Abstract
Biogenic manganese oxides (BMOs) have gained increasing attention for environmental application because of their sequestration and oxidizing abilities for various elements. Oxidation and sequestration of Cr(III) by BMOs, however, still remain unknown. We prepared BMOs in liquid cultures of Acremonium strictum strain KR21-2, and subsequently conducted single or repeated treatment experiments in Cr(NO3)3 at pH 6.0. Under aerobic conditions, newly formed BMOs exhibited a rapid production of Cr(VI) without a significant release of Mn(II), demonstrating that newly formed BMO mediates a catalytic oxidation of Cr(III) with a self-regeneration step of reduced Mn. In anaerobic solution, newly formed BMOs showed a cessation of Cr(III) oxidation in the early stage of the reaction, and subsequently had a much smaller Cr(VI) production with significant release of reduced Mn(II). Extraordinary sequestration of Cr(III) was observed during the repeated treatments under anaerobic conditions. Anaerobically sequestered Cr(III) was readily converted to Cr(VI) when the conditions became aerobic, which suggests that the surface passivation is responsible for the anaerobic cessation of Cr(III) oxidation. The results presented herein increase our understanding of the roles of BMO in Cr(III) oxidation and sequestration processes in potential application of BMOs towards the remediation of Cr(III)/Cr(VI) in contaminated sites.
1. Introduction
Chromium (Cr) is widely used in various industries such as electroplating, pigment production, wood preservation, tanning of animal hides, etc. [1,2]. Concomitant anthropogenic emissions of Cr into the environment have increasingly induced a significant environmental concern because of the potential toxicity of Cr toward humans [3], fishes [4], and plants [5,6,7]. Therefore, development of cost-effective remediation systems for Cr is urgently needed [8,9].
In natural environments, Cr exists as trivalent (Cr[III]) and hexavalent (Cr[VI]) forms which have quite different physicochemical properties. Although Cr(III) is less toxic and nutritionally essential for some organisms, including humans, large amounts of Cr(III) can cause health problems such as lung cancer [1]. Cr(VI) is more toxic and labile, and exists as hydrochromate (HCrO4−), chromate (CrO42−), and dichromate (Cr2O72−) ions, with domination of chromate (CrO42−) at neutral pH [1]. Many studies have focused on the oxidative transformation of Cr(III) to Cr(VI) by manganese oxide (MnO2), because MnO2 is considered to be the only naturally existing oxidizer of Cr(III) to Cr(VI) [1], and so affects Cr(III) cycling in the environment. Under circumneutral conditions, microbial oxidation of Mn(II) proceeds several orders of magnitude faster than abiotic oxidation (such as homogenous and mineral surface-catalytic reactions; [10,11]), participating directly in the production of Mn oxide minerals in aquatic [12,13] and soil/subsurface [14,15,16] environments. Consequently, transformation of Cr(III) to Cr(VI), although indirectly, is primarily controlled by microbial Mn oxidizing activity, as demonstrated in culture experiments using Mn(II)-oxidizing bacteria including Pseudomonas putida GB-1 [17] and MnB-1 [18], Bacillus sp. SG-1 [19] and WH4 [20], and Rosebacter sp. Azwk-3b [21]. Among these studies, higher efficiencies of oxidative transformation by biogenic Mn oxide (BMO: abbreviations used in this study are listed in Supplementary Materials) compared to chemically synthetic MnO2 particles have been shown [17,20]. Although physicochemical features of BMOs (poorly crystalline, larger density of structural Mn(IV) vacancies, small domain size, etc. [10,11]) may partly cause their higher efficiencies [17,20], detailed mechanisms for efficient oxidative Cr(III) transformation by BMOs remain to be elucidated.
Oxidative reaction of Cr(III) on the chemically synthetic MnO2 is generally susceptible to cessation during the early stage of the reaction at circumneutral pH condition (commonly at pH > 5) even when excessive amounts of unreacted MnO2 remain [22,23,24,25,26]. The cause for this cessation in Cr(III) oxidation remains uncertain [25,26]. Our previous studies using newly formed BMOs prepared by a Mn(II)-oxidizing fungus, Acremonium strictum KR21-2 [27], have demonstrated that the cessation of oxidative transformations of As(III) to As(V) [28,29] and of Co(II) to Co(III) [30] by fungal BMOs is readily recovered through the self-regeneration of reduced Mn(II) or Mn(III) by the associated Mn(II) oxidase activity in newly formed BMOs. We therefore hypothesized that the newly formed BMOs can effectively convert Cr(III) to Cr(VI) without the cessation if the Mn(II) oxidase associated in BMOs is active to reduce the surface passivation. However, the role of enzymatic activity in Cr(III) oxidation process remains unclear.
