Immobilization of Mutant Phosphotriesterase on Fuller’s Earth Enhanced the Stability of the Enzyme
Abstract
1. Introduction
2. Results and Discussion
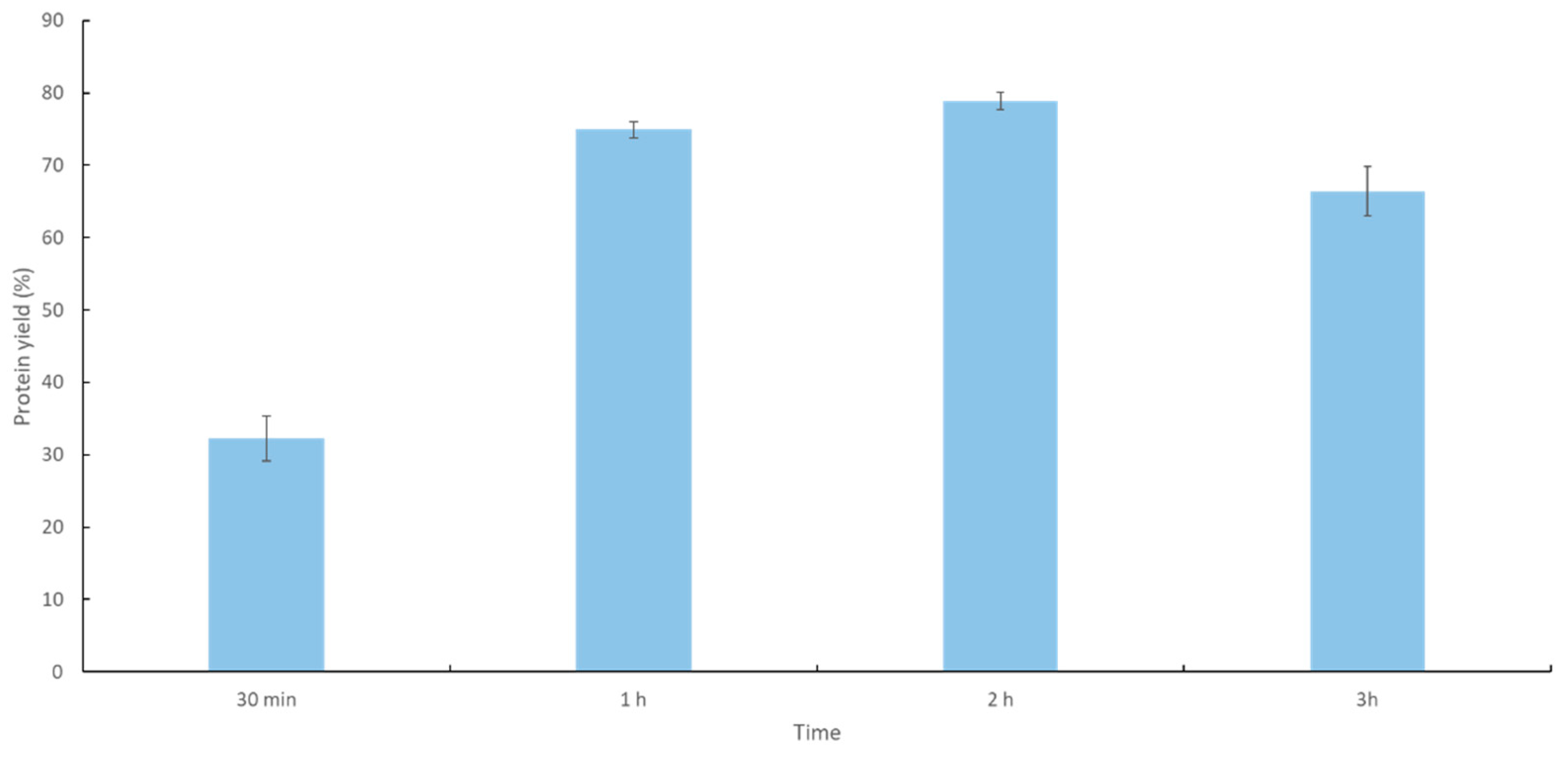
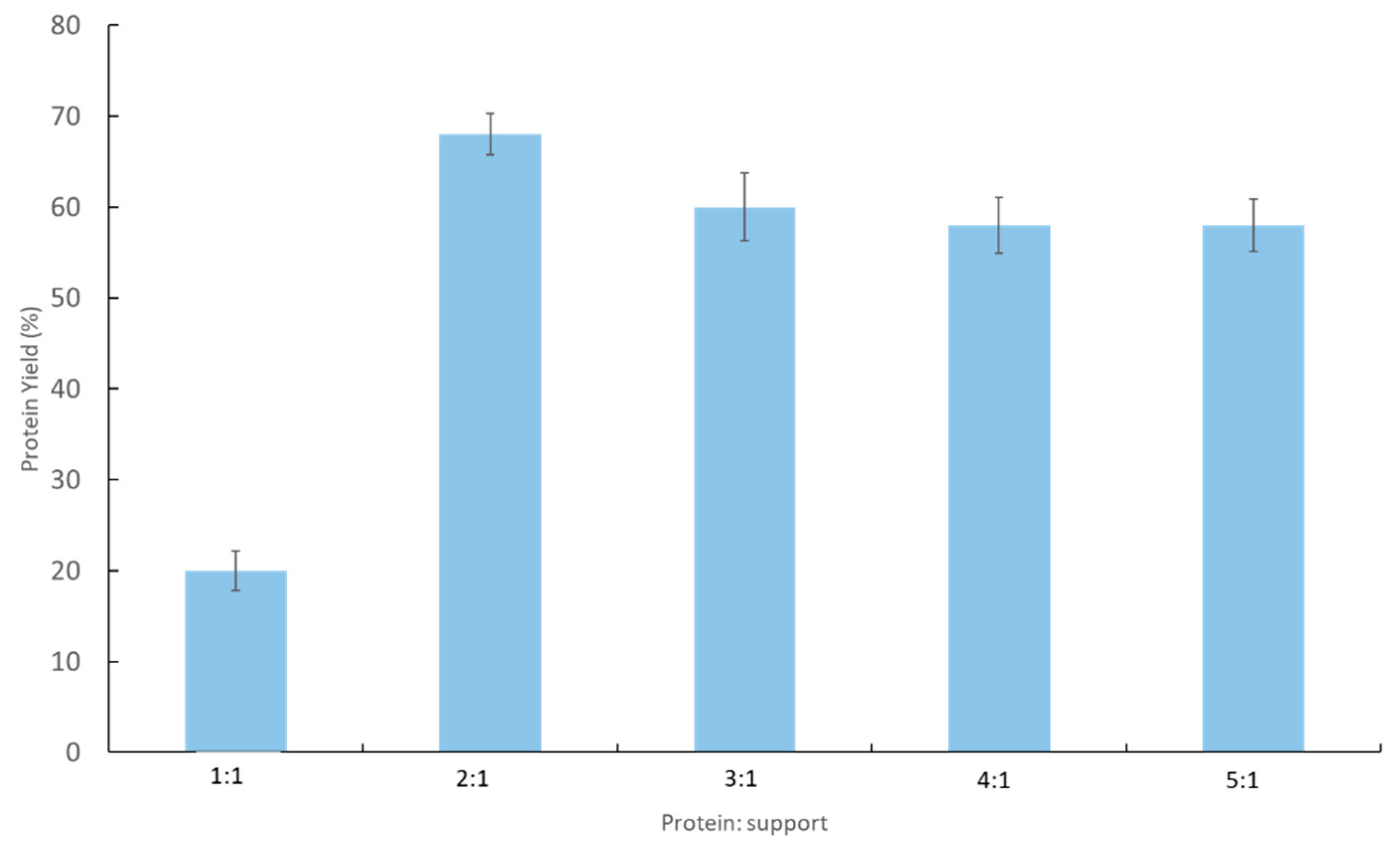
2.1. Immobilization Process of Recombinant YT PTE
2.2. Characterization of the Immobilized YT PTE
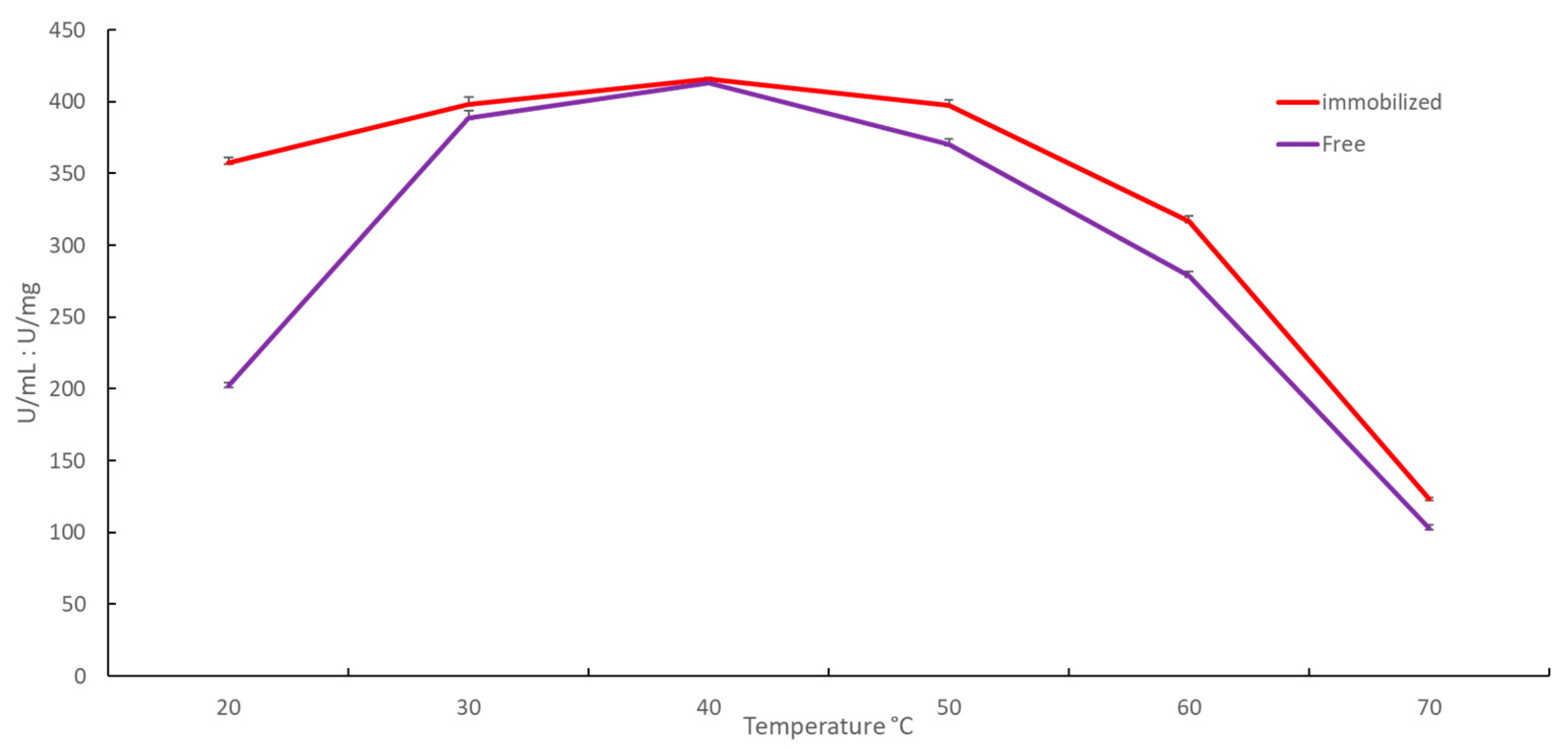
2.2.1. Effect of Temperature on Immobilized YT PTE Activity and Stability
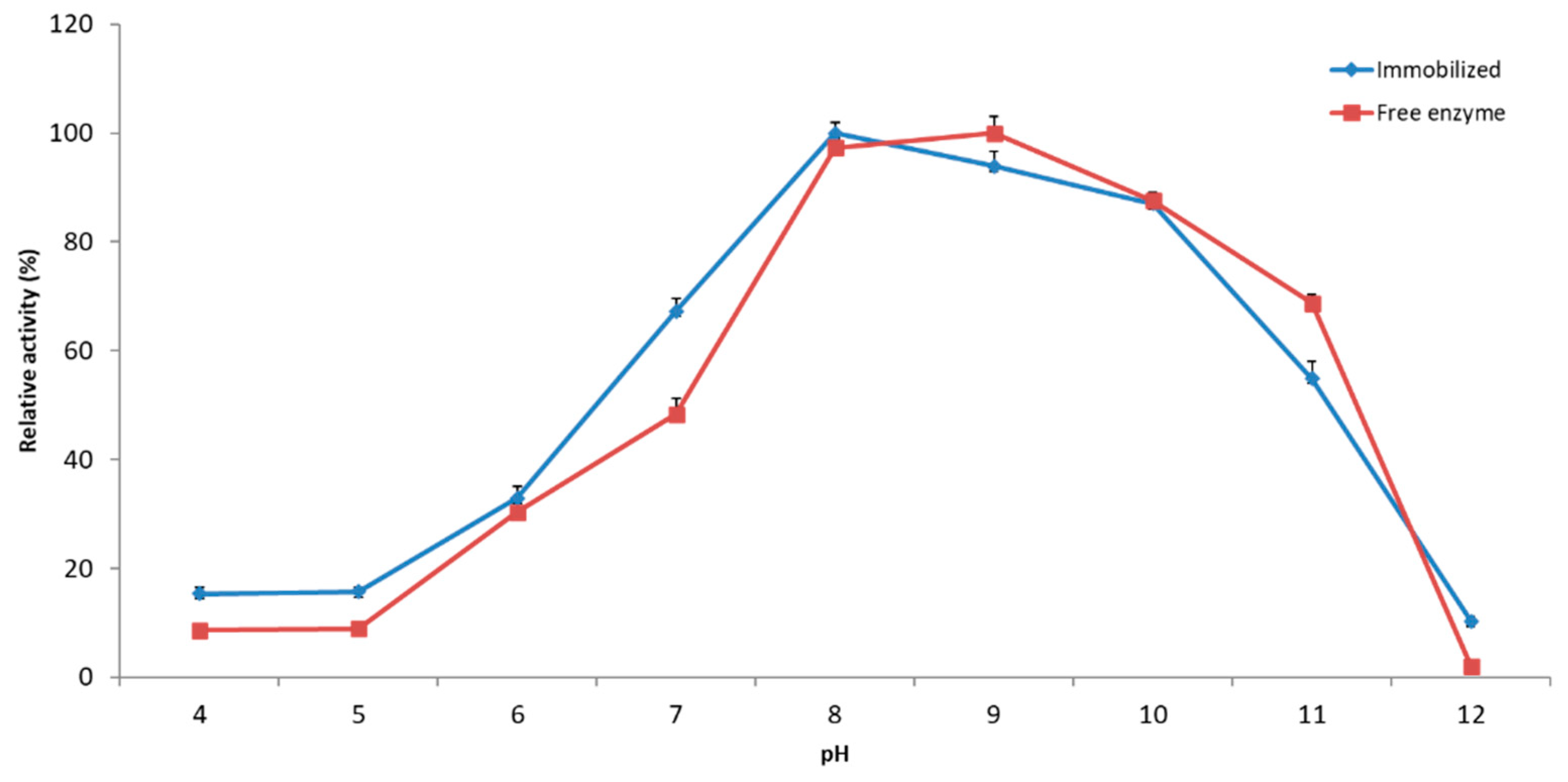
2.2.2. Effect of pH on Immobilized YT PTE Activity
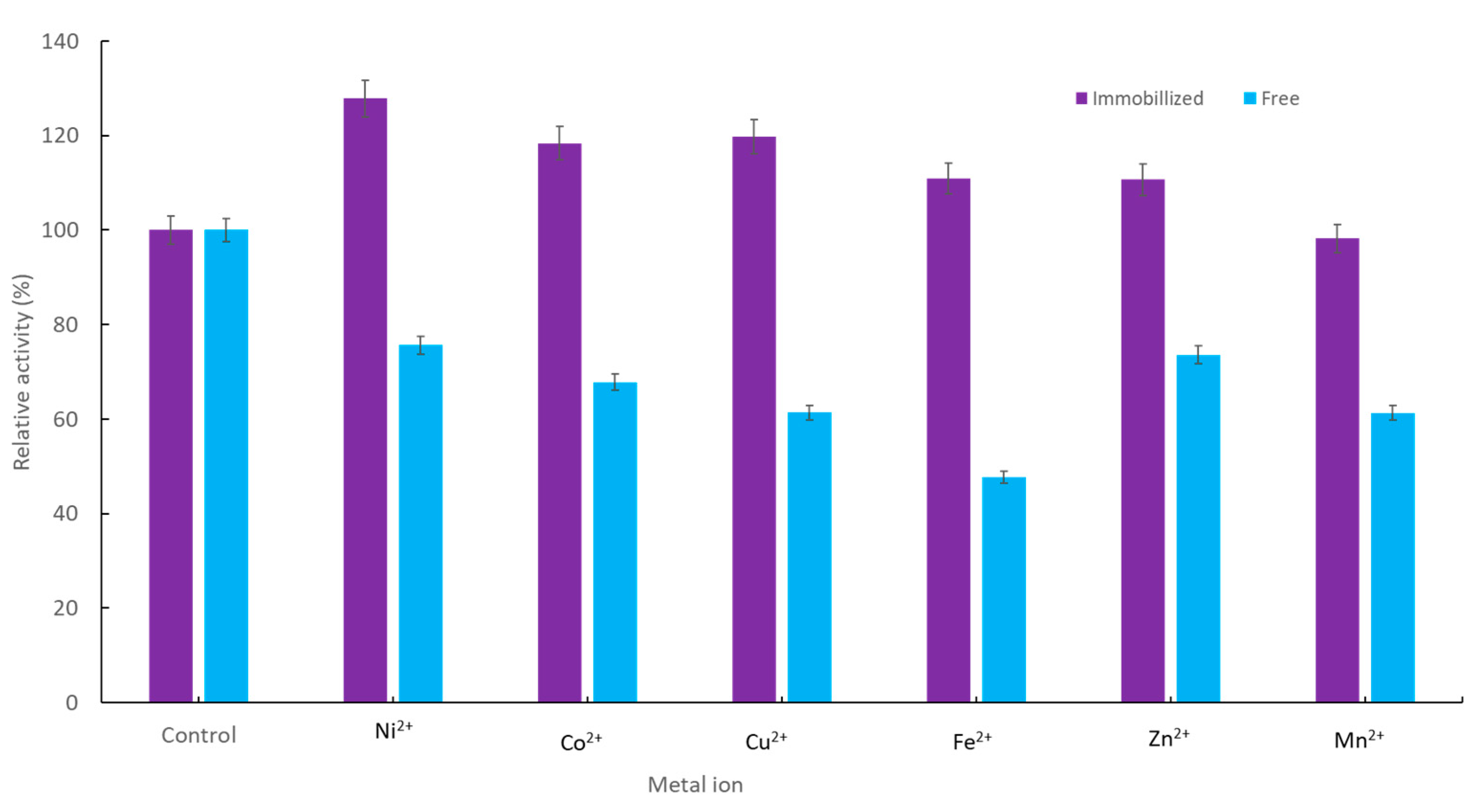
2.2.3. Effect of Metal Ions on Immobilized YT PTE Activity
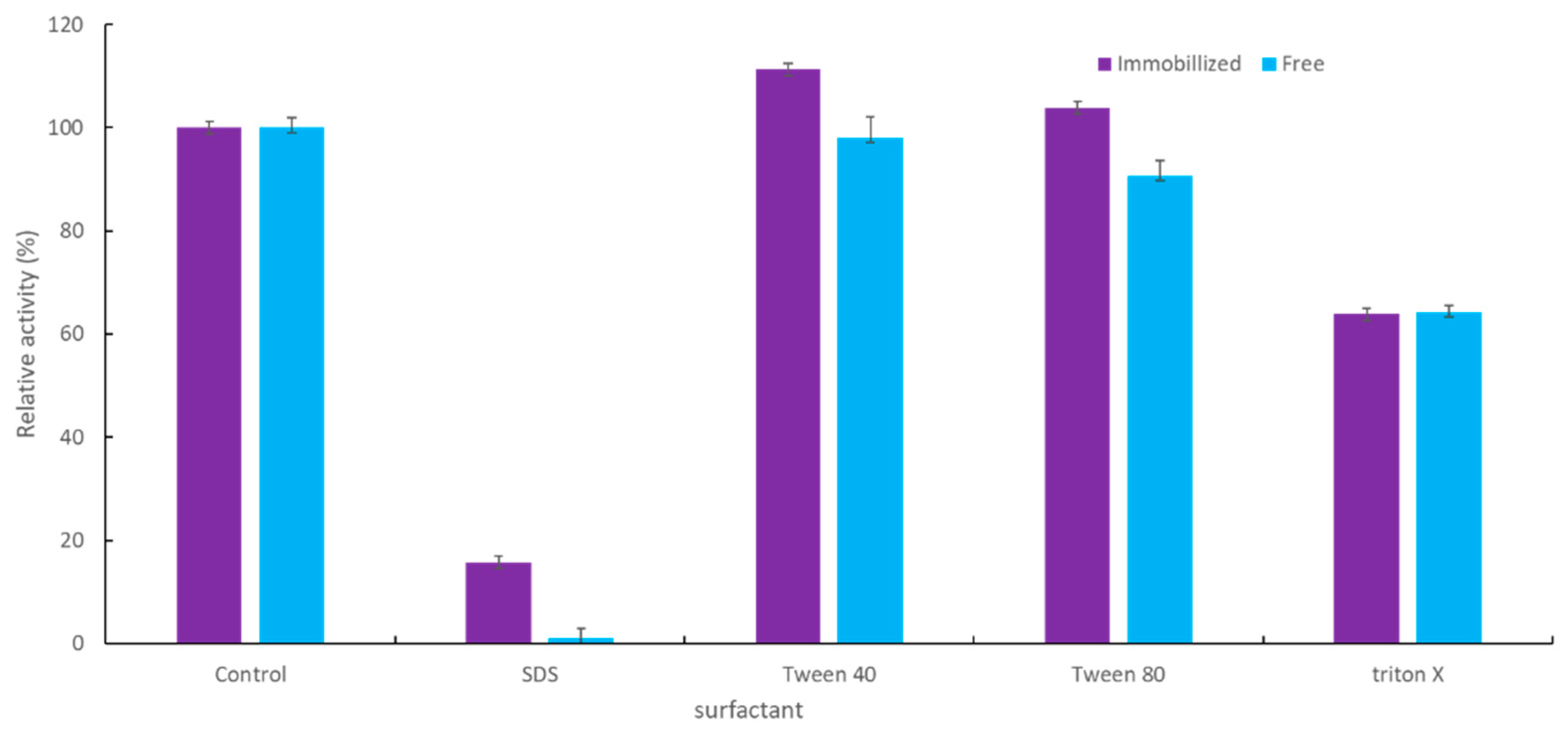
2.2.4. Effect of Surfactant on the Activity of Immobilized YT PTE
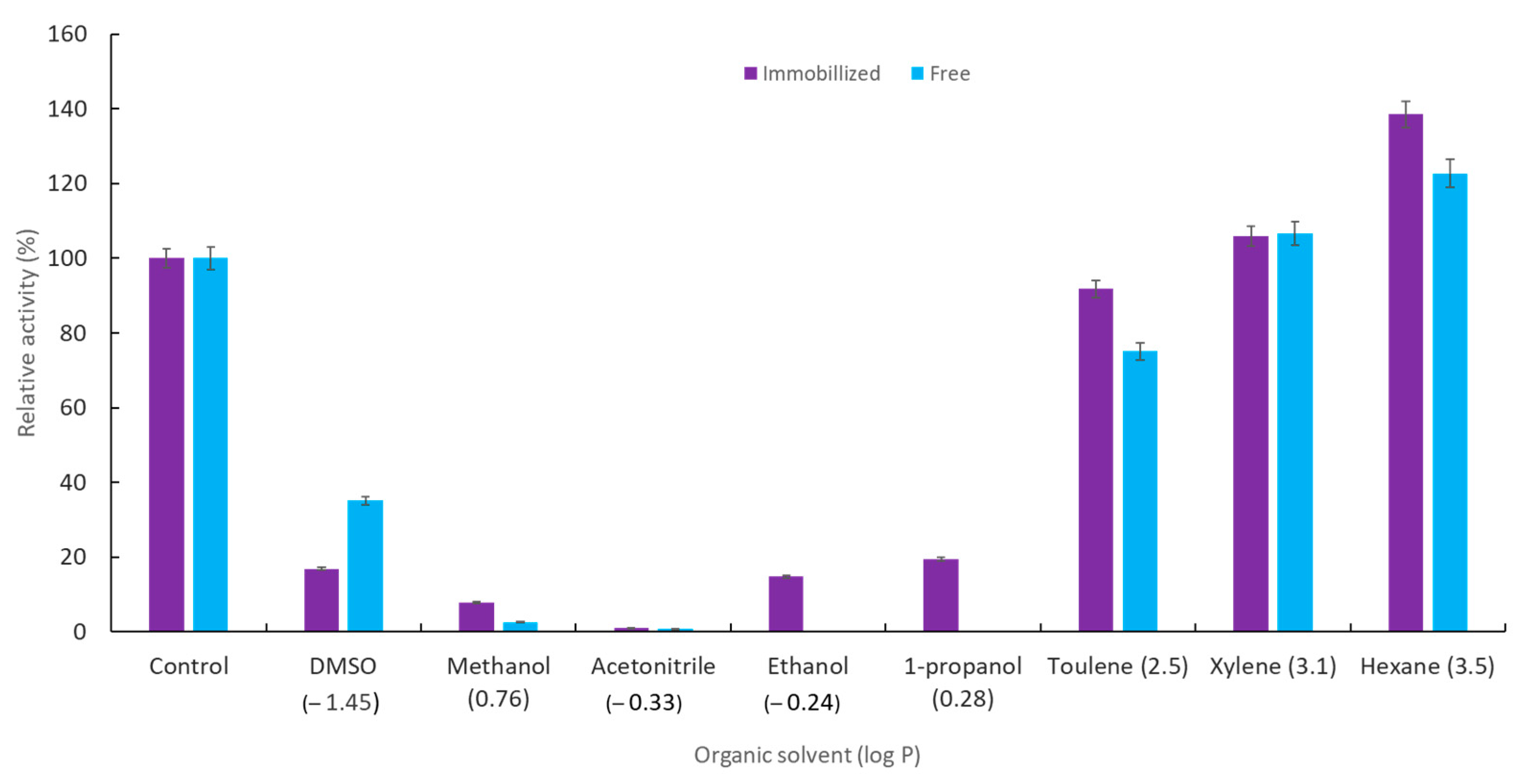
2.2.5. Effect of Different Solvents on the Activity of Immobilized YT PTE
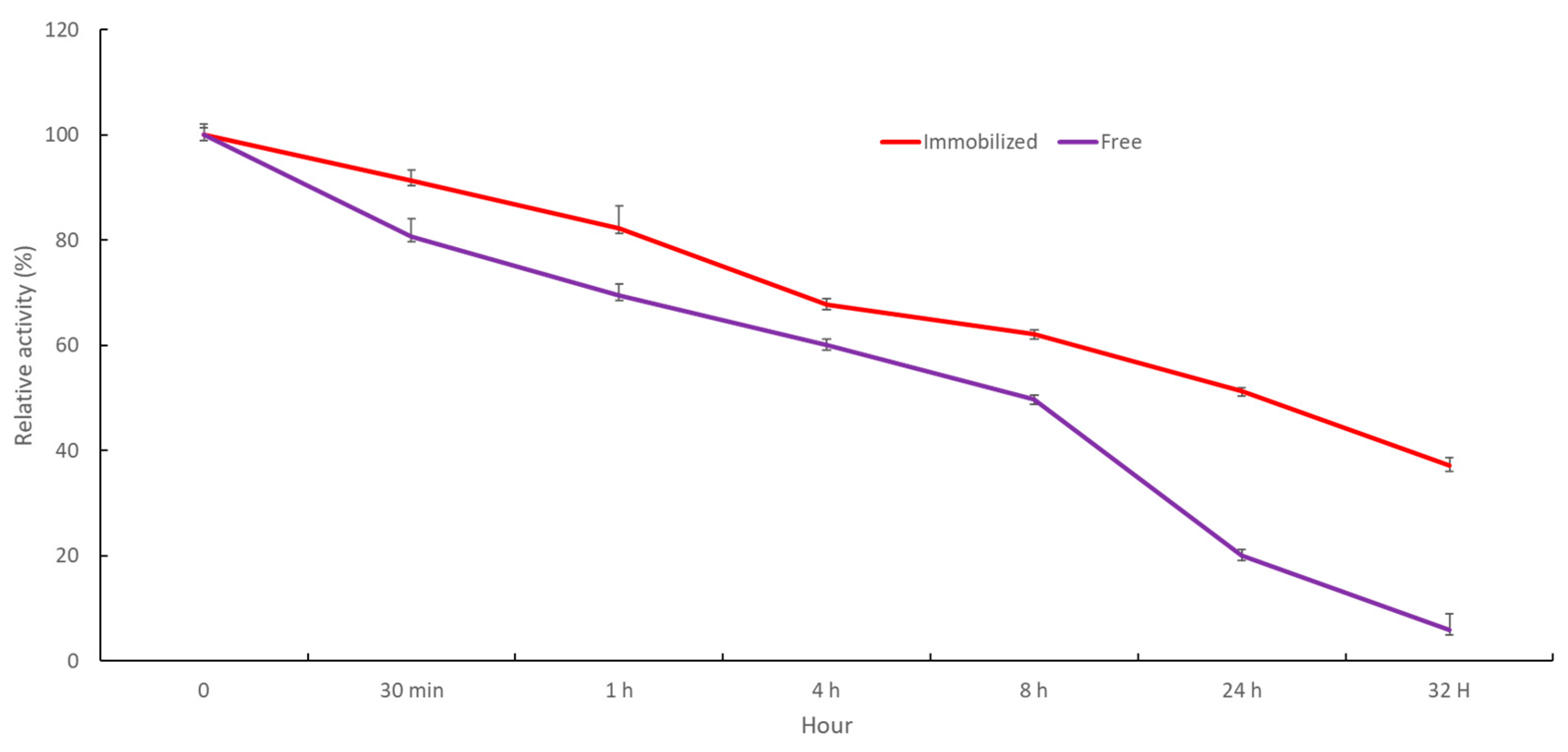
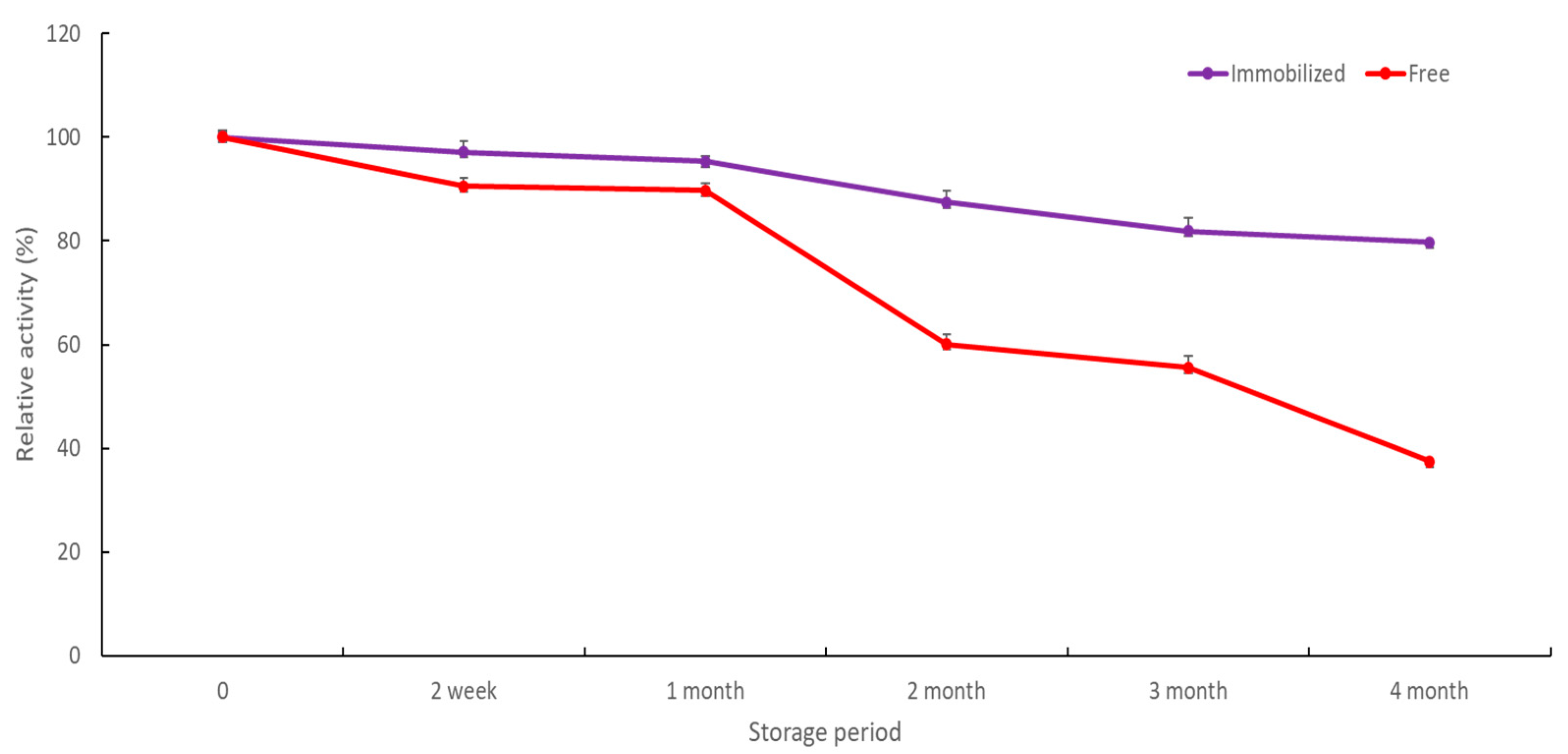
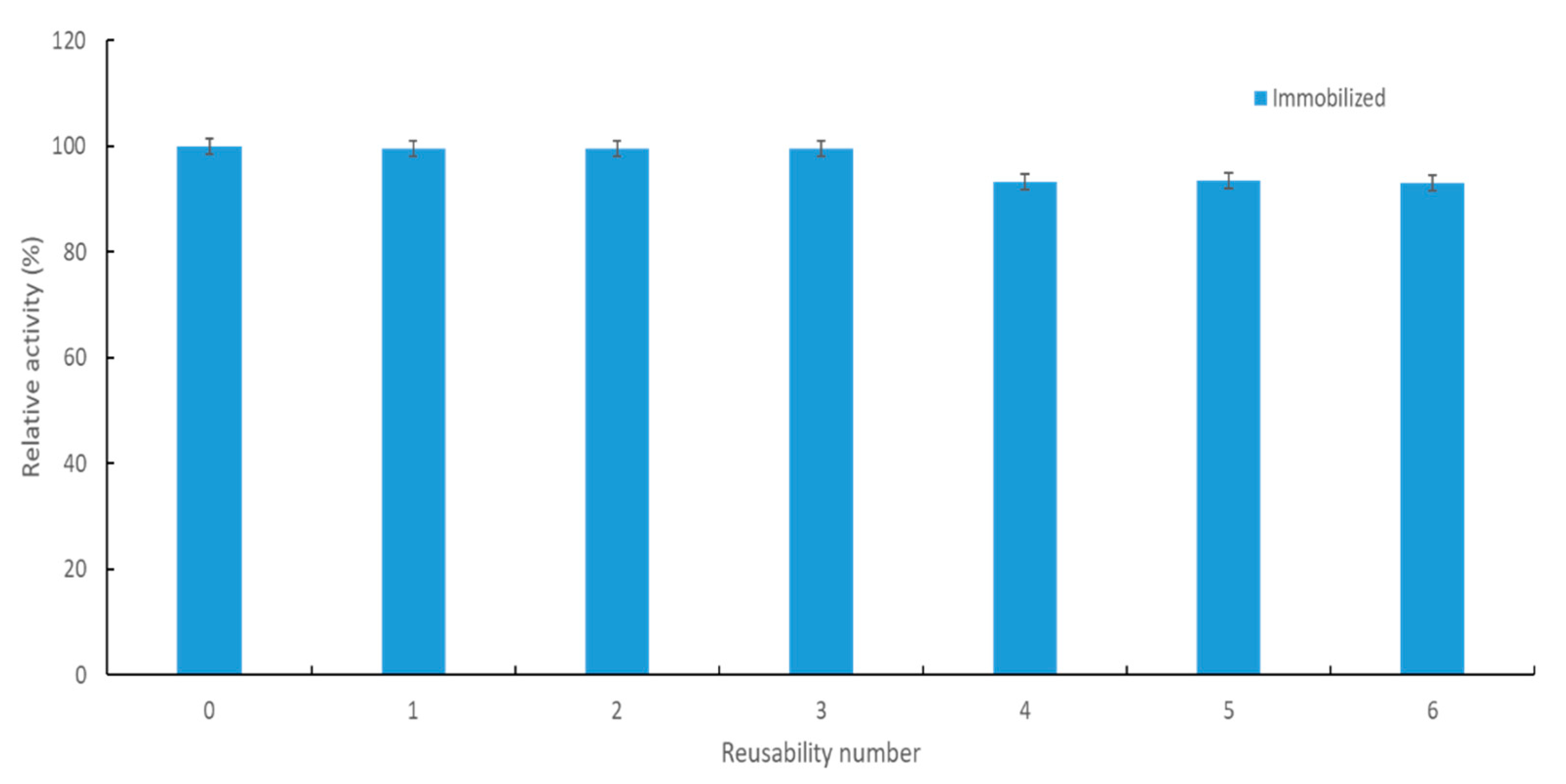
2.2.6. Storage Stability and Reusability
2.2.7. Surface Morphology of FE before and after Immobilization
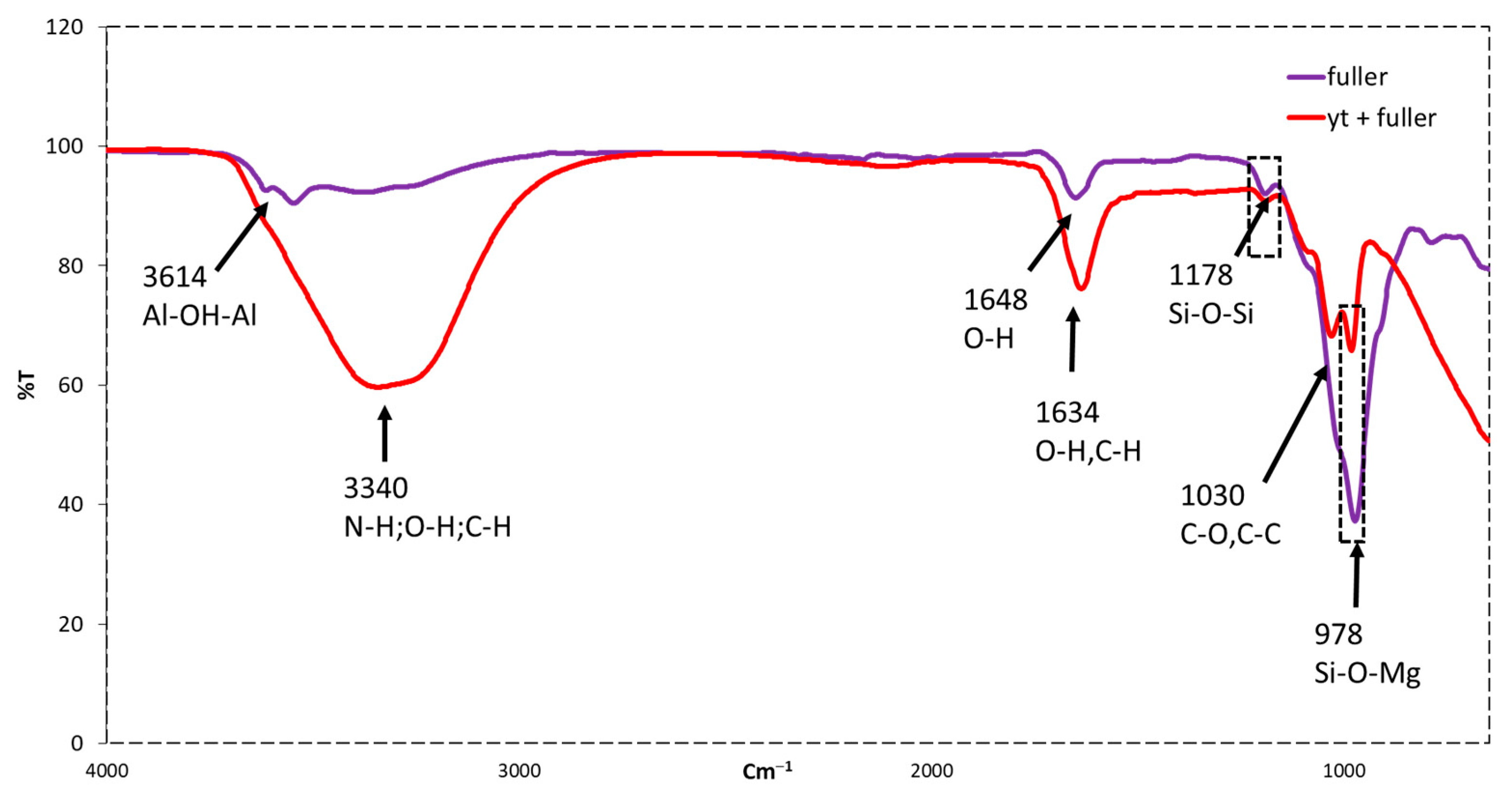
2.2.8. FTIR Spectroscopy Analysis
3. Materials and Methods
3.1. Purification and Immobilization Process of Recombinant YT PTE
3.1.1. Purification of Recombinant YT PTE
3.1.2. Immobilization Process of YT PTE
3.2. Determination of Protein Concentration
3.3. Phosphotriesterase Assay
3.4. Characterization of the Immobilized YT PTE
3.4.1. Effect of Temperature
3.4.2. Effect of pH
3.4.3. Effect of Organic Solvents
3.4.4. Effect of Surfactants
3.4.5. Storage Stability and Reusability Test
3.4.6. Field Emission Scanning Electron Microscopy (FESEM)
3.4.7. Fourier-Transform Infrared Spectroscopy (FTIR)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Del Giudice, I.; Coppolecchia, R.; Merone, L.; Porzio, E.; Carusone, T.M.; Mandrich, L.; Worek, F.; Manco, G. An Efficient Thermostable Organophosphate Hydrolase and Its Application in Pesticide Decontamination. Biotechnol. Bioeng. 2016, 113, 724–734. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme Immobilization: An Update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, E.; Raushel, F.M. Detoxification of Organophosphate Nerve Agents by Bacterial Phosphotriesterase. Toxicol. Appl. Pharmacol. 2005, 207, s459–s470. [Google Scholar] [CrossRef] [PubMed]
- Aubert, S.D.; Li, Y.; Raushel, F.M. Mechanism for the Hydrolysis of Organophosphates by the Bacterial Phosphotriesterase. Biochemistry 2004, 43, 5707–5715. [Google Scholar] [CrossRef]
- Singh, B.K. Organophosphorus-Degrading Bacteria: Ecology and Industrial Applications. Nat. Rev. Microbiol. 2009, 7, 156–164. [Google Scholar] [CrossRef]
- Latip, W.; Knight, V.F.; Halim, N.A.; Ong, K.K.; Kassim, N.A.M.; Yunus, W.M.Z.W.; Noor, S.A.M.; Mohamad Ali, M.S. Microbial Phosphotriesterase: Structure, Function, and Biotechnological Applications. Catalysts 2019, 9, 671. [Google Scholar] [CrossRef]
- Istamboulie, G.; Durbiano, R.; Fournier, D.; Marty, J.L.; Noguer, T. Biosensor-Controlled Degradation of Chlorpyrifos and Chlorfenvinfos Using a Phosphotriesterase-Based Detoxification Column. Chemosphere 2010, 78, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Andrianova, M.S.; Gubanova, O.V.; Komarova, N.V.; Kuznetsov, E.V.; Kuznetsov, A.E. Development of a Biosensor Based on Phosphotriesterase and N-Channel ISFET for Detection of Pesticides. Electroanalysis 2016, 28, 1311–1321. [Google Scholar] [CrossRef]
- Vitola, G.; Mazzei, R.; Fontananova, E.; Porzio, E.; Manco, G.; Gaeta, S.N.; Giorno, L. Polymeric Biocatalytic Membranes with Immobilized Thermostable Phosphotriesterase. J. Memb. Sci. 2016, 516, 144–151. [Google Scholar] [CrossRef]
- Musa, N.; Latip, W.; Salleh, A.; Abd Rahman, R.; Mohamad Ali, M.S. Immobilization of an Antarctic Pseudomonas AMS8 Lipase for Low Temperature Ethyl Hexanoate Synthesis. Catalysts 2018, 8, 234. [Google Scholar] [CrossRef]
- Go, L. Polyelectrolyte Complex Formation Mediated Immobilization of Chitosan-Invertase Neoglycoconjugate on Pectin-Coated Chitin. Bioprocess Biosyst. Eng. 2006, 28, 387–395. [Google Scholar] [CrossRef]
