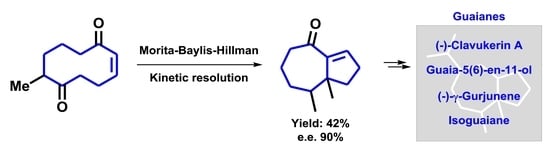
Kinetic Resolution in Transannular Morita-Baylis-Hillman Reaction: An Approximation to the Synthesis of Sesquiterpenes from Guaiane Family
Abstract
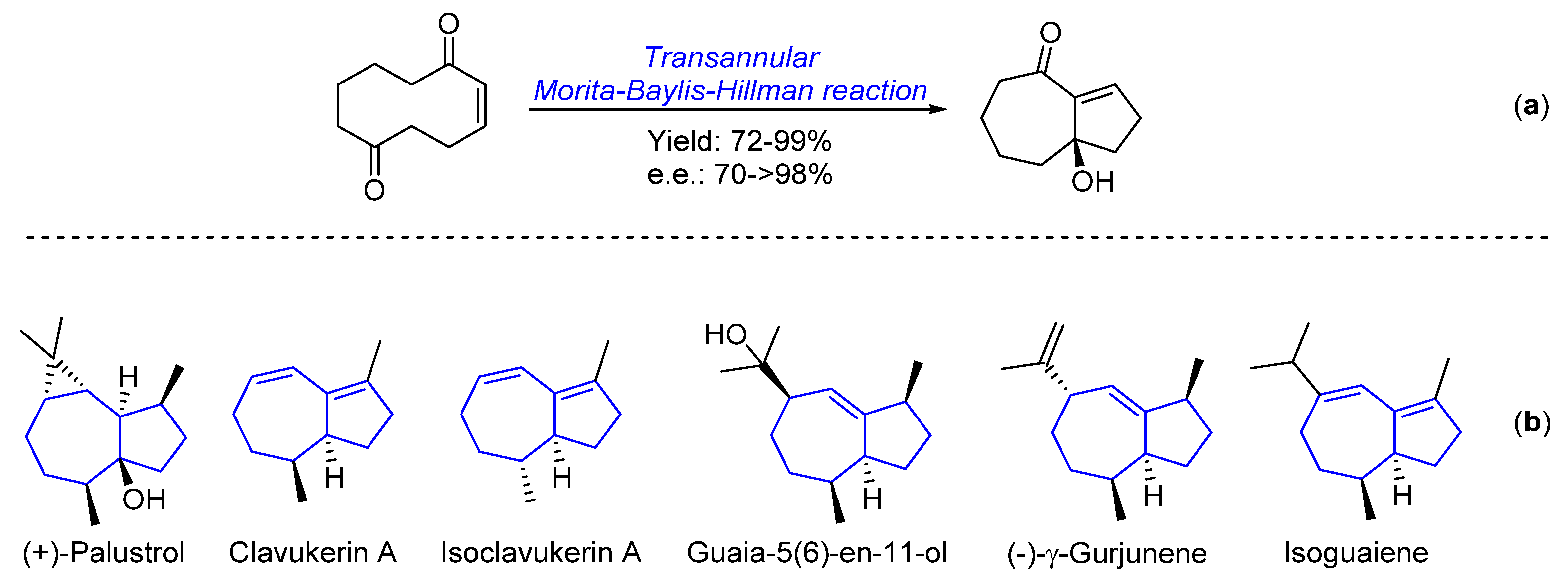
1. Introduction
2. Results and Discussion
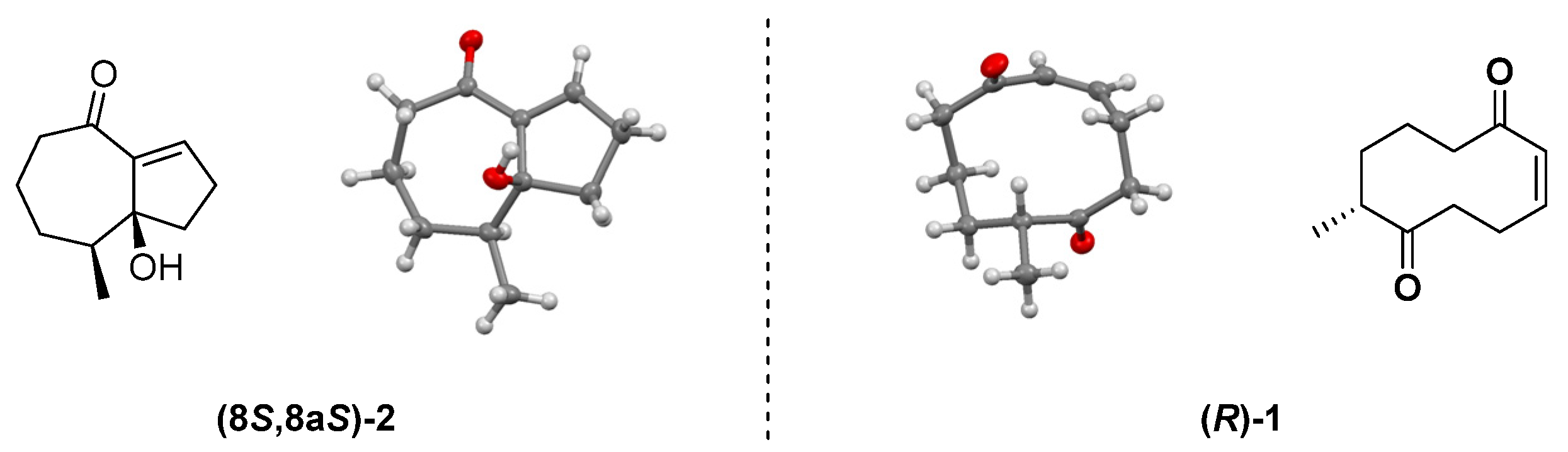
2.1. Transannular Kinetic Resolution
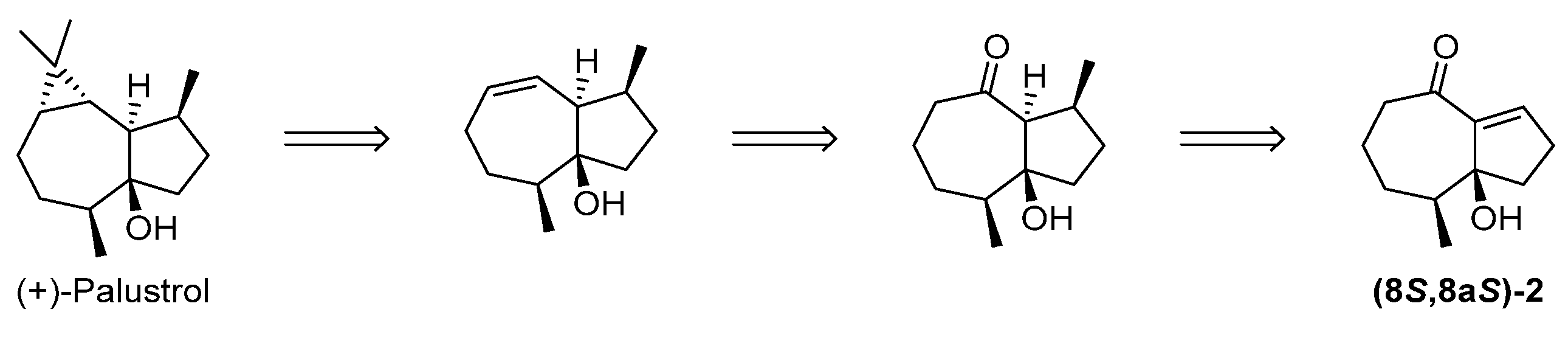
2.2. Preparation of an Advanced Intermediate in the Synthesis of Palustrol
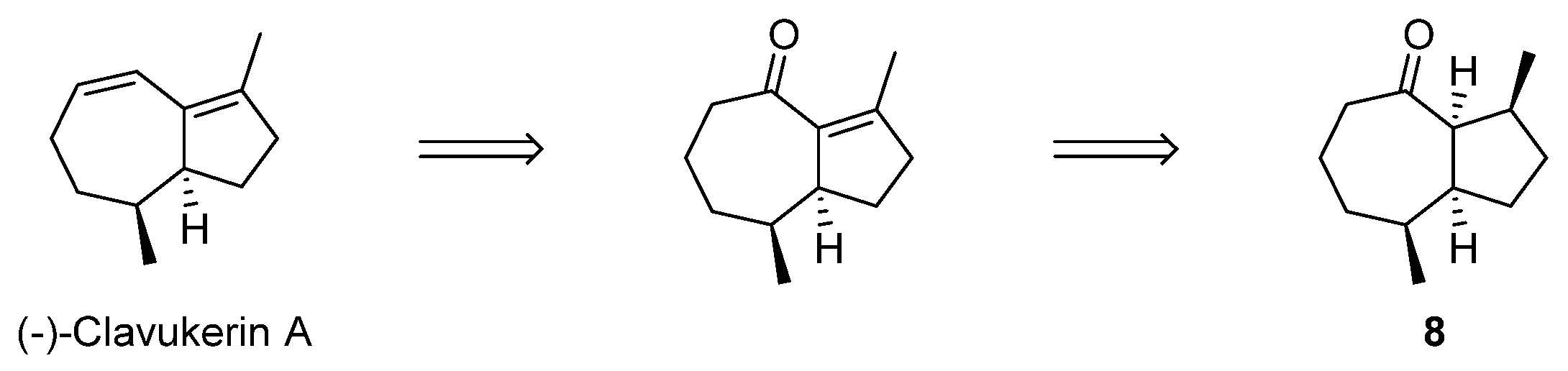
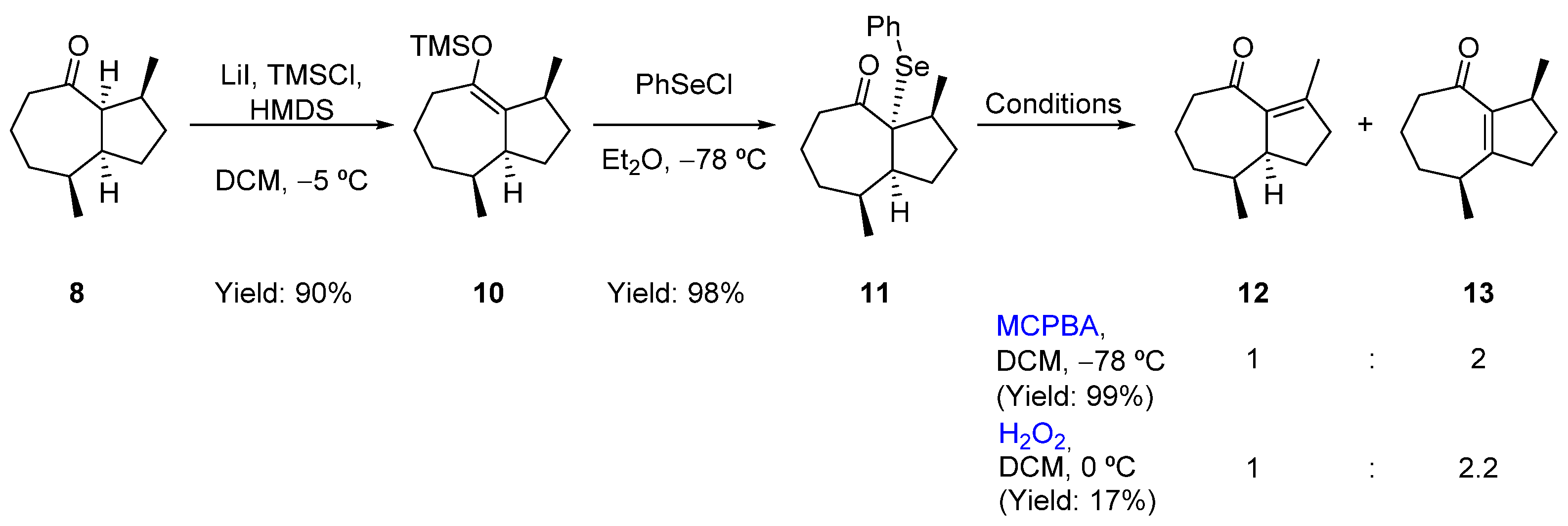
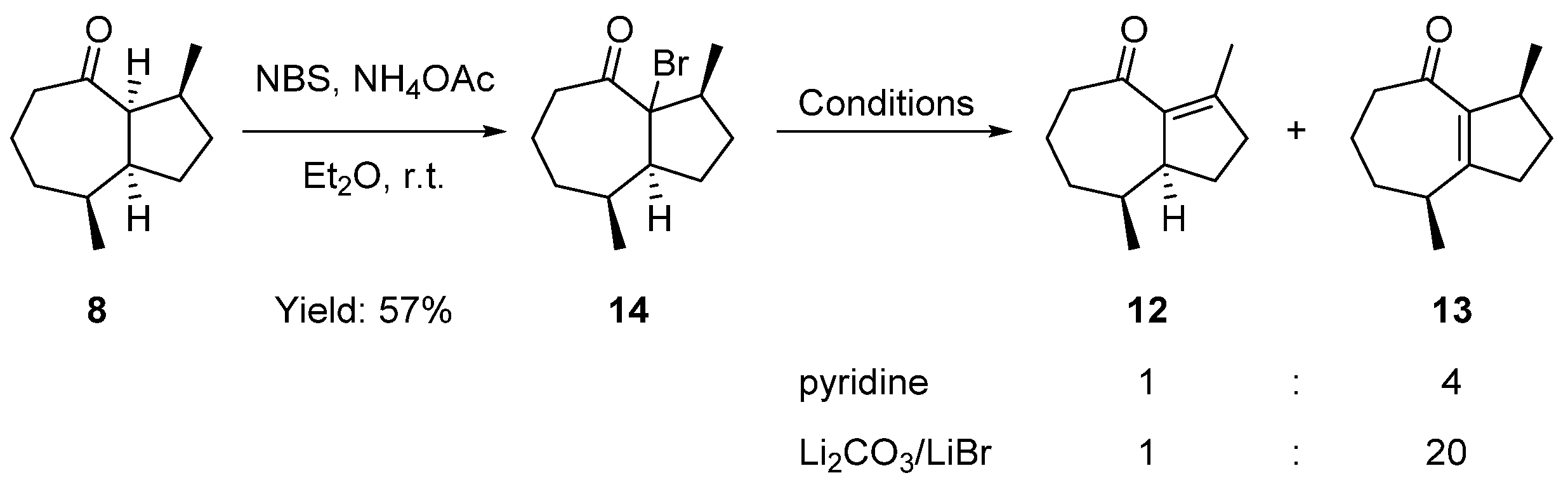
2.3. Formal Total Synthesis of Clavukerin A
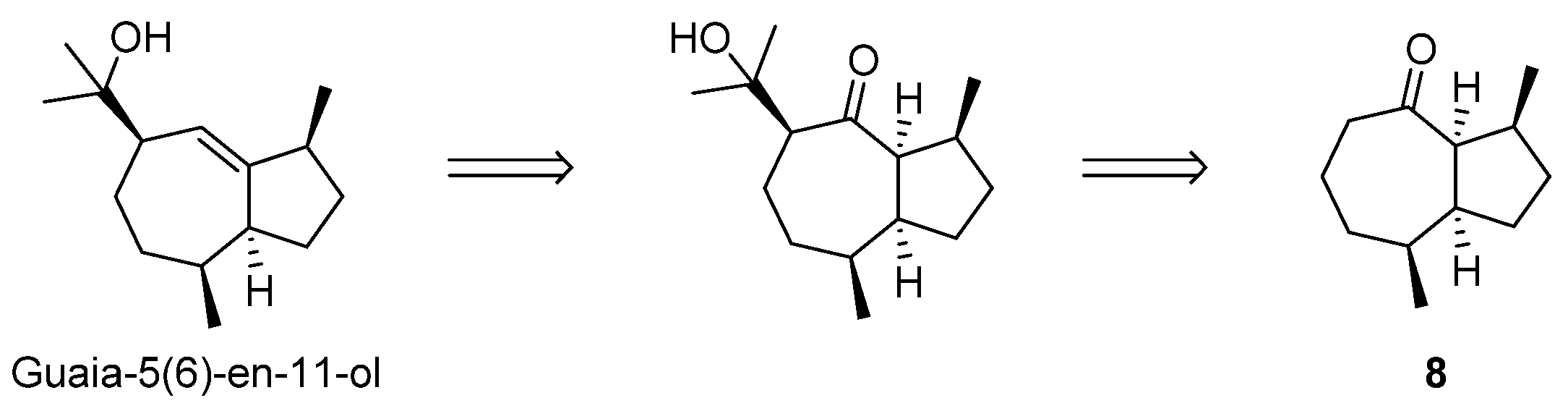
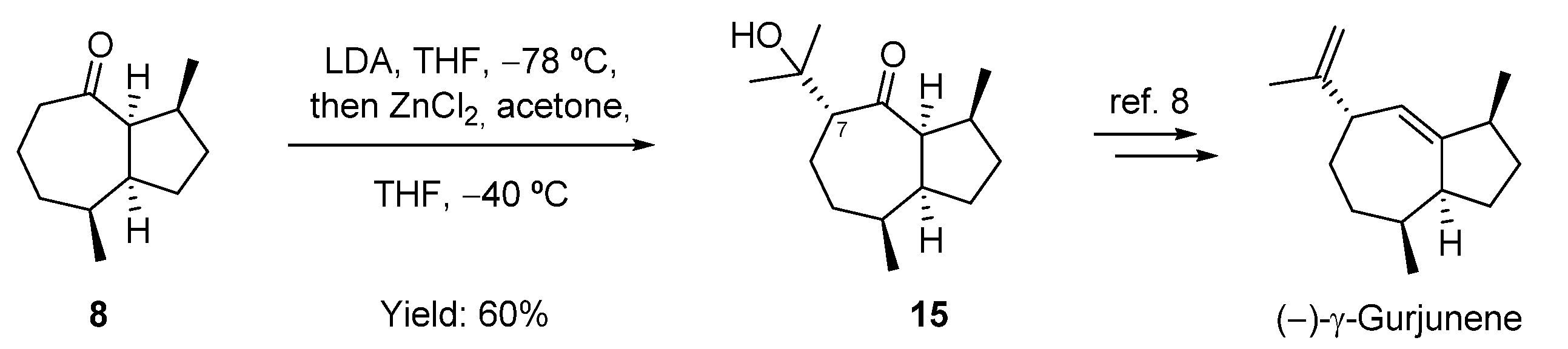
2.4. Attempt to the Synthesis Guaia-5(6)-en-11-ol; Synthesis of (−)-γ-Gurjunene and Non-Natural 1-epi-11,12-didehydro-4,5-dihydroisoguaiane
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, G. Chemistry of Terpenoids and Carotenoids; Discovery Publishing Home: New Dehli, India, 2007. [Google Scholar]
- Zhang, L.; Demain, A.L. Natural Products: Drug Discovery and Therapeutic Medicine; Human Press: Totowa, NJ, USA, 2005. [Google Scholar]
- Thomson, R.H. The Chemistry of Natural Products; Blackie Academic & Professional: Glasgow, Scotland, 1993. [Google Scholar]
- Pirrung, M.C.; Morehead, A.T.; Young, B.G. The Total Synthesis of Natural Products: Bicyclic and Tricyclic Sesquiterpenes; John Wiley & Sons: Atlanta, GA, USA, 2009. [Google Scholar]
- Overton, K.H. Terpenoids and Steroids; RSC Publishing: London, UK, 1975. [Google Scholar]
- Kornprobst, J.-M. Encyclopedia of Marine Natural Products; Wiley-Blackwell: Oxford, UK, 2010. [Google Scholar]
- Bideau, F.L.; Kousara, M.; Chen, L.; Wei, L.; Dumas, F. Tricyclic Sesquiterpenes from Marine Origin. Chem. Rev. 2017, 117, 6110–6159. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine Pharmacology in 2009–2011: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, and Antiviral Activities; Affecting the Immune and Nervous Systems, and other Miscellaneous Mechanisms of Action. Mar. Drugs 2013, 11, 2510–2573. [Google Scholar]
