Synthesis, Stereochemical and Photophysical Properties of Functionalized Thiahelicenes
Abstract
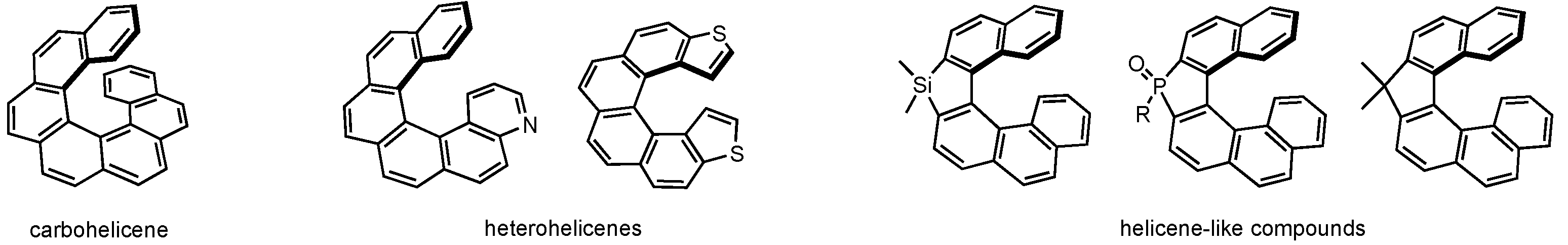
:1. Introduction
2. Results
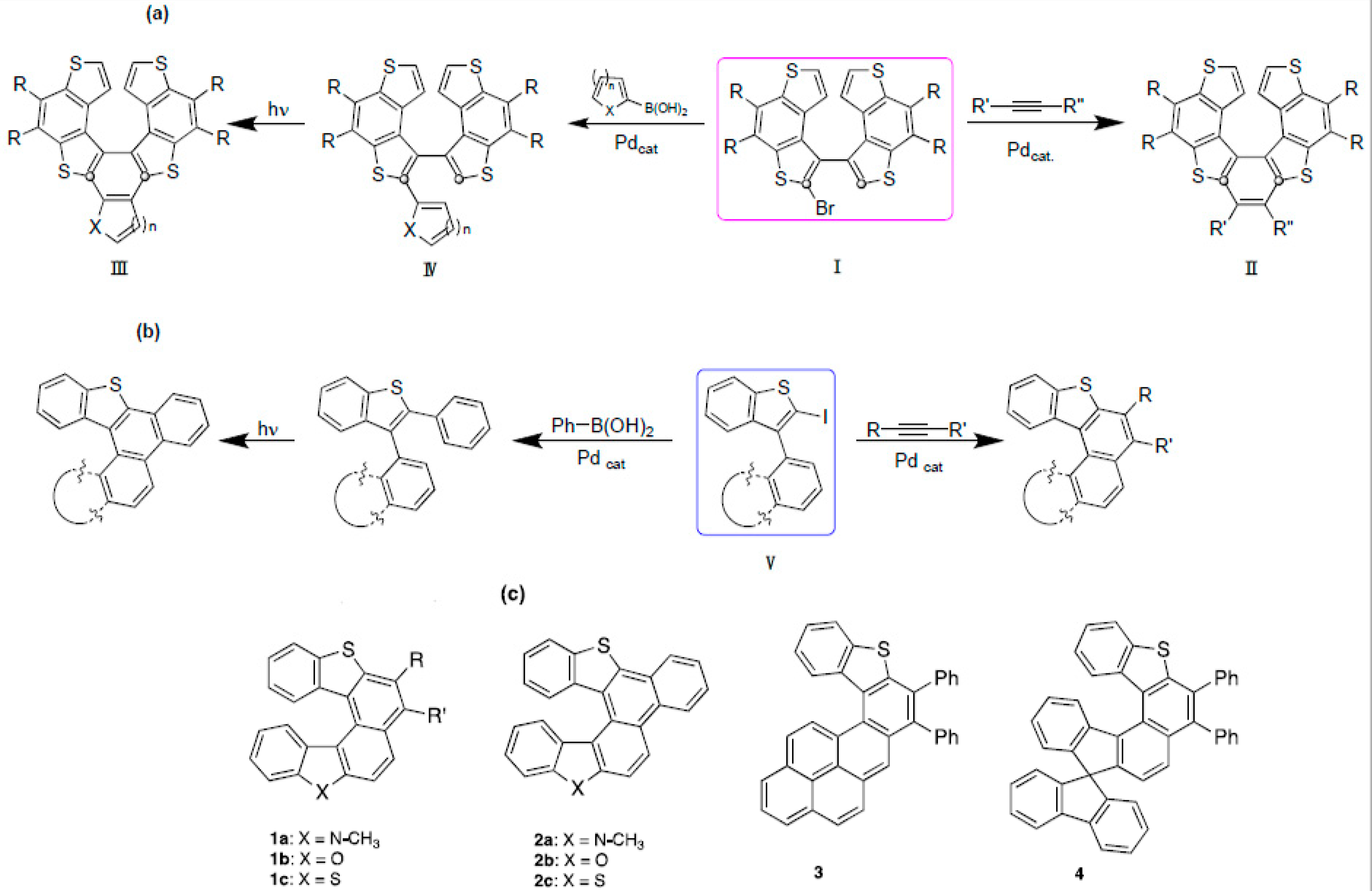
2.1. Synthesis, NMR Studies, and X-ray Characterization
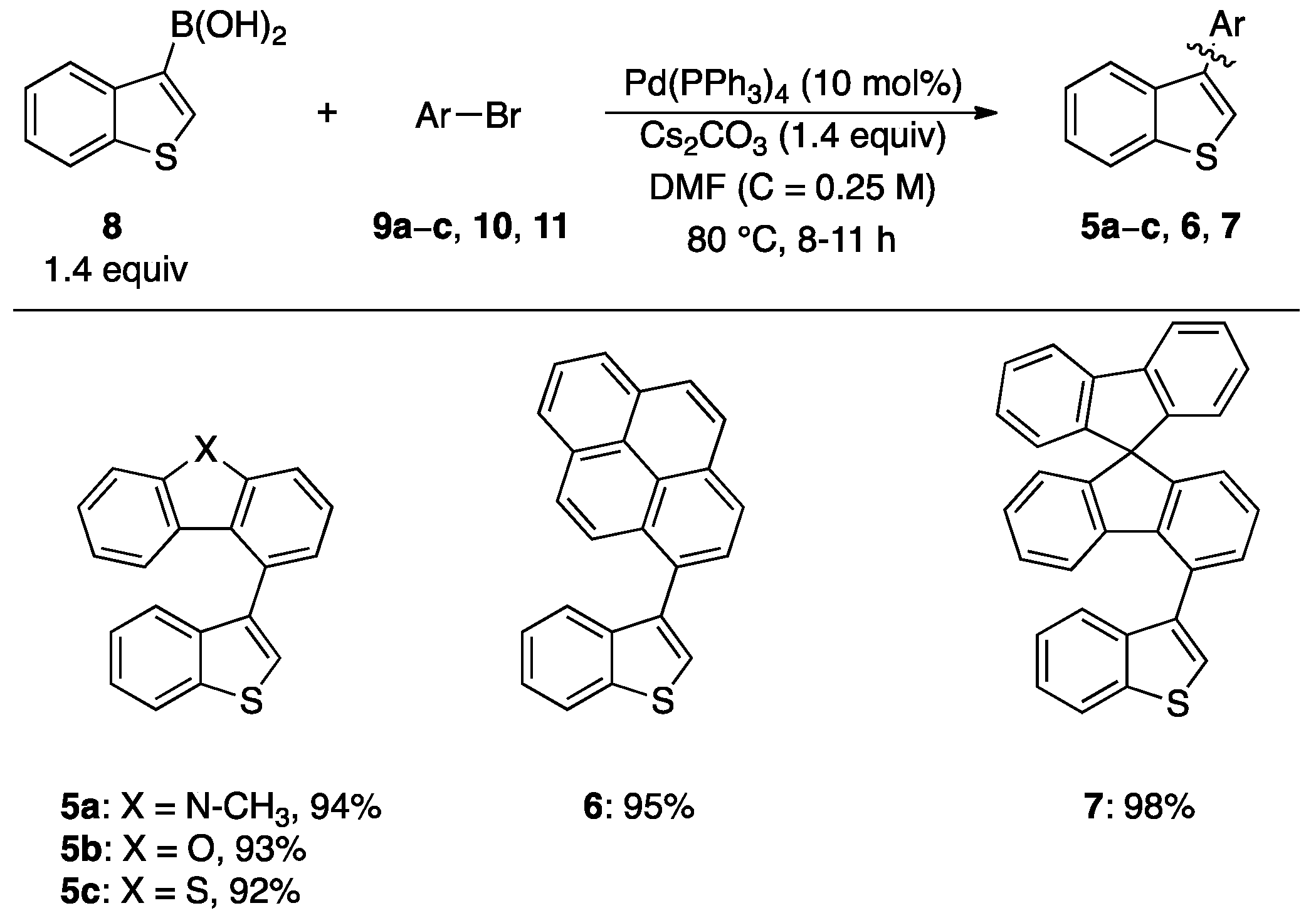
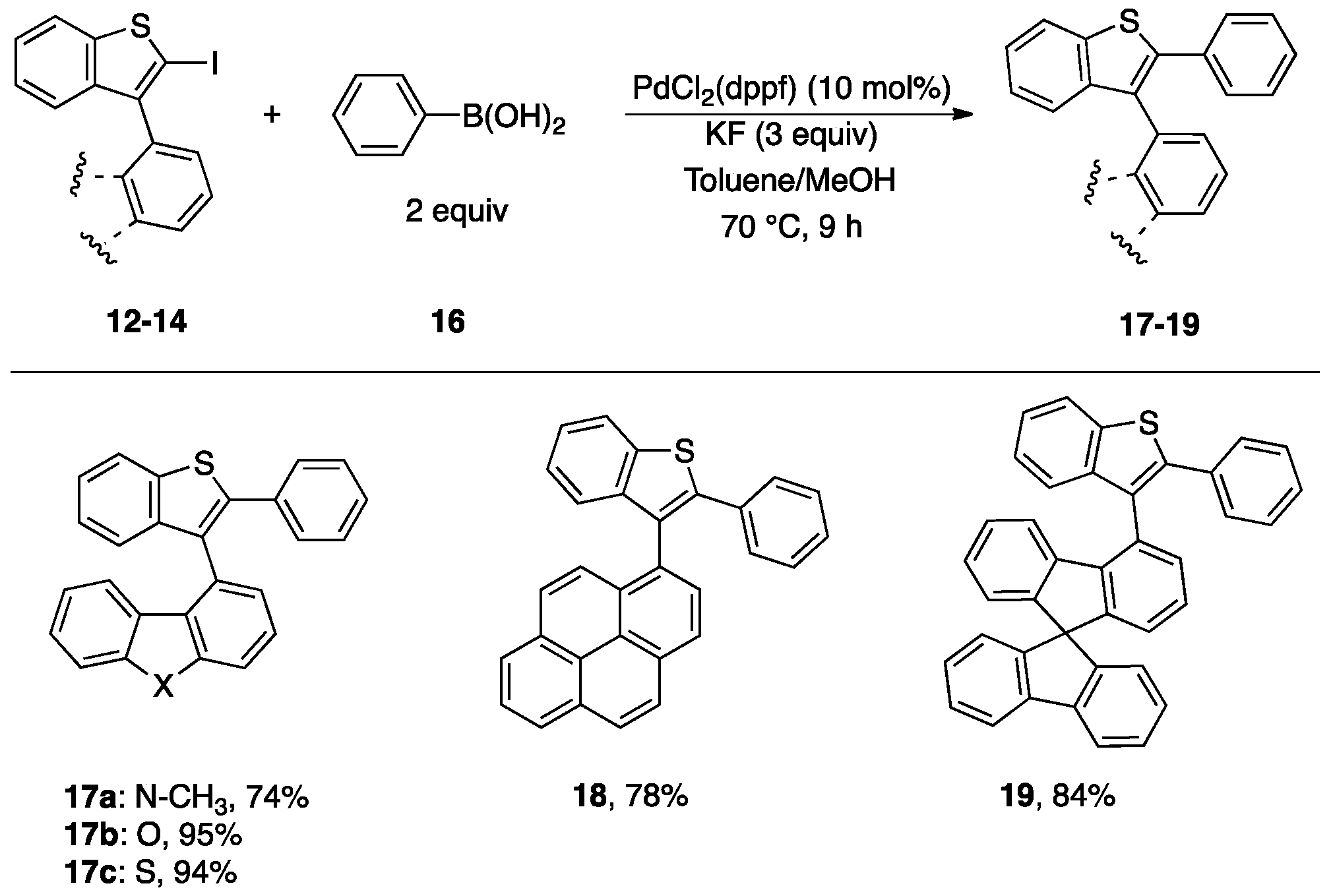
2.1.1. Synthesis of Bi(hetero)aryls 5a–c, 6, and 7
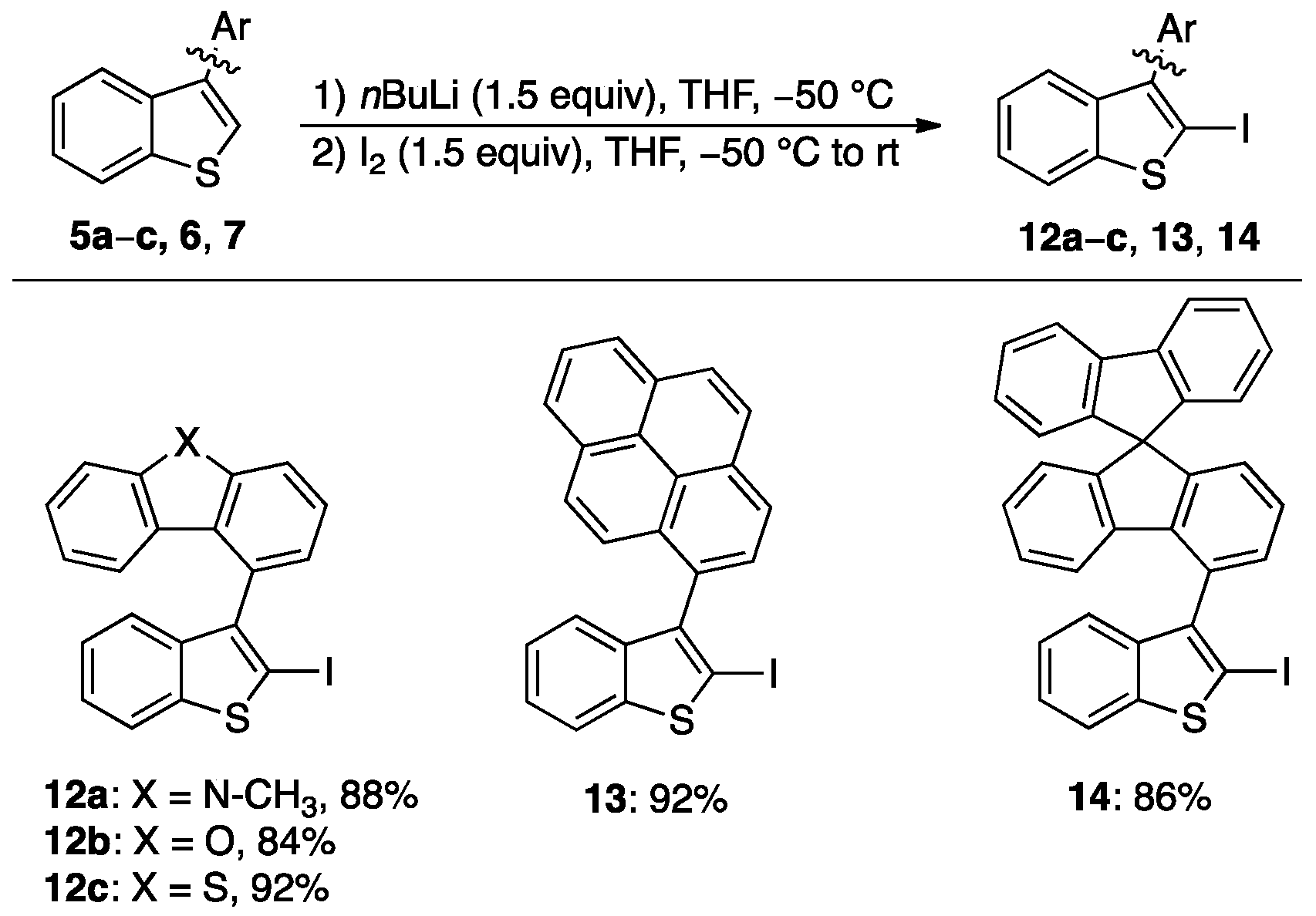
2.1.2. Synthesis of Iodides 12a–c, 13, and 14
2.1.3. Palladium-Catalyzed Annulation of Alkynes by Iodides 12a–c, 13 and 14
2.1.4. Synthesis of Benzo Fused Thiahelicenes 2a–c
2.2. Stereochemical Properties and Absolute Configuration
2.2.1. Configurational Stability
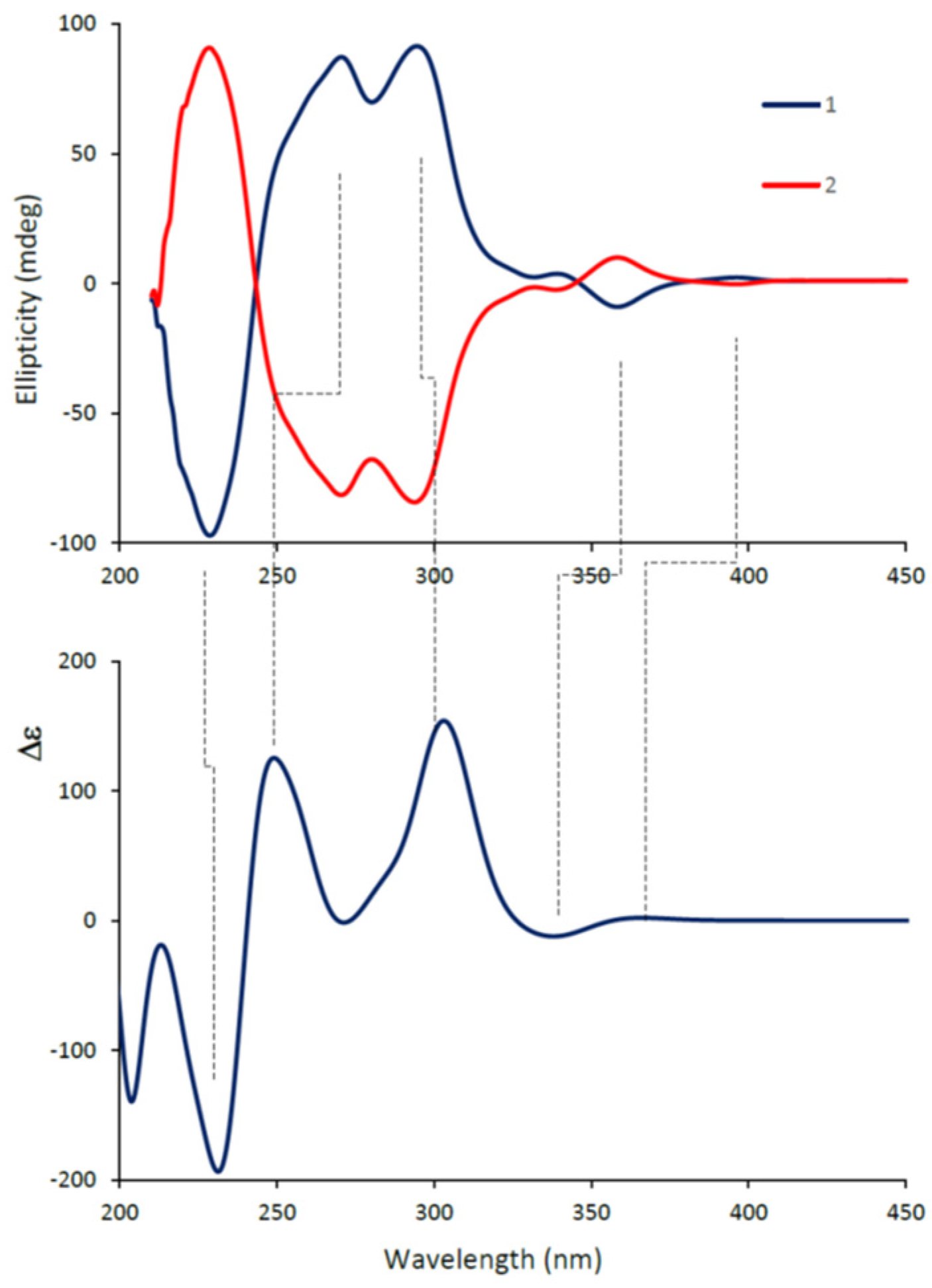
2.2.2. Assignment of Absolute Configuration of 2a
2.3. Photophysical Studies
3. Materials and Methods
3.1. General Methods
3.2. General Procedure for the Synthesis of Benzothiophene-Based Biaryls 5a–c, 6, and 7
3.3. General Procedure for the Synthesis of Iodides 12a–c, 13 and 14
3.4. General Procedure for the Carboannulation of Alkynes 15a–c with Iodides 12a–c, 13, and 14
3.5. General Procedure for the Synthesis of Intermediates 17a–c, 18, and 19
3.6. General Procedure for the Synthesis of Benzo Fused Thiahelicenes 2a–c
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ravat, P. Carbo[n]helicenes Restricted to Enantiomerize: An Insight into the Design Process of Configurationally Stable Functional Chiral PAHs. Chem. Eur. J. 2021, 27, 3957–3967. [Google Scholar] [PubMed]
- Chen, C.-F.; Shen, Y. Helicene Chemistry, from Synthesis to Applications; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Shen, Y.; Chen, C.-F. Helicenes: Synthesis and Applications. Chem. Rev. 2012, 112, 1463–1535. [Google Scholar] [PubMed]
- Nakai, Y.; Mori, T.; Inoue, Y. Theoretical and Experimental Studies on Circular Dichroism of Carbo[n]helicenes. J. Phys. Chem. A 2012, 116, 7372–7385. [Google Scholar] [PubMed]
- Zhao, W.-L.; Li, M.; Lu, H.-Y.; Chen, C.-F. Advances in helicene derivatives with circularly polarized luminescence. Chem. Commun. 2019, 55, 13793–13803. [Google Scholar]
- Dhbaibi, K.; Favereau, L.; Crassous, J. Enantioenriched Helicenes and Helicenoids Containing Main-Group Elements (B, Si, N, P). Chem. Rev. 2019, 119, 8846–8953. [Google Scholar]
- Pop, F.; Zigon, N.; Avarvari, N. Main-Group-Based Electro- and Photoactive Chiral Materials. Chem. Rev. 2019, 119, 8435–8478. [Google Scholar]
- Biet, T.; Martin, K.; Hankache, J.; Hellou, N.; Hauser, A.; Bürgi, T.; Vanthuyne, N.; Aharon, T.; Caricato, M.; Crassous, J.; et al. Triggering Emission with the Helical Turn in Thiadiazole-Helicenes. Chem. Eur. J. 2017, 23, 437–446. [Google Scholar]
