Abstract
Direct electron transfer (DET)-capable oxidoreductases are enzymes that have the ability to transfer/receive electrons directly to/from solid surfaces or nanomaterials, bypassing the need for an additional electron mediator. More than 100 enzymes are known to be capable of working in DET conditions; however, to this day, DET-capable enzymes have been mainly used in designing biofuel cells and biosensors. The rapid advance in (semi) conductive nanomaterial development provided new possibilities to create enzyme-nanoparticle catalysts utilizing properties of DET-capable enzymes and demonstrating catalytic processes never observed before. Briefly, such nanocatalysts combine several cathodic and anodic catalysis performing oxidoreductases into a single nanoparticle surface. Hereby, to the best of our knowledge, we present the first review concerning such nanocatalytic systems involving DET-capable oxidoreductases. We outlook the contemporary applications of DET-capable enzymes, present a principle of operation of nanocatalysts based on DET-capable oxidoreductases, provide a review of state-of-the-art (nano) catalytic systems that have been demonstrated using DET-capable oxidoreductases, and highlight common strategies and challenges that are usually associated with those type catalytic systems. Finally, we end this paper with the concluding discussion, where we present future perspectives and possible research directions.
1. General Overview of the Enzyme-Based Heterogeneous Catalysis
Chemical kinetics without catalysis is like “skiing without skis” [1] and every living system depends on nature’s designed catalysts to make chemical reactions proceed faster. Some enzymes catalyze reactions that otherwise would take years or decades to occur, for example, triose phosphate isomerase [1] or cytochrome c peroxidase [2], which increase the rate of catalyzed chemical processes billion-fold. Besides that, enzymes are also capable of high selectivity (e.g., differentiation between different enantiomeric forms [3,4]) and operation at mild conditions, for example, ambient temperatures and neutral pH [5]. Despite apparent advantages, the enzymatic catalysis approach inheres several flaws: enzymes can be unstable during the catalytic process and thus require stabilization solutions [6], and the enzyme–substrate/product separation comes with difficulties [7]. Various methods have been used to overcome the above-mentioned issues, typically involving the immobilization of enzymes on solid inorganic (e.g., silica, porous glass) and organic (e.g., chitosan, cellulose) carriers [8]. The recent emergence of nanomaterial production techniques has enabled many nanocatalysts-based applications [9]. Many of the newly discovered properties are attributable to their nanometer-range size, such as a very high surface area–volume ratio (>60 m2 cm−3) [10], spectrophotometric properties [11,12] or catalytic capabilities [13]. The catalytic performance of most nanomaterials (e.g., metallic nanoparticles) is also dependent on their physical or chemical parameters [14], and they can readily catalyze oxidation of organic compounds (e.g., carbohydrates) directly on their surface [15], provided they operate in relatively pure solutions—the untreated nanosurfaces are prone to contamination and catalytic passivation when used in media containing organic additives [16,17,18]. It was noticed that nanomaterials can very well, and in some cases even irreversibly, adsorb certain proteins [19,20], even for in vivo applications [21]. It was demonstrated that proteins on the nanoparticle surface form a protein corona—in some cases, the corona forms in less than a minute after exposure—and determine the pathophysiology of certain nanoparticles [22,23]. Moreover, it was also demonstrated that nanomaterial–enzyme conjugates possess certain distinct qualities such as increased activity, stability, as well as separability from reaction media of certain enzymes [24,25]. A number of various catalysts were synthesized using enzymes and nanomaterials for various applications, for example, glucose oxidase (GOx) and urease were immobilized in mesoporous silica nanoparticles in order to control the pH of target medium [26], GOx and peroxidase were immobilized using polystyrene for cascade reaction [27], and organophosphorus hydrolase was immobilized using a metal–organic framework for the degradation of organophosphate nerve agents [28], among other examples [29,30,31]. Additional attention was given to oxidoreductases, especially dehydrogenases and hydrogenases, as possible candidates for (nano) catalytic applications [32]. However, until this day, the majority of direct electron transfer (DET)-capable oxidoreductases were applied mainly for the development of biofuel cells and biosensors [33,34]. The following chapter will focus on reviewing the DET-capable oxidoreductases and their widespread application.
2. Application of Direct Electron Transfer (DET)-Capable Oxidoreductases
To the best of our knowledge, the possibility of DET reactions on solid surfaces (electrodes) was raised first in 1976, when Yaropolov et al. demonstrated a mediated electron transfer (MET) for oxygen reduction [35]. Soon after, Eddowes and Hill demonstrated a DET for a cytochrome c [36] and Kulys et al. showed that DET is possible for cytochrome b2 and peroxidase immobilized together with the organic metals [37]. After DET was achieved for cytochromes c and b2, an effort was undertaken to find more DET-capable enzymes. It was noticed that multifactor enzymes containing cytochromes (e.g., theophylline oxidase, D-fructose dehydrogenase, cellobiose dehydrogenase) can operate by DET [38,39]. Some of the heme-containing enzymes (e.g., cytochrome c peroxidase, horseradish peroxidase, lignin peroxidase, manganese peroxidase) could also transfer electrons directly to the electrode surface [40,41,42]. It was noticed that oxidoreductases containing copper ion-based cofactors (e.g., laccase and bilirubin oxidase) [43], as well as NiFe and FeS hydrogenases [44,45], are also capable of DET. However, the DET phenomenon is not easily implemented—approximately only 100 out of 1700 known oxidoreductases have been shown to operate in DET applications [46,47]. Interestingly, in general, some types of oxidoreductases tend to operate by DET (e.g., metalloenzymes), while the others do not, for example, containing flavin cofactors such as FAD [46], with some exceptions, as reported recently about FAD-dependent glucose dehydrogenases (FAD-GDH) [48]. It was also noticed that, for some FAD-dependent enzymes, the cofactor can be eliminated from the protein active site after immobilization (e.g., native GOx) and exhibit a false impression about the apparent DET [49,50,51]. For a real DET, the enzymes should be treated using more complex procedures to make a redox active site more accessible to the nanostructures of the electrode surface [52]. Those facts demonstrate that, for a successful DET, the enzyme should have distinctive features ensuring the electron transfer capabilities. Moreover, efficient DET, where enzymatic turnover is not limited by the rate of electron transfer, is rarely observed [53], and thus causes additional problems for a fast and sustainable DET-based electrocatalysis. In the next section, we will briefly review the principles of the DET and contemporary application of DET-capable oxidoreductases.
2.1. Principles of the DET Mechanism
Typically, catalysis and successful operation of oxidoreductases require two substrates that are coupled by the oxidation and reduction half-reactions. The enzyme transfers electrons from one of the substrates to another according to the reaction thermodynamics, that is, the electrons are transferred to a compound with higher redox potential [54]. Typically, oxidoreductases reduce co-enzymes (i.e., NAD+, FAD, PQQ) to drive the oxidative reaction [55,56,57]; however, in recent years, artificial electron transfer mediators have also been synthesized and applied as electron accepting/donating substrates [58,59,60]. As illustrated in Figure 1A, for the oxidative process, those electroactive and low molecular mass mediators act as electron transport shuttles from/to the electrode and oxidoreductase and result in MET. In some cases, the MET approach was successfully used for the development of highly sensitive and active biosensors [61,62,63], as well as high current bioanodes for the development of biofuel cells [64,65,66]. However, this approach has disadvantages. First of all, the electronic properties of the redox mediator must be in concert with the ones of biocatalysts, for example, in the case of an oxidative process, the potential of the redox mediator must be more positive than redox potential of the active redox site (or cofactor) of the enzyme to ensure spontaneous MET bioelectrocatalysis [49]. Also, the redox mediators typically leak from the electrode surface to a solution, resulting in decreased catalytic activity of the system [67], with some exceptions [68]. In contrast to MET, DET is a more viable and effective approach, as electrons can be directly transported from/to the redox active site of oxidoreductase to/from the electrode, as demonstrated in Figure 1B. Some advantages of the DET approach have been demonstrated: the oxidation/reduction of the substrate typically occurs at a favorable electrochemical potential, close to the potential of the redox active site of the enzyme; leakage of potentially toxic redox mediator is avoided whatsoever; and the design of the biocatalytic system can be simplified [69,70]. However, DET is usually difficult to achieve, as the typical electron transfer distance is 1.5–2.0 nm [71]. For a longer transfer, the electron should be tunneled, otherwise the rate drops exponentially and is generally not sufficient for observable catalysis [72,73]. For those reasons, the oxidoreductases with redox-active groups close to protein surface tend to operate better when immobilized on proper surfaces. However, the nature of DET is not yet fully understood and is under investigation by many research groups.
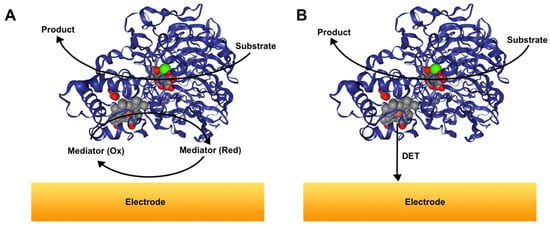
Figure 1.
Principal scheme demonstrating difference between mediated electron transfer (MET)- and direct electron transfer (DET)-based bioelectrocatalysis during the oxidative process at a solid interface (e.g., for alcohol dehydrogenase (ADH), which contains a PQQ catalytic site and heme as an electron transfer site). (A) In MET-based catalysis, a mediator is required to transfer electrons between the oxidoreductase and electrode. (B) In DET-based catalysis, oxidoreductase is capable to transfer electrons directly from/to its active redox center to/from the electrode.
