1. Introduction
Glycerol is a by-product of the biodiesel manufacturing process. Because the increase in biodiesel production caused glycerol oversupply, a selective oxidation technique is required to change glycerol into valuable compounds, including glyceric acid (GA), dihydroxyacetone (DHA), and hydroxypyruvic acid (HA) [
1,
2,
3,
4]. HA is an important intermediate in the pharmaceutical and chemical industries owing to its high functionality, that is, three different functional groups of hydroxyl, carbonyl, and carboxyl groups [
5,
6]. In addition, during the synthesis of biologically important molecules using the transketolase enzyme, HA is the widely used donor substrate [
7].
Based on previous studies [
1,
2,
3,
4], glycerol oxidation routes have been proposed, as shown in
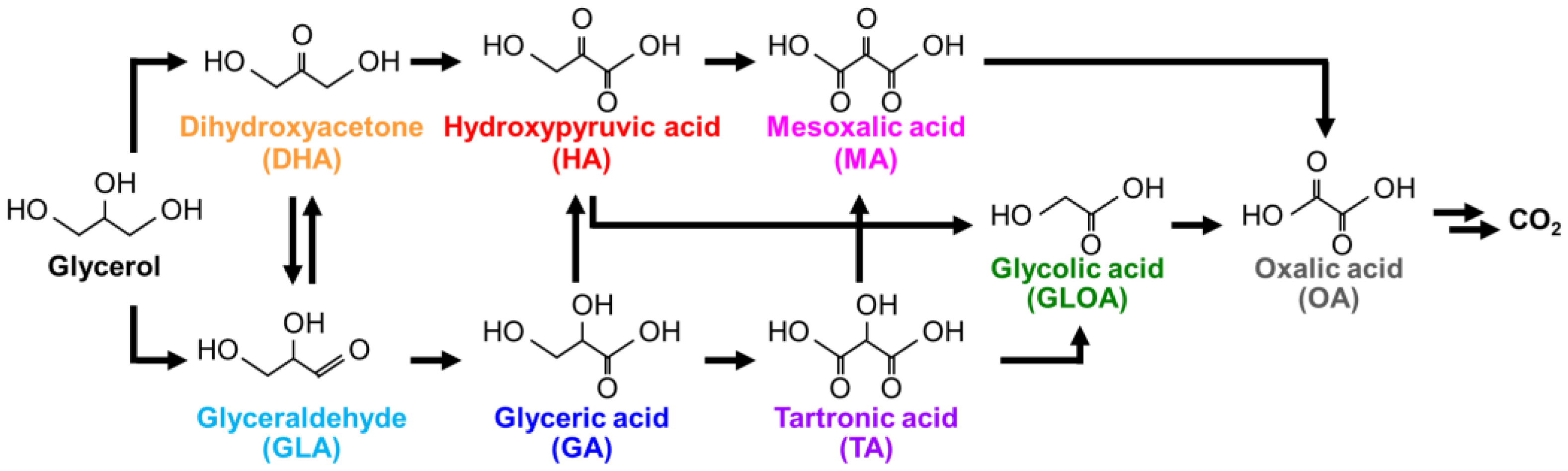
Figure 1. In the first step, the terminal and secondary OH groups in glycerol are oxidized to form glyceraldehyde (GLA) and DHA, respectively, where GLA is easily oxidized into a more chemically stable compound of GA. The selectivities of GA and DHA were affected by the pH of the reaction solution and the nature of the catalysts [
4]. In the base condition, glycerate salts were predominantly formed rather than DHA. Further, the oxidation of the secondary OH group in glycerol was facilitated in the presence of heavy metals in Group V (Bi) and IV (Pb), owing to the geometric effect [
1], and Pt-Bi based catalysts have been investigated for the production of DHA [
8,
9]. In the glycerol oxidation process (
Figure 1), further oxidation of GA or DHA results in the formation of glycolic acid (GLOA) and OA with high chemical stability via the intermediate compounds of HA, mesoxalic acid (MA), and tartronic acid (TA); the final product is carbon dioxide. Therefore, producing HA, MA, and TA intermediates is challenging. In particular, HA production requires control of the catalytic reactivity to oxidize a terminal and a secondary OH group in glycerol without the oxidation of the other terminal OH group. Further, the yield of HA was significantly lower than those of GA and DHA [