- Mori, T. Chiroptical Properties of Symmetric Double, Triple, and Multiple Helicenes. Chem. Rev. 2021, 121, 2373–2412. [Google Scholar]
- Li, C.; Yang, Y.; Miao, Q. Recent Progress in Chemistry of Multiple Helicenes. Chem. Asian J. 2018, 13, 884–894. [Google Scholar]
- Swain, A.K.; Radacki, K.; Braunschweig, H.; Ravat, P. Pyrene-Fused [7]Helicenes Connected Via Hexagonal and Heptagonal Rings: Stereospecific Synthesis and Chiroptical Properties. J. Org. Chem. 2022, 87, 993–1000. [Google Scholar]
- Swain, A.k.; Kolanji, K.; Stapper, C.; Ravat, P. C2- and C1-Symmetric Configurationally Stable Pyrene-Fused [5]Helicenes Connected via Hexagonal and Heptagonal Rings. Org. Lett. 2021, 23, 1339–1343. [Google Scholar] [PubMed]
- Wang, L.; Han, Y.; Zhang, J.; Li, X.; Liu, X.; Xiao, J. Stable Double and Quadruple [5]Helicene Derivatives: Synthesis, Structural Analysis, and Physical Properties. Org. Lett. 2020, 22, 261–264. [Google Scholar] [PubMed]
- Hu, Y.; Paternò, G.M.; Wang, X.-Y.; Wang, X.-C.; Guizzardi, M.; Chen, Q.; Schollmeyer, D.; Cao, X.-Y.; Cerullo, G.; Scotognella, F.; et al. π-Extended Pyrene-Fused Double [7]Carbohelicene as a Chiral Polycyclic Aromatic Hydrocarbon. J. Am. Chem. Soc. 2019, 141, 12797–12803. [Google Scholar] [PubMed] [Green Version]
- Bam, R.; Yang, W.; Longhi, G.; Abbate, S.; Lucotti, A.; Tommasini, M.; Franzini, R.; Villani, C.; Catalano, V.J.; Olmstead, M.M.; et al. Four-Fold Alkyne Benzannulation: Synthesis, Properties, and Structure of Pyreno[a]pyrene-Based Helicene Hybrids. Org. Lett. 2019, 21, 8652–8656. [Google Scholar]
- Wang, C.-Z.; Kihara, R.; Feng, X.; Thuéry, P.; Redshaw, C.; Yamato, T. Synthesis, Structure and Photophysical Properties of Pyrene–based [5]Helicenes: An Experimental and Theoretical Study. ChemistrySelect 2017, 2, 1436–1441. [Google Scholar]
- Buchta, M.; Rybáček, J.; Jančařík, A.; Kudale, A.A.; Buděšínský, M.; Chocholoušová, J.V.; Vacek, J.; Bednárová, L.; Císařová, I.; Bodwell, G.J.; et al. Chimerical Pyrene-Based [7]Helicenes as Twisted Polycondensed Aromatics. Chem. Eur. J. 2015, 21, 8910–8917. [Google Scholar]
- Bock, H.; Subervie, D.; Mathey, P.; Pradhan, A.; Sarkar, P.; Dechambenoit, P.; Hillard, E.A.; Durola, F. Helicenes from Diarylmaleimides. Org. Lett. 2014, 16, 1546–1549. [Google Scholar]
- Hu, J.-Y.; Feng, X.; Paudel, A.; Tomiyasu, H.; Rayhan, U.; Thuéry, P.; Elsegood, M.R.J.; Redshaw, C.; Yamato, T. Synthesis, Structural, and Photophysical Properties of the First Member of the Class of Pyrene-Based [4]Helicenes. Eur. J. Org. Chem. 2013, 2013, 5829–5837. [Google Scholar]