2.2. Development of Biosensors and Biofuel Cells
To this day, the majority of applications of the DET-capable enzymes include the development of third generation biosensors and biofuel cells, as they do not require mediators [41,74,75], can be insensitive to oxygen, and can operate in mass transfer-controlled kinetics [76]. Many biosensors based on DET-capable oxidoreductases were developed for the measurement of glucose, lactose, and lactate. Stoica et al. demonstrated a lactose biosensor with DET-capable cellobiose dehydrogenases from Trametes villosa and Phanerochaete sordida, which were highly sensitive and stable [77]. Successful biosensors for the determination of glucose were produced using DET-capable FAD-GDH, even for continuous glucose monitoring [78,79]. Moreover, some biosensors for the glucose detection were capable of operating at low potentials (e.g., −0.4 V versus standard hydrogen electrode (SHE)), thus reducing the risk of false biosensor response owing to interference [80]. Lactate biosensors based on DET-capable lactate dehydrogenase were demonstrated as well [81]. Quite an interesting application of DET-capable oxidoreductases in biosensing was demonstrated recently by Larpant et al. [82]. In this work, a wireless biosensing application of DET-based oxidoreductase with conductive nanomaterials was demonstrated, and it may open new possibilities for the development of wireless biosensors. Other biosensors based on DET-capable oxidoreductases were demonstrated as well [83,84,85,86,87]. Similarly, DET-capable oxidoreductases were also used in developing third generation bioelectrodes for biofuel cells. For biocathode development, multicopper oxidases (usually laccases and bilirubin oxidases) in a bioelectrochemical system with metallic nanoparticles are the most frequent candidates [88,89]. Multicopper oxidases are attractive because of their capability to catalyze highly exergonic oxygen reduction reaction (E = 0.774 V at pH 7.4 and 37 °C) [90]. One particularly interesting application of DET-capable laccase from Trametes hirsuta was demonstrated by Pita et al. [91]. In this work, the laccase was immobilized on the low density graphite electrode and DET was utilized for the oxygen evolution reaction with low overpotential. Another curious application of multicopper oxidase was recently demonstrated by our group [92]. In this work, laccase from Didymocrea sp. was wired to nanostructured gold nanoparticles via the T2/T3 active center, bypassing the usually intermediary electron-accepting copper center T1. This resulted in the biocathode exhibiting good performance—the system was able to operate in electrolyte solutions at a broad pH range and in the presence of high fluoride concentrations. Bioanodes with DET-capable oxidoreductases were also developed, for example, cellobiose dehydrogenase [93,94], fructose dehydrogenase [95], glucose dehydrogenase [96,97,98], and alcohol dehydrogenases [99,100]. Our group has also been working in this field—recently, we have demonstrated a DET bioanode system with glucose dehydrogenase from Ewingella americana, immobilized using gold nanoparticle and polyaniline nanocomposite, which exhibited exceptional performance towards glucose oxidation and operated in whole human blood [101].
3. Performance of DET-Capable Oxidoreductases at Nanomaterial Interface
As established in previous chapters, direct electron exchange between oxidoreductase’s active center and nanomaterials is the key prerequisite and advantage of the nanocatalysts containing DET-capable oxidoreductases. For those reasons, nanomaterials act not only as binding enzyme carriers, but their surfaces participate in electrocatalysis by transferring electrons from/to specific enzymes. Consequently, catalytic process becomes independent of the second substrate (cofactor) because nanomaterials act as a cofactor by receiving/transferring the electrons from/to the enzyme. As depicted in Figure 2, to this date, two types of nanocatalysts have been created: one incorporating a single enzyme (Figure 2A) and those involving several (Figure 2B). In the case where a single enzyme is used, electrons are transfer from/to the nanomaterial, which in turn removes/provides those electrons by additional process. When two enzymes are used, typically, the second higher redox potential oxidoreductase is added, which catalyzes oxidation of nanoparticle surface by additional process via the DET mechanism. In the next chapter, we will review nanocatalysts already developed and reported in the literature, as well as strategies used in their design.
Figure 2.
A principal scheme, demonstrating nanomaterial-based catalysts (nanocatalysts), containing DET-capable oxidoreductases. In both cases, enzymes are wired to nanomaterial and electron transfer is achieved. (A) Nanocatalysts incorporating a single enzyme. During the target reaction (oxidation), the enzyme catalyzes electron transfer to the nanomaterial surface, which is further oxidized by an additional process (e.g., oxygen reduction reaction (ORR)) (e.g., anodic enzyme is ADH, which contains a PQQ catalytic site and heme as the electron transfer site). (B) Nanocatalysts incorporating several enzymes. During the reaction of interest (oxidation), the enzyme also catalyzes electron transfer to the surface, but here, the surface is oxidized by the DET catalysis facilitated by the second enzyme (e.g., anodic enzyme is ADH, cathodic enzyme is laccase (LAC), which contains T2/T3 catalytic site and T1 as the electron accepting site).
3.1. Design Challenges of Enzyme-Nanoparticle Catalysts Containing DET-Capable Oxidoreductases
The variety of enzyme-nanoparticle systems based on DET-capable oxidoreductases, as described previously, is yet quite limited. To the best of our knowledge, only five research papers have been reported concerning the development of such systems. Those papers have reported immobilization of target enzymes on carbon-based nanomaterials (e.g., conductive graphite particles, carbon nanotubes) or conductive metal nanoparticles (e.g., gold nanoparticles). The enzymes used for the development of catalysts included hydrogenase, nitrate reductase, glucose dehydrogenase, laccase, and CO2 reductase. This section will briefly review those papers in the context of different strategies used to design enzyme-nanoparticle DET catalysts. Also, we will discuss major challenges that may be the limiting factors for the development of those types of nanocatalysts.
3.1.1. Designing Enzyme–Nanoparticle Catalysts with Enzymes Immobilized on Carbon Nanomaterials
One of the most viable and simple approaches to designing an enzyme-based catalytic material is a random immobilization of two different DET-capable oxidoreductases on a conductive carbon nano/micro particle, as carbon is protein-compatible [102,103]. Carbon nanomaterials, for example, graphite particles, were successfully used to wire various enzymes [34,104] and, to the best of our knowledge, for the first time, the concept that involves incorporation of donor and acceptor enzymes was demonstrated by Vincent et al. and was used for the reduction of nitrate (and fumarate) using hydrogen gas [105]. In this work, NiFe hydrogenase from Allochromatium vinosum was wired concordantly with nitrate reductase from Escherichia coli (or fumarate reductase from Escherichia coli) via conductive graphite particles, as demonstrated in Figure 3A. At first, the researchers demonstrated that oxidoreductases are indeed electrochemically active once immobilized on graphite electrodes. From Figure 3B, it is apparent that hydrogenase is electrochemically active and catalyzes oxidation of H2 to H+ at a potential higher than −0.5 V versus SHE. It was also demonstrated that nitrate reductase is also electrochemically active and reduces nitrate to nitrite at the potential lower than 0.1 V versus SHE. The DET ability of both enzymes at the same conditions and the potential difference between anodic and cathodic half-reactions of around 0.5 V has given a reason to wire those enzymes directly using conductive graphite particles (Figure 3C). Graphite particles were treated using diluted solutions of enzymes in a sequence and a biocatalytic process was expected to be according to the following reaction (Equation (1)):
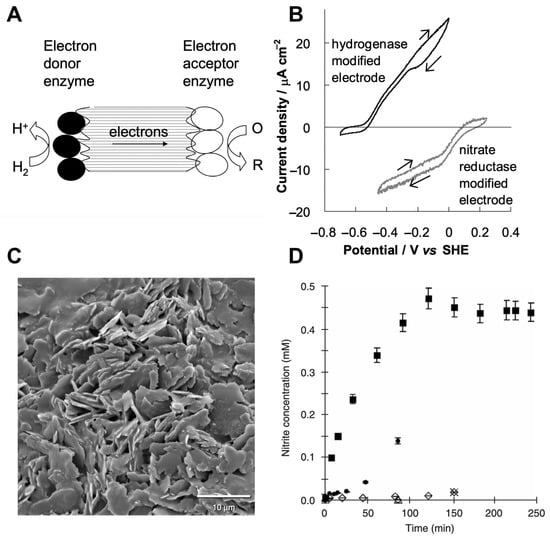
Figure 3.
Hydrogenase and nitrate/fumarate reductase wired on conductive graphite particles. (A) A scheme demonstrating the principle of wiring oxidoreductases catalyzing different redox half-reactions, where electrons are transferred from dihydrogen to nitrate/fumarate catalyzed by hydrogenase and nitrate/fumarate reductase via conducting carbon microparticles. (B) Cyclic voltamperograms demonstrating electrochemical activity of hydrogenase and nitrate reductase immobilized on graphite strip electrodes. (C) Electron microscope image of a cluster of particles immobilized on the adhesive carbon-loaded surface. (D) Graph demonstrating the activity of the designed hydrogenase-nitrate reductase system toward the production of nitrite (■). Adapted with the permission from Vincent et al. [105]. Copyright (2017) Springer Nature. SHE, standard hydrogen electrode.
To provide evidence that the reaction was undergoing according to Equation (1), the authors measured the concentration of nitrite by colorimetry. They demonstrated that, after 90 min, nearly all of the nitrate was converted to nitrite (Figure 3D), indicating that nitrate was directly reduced by H2.
The other catalysts based on carbon nanostructures and hydrogenases were demonstrated by Reeve et al. [106]. In this work, hydrogenase and diaphorase moieties of DET-capable NAD+-reducing soluble hydrogenase from Alcaligenes eutrophus were wired using pyrolytic graphite particles (Figure 4). The practical aim of this work was to create an NAD+/NADH cofactor regeneration system, which is important for a sustainable catalysis [107]. Both moieties were electrochemically active and electrons were transferred from H2 to NAD+, forming NADH.
