; Martínez-Silvestre, S. Biomass into chemicals: One-pot two- and three-step synthesis of quinoxalines from biomass-derived glycols and 1,2-dinitrobenzene derivatives using supported gold nanoparticles as catalysts. J. Catal. 2012, 292, 118–129. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S.; Martínez-Silvestre, S. Gold Catalysis Opens Up a New Route for the Synthesis of Benzimidazoylquinoxaline Derivatives from Biomass-Derived Products (Glycerol). ChemCatChem 2013, 5, 3866–3874. [Google Scholar] [CrossRef]
- Shimura, S.; Miura, H.; Wada, K.; Hosokawa, S.; Yamazoe, S.; Inoue, M. Ceria-supported ruthenium catalysts for the synthesis of indole via dehydrogenative N-heterocyclization. Catal. Sci. Technol. 2011, 1, 1340–1346. [Google Scholar] [CrossRef][Green Version]
- Maddila, S.; Rana, S.; Pagadala, R.; Kankala, S.; Maddila, S.; Jonnalagadda, S.B. Synthesis of pyrazole-4-carbonitrile derivatives in aqueous media with CuO/ZrO2 as recyclable catalyst. Catal. Commun. 2015, 61, 26–30. [Google Scholar] [CrossRef]
- Shabalala, S.; Maddila, S.; van Zyl, W.E.; Jonnalagadda, S.B. A facile, efficacious and reusable Sm2O3/ZrO2 catalyst for the novel synthesis of functionalized 1,4-dihydropyridine derivatives. Catal. Commun. 2016, 79, 21–25. [Google Scholar] [CrossRef]
- Ahmadian, H.; Veisi, H.; Karami, C.; Sedrpoushan, A.; Nouri, M.; Jamshidi, F.; Alavioon, I. Cobalt manganese oxide nanoparticles as recyclable catalyst for efficient synthesis of 2-aryl-1-arylmethyl-1H-1,3-benzimidazoles under solvent-free conditions. Appl. Organomet. Chem. 2015, 29, 266–269. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, J.; Chen, B.; Jing, Z.; Zhao, P. Copper supported on H+-modified manganese oxide octahedral molecular sieves (Cu/H-OMS-2) as a heterogeneous biomimetic catalyst for the synthesis of imidazo [1,2-a]-N-heterocycles. Catal. Sci. Technol. 2016, 6, 890–896. [Google Scholar] [CrossRef]
- Albadi, J.; Alihosseinzadeh, A.; Mansournezhad, A. Regioselective synthesis of 1,2,3-triazoles catalyzed over ZnO supported copper oxide nanocatalyst as a new and efficient recyclable catalyst in water. Acta Chim. Slov. 2015, 62, 8. [Google Scholar] [CrossRef] [PubMed]
- Moromi, S.K.; Touchy, A.S.; Hakim Siddiki, S.M.A.; Ali, M.A.; Shimizu, K.-i. Synthesis of indoles via dehydrogenative N-heterocyclization by supported platinum catalysts. RSC Adv. 2015, 5, 1059–1062. [Google Scholar] [CrossRef]
- Anil Kumar, B.S.P.; Harsha Vardhan Reddy, K.; Madhav, B.; Ramesh, K.; Nageswar, Y.V.D. Magnetically separable CuFe2O4 nano particles catalyzed multicomponent synthesis of 1,4-disubstituted 1,2,3-triazoles in tap water using ‘click chemistry’. Tetrahedron Lett. 2012, 53, 4595–4599. [Google Scholar] [CrossRef]
- Kumar, A.S.; Reddy, M.A.; Knorn, M.; Reiser, O.; Sreedhar, B. Magnetically Recoverable CuFe2O4 Nanoparticles: Catalyzed Synthesis of Aryl Azides and 1,4-Diaryl-1,2,3-triazoles from Boronic Acids in Water. Eur. J. Org. Chem. 2013, 2013, 4674–4680. [Google Scholar] [CrossRef]
- El-Remaily, M.A.E.A.A.A.; Abu-Dief, A.M. CuFe2O4 nanoparticles: An efficient heterogeneous magnetically separable catalyst for synthesis of some novel propynyl-1H-imidazoles derivatives. Tetrahedron 2015, 71, 2579–2584. [Google Scholar] [CrossRef]
- Sanasi, P.D.; Santhipriya, D.; Ramesh, Y.; Kumar, M.R.; Swathi, B.; Rao, K.J. Nano copper and cobalt ferrites as heterogeneous catalysts for the one-pot synthesis of 2,4,5-tri substituted imidazoles. J. Chem. Sci. 2014, 126, 1715–1720. [Google Scholar] [CrossRef]
- Yamane, Y.; Liu, X.; Hamasaki, A.; Ishida, T.; Haruta, M.; Yokoyama, T.; Tokunaga, M. One-Pot Synthesis of Indoles and Aniline Derivatives from Nitroarenes under Hydrogenation Condition with Supported Gold Nanoparticles. Org. Lett. 2009, 11, 5162–5165. [Google Scholar] [CrossRef]
- Moghaddam, F.M.; Koushki Foroushani, B.; Rezvani, H.R. Nickel ferrite nanoparticles: An efficient and reusable nanocatalyst for a neat, one-pot and four-component synthesis of pyrroles. RSC Adv. 2015, 5, 18092–18096. [Google Scholar] [CrossRef]
- Ravikumar Naik, T.R.; Shivashankar, S.A. Heterogeneous bimetallic ZnFe2O4 nanopowder catalyzed synthesis of Hantzsch 1,4-dihydropyridines in water. Tetrahedron Lett. 2016, 57, 4046–4049. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, C.; Wang, S.; Wan, C.; Wang, Z. A novel and efficient methodology for the construction of quinazolines based on supported copper oxide nanoparticles. Chem. Commun. 2010, 46, 5244–5246. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Padala, A.K.; Dar, B.A.; Singh, B.; Sreedhar, B.; Vishwakarma, R.A.; Bharate, S.B. Recyclable clay supported Cu (II) catalyzed tandem one-pot synthesis of 1-aryl-1,2,3-triazoles. Tetrahedron 2012, 68, 8156–8162. [Google Scholar] [CrossRef]
- Dubey, N.; Sharma, P.; Kumar, A. Clay-Supported Cu (II) Catalyst: An Efficient, Heterogeneous, and Recyclable Catalyst for Synthesis of 1,4-Disubstituted 1,2,3-Triazoles from Alloxan-Derived Terminal Alkyne and Substituted Azides Using Click Chemistry. Synth. Commun. 2015, 45, 2608–2626. [Google Scholar] [CrossRef]
- Qiao, B.; Zhang, L.; Li, R. A highly active and recyclable catalyst for the synthesis of indole and phenyl ether. RSC Adv. 2015, 5, 93463–93469. [Google Scholar] [CrossRef]
- Pagadala, R.; Maddila, S.; Dasireddy, V.D.B.C.; Jonnalagadda, S.B. Zn-VCO3 hydrotalcite: A highly efficient and reusable heterogeneous catalyst for the Hantzsch dihydropyridine reaction. Catal. Commun. 2014, 45, 148–152. [Google Scholar] [CrossRef]
- Yum, E.K.; Hong, K.B. Synthesis of pyrrolo-heterocycles via Pd-loaded zeolite catalyzed annulation of o-haloaromatic amine with terminal alkynes. Tetrahedron 2017, 73, 6581–6586. [Google Scholar] [CrossRef]
- Brindha, K.; Amutha, P.; Krishnakumar, B.; do Nascimento Sobral, A.J.F. BiCl3-modified perlite as an effective catalyst for selective organic transformations: A green protocol. Res. Chem. Intermed. 2019, 45, 4367–4381. [Google Scholar] [CrossRef]
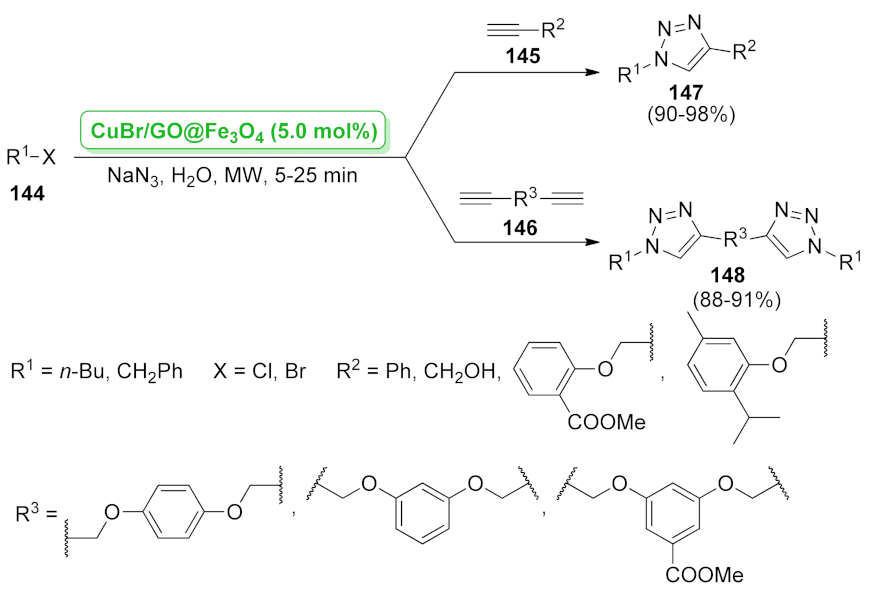
- Xiong, X.; Chen, H.; Tang, Z.; Jiang, Y. Supported CuBr on graphene oxide/Fe3O4: A highly efficient, magnetically separable catalyst for the multi-gram scale synthesis of 1,2,3-triazoles. RSC Adv. 2014, 4, 9830–9837. [Google Scholar] [CrossRef]
- Verma, S.; Verma, D.; Jain, S.L. Magnetically separable palladium-graphene nanocomposite as heterogeneous catalyst for the synthesis of 2-alkylquinolines via one pot reaction of anilines with alkenyl ethers. Tetrahedron Lett. 2014, 55, 2406–2409. [Google Scholar] [CrossRef]
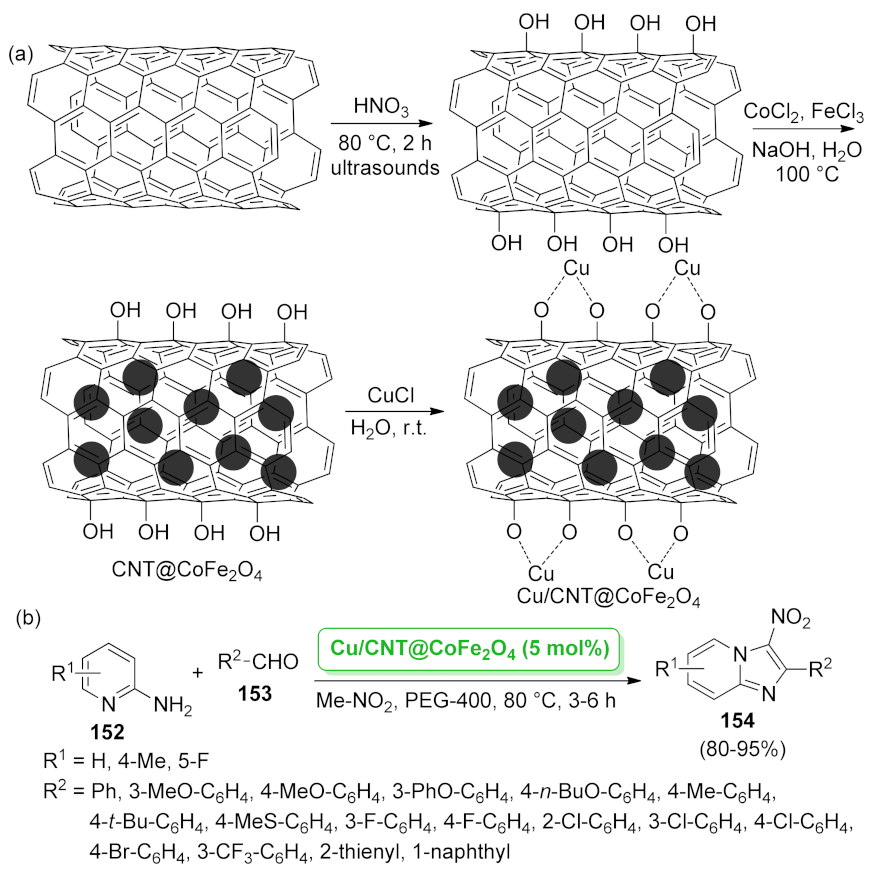
- Zhang, M.; Lu, J.; Zhang, J.-N.; Zhang, Z.-H. Magnetic carbon nanotube supported Cu (CoFe2O4/CNT-Cu) catalyst: A sustainable catalyst for the synthesis of 3-nitro-2-arylimidazo [1,2-a] pyridines. Catal. Commun. 2016, 78, 26–32. [Google Scholar] [CrossRef]
- Prakash, P.; Kumar, R.A.; Miserque, F.; Geertsen, V.; Gravel, E.; Doris, E. Carbon nanotube-copper ferrite-catalyzed aqueous 1,3-dipolar cycloaddition of in situ-generated organic azides with alkynes. Chem. Commun. 2018, 54, 3644–3647. [Google Scholar] [CrossRef]
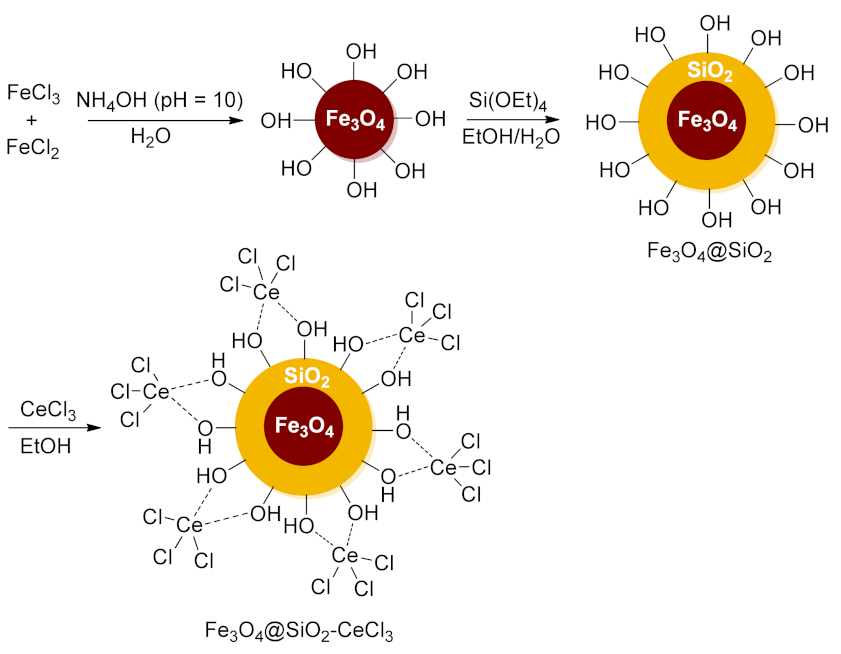
- Zhou, L.; Wang, M.; Wang, K.; Wen, T.; Wang, L. Fe3O4@SiO2-CeCl3 Catalyzed Chemoselective Synthesis of Functionalized 3-Substituted-1,5-Benzodiazepines via One-pot Multicomponent and Domino Reactions. Appl. Organomet. Chem. 2020, 34, e5707. [Google Scholar] [CrossRef]