- Wright, A.D.; König, G.M.; Angerhofer, C.K.; Greenidge, P.; Linden, A.; Desqueyroux-Faundez, R. Antimalarial Activity: The Search for Marine-Derived Natural Products with Selective Antimalarial Activity. J. Nat. Prod. 1996, 59, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Fraga, B.M. Natural sesquiterpenoids. Nat. Prod. Rep. 1998, 15, 73–92. [Google Scholar] [CrossRef]
- Mato, R.; Manzano, R.; Reyes, E.; Carrillo, L.; Uria, U.; Vicario, J.L. Catalytic Enantioselective Transannular Morita–Baylis–Hillman Reaction. J. Am. Chem. Soc. 2019, 141, 9495–9499. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.N.; Cramer, N. Biomimetic Synthesis of (+)-Ledene, (+)-Viridiflorol, (−)-Palustrol, (+)-Spathulenol, and Psiguadial A, C, and D via the Platform Terpene (+)-Bicyclogermacrene. Chem. Eur. J. 2014, 20, 10654–10660. [Google Scholar] [CrossRef]
- Barthel, A.; Kaden, F.; Jäger, A.; Metz, P. Enantioselective Synthesis of Guaianolides in the Osmitopsin Family by Domino Metathesis. Org. Lett. 2016, 18, 3298–3301. [Google Scholar] [CrossRef] [PubMed]
- Knüppel, S.; Rogachev, V.O.; Metz, P. A Concise Catalytic Route to the Marine Sesquiterpenoids (−)-Clavukerin A and (−)-Isoclavukerin A. Eur. J. Org. Chem. 2010, 6145–6148. [Google Scholar] [CrossRef]
- Srikrishna, A.; Pardehi, V.H.; Satyanarayana, G. Enantioselective formal total syntheses of Clavukerin A and Isoclavukerin A via a ring-closing metathesis reaction. Tetrahedron Asymmetry 2010, 21, 746–750. [Google Scholar] [CrossRef]
- Grimm, E.L.; Methot, J.-L.; Shamji, M. Total synthesis of ( )-Clavukerin A. Pure Appl. Chem. 2003, 75, 231–234. [Google Scholar] [CrossRef][Green Version]
- Friese, J.C.; Krause, S.; Schäfer, H.J. Formal total synthesis of the trinorguaiane sesquiterpenes (+/−)-Clavukerin A and (+/−)-Isoclavukerin. Tetrahedron Lett. 2002, 43, 2683–2685. [Google Scholar] [CrossRef]
