Investigation on the Cause of the SO2 Generation during Hot Gas Desulfurization (HGD) Process
Abstract
1. Introduction
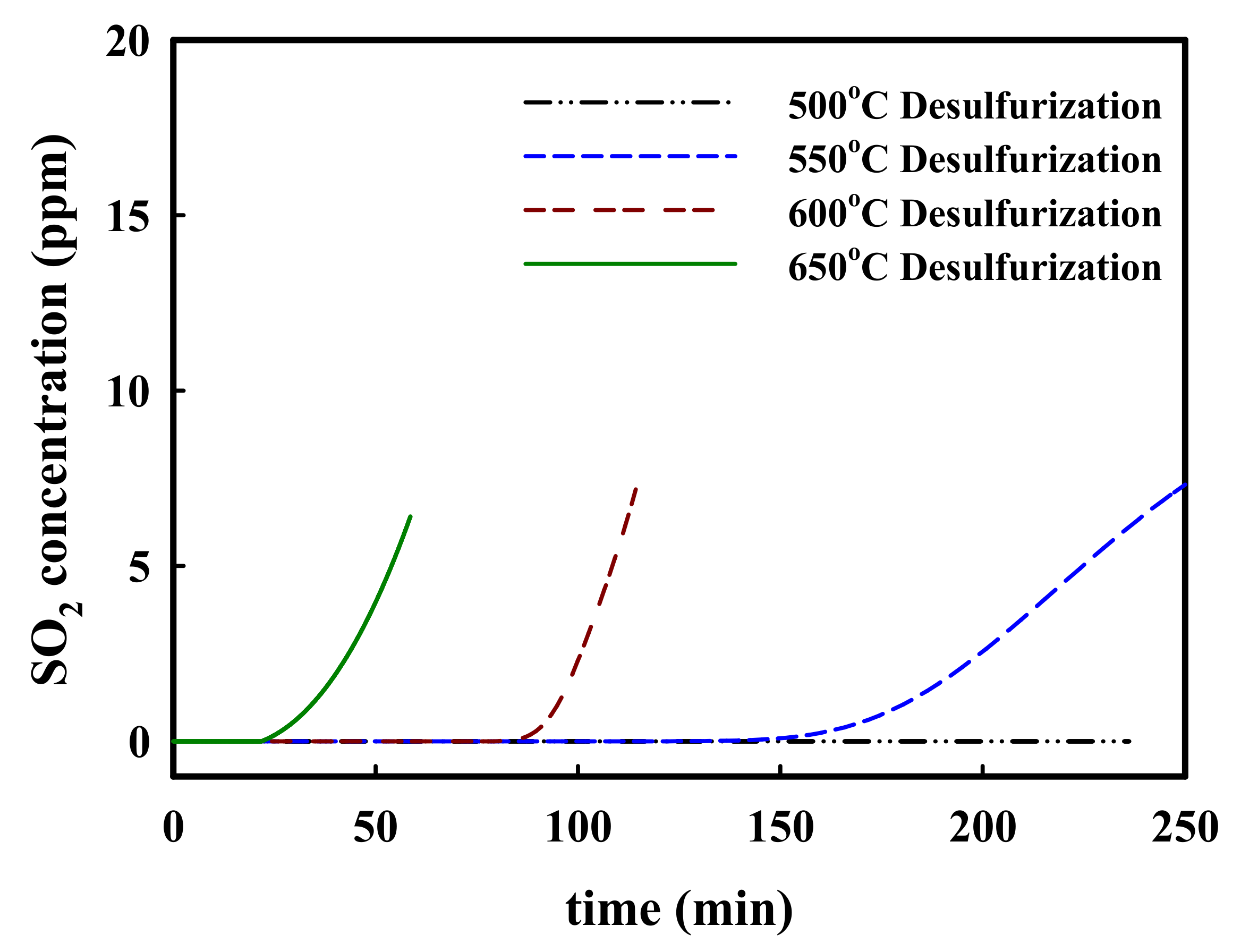
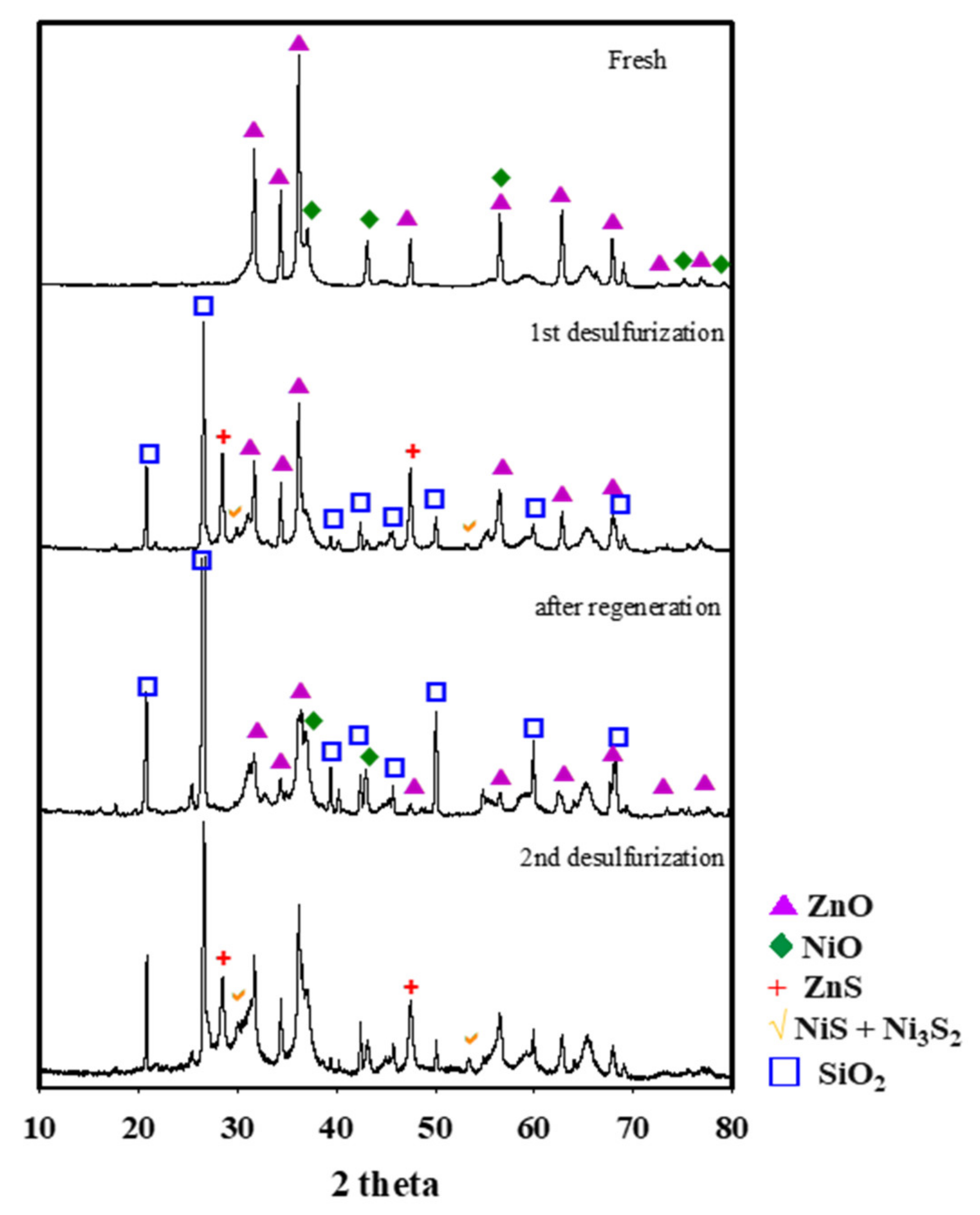
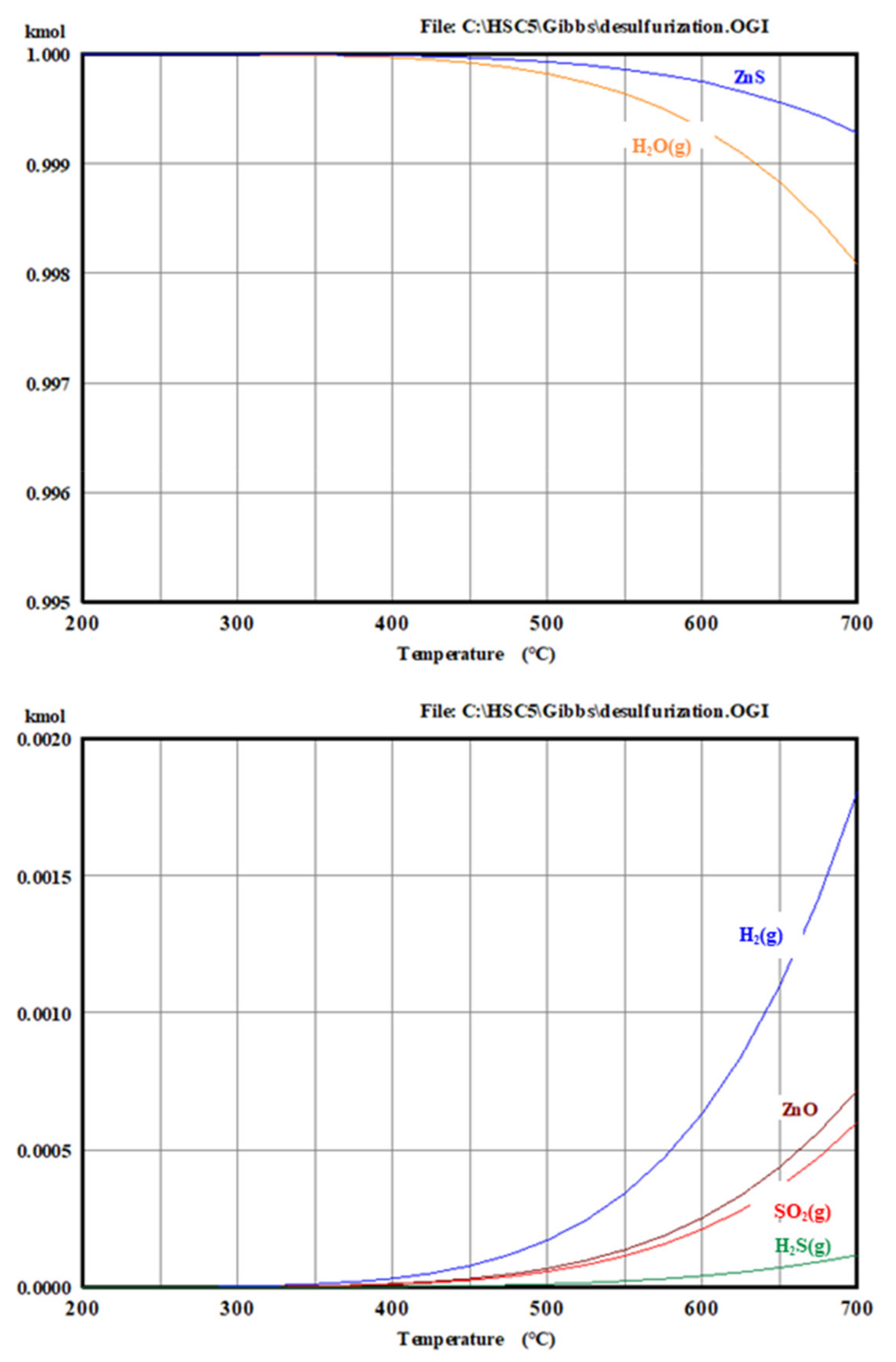
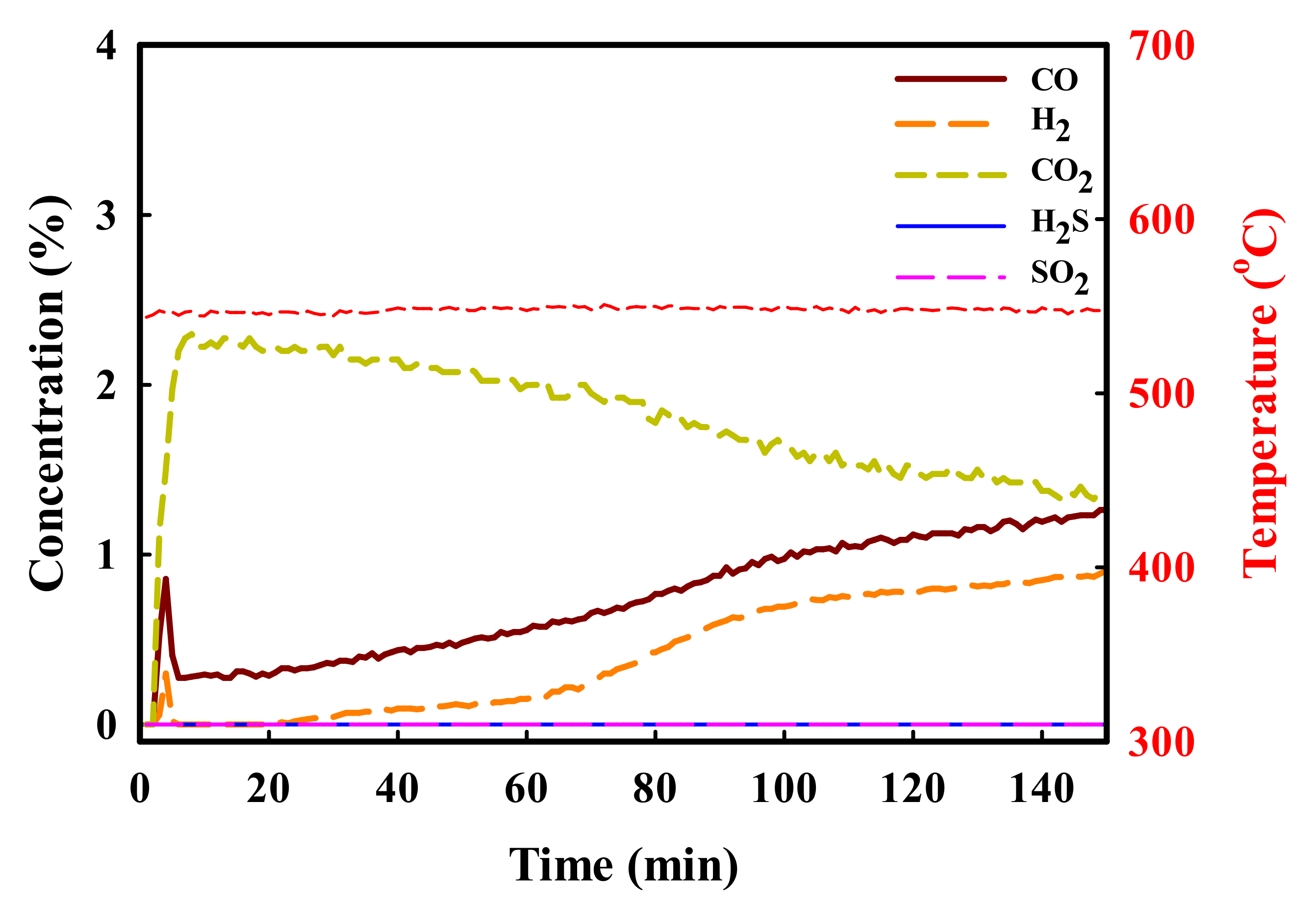
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ben-Slimane, R.; Hepworth, M.T. Desulfurization of hot coal-derived fuel gases with manganese-based regenerable sorbents. 1. Loading (sulfidation) tests. Energy Fuels 1994, 8, 1175–1183. [Google Scholar] [CrossRef]
- Cheah, S.; Carpenter, D.L.; Magrini-Bair, K.A. Review of mid- to high-temperature sulfur sorbents for desulfurization of biomass- and coal-derived syngas. Energy Fuels 2009, 23, 5291–5307. [Google Scholar] [CrossRef]
- Feng, Y.; Mi, J.; Wu, M.; Shangguan, J.; Fan, H. In situ preparation and regeneration behaviors of zinc oxide/red clay desulfurization sorbents. Energy Fuels 2017, 31, 1015–1022. [Google Scholar] [CrossRef]
- Giuffrida, A.; Romano, M.C. On the Effects of Syngas Clean-Up Temperature in Igccs, ASME Turbo Expo 2010: Power for Land, Sea, and Air, 2010; American Society of Mechanical Engineers: New York, NY, USA, 2010; pp. 661–670. [Google Scholar]