- Hu, J.-Y.; Paudel, A.; Seto, N.; Feng, X.; Era, M.; Matsumoto, T.; Tanaka, J.; Elsegood, M.R.J.; Redshaw, C.; Yamato, T. Pyrene-cored blue-light emitting [4]helicenes: Synthesis, crystal structures, and photophysical properties. Org. Biomol. Chem. 2013, 11, 2186–2197. [Google Scholar]
- Bédard, A.-C.; Vlassova, A.; Hernandez-Perez, A.C.; Bessette, A.; Hanan, G.S.; Heuft, M.A.; Collins, S.K. Synthesis, Crystal Structure and Photophysical Properties of Pyrene–Helicene Hybrids. Chem. Eur. J. 2013, 19, 16295–16302. [Google Scholar]
- Hayward, R.J.; Hopkinson, A.C.; Leznoff, C.C. Photocyclization reactions of aryl polyenes—VI: The photocyclization of 1,4-diaryl-1,3-butadienes. Tetrahedron 1972, 28, 439–447. [Google Scholar]
- Vingiello, F.A.; Henson, P.D. 7-Phenyldibenz[a,h]anthracene and Benzo[e]naphtho[1,2-b]pyrene1,2. J. Org. Chem. 1965, 30, 2842–2845. [Google Scholar]
- Stara, I.; Stary, I. Helically chiral aromatics: The synthesis of helicenes by [2+2+2] cycloisomerization of π-electron system. Acc. Chem. Res. 2020, 53, 144–158. [Google Scholar]
- Tanaka, K.; Kimura, Y.; Murayama, K. Enantioselective helicene synthesis by rhodium-catalyzed [2+2+2] cycloadditions. Bull. Chem. Soc. Jpn. 2015, 88, 375–385. [Google Scholar]
- Hoffmann, N. Photochemical reactions applied to the synthesis of helicenes and helicene-like compounds. J. Photochem. Photobiol. C 2014, 19, 1–19. [Google Scholar]
- Kaga, A.; Iida, H.; Tsuchiya, S.; Saito, H.; Nakano, K.; Yorimitsu, H. Aromatic Metamorphosis of Thiophenes by Means of Desulfurative Dilithiation. Chem. Eur. J. 2021, 27, 4567–4572. [Google Scholar] [PubMed]
- Matsuo, Y.; Tanaka, T.; Osuka, A. Diazadimethano[8]circulene: Synthesis, Structure, Properties, and Isolation of Stable Radical Cation. Chem. Lett. 2020, 49, 959–962. [Google Scholar]
- Takase, K.; Noguchi, K.; Nakano, K. Synthesis of Pyrrole-Containing Chiral Spiro Molecules and Their Optical and Chiroptical Properties. Bull. Chem. Soc. Jpn. 2019, 92, 1008–1017. [Google Scholar]
- Nagata, Y.; Kato, S.; Miyake, Y.; Shinokubo, H. Synthesis of Tetraaza[8]circulenes from Tetrathia[8]circulenes through an SNAr-Based Process. Org. Lett. 2017, 19, 2718–2721. [Google Scholar]
- Yorimitsu, H.; Vasu, D.; Bhanuchandra, M.; Murakami, K.; Osuka, A. Aromatic Metamorphosis of Dibenzothiophenes. Synlett 2016, 27, 1765–1774. [Google Scholar]
- Uematsu, K.; Hayasaka, C.; Takase, K.; Noguchi, K.; Nakano, K. Transformation of Thia[7]helicene to Aza[7]helicenes and [7]Helicene-like Compounds via Aromatic Metamorphosis. Molecules 2022, 27, 606. [Google Scholar] [PubMed]