10,
11,
12,
13,
14,
15]. Here, when the reactant is GA, in which one terminal OH group in glycerol has been oxidized, HA is reported to be effectively produced from GA; e.g., Pt-Bi/C can convert GA to HA with a yield of 64% (GA conversion: 75%) [
16]. However, considering the oxidation of glycerol, the highest HA yield was 22% (estimated from the glycerol conversion (75%) and the HA selectivity (28.9%)) for Au/graphite after the reaction at 30 °C with an applied pressure of 0.3 MPa (3 atm) (pure O
2) and the addition of NaOH. In addition, a high amount of toxic OA (yield: 12%) was formed as a by-product, probably owing to further oxidation of HA [
17]. Since the base condition and the high reaction pressure might cause the oxidation of HA, the suppression of the unsolicited reaction is an important factor in selecting the base-free and moderate reaction conditions such as room temperature and open-air atmosphere.
In our previous studies, we demonstrated the high yields of DHA and GLA over Pt/CeO
2-ZrO
2-MO
x/SBA-16 (MO
x = Bi
2O
3 and Fe
2O
3, SBA-16: Santa Barbara Amorphous No. 16) using CeO
2-ZrO
2-MO
x promoters with high oxygen release and storage abilities under moderate reaction conditions of 30 °C and atmospheric pressure [
18,
19,
20]. The oxygen supply from the promoter facilitated the oxidation ability of Pt, and the moderate conditions suppressed further oxidation of the products, resulting in a high DHA yield of 76% for Pt/CeO
2-ZrO
2-Bi
2O
3/SBA-16 [
18] and a high GLA yield of 22% for Pt/CeO
2-ZrO
2-Fe
2O
3/SBA-16 [
20].
In this study, to produce HA from glycerol, we introduced PbO into CeO
2-ZrO
2-Bi
2O