- Hajizadeh, Z.; Radinekiyan, F.; Eivazzadeh-keihan, R.; Maleki, A. Development of novel and green NiFe2O4/geopolymer nanocatalyst based on bentonite for synthesis of imidazole heterocycles by ultrasonic irradiations. Sci. Rep. 2020, 10, 11671. [Google Scholar] [CrossRef] [PubMed]
- Bergbreiter, D.E. Organic Polymers as a Catalyst Recovery Vehicle. In Chiral Catalyst Immobilization and Recycling; De Vos, D.E., Vankelecom, I.F.J., Jacobs, P.A., Eds.; Wiley-VCH: Weinheim, Germany, 2000; pp. 43–80. [Google Scholar]
- Adharvana Chari, M.; Syamasundar, K. Polymer (PVP) supported ferric chloride: An efficient and recyclable heterogeneous catalyst for high yield synthesis of 1,5-benzodiazepine derivatives under solvent free conditions and microwave irradiation. Catal. Commun. 2005, 6, 67–70. [Google Scholar] [CrossRef]
- Hashemi, E.; Beheshtiha, Y.S.; Ahmadi, S.; Heravi, M.M. In situ prepared CuI nanoparticles on modified poly (styrene-co-maleic anhydride): An efficient and recyclable catalyst for the azide-alkyne click reaction in water. Transit. Met. Chem. 2014, 39, 593–601. [Google Scholar] [CrossRef]
- Reddy, C.B.; Bharti, R.; Kumar, S.; Das, P. Supported palladium nanoparticles-catalyzed decarboxylative coupling approaches to aryl alkynes, indoles and pyrrolines synthesis. RSC Adv. 2016, 6, 71117–71121. [Google Scholar] [CrossRef]
- Abdollahi-Alibeik, M.; Moosavifard, M. FeCl3-Doped Polyaniline Nanoparticles as Reusable Heterogeneous Catalyst for the Synthesis of 2-Substituted Benzimidazoles. Synth. Commun. 2010, 40, 2686–2695. [Google Scholar] [CrossRef]
- Chopra, R.; Kumar, M.; Neelam, N.; Bhalla, V. Visible light promoted PANI@Au: CuO catalyzed sequential amination, azidation and annulation for the preparation of 2-arylbenzimidazoles. Green Chem. 2019, 21, 3666–3674. [Google Scholar] [CrossRef]
- Santra, S.; Dhara, K.; Ranjan, P.; Bera, P.; Dash, J.; Mandal, S.K. A supported palladium nanocatalyst for copper free acyl Sonogashira reactions: One-pot multicomponent synthesis of N-containing heterocycles. Green Chem. 2011, 13, 3238–3247. [Google Scholar] [CrossRef]
- Albano, G.; Evangelisti, C.; Aronica, L.A. Palladium Nanoparticles Supported on Smopex-234® as Valuable Catalysts for the Synthesis of Heterocycles. Catalysts 2021, 11, 706. [Google Scholar] [CrossRef]
- Albano, G.; Evangelisti, C.; Aronica, L.A. Hydrogenolysis of Benzyl Protected Phenols and Aniline Promoted by Supported Palladium Nanoparticles. ChemistrySelect 2017, 2, 384–388. [Google Scholar] [CrossRef]
- Albano, G.; Interlandi, S.; Evangelisti, C.; Aronica, L.A. Polyvinylpyridine-Supported Palladium Nanoparticles: A Valuable Catalyst for the Synthesis of Alkynyl Ketones via Acyl Sonogashira Reactions. Catal. Lett. 2020, 150, 652–659. [Google Scholar] [CrossRef]
- Rad, M.N.S.; Behrouz, S.; Movahedian, A.; Doroodmand, M.M.; Ghasemi, Y.; Rasoul-Amini, S.; Gandomani, A.-R.A.; Rezaie, R. Doped Nano-Sized Copper (I) Oxide (Cu2O) on Melamine—Formaldehyde Resin: A Highly Efficient Heterogeneous Nano Catalyst for ‘Click’ Synthesis of Some Novel 1H-1,2,3-Triazole Derivatives Having Antibacterial Activity. Helv. Chim. Acta 2013, 96, 688–701. [Google Scholar] [CrossRef]
- Elavarasan, S.; Bhaumik, A.; Sasidharan, M. An Efficient Mesoporous Cu-Organic Nanorod for Friedländer Synthesis of Quinoline and Click Reactions. ChemCatChem 2019, 11, 4340–4350. [Google Scholar] [CrossRef]
- Khatun, R.; Biswas, S.; Biswas, I.H.; Riyajuddin, S.; Haque, N.; Ghosh, K.; Islam, S.M. Cu-NPs@COF: A potential heterogeneous catalyst for CO2 fixation to produce 2-oxazolidinones as well as benzimidazoles under moderate reaction conditions. J. CO2 Util. 2020, 40, 101180. [Google Scholar] [CrossRef]
- Grigg, R.; York, M. Bimetallic catalytic cascade ring closing metathesis—intramolecular Heck reactions using a fluorous biphasic solvent system or a polymer-supported palladium catalyst. Tetrahedron Lett. 2000, 41, 7255–7258. [Google Scholar] [CrossRef]
- Bakherad, M.; Bahramian, B.; Nasr-Isfahani, H.; Keivanloo, A.; Doostmohammadi, N. Polystyrene-supported palladium (II) ethylenediamine complex: A highly active and recyclable catalyst for the synthesis of 2-benzylimidazo [2,1-b] pyridines through heteroannulation of acetylenic compounds. J. Heterocycl. Chem. 2009, 46, 100–104. [Google Scholar] [CrossRef]
- Molla, R.A.; Roy, A.S.; Ghosh, K.; Salam, N.; Iqubal, M.A.; Tuhina, K.; Islam, S.M. Polymer anchored ruthenium complex: A highly active and recyclable catalyst for one-pot azide-alkyne cycloaddition and transfer-hydrogenation of ketones under mild conditions. J. Organomet. Chem. 2015, 776, 170–179. [Google Scholar] [CrossRef]
- Chtchigrovsky, M.; Primo, A.; Gonzalez, P.; Molvinger, K.; Robitzer, M.; Quignard, F.; Taran, F. Functionalized Chitosan as a Green, Recyclable, Biopolymer-Supported Catalyst for the [3+2] Huisgen Cycloaddition. Angew. Chem. Int. Ed. 2009, 48, 5916–5920. [Google Scholar] [CrossRef]
- Chan, T.R.; Fokin, V.V. Polymer-Supported Copper (I) Catalysts for the Experimentally Simplified Azide-Alkyne Cycloaddition. QSAR Comb. Sci. 2007, 26, 1274–1279. [Google Scholar] [CrossRef]
- Pucci, A.; Albano, G.; Pollastrini, M.; Lucci, A.; Colalillo, M.; Oliva, F.; Evangelisti, C.; Marelli, M.; Santalucia, D.; Mandoli, A. Supported Tris-Triazole Ligands for Batch and Continuous-Flow Copper-Catalyzed Huisgen 1,3-Dipolar Cycloaddition Reactions. Catalysts 2020, 10, 434. [Google Scholar] [CrossRef]
- Alacid, E.; Nájera, C. Arylation of Allyl Alcohols in Organic and Aqueous Media Catalyzed by Oxime-Derived Palladacycles: Synthesis of β-Arylated Carbonyl Compounds. Adv. Synth. Catal. 2007, 349, 2572–2584. [Google Scholar] [CrossRef]
- Biswas, I.H.; Biswas, S.; Islam, M.S.; Riyajuddin, S.; Sarkar, P.; Ghosh, K.; Islam, S.M. Catalytic synthesis of benzimidazoles and organic carbamates using a polymer supported zinc catalyst through CO2 fixation. New J. Chem. 2019, 43, 14643–14652. [Google Scholar] [CrossRef]
- Kröll, R.M.; Schuler, N.; Lubbad, S.; Buchmeiser, M.R. A ROMP-derived, polymer-supported chiral Schrock catalyst for enantioselective ring-closing olefin metathesis. Chem. Commun. 2003, 21, 2742–2743. [Google Scholar] [CrossRef] [PubMed]
- Hlil, A.R.; Moncho, S.; Tuba, R.; Elsaid, K.; Szarka, G.; Brothers, E.N.; Grubbs, R.H.; Al-Hashimi, M.; Bazzi, H.S. Synthesis and catalytic activity of supported acenaphthoimidazolylidene N-heterocyclic carbene ruthenium complex for ring closing metathesis (RCM) and ring opening metathesis polymerization (ROMP). J. Catal. 2016, 344, 100–107. [Google Scholar] [CrossRef]
- Chtchigrovsky, M.; Lin, Y.; Ouchaou, K.; Chaumontet, M.; Robitzer, M.; Quignard, F.; Taran, F. Dramatic Effect of the Gelling Cation on the Catalytic Performances of Alginate-Supported Palladium Nanoparticles for the Suzuki-Miyaura Reaction. Chem. Mater. 2012, 24, 1505–1510. [Google Scholar] [CrossRef]
- Xiang, Z.; Chen, Y.; Liu, Q.; Lu, F. A highly recyclable dip-catalyst produced from palladium nanoparticle-embedded bacterial cellulose and plant fibers. Green Chem. 2018, 20, 1085–1094. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Z.; Chen, J. Applications of lignin-derived catalysts for green synthesis. Green Energy Environ. 2019, 4, 210–244. [Google Scholar] [CrossRef]
- Rizzo, G.; Albano, G.; Lo Presti, M.; Milella, A.; Omenetto, F.G.; Farinola, G.M. Palladium Supported on Silk Fibroin for Suzuki-Miyaura Cross-Coupling Reactions. Eur. J. Org. Chem. 2020, 2020, 6992–6996. [Google Scholar] [CrossRef]