- Boudrant, J.; Woodley, J.M.; Fernandez-lafuente, R. Parameters Necessary to Define an Immobilized Enzyme Preparation. Process Biochem. 2019, 90, 66–80. [Google Scholar] [CrossRef]
- Reis, C.L.B.; Sousa, E.Y.A.D.; Serpa, J.D.F.; Oliveira, R.C.; Santos, J.C.S.D. Design of Immobilized Enzyme Biocatalysts: Drawbacks and Opportunities. Quim. Nov. 2019, 42, 768–783. [Google Scholar] [CrossRef]
- Kaur, K.; Kaushal, P. Enzymes as Analytical Tools for the Assessment of Food Quality and Food Safety; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme Immobilization by Adsorption: A Review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Rath, P.; Tripathi, P. Immobilization of α-Amylase from Germinated Mung Beans (Vigna radiata) on Fuller’s Earth by Adsorption. J. Plant Biochem. Biotechnol. 2012, 21, 229–234. [Google Scholar] [CrossRef]
- Oubagaranadin, J.U.K.; Sathyamurthy, N.; Murthy, Z.V.P. Evaluation of Fuller’s Earth for the Adsorption of Mercury from Aqueous Solutions: A Comparative Study with Activated Carbon. J. Hazard. Mater. 2007, 142, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Roul, A.; Verrier, B.; Gustin, M.P.; Clavaud, E.; Pirot, F.; Falson, F. Comparison of Four Different Fuller’s Earth Formulations in Skin Decontamination. J. Appl. Toxicol. 2017, 37, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Taysse, L.; Daulon, S.; Delamanche, S.; Bellier, B.; Breton, P. Skin Decontamination of Mustards and Organophosphates: Comparative Efficiency of RSDL and Fuller’s Earth in Domestic Swine. Hum. Exp. Toxicol. 2007, 26, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Sharma, N.M.; Kumar, S.; Sawhney, S.K. A Novel Method for the Immobilization of Tyrosinase to Enhance Stability. Biotechnol. Appl. Biochem. 2003, 141, 137–141. [Google Scholar] [CrossRef]
- Hasan, S.; Iasir, A.R.M.; Ghosh, T.K.; Sen Gupta, B.; Prelas, M.A. Characterization and Adsorption Behavior of Strontium from Aqueous Solutions onto Chitosan-Fuller’s Earth Beads. Healthcare 2019, 7, 52. [Google Scholar] [CrossRef]