3, because Pb showed selective oxidation of the secondary OH group similar to Bi [
1]. The redox of Pb
2+/4+ may improve the oxygen supply ability, which can accelerate the oxidation of the OH group even under moderate reaction conditions. Therefore, novel catalysts of 7 wt% Pt/16 wt% Ce
0.80(1−x−y)Zr
0.20(1−x−y)Bi
yPb
xO
2−δ/SBA-16 (Pt/CZBi(
y)Pb(
x)/SBA) were prepared, and their catalytic activities for the glycerol oxidation were investigated under moderate conditions of an atmospheric open-air system and 30 °C in a base-free solution.
2. Results and Discussion
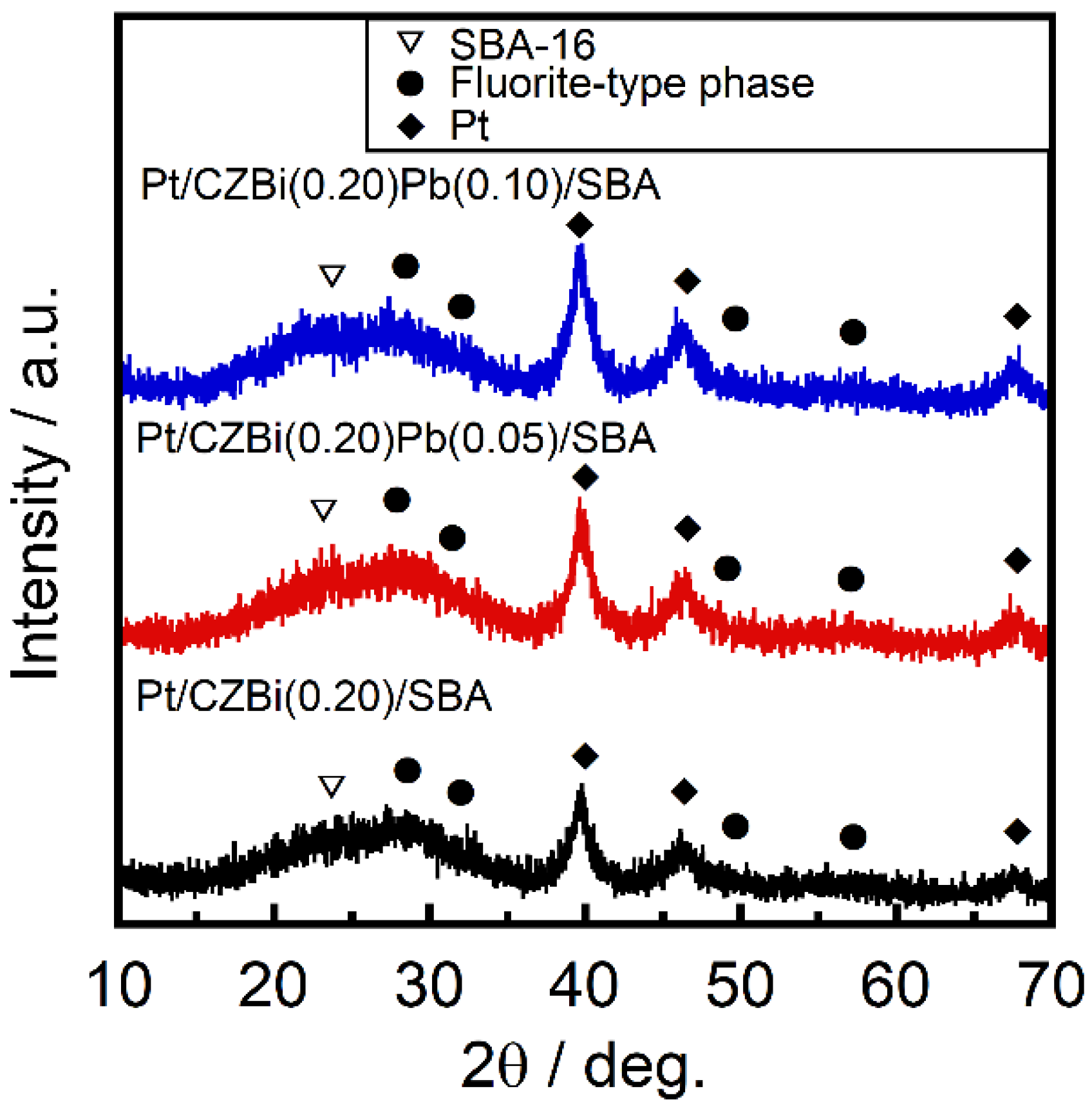
Figure 2 shows the X-ray diffraction (XRD) patterns of Pt/CZBi(0.20)Pb(
x)/SBA. Broad peaks corresponding to the cubic fluorite-type structure were observed in addition to that of the Pt metal and SBA-16. For all the catalysts, the crystallite sizes of Pt and the fluorite-type phase were almost the same—ca. 5 and ca. 1 nm, respectively, irrespective of the Pb content (
Table S1). Confirming the peak shift in the XRD patterns shown in
Figure 2 is challenging; however, the XRD measurements of CZBi(0.20)Pb(
x)/SBA (
Figure S1) revealed that the broad peaks of the fluorite-type structure were slightly shifted toward lower angles with increasing
x, suggesting the replacement of the Ce
4+ (0.111 nm [
21]) and Zr
4+ (0.098 nm [
21]) sites for larger Pb
2+ (0.143 nm [
21]). The catalyst compositions, measured by X-ray fluorescence spectroscopy, were in good agreement with the feed values (
Table S1). Scanning electron microscope-energy dispersive X-ray spectrometry analysis of Pt/CZBi(0.20)Pb(0.05)/SBA (
Figure S2), indicated that Pt and CZBi(0.20)Pb(0.05) were well dispersed on SBA-16. The surface area decreased with an increase in Pb content—Pt/CZBi(0.20)/SBA (298 m
2·g
−1) > Pt/CZBi(0.20)Pb(0.05)/SBA (236 m
2·g
−1) > Pt/CZBi(0.20)Pb(0.10)/SBA (220 m
2·g
−1).
