Progress and Trend on the Regulation Methods for Nanozyme Activity and Its Application
Abstract
1. Introduction
2. Types and Classification of Nanozymes
3. Regulation Strategy of Nanozyme Activity
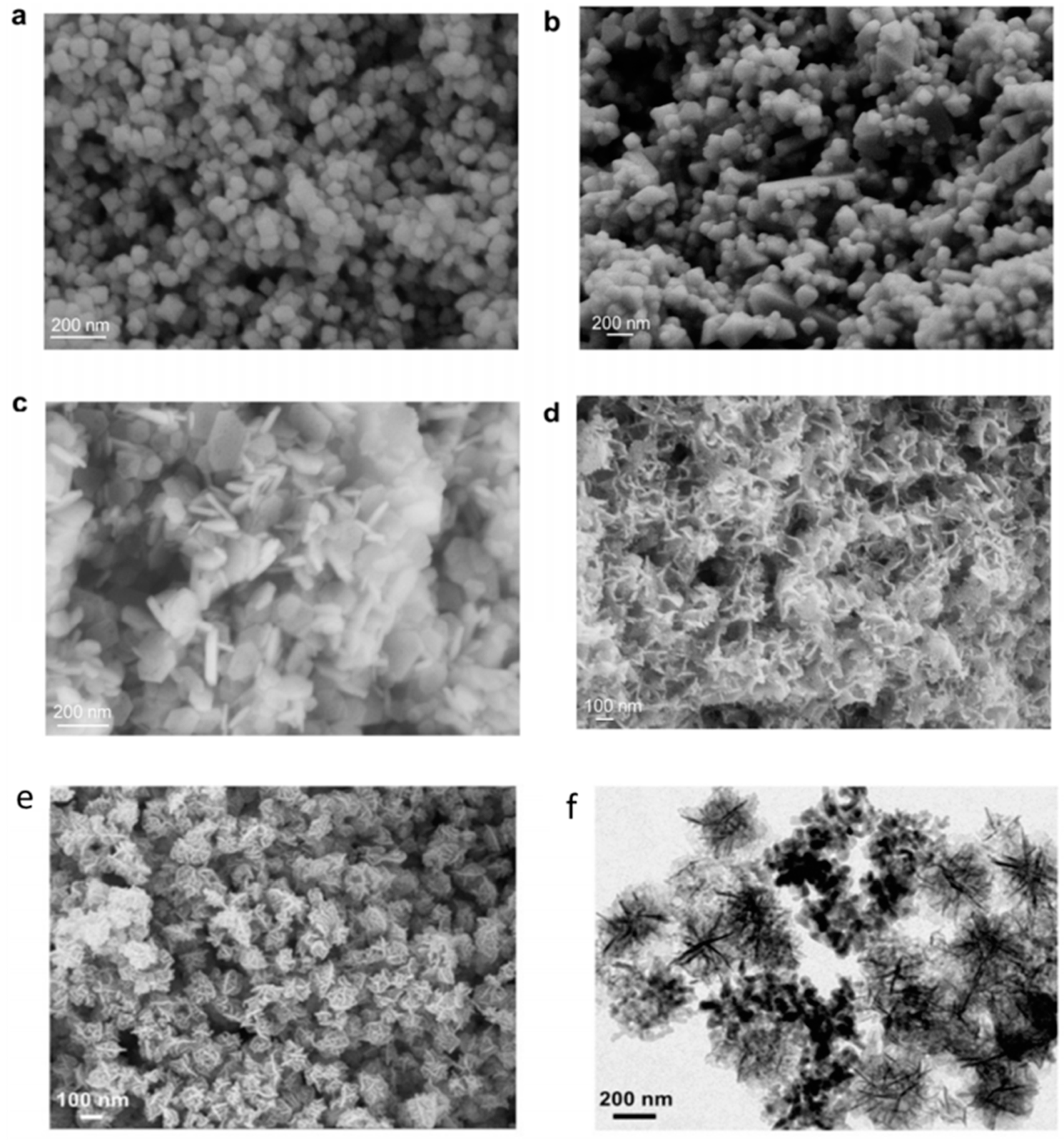
3.1. Size
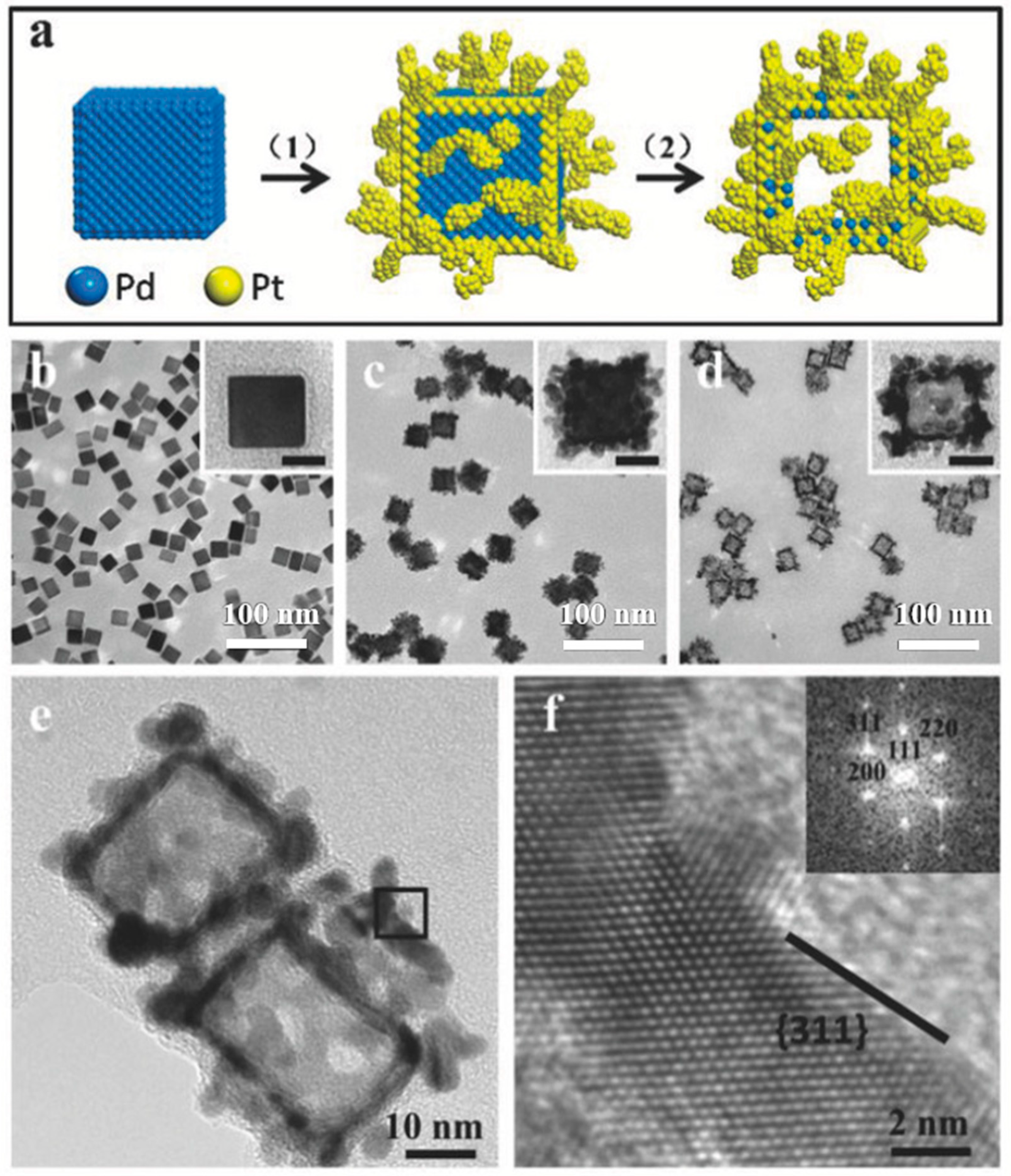
3.2. Morphology
3.3. Composition
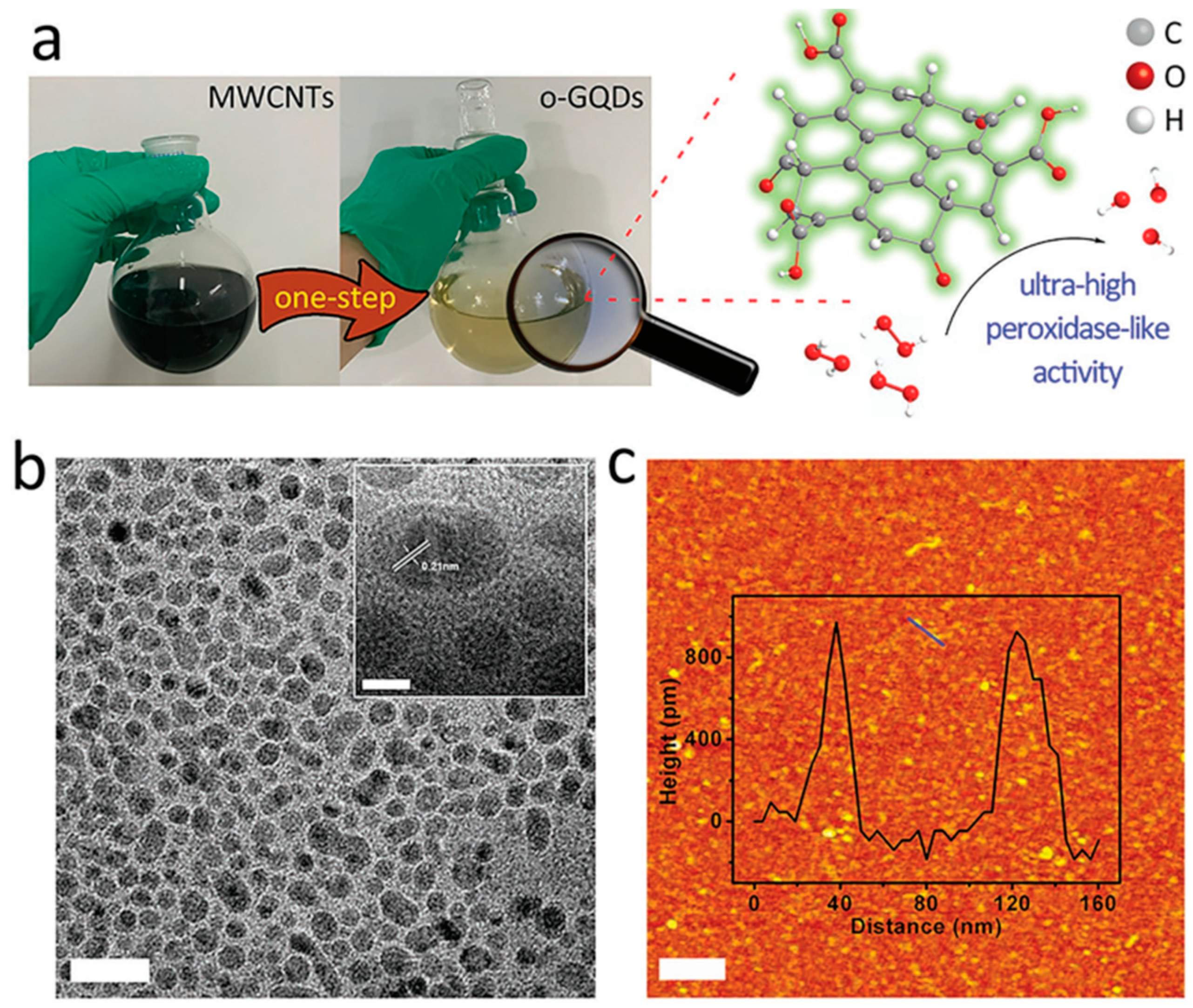
3.4. Surface Modification
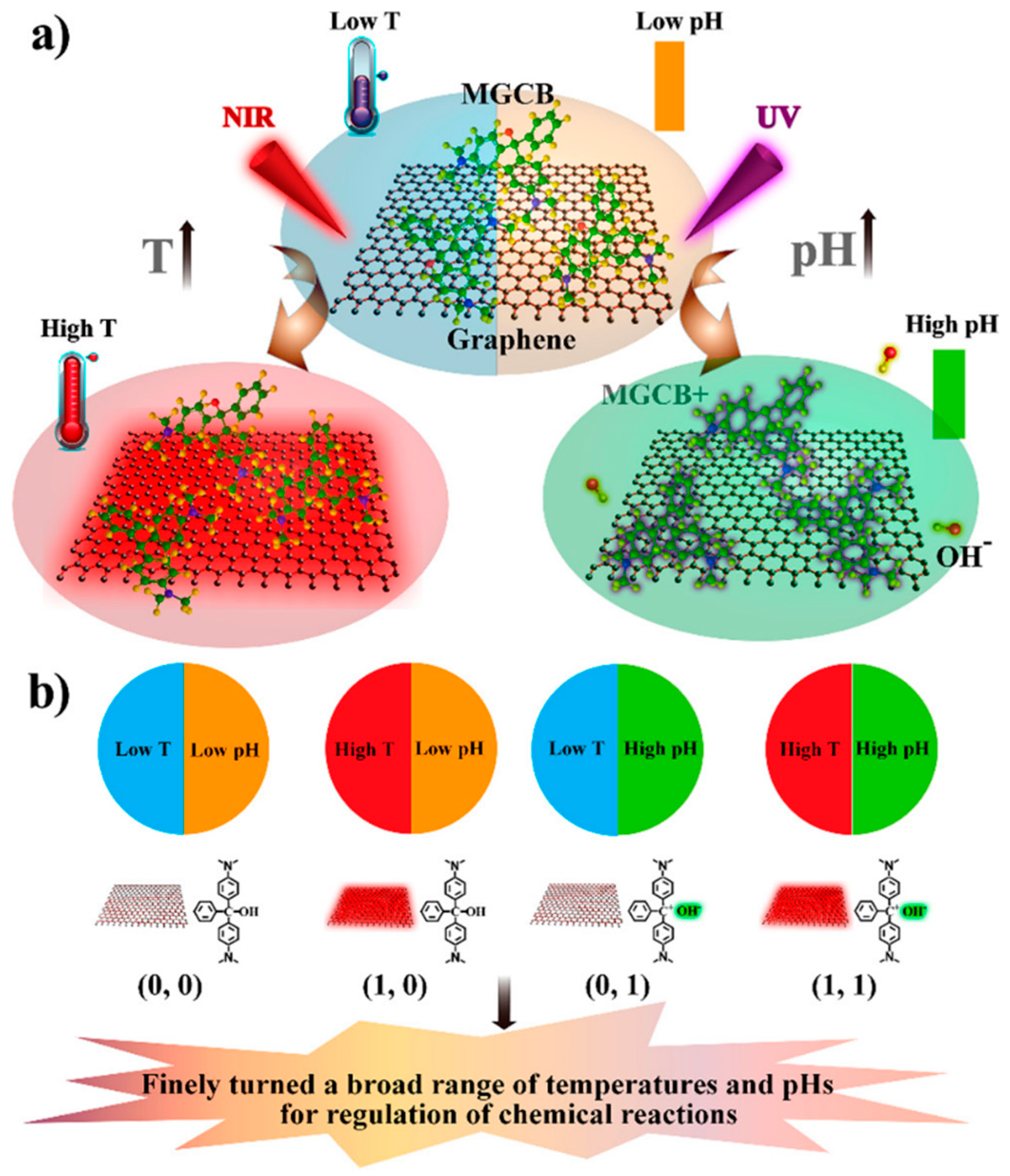
3.5. pH and Temperature
3.6. Activators and Inhibitors
4. Applications of Nanozymes
4.1. Nanozymes in Antibacteria for Topical Application
4.2. Nanozymes in Hazardous Degradation
4.3. Nanozymes in Sensing
4.4. Nanozymes in Cancer Therapy
5. Summary and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Breslow, R. Artificial enzymes; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 1–35. [Google Scholar]
- Breslow, R.; Overman, L.E. “Artificial enzyme” combining a metal catalytic group and a hydrophobic binding cavity. J. Am. Chem. Soc. 1970, 92, 1075–1077. [Google Scholar] [CrossRef]
- Xiong, X.; Huang, Y.; Lin, C.; Liu, X.Y.; Lin, Y. Recent advances in nanoparticulate biomimetic catalysts for combating bacteria and biofilms. Nanoscale 2019, 11, 22206–22215. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Wei, H. Nanozymes in bionanotechnology: From sensing to therapeutics and beyond. Inorg. Chem. Front. 2016, 3, 41–60. [Google Scholar] [CrossRef]
- Manea, F.; Houillon, F.B.; Pasquato, L.; Scrimin, P. Nanozymes: Gold-nanoparticle-based transphosphorylation catalysts. Angew. Chem. Int. Ed. 2004, 43, 6165–6169. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, H.; Shi, W.; Liu, H.; Huang, Y. Fe–Co bimetallic alloy nanoparticles as a highly active peroxidase mimetic and its application in biosensing. Chem. Commun. 2013, 49, 5013–5015. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Q.; Long, Y.; Cheng, Z.; Chen, S.; Zheng, H.; Huang, Y. Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem. Commun. 2011, 47, 6695–6697. [Google Scholar] [CrossRef]
- Long, Y.J.; Li, Y.F.; Liu, Y.; Zheng, J.J.; Tang, J.; Huang, C.Z. Visual observation of the mercury-stimulated peroxidase mimetic activity of gold nanoparticles. Chem. Commun. 2011, 47, 11939–11941. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.; Wang, L.; Luo, Y.; Sun, X. A general strategy for the production of photoluminescent carbon nitride dots from organic amines and their application as novel peroxidase-like catalysts for colorimetric detection of H2O2 and glucose. Rsc Adv. 2012, 2, 411–413. [Google Scholar] [CrossRef]
- Nirala, N.R.; Abraham, S.; Kumar, V.; Bansal, A.; Srivastava, A.; Saxena, P.S. Colorimetric detection of cholesterol based on highly efficient peroxidase mimetic activity of graphene quantum dots. Sens. Actuators B Chem. 2015, 218, 42–50. [Google Scholar] [CrossRef]
- Wang, H.; Liu, C.; Liu, Z.; Ren, J.; Qu, X. Specific oxygenated groups enriched graphene quantum dots as highly efficient enzyme mimics. Small 2018, 14, 1703710. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, M.; Zhao, J.; Peng, X.; Chen, X.; Mi, N.; Yin, B.; Li, H.; Zhang, Y.; Yao, S. Water-dispersible silicon dots as a peroxidase mimetic for the highly-sensitive colorimetric detection of glucose. Chem. Commun. 2014, 50, 6771–6774. [Google Scholar] [CrossRef]
- Lin, Y.; Ren, J.; Qu, X. Nano-gold as artificial enzymes: Hidden talents. Adv. Mater. 2014, 26, 4200–4217. [Google Scholar] [CrossRef]