- Nakhjiri, A.T.; Heydarinasab, A.; Bakhtiari, O.; Mohammadi, T. Numerical simulation of CO2/H2S simultaneous removal from natural gas using potassium carbonate aqueous solution in hollow fiber membrane contactor. J. Environ. Chem. Eng. 2020, 8, 104130. [Google Scholar] [CrossRef]
- Wang, J.; Song, Z.; Cheng, H.; Chen, L.; Deng, L.; Qi, Z. Multilevel screening of ionic liquid absorbents for simultaneous removal of CO2 and H2S from natural gas. Sep. Purif. Technol. 2020, 248, 117053. [Google Scholar] [CrossRef]
- Raabe, T.; Mehne, M.; Rasser, H.; Krause, H.; Kureti, S. Study on iron-based adsorbents for alternating removal of H2S and CO2 from natural gas and biogas. Chem. Eng. J. 2019, 371, 738–749. [Google Scholar] [CrossRef]
- Zulkefli, N.N.; Masdar, M.S.; Wan Isahak, W.N.R.; Abu Bakar, S.N.H.; Abu Hasan, H.; Mohd Sofian, N. Application of response surface methodology for preparation of znac2/cac adsorbents for hydrogen sulfide (H2S) capture. Catalysts 2021, 11, 545. [Google Scholar] [CrossRef]
- Gupta, R.P.; Gangwal, S.K.; Johnson, E.W. Integration and Testing of Hot Desulfurization and Entrained-Flow Gasification for Power Generation Systems. Volume 2, Evaluation of Zinc Loss from Zinc Titanate Sorbents during Hot Gas Desulfurization: Final Report, 1 October 1987–31 October 1993; Texaco Montebello Research Lab.: Montebello, CA, USA, 1993. [Google Scholar]