- Yanagi, T.; Tanaka, T.; Yorimitsu, H. Asymmetric systematic synthesis, structures, and (chir)optical properties of a series of dihetero[8]helicenes. Chem. Sci. 2021, 12, 2784–2793. [Google Scholar] [PubMed]
- Licandro, E.; Cauteruccio, S.; Dova, D. Thiahelicenes: From basic knowledge to applications. Adv. Heteroc. Chem. 2016, 118, 1–46. [Google Scholar]
- Collins, S.K.; Vachon, M.P. Unlocking the potential of thiaheterohelicenes: Chemical synthesis as the key. Org. Biomol. Chem. 2006, 4, 2518–2524. [Google Scholar]
- Champagne, B.; Labidi, S.N. Second-order nonlinear optical responses of heptahelicene and heptathiahelicene derivatives. Chem. Phys. Lett. 2016, 644, 195–200. [Google Scholar]
- Bossi, A.; Licandro, E.; Maiorana, S.; Rigamonti, C.; Righetto, S.; Stephenson, G.R.; Spassova, M.; Botek, E.; Champagne, B. Theoretical and Experimental Investigation of Electric Field Induced Second Harmonic Generation in Tetrathia[7]helicenes. J. Phys. Chem. C 2008, 112, 7900–7907. [Google Scholar]
- Botek, E.; André, J.-M.; Champagne, B.; Verbiest, T.; Persoons, A. Mixed electric-magnetic second-order nonlinear optical response of helicenes. J. Chem. Phys. 2005, 122, 234713. [Google Scholar]
- Botek, E.; Spassova, M.; Champagne, B.; Asselberghs, I.; Persoons, A.; Clays, K. Hyper-Rayleigh scattering of neutral and charged helicenes. Chem. Phys. Lett. 2005, 412, 274–279. [Google Scholar]
- Champagne, B.; André, J.-M.; Botek, E.; Licandro, E.; Maiorana, S.; Bossi, A.; Clays, K.; Persoons, A. Theoretical Design of Substituted Tetrathia-[7]-Helicenes with Large Second-Order Nonlinear Optical Responses. ChemPhysChem 2004, 5, 1438–1442. [Google Scholar]
- Daul, C.A.; Ciofini, I.; Weber, V. Investigation of NLO properties of substituted (M)-tetrathia-[7]-helicenes by semiempirical and DFT methods. Int. J. Quantum Chem. 2003, 91, 297–302. [Google Scholar]
- Fukumi, T.; Sakaguchi, T.; Miya, M.; Nakagawa, H.; Yamada, K.-I.; Kawazura, H. Determination of Second Molecular Hyperporalizability γ of Thiaheterohelicene by Degenerate Four-Wave Mixing Method. Rev. Laser Eng. 1994, 22, 409–414. [Google Scholar]
- Chaunchaiyakul, S.; Krukowski, P.; Tsuzuki, T.; Minagawa, Y.; Akai-Kasaya, M.; Saito, A.; Osuga, H.; Kuwahara, Y. Self-Assembly Formation of M-Type Enantiomer of 2,13-Bis(hydroxymethyl)[7]-thiaheterohelicene Molecules on Au(111) Surface Investigated by STM/CITS. J. Phys. Chem. C 2015, 119, 21434–21442. [Google Scholar]