- Shen, X.; Liu, W.; Gao, X.; Lu, Z.; Wu, X.; Gao, X. Mechanisms of oxidase and superoxide dismutation-like activities of gold, silver, platinum, and palladium, and their alloys: A general way to the activation of molecular oxygen. J. Am. Chem. Soc. 2015, 137, 15882–15891. [Google Scholar] [CrossRef]
- Yu, C.J.; Chen, T.H.; Jiang, J.Y.; Tseng, W.L. Lysozyme-directed synthesis of platinum nanoclusters as a mimic oxidase. Nanoscale 2014, 6, 9618–9624. [Google Scholar] [CrossRef]
- Luo, W.; Zhu, C.; Su, S.; Li, D.; He, Y.; Huang, Q.; Fan, C. Self-catalyzed, self-limiting growth of glucose oxidase-mimicking gold nanoparticles. ACS Nano 2010, 4, 7451–7458. [Google Scholar] [CrossRef]
- Natalio, F.; André, R.; Hartog, A.F.; Stoll, B.; Jochum, K.P.; Wever, R.; Tremel, W. Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation. Nat. Nanotechnol. 2012, 7, 530–535. [Google Scholar] [CrossRef]
- Tian, Z.; Li, J.; Zhang, Z.; Gao, W.; Zhou, X.; Qu, Y. Highly sensitive and robust peroxidase-like activity of porous nanorods of ceria and their application for breast cancer detection. Biomaterials 2015, 59, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Korschelt, K.; Schwidetzky, R.; Pfitzner, F.; Strugatchi, J.; Schilling, C.; von der Au, M.; Kirchhoff, K.; Panthöfer, M.; Lieberwirth, I.; Tahir, M. CeO2−x nanorods with intrinsic urease-like activity. Nanoscale 2018, 10, 13074–13082. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, W.; Wu, X.; Gao, X. Mechanism of pH-switchable peroxidase and catalase-like activities of gold, silver, platinum and palladium. Biomaterials 2015, 48, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, P.; Yu, D.; Zhang, Y.; Wang, Z.; Liu, C.; Qiu, H.; Liu, Z.; Ren, J.; Qu, X. Unraveling the enzymatic activity of oxygenated carbon nanotubes and their application in the treatment of bacterial infections. Nano Lett. 2018, 18, 3344–3351. [Google Scholar] [CrossRef] [PubMed]
- André, R.; Natálio, F.; Humanes, M.; Leppin, J.; Heinze, K.; Wever, R.; Schröder, H.C.; Müller, W.E.; Tremel, W. V2O5 nanowires with an intrinsic peroxidase-like activity. Adv. Funct. Mater. 2011, 21, 501–509. [Google Scholar] [CrossRef]
- Vernekar, A.A.; Sinha, D.; Srivastava, S.; Paramasivam, P.U.; D’Silva, P.; Mugesh, G. An antioxidant nanozyme that uncovers the cytoprotective potential of vanadia nanowires. Nat. Commun. 2014, 5, 5301. [Google Scholar] [CrossRef]
- Song, Y.; Qu, K.; Zhao, C.; Ren, J.; Qu, X. Graphene oxide: Intrinsic peroxidase catalytic activity and its application to glucose detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef]
- Lin, T.; Zhong, L.; Wang, J.; Guo, L.; Wu, H.; Guo, Q.; Fu, F.; Chen, G. Graphite-like carbon nitrides as peroxidase mimetics and their applications to glucose detection. Biosens. Bioelectron. 2014, 59, 89–93. [Google Scholar] [CrossRef]
- Zeb, A.; Xie, X.; Yousaf, A.B.; Imran, M.; Wen, T.; Wang, Z.; Guo, H.L.; Jiang, Y.F.; Qazi, I.A.; Xu, A.W. Highly efficient fenton and enzyme-mimetic activities of mixed-phase VOx nanoflakes. ACS Appl. Mater. Interfaces 2016, 8, 30126–30132. [Google Scholar] [CrossRef]
- Lin, T.; Zhong, L.; Guo, L.; Fu, F.; Chen, G. Seeing diabetes: Visual detection of glucose based on the intrinsic peroxidase-like activity of MoS2 nanosheets. Nanoscale 2014, 6, 11856–11862. [Google Scholar] [CrossRef]