- Lee, E.; Yoon, C.H. 8-endo Cyclization of (alkoxycarbonyl)methyl radicals: Stereoselective synthesis of (−)-Clavukerin A and (−)-11-hydroxyguaiene. Tetrahedron Lett. 1996, 37, 5929–5930. [Google Scholar] [CrossRef]
- Shimizu, I.; Ishikawa, T. Stereoselective synthesis of (±)-Clavukerin A and (±)-Isoclavukerin A based on palladium-catalyzed reductive cleavage of alkenylcyclopropanes with formic acid. Tetrahedron Lett. 1994, 35, 1905–1908. [Google Scholar] [CrossRef]
- Honda, T.; Ishige, H.; Nagase, H. Chiral synthesis of a trinorguaiane sesquiterpene, Clavukerin A. J. Chem. Soc. Perkin Trans 1994, 3305–3310. [Google Scholar] [CrossRef]
- Kim, S.K.; Pak, C.S. Total synthesis of (.+-.)-Clavukerin A: A new trinorguaiane sesquiterpene. Biomimetic synthesis of (.+-.)-clavularin A from (.+-.)-Clavukerin A. J. Org. Chem. 1991, 56, 6829–6832. [Google Scholar] [CrossRef]
- Asaoka, M.; Kosaka, T.; Itahana, H.; Takei, H. Total Synthesis of Clavukerin A and Its Epimer. Chem. Lett. 1991, 1295–1298. [Google Scholar] [CrossRef]
- Trost, B.M.; Higuchi, R.I. On the Diastereoselectivity of Intramolecular Pd-Catalyzed TMM Cycloadditions. An Asymmetric Synthesis of the Perhydroazulene (−)-Isoclavukerin A. J. Am. Chem. Soc. 1996, 118, 10094–10105. [Google Scholar] [CrossRef]
- Huang, A.-C.; Sumby, C.J.; Tiekink, E.R.T.; Taylor, D.K. Synthesis of guaia-4(5)-en-11-ol, guaia-5(6)-en-11-ol, aciphyllene, 1-epi-melicodenones C and E, and other guaiane-type sesquiterpenoids via the diastereoselective epoxidation of guaiol. J. Nat. Prod. 2014, 77, 2522–2536. [Google Scholar] [CrossRef] [PubMed]
- Rienäcker, R.; Graefe, J. Catalytic Transformations of Sesquiterpene Hydrocarbons on Alkali Metal/Aluminum Oxide. Angew. Chem. Int. Ed. Engl. 1985, 24, 320–321. [Google Scholar] [CrossRef]