- Krukowski, P.; Chaunchaiyakul, S.; Akai-Kasaya, M.; Saito, A.; Osuga, H.; Kuwahara, Y. Adsorption and Light Emission of a Racemic Mixture of [7]thiaheterohelicene-2,13-carboxaldehyde on Au(111), Cu(001), and NiAl(110) Surfaces Investigated Using a Scanning Tunneling Microscope. J. Phys. Chem. C 2021, 125, 9419–9427. [Google Scholar]
- Nakamura, T.; Kondoh, H.; Matsumoto, M. Scanning Tunneling Microscopy Observations of [7]Thiahelicene Adsorbed on Au(111). Mol. Cryst. Liq. Cryst. 1999, 337, 273–276. [Google Scholar]
- Arnaboldi, S.; Cauteruccio, S.; Grecchi, S.; Benincori, T.; Marcaccio, M.; Biroli, A.O.; Longhi, G.; Licandro, E.; Mussini, P.R. Thiahelicene-based inherently chiral films for enantioselective electroanalysis. Chem. Sci. 2019, 10, 1539–1548. [Google Scholar]
- Dova, D.; Viglianti, L.; Mussini, P.R.; Prager, S.; Dreuw, A.; Voituriez, A.; Licandro, E.; Cauteruccio, S. Tetrathia[7]helicene Phosphorus Derivatives: Experimental and Theoretical Investigations of Electronic Properties, and Preliminary Applications as Organocatalysts. Asian J. Org. Chem. 2016, 5, 537–549. [Google Scholar]
- Cauteruccio, S.; Dova, D.; Benaglia, M.; Genoni, A.; Orlandi, M.; Licandro, E. Synthesis, Characterisation, and Organocatalytic Activity of Chiral Tetrathiahelicene Diphosphine Oxides. Eur. J. Org. Chem. 2014, 2014, 2694–2702. [Google Scholar]
- Cauteruccio, S.; Loos, A.; Bossi, A.; Blanco Jaimes, M.C.; Dova, D.; Rominger, F.; Prager, S.; Dreuw, A.; Licandro, E.; Hashmi, A.S.K. Gold(I) Complexes of Tetrathiaheterohelicene Phosphanes. Inorg. Chem. 2013, 52, 7995–8004. [Google Scholar]
- Monteforte, M.; Cauteruccio, S.; Maiorana, S.; Benincori, T.; Forni, A.; Raimondi, L.; Graiff, C.; Tiripicchio, A.; Stephenson, G.R.; Licandro, E. Tetrathiaheterohelicene Phosphanes as Helical-Shaped Chiral Ligands for Catalysis. Eur. J. Org. Chem. 2011, 2011, 5649–5658. [Google Scholar]
- Taroni, T.; Cauteruccio, S.; Vago, R.; Franchi, S.; Barbero, N.; Licandro, E.; Ardizzone, S.; Meroni, D. Thiahelicene-grafted halloysite nanotubes: Characterization, biological studies and pH triggered release. Appl. Surf. Sci. 2020, 520, 146351. [Google Scholar]
- Cauteruccio, S.; Bartoli, C.; Carrara, C.; Dova, D.; Errico, C.; Ciampi, G.; Dinucci, D.; Licandro, E.; Chiellini, F. A Nanostructured PLGA System for Cell Delivery of a Tetrathiahelicene as a Model for Helical DNA Intercalators. ChemPlusChem 2015, 80, 490–493. [Google Scholar] [PubMed]
- Shinohara, K.-I.; Sannohe, Y.; Kaieda, S.; Tanaka, K.-I.; Osuga, H.; Tahara, H.; Xu, Y.; Kawase, T.; Bando, T.; Sugiyama, H. A Chiral Wedge Molecule Inhibits Telomerase Activity. J. Am. Chem. Soc. 2010, 132, 3778–3782. [Google Scholar] [PubMed]