- Huang, L.; Wang, G.; Qin, Z.; Dong, M.; Du, M.; Ge, H.; Li, X.; Zhao, Y.; Zhang, J.; Hu, T. In situ xas study on the mechanism of reactive adsorption desulfurization of oil product over Ni/ZnO. Appl. Catal. B Environ. 2011, 106, 26–38. [Google Scholar] [CrossRef]
- Jung, S.Y.; Jun, H.K.; Lee, S.J.; Lee, T.J.; Ryu, C.K.; Kim, J.C. Improvement of the desulfurization and regeneration properties through the control of pore structures of the Zn−Ti-based H2S removal sorbents. Environ. Sci. Technol. 2005, 39, 9324–9330. [Google Scholar] [CrossRef]
- Jung, S.Y.; Lee, S.J.; Lee, T.J.; Ryu, C.K.; Kim, J.C. H2S removal and regeneration properties of zn–al-based sorbents promoted with various promoters. Catal. Today 2006, 111, 217–222. [Google Scholar] [CrossRef]
- Jung, S.Y.; Lee, S.J.; Park, J.J.; Lee, S.C.; Jun, H.K.; Lee, T.J.; Ryu, C.K.; Kim, J.C. The simultaneous removal of hydrogen sulfide and ammonia over zinc-based dry sorbent supported on alumina. Sep. Purif. Technol. 2008, 63, 297–302. [Google Scholar] [CrossRef]
- Kim, H.-N.; Lee, D.-H.; Lee, S.-Y.; Hwang, T.-S.; Ryu, H.-J. Reaction characteristics of wgs catalyst for sewgs process in a pressurized fluidized bed reactor. Trans. Korean Hydrog. New Energy Soc. 2012, 23, 337–345. [Google Scholar] [CrossRef][Green Version]