- Wang, Y.; Darweesh, A.F.; Zimdars, P.; Metz, P. An efficient synthesis of the guaiane sesquiterpene (−)-isoguaiene by domino metathesis. Belstein J. Org. Chem. 2019, 15, 858–862. [Google Scholar] [CrossRef]
- Blay, G.; Garcia, B.; Molina, E.; Pedro, J.R. Total Syntheses of Four Stereoisomers of 4α-Hydroxy-1β,7β-peroxy- 10βH-guaia-5-ene. Org. Lett. 2005, 7, 3291–3294. [Google Scholar] [CrossRef]
- Sasaki, S.; Sutoh, K.; Shimizu, Y.; Kato, K.; Yoshifuji, M. Oxidation of tris(2,4,6-triisopropylphenyl)phosphine and arsine. Tetrahedron Lett. 2014, 55, 322–325. [Google Scholar] [CrossRef]
- Hilliard, C.R.; Bhuvanesh, N.; Gladysz, J.A.; Blümel, J. Synthesis, purification, and characterization of phosphine oxides and their hydrogen peroxide adducts. Dalton Trans. 2012, 41, 1742–1754. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, P.; Slawin, A.M.Z.; Smith, M.B.; Woolins, J.D. Palladium(II) and Platinum(II) Complexes of the Heterodifunctional Ligand Ph2PNHP(O)Ph2. Inorg. Chem. 1996, 35, 3675–3682. [Google Scholar] [CrossRef]
- Whitaker, C.M.; Kott, K.L.; McMahon, R.J. Synthesis and Solid-State Structure of Substituted Arylphosphine Oxides. J. Org. Chem. 1995, 60, 3499–3508. [Google Scholar] [CrossRef]
- Relles, H.M.; Schluenz, R.W. Chemical Transformations with Regenerable, Polymer-Supported Trisubstituted Phosphine Dichlorides. Efficacious Incorporation of Phosphorus Reagents on Polymer Supports. J. Am. Chem. Soc. 1974, 96, 6469–6475. [Google Scholar] [CrossRef]
- Kociensky, P.J. Protecting Groups; Thieme: Stuttgart, Germany, 2005. [Google Scholar]
- Wuts, P.G.M.; Greene, T.W. Protective Groups in Organic Synthesis; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Kerwin, S.M.; Paul, A.G.; Heathcock, C.H. Quassinoid synthesis. 2. Preparation of a tetracyclic intermediate having the bruceantin tetrahydrofuran ring. J. Org. Chem. 1987, 52, 1686–1695. [Google Scholar] [CrossRef]
- Kumar, J.S.R.; O’Sullivan, M.; Resiman, S.E.; Hulford, C.A.; Ovaska, T.V. Facile approach to the bicyclo[5.3.0]decane ring system; efficient synthesis of (±)-7-epi-β-bulnesene. Tetrahedron Lett. 2002, 43, 1939–1941. [Google Scholar] [CrossRef]