Kinetics of oxidative transformation of Cr(III) may be affected by the sorption affinity of Cr(III) on BMO because it requires electron transfer from Cr(III) to the structural Mn(IV) in BMO. Because BMOs possess sheet (vernadite/birnessite-like) structures with nano-sized domains and high vacant site density for structural Mn(IV) [31,32], subsequently they show high sorption affinity for, especially, various cationic species such as Pb2+ [33,34,35], Zn2+ [36,37,38,39], Cd2+ [40,41], Ni2+ [41,42], Co2+ [30,43], and rare earth element (REE3+) ions [44,45]. Sorption of Mn2+ and Zn2+ was demonstrated to lower the oxidation kinetics of As(III) to As(V) on BMO, likely due to the blocking of the redox-active sites on BMOs [28,29]. At circumneutral pH in aqueous solutions, Cr3+ can be readily hydrolyzed to form monomers such as [Cr(OH)]2+ and [Cr(OH)2]+ as well as oligomers as dimeric [Cr2(OH)2]4+, trimeric [Cr3(OH)4]5+, and tetrametric [Cr4(OH)6]6+ [46,47,48,49], and may be sequestered on BMO surface. Although these polynuclear Cr(III) species may eventually precipitate as Cr(OH)3, Saleh et al. [48] and Hu et al. [50] demonstrated that such polymeric Cr(III) complexes exist in aqueous solution for relatively long time periods. Sequestration (sorption/precipitation) properties of such polymetric Cr(III) species on BMOs remain unknown and may be a key to remediating Cr contamination by BMOs.
The aim of this study was to clarify the role of fungal Mn(II) oxidase activity in newly formed BMOs on oxidative transformation of Cr(III) to Cr(VI) as well as in Cr(III) sequestration, which were generated in batch cultures of A. strictum KR21-2 [51,52]. The enzymatically inactive BMOs [27,53] were prepared by heat treatment, or by deaeration to remove dissolved oxygen, a terminal electron acceptor for enzymatic Mn(II) oxidation, and were used for the experiments to determine whether the enzymatic activity affected the oxidation of Cr(III) to Cr(VI) and concomitant Cr sequestration.
2. Results and Discussion
2.1. Cr(III) Oxidation Efficiency of Newly Formed BMOs with an Enzymatic Mn(II)-Oxidizing Activity
We conducted the treatment experiments of Cr(NO3)3 by newly formed BMO under aerobic condition to clear Cr(III) oxidation efficiency by newly formed BMO at pH 6.0 where the enzymatic Mn(II)-oxidizing activity is active to readily oxidize Mn2+ to insoluble Mn oxide [54]. When newly formed BMOs (1 mM as Mn) were reacted with 0.1–0.5 mM Cr(NO3)3 in 100 mM 2-morpholinoethanesulfonic acid (MES) buffer (pH at 6.0) under aerobic conditions, rapid production of Cr(VI) dissolved (CrVIdiss) was observed within the first 4-h reaction (Figure 1), and then gradually increased to 79%–86% of the initial Cr(III) (CrIIIInt) at 24 h (Figure 1E and Table S1). This indicates that they readily convert Cr(III) to Cr(VI) at pH 6.0, whereas chemically synthesized MnO2 commonly ceases the Cr(III) oxidation reaction at pH > 5 [22,23]. At pH 6.0, HCrVIO4- (76%) and CrVIO42− (24%) coexists [55] according to the following acid dissociation equilibrium [56].
H2CrO4(aq) → H+(aq) + HCrO4−(aq) pKa1 = 0.2
HCrO4−(aq) → H+(aq) + CrO42−(aq) pKa2 = 6.5
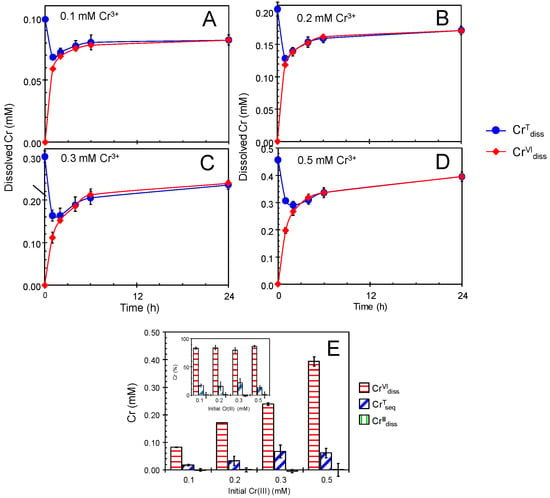
Figure 1.