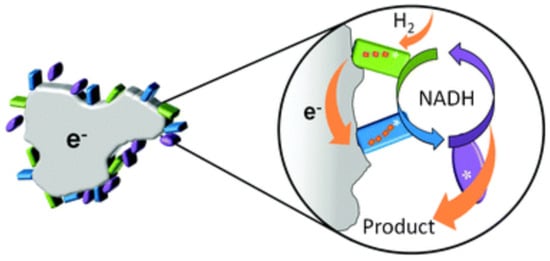
Figure 4.
A principal design scheme of biocatalyst for NADH regeneration. Hydrogenase and diaphorase moieties were wired via graphite particles and the electrons were transferred from H2 to NAD+. Adapted with permission from Reeve et al. [106]. Copyright (2012) Royal Society of Chemistry.
Following previously synthesized and modelled carbon-based bioelectrode designs [108,109], an attempt to wire two DET-capable oxidoreductases was conducted using single-walled carbon nanotubes (SWCNTs) by a study of our group [110]. In this work, a DET-capable glucose dehydrogenase from Ewangella americana and a laccase from Trichaptum abietinum were immobilized on graphite electrode modified with SWCNTs (Figure 5A). It was expected that GDH should oxidize substrate (glucose or lactose) by transferring electrons directly to SWCNT, while laccase should reduce oxygen to water receiving the electrons from SWCNT. Thus, the net reaction could be described as follows (Equation (2)):
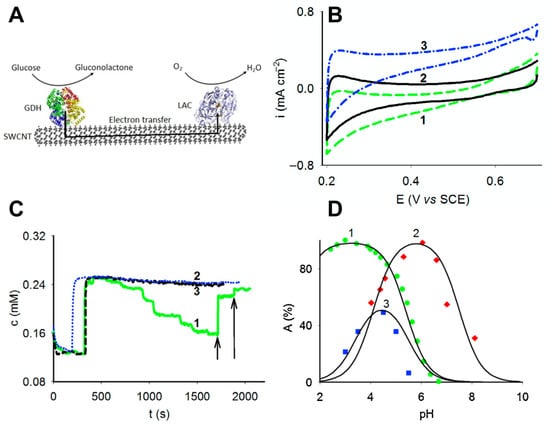
Figure 5.
A mediatorless system composed of glucose dehydrogenase form Ewingella americana and laccase from Trichaptum abietinum. (A) A principal design scheme of nanocatalyst. (B) Cyclic voltamperograms demonstrating electrochemical activity of glucose dehydrogenase and laccase immobilized on graphite electrode. (C) Oxygen consumption measurements of the designed system in the presence of substrate (glucose). (D) Activity dependence on pH of different enzymes (glucose dehydrogenase and laccase) and nanocatalysts. Adapted with permission from Ratautas et al. [110]. Copyright (2015) Elsevier. SWCNT, single-walled carbon nanotube; LAC, laccase; GDH, glucose dehydrogenases; SCE, saturated calomel electrode.
To provide evidence that the catalyzed reaction proceeded according to Equation (2), both enzymes were immobilized on electrode modified with SWCNTs, and electrochemical analysis using cyclic voltammetry was carried out (Figure 5B). It was determined that LAC was electrochemically active on SWCNT and reduced oxygen to water at the potential lower than around 0.5 V versus saturated calomel electrode (SCE). It was also determined that GDH was electrochemically active on the same SWCNT electrode at a potential higher than 0.2 V versus SCE and oxidized glucose. Finally, to provide evidence that the reaction proceeded as suggested, the SWCNT, laccase, and glucose dehydrogenase system was immobilized on the surface of the Clark oxygen electrode and the oxygen concentration in the solution was measured following varying addition of substrate (glucose or lactose). It was demonstrated that the addition of substrate resulted in a direct decrease of oxygen concentration (Figure 5C). Also, the activity dependence on pH was analyzed, and it was determined that the dependence of activity on pH of completed nanocatalytic system was related to the activity dependence on the pH of both enzymes (Figure 5D).
3.1.2. Designing Enzyme–Nanoparticle Catalysts with Enzymes Immobilized on Metallic Nanoparticles
Nanocatalysts containing DET-capable oxidoreductases can be also designed utilizing conductive or semi-conductive particles. It was reported in many previous works that enzymes can easily and, in some cases, almost irreversibly adsorb on metallic nanoparticles [111,112]. One of the most important catalytic reactions involves the generation of CO from CO2 [113]. CO may be readily used for the various synthetic processes for the production of hydrocarbons using Fischer–Tropsch [114] or for the production of acetic acid using Monsanto processes [115]. Though CO2 is generally abundant, it is difficult convert those gases to CO at high yields without many additional byproducts [116]. For this reason, nanocatalytic systems could be designed containing enzyme–metallic nanoparticle conjugates, which could produce CO from CO2. Woolerton et al. demonstrated hybrid nanocatalysts composed of TiO2 particles with immobilized CO2-reducing enzyme (CODH) I from the anaerobic microbe Carboxydothermus hydrogenoformans and photosensitizer (ruthenium bipyridine-based compound) [117]. In this work, CODH catalyzed the reduction of CO2 to CO using MES buffer solution as a sacrificial electron donor when the system was exposed to visible light (Figure 6).

Figure 6.
A principal design scheme of nanocatalyst for CO production. CO2-reducing enzyme (CODH) was immobilized on TiO2 nanoparticles together with photosensitizer (RuP). Electrons were transferred from sacrificial electron donor MES to CODH when the system was affected by visible light. Adapted with permission from Woolerton et al. [117]. Copyright (2010) American Chemical Society.
In this regard, our group has recently published a study, where metallic nanoparticles and DET-capable enzymes were incorporated, producing novel nanocatalysts [118]. In this work, efforts were made to produce a clean and efficient synthetic route towards the production of highly desired and economically valuable aldonic acids (e.g., lactobionic acid) [119]. Glucose dehydrogenase from Ewangella americana was wired directly with the laccase from Didymocrea sp. J6 (Figure 7A). It was expected that two enzymes could transfer electrons directly from one to another via highly conductive gold nanoparticles (AuNPs). Thus, a novel developed catalyst could operate and oxidize carbohydrates to their aldonic acids directly with the molecular oxygen. At first, both enzymes were immobilized on gold nanoparticles and electrochemical analysis was carried out to observe that both enzymes are electrochemically active on the same electrode and receive/transfer electrons by the DET (Figure 7B). Afterwards, nanocatalysts were synthesized and their performance was tested in the cell isolated from the atmosphere by measuring oxygen concentration in a solution (Figure 7C). It was demonstrated that nanocatalysts were capable of oxidizing sugars directly with the molecular oxygen and the kinetic model of nanocatalysis was developed, incorporating the catalytic parameters of both enzymes (Figure 7D).

Figure 7.
Enzyme–nanoparticle (NP) catalysts containing glucose dehydrogenase and laccase immobilized on gold nanoparticles (AuNPs) for the oxidation of carbohydrates with the molecular oxygen. (A) Principal design scheme of nanocatalysts. (B) Cyclic voltamperograms demonstrating electrochemical activity of glucose dehydrogenase and laccase immobilized on gold electrode modified with AuNPs. (C) The activity dependence of the designed nanocatalysts on lactose concentration. (D) Kinetic model on the nanocatalysts’ catalyzed process. Adapted with permission from Ratautas et al. [118]. Copyright (2018) John Wiley and Sons. GDH, glucose dehydrogenase; LAC, laccase.
A follow-up work has been published by our group [120], where a practical improvement of the previously described work has been done using novel conductive nanocomposite particles for the immobilization of enzymes (Figure 8). Those particles were made using Fe3O4 cores covered with a thin layer of gold. The most important advantage over the previous work was the ability to separate nanocatalysts from the reaction mixture using the magnetic field. All catalytic systems involving DET-capable oxidoreductases are summarized in Table 1.

Figure 8.
Enzyme–nanoparticle catalysts containing glucose dehydrogenase and laccase immobilized on Fe3O4 and gold-coated Fe3O4 particles for the oxidation of lactose and glucose with the molecular oxygen. Adapted with permission from Gružauskaitė et al. [120]. Copyright (2019) Elsevier. GDH, glucose dehydrogenase.

Table 1.
A summary of the developed enzymatic (nano)catalysts based on direct electron transfer (DET)-capable oxidoreductases. AuNPs, gold nanoparticles; GDH, glucose dehydrogenase; LAC, laccase; CODH, CO2-reducing enzyme.
3.2. Challenges for Enzyme–Nanoparticle Catalysts Based on DET-Capable Enzymes
In previous chapters, we described the potential benefits of catalytic systems based on DET-capable oxidoreductases; however, there are many unresolved challenges that limit their possible development. Some of them are fundamentally inextricable (e.g., reaction thermodynamics), while the others can be solved by choosing a specific design strategy. Here, based on our previously depicted experience, we will review some of the common challenges that may arise while designing heterogeneous catalytic systems based on DET-capable oxidoreductase, as well as present the possible solutions.
3.2.1. Compatibility of the Oxidoreductase Activity Dependence on pH
To begin with, one of the important requirements for the development of a bienzymatic nanocatalyst is common pH compatibility. Activity dependence on pH is one of the most important properties of oxidoreductase catalysis [121]. Typically, the dependence of activity on pH can be described by three protonated and/or deprotonated enzyme-forms model involving two values of pKa, as described by Equation (3) [122]:
where A is relative activity (0–1), pKa1–pKa of acidic, and pKa2–pKa of basic amino acid residues. As demonstrated in Figure 9A, some oxidoreductases may operate well according to DET, but also could be incompatible for design of the nanocatalysts. Here, the first oxidoreductase has pH optimum of 4.0, while that of the second one is 8.0. In this case, the optimum pH of the designed nanocatalysts involving both enzymes should be around 6.0, as demonstrated by a purple cross section in Figure 9A. However, the designed nanocatalysts may demonstrate a very low activity (less than 10% of the initial activity of separate enzymes). When oxidoreductases with more similar pKa values are used, as demonstrated by Figure 9B,C, the activity of designed (nano) catalysts may be acceptable and even comparable to the activity of the separate enzymes. In the case that enzyme activity depends on a single amino acid residue [122], the model in Equation (3) becomes simpler, yet similar difficulties still exist.