- Xu, Y.; Zhang, Y.X.; Sugiyama, H.; Umano, T.; Osuga, H.; Tanaka, K. (P)-Helicene Displays Chiral Selection in Binding to Z-DNA. J. Am. Chem. Soc. 2004, 126, 6566–6567. [Google Scholar]
- Nakagawa, H.; Gomi, K.; Yamada, K.-I. Chiral Recognition of Thiaheterohelicenes by Alkyl beta;-D-Pyranoside Micelles. Influence of Extension of Helix. Chem. Pharm. Bull. 2001, 49, 49–53. [Google Scholar]
- Pelliccioli, V.; Dova, D.; Baldoli, C.; Graiff, C.; Licandro, E.; Cauteruccio, S. Diversified Syntheses of Tetrathia[7]helicenes by Metal-Catalyzed Cross-Coupling Reactions. Eur. J. Org. Chem. 2021, 2021, 383–395. [Google Scholar]
- Shen, D.; Xu, Y.; Shi, S.-L. A Bulky Chiral N-Heterocyclic Carbene Palladium Catalyst Enables Highly Enantioselective Suzuki–Miyaura Cross-Coupling Reactions for the Synthesis of Biaryl Atropisomers. J. Am. Chem. Soc. 2019, 141, 14938–14945. [Google Scholar]
- Schubert, M.; Trosien, S.; Schulz, L.; Brandscheid, C.; Schollmeyer, D.; Waldvogel, S.R. Oxidative (Cross-)Coupling Reactions Mediated by C–H Activation of Thiophene Derivatives by Using Molybdenum(V) Reagents. Eur. J. Org. Chem. 2014, 2014, 7091–7094. [Google Scholar]
- Worlikar, S.A.; Larock, R.C. Pd-catalyzed Reactions Involving Arynes. Curr. Org. Chem. 2011, 15, 3214–3232. [Google Scholar]
- Larock, R.C. Palladium-Catalyzed Annulation of Alkynes. In Palladium in Organic Synthesis; Tsuji, J., Ed.; Springer GmbH: Berlin/Heidelberg, Germany, 2005; pp. 147–182. [Google Scholar]
- Larock, R.C.; Doty, M.J.; Tian, Q.; Zenner, J.M. Synthesis of Polycyclic Aromatic Hydrocarbons by the Pd-Catalyzed Annulation of Alkynes. J. Org. Chem. 1997, 62, 7536–7537. [Google Scholar]
- Larock, R.C.; Yum, E.K. Synthesis of indoles via palladium-catalyzed heteroannulation of internal alkynes. J. Am. Chem. Soc. 1991, 113, 6689–6690. [Google Scholar]
- Larock, R.C.; Yum, E.K.; Doty, M.J.; Sham, K.K.C. Synthesis of Aromatic Heterocycles via Palladium-Catalyzed Annulation of Internal Alkynes. J. Org. Chem. 1995, 60, 3270–3271. [Google Scholar]
- Larock, R.C.; Doty, M.J.; Cacchi, S. Synthesis of indenones via palladium-catalyzed annulation of internal alkynes. J. Org. Chem. 1993, 58, 4579–4583. [Google Scholar]
- Bossi, A.; Maiorana, S.; Graiff, C.; Tiripicchio, A.; Licandro, E. Silyl-Substituted Tetrathia[7]helicenes: Synthesis, X-ray Characterization and Reactivity. Eur. J. Org. Chem. 2007, 2007, 4499–4509. [Google Scholar]
- Dova, D.; Cauteruccio, S.; Prager, S.; Dreuw, A.; Graiff, C.; Licandro, E. Chiral Thiahelicene-Based Alkyl Phosphine–Borane Complexes: Synthesis, X-ray Characterization, and Theoretical and Experimental Investigations of Optical Properties. J. Org. Chem. 2015, 80, 3921–3928. [Google Scholar] [PubMed]