- Lin, T.; Zhong, L.; Song, Z.; Guo, L.; Wu, H.; Guo, Q.; Chen, Y.; Fu, F.; Chen, G. Visual detection of blood glucose based on peroxidase-like activity of WS2 nanosheets. Biosens. Bioelectron. 2014, 62, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-W.; Wei, J.-J.; Liu, T.; Zhang, X.L.; Bai, S.M.; Yang, H.H. Silk fibroin-assisted exfoliation and functionalization of transition metal dichalcogenide nanosheets for antibacterial wound dressings. Nanoscale 2017, 9, 17193–17198. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, X.; Wang, J.; Yang, G. WSe2 few layers with enzyme mimic activity for high-sensitive and high-selective visual detection of glucose. Nanoscale 2017, 9, 11806–11813. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, T.; Wang, J.; Yang, G. Few-layered MoSe2 nanosheets as an efficient peroxidase nanozyme for highly sensitive colorimetric detection of H2O2 and xanthine. J. Mater. Chem. B 2018, 6, 105–111. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, S.; Ye, F.; Su, L.; Zhang, C.; Shen, S.; Zhao, S. Synthesis of a mixed valence state Ce-MOF as an oxidase mimetic for the colorimetric detection of biothiols. Chem. Commun. 2015, 51, 4635–4638. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, R.; Zhang, P.; Qin, Y.; Chen, T.; Ye, F. A bimetallic (Co/2Fe) metal-organic framework with oxidase and peroxidase mimicking activity for colorimetric detection of hydrogen peroxide. Microchim. Acta 2017, 184, 4629–4635. [Google Scholar] [CrossRef]
- Lin, T.; Qin, Y.; Huang, Y.; Yang, R.; Hou, L.; Ye, F.; Zhao, S. A label-free fluorescence assay for hydrogen peroxide and glucose based on the bifunctional MIL-53 (Fe) nanozyme. Chem. Commun. 2018, 54, 1762–1765. [Google Scholar] [CrossRef]
- Zhang, J.W.; Zhang, H.T.; Du, Z.Y.; Wang, X.; Yu, S.H.; Jiang, H.L. Water-stable metal–organic frameworks with intrinsic peroxidase-like catalytic activity as a colorimetric biosensing platform. Chem. Commun. 2014, 50, 1092–1094. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Binyam, A.; Liu, M.; Wu, Y.; Li, F. Discovering the enzyme mimetic activity of metal-organic framework (MOF) for label-free and colorimetric sensing of biomolecules. Biosens. Bioelectron. 2016, 86, 432–438. [Google Scholar] [CrossRef]
- Tan, H.; Li, Q.; Zhou, Z.; Ma, C.; Song, Y.; Xu, F.; Wang, L. A sensitive fluorescent assay for thiamine based on metal-organic frameworks with intrinsic peroxidase-like activity. Anal. Chim. Acta 2015, 856, 90–95. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhao, X.J.; Yang, X.X.; Li, Y.F. A nanosized metal–organic framework of Fe-MIL-88NH2 as a novel peroxidase mimic used for colorimetric detection of glucose. Analyst 2013, 138, 4526–4531. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, W.; Qin, L.; Lin, T.; Chen, W.; Lin, S.; Yao, J.; Zhao, X.; Zhou, M.; Hang, C. Boosting the peroxidase-like activity of nanostructured nickel by inducing its 3+ oxidation state in LaNiO3 perovskite and its application for biomedical assays. Theranostics 2017, 7, 2277. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Li, L.; Zhang, C.; Fu, J.; Jiang, J. MIL-53 (Fe): A metal–organic framework with intrinsic peroxidase-like catalytic activity for colorimetric biosensing. Chem. Eur. J. 2013, 19, 15105–15108. [Google Scholar] [CrossRef] [PubMed]