- Banwell, M.G.; Hockless, D.C.R.; McLeod, M.D. Chemoenzymatic total syntheses of the sesquiterpene (−)-patchoulenone. New J. Chem. 2003, 27, 50–59. [Google Scholar] [CrossRef]
- Bertz, S.H.; Miao, G.; Rossiter, B.E.; Snyder, J.P. New Copper Chemistry. 25. Effect of TMSCl on the Conjugate Addition of Organocuprates to alpha.-Enones: A New Mechanism. J. Am. Chem. Soc. 1995, 117, 11023–11024. [Google Scholar] [CrossRef]
- Reuss, R.H.; Hassner, A. Synthetic methods. IV. Halogenation of carbonyl compounds via silyl enol ethers. J. Org. Chem. 1974, 39, 1785–1787. [Google Scholar] [CrossRef]
- Jeganathan, A.; Richardson, S.K.; Watt, D.S. Manganese Triacetate Oxidation of 3β, 3aβ, 6-Trimethyl-3a, 7aβ-dihydro-2(3H), 5(4H)-benzofurandione. Synth. Commun. 1989, 19, 1091–1100. [Google Scholar] [CrossRef]
- Roosen, P.C.; Vanderwal, C.D. Investigations into an Anionic Oxy-Cope/Transannular Conjugate Addition Approach to 7,20-Diisocyanoadociane. Org. Lett. 2014, 16, 4368–4371. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Katoh, S.-I.; Kato, T.; Urabe, D.; Inoue, M. Total Synthesis of Resiniferatoxin Enabled by Radical-Mediated Three-Component Coupling and 7-endo Cyclization. J. Am. Chem. Soc. 2017, 139, 16420–16429. [Google Scholar] [CrossRef] [PubMed]
- Shenvi, R.A.; Guerrero, C.A.; Shi, J.; Li, C.-C.; Baran, P.S. Synthesis of (+)-cortistatin A. J. Am. Chem. Soc. 2008, 130, 7241–7243. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ikawa, T.; Hattori, K.; Sajiki, H.; Hirota, K. Solvent-modulated Pd/C-catalyzed deprotection of silyl ethers and chemoselective hydrogenation. Tetrahedron 2004, 60, 6901–6911. [Google Scholar] [CrossRef]
- Mahoney, W.S.; Brestensky, D.M.; Stryker, J.M.J. Selective hydride-mediated conjugate reduction of .alpha.,.beta.-unsaturated carbonyl compounds using [(Ph3P)CuH]6. Am. Chem. Soc. 1988, 110, 291. [Google Scholar] [CrossRef]