- Bossi, A.; Falciola, L.; Graiff, C.; Maiorana, S.; Rigamonti, C.; Tiripicchio, A.; Licandro, E.; Mussini, P.R. Electrochemical activity of thiahelicenes: Structure effects and electrooligomerization ability. Electrochim. Acta 2009, 54, 5083–5097. [Google Scholar]
- Grimme, S.; Peyerimhoff, S.D. Theoretical study of the structures and racemization barriers of [n]helicenes (n = 3–6, 8). Chem. Phys. 1996, 204, 411–417. [Google Scholar]
- Lebon, F.; Longhi, G.; Gangemi, F.; Abbate, S.; Priess, J.; Juza, M.; Bazzini, C.; Caronna, T.; Mele, A. Chiroptical Properties of Some Monoazapentahelicenes. J. Phys. Chem. A 2004, 108, 11752–11761. [Google Scholar]
- Hua, W.; Liu, Z.; Duan, L.; Dong, G.; Qiu, Y.; Zhang, B.; Cui, D.; Tao, X.; Cheng, N.; Liu, Y. Deep-blue electroluminescence from nondoped and doped organic light-emitting diodes (OLEDs) based on a new monoaza[6]helicene. RSC Adv. 2015, 5, 75–84. [Google Scholar]
- Kaiser, J.; Mekic, A.; Parham, A.H.; Buchholz, H.; König, B. Synthesis and Characterization of Naphtho[2,1-b:7,8-b′]bis[1]benzothiophene. Eur. J. Org. Chem. 2020, 2020, 66–69. [Google Scholar]
- Brouwer, A.M. Standards for photoluminescence quantum yield measurements in solution. Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar]
- Carmody, M.P.; Cook, M.J.; Tack, R.D. Aromaticity and tautomerism—V: The basicity and aromaticity of pyrrole, furan, thiophen and their benzo derivatives. Tetrahedron 1976, 32, 1767–1771. [Google Scholar]
- Horner, K.E.; Karadakov, P.B. Chemical Bonding and Aromaticity in Furan, Pyrrole, and Thiophene: A Magnetic Shielding Study. J. Org. Chem. 2013, 78, 8037–8043. [Google Scholar] [PubMed]
- Bruker. APEX3 and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2015. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of Silver and Molybdenum Microfocus X-ray Sources for Single-Crystal Structure Determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. SHELXT−Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comp. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16 Revision B.0.1.; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]





| Entry | λabs1 | logε | λem1 | ΦF 2 |
|---|---|---|---|---|
| Dibenzofuran series | ||||
| 5b | 289 | 4.13 | 355 | 0.06 |
| 12b | 286 | 4.39 | 354 | 0.01 |
| 17b | 289 | 4.35 | 401 | 0.02 |
| 1ba | 305, 317, 343 | 4.44, 4.37, 4.15 | 392 | 0.03 |
| 1bb | 296, 307, 321, 348 | 4.03, 4.14, 4.14, 3.87 | 417 | 0.01 |
| 2b | 287, 341 | 4.50, 4.16 | 386 | 0.03 |
| Pyrene series | ||||
| 6 | 278, 345 | 4.45, 4.37 | 391 | 0.18 |