- Baldim, V.; Bedioui, F.; Mignet, N.; Margaill, I.; Berret, J.F. The enzyme-like catalytic activity of cerium oxide nanoparticles and its dependency on Ce(3+) surface area concentration. Nanoscale 2018, 10, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dong, J.; Wu, Y.; Cao, P.; Song, L.; Ma, M.; Gu, N.; Zhang, Y. Shape-dependent enzyme-like activity of Co3O4 nanoparticles and their conjugation with his-tagged EGFR single-domain antibody. Colloid. Surf. B 2017, 154, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Klet, R.C.; Moon, S.-Y.; Wang, T.C.; Deria, P.; Peters, A.W.; Klahr, B.M.; Park, H.J.; Al-Juaid, S.S.; Hupp, J.T. Synthesis of nanocrystals of Zr-based metal–organic frameworks with csq-net: Significant enhancement in the degradation of a nerve agent simulant. Chem. Commun. 2015, 51, 10925–10928. [Google Scholar] [CrossRef]
- Peng, F.F.; Zhang, Y.; Gu, N. Size-dependent peroxidase-like catalytic activity of Fe3O4 nanoparticles. Chin. Chem. Lett. 2008, 19, 730–733. [Google Scholar] [CrossRef]
- Asati, A.; Santra, S.; Kaittanis, C.; Nath, S.; Perez, J.M. Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 2308–2312. [Google Scholar] [CrossRef]
- Ge, C.; Fang, G.; Shen, X.; Chong, Y.; Wamer, W.G.; Gao, X.; Chai, Z.; Chen, C.; Yin, J.J. Facet energy versus enzyme-like activities: The unexpected protection of palladium nanocrystals against oxidative damage. ACS Nano 2016, 10, 10436–10445. [Google Scholar] [CrossRef]
- Peterson, G.W.; Lu, A.X.; Epps, T.H., III. Tuning the morphology and activity of electrospun polystyrene/UiO-66-NH2 metal–organic framework composites to enhance chemical warfare agent removal. ACS Appl. Mater. Interfaces 2017, 9, 32248–32254. [Google Scholar] [CrossRef]
- Singh, N.; Geethika, M.; Eswarappa, S.M.; Mugesh, G. Manganese-based nanozymes: Multienzyme redox activity and effect on the nitric oxide produced by endothelial nitric oxide synthase. Chem. Eur. J. 2018, 24, 8393–8403. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Li, W.; Shen, X.; Perez-Aguilar, J.M.; Chong, Y.; Gao, X.; Chai, Z.; Chen, C.; Ge, C.; Zhou, R. Differential Pd-nanocrystal facets demonstrate distinct antibacterial activity against Gram-positive and Gram-negative bacteria. Nat. Commun. 2018, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Sun, J.; Qi, Y.; Zhang, B.; Guo, S.; Zhao, M. Influence of VO2 nanoparticle morphology on the colorimetric assay of H2O2 and glucose. Nanomaterials 2017, 7, 347. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Savanur, M.A.; Srivastava, S.; D’Silva, P.; Mugesh, G. A redox modulatory Mn3O4 nanozyme with multi-enzyme activity provides efficient cytoprotection to human cells in a Parkinson’s disease model. Angew. Chem. Int. Ed. 2017, 56, 14267–14271. [Google Scholar] [CrossRef]
- Mu, J.; Zhang, L.; Zhao, M.; Wang, Y. Catalase mimic property of Co3O4 nanomaterials with different morphology and its application as a calcium sensor. ACS Appl. Mater. Interfaces 2014, 6, 7090–7098. [Google Scholar] [CrossRef]
- Liu, S.; Lu, F.; Xing, R.; Zhu, J.J. Structural effects of Fe3O4 nanocrystals on peroxidase-like activity. Chem. Eur. J. 2011, 17, 620–625. [Google Scholar] [CrossRef]
- Wan, Y.; Qi, P.; Zhang, D.; Wu, J.; Wang, Y. Manganese oxide nanowire-mediated enzyme-linked immunosorbent assay. Biosens. Bioelectron. 2012, 33, 69–74. [Google Scholar] [CrossRef]
- Zhang, K.; Zuo, W.; Wang, Z.; Liu, J.; Li, T.; Wang, B.; Yang, Z. A simple route to CoFe2O4 nanoparticles with shape and size control and their tunable peroxidase-like activity. Rsc Adv. 2015, 5, 10632–10640. [Google Scholar] [CrossRef]