Single treatment of newly formed biogenic manganese oxides (BMOs; 1 mM as Mn) with 0.1–0.5 mM Cr(NO3)3 under aerobic conditions at pH 6.0 (100 mM morpholinoethanesulfonic acid [MES] buffer). (A–E) Concentations of total Cr dissolved (CrTdiss) and Cr(VI) dissolved (CrVIdiss). Initial Cr(NO3)3 concentrations were: (A) 0.1 mM, (B) 0.2 mM, (C) 0.3 mM, and (D) 0.5 mM. (E) Concentrations (inset: percentages) of total Cr sequestered (CrTseq), Cr(VI) dissolved (CrVIdiss), and Cr(III) dissolved (CrIIIdiss) after the treatments. Data are shown as mean ± standard deviation (n = 3).
Thus, oxidation reactions of Cr(III) to Cr(VI) by MnO2 can be expressed as Equations (3) and (4) and their stoichiometry expects CrVIdiss with the molar ratio relative to Mn2+ release (MnIIrel) at CrVIdiss/MnIIrel = 0.67.
Cr3+(aq) + 1.5 MnIVO2(s) + H2O(aq) → HCrVIO4−(aq) + 1.5Mn2+(aq) + H+(aq)
Cr3+(aq) + 1.5MnIVO2(s) + H2O(aq) → CrVIO42−(aq) + 1.5Mn2+(aq) + 2H+(aq)
However, throughout the treatments, the Mn(II) concentrations released (MnIIrel) from the BMO phase were consistently lower than 0.005 mM (Figure S1 and Table S1), which is two orders of magnitude less than the calculated values from Equations (3) and (4). In the case of the aerobic treatments, in 0.5 mM CrIIIInt (i.e., the highest concentration in this experiment) the observed CrVIdiss and MnIIrel were 0.39 mM and <0.005 mM (Table S1), respectively, giving the CrVIdiss/MnIIrel ratio of >78. Newly formed BMOs effectively oxidize exogenous Mn2+ through the enzymatic Mn(II)-oxidizing activity maintained in the BMO under aerobic conditions at pH 6.0 [27,45,54] as:
2Mn2+(aq) + O2(aq) + 2H2O(aq) → 2MnIVO2(s) + 4H+(aq)
The observed CrVIdiss/MnIIrel ratio suggests the effective reoxidation of Mn2+ (and intermediate MnIII) that was produced by the redox reaction with Cr(III), through Mn(II)-oxidizing enzyme activity. Thus, the enzymatically active BMOs act as a catalytic agent for Cr(III) oxidation with the terminal electron acceptor of dissolved O2, by which homogeneous oxidation of Cr(III) does not proceed under the same conditions (Figure S2A). Our previous results have demonstrated such catalytic oxidation processes of As(III) to As(V) [29] and of Co(II) to Co(III) [30] (as the terminal electron acceptor of dissolved O2) using newly formed BMOs, which continuously mediates the oxidation reactions without release of reduced Mn. Enzymatically active BMOs also performed, through catalytic mediation, effective Ce(III) to Ce(IV) oxidation with a lower release of Mn(II), where the Ce oxidized/Mn released molar of >200 [54].