| 13 | 268, 278, 331, 346 | 4.52, 4.61, 4.34, 4.49 | 388 | 0.01 |
| 18 | 268, 279, 330, 346 | 4.43, 4.57, 4.25, 4.37 | 411 | 0.42 |
| 3 | 288, 346, 381, 402, 440 | 4.46, 4.65, 4.18, 4.26, 3.50 | 457 | 0.05 |
| Dibenzothiophene series | ||||
| 5c | 287, 301, 330 | 3.98, 3.71, 3.56 | 373 | 0.02 |
| 12c | 288, 330 | 4.11, 3.61 | 394 | 0.02 |
| 17c | 287, 310, 402 | 4.46, 4.45, 3.59 | 420 | 0.01 |
| 1ca | 279, 293, 318, 343, 386 | 4.21, 4.32, 4.39, 3.99, 3.71 | 402 | 0.02 |
| 1cb | 321, 357, 391 | 4.29, 3.91, 3.68 | 434 | 0.01 |
| 2c | 298, 353, 390 | 4.70, 4.17, 3.80 | 399 | 0.02 |
| Carbazole series | ||||
| 5a | 265, 295, 349 | 4.24, 3.96, 3.58 | 372 | 0.10 |
| 12a | 264, 296, 351 | 4.47, 4.10, 3.71 | 371 | 0.01 |
| 17a | 264, 296, 350 | 4.45, 4.30, 3.68 | 415 | 0.03 |
| 1aa | 321, 343, 348, 361, 397 | 4.53, 4.02, 4.01, 4.01, 3.90 | 413 | 0.03 |
| 1ab | 325, 402 | 4.54, 3.91 | 439 | 0.01 |
| 1ac | 319, 343, 358, 376, 396 | 4.51, 4.01, 4.04, 3.84, 3.92 | 408 | 0.03 |
| 2a | 305, 359, 380, 400 | 4.73, 4.03, 3.77, 3.75 | 414 | 0.08 |
| Spirobifluorene series | ||||
| 7 | 300, 309 | 4.01, 4.06 | 363 | 0.06 |
| 14 | 297, 309 | 4.09, 4.13 | 364 | 0.02 |
| 19 | 297, 309 | 4.33, 4.35 | 399 | 0.03 |
| 4 | 279, 298, 310, 322, 356 | 4.57, 4.56, 4.46, 4.54, 4.22 | 417 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelliccioli, V.; Cardano, F.; Renno, G.; Vasile, F.; Graiff, C.; Mazzeo, G.; Fin, A.; Longhi, G.; Abbate, S.; Rosetti, A.; et al. Synthesis, Stereochemical and Photophysical Properties of Functionalized Thiahelicenes. Catalysts 2022, 12, 366. https://doi.org/10.3390/catal12040366
Pelliccioli V, Cardano F, Renno G, Vasile F, Graiff C, Mazzeo G, Fin A, Longhi G, Abbate S, Rosetti A, et al. Synthesis, Stereochemical and Photophysical Properties of Functionalized Thiahelicenes. Catalysts. 2022; 12(4):366. https://doi.org/10.3390/catal12040366
Chicago/Turabian StylePelliccioli, Valentina, Francesca Cardano, Giacomo Renno, Francesca Vasile, Claudia Graiff, Giuseppe Mazzeo, Andrea Fin, Giovanna Longhi, Sergio Abbate, Alessia Rosetti, and et al. 2022. "Synthesis, Stereochemical and Photophysical Properties of Functionalized Thiahelicenes" Catalysts 12, no. 4: 366. https://doi.org/10.3390/catal12040366
APA StylePelliccioli, V., Cardano, F., Renno, G., Vasile, F., Graiff, C., Mazzeo, G., Fin, A., Longhi, G., Abbate, S., Rosetti, A., Villani, C., Viscardi, G., Licandro, E., & Cauteruccio, S. (2022). Synthesis, Stereochemical and Photophysical Properties of Functionalized Thiahelicenes. Catalysts, 12(4), 366. https://doi.org/10.3390/catal12040366