- Khalafi-Nezhad, A.; Mohammadi, S. Highly efficient synthesis of 1- and 5-substituted 1H-tetrazoles using chitosan derived magnetic ionic liquid as a recyclable biopolymer-supported catalyst. RSC Adv. 2013, 3, 4362–4371. [Google Scholar] [CrossRef]
- Baig, R.B.N.; Varma, R.S. Copper on chitosan: A recyclable heterogeneous catalyst for azide-alkyne cycloaddition reactions in water. Green Chem. 2013, 15, 1839–1843. [Google Scholar] [CrossRef]
- Lai, B.; Ye, M.; Liu, P.; Li, M.; Bai, R.; Gu, Y. A novel and robust heterogeneous Cu catalyst using modified lignosulfonate as support for the synthesis of nitrogen-containing heterocycles. Beilstein J. Org. Chem. 2020, 16, 2888–2902. [Google Scholar] [CrossRef] [PubMed]
- Sharghi, H.; Ebrahimpourmoghaddam, S.; Doroodmand, M.M.; Purkhosrow, A. Synthesis of Vasorelaxaing 1,4-Disubstituted 1,2,3-Triazoles Catalyzed by a 4′-Phenyl-2,2′:6′,2′′-Terpyridine Copper (II) Complex Immobilized on Activated Multiwalled Carbon Nanotubes. Asian J. Org. Chem. 2012, 1, 377–388. [Google Scholar] [CrossRef]
- Sharghi, H.; Ebrahimpourmoghaddam, S.; Doroodmand, M.M. Facile synthesis of 5-substituted-1H-tetrazoles and 1-substituted-1H-tetrazoles catalyzed by recyclable 4′-phenyl-2,2′:6′,2″-terpyridine copper (II) complex immobilized onto activated multi-walled carbon nanotubes. J. Organomet. Chem. 2013, 738, 41–48. [Google Scholar] [CrossRef]
- Liu, G.; Wu, B.; Zhang, J.; Wang, X.; Shao, M.; Wang, J. Controlled Reversible Immobilization of Ru Carbene on Single-Walled Carbon Nanotubes: A New Strategy for Green Catalytic Systems Based on a Solvent Effect on π-π Interaction. Inorg. Chem. 2009, 48, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Nasrallah, H.; Germain, S.; Queval, P.; Bouvier, C.; Mauduit, M.; Crévisy, C.; Schulz, E. Non covalent immobilization of pyrene-tagged ruthenium complexes onto graphene surfaces for recycling in olefin metathesis reactions. J. Mol. Catal. A Chem. 2016, 425, 136–146. [Google Scholar] [CrossRef]
- Shaygan Nia, A.; Rana, S.; Döhler, D.; Jirsa, F.; Meister, A.; Guadagno, L.; Koslowski, E.; Bron, M.; Binder, W.H. Carbon-Supported Copper Nanomaterials: Recyclable Catalysts for Huisgen [3+2] Cycloaddition Reactions. Chem. Eur. J. 2015, 21, 10763–10770. [Google Scholar] [CrossRef]
- Collinson, J.-M.; Wilton-Ely, J.D.E.T.; Díez-González, S. Reusable and highly active supported copper (i)-NHC catalysts for Click chemistry. Chem. Commun. 2013, 49, 11358–11360. [Google Scholar] [CrossRef][Green Version]
- Sharghi, H.; Shiri, P.; Aberi, M. Five-membered N-Heterocycles Synthesis Catalyzed by Nano-silica Supported Copper (II)-2-imino-1,2-diphenylethan-1-ol Complex. Catal. Lett. 2017, 147, 2844–2862. [Google Scholar] [CrossRef]
- Sharghi, H.; Aberi, M.; Shiri, P. Supported benzimidazole-salen Cu (II) complex: An efficient, versatile and highly reusable nanocatalyst for one-pot synthesis of hybrid molecules. Appl. Organomet. Chem. 2018, 32, e4446. [Google Scholar] [CrossRef]
- Sharghi, H.; Aberi, M.; Shiri, P. Silica-supported Cu (II)-quinoline complex: Efficient and recyclable nanocatalyst for one-pot synthesis of benzimidazolquinoline derivatives and 2H-indazoles. Appl. Organomet. Chem. 2019, 33, e4974. [Google Scholar] [CrossRef]
- Veerakumar, P.; Velayudham, M.; Lu, K.-L.; Rajagopal, S. Highly dispersed silica-supported nanocopper as an efficient heterogeneous catalyst: Application in the synthesis of 1,2,3-triazoles and thioethers. Catal. Sci. Technol. 2011, 1, 1512–1525. [Google Scholar] [CrossRef]
- Nasr-Esfahani, M.; Mohammadpoor-Baltork, I.; Khosropour, A.R.; Moghadam, M.; Mirkhani, V.; Tangestaninejad, S. Synthesis and characterization of Cu (II) containing nanosilica triazine dendrimer: A recyclable nanocomposite material for the synthesis of benzimidazoles, benzothiazoles, bis-benzimidazoles and bis-benzothiazoles. J. Mol. Catal. A Chem. 2013, 379, 243–254. [Google Scholar] [CrossRef]
- Jumde, R.P.; Evangelisti, C.; Mandoli, A.; Scotti, N.; Psaro, R. Aminopropyl-silica-supported Cu nanoparticles: An efficient catalyst for continuous-flow Huisgen azide-alkyne cycloaddition (CuAAC). J. Catal. 2015, 324, 25–31. [Google Scholar] [CrossRef]
- Sarmiento, J.T.; Suárez-Pantiga, S.; Olmos, A.; Varea, T.; Asensio, G. Silica-Immobilized NHC-Gold (I) Complexes: Versatile Catalysts for the Functionalization of Alkynes under Batch and Continuous Flow Conditions. ACS Catal. 2017, 7, 7146–7155. [Google Scholar] [CrossRef]
- Tyrrell, E.; Whiteman, L.; Williams, N. Sonogashira Cross-Coupling Reactions and Construction of the Indole Ring System Using a Robust, Silica-Supported Palladium Catalyst. Synthesis 2009, 2009, 829–835. [Google Scholar] [CrossRef]
- Abarghooei, M.A.; Mohebat, R.; Karimi-Jaberi, Z.; Mosslemin, M.H. Nano-silica supported palladium nanoparticles: A sustainable nanocatalyst for efficient synthesis of 2,3-diarylimidazo [1,2-a] pyridines at low catalyst loading. Catal. Commun. 2018, 105, 59–64. [Google Scholar] [CrossRef]
- Michalek, F.; Bannwarth, W. Application of a Grubbs-Hoveyda Metathesis Catalyst Noncovalently Immobilized by Fluorous-Fluorous Interactions. Helv. Chim. Acta 2006, 89, 1030–1037. [Google Scholar] [CrossRef]
- Allen, D.P.; Van Wingerden, M.M.; Grubbs, R.H. Well-Defined Silica-Supported Olefin Metathesis Catalysts. Org. Lett. 2009, 11, 1261–1264. [Google Scholar] [CrossRef]
- Monge-Marcet, A.; Pleixats, R.; Cattoën, X.; Wong Chi Man, M. Sol-gel immobilized Hoveyda-Grubbs complex through the NHC ligand: A recyclable metathesis catalyst. J. Mol. Catal. A Chem. 2012, 357, 59–66. [Google Scholar] [CrossRef]
- Sharma, R.K.; Sharma, C. Zirconium (IV)-modified silica gel: Preparation, characterization and catalytic activity in the synthesis of some biologically important molecules. Catal. Commun. 2011, 12, 327–331. [Google Scholar] [CrossRef]
- Hajjami, M.; Ghorbani, F.; Yousofvand, Z. Copper (I) complex of 1,3-DimethylBarbituric acid modified SBA-15 and its catalytic role for the synthesis of 2,3-Dihydroquinazolin-4(1H)-ones and Imidazoles. Appl. Organomet. Chem. 2017, 31, e3843. [Google Scholar] [CrossRef]
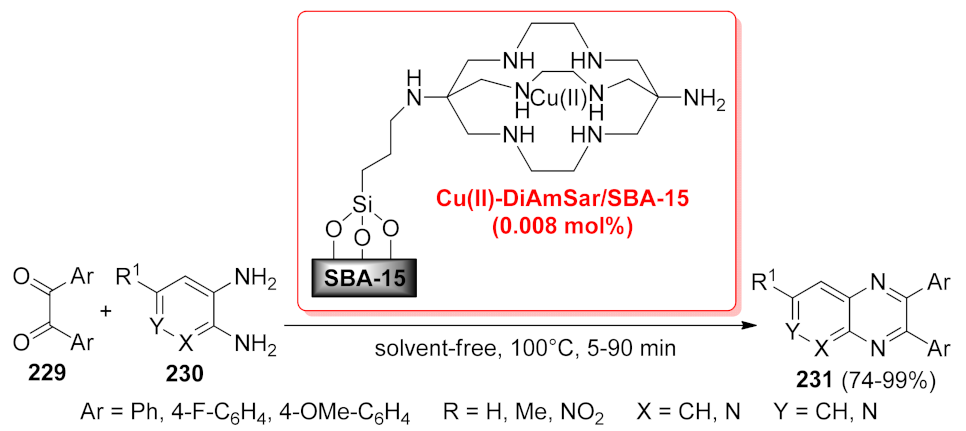
- Mohammadi, M.; Bardajee, G.R.; Pesyan, N.N. Efficient solvent-free synthesis of pyridopyrazine and quinoxaline derivatives using copper-DiAmSar complex anchored on SBA-15 as a reusable catalyst. Chin. J. Catal. 2015, 36, 1379–1386. [Google Scholar] [CrossRef]
- Malakooti, R.; Bardajee, G.R.; Hadizadeh, S.; Atashin, H.; Khanjari, H. An iron Schiff base complex loaded mesoporous silica nanoreactor as a catalyst for the synthesis of pyrazine-based heterocycles. Transit. Met. Chem. 2014, 39, 47–54. [Google Scholar] [CrossRef]
- Malakooti, R.; Bardajee, G.R.; Mahmoudi, H.; Kakavand, N. Zirconium Schiff-Base Complex Modified Mesoporous Silica as an Efficient Catalyst for the Synthesis of Nitrogen Containing Pyrazine Based Heterocycles. Catal. Lett. 2013, 143, 853–861. [Google Scholar] [CrossRef]
- Bardajee, G.R.; Malakooti, R.; Abtin, I.; Atashin, H. Palladium Schiff-base complex loaded SBA-15 as a novel nanocatalyst for the synthesis of 2,3-disubstituted quinoxalines and pyridopyrazine derivatives. Microporous Mesoporous Mater. 2013, 169, 67–74. [Google Scholar] [CrossRef]
- Batail, N.; Bendjeriou, A.; Djakovitch, L.; Dufaud, V. Larock indole synthesis using palladium complexes immobilized onto mesoporous silica. Appl. Catal. A 2010, 388, 179–187. [Google Scholar] [CrossRef]