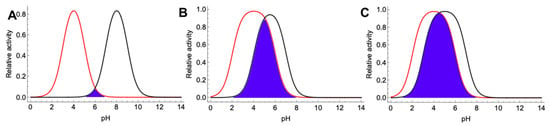
Figure 9.
Theoretical models of an activity dependence on pH of different oxidoreductases. (A) First oxidoreductase (red) has pKa values of 3.0 and 5.0, while those of the second one (black) are 7.0 and 9.0. (B) First oxidoreductase (red) has pKa values of 2.0 and 5.0, while those of the second one (black) are 4.0 and 7.0. (C) First oxidoreductase (red) has pKa values of 2.0 and 6.0, while those of the second one (black) are 3.0 and 7.0. Purple cross section demonstrates the theoretical activity dependence on pH of the (nano)catalytic system composed of those oxidoreductases.
Thus, it is important, when designing enzymatic nanocatalysts, to choose “pH compatible” enzymes, to ensure maximal activity of the newly formed nanocatalysts. For example, Ratautas et al. [118] and Gružauskaitė et al. [120] used GDH and LAC, which had similar activity dependence on pH (Figure 5D). Thus, newly formed catalysts were relatively active in a solution that had a pH value relatively suitable for both oxidoreductases. In the case in which it is not possible to use enzymes, which have similar activity dependence on pH, several methods could be used to engineer a more suitable environment for a catalytic process. The first method involves the production of similar oxidoreductase, which has different activity dependence on pH. It could be achieved by modifying the growth conditions for the microorganism, which was used for the production of the enzyme [123]. As many enzymatic reactions either generate or consume protons, the other method could be the utilization of pH-altering reaction, near the target oxidoreductase. It has been demonstrated previously that the usage of pH-altering reactions could have a substantial effect on apparent enzymatic activity dependence on pH for heterogeneous catalysis [124]. Finally, it has been reported that enzymatic activity dependence on pH during heterogeneous catalysis could be altered by rational engineering of the surface charge of the enzyme carrier [125]. However, we would like to point out that all of the above-mentioned approaches could affect the DET capability of the oxidoreductase, and thus should be used with additional care.
3.2.2. Compatibility of the Operational Electrochemical Potential of Enzymes
The second requirement for the nanocatalysts combining two redox reactions is a redox potential compatibility. This requirement is related to the potential difference and the thermodynamics of different half-reactions. Briefly, the redox potential of the reduction half-reaction must be more positive than the oxidation half-reaction for a process to occur [90,126]. The standard redox potentials at pH 7.0 and 25 °C for some catalytically relevant half-reactions are given in Figure 10 [90,126].
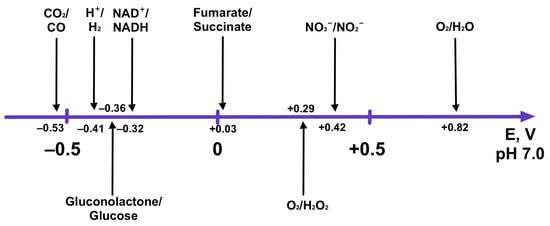
Figure 10.
The standard reduction potentials of some catalytically relevant reactions at pH 7.0 and 25 °C.
From Figure 10, one can see the importance of oxygen reduction reaction (ORR) [127]. Because oxygen reduction has a very high thermodynamic potential at pH 7.0, this process is sufficient to drive many other catalytically relevant reactions, and thus it comes as a no surprise why multicopper oxidases are usually used for biocathodes in biofuel cells. In all of the reviewed works (Table 1), the bienzymatic nanocatalysts were demonstrated to catalyze highly exergonic electrochemical reactions when the standard electrochemical potential reached more than 0.5 V (with the exception in the work reported by Reeve et al. [106]). However, the reaction thermodynamics is not the only factor determining the possibility of a catalytic process. Owing to electron transfer inefficiency, in some cases, enzymes require a significant overpotential for a reaction to occur [128]. Even the most effective bioanodes and biocathodes need at least 0.1–0.2 V of overpotential for a reaction to occur [92] and, in some cases, the overpotential may be as high as 0.8 V [129], making the development of sustainable catalysts a challenge. In recent works, it was demonstrated that overpotential of oxidoreductase catalyzed half-reaction could be reduced by a rational immobilization of the enzymes [130]. We would like to note that significant attention should be given to a highly important hydrogen evolution reaction [131] using glucose both as a reducer and proton source for hydrogen evolution reaction (HER) (Equation (4)):
Even though the standard potential for this reaction (E0 at pH 7.0) is equal to −0.05 V, this reaction becomes thermodynamically possible, with the E value higher than 0 V when glucose concentration is kept significantly higher than gluconolactone or when the produced hydrogen gas is constantly removed from the solution, that is, p(H2) is kept very low. However, to this day, no one successfully demonstrated enzymatic (nano) catalysts capable of producing hydrogen from carbohydrates and HER using carbohydrates has been demonstrated using H2 producing bacteria cultures only [132]. If the reaction is thermodynamically possible (i.e., E ≥ 0 at reaction conditions), the potential incompatibility could be solved using various methods, which improves the DET contact between oxidoreductase and nanomaterial, as a fast DET usually results in low overpotential [97,100]. The first approach could be a rational engineering of the enzyme to produce novel enzyme homologues, which could operate in DET reactions faster or with lower overpotentials. For example, this approach was successfully used to produce a downsized variant of D-fructose dehydrogenase with favorable DET properties when compared with the native enzyme [133]. The genetically engineered enzyme homologue catalyzed DET-based oxidation of D-fructose more efficiently, and operational overpotential was lowered by around 0.2 V. The second way to improve DET between oxidoreductase and nanomaterials and to overcome redox potential incompatibility is the immobilization of enzymes on specifically modified nanomaterial surface. It is known that the efficiency of the DET and an overpotential of the enzyme catalyzed reaction depends on the surface charge of the nanomaterial [134]. For example, our group demonstrated that the modification of AuNPs using positive charge inducing molecules (e.g., 4-aminothiophenol, cysteamine) results in a significantly improved DET for GDH and overpotential decrease in 0.3 V [97]. However, when several enzymes are used to design the nanocatalysts, it may be difficult to use specific surface modifications—one surface modification may increase the efficiency of DET for one enzyme, but decrease it for another. A possible solution may come from recent advances in a nanomaterial development approach known as Janus nanomaterials [135]. Those types of nanomaterials emerged recently and are important for the unique property—one side of the nanomaterial can be modified using one type of compound and the other using different compounds (e.g., alkyne and azide [136]). As a result, those particles could be selectively modified to ensure the most effective DET of different types of oxidoreductases. Thus, when designing nanocatalysts, attention should be given for immobilization techniques of oxidoreductases and nanomaterial surface charge to overcome electrochemical potential-related issues.
4. Concluding Discussion and Future Perspectives
The DET-capable oxidoreductases are highly important enzymes for the ability to conduct electron transfer with nanomaterials. However, to this day, the application of those enzymes is mainly involved in the development of biofuel cells and biosensors. Recently, a new application possibility to develop nanocatalysts, by incorporating anodic and cathodic reaction DET-capable enzymes on a single conductive nanoparticle, was demonstrated. In the reviewed papers, researchers managed to wire hydrogenase, nitrate/fumarate reductase, glucose dehydrogenase, and laccase oxidoreductases using various nanostructured materials. It resulted in the production of novel nanocatalysts that were able to catalyze interesting and highly desired reactions, that is, NAD+ regeneration to NADH using hydrogen gas as a reducer, the production of lactobionic acid from lactose using dioxygen as an oxidizer, and so on. Those and similar reactions are quite difficult and/or expensive to achieve using standard methods, thus the development of those type of nanocatalysts opens new possibilities for nanomaterial-based catalysis. On the other hand, the developed nanocatalytic systems at this moment have significant flaws: some are sensitive to oxygen, while others have low activity, stability, or use quite expensive enzymes. Some of those disadvantages come from the reason that the idea of wiring DET-capable oxidoreductases via conductive nanomaterials is relatively young. We can expect that improvements to the current nanocatalytic biotechnologies will be made in the future.
The possible research directions involve the further development of nanocatalysts involving DET-capable oxidoreductases, and there are many directions. Significant attention should be given to designing nanocatalysts for hydrogen evolution reaction using abundant carbohydrates as an electron source for H+ reduction. Another research direction could involve the development of enzymatic cascade reactions, where several DET-capable oxidoreductases are immobilized on nanomaterials and produce desired products from abundant substrates. In addition, new robotic nanomaterials (e.g., nanodiodes) could be developed. At this moment, the transfer of electrons via conductive nanoparticles does not produce any work and the energy related to the electrochemical reactions is lost to the surroundings as heat. We envision that this energy could be utilized to perform work or to generate light, as demonstrated in Figure 11. Here, electrons from one DET-capable enzyme are transferred via semi-conductive quantum dot to the other, and the electrochemical potential is used to produce light. Although designing the system demonstrated in Figure 11 would constitute a significant technological challenge, it is doable in principle. To conclude, we believe that DET-capable oxidoreductases should be used for more than just in biofuel cells and biosensors—the nanocatalysts designed with those enzymes could open new roads for future electrocatalysis as well semi-conductive technologies.