- Malacas, M.; Balberan, M.C.; Amal, N.; Bederi, J.; Ramos, C.J.; Rato, M.; Salazar, G.; Roque, E. The Removal of Copper (II) and Lead (II) from Aqueous Solution Using Fuller’s e Arth and Fuller’s Earth-Immobilized Nanoscale Zero Valent Iron (FE-NZVI) by Adsorption. MATEC Wed Conf. 2019, 268, 05006. [Google Scholar] [CrossRef][Green Version]
- Tsai, P.; Fox, N.; Bigley, A.N.; Harvey, S.P.; David, P.; Raushel, F.M. Enzymes for the Homeland Defense: Optimizing Phosphotriesterase for the Hydrolysis of Organophosphate Nerve Agents. Biochemistry 2013, 51, 6463–6475. [Google Scholar] [CrossRef] [PubMed]
- Bigley, A.N.; Raushel, F.M. The Evolution of Phosphotriesterase for Decontamination and Detoxification of Organophosphorus Chemical Warfare Agents. Chem. Biol. Interact. 2019, 308, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Seleci, M.; Ag, D.; Yalcinkaya, E.; Demirkol, O. RSC Advances Amine-Intercalated Montmorillonite Matrices for Enzyme Immobilization and Biosensing Applications. RSC Adv. 2012, 2, 2112–2118. [Google Scholar] [CrossRef]
- Suzuki, M.; Matsumoto, Y.; Sekikawa, T. Adsorption and Enzymatic Activity of Immobilized Catalase on Calcined Montmorillonite and Pillared Interlayer Clays. Jpn. J. Water Treat. Biol. 2008, 44, 161–167. [Google Scholar] [CrossRef][Green Version]
- Caldwell, S.R.; Raushel, F.M. Detoxification of Organophosphate Pesticides Using an Immobilized Phosphotriesterase from Pseudomonas diminuta. Biotechnol. Bioeng. 1991, 37, 103–109. [Google Scholar] [CrossRef]
- Chouyyok, W.; Panpranot, J.; Thanachayanant, C.; Prichanont, S. Effects of PH and Pore Characters of Mesoporous Silicas on Horseradish Peroxidase Immobilization. J. Mol. Catal. B Enzym. 2009, 56, 246–252. [Google Scholar] [CrossRef]
- Abada, E.A. Production and Purification of Lipase from Pseudomonas sp. AB2 with Potential Application in Biodiesel Production. J. Pure Appl. Microbilo. 2014, 8, 133–142. [Google Scholar]