- Skowerski, K.; Pastva, J.; Czarnocki, S.J.; Janoscova, J. Exceptionally Stable and Efficient Solid Supported Hoveyda-Type Catalyst. Org. Process Res. Dev. 2015, 19, 872–877. [Google Scholar] [CrossRef]
- Nie, Q.; Yao, F.; Yi, F.; Cai, M. A heterogeneous gold (I)-catalyzed cascade annulation of aldehydes with propargylamine leading to 3-substituted 2,5-dimethylpyrazines. J. Organomet. Chem. 2017, 846, 343–350. [Google Scholar] [CrossRef]
- Wei, L.; Yao, F.; Yi, F.; Cai, M. Heterogeneous Gold (I)-Catalysed Annulation between 2-Aminopyridines and Propiolaldehydes Leading to 3-Acylimidazo [1,2-A] Pyridines. J. Chem. Res. 2018, 42, 341–346. [Google Scholar] [CrossRef]
- Jiang, G.; Lin, Y.; Cai, M.; Zhao, H. Recyclable Heterogeneous Copper (II)-Catalyzed Oxidative Cyclization of 2-Pyridine Ketone Hydrazones Towards [1,2,3] Triazolo [1,5-a] pyridines. Synthesis 2019, 51, 4487–4497. [Google Scholar] [CrossRef]
- Garcés, K.; Fernández-Alvarez, F.J.; García-Orduña, P.; Lahoz, F.J.; Pérez-Torrente, J.J.; Oro, L.A. Grafting of Copper (I)-NHC Species on MCM-41: Homogeneous versus Heterogeneous Catalysis. ChemCatChem 2015, 7, 2501–2507. [Google Scholar] [CrossRef]
- Nikoorazm, M.; Tahmasbi, B.; Gholami, S.; Moradi, P. Copper and nickel immobilized on cytosine@MCM-41: As highly efficient, reusable and organic-inorganic hybrid nanocatalysts for the homoselective synthesis of tetrazoles and pyranopyrazoles. Appl. Organomet. Chem. 2020, 34, e5919. [Google Scholar] [CrossRef]
- Deiana, L.; Afewerki, S.; Palo-Nieto, C.; Verho, O.; Johnston, E.V.; Córdova, A. Highly Enantioselective Cascade Transformations by Merging Heterogeneous Transition Metal Catalysis with Asymmetric Aminocatalysis. Sci. Rep. 2012, 2, 851. [Google Scholar] [CrossRef]
- Deiana, L.; Jiang, Y.; Palo-Nieto, C.; Afewerki, S.; Incerti-Pradillos, C.A.; Verho, O.; Tai, C.-W.; Johnston, E.V.; Córdova, A. Combined Heterogeneous Metal/Chiral Amine: Multiple Relay Catalysis for Versatile Eco-Friendly Synthesis. Angew. Chem. Int. Ed. 2014, 53, 3447–3451. [Google Scholar] [CrossRef]
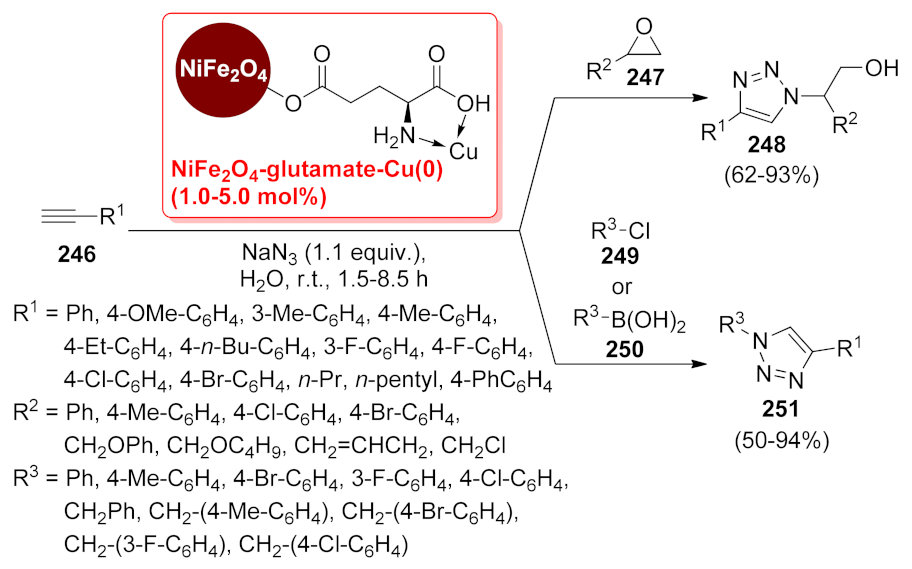
- Lu, J.; Ma, E.-Q.; Liu, Y.-H.; Li, Y.-M.; Mo, L.-P.; Zhang, Z.-H. One-pot three-component synthesis of 1,2,3-triazoles using magnetic NiFe2O4-glutamate-Cu as an efficient heterogeneous catalyst in water. RSC Adv. 2015, 5, 59167–59185. [Google Scholar] [CrossRef]
- Norouzi, M.; Ghorbani-Choghamarani, A.; Nikoorazm, M. Heterogeneous Cu (ii)/l-His@Fe3O4 nanocatalyst: A novel, efficient and magnetically-recoverable catalyst for organic transformations in green solvents. RSC Adv. 2016, 6, 92387–92401. [Google Scholar] [CrossRef]
- Habibi, D.; Pakravan, N.; Arabi, A.; Kaboudvand, Z. Preparation of Fe3O4@5,10-dihydropyrido [2,3-b] quinoxaline-7,8-diol copper complex: A capable nanocatalyst for the green synthesis of 1-substituted 1H-tetrazoles. Appl. Organomet. Chem. 2018, 32, e3988. [Google Scholar] [CrossRef]
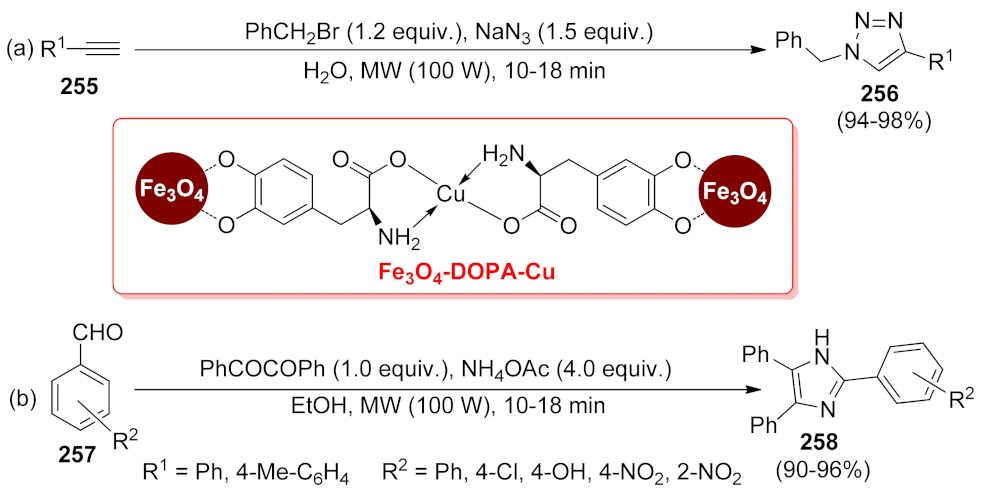
- Kumari, M.; Jain, Y.; Yadav, P.; Laddha, H.; Gupta, R. Synthesis of Fe3O4-DOPA-Cu Magnetically Separable Nanocatalyst: A Versatile and Robust Catalyst for an Array of Sustainable Multicomponent Reactions under Microwave Irradiation. Catal. Lett. 2019, 149, 2180–2194. [Google Scholar] [CrossRef]
- Darabi, M.; Tamoradi, T.; Ghadermazi, M.; Ghorbani-Choghamarani, A. A magnetically retrievable heterogeneous copper nanocatalyst for the synthesis of 5-substituted tetrazoles and oxidation reactions. Transit. Met. Chem. 2017, 42, 703–710. [Google Scholar] [CrossRef]
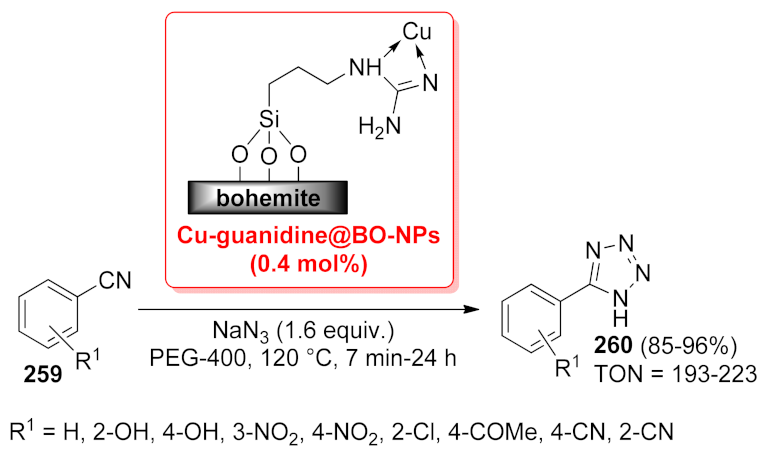
- Jafari, F.; Ghorbani-Choghamarani, A.; Hasanzadeh, N. Guanidine complex of copper supported on boehmite nanoparticles as practical, recyclable, chemo and homoselective organic-inorganic hybrid nanocatalyst for organic reactions. Appl. Organomet. Chem. 2020, 34, e5901. [Google Scholar] [CrossRef]
- Dehghani, F.; Sardarian, A.R.; Esmaeilpour, M. Salen complex of Cu (II) supported on superparamagnetic Fe3O4@SiO2 nanoparticles: An efficient and recyclable catalyst for synthesis of 1- and 5-substituted 1H-tetrazoles. J. Organomet. Chem. 2013, 743, 87–96. [Google Scholar] [CrossRef]
- Xiong, X.; Cai, L. Application of magnetic nanoparticle-supported CuBr: A highly efficient and reusable catalyst for the one-pot and scale-up synthesis of 1,2,3-triazoles under microwave-assisted conditions. Catal. Sci. Technol. 2013, 3, 1301–1307. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Jamwal, B.; Paul, S. Novel Cu (0)-Fe3O4@SiO2/NH2cel as an Efficient and Sustainable Magnetic Catalyst for the Synthesis of 1,4-Disubstituted-1,2,3-triazoles and 2-Substituted-Benzothiazoles via One-Pot Strategy in Aqueous Media. Catal. Lett. 2016, 146, 629–644. [Google Scholar] [CrossRef]
- Esmaeilpour, M.; Sardarian, A.R.; Firouzabadi, H. Dendrimer-encapsulated Cu (II) nanoparticles immobilized on superparamagnetic Fe3O4@SiO2 nanoparticles as a novel recyclable catalyst for N-arylation of nitrogen heterocycles and green synthesis of 5-substituted 1H-tetrazoles. Appl. Organomet. Chem. 2018, 32, e4300. [Google Scholar] [CrossRef]
- Wang, D.; Etienne, L.; Echeverria, M.; Moya, S.; Astruc, D. A Highly Active and Magnetically Recoverable Tris (triazolyl)-CuI Catalyst for Alkyne-Azide Cycloaddition Reactions. Chem. Eur. J. 2014, 20, 4047–4054. [Google Scholar] [CrossRef]
- Moghaddam, F.M.; Ayati, S.E. Copper immobilized onto a triazole functionalized magnetic nanoparticle: A robust magnetically recoverable catalyst for “click” reactions. RSC Adv. 2015, 5, 3894–3902. [Google Scholar] [CrossRef]
- Moghaddam, F.M.; Ayati, S.E.; Firouzi, H.R.; Ghorbani, F. Immobilization of copper ions onto α-amidotriazole-functionalized magnetic nanoparticles and their application in the synthesis of triazole derivatives in water. Appl. Organomet. Chem. 2016, 30, 488–493. [Google Scholar] [CrossRef]
- Ariannezhad, M.; Habibi, D.; Heydari, S. Copper nanoparticles: A capable and versatile catalyst for the synthesis of diverse 1-phenyl-1H-tetrazoles from amino acids. Polyhedron 2019, 160, 170–179. [Google Scholar] [CrossRef]
- Vibhute, S.P.; Mhaldar, P.M.; Korade, S.N.; Gaikwad, D.S.; Shejawal, R.V.; Pore, D.M. Synthesis of magnetically separable catalyst Cu-ACP-Am-Fe3O4@SiO2 for Huisgen 1,3-dipolar cycloaddition. Tetrahedron Lett. 2018, 59, 3643–3652. [Google Scholar] [CrossRef]
- Tajbakhsh, M.; Farhang, M.; Hosseinzadeh, R.; Sarrafi, Y. Nano Fe3O4 supported biimidazole Cu (i) complex as a retrievable catalyst for the synthesis of imidazo [1,2-a] pyridines in aqueous medium. RSC Adv. 2014, 4, 23116–23124. [Google Scholar] [CrossRef]
- Tajbakhsh, M.; Farhang, M.; Baghbanian, S.M.; Hosseinzadeh, R.; Tajbakhsh, M. Nano magnetite supported metal ions as robust, efficient and recyclable catalysts for green synthesis of propargylamines and 1,4-disubstituted 1,2,3-triazoles in water. New J. Chem. 2015, 39, 1827–1839. [Google Scholar] [CrossRef]
- Esmaeilpour, M.; Javidi, J.; Zahmatkesh, S. One-pot synthesis of 1- and 5-substituted 1H-tetrazoles using 1,4-dihydroxyanthraquinone-copper (II) supported on superparamagnetic Fe3O4@SiO2 magnetic porous nanospheres as a recyclable catalyst. Appl. Organomet. Chem. 2016, 30, 897–904. [Google Scholar] [CrossRef]
- Zahmatkesh, S.; Esmaeilpour, M.; Javidi, J. 1,4-Dihydroxyanthraquinone-copper (ii) supported on superparamagnetic Fe3O4@SiO2: An efficient catalyst for N-arylation of nitrogen heterocycles and alkylamines with aryl halides and click synthesis of 1-aryl-1,2,3-triazole derivatives. RSC Adv. 2016, 6, 90154–90164. [Google Scholar] [CrossRef]