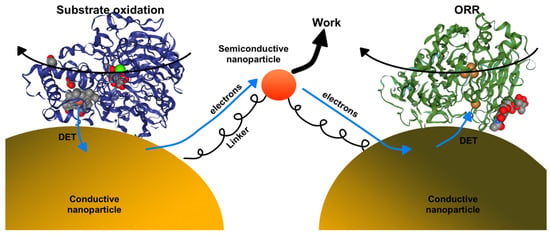
Figure 11.
The conceptual proposition of two DET-capable oxidoreductases immobilized on different conductive nanoparticles and linked via semi-conductive nanoparticle. Electron flow from one enzyme to another is used to carry out work (e.g., emit light).
Author Contributions
Conceptualization, D.R. and M.D.; methodology, D.R. and M.D.; software, D.R.; validation, D.R.; formal analysis, D.R.; investigation, D.R. and M.D.; resources, D.R.; data curation, D.R; writing—original draft preparation, D.R.; writing—review and editing, D.R. and M.D.; visualization, D.R.; supervision, D.R. and M.D.; project administration, D.R. and M.D.; funding acquisition, M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ADH | Alcohol dehydrogenase |
| AuNP(s) | Gold nanoparticle(s) |
| CODH | CO2-reducing enzyme |
| DET | Direct electron transfer |
| FAD-GDH | FAD-depended glucose dehydrogenase |
| GDH | Glucose dehydrogenase |
| GOx | Glucose oxidase |
| HER | Hydrogen evolution reaction |
| LAC | Laccase |
| MET | Mediated electron transfer |
| ORR | Oxygen reduction reaction |
| SCE | Saturated calomel electrode (0.244 V vs. SHE) |
| SHE | Standard hydrogen electrode |
| SWCNT | Single-walled carbon nanotubes |
References
- Albery, W.J.; Knowles, J.R. Efficiency and Evolution of Enzyme Catalysis. Angew. Chem. Int. Ed. Engl. 1977, 16, 285–293. [Google Scholar] [CrossRef]
- Northrup, S.H.; Reynolds, J.C.L.; Miller, C.M.; Forrest, K.J.; Boles, J.O. Diffusion-controlled association rate of cytochrome c and cytochrome c peroxidase in a simple electrostatic model. J. Am. Chem. Soc. 1986, 108, 8162–8170. [Google Scholar] [CrossRef]
- Reetz, M.T.; Wiesenhöfer, W.; Franciò, G.; Leitner, W. Continuous Flow Enzymatic Kinetic Resolution and Enantiomer Separation using Ionic Liquid/Supercritical Carbon Dioxide Media. Adv. Synth. Catal. 2003, 345, 1221–1228. [Google Scholar] [CrossRef]
- Bodnár, J.; Gubicza, L.; Szabó, L.P. Enantiomeric separation of 2-chloropropionic acid by enzymatic esterification in organic solvents. J. Mol. Catal. 1990, 61, 353–361. [Google Scholar] [CrossRef]
- Cornish-Bowden, A. Fundamentals of Enzyme Kinetics; Elsevier Science: 2014; Wiley-Blackwell: Weinheim, Germany, 2012; ISBN 978-1-322-33429-5. [Google Scholar]
- Iyer, P.V.; Ananthanarayan, L. Enzyme stability and stabilization—Aqueous and non-aqueous environment. Process Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- Larsson, K.M.; Adlercreutz, P.; Mattiasson, B.; Olsson, U. Enzymatic catalysis in microemulsions: Enzyme reuse and product recovery. Biotechnol. Bioeng. 1990, 36, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, H.; Campbell, C.T. Nanoparticles for Catalysis. Acc. Chem. Res. 2013, 46, 1671–1672. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Semmler-Behnke, M.; Chaudhry, Q. A complementary definition of nanomaterial. Nano Today 2010, 5, 165–168. [Google Scholar] [CrossRef]
- Uddin, J. Macro to Nano Spectroscopy; Intech: Rijeka, Croatia, 2012; ISBN 978-953-51-0664-7. [Google Scholar]
- Gruskiene, R.; Krivorotova, T.; Staneviciene, R.; Ratautas, D.; Serviene, E.; Sereikaite, J. Preparation and characterization of iron oxide magnetic nanoparticles functionalized by nisin. Colloids Surf. B Biointerfaces 2018, 169, 126–134. [Google Scholar] [CrossRef]
- Sharma, N.; Ojha, H.; Bharadwaj, A.; Pathak, D.P.; Sharma, R.K. Preparation and catalytic applications of nanomaterials: A review. RSC Adv. 2015, 5, 53381–53403. [Google Scholar] [CrossRef]
- Lin, C.; Compton, R.G. Size Effects in Nanoparticle Catalysis at Nanoparticle Modified Electrodes: The Interplay of Diffusion and Chemical Reactions. J. Phys. Chem. C 2017, 121, 2521–2528. [Google Scholar] [CrossRef]
- Luo, W.; Zhu, C.; Su, S.; Li, D.; He, Y.; Huang, Q.; Fan, C. Self-Catalyzed, Self-Limiting Growth of Glucose Oxidase-Mimicking Gold Nanoparticles. ACS Nano 2010, 4, 7451–7458. [Google Scholar] [CrossRef] [PubMed]
- Lee, H. Utilization of shape-controlled nanoparticles as catalysts with enhanced activity and selectivity. RSC Adv. 2014, 4, 41017–41027. [Google Scholar] [CrossRef]
- Tominaga, M.; Shimazoe, T.; Nagashima, M.; Taniguchi, I. Electrocatalytic oxidation of glucose at gold nanoparticle-modified carbon electrodes in alkaline and neutral solutions. Electrochem. Commun. 2005, 7, 189–193. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, N.; Yu, H.; Niu, Y.; Sun, C. Covalent attachment of glucose oxidase to an Au electrode modified with gold nanoparticles for use as glucose biosensor. Bioelectrochemistry 2005, 67, 15–22. [Google Scholar] [CrossRef]
- Lynch, I.; Dawson, K.A. Protein-nanoparticle interactions. Nano Today 2008, 3, 40–47. [Google Scholar] [CrossRef]
- Cagliani, R.; Gatto, F.; Bardi, G. Protein Adsorption: A Feasible Method for Nanoparticle Functionalization? Materials 2019, 12, 1991. [Google Scholar] [CrossRef]
- Barbero, F.; Russo, L.; Vitali, M.; Piella, J.; Salvo, I.; Borrajo, M.L.; Busquets-Fité, M.; Grandori, R.; Bastús, N.G.; Casals, E.; et al. Formation of the Protein Corona: The Interface between Nanoparticles and the Immune System. Semin. Immunol. 2017, 34, 52–60. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef]
- Vilanova, O.; Mittag, J.J.; Kelly, P.M.; Milani, S.; Dawson, K.A.; Rädler, J.O.; Franzese, G. Understanding the Kinetics of Protein-Nanoparticle Corona Formation. ACS Nano 2016, 10, 10842–10850. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zeng, G.; Xu, P.; Lai, C.; Tang, L. How Do Enzymes ‘Meet’ Nanoparticles and Nanomaterials? Trends Biochem. Sci. 2017, 42, 914–930. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.J.; Russ Algar, W.; Malanoski, A.P.; Ancona, M.G.; Medintz, I.L. Understanding enzymatic acceleration at nanoparticle interfaces: Approaches and challenges. Nano Today 2014, 9, 102–131. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, Y.; Ran, X.; Ren, J.; Qu, X. A semipermeable enzymatic nanoreactor as an efficient modulator for reversible pH regulation. Nanoscale 2014, 6, 11328–11335. [Google Scholar] [CrossRef]
- Delaittre, G.; Reynhout, I.; Cornelissen, J.J.L.M.; Nolte, R.J.M. Cascade Reactions in an All-Enzyme Nanoreactor. Chem. A Eur. J. 2009, 15, 12600–12603. [Google Scholar] [CrossRef]
- Li, H.; Ma, L.; Zhou, L.; Gao, J.; Huang, Z.; He, Y.; Jiang, Y. An integrated nanocatalyst combining enzymatic and metal-organic framework catalysts for cascade degradation of organophosphate nerve agents. Chem. Commun. 2018, 54, 10754–10757. [Google Scholar] [CrossRef]
- Aquino Neto, S.; Almeida, T.S.; Palma, L.M.; Minteer, S.D.; de Andrade, A.R. Hybrid nanocatalysts containing enzymes and metallic nanoparticles for ethanol/O2 biofuel cell. J. Power Sources 2014, 259, 25–32. [Google Scholar] [CrossRef]
- Parolo, C.; de la Escosura-Muñiz, A.; Merkoçi, A. Enhanced lateral flow immunoassay using gold nanoparticles loaded with enzymes. Biosens. Bioelectron. 2013, 40, 412–416. [Google Scholar] [CrossRef]
- Phadtare, S.; Kumar, A.; Vinod, V.P.; Dash, C.; Palaskar, D.V.; Rao, M.; Shukla, P.G.; Sivaram, S.; Sastry, M. Direct Assembly of Gold Nanoparticle “Shells” on Polyurethane Microsphere “Cores” and Their Application as Enzyme Immobilization Templates. Chem. Mater. 2003, 15, 1944–1949. [Google Scholar] [CrossRef]
- May, S.W. Applications of oxidoreductases. Curr. Opin. Biotechnol. 1999, 10, 370–375. [Google Scholar] [CrossRef]
- Razumiene, J.; Barkauskas, J.; Kubilius, V.; Meskys, R.; Laurinavicius, V. Modified graphitized carbon black as transducing material for reagentless H2O2 and enzyme sensors. Talanta 2005, 67, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Razumiene, J.; Vilkanauskyte, A.; Gureviciene, V.; Barkauskas, J.; Meskys, R.; Laurinavicius, V. Direct electron transfer between PQQ dependent glucose dehydrogenases and carbon electrodes: An approach for electrochemical biosensors. Electrochim. Acta 2006, 51, 5150–5156. [Google Scholar] [CrossRef]