Repeated treatments of newly formed BMO in 0.1 mM Cr(NO3)3 (100 mM MES at pH 6.0) solution with renewal of the bathing solution every 24 h confirmed that the catalytic Cr(III) oxidation by newly formed BMO continued for least 18 days (Figure 2). Following each treatment, the CrVIdiss concentration ranged from 0.097 ± 0.003 mM for the 5th treatment to 0.068 ± 0.002 mM for the last treatments, whereas MnIIrel was <0.008 mM throughout, resulting in CrVIdiss/MnIIrel molar ratios from 150 ± 9 (1st treatment) to 8.6 ± 0.8 (last treatment). Decrease in CrVIdiss/MnIIrel molar ratios suggests that the Mn(II) oxidizing activity in BMOs gradually weakened as the treatment was repeatedly conducted. Even in the last treatment, the CrVIdiss/MnIIrel molar ratios were still higher than was expected from the redox stoichiometry of CrVIdiss/MnIIrel at 0.67 (Equations (3) and (4)). In this repeated treatment, the oxidative conversion (CrVIdiss relative to CrIIIInt) was higher than 66% ± 1% for each treatment with the cumulative conversion of 86% ± 1% for 18 days (Figure 2). When newly formed BMO (1 mM as Mn) was repeatedly treated in a higher concentration of Cr(NO3)3 at 0.5 mM (100 mM MES at pH 6.0), conversion of CrIIIInt to CrVIdiss was high at 86% ± 2% and 90% ± 2% for the 1st and 2nd treatments, respectively, without significant release of Mn(II) (MnIIrel) (Figure 3 and Table S2). This shows the ability of newly formed BMOs to oxidize Cr(III) in these CrIIIInt concentration ranges, in which some Mn(II)-oxidizing bacteria commonly lost growth and/or Mn(II)-oxidizing abilities; Psedomonas putida MnB-1 growth is partly inhibited at 0.2 mM and totally inhibited at 0.5 mM Cr(III) [18]. Murray et al. [17], also reported Cr(III) and Cr(VI) toxicity on Psedomonas putida GB-1 growth at concentrations ≥0.01 mM. Preformation of fungal BMOs in the culture without any Cr(III)/Cr(VI) can prevent the inhibitory effect of Cr and subsequently make the microbially-mediated Cr(III) oxidation sufficient, even at a high Cr(III) concentration. In the 3rd treatment, however, a smaller conversion (19% ± 0%) was observed (Figure 3), which was likely due to inhibitory effects through the exposure of higher Cr(III) and/or Cr(VI) concentrations on enzymatic Mn(II) oxidation.
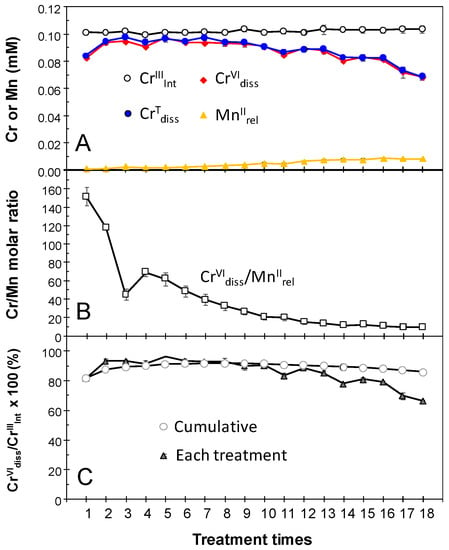
Figure 2.
Repeated treatments of newly formed biogenic manganese oxides (BMOs; 1 mM as Mn) with 0.1 mM Cr(NO3)3 under aerobic conditions at pH 6.0 (100 mM MES buffer). (A) Concentrations of initial Cr(III) (CrIIIInt), total Cr dissolved (CrTdiss), Cr(VI) dissolved (CrVIdiss), and Mn(II) released (MnIIrel). (B) Molar ratio of CrVIdiss/MnIIrel. (C) Percentages of conversion to CrVIdiss. Bathing solutions were renewed every 24 h for 18 days. Data are shown as mean ± standard deviation (n = 3).
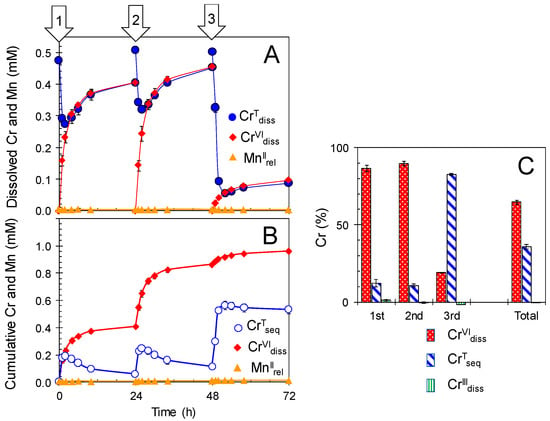
Figure 3.