- Sardarian, A.R.; Eslahi, H.; Esmaeilpour, M. Copper (II) Complex Supported on Fe3O4@SiO2 Coated by Polyvinyl Alcohol as Reusable Nanocatalyst in N-Arylation of Amines and N (H)-Heterocycles and Green Synthesis of 1H-Tetrazoles. ChemistrySelect 2018, 3, 1499–1511. [Google Scholar] [CrossRef]
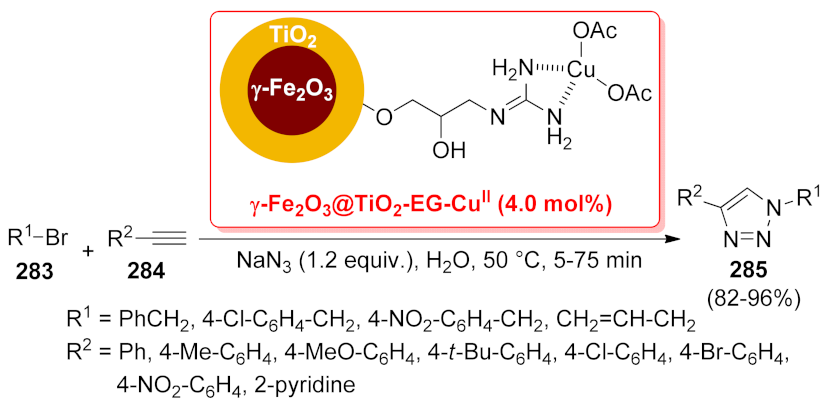
- Jahanshahi, R.; Akhlaghinia, B. CuII immobilized on guanidinated epibromohydrin functionalized γ-Fe2O3@TiO2 (γ-Fe2O3@TiO2-EG-CuII): A novel magnetically recyclable heterogeneous nanocatalyst for the green one-pot synthesis of 1,4-disubstituted 1,2,3-triazoles through alkyne-azide cycloaddition in water. RSC Adv. 2016, 6, 29210–29219. [Google Scholar]
- Chen, S.-W.; Zhang, Z.-C.; Zhai, N.-N.; Zhong, C.-M.; Lee, S.-G. The effect of silica-coating on catalyst recyclability in ionic magnetic nanoparticle-supported Grubbs-Hoveyda catalysts for ring-closing metathesis. Tetrahedron 2015, 71, 648–653. [Google Scholar] [CrossRef]
- Rakhtshah, J.; Salehzadeh, S.; Gowdini, E.; Maleki, F.; Baghery, S.; Zolfigol, M.A. Synthesis of pyrazole derivatives in the presence of a dioxomolybdenum complex supported on silica-coated magnetite nanoparticles as an efficient and easily recyclable catalyst. RSC Adv. 2016, 6, 104875–104885. [Google Scholar] [CrossRef]
- Li, B.-L.; Zhang, M.; Hu, H.-C.; Du, X.; Zhang, Z.-H. Nano-CoFe2O4 supported molybdenum as an efficient and magnetically recoverable catalyst for a one-pot, four-component synthesis of functionalized pyrroles. New J. Chem. 2014, 38, 2435–2442. [Google Scholar] [CrossRef]
- Li, B.-L.; Hu, H.-C.; Mo, L.-P.; Zhang, Z.-H. Nano CoFe2O4 supported antimony (iii) as an efficient and recyclable catalyst for one-pot three-component synthesis of multisubstituted pyrroles. RSC Adv. 2014, 4, 12929–12943. [Google Scholar] [CrossRef]
- Ma, F.-P.; Li, P.-H.; Li, B.-L.; Mo, L.-P.; Liu, N.; Kang, H.-J.; Liu, Y.-N.; Zhang, Z.-H. A recyclable magnetic nanoparticles supported antimony catalyst for the synthesis of N-substituted pyrroles in water. Appl. Catal. A 2013, 457, 34–41. [Google Scholar] [CrossRef]
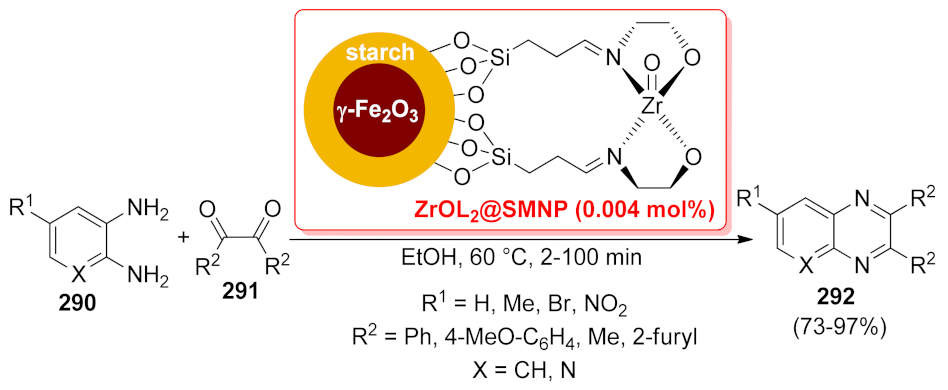
- Jafarpour, M.; Rezaeifard, A. A zirconium Schiff base complex immobilized on starch-coated maghemite nanoparticles catalyzes heterogeneous condensation of 1,2-diamines with 1,2-dicarbonyl compounds. Transit. Met. Chem. 2016, 41, 205–211. [Google Scholar] [CrossRef]
- Maleki, A.; Movahed, H.; Paydar, R. Design and development of a novel cellulose/γ-Fe2O3/Ag nanocomposite: A potential green catalyst and antibacterial agent. RSC Adv. 2016, 6, 13657–13665. [Google Scholar] [CrossRef]
- Mahdavi, M.; Lijan, H.; Bahadorikhalili, S.; Ma’mani, L.; Rashidi Ranjbar, P.; Shafiee, A. Copper supported β-cyclodextrin grafted magnetic nanoparticles as an efficient recyclable catalyst for one-pot synthesis of 1-benzyl-1H-1,2,3-triazoldibenzodiazepinone derivatives via click reaction. RSC Adv. 2016, 6, 28838–28843. [Google Scholar] [CrossRef]
- Chetia, M.; Ali, A.A.; Bhuyan, D.; Saikia, L.; Sarma, D. Magnetically recoverable chitosan-stabilised copper-iron oxide nanocomposite material as an efficient heterogeneous catalyst for azide-alkyne cycloaddition reactions. New J. Chem. 2015, 39, 5902–5907. [Google Scholar] [CrossRef]
- Zarnegar, Z.; Safari, J. Catalytic activity of Cu nanoparticles supported on Fe3O4-polyethylene glycol nanocomposites for the synthesis of substituted imidazoles. New J. Chem. 2014, 38, 4555–4565. [Google Scholar] [CrossRef]
- Zohreh, N.; Hosseini, S.H.; Pourjavadi, A.; Bennett, C. Immobilized copper (II) on nitrogen-rich polymer-entrapped Fe3O4 nanoparticles: A highly loaded and magnetically recoverable catalyst for aqueous click chemistry. Appl. Organomet. Chem. 2016, 30, 73–80. [Google Scholar] [CrossRef]
- Dang, H.V.; Le, H.T.B.; Tran, L.T.B.; Ha, H.Q.; Le, H.V.; Phan, N.T.S. Copper-catalyzed one-pot domino reactions via C-H bond activation: Synthesis of 3-aroylquinolines from 2-aminobenzylalcohols and propiophenones under metal-organic framework catalysis. RSC Adv. 2018, 8, 31455–31464. [Google Scholar] [CrossRef]
- Martín, N.; Dusselier, M.; De Vos, D.E.; Cirujano, F.G. Metal-Organic Framework Derived Metal Oxide Clusters in Porous Aluminosilicates: A Catalyst Design for the Synthesis of Bioactive aza-Heterocycles. ACS Catal. 2019, 9, 44–48. [Google Scholar] [CrossRef]
- Gupta, A.; Sarkar, F.K.; Sarkar, R.; Jamatia, R.; Lee, C.Y.; Gupta, G.; Pal, A.K. Development of a new catalytic and sustainable methodology for the synthesis of benzodiazepine triazole scaffold using magnetically separable CuFe2O4@MIL-101 (Cr) nano-catalyst in aqueous medium. Appl. Organomet. Chem. 2020, 34, e5782. [Google Scholar] [CrossRef]


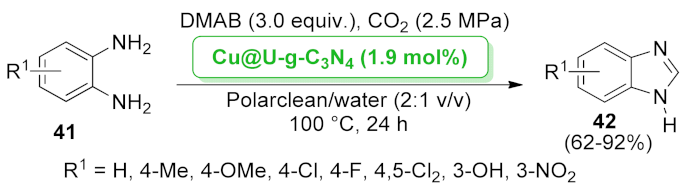












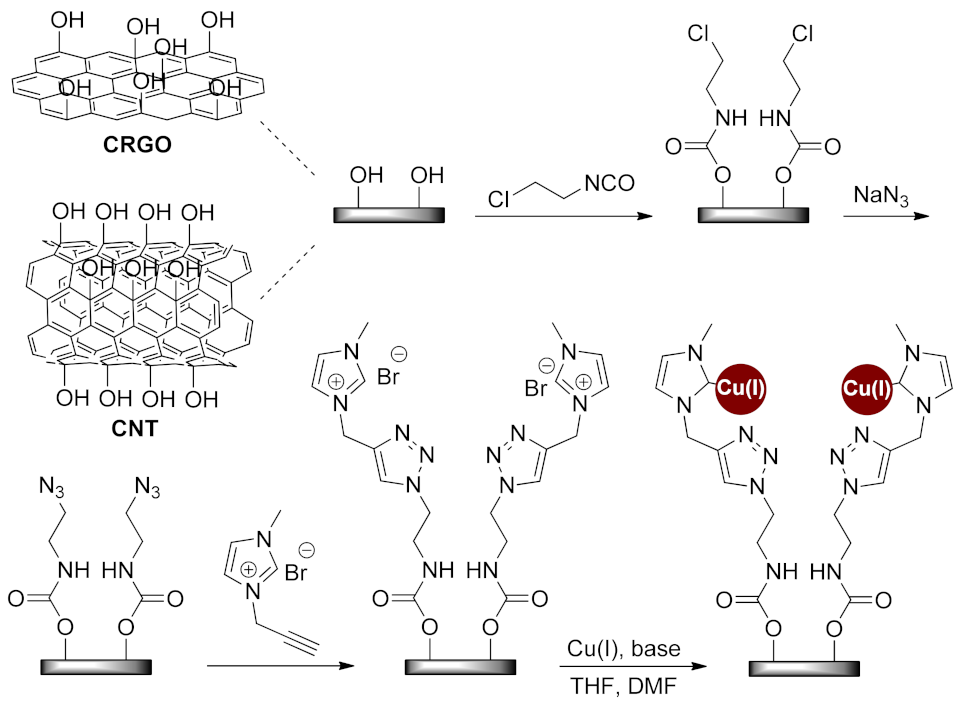
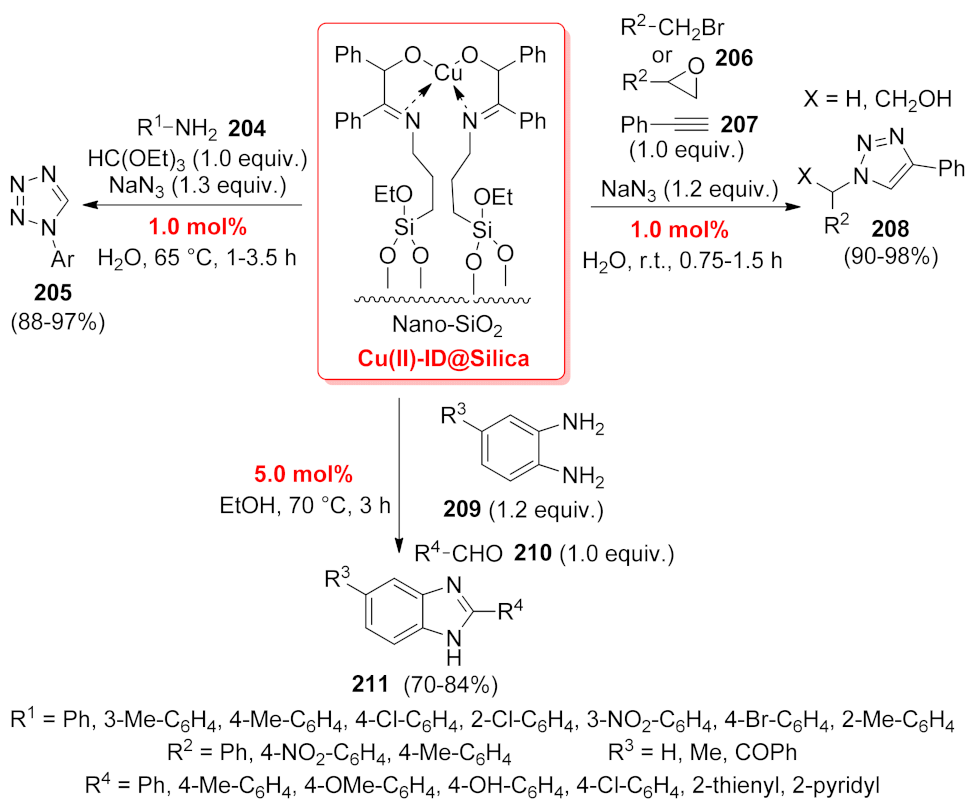
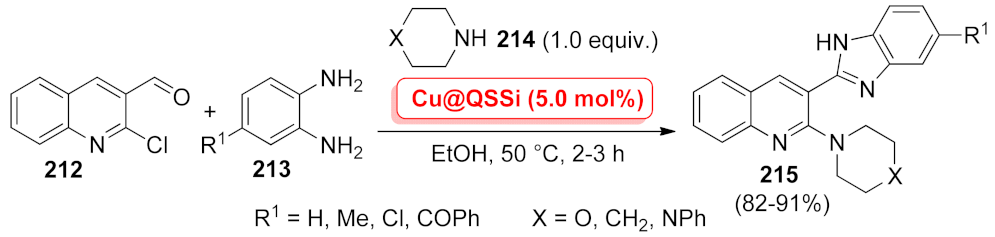
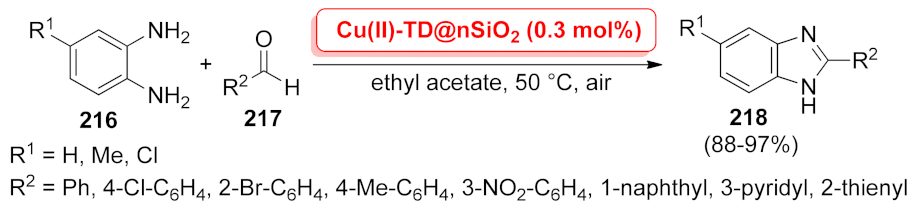


| N-Heterocycle | Catalyst | Reaction Type | Metal Loading | Product Yields | Recycling | TON/TOF | Ref. |
|---|---|---|---|---|---|---|---|
| aziridine | Pd(0)-AmP-MCF | asymmetric cascade reaction | 5 mol% | 61% | 8 runs | not calculated | [180] |
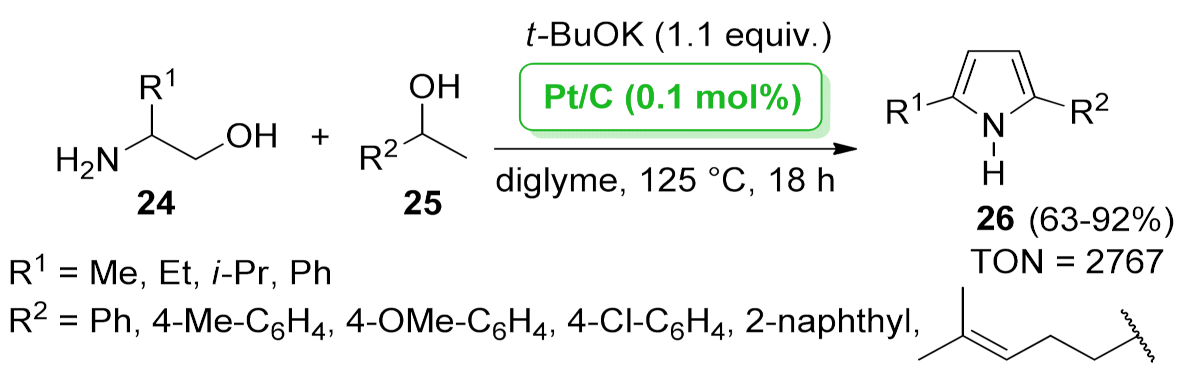
| 1H-pyrrole | Pt/C | dehydrogenative condensation | 0.1 mol% | 63–92% | 4 runs | TON = 2767 | [46] |
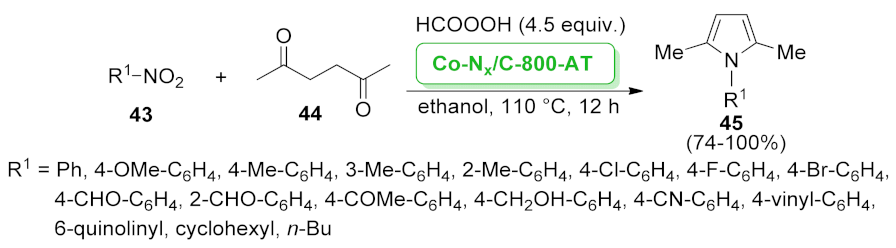
| Co–Nx/C-800-AT | Paal-Knorr condensation | n.a. | 74–100% | 5 runs | not calculated | [56] | |
| Fe(ClO4)3/SiO2 | Paal-Knorr condensation | 2 mol% | 70–98% | 5 runs | not calculated | [63] | |
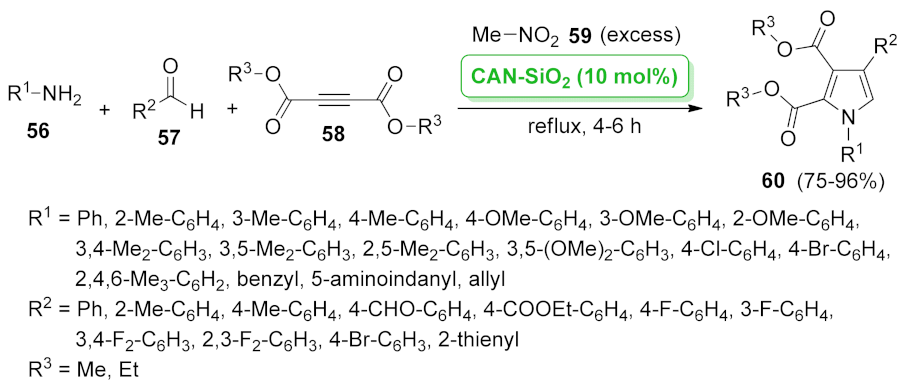
| CAN-SiO2 | four component cyclocondensation | 10 mol% | 75–96% | 4 runs | not calculated | [66] | |
| NiFe2O4 | four-component reaction | 5.0 mol% | 80–96% | 9 runs | not calculated | [103] | |
| CoFe2O4@SiO2- PrNH2-Mo(acac)2 | multi-component reaction | 1 mol% | 48–91% | 5 runs | TON = 48–91 | [204] | |
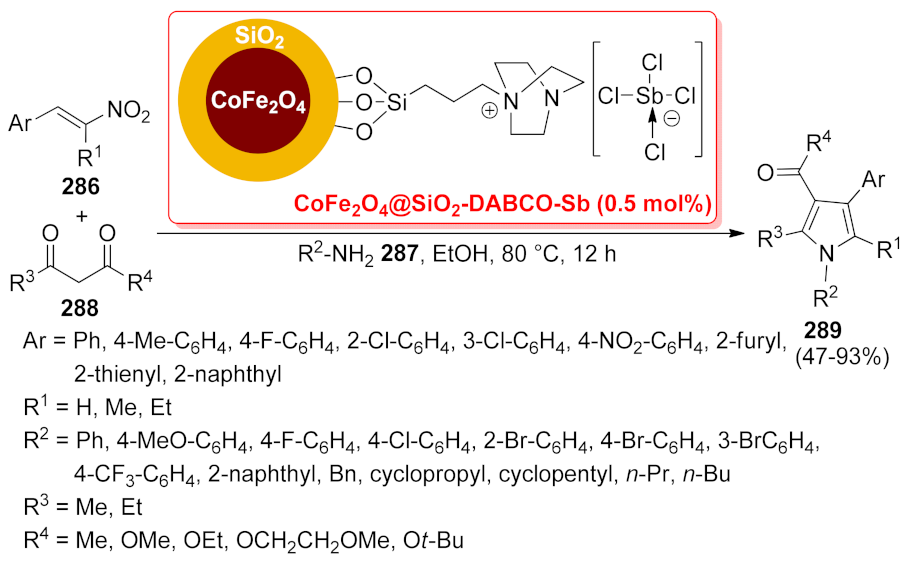
| CoFe2O4@SiO2- DABCO-Sb | multi-component reaction | 0.5 mol% | 47–93% | 5 runs | not calculated | [205] | |
| γ-Fe2O3@SiO2-Sb-IL | Clauson-Kaas reaction | 5 mol% | 81–96% | 6 runs | not calculated | [206] | |
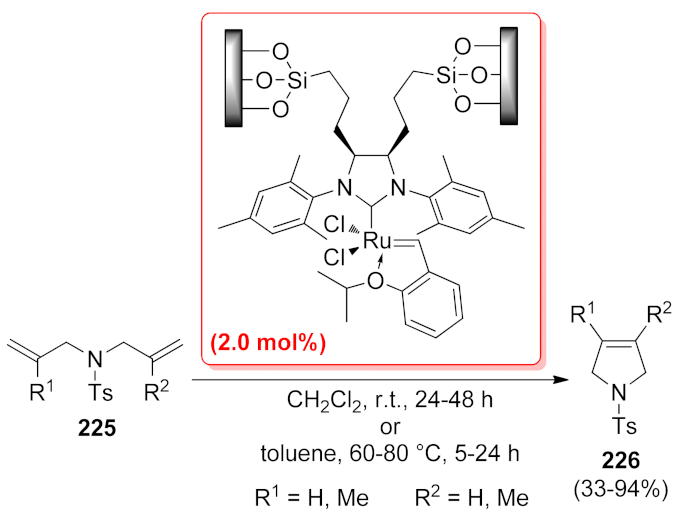
| 2,5-dihydro-1H-pyrrole | PIB-BIAN-NHC-Ru | ring closing metathesis | 1.0 mol% | 95–99% | 8 runs | not calculated | [140] |
| SWNTs-Pyr-Ru | ring closing metathesis | 0.2 mol% | 93–99% | 7 runs | not calculated | [150] | |
| rGO-Pyr-Ru | ring closing metathesis | 1.0 mol% | 94% | 3 runs | not calculated | [151] | |
| SiO2-supported Grubbs-Hoveyda Ru catalyst | ring closing metathesis | 2.5 mol% | 98% | 2 runs | TON = 169 | [163] | |
| SiO2-supported Grubbs Ru catalyst | ring closing metathesis | 0.4 mol% | 84–85% | n.a. | not calculated | [164] | |
| SiO2-supported Heoveyda-Grubbs Ru complex | ring closing metathesis | 2.0 mol% | 33–94% | 3 runs | not calculated | [165] | |
| SBA-15-supported Hoveyda-type Ru catalyst | ring closing metathesis | 0.1 mol% | 69–89% | 23 runs | TON = 3450–17,400 | [173] | |
| Pd(0)-AmP-MCF | asymmetric cascade reaction | 3 mol% | 53–84% | 8 runs | not calculated | [179] | |
| Fe3O4@SiO2-supported Grubbs-Hoveyda- type Ru catalyst | ring closing metathesis | 0.85 mol% | 91–99% | 6 runs | not calculated | [202] | |
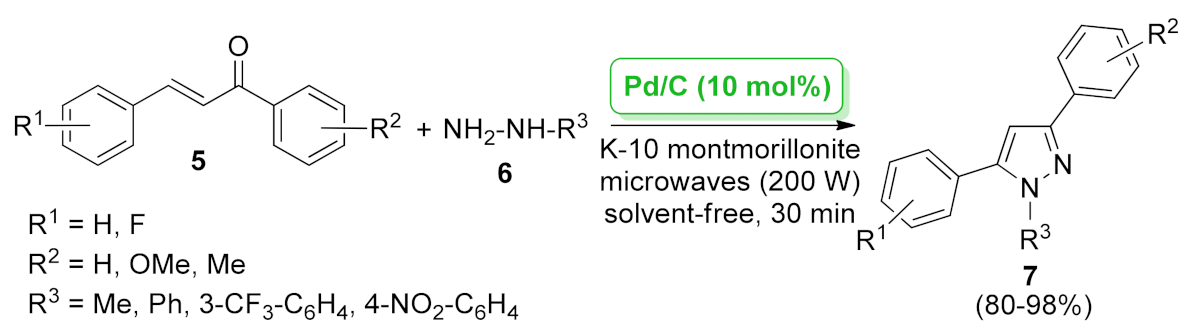
| 1H-pyrazole | Pd/C + K-10 montmorillonite | cyclization/dehydrogenation | 10 mol% | 80–98% | n.a. | not calculated | [38] |
| CuO/ZrO2 | multicomponent reaction | 0.5 mol% | 88–92% | 5 runs | not calculated | [92] | |