- Ko, T.-H.; Chu, H.; Chaung, L.-K. The sorption of hydrogen sulfide from hot syngas by metal oxides over supports. Chemosphere 2005, 58, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.W.; Lim, J.H.; Chae, H.J.; Ryu, H.-J.; Lee, D.; Lee, J.B.; Kim, H.; Lee, S.C.; Kim, J.C. CO2 capture and regeneration properties of mgo-based sorbents promoted with alkali metal nitrates at high pressure for the sorption enhanced water gas shift process. Process Saf. Environ. Prot. 2018, 116, 219–227. [Google Scholar] [CrossRef]
- Barba, D. Catalysts and processes for H2S conversion to sulfur. Catalysts 2021, 11, 903. [Google Scholar] [CrossRef]
- Lee, J.B.; Ryu, C.K.; Yi, C.K.; Jo, S.H.; Kim, S.H. Screening of zinc-based sorbents for hot-gas desulfurization. Energy Fuels 2008, 22, 1021–1026. [Google Scholar] [CrossRef]
- Lee, T.J.; Kwon, W.T.; Chang, W.C.; Kim, J.C. A study on regeneration of zinc titanate sorbents for H2S removal. Korean J. Chem. Eng. 1997, 14, 513–518. [Google Scholar] [CrossRef]
- Park, N.-K.; Jung, Y.-K.; Kim, B.-S.; Ryu, S.-O.; Lee, T.-J.; Kim, K.-S. Behavior of zinc based sorbents for hot gas desulfurization. Theor. Appl. Chem. Eng. 2002, 8, 4493–4496. [Google Scholar]