- Yaropolov, A.I.; Varfolomeev, S.D.; Berezin, I.V. Bioelectrocatalysis. Activation of a cathode oxygen reduction in the peroxidase-mediator carbon electrode system. FEBS Lett. 1976, 71, 306–308. [Google Scholar] [CrossRef][Green Version]
- Eddowes, M.J.; Hill, H.A.O. Novel method for the investigation of the electrochemistry of metalloproteins: Cytochrome c. J. Chem. Soc. Chem. Commun. 1977, 771b. [Google Scholar] [CrossRef]
- Kulys, J.J.; Švirmickas, G.J.S. Reagentless lactate sensor based on cytochrome b2. Anal. Chim. Acta 1980, 117, 115–120. [Google Scholar] [CrossRef]
- Ferapontova, E.E.; Gorton, L. Direct electrochemistry of heme multicofactor-containing enzymes on alkanethiol-modified gold electrodes. Bioelectrochemistry 2005, 66, 55–63. [Google Scholar] [CrossRef]
- Sakinyte, I.; Barkauskas, J.; Gaidukevic, J.; Razumiene, J. Thermally reduced graphene oxide: The study and use for reagentless amperometric d-fructose biosensors. Talanta 2015, 144, 1096–1103. [Google Scholar] [CrossRef]
- Christenson, A.; Dimcheva, N.; Ferapontova, E.E.; Gorton, L.; Ruzgas, T.; Stoica, L.; Shleev, S.; Yaropolov, A.I.; Haltrich, D.; Thorneley, R.N.F.; et al. Direct Electron Transfer Between Ligninolytic Redox Enzymes and Electrodes. Electroanalysis 2004, 16, 1074–1092. [Google Scholar] [CrossRef]
- Gorton, L.; Lindgren, A.; Larsson, T.; Munteanu, F.D.; Ruzgas, T.; Gazaryan, I. Direct electron transfer between heme-containing enzymes and electrodes as basis for third generation biosensors. Anal. Chim. Acta 1999, 400, 91–108. [Google Scholar] [CrossRef]
- Razumiene, J.; Niculescu, M.; Ramanavicius, A.; Laurinavicius, V.; Csöregi, E. Direct bioelectrocatalysis at carbon electrodes modified with quinohemoprotein alcohol dehydrogenase from Gluconobacter sp. 33. Electroanalysis 2002, 14, 43–49. [Google Scholar] [CrossRef]
- Sakurai, T.; Kataoka, K. Basic and applied features of multicopper oxidases, CueO, bilirubin oxidase, and laccase. Chem. Rec. 2007, 7, 220–229. [Google Scholar] [CrossRef]
- Lojou, É.; Luo, X.; Brugna, M.; Candoni, N.; Dementin, S.; Giudici-Orticoni, M.T. Biocatalysts for fuel cells: Efficient hydrogenase orientation for H2 oxidation at electrodes modified with carbon nanotubes. JBIC J. Biol. Inorg. Chem. 2008, 13, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Lubitz, W.; Ogata, H.; Rüdiger, O.; Reijerse, E. Hydrogenases. Chem. Rev. 2014, 114, 4081–4148. [Google Scholar] [CrossRef] [PubMed]
- Shleev, S.; Andoralov, V.; Pankratov, D.; Falk, M.; Aleksejeva, O.; Blum, Z. Oxygen Electroreduction versus Bioelectroreduction: Direct Electron Transfer Approach. Electroanalysis 2016, 28, 2270–2287. [Google Scholar] [CrossRef]
- Ruzgas, T.; Larpant, N.; Shafaat, A.; Sotres, J. Wireless, Battery-Less Biosensors Based on Direct Electron Transfer Reactions. ChemElectroChem 2019, 6, 5167–5171. [Google Scholar] [CrossRef]
- Okuda-Shimazaki, J.; Yoshida, H.; Sode, K. FAD dependent glucose dehydrogenases—Discovery and engineering of representative glucose sensing enzymes-. Bioelectrochemistry 2019, 107414. [Google Scholar] [CrossRef]
- Milton, R.D.; Minteer, S.D. Direct enzymatic bioelectrocatalysis: Differentiating between myth and reality. J. R. Soc. Interface 2017, 14, 20170253. [Google Scholar] [CrossRef]
- Wilson, G.S. Native glucose oxidase does not undergo direct electron transfer. Biosens. Bioelectron. 2016, 82, vii–viii. [Google Scholar] [CrossRef]
- Bartlett, P.N.; Al-Lolage, F.A. There is no evidence to support literature claims of direct electron transfer (DET) for native glucose oxidase (GOx) at carbon nanotubes or graphene. J. Electroanal. Chem. 2018, 819, 26–37. [Google Scholar] [CrossRef]
- Courjean, O.; Gao, F.; Mano, N. Deglycosylation of Glucose Oxidase for Direct and Efficient Glucose Electrooxidation on a Glassy Carbon Electrode. Angew. Chem. Int. Ed. 2009, 48, 5897–5899. [Google Scholar] [CrossRef]
- Ma, S.; Ludwig, R. Direct Electron Transfer of Enzymes Facilitated by Cytochromes. ChemElectroChem 2019, 6, 958–975. [Google Scholar] [CrossRef]
- Rottenberg, H. The Thermodynamic Description of Enzyme-Catalyzed Reactions. Biophys. J. 1973, 13, 503–511. [Google Scholar] [CrossRef]
- Gallop, P.M.; Paz, M.A.; Flückiger, R.; Kagan, H.M. PQQ, the elusive coenzyme. Trends Biochem. Sci. 1989, 14, 343–346. [Google Scholar] [CrossRef]
- Walsh, C. Flavin coenzymes: At the crossroads of biological redox chemistry. Acc. Chem. Res. 1980, 13, 148–155. [Google Scholar] [CrossRef]
- Saleh, F.S.; Rahman, M.R.; Okajima, T.; Mao, L.; Ohsaka, T. Determination of formal potential of NADH/NAD+ redox couple and catalytic oxidation of NADH using poly(phenosafranin)-modified carbon electrodes. Bioelectrochemistry 2011, 80, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Ansell, R.J.; Lowe, C.R. Artificial redox coenzymes: Biomimetic analogues of NAD+. Appl. Microbiol. Biotechnol. 1999, 51, 703–710. [Google Scholar] [CrossRef]
- Vaitkutė, G.; Bratkovskaja, I.; Časaitė, V.; Stankevičiūtė, J.; Meškys, R.; Tetianec, L. Electron transfer mediators for PQQ dependent soluble glucose dehydrogenase catalyzed lactose oxidation reaction. Chemija 2019, 30, 194–200. [Google Scholar] [CrossRef]
- Tetianec, L.; Chaleckaja, A.; Kulys, J.; Janciene, R.; Marcinkeviciene, L.; Meskiene, R.; Stankeviciute, J.; Meskys, R. Characterization of methylated azopyridine as a potential electron transfer mediator for electroenzymatic systems. Process Biochem. 2017, 54, 41–48. [Google Scholar] [CrossRef]
- Ramonas, E.; Ratautas, D.; Dagys, M.; Meškys, R.; Kulys, J. Highly sensitive amperometric biosensor based on alcohol dehydrogenase for determination of glycerol in human urine. Talanta 2019, 200, 333–339. [Google Scholar] [CrossRef]
- Bartlett, P.N.; Bradford, V.Q.; Whitaker, R.G. Enzyme electrode studies of glucose oxidase modified with a redox mediator. Talanta 1991, 38, 57–63. [Google Scholar] [CrossRef]
- Besharati, M.; Hamedi, J.; Hosseinkhani, S.; Saber, R. A novel electrochemical biosensor based on TetX2 monooxygenase immobilized on a nano-porous glassy carbon electrode for tetracycline residue detection. Bioelectrochemistry 2019, 128, 66–73. [Google Scholar] [CrossRef]
- Tsujimura, S.; Murata, K.; Akatsuka, W. Exceptionally High Glucose Current on a Hierarchically Structured Porous Carbon Electrode with “Wired” Flavin Adenine Dinucleotide-Dependent Glucose Dehydrogenase. J. Am. Chem. Soc. 2014, 136, 14432–14437. [Google Scholar] [CrossRef] [PubMed]
- Meredith, M.T.; Kao, D.Y.; Hickey, D.; Schmidtke, D.W.; Glatzhofer, D.T. High Current Density Ferrocene-Modified Linear Poly(ethylenimine) Bioanodes and Their Use in Biofuel Cells. J. Electrochem. Soc. 2011, 158, B166–B174. [Google Scholar] [CrossRef]
- Shao, M.; Zafar, M.N.; Falk, M.; Ludwig, R.; Sygmund, C.; Peterbauer, C.K.; Guschin, D.A.; MacAodha, D.; Ó Conghaile, P.; Leech, D.; et al. Optimization of a Membraneless Glucose/Oxygen Enzymatic Fuel Cell Based on a Bioanode with High Coulombic Efficiency and Current Density. ChemPhysChem 2013, 14, 2260–2269. [Google Scholar] [CrossRef] [PubMed]
- Schuhmann, W. Functionalized polypyrrole. A new material for the construction of biosensors. Synth. Met. 1991, 41, 429–432. [Google Scholar] [CrossRef]
- Schuhmann, W. Non-leaking amperometric biosensors based on high-molecular ferrocene derivatives. Biosens. Bioelectron. 1993, 8, 191–196. [Google Scholar] [CrossRef]