| Fe3O4@Si@MoO2 | multi-component reaction | 20 mg/mmol | 85–95% | 8 runs | not calculated | [203] | |
| 1H-imidazole | Bi2O3-ZnO/fly ash | condensation/cyclization | 5 wt.% | 89–98% | 4 runs | not calculated | [53] |
| SbCl3/SiO2 | multi-component cyclocondensation | 44 mol% | 58–95% | n.a. | not calculated | [64] | |
| SbCl3/SiO2 | multi-component cyclocondensation | 44 mol% | 76–97% | 5 runs | not calculated | [65] | |
| CuFe2O4 | condensation/cyclization | 10 mol% | 84–91% | 6 runs | not calculated | [100] | |
| CuFe2O4 | three-component reaction | 50 mg/mmol | 92–96% | 5 runs | not calculated | [101] | |
| CoFe2O4 | three-component reaction | 50 mg/mmol | 94–98% | 5 runs | not calculated | [101] | |
| Ni-bentonite@ferrite | condensation/cyclization | 37 mg/mmol | 81–92% | 8 runs | not calculated | [117] | |
| Zr-CAP-SG | condensation | 10 wt.% | 71–87% | 5 runs | not calculated | [166] | |
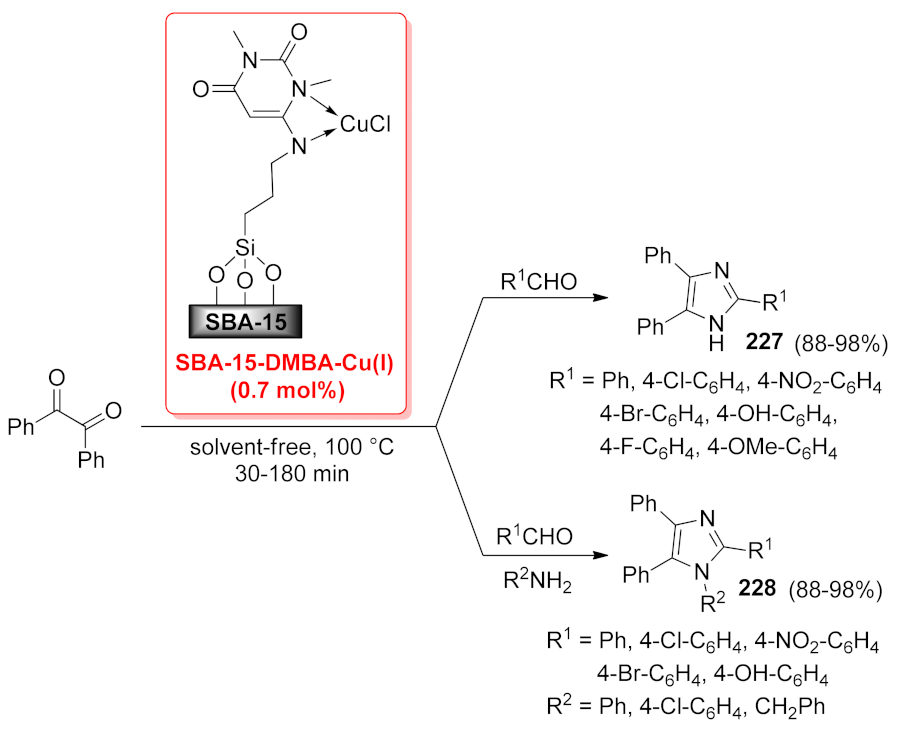
| SBA-15-DMBA-Cu(I) | condensation/cylization | 0.7 mol% | 88–98% | 5 runs | not calculated | [167] | |
| Fe3O4-DOPA-Cu | tandem SN2/[3 + 2] cycloaddition | 30 mg/mmol | 90–96% | 6 runs | not calculated | [184] | |
| cellulose/γ-Fe2O3/Ag | multi-component reaction | 15 mg/mmol | 82–95% | 5 runs | not calculated | [208] | |
| Fe3O4-PEG-Cu | multi-component reaction | 10 mol% | 88–98% | 6 runs | not calculated | [211] | |
| 1,2,3-1H-triazole | Pd-Cu/C | Sonogashira/desilylation/[3 + 2] cycloaddition | 5.0 mol% | 52–76% | 1 run | not calculated | [40] |
| Cu/C | [3 + 2] cycloaddition | 5.0 mol% | 92–99% | 3 runs | not calculated | [41] | |
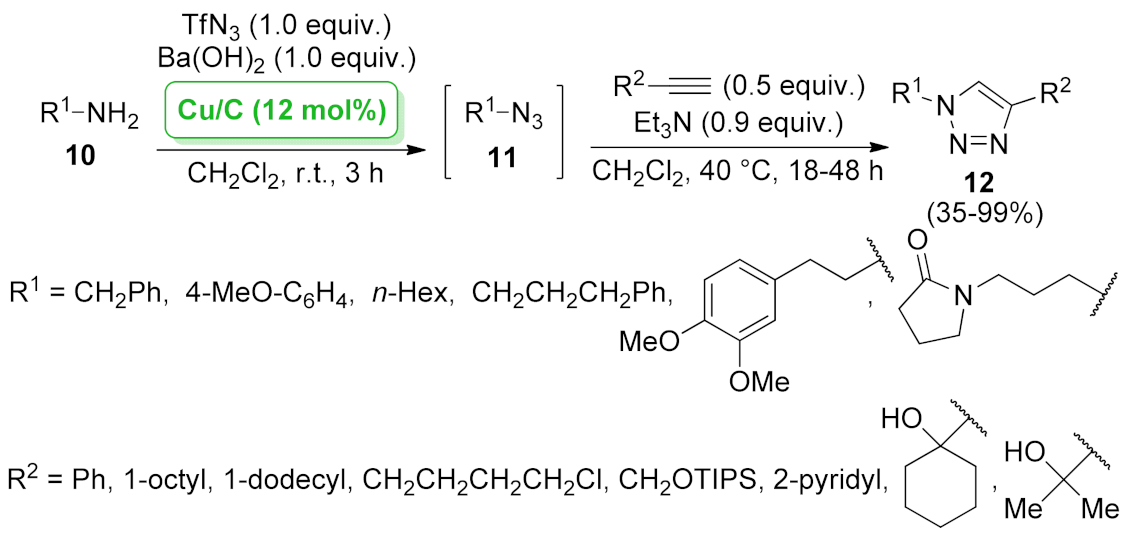
| Cu/C | diazo transfer/[3 + 2] cycloaddition | 12 mol% | 35–99% | 2 runs | not calculated | [42] | |
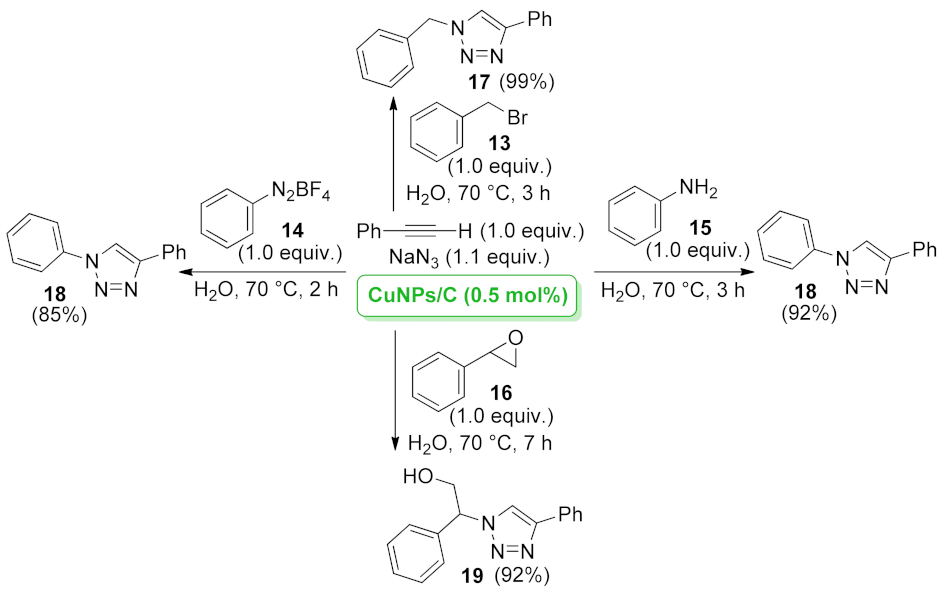
| CuNPs/C | three-component [3 + 2] cycloaddition | 0.5 mol% | 85–99% | 2 runs | not calculated | [43] | |
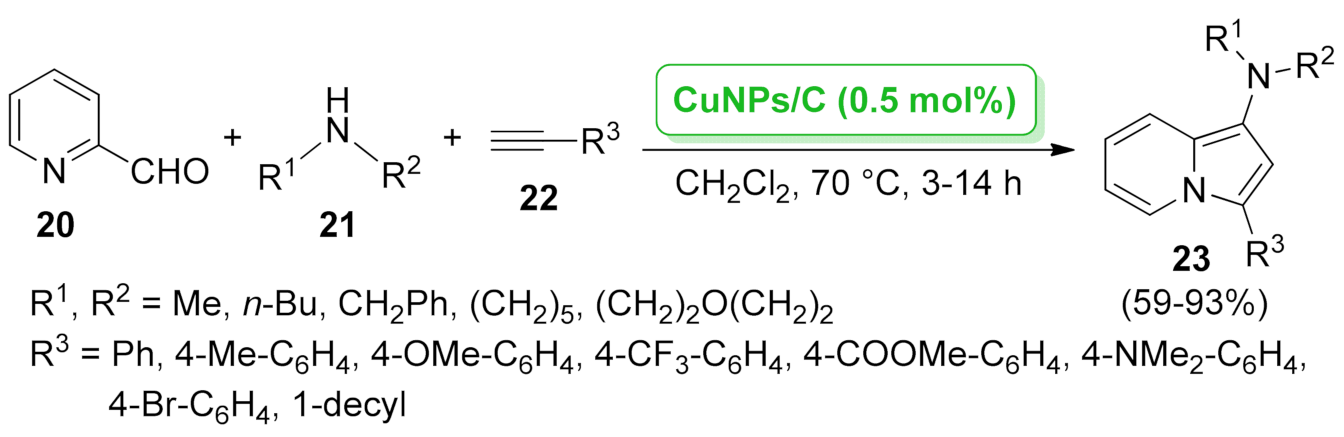
| CuNPs/C | three-component [3 + 2] cycloaddition | 0.5 mol% | 64–99% | 5 runs | not calculated | [44] | |
| TRGO/Cu(I) | [3 + 2] cycloaddition | 2 mol% | 80–99% | 4 runs | not calculated | [48] | |
| Cu NPs-MCN | three-component [3 + 2] cycloaddition | 0.5 mol% | 78–98% | 8 runs | not calculated | [54] | |
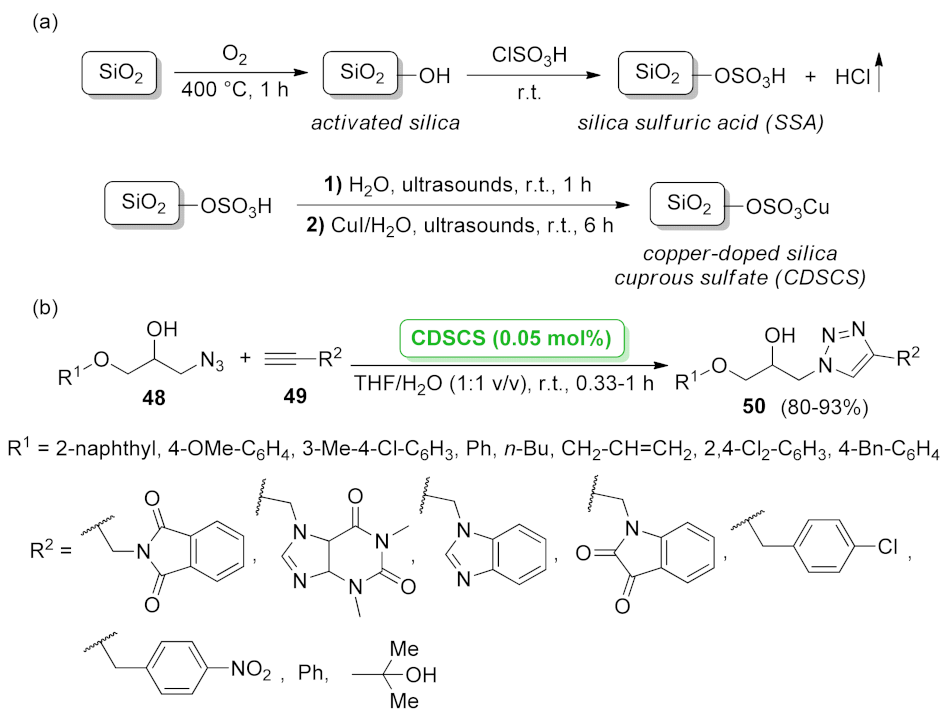
| CDSCS | [3 + 2] cycloaddition | 0.05 mol% | 80–93% | 5 runs | not calculated | [60] | |
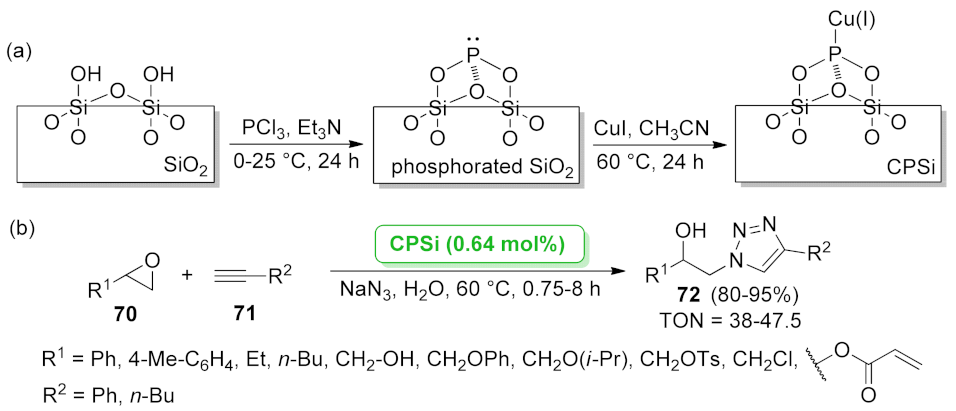
| CPSi | three-component reaction | 0.64 mol% | 80–95% | 5 runs | TON = 38–47.5 | [72] | |
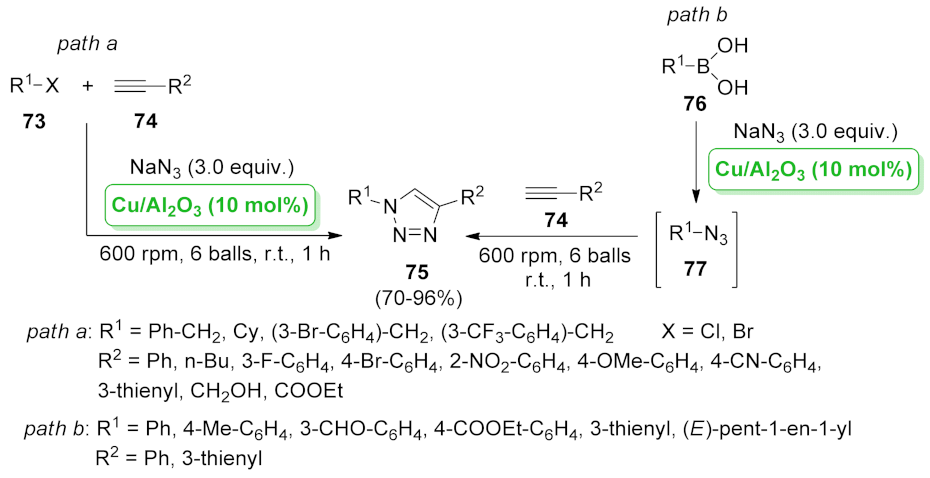
| Cu/Al2O3 | nucleophilic substitution/[3 + 2] cycloaddition | 10 mol% | 70–96% | 8 runs | not calculated | [74] | |
| CuI/Al2O3 | azide formation/[3 + 2] cycloaddition | 3 mol% | 70–98% | 8 runs | TON = 495 TOF = 1.37 s−1 | [75] | |
| CuO/ZnO | azide formation/[3 + 2] cycloaddition | 22 mol% | 89–92% | 5 runs | not calculated | [96] | |
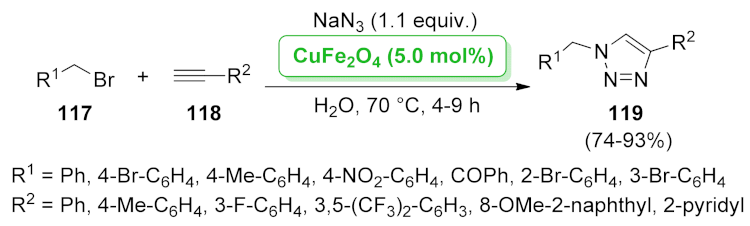
| CuFe2O4 | nucleophilic substitution/[3 + 2] cycloaddition | 5.0 mol% | 74–93% | 4 runs | not calculated | [98] | |
| CuFe2O4 | multicomponent reaction | 10 mol% | 74–92% | 3 runs | not calculated | [99] | |
| clay-Cu(II) | azidonation/[3 + 2] cycloaddition | 10 mol% | 83–98% | 5 runs | not calculated | [106] | |
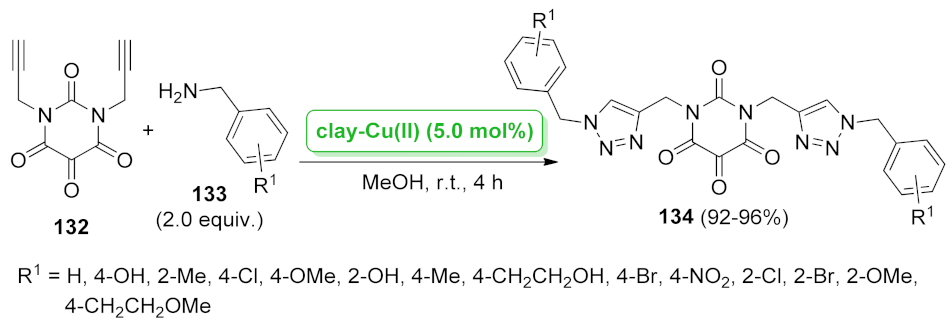
| clay-Cu(II) | [3 + 2] cycloaddition | 5.0 mol% | 92–96% | 5 runs | not calculated | [107] | |
| CuBr/GO@Fe3O4 | azide formation/[3 + 2] cycloaddition | 5.0 mol% | 88–98% | 6 runs | not calculated | [112] | |
| Cu/CNT@CuFe2O4 | azide formation/[3 + 2] cycloaddition | 0.4 mol% | 57–92% | 4 runs | not calculated | [115] | |
| SMI–Cu(I) | azide formation/[3 + 2] cycloaddition | 35.1 mol% | 71–91% | 5 runs | not calculated | [120] | |
| nano-Cu2O-MFR | [3 + 2] cycloaddition | 0.072 mol% | 80–93% | 5 runs | not calculated | [128] | |
| Cu-HMOP | azidation/[3 + 2] cycloaddition | 10 wt.% | 63–99% | 5 runs | not calculated | [129] | |
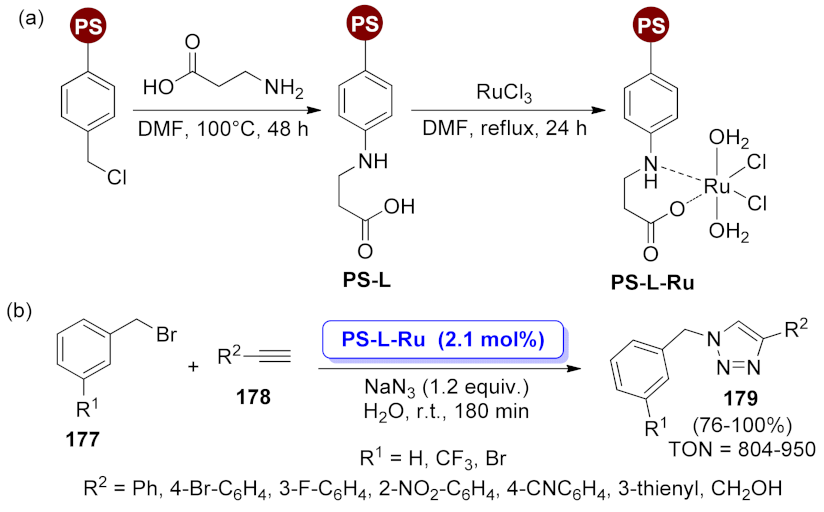
| PS-L-Ru | azide formation/[3 + 2] cycloaddition | 2.1 mol% | 76–100% | 6 runs | TON = 804–950 | [133] | |
| Chitosan-Phen-Cu(I) | [3 + 2] cycloaddition | 0.1 mol% | 64–99% | 4 runs | not calculated | [134] | |
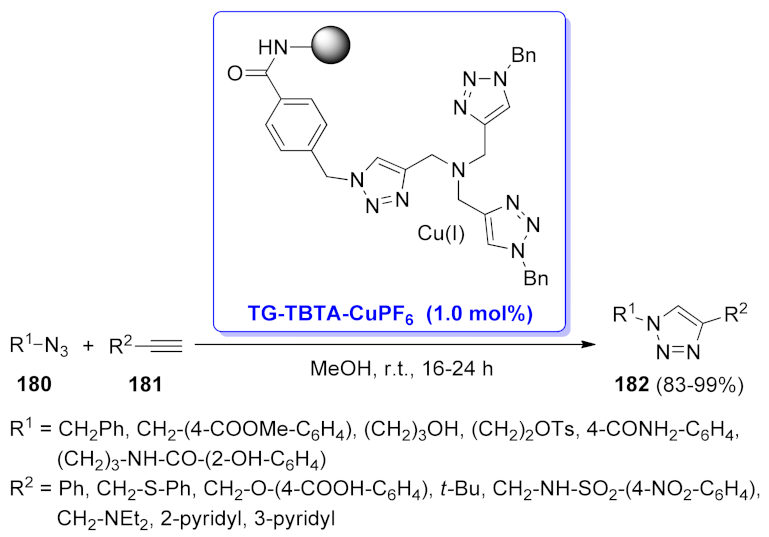
| TG-TBTA-CuPF6 | [3 + 2] cycloaddition | 1.0 mol% | 83–99% | 10 runs | not calculated | [135] | |
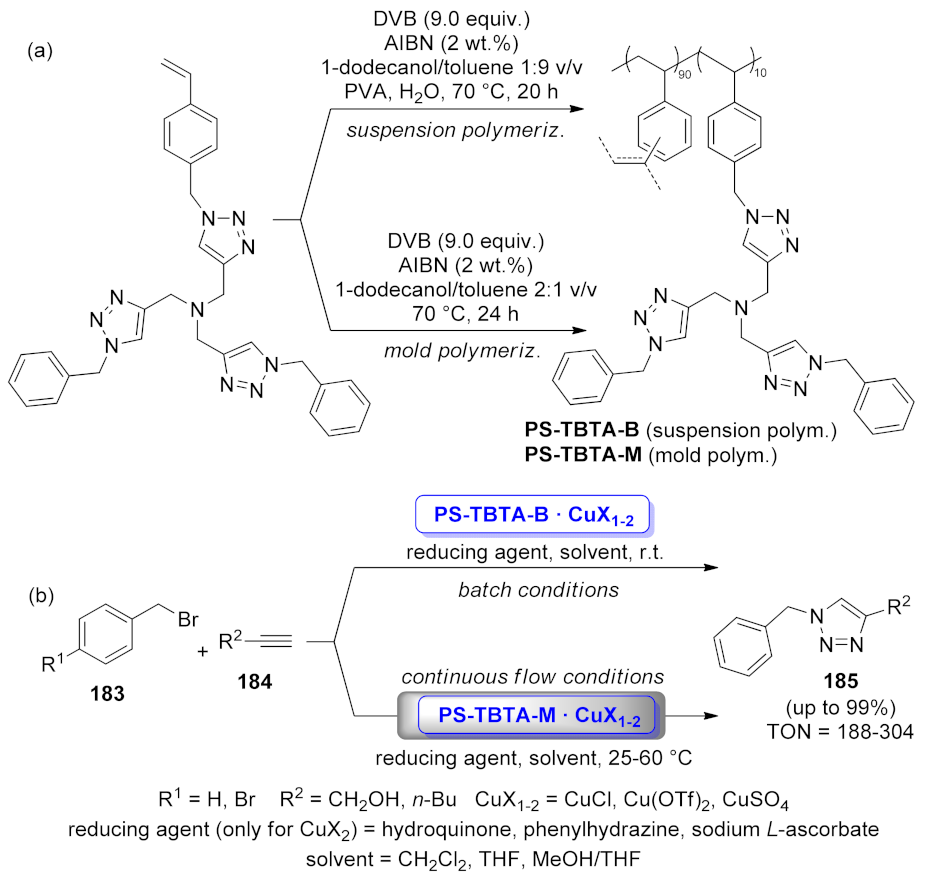
| PS-TBTA · CuX1-2 beads and flow | [3 + 2] cycloaddition | 0.6–20 mol% | Up to 99% | up to 8 runs | TON = 188–304 | [136] | |
| Chit-CuSO4 | [3 + 2] cycloaddition | 0.4 mol% | 93–99% | 5 runs | not calculated | [146] | |
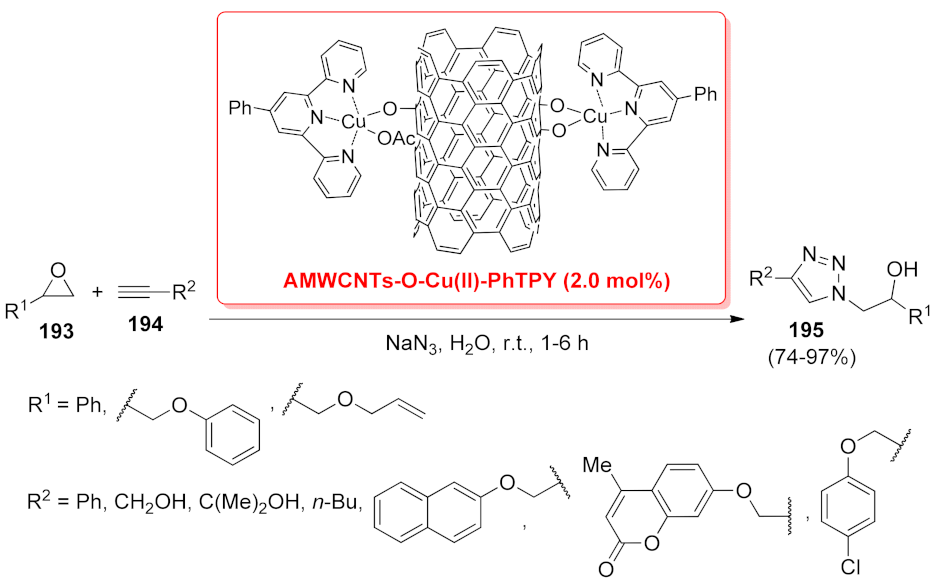
| AMWCNTs-O-Cu(II)-PhTPY | [3 + 2] cycloaddition | 2 mol% | 74–97% | 5 runs | not calculated | [148] | |
| CRGO-Ima-Cu(I) | [3 + 2] cycloaddition | 2.0 mol% | 85–99% | 10 runs | not calculated | [152] | |
| SiFKIAd/Cu(I) SiNPIAd/Cu(I) | [3 + 2] cycloaddition | 1.0 mol% | 56–68% | 3 runs | not calculated | [153] | |
| Cu(II)-ID@Silica | azidation/[3 + 2] cycloaddition | 1.0 mol% | 90–98% | 12 runs | not calculated | [154] | |
| BS-Cu(II)@SiO2 | four component C–H bond activation/[3 + 2] cycloaddition | 5.0 mol% | 87–94% | 7 runs | not calculated | [155] | |
| CuNPs-PEI/SiO2 | [3 + 2] cycloaddition | 0.05 mol% | 60–98% | 3 runs | not calculated | [157] | |
| Cu/APSiO2 | [3 + 2] cycloaddition | 0.5 mol% | 99% | 5 runs | not calculated | [159] | |
| Cu-NHC-MCM-41 | [3 + 2] cycloaddition | 5 mol% | 99% | 4 runs | not calculated | [177] | |
| NiFe2O4-glutamate- Cu(0) | tandem SN2/[3 + 2] cycloaddition | 1.0–5.0 mol% | 50–94% | 10 runs | not calculated | [181] | |
| Fe3O4-DOPA-Cu | tandem SN2/[3 + 2] cycloaddition | 0.1 g/mmol | 94–98% | 6 runs | not calculated | [184] | |
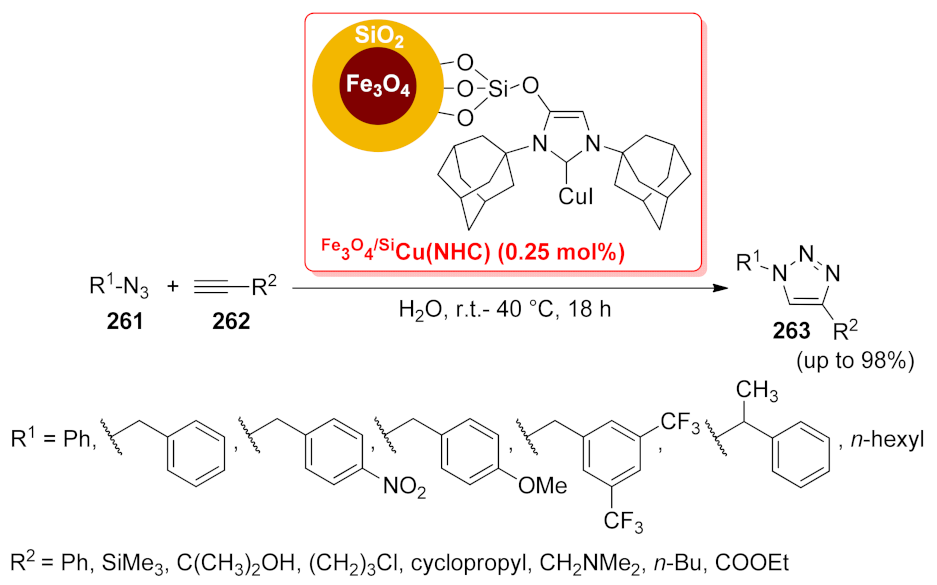
| Fe3O4/SiCu(NHC) | [3 + 2] cycloaddition | 0.25 mol% | up to 98% | 10 runs | not calculated | [153] | |
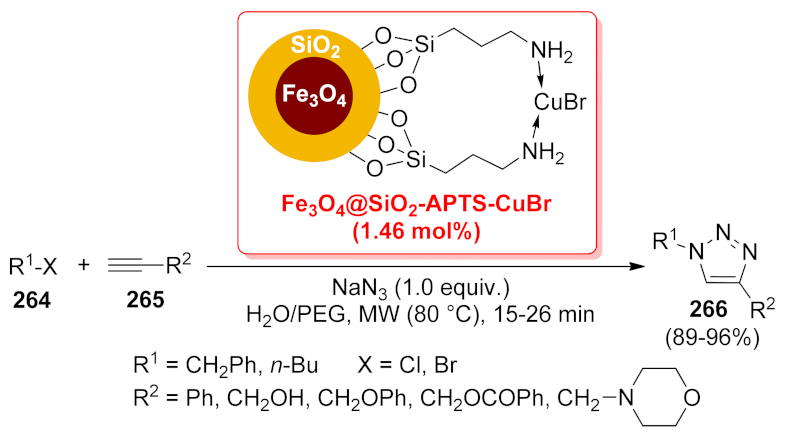
| Fe3O4@SiO2-APTS- CuBr | tandem SN2/[3 + 2] cycloaddition | 1.46 mol% | 89–96% | 7 runs | not calculated | [188] | |
| Cu(0)-Fe3O4@SiO2/NH2cel | multi-component reaction | 0.25 mol% | 90–95% | 6 runs | not calculated | [189] | |
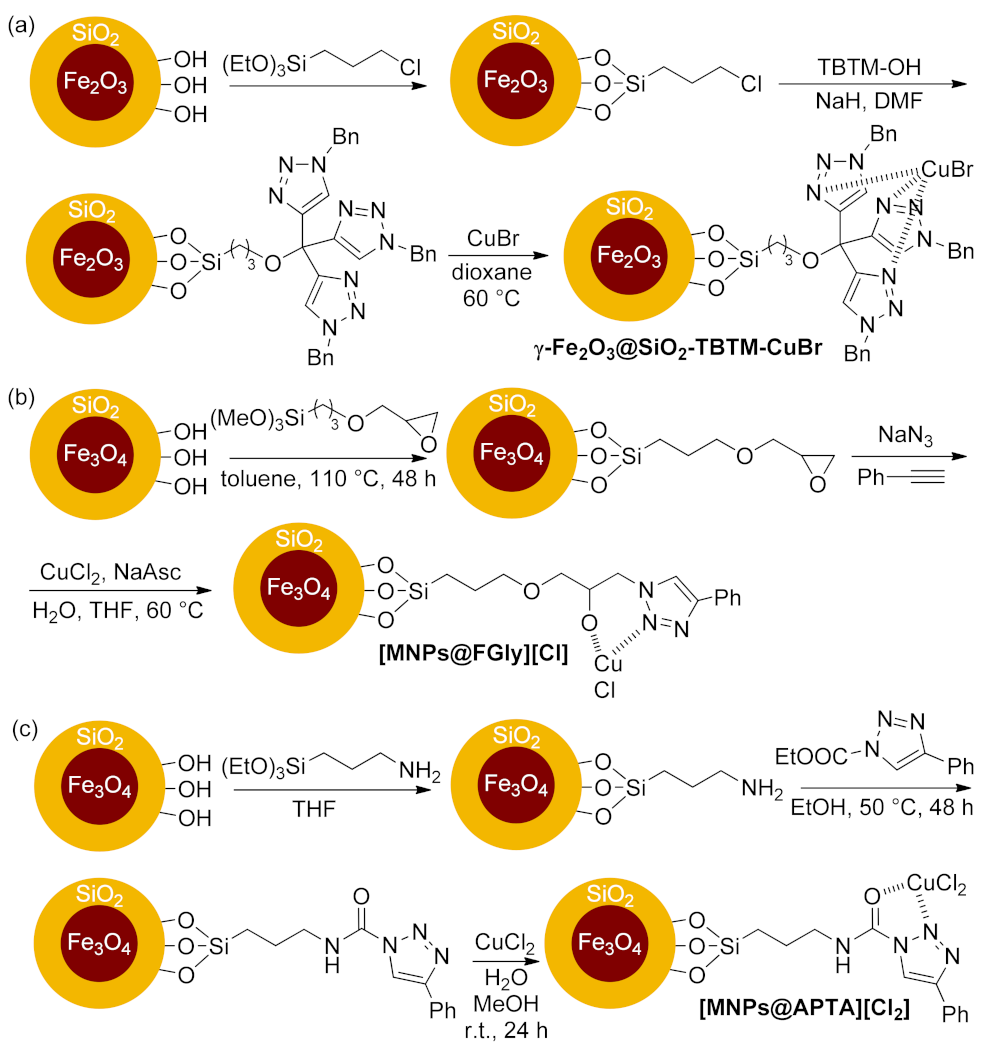
| γ-Fe2O3@SiO2-TBTM- CuBr | [3 + 2] cycloaddition | 0.5 mol% | 82–96% | 3 runs | not calculated | [191] | |
| [MNPs@FGly][Cl] | [3 + 2] cycloaddition | 2.0 mol% | 85–99% | 10 runs | not calculated | [192] | |
| [MNPs@APTA][Cl2] | multi-component reaction | 0.5 mol% | 82–98% | 10 runs | not calculated | [193] | |
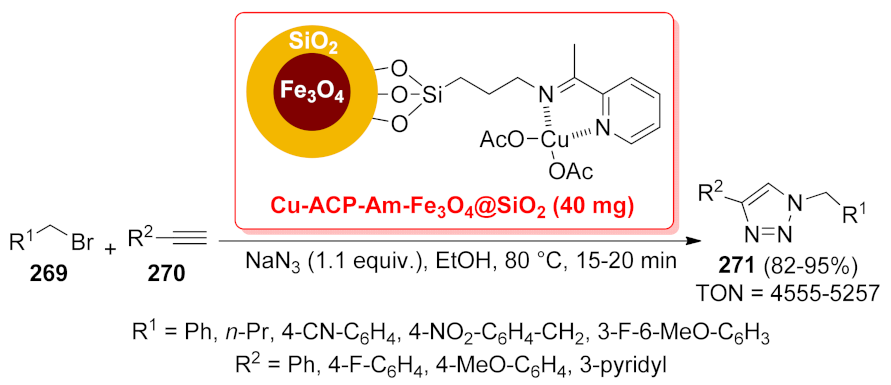
| Cu-ACP-Am- Fe3O4@SiO2 | SN2 substitution/[3 + 2] cycloaddition | 40 mg/mmol | 82–95% | 6 runs | TON = 4555–5257 | [195] | |
| MNP@BiimCu(I) | multi-component reaction | 0.85 mol% | 65–99% | 10 runs | TON = 147–165 | [197] | |
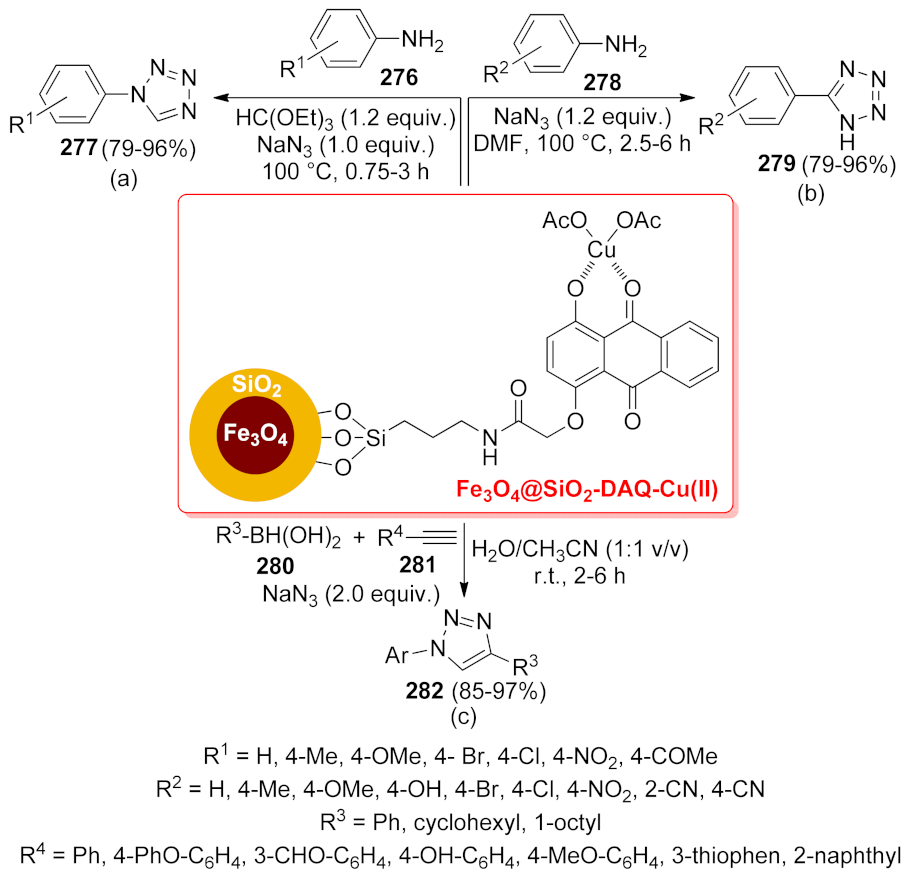
| Fe3O4@SiO2-DAQ- Cu(II) | multi-component reaction | 0.5 mol% | 85–97% | 6 runs | not calculated | [199] | |
| γ-Fe2O3@TiO2- EG-CuII | [3 + 2] cycloaddition | 4 mol% | 82–96% | 6 runs | not calculated | [201] | |
| Cu@β-CD@SPIONs | condensation/cyclocondensation/[3 + 2] cycloaddition | 1.0 mol% | 71–87% | 5 runs | not calculated | [209] | |
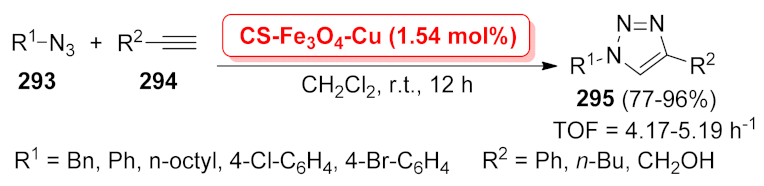
| CS-Fe3O4-Cu | [3 + 2] cycloaddition | 1.54 mol% | 77–96% | 4 runs | TOF = 4.17–5.19 h−1 | [210] | |
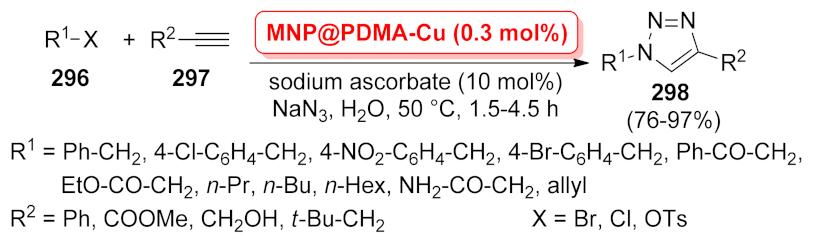
| MNP@PDMA-Cu | multi-component reaction | 0.3 mol% | 76–97% | 7 runs | not calculated | [212] | |
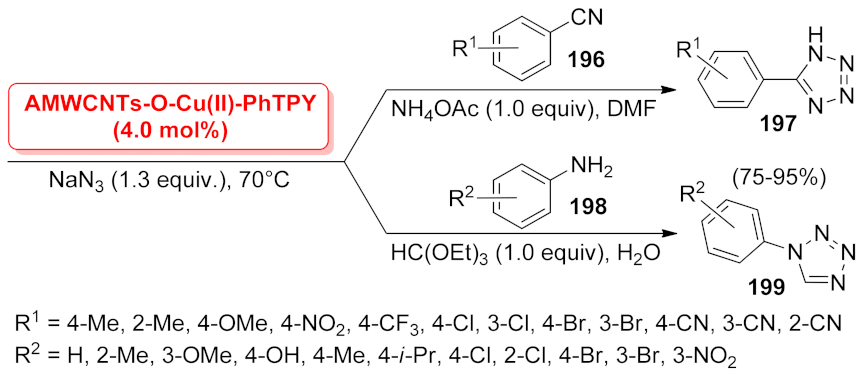