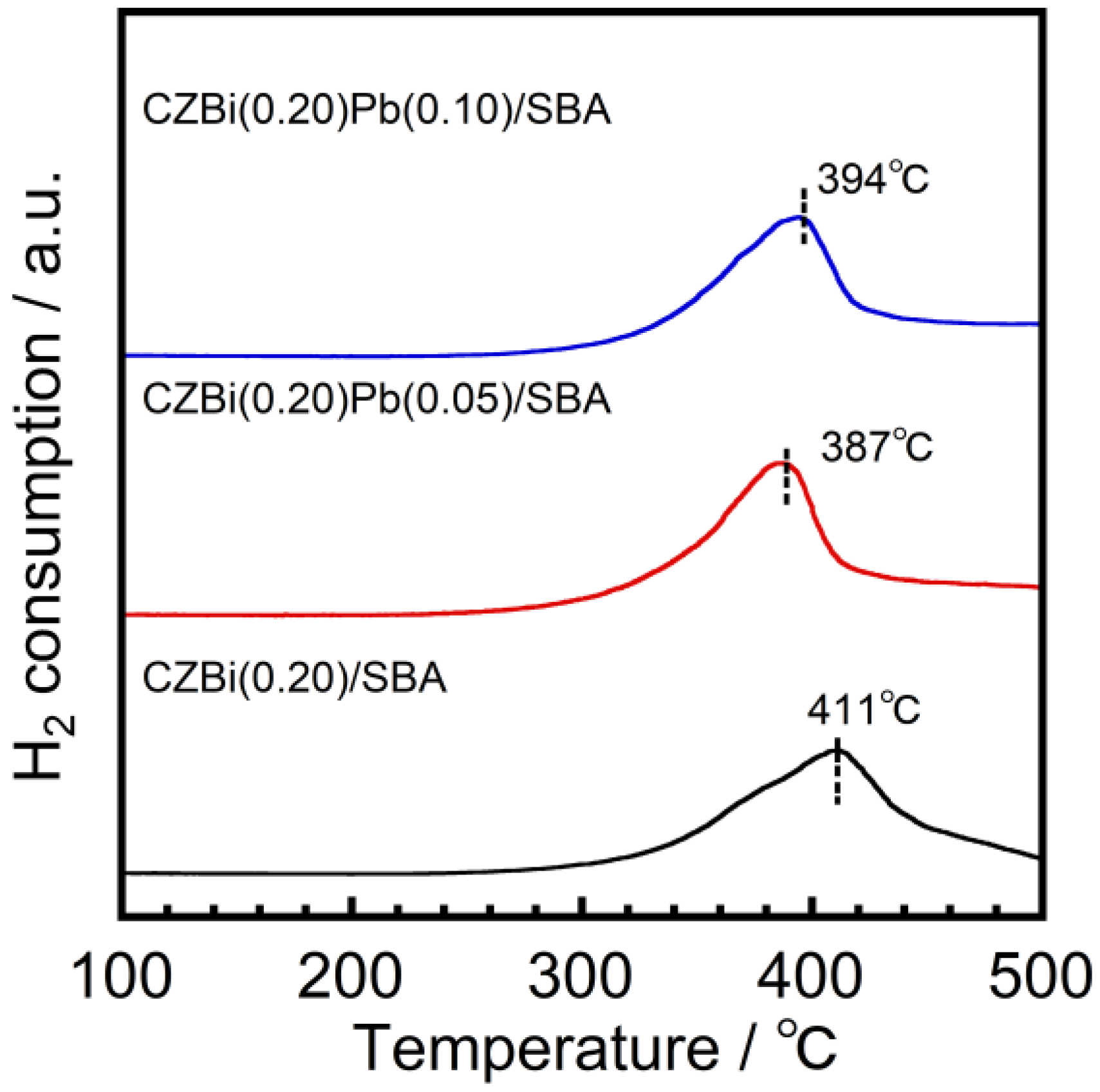
The effect of introducing Pb
2+/4+ on oxygen release and storage abilities was investigated using temperature-programmed reduction (TPR) measurements. The TPR profiles of CZBi(0.20)Pb(
x)/SBA are shown in
Figure 3. The Pb
2+/4+ introduction lowered the reduction temperature, and CZBi(0.20)Pb(0.05)/SBA showed the lowest peak temperature among CZBi(0.20)Pb(
x)/SBA. In addition, the total oxygen storage capacity of CZBi(0.20)Pb(0.05)/SBA was the highest (243 μmol·g
−1), followed by CZBi(0.20)Pb(0.10)/SBA (237 μmol·g
−1) and CZBi(0.20)/SBA (236 μmol·g
−1). Therefore, CZBi(0.20)Pb(0.05)/SBA exhibited the highest oxygen release and storage abilities owing to the synergistic redox reaction between Ce
3+/4+ and Pb
2+/4+. Further, the decrease in the abilities of CZBi(0.20)Pb(0.10)/SBA might be caused by the decrease in its surface area.
The glycerol oxidation test was performed using Pt/CZBi(0.20)Pb(
x)/SBA (0.3 g, glycerol/Pt = 10 mol/mol) at 30 °C for 4 h in atmospheric air, and the results are tabulated in
Table 1, wherein, MA was not detected. Considering Pt/CZBi(0.20)Pb(0.05)/SBA, the glycerol conversion and the yields of HA and DHA significantly enhanced compared with those of Pt/CZBi(0.20)/SBA. The high glycerol conversion can be explained by the high oxygen release and storage abilities; that is, the effective oxygen supply