- Singh, S.; Dosani, T.; Karakoti, A.S.; Kumar, A.; Seal, S.; Self, W.T. A phosphate-dependent shift in redox state of cerium oxide nanoparticles and its effects on catalytic properties. Biomaterials 2011, 32, 6745–6753. [Google Scholar] [CrossRef]
- Lee, Y.; Garcia, M.A.; Frey Huls, N.A.; Sun, S. Synthetic tuning of the catalytic properties of Au-Fe3O4 nanoparticles. Angew. Chem. Int. Ed. 2010, 49, 1271–1274. [Google Scholar] [CrossRef]
- Fernandez-Garcia, S.; Jiang, L.; Tinoco, M.; Hungria, A.B.; Han, J.; Blanco, G.; Calvino, J.J.; Chen, X. Enhanced hydroxyl radical scavenging activity by doping lanthanum in ceria nanocubes. J. Phys. Chem. C 2016, 120, 1891–1901. [Google Scholar] [CrossRef]
- Long, L.; Liu, J.; Lu, K.; Zhang, T.; Xie, Y.; Ji, Y.; Wu, X. Highly sensitive and robust peroxidase-like activity of Au–Pt core/shell nanorod-antigen conjugates for measles virus diagnosis. J. Nanobiotechnol. 2018, 16, 46. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Guan, Z.; Jia, S.; Lei, Z.; Lin, S.; Zhang, H.; Ma, Y.; Tian, Z.Q.; Yang, C.J. Au@ Pt nanoparticle encapsulated target-responsive hydrogel with volumetric bar-chart chip readout for quantitative point-of-care testing. Angew. Chem. Int. Ed. 2014, 53, 12503–12507. [Google Scholar]
- Wu, J.; Qin, K.; Yuan, D.; Tan, J.; Qin, L.; Zhang, X.; Wei, H. Rational design of Au@ Pt multibranched nanostructures as bifunctional nanozymes. ACS Appl. Mater. Interfaces 2018, 10, 12954–12959. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Sun, K.; Petty, H.R. Titanium doping reduces superoxide dismutase activity, but not oxidase activity, of catalytic CeO2 nanoparticles. Inorg. Chem. Commun. 2012, 15, 235–237. [Google Scholar] [CrossRef]
- Sun, H.; Jiao, X.; Han, Y.; Jiang, Z.; Chen, D. Synthesis of Fe3O4-Au Nanocomposites with Enhanced Peroxidase-Like Activity. Eur. J. Inorg. Chem. 2013, 2013, 109–114. [Google Scholar] [CrossRef]
- Wang, C.; Qian, J.; Wang, K.; Yang, X.; Liu, Q.; Hao, N.; Wang, C.; Dong, X.; Huang, X. Colorimetric aptasensing of ochratoxin A using Au@ Fe3O4 nanoparticles as signal indicator and magnetic separator. Biosens. Bioelectron. 2016, 77, 1183–1191. [Google Scholar] [CrossRef]
- Ge, C.; Wu, R.; Chong, Y.; Fang, G.; Jiang, X.; Pan, Y.; Chen, C.; Yin, J.J. Synthesis of Pt hollow nanodendrites with enhanced peroxidase-like activity against bacterial infections: Implication for wound healing. Adv. Funct. Mater. 2018, 28, 1801484. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Gong, S.-W.; Zhang, Y.; Yang, T.; Wang, C.-Y.; Gu, N. Prussian blue modified iron oxide magnetic nanoparticles and their high peroxidase-like activity. J. Mater. Chem. 2010, 20, 5110–5116. [Google Scholar] [CrossRef]
- Sobańska, K.; Pietrzyk, P.; Sojka, Z. Generation of reactive oxygen species via electroprotic interaction of H2O2 with ZrO2 gel: Ionic sponge effect and pH-switchable peroxidase-and catalase-like activity. ACS Catal. 2017, 7, 2935–2947. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, S.; Yin, J.J.; He, W.; Lu, W.; Ma, M.; Gu, N.; Zhang, Y. Prussian blue nanoparticles as multienzyme mimetics and reactive oxygen species scavengers. J. Am. Chem. Soc. 2016, 138, 5860–5865. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Su, P.; Li, H.; Yang, Y.; Yang, Y. Triple-enzyme mimetic activity of Co3O4 nanotubes and their applications in colorimetric sensing of glutathione. New J. Chem. 2016, 40, 10056–10063. [Google Scholar] [CrossRef]