Repeated treatments of newly formed biogenic manganese oxides (BMOs; 1 mM as Mn) with 0.5 mM Cr(NO3)3 under aerobic conditions at pH 6.0 (100 mM MES buffer). (A) Concentrations of total Cr dissolved (CrTdiss), Cr(VI) dissolved (CrVIdiss), and Mn(II) released (MnIIrel). (B) Cumulative concentrations of total Cr sequestered (CrTseq), CrVIdiss, and MnIIrel. (C) Percentages of CrVIdiss, CrTseq, and Cr(III) dissolved (CrIIIdiss) during the repeated treatments. Bathing solutions were renewed every 24 h for 3 days (indicated by arrows). Data are shown as mean ± standard deviation (n = 3).
2.2. Cessation of Cr(III) Oxidation by BMOs under Anaerobic Condition
Under anaerobic condition, enzymatic Mn(II) oxidation is suppressed due to the lack of dissolved O2 as the terminal electron acceptor [27]. Anaerobic treatments of newly formed BMO in Cr(NO3)3 were conducted to deduce the Cr(III) oxidation efficiency when the catalytic mediation for Cr(III) oxidation by BMOs was inactive. Single treatment of newly formed BMOs (1 mM as Mn) with anaerobic 0.1–0.5 mM Cr(NO3)3 (100 mM MES at pH 6.0) showed that CrVIdiss production ceased within the first 2 h of the treatment (Figure 4) and accompanied significant release of Mn(II) (MnIIrel) (0.02–0.03 mM), because BMOs failed to reoxidize MnIIrel (Figure S1B). The anaerobic cessation of Cr(III) oxidation resulted in much lower CrVIdiss concentrations, ranging from 0.03 (0.1 mM CrIIIInt) to 0.05 mM (0.5 mM CrIIIInt) (Figure 4E and Table S1), demonstrating an important role of enzymatic Mn(II) oxidation for continuous mediation of Cr(III) oxidation by BMOs. Cessation of Cr(III) oxidation has been found for chemically synthesized Mn oxides [22,23,24,25,26], even under aerobic condition at pH > 5. In fact, heated BMOs, in which the enzymatic Mn(II) oxidizing activities were inactivated [27], showed cessation of Cr(III) oxidation (Figure 5) and concomitant Mn(II) release (Figure S1C) even under aerobic conditions, in a similar manner as abiotic Mn oxide phases.
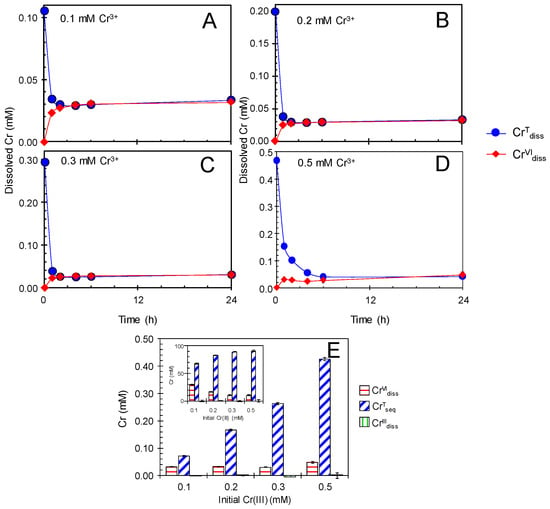
Figure 4.
Single treatment of newly formed biogenic manganese oxides (BMOs; 1 mM as Mn) with 0.1–0.5 mM Cr(NO3)3 under anaerobic conditions at pH 6.0 (100 mM MES buffer). (A–E) Concentrations of total Cr dissolved (CrTdiss) and Cr(VI) dissolved (CrVIdiss). Initial Cr(NO3)3 concentrations were: (A) 0.1 mM, (B) 0.2 mM, (C) 0.3 mM, and (D) 0.5 mM. (E) Concentrations (inset: percentages) of total Cr sequestered (CrTseq), Cr(VI) dissolved (CrVIdiss), and Cr(III) dissolved (CrIIIdiss) after the treatments. Data are shown as mean ± standard deviation (n = 3).