| 1H-tetrazole | Sm@ l-MSNs | [3 + 2] cycloaddition | 1.4 mol% | 70–98% | 5 runs | not calculated | [71] |
| CSMIL | [3 + 2] cycloaddition | 2.5 mol% | 81–90% | 5 runs | not calculated | [145] | |
| AMWCNTs-O-Cu(II)-PhTPY | [3 + 2] cycloaddition | 4 mol% | 75–95% | 5 runs | not calculated | [149] | |
| Cu(II)-ID@Silica | three-component reaction | 1.0 mol% | 88–97% | n.a. | not calculated | [154] | |
| Ni(II)-cytosine- MCM-41 | multi-component reaction | 0.1 mol% | 90–97% | 6 runs | TOF = 900–1940 h−1 | [178] | |
| Fe3O4@Quinindiol @Cu | multi-component reaction | 10 mg/mmol | 84–93% | 6 runs | not calculated | [183] | |
| Fe3O4-AMPD-Cu | [3 + 2] cycloaddition | 4 mg/mmol | 86–97% | n.a. | not calculated | [185] | |
| Cu-guanidine@BO- NPs | [3 + 2] cycloaddition | 0.44 mol% | 85–96% | 6 runs | TON = 193–223 | [186] | |
| Fe3O4@SiO2/salen- Cu(II) | multi-component reaction | 0.4 mol% | 75–96% | 7 runs | not calculated | [187] | |
| Fe3O4@SiO2-TCT-NH2-dendrimer-Cu(II) | multi-component reaction | 0.4 mol% | 81–96% | 7 runs | not calculated | [190] | |
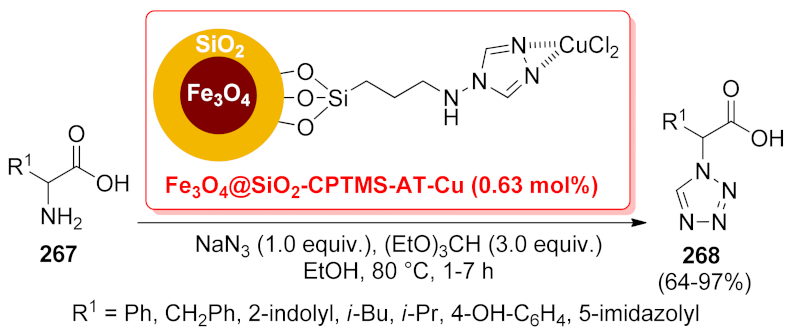
| Fe3O4@SiO2-CPTMS- AT-Cu | multicomponent reaction | 6.6 mol% | 64–97% | 5 runs | not calculated | [194] | |
| Fe3O4@SiO2-DAQ- Cu(II) | multi-component reaction | 0.8 mol% | 79–96% | 6 runs | not calculated | [198] | |
| Fe3O4@SiO2-PVA-Cu2+ | multi-component reaction | 0.5 mol% | 82–96% | 7 runs | not calculated | [200] | |
| pyridine | Bi(III)-Al2O3 | one-pot synthesis | 5 wt.% | 25–82% | n.a. | not calculated | [73] |
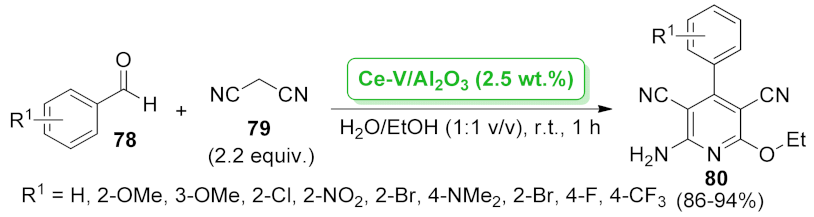
| Ce-V/Al2O3 | multicomponent reaction | 2.5 wt.% | 86–94% | 5 runs | not calculated | [76] | |
| LS-FAS-Cu | oxidative cyclization | 40 mol% | 39–76% | 3 runs | not calculated | [147] | |
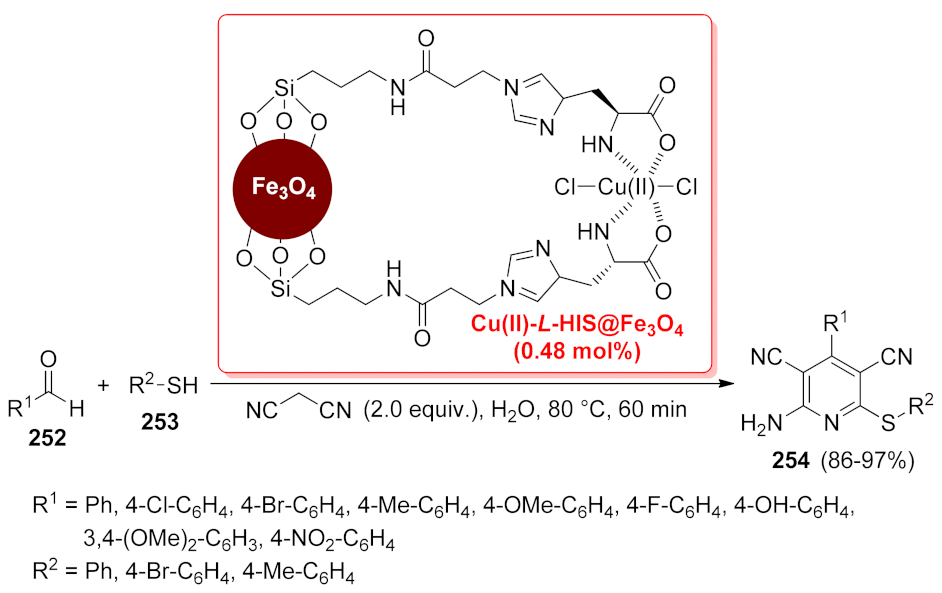
| Cu(II)-L-HIS@Fe3O4 | multi-component reaction | 0.48 mol% | 86–97% | 6 runs | not calculated | [182] | |
| 1,4- dihydropyridine | PdRuNi@GO | multicomponent condensation | 9.3 wt.% | 86–96% | 5 runs | TON = 149.6–167 TOF = 199–223 h−1 | [50] |
| Sm2O3/ZrO2 | multicomponent reaction | 0.4 mol% | 87–96% | 7 runs | not calculated | [93] | |
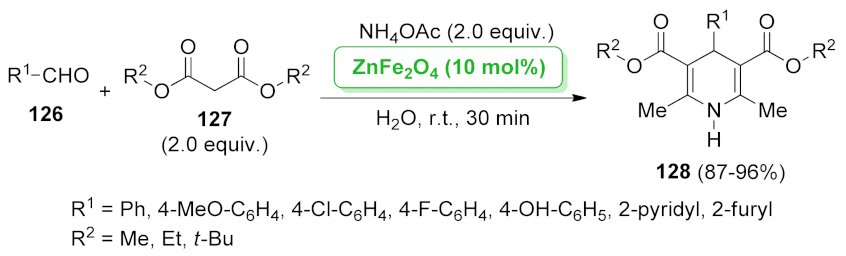
| ZnFe2O4 | multi-component reaction | 3.3 mol% | 87–96% | 5 runs | not calculated | [104] | |
| Zn-VCO3 HT | Hantzsch reaction | not indicated | 85–93% | 5 runs | not calculated | [109] | |
| 1,2,3,6-tetrahydropyridine | Supported Schrock catalyst | ring closing metathesis | 2.8–5.2 mol% | 21–35% | 3 runs | not calculated | [139] |
| PIB-BIAN-NHC-Ru | ring closing metathesis | 1.0 mol% | 95–97% | 3 runs | not calculated | [140] | |
| SWNTs-Pyr-Ru | ring closing metathesis | 0.2 mol% | 93–98% | 7 runs | not calculated | [150] | |
| Fe3O4@SiO2- supported Grubbs- Hoveyda-type Ru catalyst | ring closing metathesis | 0.85 mol% | 99% | 6 runs | not calculated | [202] | |
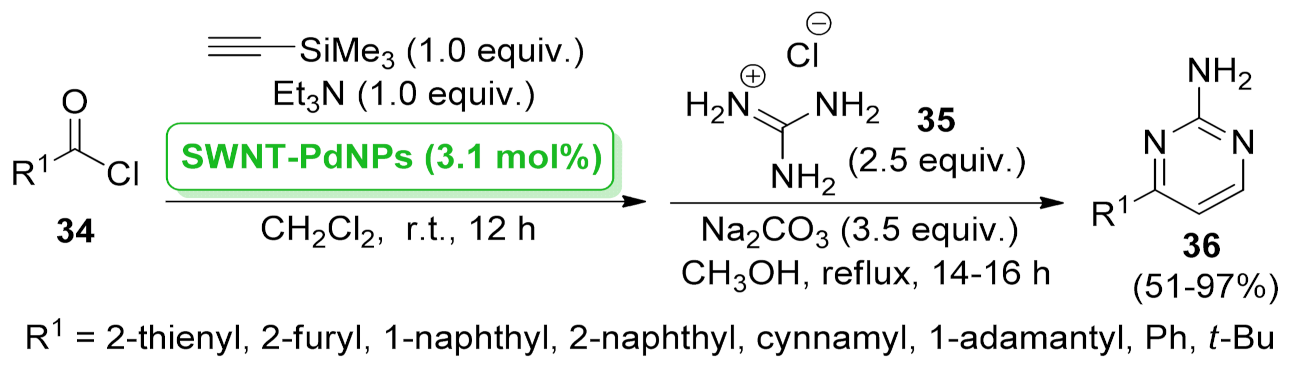
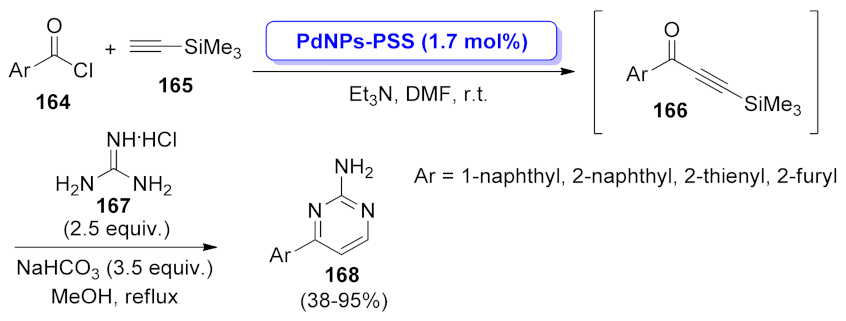
| pyrimidine | SWNT–PdNPs | acyl Sonogashira coupling/cyclization | 3.1 mol% | 51–97% | 7 runs | not calculated | [51] |
| PdNPs-PSS | acyl Sonogashira coupling/cyclization | 1.7 mol% | 38–95% | 6 runs | not calculated | [124] | |
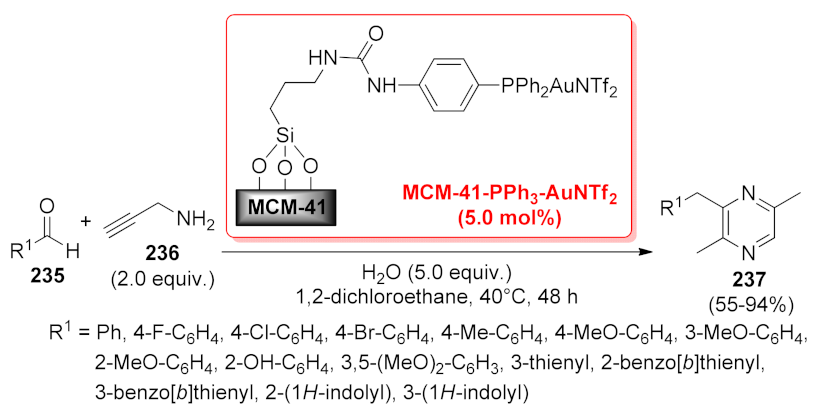
| pyrazine | MCM-41-PPh3- AuNTf2 | cascade annulation | 5 mol% | 55–94% | 8 runs | not calculated | [174] |
| piperazine | Pd/Al2O3 | reductive cycloamination | 0.17 wt.% | 83% | 3 runs + regenerated | TON = 818 | [79] |
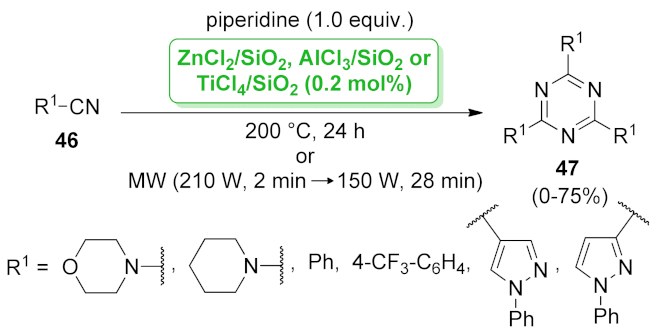
| 1,3,5-triazine | ZnCl2/SiO2 AlCl3/SiO2 TiCl4/SiO2 | cyclotrimerization | 2 mol% | 0–75% 0–72% 0–63% | n.a. | not calculated | [59] |
| 2,3,6,7-tetrahydro-1H-azepine | Fe3O4@SiO2-supported Grubbs-Hoveyda- type Ru catalyst | ring closing metathesis | 0.85 mol% | 99% | 6 runs | not calculated | [202] |
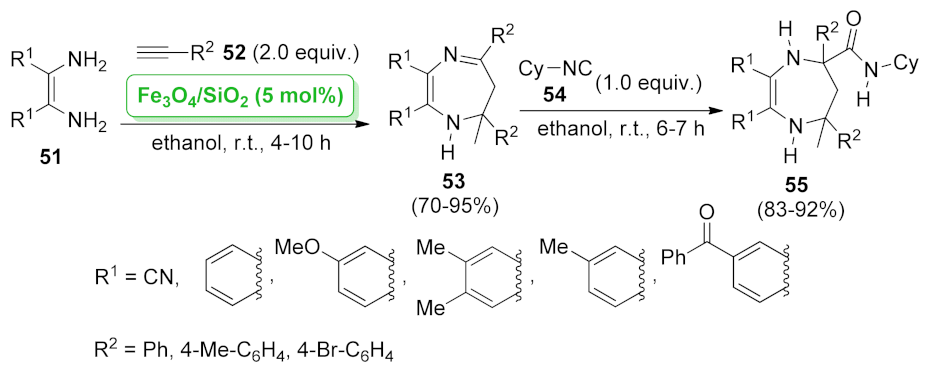
| 1H-1,4-diazepine | Fe3O4/SiO2 | multicomponent reaction | 5.0 mol% | 70–95% | 5 runs | not calculated | [62] |
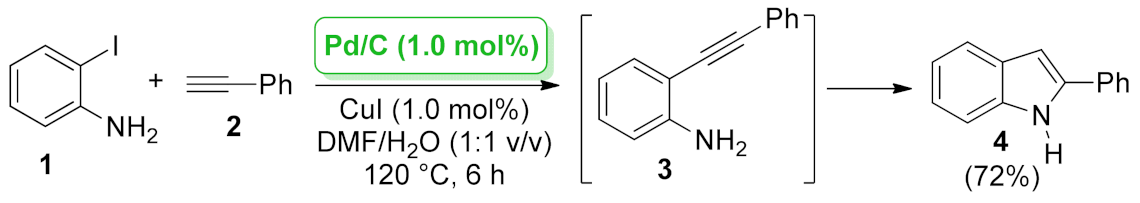
| 1H-indole | Pd/C | Sonogashira/intramolecular heteroannulation | 1.0 mol% | 72% | 3 runs | not calculated | [37] |
| Pt/Al2O3 + ZnO | dehydrogenative condensation | 1.7 mol% Pt 4.5 mol% Zn | 30–99% | n.a. | not calculated | [80] | |
| Ru/CeO2 | intramolecular dehydrogenative heterocyclization | 2.5 mol% | 84% | 2 runs | not calculated | [91] | |
| Pt/Nb2O5 | dehydrogenative N-heterocyclization | 0.2 mol% | 76% | 3 runs | TON = 380 | [97] | |
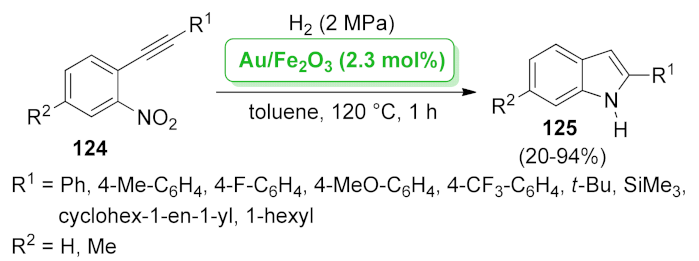
| Au/Fe2O3 | hydrogenation/hydroamination | 2.3 mol% | 20–94% | 1 run | not calculated | [102] | |
| CuAl-HT | intramolecular dehydrogenative N-heterocyclization | 0.2 g/mmol | 94–100% | 6 runs | not calculated | [108] | |
| Pt/HBEA | dehydrogenative N-heterocyclization | 1.0 mol% | 68–90% | 3 runs | TON = 450 | [97] | |
| Pd@PS | domino decarboxylative coupling/5-exo-dig cyclization | 3 mol% | 63–76% | 5 runs | not calculated | [121] | |
| LS-FAS-Cu | multi-component reaction | 20 mol% | 37–80% | 3 runs | not calculated | [147] | |
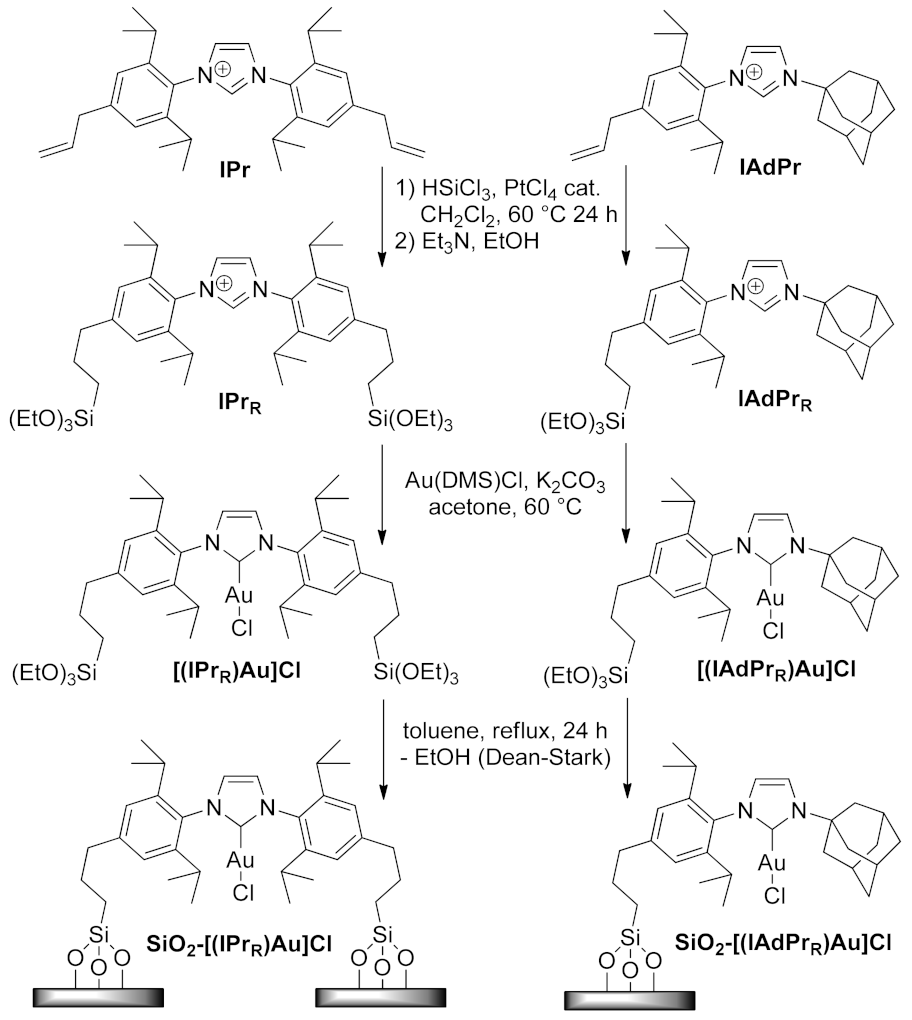
| SiO2-[(IPrR)Au]Cl SiO2-[(IAdPrR)Au]Cl | cyclization | 1.0 mol% | 99% | 5 runs | not calculated | [160] | |
| [PdCl2(NCPh)2] | cyclization | 5.0 mol% | 89–96% | 4 runs | not calculated | [161] | |
| SBA-15-anchored PdCl2L2 | Larock synthesis | 1.0 mol% | 80–85% | 4 runs | not calculated | [172] | |
| CuOMOFY | multi-component reaction | 0.7 mol% | 91–94% | n.a. | TOF = 67–104 h−1 | [214] | |
| indolizine | CuNPs/C | multicomponent reaction | 0.5 mol% | 59–93% | 1 run | not calculated | [45] |
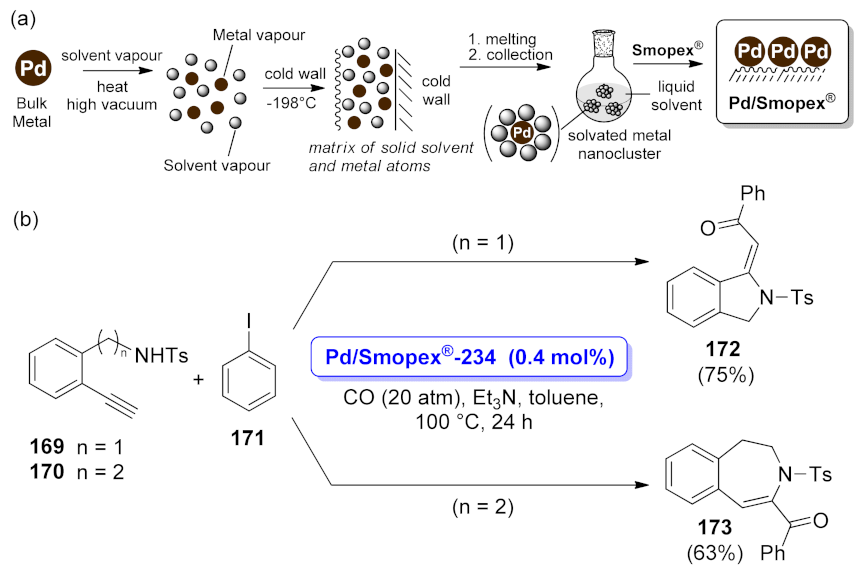
| isoindoline | Pd/Smopex®-234 | cyclocarbonylative Sonogashira coupling | 0.4 mol% | 75% | n.a. | not calculated | [125] |
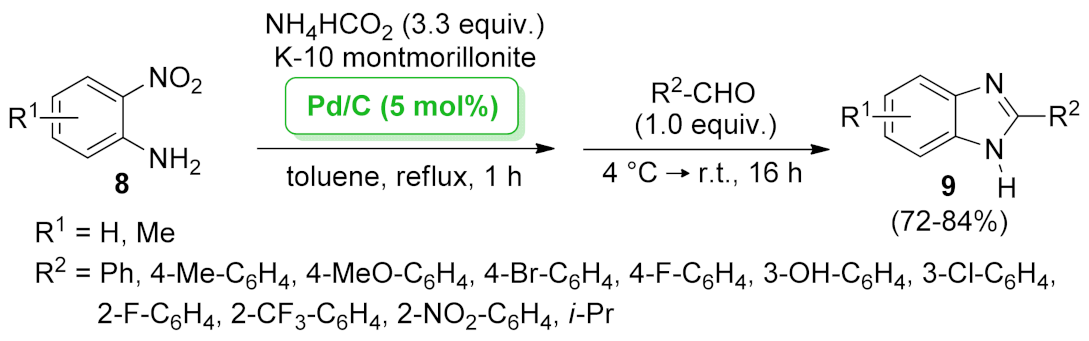
| 1H-benzo[d] imidazole | Pd/C + K-10 montmorillonite | transfer hydrogenation/condensation/dehydrogenation | 5.0 mol% | 72–84% | n.a. | not calculated | [39] |
| Cu@U-g-C3N4 | CO2-fixation/cyclization | 1.9 mol% | 62–92% | 5 runs | not calculated | [55] | |
| Ni2P/N,P-C-800 | oxidative cross- dehydrogenative coupling | 7.5 mol% | 73–93% | 5 runs | not calculated | [58] | |
| CuO-np/SiO2 | condensation/dehydrogenation | 10 mol% | 76–93% | 5 runs | not calculated | [61] | |
| Yb@l-MSNs | condensation/dehydrogenation | 2.25 mol% | 80–92% | 5 runs | TON = 27–34 | [70] | |
| Pd/Al2O3 | isomerization/imine formation/ring closing/dehydrogenation | 2.5 mol% | 50% | 4 runs | not calculated | [78] | |
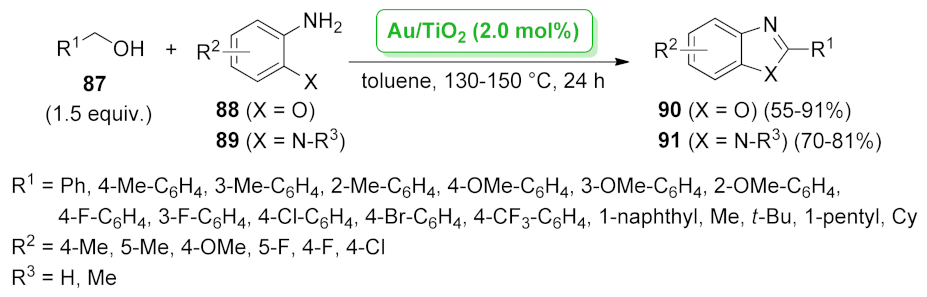
| Au/TiO2 | hydrogenation/CO2- fixation/cyclization | 1.0 mol% | 28–86% | 1 run | not calculated | [81] | |
| Au/TiO2 | two hydrogen- transfer reaction | 2.0 mol% | 70–81% | 7 runs | not calculated | [82] | |
| Au/TiO2 | condensation/dehydrogenation | 1 mol% | 51–99% | 5 runs | not calculated | [84] | |
| Au/CeO2 | one-pot four step protocol | 0.5 mol% | 13–91% | 2 runs | not calculated | [88] | |
| Co/MnO | condensation/dehydrogenation | 100 mg/mmol | 89–97% | 4 runs | not calculated | [94] | |
| FeCl3/PANI | condensation/dehydrogenation | 19 wt.% | 70–97% | 4 runs | not calculated | [122] | |
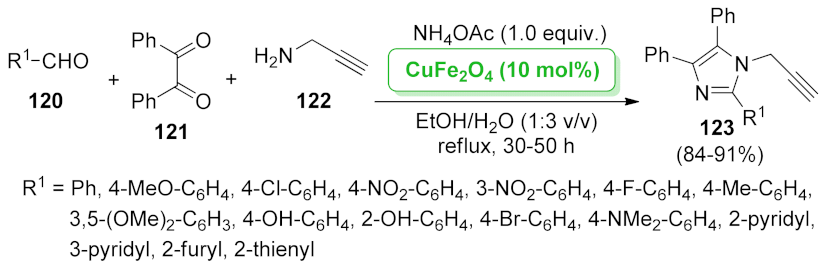
| PANI@Au:CuO | amination/azidation/annulation | 1.0 mol% | 61–87% | 7 runs | not calculated | [123] | |
| Cu-NPs@COF | CO2-fixation/cyclization | 30 mg/mmol | 71–96% | 6 runs | not calculated | [130] | |
| PS-Zn(II)SALTETA | CO2-fixation/cyclization | 3.0 mol% | 68–95% | 7 runs | not calculated | [138] | |