- Siriwardane, R.V.; Gardner, T.; Poston, J.A.; Fisher, E.P.; Miltz, A. Spectroscopic characterization of nickel containing desulfurization sorbents. Ind. Eng. Chem. Res. 2000, 39, 1106–1110. [Google Scholar] [CrossRef]
- Seo, J.-H.; Beak, C.; Kown, W.; Ahn, J.; Cho, K. Trends in research and technical development of sorbents for hot gas desulfurization for H 2S removal. J. Korean Inst. Resour. Recycl. 2016, 25, 14–27. [Google Scholar]
- Sasaoka, E.; Hatori, M.; Yoshimura, H.; Su, C.; Uddin, M.A. Role of H2O in oxidation of spent high-temperature desulfurization sorbent Fe2O3 and cuo in the presence of O2. Ind. Eng. Chem. Res. 2001, 40, 2512–2517. [Google Scholar] [CrossRef]
- Sasaoka, E.; Hatori, M.; Sada, N.; Uddin, M.A. Role of H2O in oxidative regeneration of zns formed from high-temperature desulfurization zno sorbent. Ind. Eng. Chem. Res. 2000, 39, 3844–3848. [Google Scholar] [CrossRef]
- Park, N.-K.; Lee, Y.J.; Han, G.B.; Ryu, S.O.; Lee, T.J. Preparation and reactivity test of nano size ZnO for hot gas desulfurization. Theor. Appl. Chem. Eng. 2006, 12, 540–543. [Google Scholar]
- Park, N.-K.; Lee, D.-H.; Lee, J.-D.; Chang, W.C.; Ryu, S.-O.; Lee, T.-J. Effects of reduction of metal oxide sorbents on reactivity and physical properties during hot gas desulphurization in igcc. Fuel 2005, 84, 2158–2164. [Google Scholar] [CrossRef]