- Meiries, S.; Bartoli, A.; Decostanzi, M.; Parrain, J.-L.; Commeiras, L. Directed studies towards the total synthesis of (+)-13-deoxytedanolide: Simple and convenient synthesis of the C8–C16 fragment. Org. Biomol. Chem. 2013, 11, 4882–4890. [Google Scholar] [CrossRef]
- Ojima, I.; Kogure, T. Reduction of carbonyl compounds via hydrosilylation. 4. Highly regioselective reductions of. alpha.,. beta.-unsaturated carbonyl compounds. Organometallics 1982, 1, 1390–1399. [Google Scholar] [CrossRef]
- Ito, H.; Ishizuka, T.; Arimoto, K.; Miura, K.; Hosomi, A. Generation of a reducing reagent from copper (I) salt and hydrosilane. New practical method for conjugate reduction. Tetrahedron Lett. 1997, 38, 8887–8890. [Google Scholar] [CrossRef]
- Miller, R.D.; McKean, D.R. The Facile Silylation of Aldehydes and Ketones using Trimethylsilyl Iodide: An Exceptionally Simple Procedure for the Generation of Thermodynamically Equilibrated Trimethylsilylenol Ethers. Synthesis 1979, 730–732. [Google Scholar] [CrossRef]
- Ryu, I.; Murai, S.; Niwa, I.; Sonoda, N. A Convenient Synthesis of α-Phenylseleno Ketones and Aldehydes from Enol Silyl Ethers and Phenylselenenyl Bromide. Synthesis 1977, 12, 874–876. [Google Scholar] [CrossRef]
- Kamikubo, T.; Ogasawara, K. Preparation of (+)-Tricyclo[6.2.1.02,7]undec-2(7)-en-3-one and Its Conversion into (+)-epi-β-Santalene. Chem. Lett. 1995, 2, 95–96. [Google Scholar] [CrossRef]
- Fernández, B.; Pérez, J.A.M.; Granja, J.R.; Castedo, L.; Mouriño, A. Synthesis of hydrindan derivatives related to vitamin D. J. Org. Chem. 1992, 57, 3173–3178. [Google Scholar] [CrossRef]
- Sharpless, K.B.; Younh, M.W.; Lauer, R.F. Reactions of selenoxides: Thermal syn-elimination and H218O exchange. Tetrahedron Lett. 1973, 22, 1979–1982. [Google Scholar] [CrossRef]