- Datta, S.; Christena, L.R. Enzyme Immobilization: An Overview on Techniques and Support Materials. 3 Biotech 2013, 6, 1–9. [Google Scholar] [CrossRef]
- Wang, Q.; Peng, L.; Li, G.; Zhang, P.; Li, D.; Huang, F. Activity of Laccase Immobilized on TiO2− Montmorillonite Complexes. Int. J. Mol. Sci. 2013, 14, 12520–12532. [Google Scholar] [CrossRef]
- Lei, Z.; Jiang, Q. Synthesis and Properties of Immobilized Pectinase onto the Macroporous Polyacrylamide Microspheres. J. Agric. Food Chem. 2011, 59, 2592–2599. [Google Scholar] [CrossRef] [PubMed]
- Jamil, N.; Man, R.C.; Suhaimi, S.; Shaarani, S.; Iffah, Z.; Arshad, M.; Kholijah, S.; Mudalip, A.; Sulaiman, S.Z. Effect of Enzyme Concentration and Temperature on the Immobilization of Cyclodextrin Glucanotransferase (CGTase) on Hollow Fiber Membrane. Mater. Today Proc. 2018, 5, 22036–22042. [Google Scholar] [CrossRef]
- Arana-peña, S.; Rios, N.S.; Carballares, D.; Mendez-sanchez, C.; Fernandez-lafuente, R. Effects of Enzyme Loading and Immobilization Conditions on the Catalytic Features of Lipase From Pseudomonas Fluorescens Immobilized on Octyl-Agarose Beads. Front. Bioeng. Biotechnol. 2020, 8, 36. [Google Scholar] [CrossRef]
- Secundo, F. Conformational Changes of Enzymes upon Immobilisation. Chem. Soc. Rev. 2013, 42, 6250–6261. [Google Scholar] [CrossRef]
- Wong, K.Y.; Gao, J. The Reaction Mechanism of Paraoxon Hydrolysis by Phosphotriesterase from Combined QM/MM Simulations. Biochemistry 2007, 46, 13352–13369. [Google Scholar] [CrossRef]
- Gopinath, S.; Sugunan, S. Enzymes Immobilized on Montmorillonite K 10: Effect of Adsorption and Grafting on the Surface Properties and the Enzyme Activity. Appl. Clay Sci. 2007, 35, 67–75. [Google Scholar] [CrossRef]
- Babavatan, E.O.; Yildirim, D.; Peksel, A.; Binay, B. Immobilization of Rhizomucor miehei Lipase onto Montmorillonite K-10 and Polyvinyl Alcohol Gel. Biocatal. Biotransform. 2019, 38, 274–282. [Google Scholar] [CrossRef]