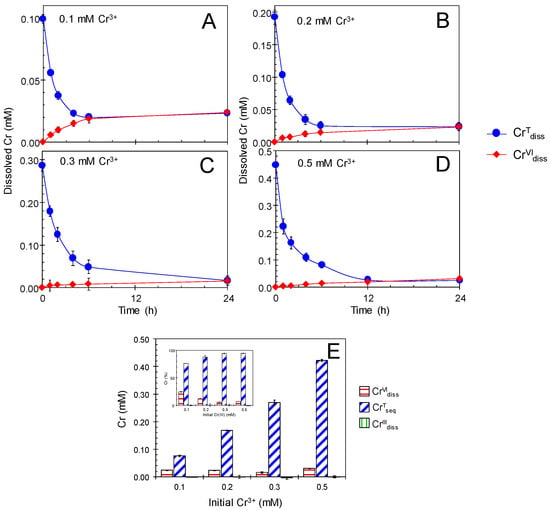
Figure 5.
Single treatment of heated biogenic manganese oxides (BMOs; 1 mM as Mn) with 0.1–0.5 mM Cr(NO3)3 under aerobic conditions at pH 6.0 (100 mM MES buffer). (A–E) Concentrations of total Cr dissolved (CrTdiss) and Cr(VI) dissolved (CrVIdiss). Initial Cr(NO3)3 concentrations were: (A) 0.1 mM, (B) 0.2 mM, (C) 0.3 mM, and (D) 0.5 mM. (E) Concentrations (inset: percentages) of total Cr sequestered (CrTseq), Cr(VI) dissolved (CrVIdiss), and Cr(III) dissolved (CrIIIdiss) after the treatments. Data are shown as mean ± standard deviation (n = 3).
Interestingly, this first phase (~2 h) of the anaerobic reaction involved the sequestration process of total Cr concentration (CrTdiss) until CrTdiss became equal mostly to CrVIdiss (Figure 4). Cr(VI) does not have significant sorption affinity to either Mn oxide phase or fungal hyphae in BMOs in 100 mM MES at pH 6.0 (Figure S2B,C). Therefore, CrTdiss sequestration in the anaerobic treatments was mostly attributable to sorption and/or precipitation of Cr(III) on BMOs. At circumneutral pH in aqueous solutions, Cr3+ can be readily hydrolyzed to form monomers such as [Cr(OH)]2+ and [Cr(OH)2]+ as well as oligomers as [Cr2(OH)2]4+, [Cr3(OH)4]5+, and [Cr4(OH)6]6+ [46,47,48,49]. These polynuclear Cr(III) species may eventually precipitate as Cr(OH)3. Fendorf et al. [22] proposed that Cr(OH)3 precipitate on Mn oxide surface may be a causative factor for cessation of Cr(III) oxidation by acting as a barrier to the charge transfer between Cr(III) and structural Mn(III)/Mn(V), and as a redox stable sink for Cr(III). In contrast, Landrot et al. [25,26] observed no Cr precipitation on Mn oxide surfaces reacted with Cr(III) for 1 h, at which time the Cr(III) oxidation had already ceased, suggesting inhibition mechanisms other than Cr(OH)3 precipitation were the cause for the cessation.
To check the reversibility of the cessation of Cr(III) oxidation by newly formed BMOs with respect to enzymatic Mn(II) activity, we conducted the single treatment of newly formed BMO (1 mM as Mn) in 0.5 mM Cr(NO3)3 (100 mM MES at pH 6.0) under anaerobic, and subsequently, aerobic conditions. During the initial anaerobic treatment, CrVIdiss production and significant MnIIrel concomitantly ceased within the first 2 h, when the majority of the CrIIIInt was sequestered (CrTseq) in the solid phase (Figure 6). When the condition was switched to aerobic, CrVIdiss was rapidly produced and concomitantly MnIIrel disappeared from the solution phase (Figure 6). The reversible production of CrVIdiss with respect to enzymatic Mn(II) oxidation activity may deny the hypothesis that Cr(OH)3 precipitation is responsible for anaerobic cessation of Cr(III) oxidation by BMOs. Our previous studies demonstrated the anaerobic cessation of oxidative reactions of As(III) to As(V) [29] and Co(II) to Co(III) [30] by newly formed BMOs, whereas aerobic conditions prevented the cessation of As(III)- and Co(II)-oxidation reactions. We assumed the anaerobic cessation of Cr(III) oxidation was due to the surface passivation by the accumulation of reduced Mn(II)/Mn(III) species at the redox reactive sites on BMOs, resulting in insufficient electron transfer from the BMO phase to the reductant species including Cr(III).