from CZBi(0.20)Pb(0.05) promoted the oxidation reaction on the Pt activator. In addition, the predominant product of Pt/CZBi(0.20)Pb(0.05)/SBA was DHA, indicating that the geometric effects of glycerol, Pt, and Bi
3+, or Pb
2+/4+ in CZBi(0.20)Pb(0.05) facilitated the oxidation of the secondary OH group rather than the terminal OH group. Although HA, which is easy to oxidize into chemically stable OA with high toxicity, is considered as an intermediate, the moderate reaction conditions enabled the suppression of the further oxidation of HA. Due to these reasons, the high HA and DHA yields for Pt/CZBi(0.20)Bi(0.05)/SBA might be obtained. In general, catalytic activity is dependent on the surface area; however, Pt/CZBi(0.20)Pb(0.05)/SBA exhibited the high glycerol conversion compared to the Pt/SBA [
20] case, regardless of the low surface area (Pt/CZBi(0.20)Pb(0.05)/SBA: 236 m
2·g
−1, Pt/SBA: 476 m
2·g
−1 [
20]). This result supports that the oxidation ability of Pt was enhanced by the CZBi(0.20)Bi(0.05) promoter. However, Pt/CZBi(0.20)Pb(0.10)/SBA showed a lower catalytic activity than Pt/CZBi(0.20)Pb(0.05)/SBA, maybe because of its low surface area. Therefore, the optimum Pb
2+/4+ content (
x) was determined to be 0.05. The Bi
3+ content (
y) was also optimized (
Table S2); consequently, Pt/CZBi(0.20)Pb(0.05)/SBA exhibited the highest activity for HA production.