- Freire, R.S.; Pessoa, C.A.; Mello, L.D.; Kubota, L.T. Direct electron transfer: An approach for electrochemical biosensors with higher selectivity and sensitivity. J. Braz. Chem. Soc. 2003, 14, 230–243. [Google Scholar] [CrossRef]
- Bollella, P.; Gorton, L.; Antiochia, R. Direct Electron Transfer of Dehydrogenases for Development of 3rd Generation Biosensors and Enzymatic Fuel Cells. Sensors 2018, 18, 1319. [Google Scholar] [CrossRef]
- Hush, N.S. Distance Dependence of Electron Transfer Rates. Coord. Chem. Rev. 1985, 64, 135–157. [Google Scholar] [CrossRef]
- Gray, H.B.; Winkler, J.R. Electron Transfer in Proteins. Annu. Rev. Biochem. 1996, 65, 537–561. [Google Scholar] [CrossRef]
- Gray, H.B.; Winkler, J.R. Long-range electron transfer. Proc. Natl. Acad. Sci. USA 2005, 102, 3534–3539. [Google Scholar] [CrossRef]
- Zhang, W.; Li, G. Third-Generation Biosensors Based on the Direct Electron Transfer of Proteins. Anal. Sci. 2004, 20, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Falk, M.; Blum, Z.; Shleev, S. Direct electron transfer based enzymatic fuel cells. Electrochim. Acta 2012, 82, 191–202. [Google Scholar] [CrossRef]
- Koopal, C.G.J.; Nolte, R.J.M. Kinetic study of the performance of third-generation biosensors. Bioelectrochem. Bioenerg. 1994, 33, 45–53. [Google Scholar] [CrossRef]
- Stoica, L.; Ludwig, R.; Haltrich, D.; Gorton, L. Third-Generation Biosensor for Lactose Based on Newly Discovered Cellobiose Dehydrogenase. Anal. Chem. 2006, 78, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Loew, N.; Tsugawa, W.; Ikebukuro, K.; Sode, K. Development of a third-generation glucose sensor based on the open circuit potential for continuous glucose monitoring. Biosens. Bioelectron. 2019, 124–125, 216–223. [Google Scholar] [PubMed]
- Ito, Y.; Okuda-Shimazaki, J.; Tsugawa, W.; Loew, N.; Shitanda, I.; Lin, C.E.; La Belle, J.; Sode, K. Third generation impedimetric sensor employing direct electron transfer type glucose dehydrogenase. Biosens. Bioelectron. 2019, 129, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Liu, Y.; Xu, Y.; Shi, L.; Peng, J.; Jin, W. In-situ fabrication of well-distributed gold nanocubes on thiol graphene as a third-generation biosensor for ultrasensitive glucose detection. Electrochim. Acta 2015, 176, 162–171. [Google Scholar] [CrossRef]
- Treu, B.L.; Minteer, S.D. Isolation and purification of PQQ-dependent lactate dehydrogenase from Gluconobacter and use for direct electron transfer at carbon and gold electrodes. Bioelectrochemistry 2008, 74, 73–77. [Google Scholar] [CrossRef]
- Larpant, N.; Pham, A.D.; Shafaat, A.; Gonzalez-Martinez, J.F.; Sotres, J.; Sjöholm, J.; Laiwattanapaisal, W.; Faridbod, F.; Ganjali, M.R.; Arnebrant, T.; et al. Sensing by wireless reading Ag/AgCl redox conversion on RFID tag: Universal, battery-less biosensor design. Sci. Rep. 2019, 9, 12948. [Google Scholar] [CrossRef]
- Musameh, M.M.; Dunn, C.J.; Uddin, M.H.; Sutherland, T.D.; Rapson, T.D. Silk provides a new avenue for third generation biosensors: Sensitive, selective and stable electrochemical detection of nitric oxide. Biosens. Bioelectron. 2018, 103, 26–31. [Google Scholar] [CrossRef]
- Das, P.; Das, M.; Chinnadayyala, S.R.; Singha, I.M.; Goswami, P. Recent advances on developing 3rd generation enzyme electrode for biosensor applications. Biosens. Bioelectron. 2016, 79, 386–397. [Google Scholar] [CrossRef]
- Díaz Nieto, C.H.; Granero, A.M.; Lopez, J.C.; Pierini, G.D.; Levin, G.J.; Fernández, H.; Zon, M.A. Development of a third generation biosensor to determine hydrogen peroxide based on a composite of soybean peroxidase/chemically reduced graphene oxide deposited on glassy carbon electrodes. Sens. Actuators B Chem. 2018, 263, 377–386. [Google Scholar] [CrossRef]
- Ghosh, T.; Sarkar, P.; Turner, A.P.F. A novel third generation uric acid biosensor using uricase electro-activated with ferrocene on a Nafion coated glassy carbon electrode. Bioelectrochemistry 2015, 102, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Sanchez, C.; Shleev, S.; De Lacey, A.L.; Pita, M. Third-generation oxygen amperometric biosensor based on Trametes hirsuta laccase covalently bound to graphite electrode. Chem. Pap. 2015, 69, 237–240. [Google Scholar] [CrossRef][Green Version]
- Dagys, M.; Haberska, K.; Shleev, S.; Arnebrant, T.; Kulys, J.; Ruzgas, T. Laccase-gold nanoparticle assisted bioelectrocatalytic reduction of oxygen. Electrochem. Commun. 2010, 12, 933–935. [Google Scholar] [CrossRef]
- Gutiérrez-Sánchez, C.; Pita, M.; Vaz-Domínguez, C.; Shleev, S.; De Lacey, A.L. Gold Nanoparticles as Electronic Bridges for Laccase-Based Biocathodes. J. Am. Chem. Soc. 2012, 134, 17212–17220. [Google Scholar] [CrossRef]
- Shleev, S. Quo Vadis, Implanted Fuel Cell? ChemPlusChem 2017, 82, 522–539. [Google Scholar] [CrossRef]
- Pita, M.; Mate, D.M.; Gonzalez-Perez, D.; Shleev, S.; Fernandez, V.M.; Alcalde, M.; De Lacey, A.L. Bioelectrochemical Oxidation of Water. J. Am. Chem. Soc. 2014, 136, 5892–5895. [Google Scholar] [CrossRef]
- Dagys, M.; Laurynėnas, A.; Ratautas, D.; Kulys, J.; Vidžiūnaitė, R.; Talaikis, M.; Niaura, G.; Marcinkevičienė, L.; Meškys, R.; Shleev, S. Oxygen electroreduction catalysed by laccase wired to gold nanoparticles via the trinuclear copper cluster. Energy Environ. Sci. 2017, 10, 498–502. [Google Scholar] [CrossRef]
- Tasca, F.; Gorton, L.; Harreither, W.; Haltrich, D.; Ludwig, R.; Nöll, G. Direct Electron Transfer at Cellobiose Dehydrogenase Modified Anodes for Biofuel Cells. J. Phys. Chem. C 2008, 112, 9956–9961. [Google Scholar] [CrossRef]
- Ludwig, R.; Harreither, W.; Tasca, F.; Gorton, L. Cellobiose Dehydrogenase: A Versatile Catalyst for Electrochemical Applications. ChemPhysChem 2010, 11, 2674–2697. [Google Scholar] [CrossRef]
- Bollella, P.; Hibino, Y.; Kano, K.; Gorton, L.; Antiochia, R. Enhanced Direct Electron Transfer of Fructose Dehydrogenase Rationally Immobilized on a 2-Aminoanthracene Diazonium Cation Grafted Single-Walled Carbon Nanotube Based Electrode. ACS Catal. 2018, 8, 10279–10289. [Google Scholar] [CrossRef]
- Flexer, V.; Durand, F.; Tsujimura, S.; Mano, N. Efficient Direct Electron Transfer of PQQ-glucose Dehydrogenase on Carbon Cryogel Electrodes at Neutral pH. Anal. Chem. 2011, 83, 5721–5727. [Google Scholar] [CrossRef] [PubMed]
- Ratautas, D.; Laurynėnas, A.; Dagys, M.; Marcinkevičienė, L.; Meškys, R.; Kulys, J. High current, low redox potential mediatorless bioanode based on gold nanoparticles and glucose dehydrogenase from Ewingella Americana. Electrochim. Acta 2016, 199, 254–260. [Google Scholar] [CrossRef]
- Muguruma, H.; Iwasa, H.; Hidaka, H.; Hiratsuka, A.; Uzawa, H. Mediatorless Direct Electron Transfer between Flavin Adenine Dinucleotide-Dependent Glucose Dehydrogenase and Single-Walled Carbon Nanotubes. ACS Catal. 2017, 7, 725–734. [Google Scholar] [CrossRef]
- Aquino Neto, S.; Suda, E.L.; Xu, S.; Meredith, M.T.; De Andrade, A.R.; Minteer, S.D. Direct electron transfer-based bioanodes for ethanol biofuel cells using PQQ-dependent alcohol and aldehyde dehydrogenases. Electrochim. Acta 2013, 87, 323–329. [Google Scholar] [CrossRef]
- Ratautas, D.; Tetianec, L.; Marcinkevičienė, L.; Meškys, R.; Kulys, J. Bioanode with alcohol dehydrogenase undergoing a direct electron transfer on functionalized gold nanoparticles for an application in biofuel cells for glycerol conversion. Biosens. Bioelectron. 2017, 98, 215–221. [Google Scholar] [CrossRef]
- Gineitytė, J.; Meškys, R.; Dagys, M.; Ratautas, D. Highly efficient direct electron transfer bioanode containing glucose dehydrogenase operating in human blood. J. Power Sources 2019, 441, 227163. [Google Scholar] [CrossRef]