| Cu(II)-ID@Silica | one pot cyclization | 5.0 mol% | 70–84% | n.a. | not calculated | [154] | |
| BS-Cu(II)@SiO2 | condensation/cyclization/aromatization | 5.0 mol% | 87–94% | 5 runs | not calculated | [155] | |
| Cu@QSSi | multi-component reaction | 5.0 mol% | 82–91% | 7 runs | not calculated | [156] | |
| Cu(II)-TD@nSiO2 | condensation/cyclization | 0.3 mol% | 88–97% | 8 runs | TOF = 178–856 h−1 | [158] | |
| benzo[d]oxazole | CuO-np/SiO2 | condensation/dehydrogenation | 10 mol% | 72–85% | 5 runs | not calculated | [61] |
| Au/TiO2 | two hydrogen-transfer reaction | 2.0 mol% | 55–91% | 7 runs | not calculated | [82] | |
| benzo[d]thiazole | CuO-np/SiO2 | condensation/dehydrogenation | 10 mol% | 80–88% | 5 runs | not calculated | [61] |
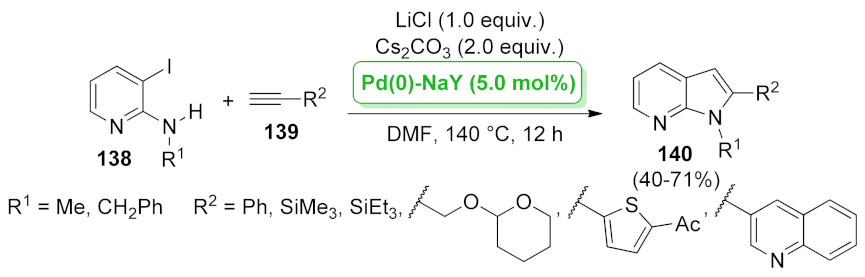
| 1H-pyrrolo[2,3-b] pyridine | Pd(0)–NaY | heteroannulation | 5.0 mol% | 40–71% | 4 runs | not calculated | [110] |
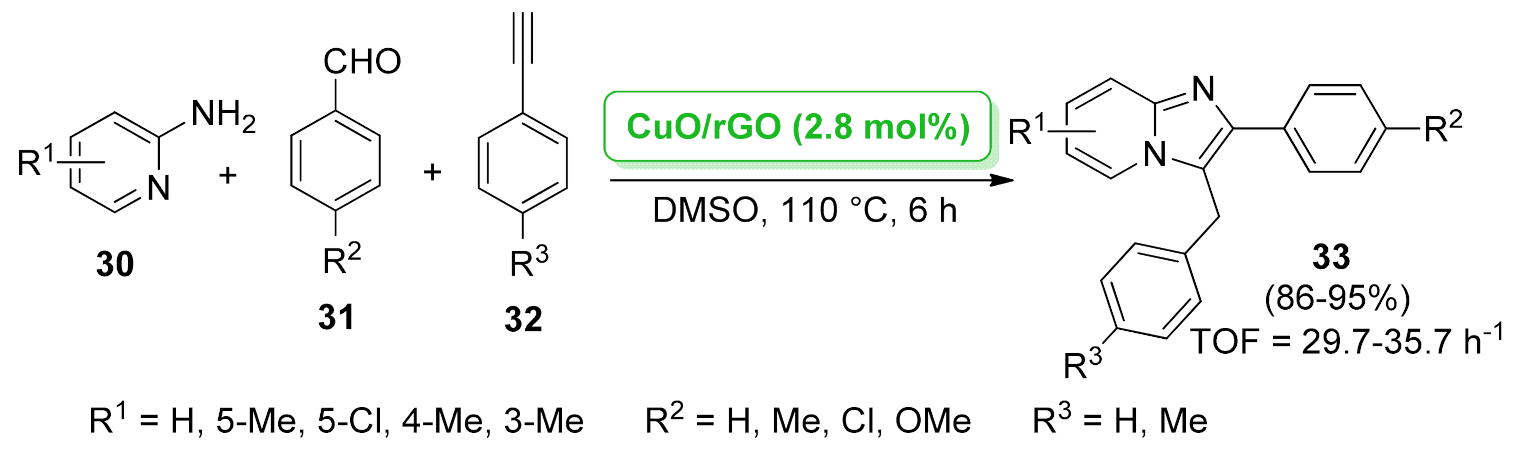
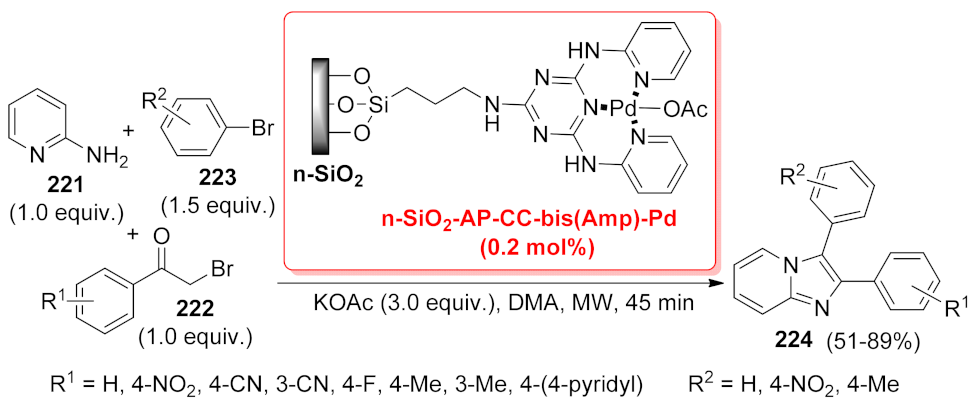
| imidazo[1,2-a] pyridine | CuO/rGO | three-component reaction | 2.8 mol% | 86–95% | 5 runs | TOF = 29.7–35.7 h−1 | [49] |
| BF3/MCM-41 | multi-component cyclocondensation | 20 wt.% | 75–95% | 5 runs | TON = 7.14 | [69] | |
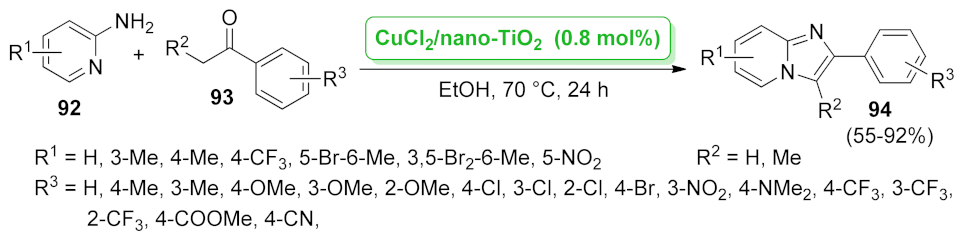
| CuCl2/nano-TiO2 | condensation/dehydrogenation | 0.8 mol% | 55–92% | 4 runs | not calculated | [85] | |
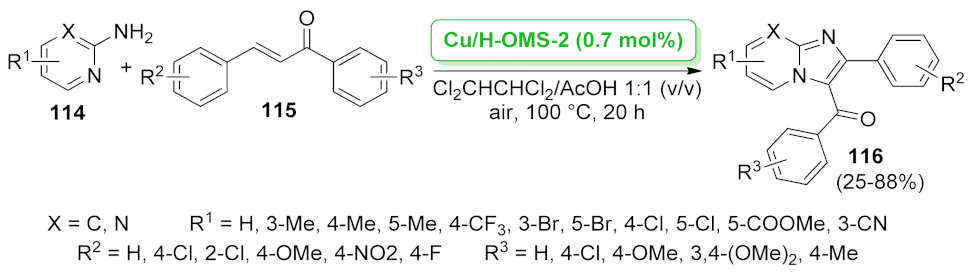
| Cu/H-OMS-2 | Michael addition/oxidative cyclization | 0.7 mol% | 39–88% | 3 runs | not calculated | [95] | |
| Cu/CNT@CoFe2O4 | multi-component reaction | 5.0 mol% | 80–95% | 8 runs | not calculated | [114] | |
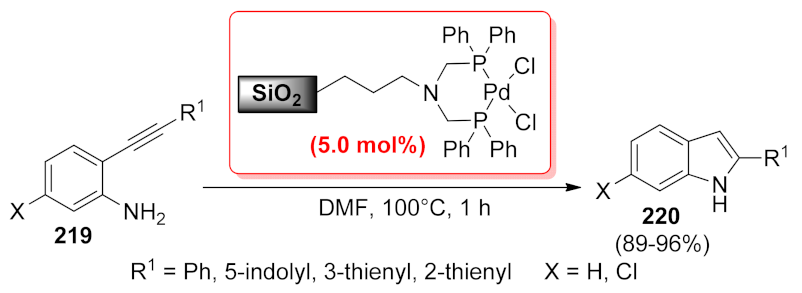
| n-SiO2-AP-CC- bis(Amp)-Pd | multi-component reaction | 0.2 mol% | 51–89% | 6 runs | not calculated | [162] | |
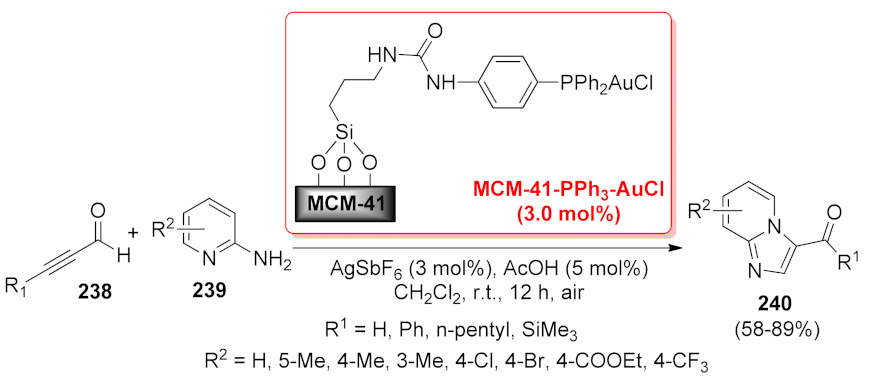
| MCM-41-PPh3-AuCl | cascade annulation | 3 mol% | 58–89% | 8 runs | not calculated | [175] | |
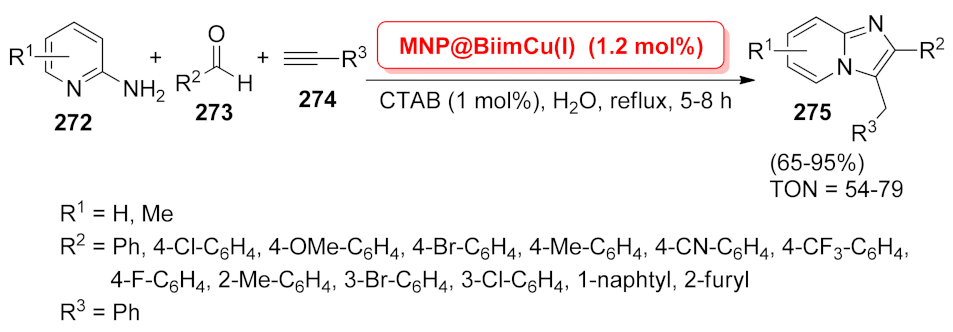
| MNP@BiimCu(I) | multi-component reaction | 1.2 mol% | 65–95% | 10 runs | TON = 54–79 | [196] | |
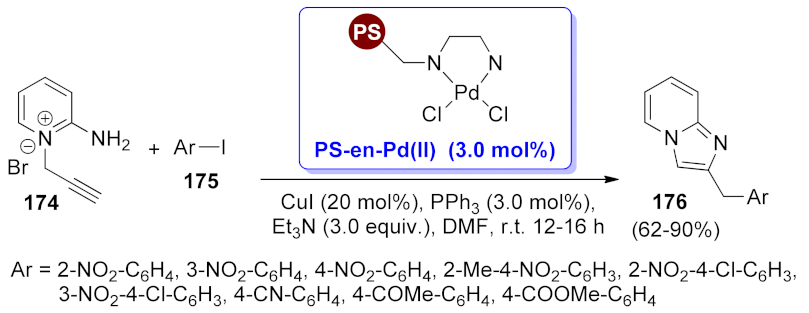
| imidazo[2,1-b] pyridine | PS-en-Pd(II) | Sonogashira coupling/heteroannulation | 3 mol% | 62–90% | 5 runs | not calculated | [132] |
| imidazo[1,2-a] pyrimidine | Cu/H-OMS-2 | Michael addition/oxidative cyclization | 0.7 mol% | 25–81% | 3 runs | not calculated | [95] |
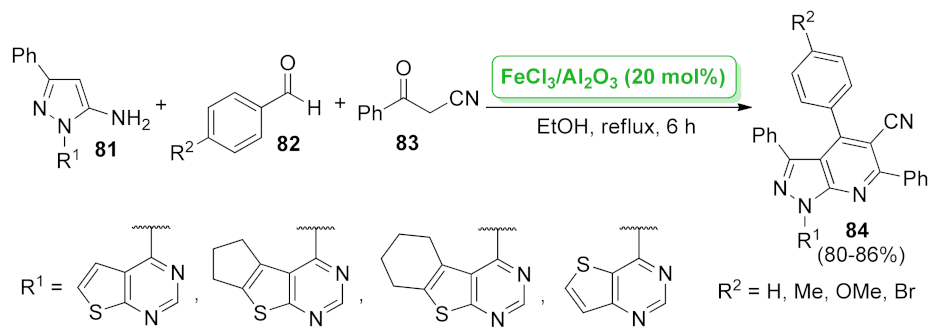
| pyrazolo[3,4-b] pyridine | FeCl3/Al2O3 | multicomponent reaction | 20 mol% | 80–86% | n.a. | not calculated | [77] |
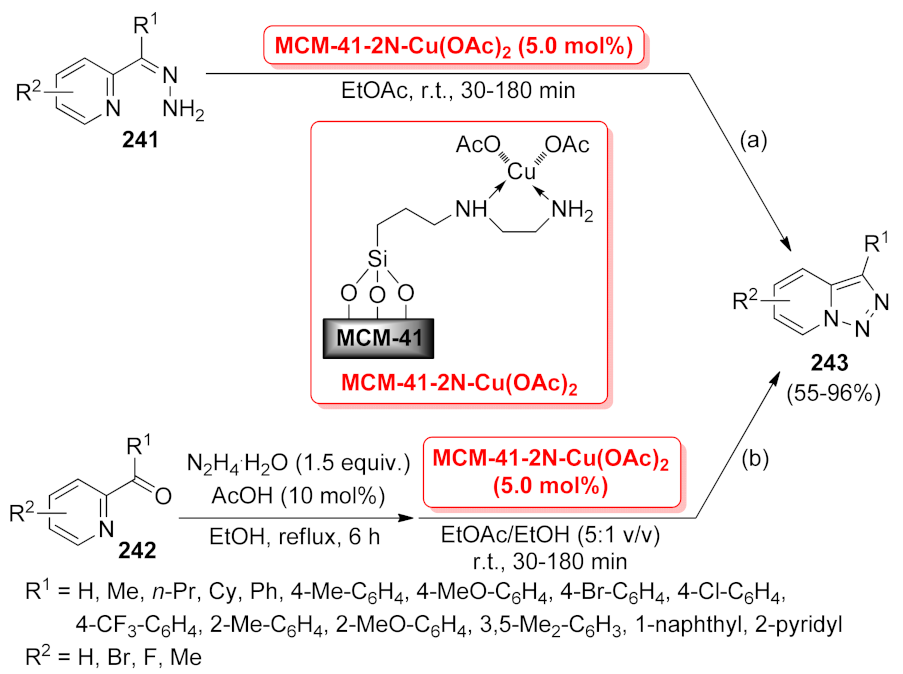
| 1,2,3-triazolo [1,5-a]pyridine | MCM-41–2N- Cu(OAc)2 | one-pot reaction | 5 mol% | 55–96% | 7 runs | not calculated | [176] |
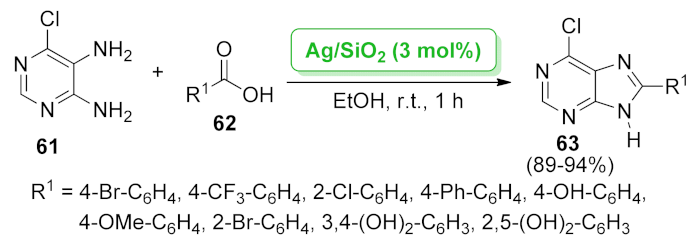
| 9H-purine | Ag/SiO2 | condensation/dehydrogenation | 3 mol% | 89–94% | 5 runs | not calculated | [67] |
| quinoline | CoO/MWCNTs | Friedländer condensation | 0.86–4.2 mol% | 51–86% | 5 runs | TOF = 0.36–0.55 s−1 | [52] |
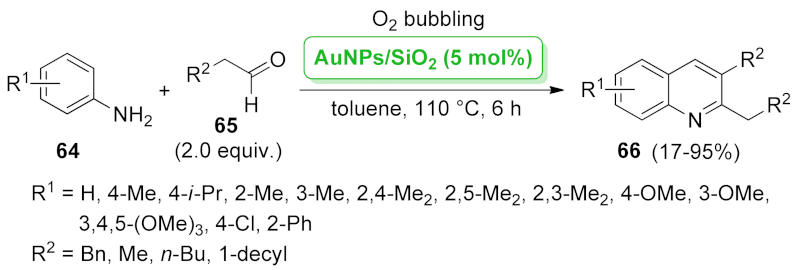
| AuNPs/SiO2 | oxidative cyclization | 5 mol% | 17–95% | 7 runs | not calculated | [68] | |
| Pt/Al2O3 + ZnO | dehydrogenative condensation | 1.7 mol% Pt 4.5 mol% Zn | 18–62% | n.a. | not calculated | [80] | |
| Pd(II)/GO@Fe-FeO | one-pot reaction | 10 mol% | 60–90% | 6 runs | not calculated | [113] | |
| Pd/Kaiser oxime resin | Mizoroki-Heck vinylation | 1.0 mol% | 34–44% | not specified | not calculated | [137] | |
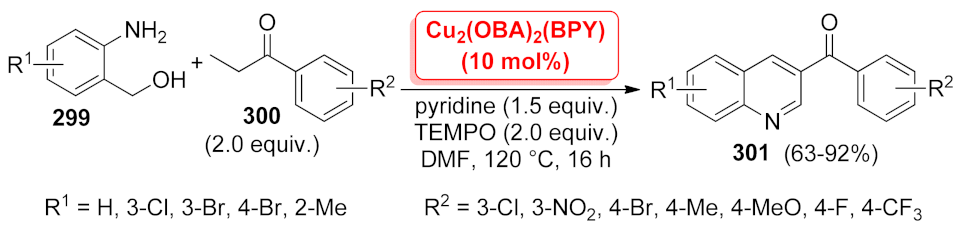
| Cu2(OBA)2(BPY)-MOF | one-pot domino reaction | 10 mol% | 63–92% | 8 runs | not calculated | [213] | |
| CuOMOFY | multi-component reaction | 0.7 mol% | 91–94% | n.a. | TOF = 67–104 h−1 | [214] | |
| isoquinoline | LS-FAS-Cu | cyclocondensation | 10 mol% | 85% | 3 runs | not calculated | [147] |
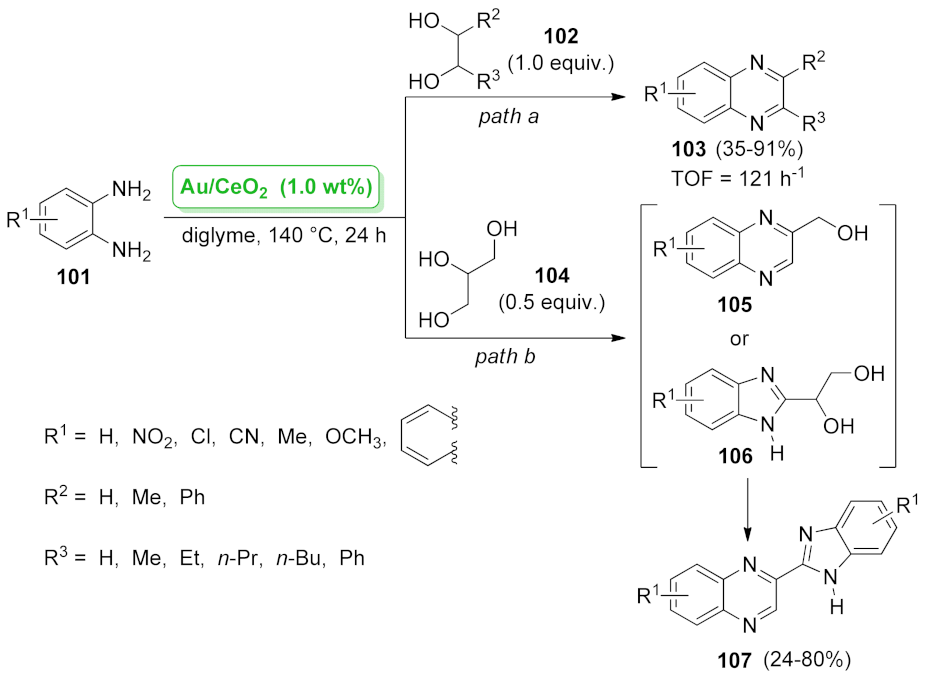
| quinoxaline | Au/CeO2 | oxidative coupling | 1.0 wt.% | 35–91% | 4 runs | TOF = 121 h−1 | [89] |
| Au/CeO2 | oxidative coupling | 1.0 wt.% | 24–80% | 3 runs | not calculated | [90] | |
| Au/HT | oxidative coupling | 0.7 wt.% | 80–85% | 4 runs + calcination | TOF = 132 h−1 | [89] | |
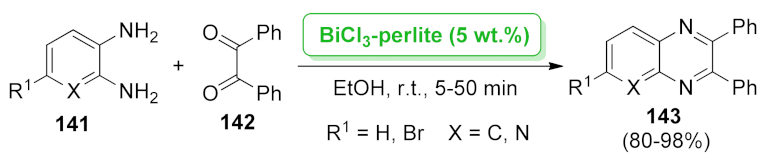
| BiCl3-perlite | condensation | 5 wt.% | 80–98% | 5 runs | not calculated | [111] | |
| Zr-CAP-SG | condensation | 10 wt.% | 83–95% | 5 runs | not calculated | [166] | |
| Cu(II)-DiAmSar/SBA-15 | condensation | 0.008 mol% | 88–99% | 7 runs | not calculated | [168] | |
| Fe-Schiff base/SBA-15 | condensation | 0.14 mol% | 98–99% | 6 runs | not calculated | [169] | |
| Zr(IV)-Schiff base/SBA-15 | condensation | 0.2 mol% | 98–99% | 7 runs | not calculated | [170] | |
| Pd(II)-Schiff base/SBA-15 | condensation | 0.5 mol% | 95–99% | 8 runs | not calculated | [171] | |
| ZrOL2@SMNP | condensation | 0.004 mol% | 78–97% | 4 runs | not calculated | [207] | |
| quinazoline | Fe-Fe3C/CN-800 | oxidative coupling | 4 mol% | 78–98% | 6 runs | not calculated | [57] |
| Ni2P/N,P-C-800 | oxidative cross- dehydrogenative coupling | 7.5 mol% | 71–94% | 5 runs | not calculated | [58] | |
| Au/TiO2 | two hydrogen-transfer reaction | 0.8 mol% | 31–99% | 8 runs | not calculated | [83] | |
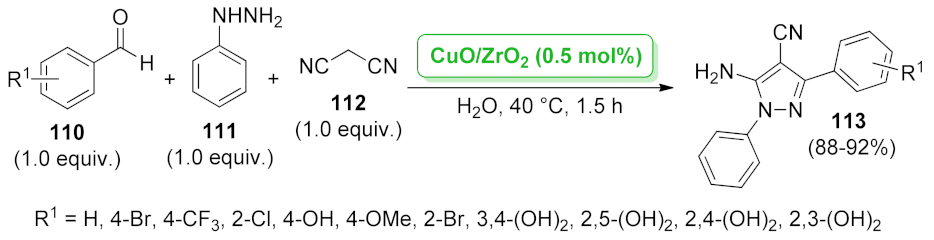
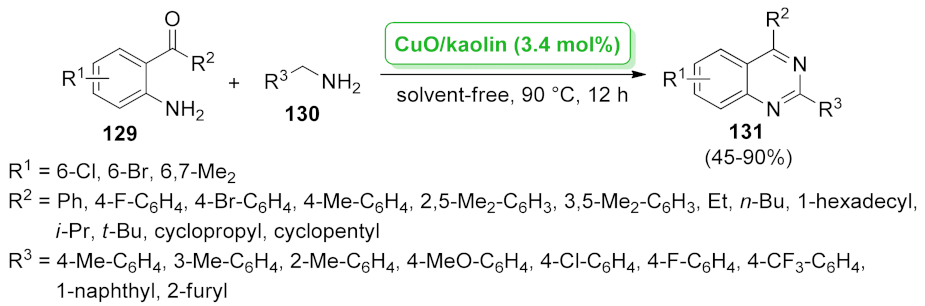
| CuO/kaolin | not specified | 3.4 mol% | 45–90% | 4 runs | not calculated | [105] | |
| pyrido[2,3-d] pyrimidine | Cu-TiO2 | not specified | 1.0 wt.% | 58–94% | n.a. | not calculated | [86] |
| pyrido[2,3-b] pyrazine | Cu(II)-DiAmSar/SBA-15 | condensation | 0.008 mol% | 74–85% | 7 runs | not calculated | [168] |
| Fe-Schiff base/SBA-15 | condensation | 0.14 mol% | 95–98% | 6 runs | not calculated | [169] | |
| Zr(IV)-Schiff base/SBA-15 | condensation | 0.2 mol% | 94–99% | 7 runs | not calculated | [170] | |
| Pd(II)-Schiff base/SBA-15 | condensation | 0.5 mol% | 95–98% | 8 runs | not calculated | [171] | |
| ZrOL2@SMNP | condensation | 0.004 mol% | 73–95% | 4 runs | not calculated | [207] | |
| 2,3-dihydro-1H- benzazepine | Pd/Smopex®-234 | cyclocarbonylative Sonogashira coupling | 0.4 mol% | 63% | n.a. | not calculated | [125] |
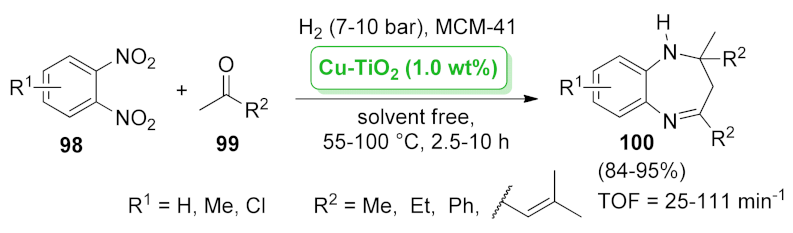
| 2,3-dihydro-1H-1,4-benzodiazepine | Pt/TiO2 + MCM-41 | hydrogenation/cyclocondensation | 1.0 wt.% | 84–95% | n.a. | TOF = 25–111 min−1 | [87] |
| Cu@β-CD@SPIONs | condensation/cyclocondensation/[3 + 2] cycloaddition | 1.0 mol% | 71–87% | 5 runs | not calculated | [209] | |
| CuFe2O4@MIL- 101(Cr) | cyclocondensation | 0.005 mol% | 87–95% | 6 runs | not calculated | [215] | |
| 2,3-dihydro-1H-1,5-benzodiazepine | Zr/Norit RX3Zr/xerogel | cyclocondensation | 100 mg | up to 99% | n.a. | not calculated | [47] |
| Fe3O4@SiO2-CeCl3 | one-pot three-component reaction | 8 mg/mmol | 71–91% | 8 runs | not calculated | [116] | |
| FeCl3/PVP | condensation/cyclization | 5.0 mol% | 85–98% | 4 runs | not calculated | [119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aronica, L.A.; Albano, G. Supported Metal Catalysts for the Synthesis of N-Heterocycles. Catalysts 2022, 12, 68. https://doi.org/10.3390/catal12010068
Aronica LA, Albano G. Supported Metal Catalysts for the Synthesis of N-Heterocycles. Catalysts. 2022; 12(1):68. https://doi.org/10.3390/catal12010068
Chicago/Turabian StyleAronica, Laura Antonella, and Gianluigi Albano. 2022. "Supported Metal Catalysts for the Synthesis of N-Heterocycles" Catalysts 12, no. 1: 68. https://doi.org/10.3390/catal12010068
APA StyleAronica, L. A., & Albano, G. (2022). Supported Metal Catalysts for the Synthesis of N-Heterocycles. Catalysts, 12(1), 68. https://doi.org/10.3390/catal12010068