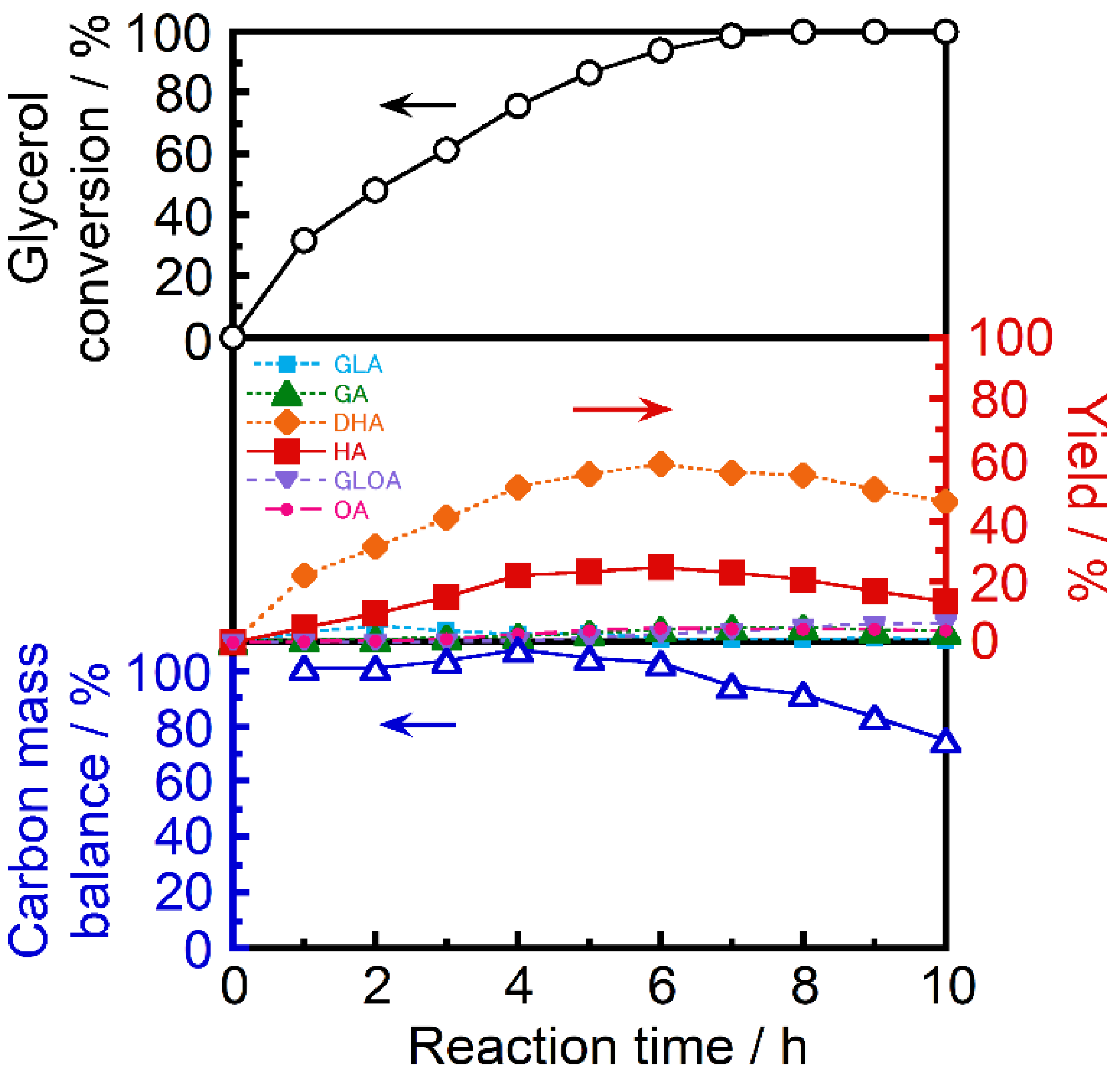
Figure 4 shows the glycerol oxidation activity as a function of reaction time over Pt/CZBi(0.20)Pb(0.05)/SBA at 30 °C in atmospheric air, where TA and MA were not detected. DHA was predominantly produced, and its yield increased with an increase in reaction time up to 6 h. The HA yield simultaneously increased, suggesting the conversion of DHA into HA (
Figure 1). Interestingly, even though GLOA and OA are chemically stable than HA, the yields of the oxidation products of HA—GLOA and OA, remained low (<5%), maybe because of the moderate reaction conditions. However, as the reaction time increased over 6 h, the HA yield decreased because of the decrease in the DHA yield and the further oxidation of HA. Therefore, the highest HA yield of 24.6% was obtained after the reaction for 6 h, where the glycerol conversion and the HA selectivity were 93.9% and 26.2%, respectively. Here, carbon mass balance maintained ca. 100% up to 6 h, and then, decreased. This result also implies that further oxidation caused the generation of CO
2. In addition, moderate reaction conditions enabled the low yield of toxic OA (4.8%). The HA yield of the catalysts in this study was higher than that of Au/graphite reported earlier [
17] (22%), where OA yield was 12%, at 30 °C by applying 0.3 MPa (3 atm) (pure O
2), with the addition of NaOH. For Pt/CZBi(0.20)Pb(0.05)/SBA even after the reaction for 10 h, the crystal structural change was not observed from the XRD measurement (
Figure S3). In addition, no metal leaching was confirmed from the XRF analysis of the liquid-phase after the 10 h reaction.