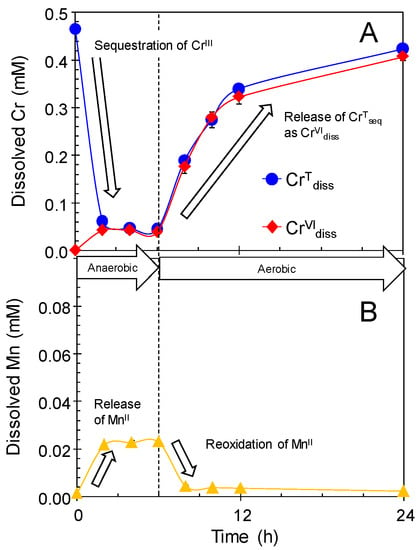
Figure 6.
Concentrations of (A) total Cr dissolved (CrTdiss), Cr(VI) dissolved (CrVIdiss), and (B) Mn(II) released (MnIIrel) in single treatment of newly formed BMO (1 mM as Mn) in 0.5 mM Cr(NO3)3 (100 mM MES at pH 6.0) under anaerobic and subsequently aerobic conditions.
2.3. Anaerobic Sequestration of Cr(III) by BMOs
When the newly formed BMOs (1 mM as Mn) were repeatedly treated in anaerobic 0.5 mM Cr(NO3)3 in 100 mM MES with the renewal of the bathing solutions every 24 h (Figure 7), the cumulative CrVIdiss concentration was 0.06 ± 0.00 mM (0.03 ± 0.00, 0.01 ± 0.00, and 0.01 ± 0.00 mM for the 1st, 2nd, and 3rd treatments, respectively), resulting in only 4% of cumulative CrIIIInt oxidation (Figure 7 and Table S2). In contrast to very low oxidation efficiency, the cumulative CTseq concentration was high at 1.18 mM (0.43 ± 0.01, 0.46 ± 0.01, and 0.14 ± 0.00 mM for the 1st, 2nd, and 3rd treatments, respectively), corresponding to 83% ± 1% of the cumulative CrIIIInt (Figure 7 and Table S2). The molar ratio of CTseq relative to solid Mn was abnormally high (~120 mol%), implying a sequestration mechanism specific to Cr other than the simple sorption process of common divalent heavy metal ions, Zn2+ and Cd2+ (their maximum sorption relative to solid Mn was 20–25 mol%; [39,41]) or trivalent La3+ ions (~30 mol% [45]). Heated BMOs possessed similar Cr sequestration capacity (Figure S3) even under aerobic conditions with the domination of Cr(III) in the solid phase, which was revealed by X-ray Absorption Near-Edge Structure (XANES) (Figure 8). The XANES spectrum for the reference compound of K2CrVI2O7 showed a sharp pre-edge and a broad peak at 5988 eV and around 6030 eV, respectively. The former peak is assigned at the 1s→3d-4p hybrid orbital transition and characteristic of Cr(VI) [57]. The XANES spectrum for CrIII(NO3)3 9H2O has a main peak around 6005 eV, along with a pre-edge peak (the 1s→3d-4p hybrid orbital transition of Cr(III)) at 5985 eV with a much smaller intensity (Figure 8) [57]. The linear combination fit showed that BMO had >95% Cr(III) content after treatment in 0.5 mM Cr(NO3)3 for 24 h. Consequently, it is likely that sorption as the polynuclear Cr(III) species would lead to abnormally high Cr sequestration capacity of BMO when enzymatically inactivated. The XANES spectrum also showed that domination of Cr(III) remained in newly formed BMOs at 24 h of the aerobic Cr(III) treatments where >80% of CrIIIInt was converted to CrVIdiss, strongly indicating a preferential sorption of Cr(III) over Cr(VI) on BMO. Once Cr(III) is converted to Cr(VI) on BMO surface, it is readily leached to the solution phase as demonstrated in Figure 6.
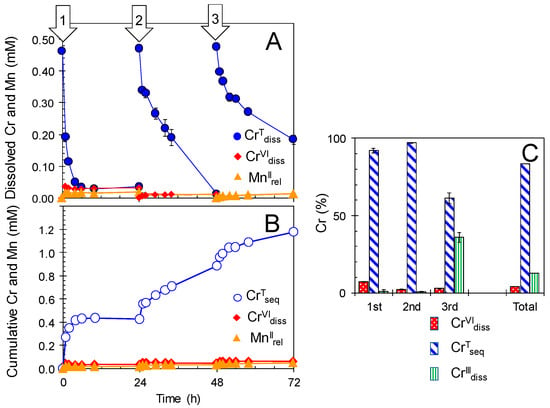
Figure 7.