- Sharpless, K.B.; Lauer, R.F.; Teranishi, A.Y. Electrophilic and nucleophilic organoselenium reagents. New routes to .alpha.,.beta.-unsaturated carbonyl compounds. J. Am. Chem. Soc. 1973, 95, 6137–6139. [Google Scholar] [CrossRef]
- Reich, H.J.; Renga, J.M.; Reich, I.L. Organoselenium chemistry. Conversion of ketones to enones by selenoxide syn elimination. J. Am. Chem. Soc. 1975, 97, 5434–5447. [Google Scholar] [CrossRef]
- Angeles, A.R.; Waters, S.P.; Danishefsky, S.J. Total Syntheses of (+)- and (−)-Peribysin E. J. Am. Chem. Soc. 2008, 130, 13765–13770. [Google Scholar] [CrossRef]
- Uchida, K.; Yokoshima, S.; Kan, T.; Fukuyama, T. Total Synthesis of (±)-Morphine. Org. Lett. 2006, 8, 5311–5313. [Google Scholar] [CrossRef]
- Hu, X.-D.; Tu, Y.Q.; Zhang, E.; Gao, S.; Wang, S.; Wang, A.; Fan, C.-A.; Wang, M. Total Synthesis of (±)-Galanthamine. Org. Lett. 2006, 8, 1823–1825. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Hirao, T.; Saegusa, T. Synthesis of .alpha.,.beta.-unsaturated carbonyl compounds by palladium(II)-catalyzed dehydrosilylation of silyl enol ethers. J. Org. Chem. 1978, 43, 1011–1013. [Google Scholar] [CrossRef]
- Tanemura, K.; Suzuki, T.; Nishida, Y.; Satsumabayashi, K.; Horaguchi, T. A mild and efficient procedure for α-bromination of ketones using N-bromosuccinimide catalysed by ammonium acetate. Chem. Commun. 2004, 470–471. [Google Scholar] [CrossRef] [PubMed]
- Ushakov, D.B.; Navickas, V.; Ströbele, M.; Maichle-Mössmer, C.; Sasse, F.; Maier, M.E. Total Synthesis and Biological Evaluation of (−)-9-Deoxy-englerin A. Org. Lett. 2011, 13, 2090–2093. [Google Scholar] [CrossRef] [PubMed]
- Gijsen, H.J.M.; Wijnberg, J.B.P.A.; Stork, G.A.; Groot, A. The synthesis of (−)-kessane, starting from natural (+)-aromadendrene-II. Tetrahedron 1991, 47, 4409–4416. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 7th ed.; Elsevier: Oxford, UK, 2012. [Google Scholar]
- Williams, D.B.G.; Lawton, M. Drying of Organic Solvents: Quantitative Evaluation of the Efficiency of Several Desiccants. J. Org. Chem. 2010, 75, 8351–8354. [Google Scholar] [CrossRef]