- Fan, Y.; Shi, W.; Zhang, X.; Huang, Y. Mesoporous material-based manipulation of the enzyme-like activity of CoFe2O4 nanoparticles. J. Mater. Chem. A 2014, 2, 2482–2486. [Google Scholar] [CrossRef]
- Huang, Y.; Ran, X.; Lin, Y.; Ren, J.; Qu, X. Self-assembly of an organic–inorganic hybrid nanoflower as an efficient biomimetic catalyst for self-activated tandem reactions. Chem. Commun. 2015, 51, 4386–4389. [Google Scholar] [CrossRef]
- Xu, C.; Bing, W.; Wang, F.; Ren, J.; Qu, X. Versatile dual photoresponsive system for precise control of chemical reactions. ACS Nano 2017, 11, 7770–7780. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, N.; Si, Y.; Li, S.; Wen, J.; Zhu, X.; Wang, H. High-throughput colorimetric assays for mercury (II) in blood and wastewater based on the mercury-stimulated catalytic activity of small silver nanoparticles in a temperature-switchable gelatin matrix. Chem. Commun. 2014, 50, 9196–9199. [Google Scholar] [CrossRef]
- Wu, G.W.; He, S.B.; Peng, H.P.; Deng, H.H.; Liu, A.L.; Lin, X.H.; Xia, X.H.; Chen, W. Citrate-capped platinum nanoparticle as a smart probe for ultrasensitive mercury sensing. Anal. Chem. 2014, 86, 10955–10960. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Li, W.; Zhang, J.; Zhang, Y.; Fu, Y. DNA-stabilized bimetallic nanozyme and its application on colorimetric assay of biothiols. Biosens. Bioelectron. 2015, 74, 1038–1046. [Google Scholar] [CrossRef]
- Carmona, U.; Zhang, L.; Li, L.; Münchgesang, W.; Pippel, E.; Knez, M. Tuning, inhibiting and restoring the enzyme mimetic activities of Pt–apoferritin. Chem. Commun. 2014, 50, 701–703. [Google Scholar] [CrossRef]
- Liu, J.; Hu, X.; Hou, S.; Wen, T.; Liu, W.; Zhu, X.; Wu, X. Screening of inhibitors for oxidase mimics of Au@ Pt nanorods by catalytic oxidation of OPD. Chem. Commun. 2011, 47, 10981–10983. [Google Scholar] [CrossRef]
- Bing, W.; Sun, H.; Yan, Z.; Ren, J.; Qu, X. Programmed bacteria death induced by carbon dots with different surface charge. Small 2016, 12, 4713–4718. [Google Scholar] [CrossRef]
- Cormode, D.P.; Gao, L.; Koo, H. Emerging biomedical applications of enzyme-like catalytic nanomaterials. Trends Biotechnol. 2018, 36, 15–29. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Ren, J.; Qu, X. Enzyme mimicry for combating bacteria and biofilms. Acc. Chem. Res. 2018, 51, 789–799. [Google Scholar] [CrossRef]
- Karim, M.N.; Singh, M.; Weerathunge, P.; Bian, P.; Zheng, R.; Dekiwadia, C.; Ahmed, T.; Walia, S.; Della Gaspera, E.; Singh, S. Visible-light-triggered reactive-oxygen-species-mediated antibacterial activity of peroxidase-mimic CuO nanorods. ACS Appl. Nano Mater. 2018, 1, 1694–1704. [Google Scholar] [CrossRef]
- Xu, B.; Wang, H.; Wang, W.; Gao, L.; Li, S.; Pan, X.; Wang, H.; Yang, H.; Meng, X.; Wu, Q. A single-atom nanozyme for wound disinfection applications. Angew. Chem. Int. Ed. 2019, 58, 4911–4916. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, C.; Zeng, G.; Chen, G.; Wan, J.; Guo, Z.; Wu, H.; Yu, Z.; Zhou, Y.; Liu, J. Metal-based quantum dots: Synthesis, surface modification, transport and fate in aquatic environments and toxicity to microorganisms. Rsc Adv. 2016, 6, 78595–78610. [Google Scholar] [CrossRef]