- Park, N.-K.; Lee, D.-H.; Jun, J.H.; Lee, J.D.; Ryu, S.O.; Lee, T.J.; Kim, J.-C.; Chang, C.H. Two-stage desulfurization process for hot gas ultra cleanup in igcc. Fuel 2006, 85, 227–234. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Woodruff, S. Ftir characterization of the interaction of oxygen with zinc sulfide. Ind. Eng. Chem. Res. 1995, 34, 699–702. [Google Scholar] [CrossRef]
- Yu, J.; Chang, L.; Li, F.; Xie, K. A review on research and development of iron-based sorbents for removal of hydrogen sulfide from hot coal gases. Front. Chem. Eng. China 2010, 4, 529–535. [Google Scholar] [CrossRef]
- Westmoreland, P.R.; Gibson, J.B.; Harrison, D.P. Comparative kinetics of high-temperature reaction between hydrogen sulfide and selected metal oxides. Environ. Sci. Technol. 1977, 11, 488–491. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, G.; Wang, Q.; Xu, C.; Gao, J. Regeneration characteristics and kinetics of Ni/ZnO–SiO2–Al2O3 adsorbent for reactive adsorption desulfurization. Ind. Eng. Chem. Res. 2012, 51, 3939–3950. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Poston, J.A. Interaction of H2S with zinc titanate in the presence of H2 and CO. Appl. Surf. Sci. 1990, 45, 131–139. [Google Scholar] [CrossRef]



| H2S Desulfurization | Regeneration | |