- Still, W.C.; Kahn, H.; Mitra, A.J. Rapid chromatographic technique for preparative separations with moderate resolution. J. Org. Chem. 1978, 43, 2923–2925. [Google Scholar] [CrossRef]


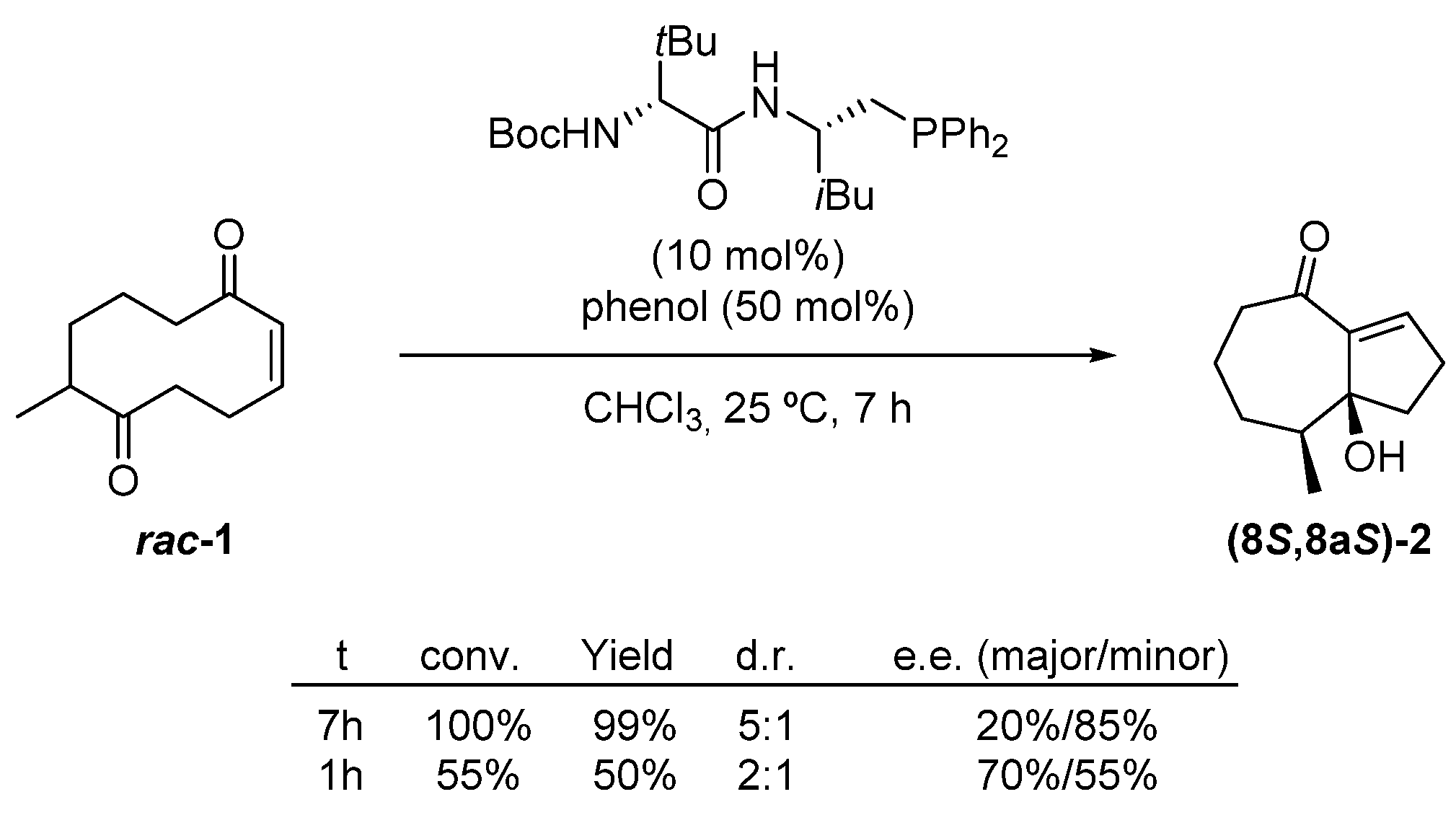



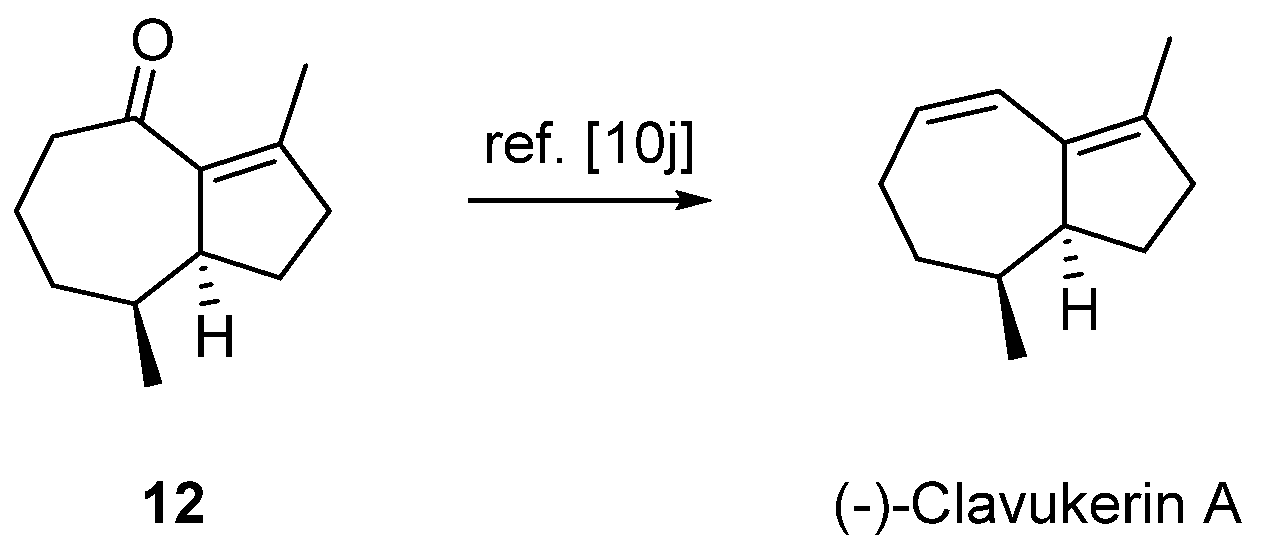

| Entry | Solvent | T (°C) | Time (min) | Recovered 1 (%) | e.e. 1 (%) 2 | Yield 2 (%) | e.e. 2 (%) 2 |
|---|---|---|---|---|---|---|---|
| 1 | CHCl3 | 25 | 40 | 61 | 50 | 35 | 88 |
| 2 | CHCl3 | 25 | 45 | 62 | 54 | 37 | 88 |
| 3 | CHCl3 | 25 | 50 | 57 | 54 | 39 | 88 |
| 4 | CHCl3 | 25 | 55 | 50 | 66 | 42 | 86 |
| 5 | CCl4 | 5 | 20 | - | - | 46 | 90 |
| 6 3 | CCl4 | 5 | 30 | 56 | 76 | 42 | 90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mato, R.; Manzano, R.; Reyes, E.; Prieto, L.; Uria, U.; Carrillo, L.; Vicario, J.L. Kinetic Resolution in Transannular Morita-Baylis-Hillman Reaction: An Approximation to the Synthesis of Sesquiterpenes from Guaiane Family. Catalysts 2022, 12, 67. https://doi.org/10.3390/catal12010067
Mato R, Manzano R, Reyes E, Prieto L, Uria U, Carrillo L, Vicario JL. Kinetic Resolution in Transannular Morita-Baylis-Hillman Reaction: An Approximation to the Synthesis of Sesquiterpenes from Guaiane Family. Catalysts. 2022; 12(1):67. https://doi.org/10.3390/catal12010067
Chicago/Turabian StyleMato, Raquel, Rubén Manzano, Efraím Reyes, Liher Prieto, Uxue Uria, Luisa Carrillo, and Jose L. Vicario. 2022. "Kinetic Resolution in Transannular Morita-Baylis-Hillman Reaction: An Approximation to the Synthesis of Sesquiterpenes from Guaiane Family" Catalysts 12, no. 1: 67. https://doi.org/10.3390/catal12010067
APA StyleMato, R., Manzano, R., Reyes, E., Prieto, L., Uria, U., Carrillo, L., & Vicario, J. L. (2022). Kinetic Resolution in Transannular Morita-Baylis-Hillman Reaction: An Approximation to the Synthesis of Sesquiterpenes from Guaiane Family. Catalysts, 12(1), 67. https://doi.org/10.3390/catal12010067