- Di Russo, N.V.; Estrin, D.A.; Martí, M.A.; Roitberg, A.E. PH-Dependent Conformational Changes in Proteins and Their Effect on Experimental PKas: The Case of Nitrophorin 4. PLoS Comput. Biol. 2012, 8, e1002761. [Google Scholar] [CrossRef] [PubMed]
- Maiangwa, J.; Mohamad Ali, M.S.; Salleh, A.B.; Rahman, R.N.Z.R.A.; Normi, Y.M.; Mohd Shariff, F.; Leow, T.C. Lid Opening and Conformational Stability of T1 Lipase Is Mediated by Increasing Chain Length Polar Solvents. PeerJ 2017, 5, e3341. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Wu, Z.; Huang, R.; Qi, W.; Su, R.; He, Z. Adsorptive Removal of Ni (II) Ions from Aqueous Solution and the Synthesis of a Ni-Doped Ceramic: An Efficient Enzyme Carrier Exhibiting Enhanced Activity of Immobilized Lipase. RSC Adv. 2016, 6, 64581–64588. [Google Scholar] [CrossRef]
- Khrustaleva, T.A. Secondary Structure Preferences of Mn2+ Binding Sites in Bacterial Proteins. Adv. Bioinform. 2014, 2014, 14. [Google Scholar] [CrossRef]
- Rosas, A.; De, M.; Mora, L.; Jara, A.A.; López, R.; Rao, M.A.; Gianfreda, L. Geoderma Catalytic Behaviour of Acid Phosphatase Immobilized on Natural Supports in the Presence of Manganese or Molybdenum. Geoderma 2008, 145, 77–83. [Google Scholar] [CrossRef]
- Haddar, A.; Agrebi, R.; Bougatef, A.; Hmidet, N.; Sellami-Kamoun, A.; Nasri, M. Two Detergent Stable Alkaline Serine-Proteases from Bacillus mojavensis A21: Purification, Characterization and Potential Application as a Laundry Detergent Additive. Bioresour. Technol. 2009, 100, 3366–3373. [Google Scholar] [CrossRef] [PubMed]
- Bussamara, R.; Agnol, L.D.; Schrank, A.; Vainstein, M.H. Optimal Conditions for Continuous Immobilization of Pseudozyma Hubeiensis (Strain HB85A) Lipase by Adsorption in a Packed-Bed Reactor by Response Surface Methodology. Enzym. Res. 2012, 2012, 329178. [Google Scholar] [CrossRef]
- Nooh, H.; Rahman, R.N.; Ali, M.; Masomian, M.; Naganthran, A. Improving the Efficiency of New Automatic Dishwashing Detergent Formulation by Addition of Thermostable Lipase, Protease and Amylase. Molecules 2017, 22, 1577. [Google Scholar] [CrossRef]