3. Materials and Methods
Mesoporous silica SBA-16 (SBA) was prepared as described in previous studies [
22,
23]. Pluronic F-127 (Sigma-Aldrich, Burlington, MA, USA) (1.6 g), 1, 3, 5-trimethylbenzene (Kishida Chemical, Osaka, Japan, ≥98.0%) (1.1 mL), and 90 mL of 0.2 mol·L
−1 hydrochloric acid (prepared by diluting concentrated hydrochloric acid (Kishida Chemical, 35%) with deionized water) were mixed, and then 7.1 mL of tetraethoxysilane (Kishida Chemical, ≥99.0%) was added to the solution. After stirring at 35 °C for 24 h, the mixture was hydrothermally heated at 140 °C for 24 h in a sealed brass vessel using a Teflon bottle. The resulting precipitates were filtered by suction filtration, washed with water and ethanol, subsequently dried at 80 °C for 12 h, and finally calcined at 600 °C for 4 h under a flow of air (15 mL·min
−1).
The chemical—16 wt% Ce0.80(1−x−y)Zr0.20(1−x−y)BiyPbxO2−δ/SBA-16 (CZBi(y)Pb(x)/SBA) was synthesized by a co-precipitation method. Solutions of 1.0 mol·L−1 Ce(NO3)3, 0.10 mol·L−1 ZrO(NO3)2, 0.10 mol·L−1 Pb(NO3)2 and 0.50 mol·L−1 Bi(NO3)3, were prepared by dissolving Ce(NO3)3·6H2O (Kojundo, 99.9%), ZrO(NO3)2·2H2O (Kishida Chemical, ≥99.0%), and Pb(NO3)2 (FUJIFILM Wako Pure Chemical, 99.5%) in deionized water; and by dissolving Bi2O3 (Kishida Chemical, ≥99.9%) in 3 mol·L−1 nitric acid (prepared by diluting nitric acid (Kishida Chemical, 60%) with deionized water), respectively. The SBA-16 powder (0.4 g) was dispersed in the mixed solutions of 1.0 mol·L−1 Ce(NO3)3, 0.10 mol·L−1 ZrO(NO3)2, 0.50 mol·L−1 Bi(NO3)3, and 0.10 mol·L−1 Pb(NO3)2 in the stoichiometric ratio, and deionized water (30 mL) was added, followed by stirring for 30 min at room temperature. A solution of 5 vol% NH3 (prepared by diluting aqueous NH3 (Kishida Chemical, 28%) with deionized water) was added dropwise under vigorous stirring until the pH reached 11. After stirring for 12 h at room temperature, the precipitates were collected by suction filtration and dried at 80 °C for 12 h, followed by calcination at 600 °C for 1 h in atmospheric air.