- Zhou, C.; Lai, C.; Xu, P.; Zeng, G.; Huang, D.; Zhang, C.; Cheng, M.; Hu, L.; Wan, J.; Liu, Y. In situ grown AgI/Bi12O17Cl2 heterojunction photocatalysts for visible light degradation of sulfamethazine: Efficiency, pathway, and mechanism. ACS Sustain. Chem. Eng. 2018, 6, 4174–4184. [Google Scholar] [CrossRef]
- Wan, J.; Zeng, G.; Huang, D.; Hu, L.; Xu, P.; Huang, C.; Deng, R.; Xue, W.; Lai, C.; Zhou, C. Rhamnolipid stabilized nano-chlorapatite: Synthesis and enhancement effect on Pb-and Cd-immobilization in polluted sediment. J. Hazard. Mater. 2018, 343, 332–339. [Google Scholar] [CrossRef]
- Jiang, J.; He, C.; Wang, S.; Jiang, H.; Li, J.; Li, L. Recyclable ferromagnetic chitosan nanozyme for decomposing phenol. Carbohydr. Polym. 2018, 198, 348–353. [Google Scholar] [CrossRef]
- Wang, J.; Huang, R.; Qi, W.; Su, R.; Binks, B.P.; He, Z. Construction of a bioinspired laccase-mimicking nanozyme for the degradation and detection of phenolic pollutants. Appl. Catal. B 2019, 254, 452–462. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Surface modification of nanozymes. Nano Res. 2017, 10, 1125–1148. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, F.; Yuan, Q.; Liu, J.; Li, Y.; Liang, H. Robust magnetic laccase-mimicking nanozyme for oxidizing o-phenylenediamine and removing phenolic pollutants. J. Environ. Sci. (China) 2020, 88, 103–111. [Google Scholar] [CrossRef]
- Miller, E.B.; Zahran, E.M.; Knecht, M.R.; Bachas, L.G. Metal oxide semiconductor nanomaterial for reductive debromination: Visible light degradation of polybrominated diphenyl ethers by Cu2O@ Pd nanostructures. Appl. Catal. B 2017, 213, 147–154. [Google Scholar] [CrossRef]
- Fan, G.; Zhan, J.; Luo, J.; Zhang, J.; Chen, Z.; You, Y. Photocatalytic degradation of naproxen by a H2O2-modified titanate nanomaterial under visible light irradiation. Catal. Sci. Technol. 2019, 9, 4614–4628. [Google Scholar] [CrossRef]
- Qiu, H.; Pu, F.; Ran, X.; Liu, C.; Ren, J.; Qu, X. Nanozyme as artificial receptor with multiple readouts for pattern recognition. Anal. Chem. 2018, 90, 11775–11779. [Google Scholar] [CrossRef]
- Hou, L.; Qin, Y.; Li, J.; Qin, S.; Huang, Y.; Lin, T.; Guo, L.; Ye, F.; Zhao, S. A ratiometric multicolor fluorescence biosensor for visual detection of alkaline phosphatase activity via a smartphone. Biosens. Bioelectron. 2019, 143, 111605. [Google Scholar] [CrossRef]
- Wang, X.; Qin, L.; Lin, M.; Xing, H.; Wei, H. Fluorescent graphitic carbon nitride-based nanozymes with peroxidase-Like activities for ratiometric biosensing. Anal. Chem. 2019, 91, 10648–10656. [Google Scholar] [CrossRef]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef]
- Li, S.; Shang, L.; Xu, B.; Wang, S.; Gu, K.; Wu, Q.; Sun, Y.; Zhang, Q.; Yang, H.; Zhang, F. A Nanozyme with Photo-Enhanced Dual Enzyme-Like Activities for Deep Pancreatic Cancer Therapy. Angew. Chem. Int. Ed. 2019, 58, 12624–12631. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Ju, E.; Liu, Z.; Cao, F.; Chen, Z.; Ren, J.; Qu, X. Biomimetic nanoflowers by self-assembly of nanozymes to induce intracellular oxidative damage against hypoxic tumors. Nat. Commun. 2018, 9, 3334. [Google Scholar] [CrossRef]
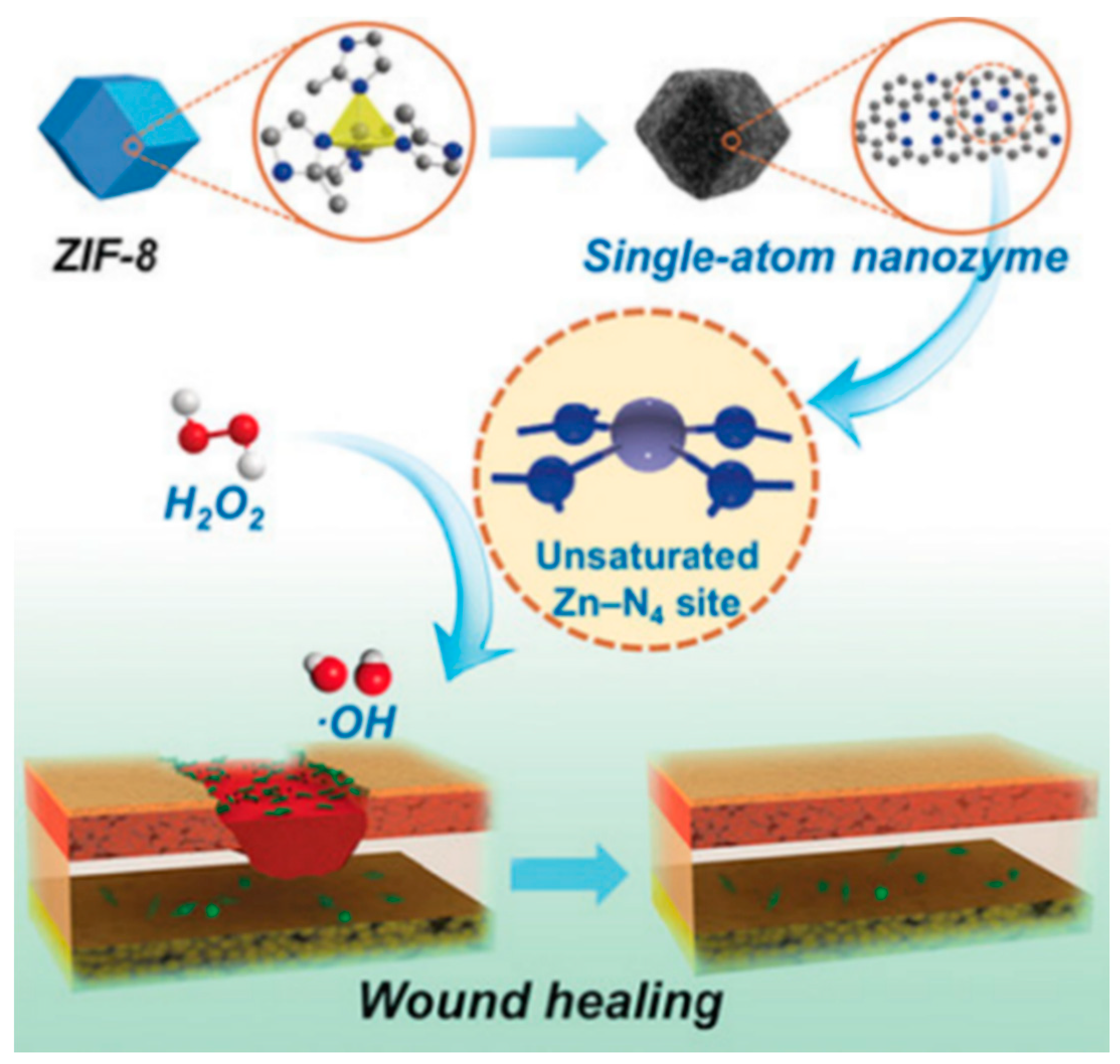

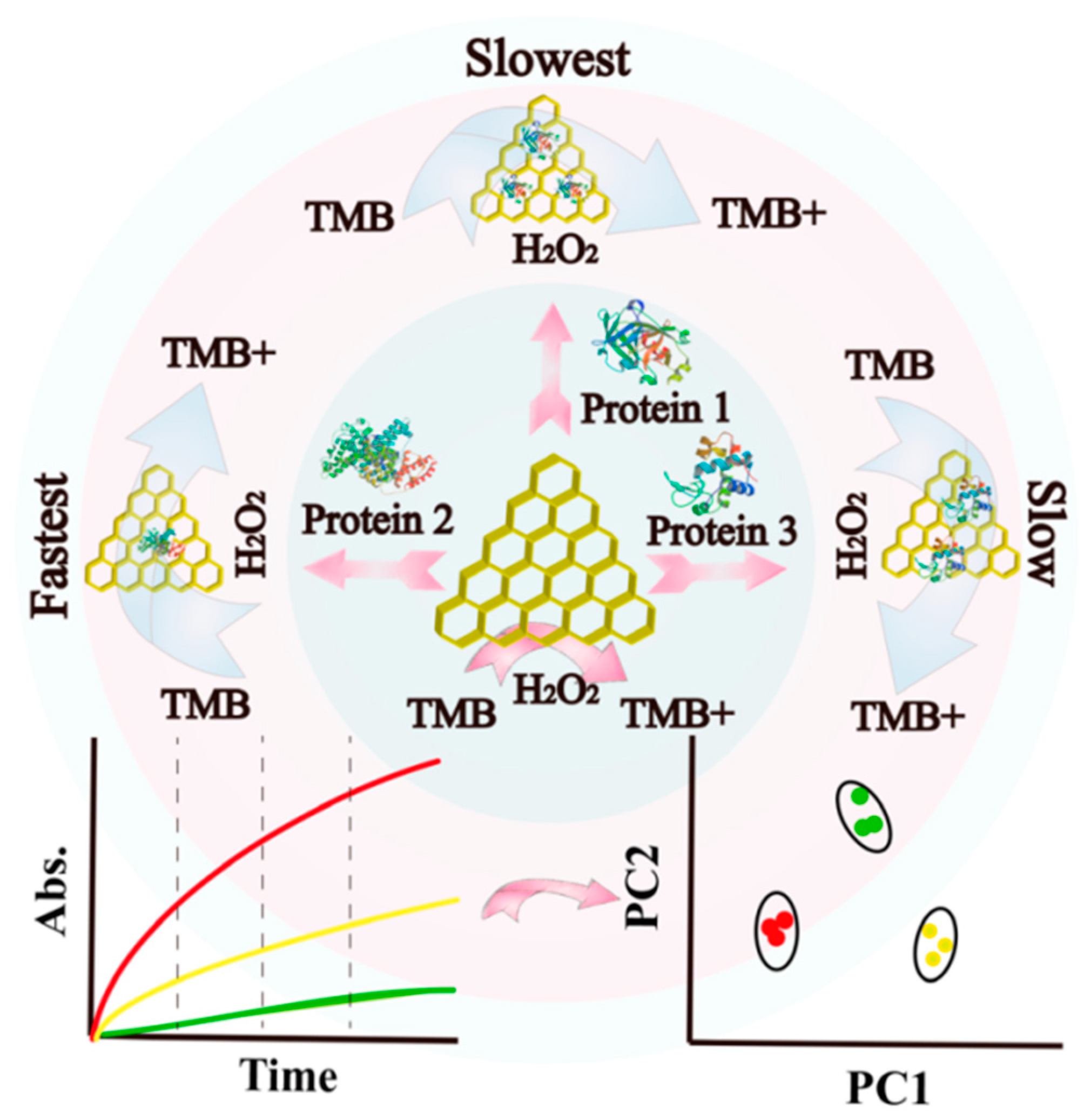
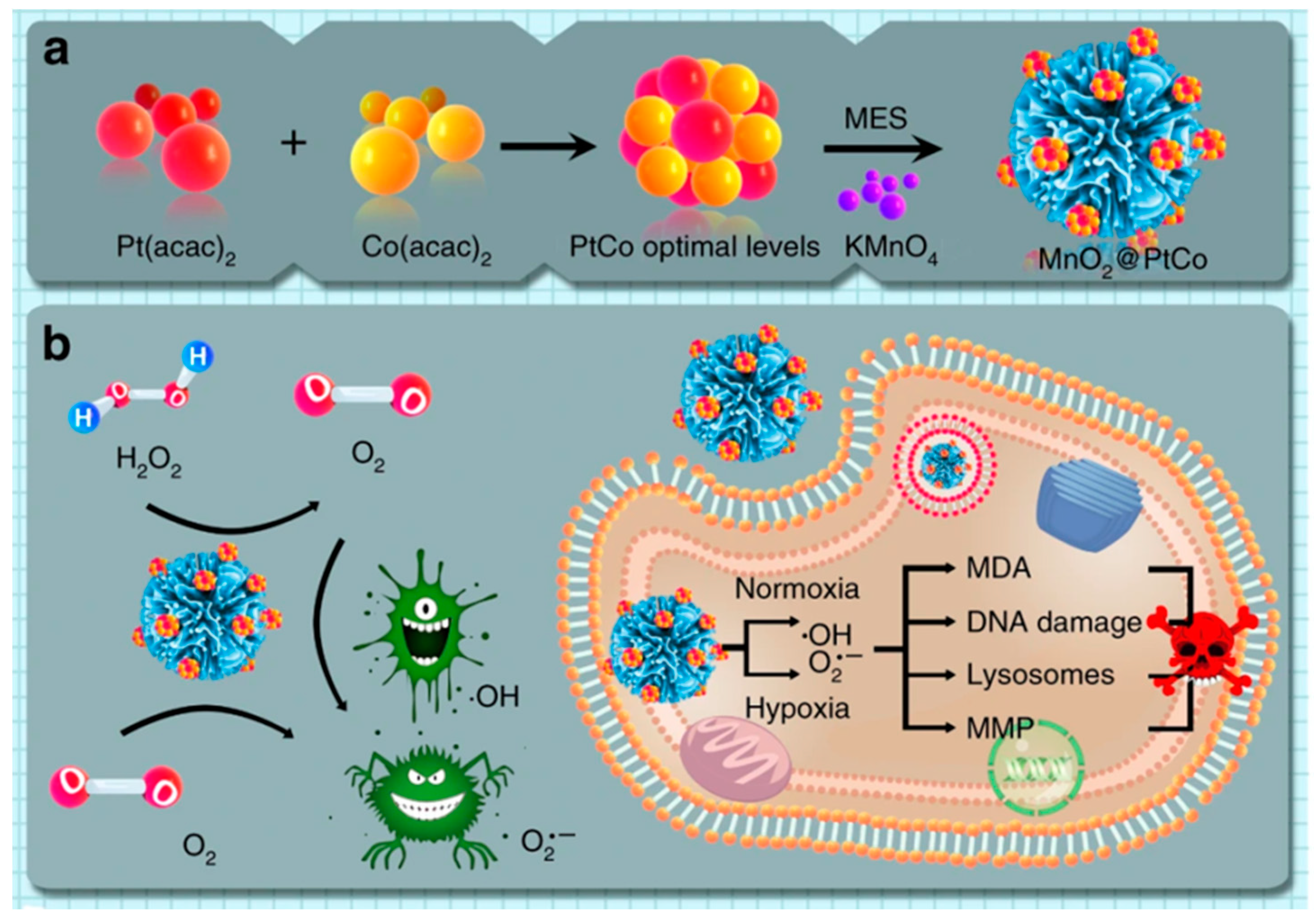
| Types of Nanozymes | Nanomaterial | Regulation Strategy | Application | Reference |
|---|---|---|---|---|