|---|---|---|
| Temperature (°C) | 450–650 | 650 |
| Pressure | ambient | ambient |
| Flow rate (mL/min) | 2000 | 2000 |
| Gas composition (Vol.%) | H2S: 0.4, or H2S: 0.36 CO: 6.5, H2: 2.95, CO2: 0.15, N2: balance | O2: 3, N2: balance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, B.; Kim, J.H.; Lee, D.; Nam, H.; Kim, H.N.; Baek, J.I.; Ryu, H.-J. Investigation on the Cause of the SO2 Generation during Hot Gas Desulfurization (HGD) Process. Catalysts 2021, 11, 985. https://doi.org/10.3390/catal11080985
Hwang B, Kim JH, Lee D, Nam H, Kim HN, Baek JI, Ryu H-J. Investigation on the Cause of the SO2 Generation during Hot Gas Desulfurization (HGD) Process. Catalysts. 2021; 11(8):985. https://doi.org/10.3390/catal11080985
Chicago/Turabian StyleHwang, Byungwook, Jung Hwan Kim, Doyeon Lee, Hyungseok Nam, Ha Na Kim, Jeom In Baek, and Ho-Jung Ryu. 2021. "Investigation on the Cause of the SO2 Generation during Hot Gas Desulfurization (HGD) Process" Catalysts 11, no. 8: 985. https://doi.org/10.3390/catal11080985
APA StyleHwang, B., Kim, J. H., Lee, D., Nam, H., Kim, H. N., Baek, J. I., & Ryu, H.-J. (2021). Investigation on the Cause of the SO2 Generation during Hot Gas Desulfurization (HGD) Process. Catalysts, 11(8), 985. https://doi.org/10.3390/catal11080985