- Andrunik, M. Modification of Bentonite with Cationic and Nonionic Surfactants: Structural and Textural Features. Material 2019, 12, 3772. [Google Scholar] [CrossRef] [PubMed]
- Rahman, R.N.Z.R.A.; Salleh, A.B.; Basri, M.; Wong, C.F. Role of α-Helical Structure in Organic Solvent-Activated Homodimer of Elastase Strain, K. Int. J. Mol. Sci. 2011, 12, 5797–5814. [Google Scholar] [CrossRef]
- Latip, W.; Raja Abd Rahman, R.N.Z.; Leow, A.T.C.; Mohd Shariff, F.; Kamarudin, N.H.A.; Mohamad Ali, M.S. The Effect of N-Terminal Domain Removal towards the Biochemical and Structural Features of a Thermotolerant Lipase from an Antarctic Pseudomonas sp. Strain AMS3. Int. J. Mol. Sci. 2018, 19, 560. [Google Scholar] [CrossRef]
- Arifi, F.A.M.; Salleh, A.B.; Abd Rahman, R.N.Z.R.; Leow, A.T.C.; Latip, W.; Ali, M.S.M. Unravelling Protein-Organic Solvent Interaction of Organic Solvent Tolerant Elastase from Pseudomonas aeruginosa Strain K Crystal Structure. Int. J. Biol. Macromol. 2019, 127, 575–584. [Google Scholar] [CrossRef]
- Doukyu, N.; Ogino, H. Organic Solvent-Tolerant Enzymes. Biochem. Eng. J. 2010, 48, 270–282. [Google Scholar] [CrossRef]
- Yaacob, N.; Hafizah, N.; Kamarudin, A.; Thean, A.; Leow, C.; Salleh, A.B.; Noor, R.; Raja, Z.; Rahman, A.; Shukuri, M.; et al. The Role of Solvent-Accessible Leu-208 of Esterification in the Presence of Toluene. Molecules 2017, 22, 1312. [Google Scholar] [CrossRef]
- Hiblot, J.; Gotthard, G.; Chabriere, E.; Elias, M. Characterisation of the Organophosphate Hydrolase Catalytic Activity of SsoPox. Sci. Rep. 2012, 2, 1–8. [Google Scholar] [CrossRef]
- Yan, W.; Liu, D.; Tan, D.; Yuan, P.; Chen, M. FTIR Spectroscopy Study of the Structure Changes of Palygorskite under Heating. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 1052–1057. [Google Scholar] [CrossRef]
- Mardani, T.; Khiabani Sowti, M.; Rezaei, R.; Hamishehkar, H. International Journal of Biological Macromolecules Immobilization of α-Amylase on Chitosan-Montmorillonite Nanocomposite Beads. Int. J. Biol. Macromol. 2018, 120, 354–360. [Google Scholar] [CrossRef]