| zero dimension | CeO2-X | size (4.5 nm, 7.8 nm, 23 nm, and 28 nm); composition (surface area concentration of Ce3+) | decomposition of H2O2 | 44 |
| Fe3O4 NPs | size (11 nm, 20 nm, 150 nm) | catalyze the oxidation of the substrate TMB by H2O2 | 47 | |
| dextran-coated nanoceria | size (5 nm, 12 nm, 14 nm, 100 nm) | enzyme-linked immunosorbent assay | 48 | |
| Au-Fe3O4 NPs | composition (complex) | detection of H2O2 | 60 | |
| Ti-doped CeO2 | composition (doping) | biological applications | 65 | |
| Pt hollow nanodendrites | composition (etching) | antibacteria | 68 | |
| Fe3O4 | surface modification (3-aminopropyltriethoxysilane, polyethylene glycol, dextran, and SiO2) | immunoassay | 8 | |
| graphene quantum dots | surface modification (phenylhydrazine, benzoic anhydride, and 2-bromo-1-phenylethyl ketone) | glucose detection | 14 | |
| γ-Fe2O3 NPs | surface modification (Prussian blue) | immunoassay | 69 | |
| Ag | activator (mercury (II)) | colorimetric assays for mercury (II) | 76 | |
| Pt | inhibitor (mercury (II)) | colorimetric assays for mercury (II) | 77 | |
| AuxPty -DNA | inhibitor (biothiol) | colorimetric assay of biothiols | 78 | |
| Pt–apoferritin | inhibitor of catalase activity (NaN3), inhibitor of catalase and superoxide dismutase activities (3-amino-1,2,4-triazole) | engineering targeting enzyme mimetics | 79 | |
| CeO2 | inhibitor (phosphate) | catalyst | 59 | |
| Prussian Blue | pH | multienzyme mimetics and reactive oxygen species scavengers | 71 | |
| CoFe2O4 | pH, surface modification | chemiluminescence without the need for H2O2 | 73 | |
| one dimension | VO2 | morphology (nanofibers, nanosheets, and nanorods) | detection of H2O2 and glucose | 53 |
| Mn3O4 nanoflower | morphology (nanoflowers, cubes, polyhedron, hexagonal plates, and flakes) | preventing the cells from oxidative damage | 54 | |
| Co3O4 nanoplates | morphology (nanoplates, nanorods, and nanocubes) | determination of calcium ion | 55 | |
| Au@Pt nanorods | inhibitor (Fe2+, Cu2+, and NaN3) | screening of inhibitors for oxidase mimics | 80 | |
| two dimension | graphene oxide | pH, temperature | programmable wound healing | 75 |
| WS2 | temperature | glucose detection | 31 | |
| three dimension | ZrO2 gel | pH | nonredox system construction | 70 |
| BSA–Cu3(PO4)·3H2O | temperature | decompose organic pollutants | 74 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, L.; Jiang, G.; Sun, Y.; Zhang, X.; Huang, J.; Liu, S.; Lin, T.; Ye, F.; Zhao, S. Progress and Trend on the Regulation Methods for Nanozyme Activity and Its Application. Catalysts 2019, 9, 1057. https://doi.org/10.3390/catal9121057
Hou L, Jiang G, Sun Y, Zhang X, Huang J, Liu S, Lin T, Ye F, Zhao S. Progress and Trend on the Regulation Methods for Nanozyme Activity and Its Application. Catalysts. 2019; 9(12):1057. https://doi.org/10.3390/catal9121057
Chicago/Turabian StyleHou, Li, Gaoyan Jiang, Ying Sun, Xuanhan Zhang, Juanjuan Huang, Shendong Liu, Tianran Lin, Fanggui Ye, and Shulin Zhao. 2019. "Progress and Trend on the Regulation Methods for Nanozyme Activity and Its Application" Catalysts 9, no. 12: 1057. https://doi.org/10.3390/catal9121057
APA StyleHou, L., Jiang, G., Sun, Y., Zhang, X., Huang, J., Liu, S., Lin, T., Ye, F., & Zhao, S. (2019). Progress and Trend on the Regulation Methods for Nanozyme Activity and Its Application. Catalysts, 9(12), 1057. https://doi.org/10.3390/catal9121057