Platinum was loaded onto CZBi(y)Pb(x)/SBA using the impregnation technique. The CZBi(y)Pb(x)/SBA powder (0.4 g) was dispersed into a Pt colloid stabilized with polyvinylpyrrolidone (Pt: 4.0 wt%; Tanaka Kikinzoku Kogyo, Tokyo, Japan) with the Pt amount adjusted to 7 wt%, and ethanol (Kishida Chemical, 99.5%) (40 mL) was added. The mixture was dried at 90 °C for 2 h and calcined at 500 °C for 4 h in atmospheric air to obtain 7 wt% Pt/16 wt% Ce0.80(1−x−y)Zr0.20(1−x−y)BiyPbxO2−δ/SBA-16 (Pt/CZBi(y)Pb(x)/SBA); that is, the molar ratio of Pt in the catalyst was ca. 0.9 mol%.
X-ray powder diffraction (XRD; SmartLab, Rigaku, Tokyo, Japan) was used to identify the crystal phase using Cu-Kα radiation (40 kV, 30 mA). The XRD data were analyzed by whole powder pattern fitting using PDXL software (Rigaku) to estimate the crystallite size. The sample composition was examined using X-ray fluorescence spectrometry (XRF; Supermini200, Rigaku). Scanning electron microscope-energy dispersive X-ray spectrometry (SEM-EDX; SSX-550, Shimadzu, Kyoto, Japan) was performed to observe the chemical composition of the elements in the catalyst. The Brunauer–Emmett–Teller (BET) specific surface area was measured at −196 °C using N2 gas (Micromeritics Tristar 3000, Shimadzu). The oxygen release ability was assessed by temperature-programmed reduction (TPR) measurements using H2, performed using 0.2 g of the sample at a heating rate of 5 °C·min−1 under a reducing gas (5 vol% H2-95 vol% Ar) flow at 50 mL·min−1 (BELCAT-B, MicrotracBEL, Osaka, Japan). After the H2-TPR analysis, the total oxygen storage capacity was measured using the pulse injection method at 500 °C.
Glycerol oxidation was performed in an open-air system. The catalyst (0.3 g) and 10 mL of 1 wt% aqueous glycerol (prepared by dissolving glycerol (Kishida Chemical, 99.0%) in deionized water) were mixed in a flask equipped with a condenser (4 °C) under stirring in an oil bath at 30 °C, without applying pressure or gas bubbling. After the reaction, the liquid-phase (10 μL) was analyzed to obtain the concentrations of glycerol and products by high-performance liquid chromatography (HPLC, JASCO, Tokyo, Japan) system, where, the product was separated by Shodex Sugar SH-1011 column operating at 50 °C with 5 mmol·L
−1 sulfuric acid eluent (prepared by diluting concentrated sulfuric acid (Kishida Chemical, 98%) with deionized water) (0.75 mL·min
−1), and detected using a refractive index and a photodiode array. The concentrations of the samples were determined using the calibration curve method, where, standard solutions were prepared by diluting DL-glyceric acid (Tokyo Chemical Industry, Tokyo, Japan, 20% in water) in deionized water, by dissolving 1,3-dihydroxyacetone dimer (Sigma-Aldrich, Burlington, MA, USA, 97%), DL-glyceraldehyde (Sigma-Aldrich, ≥90%), glycolic acid (FUJIFILM Wako Pure Chemical, Osaka, Japan, 97%), oxalic acid dihydrate (Kishida Chemical, 99.5%), and tartronic acid (Alfa Aesar, Lancashire, UK, 98%) in deionized water, and by dissolving sodium β-hydroxypyruvate hydrate (Sigma-Aldrich, ≥97%) and sodium mesoxalate monohydrate (Sigma-Aldrich, ≥98%) in dilute sulfuric acid (5 mmol·L
−1). The glycerol conversion, product yield, and product selectivity were calculated using the following equations (Equations (1)–(3)):
where, [Glycerol] and [Glycerol]
0 are the glycerol concentrations (mol·L
−1) before and after the reaction, respectively. [Product] is the product concentration (mol·L
−1) and
nc(product) and
nc(glycerol) values are the number of carbon atoms in the product and glycerol (
nc(glycerol) = 3), respectively. In order to investigate the metal leaching during the catalytic reaction, the liquid-phase after the reaction was analyzed by XRF (EDX-720, Shimadzu).