- Santos, D.I.; Correia, M.J.N.; Mateus, M.M.; Saraiva, J.A.; Vicente, A.A.; Moldão, M. Fourier Transform Infrared (FT-IR) Spectroscopy as a Possible Rapid Tool to Evaluate Abiotic Stress Effects on Pineapple by-Products. Appl. Sci. 2019, 9, 4141. [Google Scholar] [CrossRef]
- Studier, F.W. Protein Production by Auto-Induction in High Density Shaking Cultures. Protein Expr. Purif. 2005, 41, 207–234. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Restaino, O.F.; Borzacchiello, M.G.; Scognamiglio, I.; Fedele, L.; Alfano, A.; Porzio, E.; Manco, G.; De Rosa, M.; Schiraldi, C. High Yield Production and Purification of Two Recombinant Thermostable Phosphotriesterase-like Lactonases from Sulfolobus acidocaldarius and Sulfolobus solfataricus Useful as Bioremediation Tools and Bioscavengers. BMC Biotechnol. 2018, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latip, W.; Knight, V.F.; Khim, O.K.; Mohd Kasim, N.A.; Wan Yunus, W.M.Z.; Mohamad Ali, M.S.; Mohd Noor, S.A. Immobilization of Mutant Phosphotriesterase on Fuller’s Earth Enhanced the Stability of the Enzyme. Catalysts 2021, 11, 983. https://doi.org/10.3390/catal11080983
Latip W, Knight VF, Khim OK, Mohd Kasim NA, Wan Yunus WMZ, Mohamad Ali MS, Mohd Noor SA. Immobilization of Mutant Phosphotriesterase on Fuller’s Earth Enhanced the Stability of the Enzyme. Catalysts. 2021; 11(8):983. https://doi.org/10.3390/catal11080983
Chicago/Turabian StyleLatip, Wahhida, Victor Feizal Knight, Ong Keat Khim, Noor Azilah Mohd Kasim, Wan Md Zin Wan Yunus, Mohd Shukuri Mohamad Ali, and Siti Aminah Mohd Noor. 2021. "Immobilization of Mutant Phosphotriesterase on Fuller’s Earth Enhanced the Stability of the Enzyme" Catalysts 11, no. 8: 983. https://doi.org/10.3390/catal11080983
APA StyleLatip, W., Knight, V. F., Khim, O. K., Mohd Kasim, N. A., Wan Yunus, W. M. Z., Mohamad Ali, M. S., & Mohd Noor, S. A. (2021). Immobilization of Mutant Phosphotriesterase on Fuller’s Earth Enhanced the Stability of the Enzyme. Catalysts, 11(8), 983. https://doi.org/10.3390/catal11080983