- Vaitheeswaran, S.; Garcia, A.E. Protein stability at a carbon nanotube interface. J. Chem. Phys. 2011, 134, 125101. [Google Scholar] [CrossRef]
- Razumiene, J.; Sakinyte, I.; Barkauskas, J.; Baronas, R. Nano-structured carbon materials for improved biosensing applications. Appl. Surf. Sci. 2015, 334, 185–191. [Google Scholar] [CrossRef]
- Setkus, A.; Galdikas, A.; Laurinavicius, V.; Meskys, R.; Mironas, A.; Razumiene, J. Electric charge transport in the symmetric metal-enzyme junctions affected by biochemical interaction. Colloids Surf. A Physicochem. Eng. Asp. 2004, 249, 141–143. [Google Scholar]
- Vincent, K.A.; Li, X.; Blanford, C.F.; Belsey, N.A.; Weiner, J.H.; Armstrong, F.A. Enzymatic catalysis on conducting graphite particles. Nat. Chem. Biol. 2007, 3, 761–762. [Google Scholar] [CrossRef]
- Reeve, H.A.; Lauterbach, L.; Ash, P.A.; Lenz, O.; Vincent, K.A. A modular system for regeneration of NADcofactors using graphite particles modified with hydrogenase and diaphorase moieties. Chem. Commun. 2012, 48, 1589–1591. [Google Scholar] [CrossRef] [PubMed]
- Kragl, U.; Kruse, W.; Hummel, W.; Wandrey, C. Enzyme engineering aspects of biocatalysis: Cofactor regeneration as example. Biotechnol. Bioeng. 2000, 52, 309–319. [Google Scholar] [CrossRef]
- Baronas, R.; Kulys, J.; Petrauskas, K.; Razumiene, J. Modelling carbon nanotubes-based mediatorless biosensor. Sensors (Switzerland) 2012, 12, 9146–9160. [Google Scholar] [CrossRef] [PubMed]
- Razumiene, J.; Gureviciene, V.; Sakinyte, I.; Barkauskas, J.; Petrauskas, K.; Baronas, R. Modified SWCNTs for Reagentless Glucose Biosensor: Electrochemical and Mathematical Characterization. Electroanalysis 2013, 25, 166–173. [Google Scholar] [CrossRef]
- Ratautas, D.; Marcinkevičienė, L.; Meškys, R.; Kulys, J. Mediatorless electron transfer in glucose dehydrogenase/laccase system adsorbed on carbon nanotubes. Electrochim. Acta 2015, 174, 940–944. [Google Scholar] [CrossRef]
- Casals, E.; Pfaller, T.; Duschl, A.; Oostingh, G.J.; Puntes, V. Time Evolution of the Nanoparticle Protein Corona. ACS Nano 2010, 4, 3623–3632. [Google Scholar] [CrossRef]
- Galdikas, A.; Bukauskas, V.; Kaciulis, S.; Laurinavicius, V.; Meskys, R.; Mezzi, A.; Mironas, A.; Razumiene, J.; Setkus, A. Properties of the planar ADH-dry-layer structures based on electrically controlled coupling between enzyme molecules and metal surfaces. Sens. Actuators B Chem. 2006, 118, 60–66. [Google Scholar] [CrossRef]
- Agarwal, J.; Fujita, E.; Schaefer, H.F.; Muckerman, J.T. Mechanisms for CO Production from CO2 Using Reduced Rhenium Tricarbonyl Catalysts. J. Am. Chem. Soc. 2012, 134, 5180–5186. [Google Scholar] [CrossRef]
- Schulz, H. Short history and present trends of Fischer-Tropsch synthesis. Appl. Catal. A Gen. 1999, 186, 3–12. [Google Scholar] [CrossRef]
- Yoneda, N.; Kusano, S.; Yasui, M.; Pujado, P.; Wilcher, S. Recent advances in processes and catalysts for the production of acetic acid. Appl. Catal. A Gen. 2001, 221, 253–265. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Balamurugan, M.; Choutipalli, V.S.K.; Jeong, E.; Subramanian, V.; Sim, U.; Nam, K.T. Achieving highly efficient CO2 to CO electroreduction exceeding 300 mA cm−2 with single-atom nickel electrocatalysts. J. Mater. Chem. A 2019, 7, 10651–10661. [Google Scholar] [CrossRef]
- Woolerton, T.W.; Sheard, S.; Reisner, E.; Pierce, E.; Ragsdale, S.W.; Armstrong, F.A. Efficient and Clean Photoreduction of CO2 to CO by Enzyme-Modified TiO2 Nanoparticles Using Visible Light. J. Am. Chem. Soc. 2010, 132, 2132–2133. [Google Scholar] [CrossRef]
- Ratautas, D.; Ramonas, E.; Marcinkevičienė, L.; Meškys, R.; Kulys, J. Wiring Gold Nanoparticles and Redox Enzymes: A Self-Sufficient Nanocatalyst for the Direct Oxidation of Carbohydrates with Molecular Oxygen. ChemCatChem 2018, 10, 971–974. [Google Scholar] [CrossRef]
- Gutiérrez, L.F.; Hamoudi, S.; Belkacemi, K. Lactobionic acid: A high value-added lactose derivative for food and pharmaceutical applications. Int. Dairy J. 2012, 26, 103–111. [Google Scholar] [CrossRef]
- Gružauskaitė, J.; Jasinskaitė, J.; Meškys, R.; Gaidamavičienė, G.; Žalga, A.; Laurynėnas, A.; Tetianec, L.; Dagys, M. Gold-coated magnetic nanocatalyst containing wired oxidoreductases for mediatorless catalysis of carbohydrate oxidation by oxygen. Catal. Commun. 2020, 135, 105848. [Google Scholar] [CrossRef]
- Thomas, P.G.; Russell, A.J.; Fersht, A.R. Tailoring the pH dependence of enzyme catalysis using protein engineering. Nature 1985, 318, 375–376. [Google Scholar] [CrossRef]
- Marangoni, A.G. Enzyme Kinetics: A Modern Approach; Wiley-Interscience: Hoboken, NJ, USA, 2003; ISBN 978-0-471-15985-8. [Google Scholar]
- McDermid, A.S.; McKee, A.S.; Marsh, P.D. Effect of environmental pH on enzyme activity and growth of Bacteroides gingivalis W50. Infect. Immun. 1988, 56, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.E.; Chow, M.T.C. Immobilized enzyme catalysis with reaction-generated pH change. Biotechnol. Bioeng. 1974, 16, 1345–1357. [Google Scholar] [CrossRef]
- Russell, A.J.; Fersht, A.R. Rational modification of enzyme catalysis by engineering surface charge. Nature 1987, 328, 496–500. [Google Scholar] [CrossRef]
- Reeve, H.A.; Ash, P.A.; Park, H.; Huang, A.; Posidias, M.; Tomlinson, C.; Lenz, O.; Vincent, K.A. Enzymes as modular catalysts for redox half-reactions in H2-powered chemical synthesis: From biology to technology. Biochem. J. 2017, 474, 215–230. [Google Scholar] [CrossRef]
- Song, C.; Zhang, J. Electrocatalytic Oxygen Reduction Reaction. In PEM Fuel Cell Electrocatalysts and Catalyst Layers; Zhang, J., Ed.; Springer: London, UK, 2008; pp. 89–134. ISBN 978-1-84800-935-6. [Google Scholar]
- Armstrong, F.A.; Hirst, J. Reversibility and efficiency in electrocatalytic energy conversion and lessons from enzymes. Proc. Natl. Acad. Sci. USA 2011, 108, 14049–14054. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Patolsky, F.; Katz, E.; Hainfeld, J.F.; Willner, I. “Plugging into Enzymes”: Nanowiring of redox enzymes by a gold nanoparticle. Science 2003, 299, 1877–1881. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, O.; Pasi, M.; Dandela, R.; Meijler, M.M.; Alfonta, L. Electron transfer rate analysis of a site-specifically wired copper oxidase. Phys. Chem. Chem. Phys. 2018, 20, 6159–6166. [Google Scholar] [CrossRef] [PubMed]
- Zeradjanin, A.R.; Grote, J.P.; Polymeros, G.; Mayrhofer, K.J.J. A Critical Review on Hydrogen Evolution Electrocatalysis: Re-exploring the Volcano-relationship. Electroanalysis 2016, 28, 2256–2269. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, W.Q.; Guo, X.C.; Ren, H.Y.; Wu, J.T.; Cao, G.L.; Wang, A.J.; Ren, N.Q. Continuous hydrogen production from glucose/xylose by an anaerobic sequential batch reactor to maximize the energy recovery efficiency. RSC Adv. 2018, 8, 20712–20718. [Google Scholar] [CrossRef]
- Kaida, Y.; Hibino, Y.; Kitazumi, Y.; Shirai, O.; Kano, K. Ultimate downsizing of D-fructose dehydrogenase for improving the performance of direct electron transfer-type bioelectrocatalysis. Electrochem. Commun. 2019, 98, 101–105. [Google Scholar] [CrossRef]
- Wang, G.X.; Wang, M.; Wu, Z.Q.; Bao, W.J.; Zhou, Y.; Xia, X.H. Dependence of the direct electron transfer activity and adsorption kinetics of cytochrome c on interfacial charge properties. Analyst 2013, 138, 5777. [Google Scholar] [CrossRef]
- Lattuada, M.; Hatton, T.A. Synthesis, properties and applications of Janus nanoparticles. Nano Today 2011, 6, 286–308. [Google Scholar] [CrossRef]
- Staff, R.H.; Willersinn, J.; Musyanovych, A.; Landfester, K.; Crespy, D. Janus nanoparticles with both faces selectively functionalized for click chemistry. Polym. Chem. 2014, 5, 4097. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).