Repeated treatments of newly formed biogenic manganese oxides (BMOs; 1 mM as Mn) with 0.5 mM Cr(NO3)3 under anaerobic conditions at pH 6.0 (100 mM MES buffer). (A) Concentrations of total Cr dissolved (CrTdiss), Cr(VI) dissolved (CrVIdiss), and Mn(II) released (MnIIrel). (B) Cumulative concentrations of total Cr sequestered (CrTseq), CrVIdiss, and MnIIrel. (C) Percentages of CrVIdiss, CrTseq, and Cr(III) dissolved (CrIIIdiss) during the repeated treatments. Bathing solutions were renewed every 24 h for 3 days (indicated by arrows). Data are shown as mean ± standard deviation (n = 3).
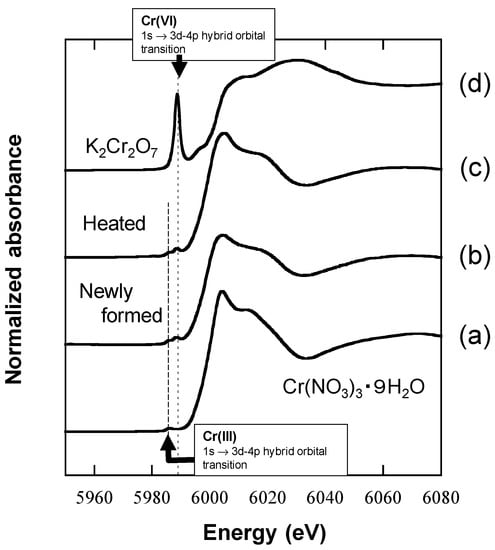
Figure 8.
Cr K-edge X-ray Absorption Near-Edge Structure (XANES) of newly formed and heated BMOs treated in 0.5 mM Cr(NO3)3 solution/100 mM MES (pH 6.0) for 24 h under aerobic condition. Cr(NO3)3·9H2O and K2Cr2O7 represent Cr(III) and Cr(VI) references, respectively. (a) Cr(NO3)3·9H2O, (b) newly formed and (c) heated BMOs treated in Cr(NO3)3, and (d) K2Cr2O7.
Newly formed BMOs produced by A. strictum KR21-2 are layered analogously to vernadite, a natural nanostructured and turbostratic variety of birnessite [32]. Prior to 0.5 mM Cr(NO3)3 treatments, no apparent changes in X-ray Diffraction (XRD) patterns were observed between newly formed and heated BMOs (Figure 9), for which the XRD peaks at ~7.3, 2.4, and 1.4 Å were assigned to (001), (11,20), and (31,02), respectively [39]. Following the treatment, heated BMO exhibited a decline in XRD intensity arising from (001) basal reflection (Figure 9), suggesting that the ordering of the vernadite (birnessite) sheet stacking was mostly disturbed by sorption of polynuclear Cr(III) species with a high proportion of CrTseq (0.42 ± 0.00 mM) relative to solid Mn (1 mM as Mn). In contrast, newly formed BMOs maintained a (001) XRD intensity even after the treatments (Figure 9) where much less CrTseq remained in the solid (0.06 ± 0.02 mM). High sorption affinity of BMO for polynuclear Cr(III) species should play an as important role in efficient Cr(III) oxidation in aerobic treatments because significant amounts of Cr sequestration (i.e., a decrease in CrTdiss in Figure 1) progressed concomitantly with CrVIdiss production.
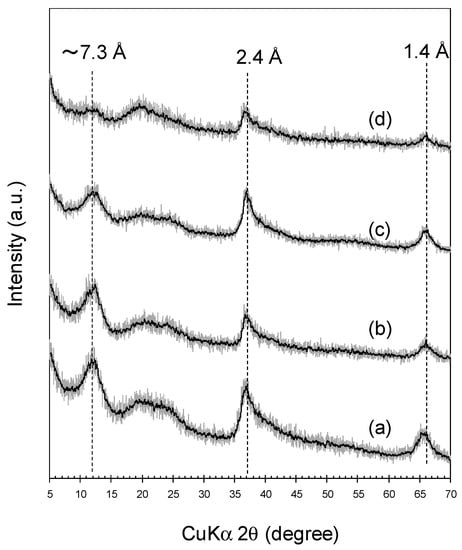
Figure 9.
X-ray Diffraction (XRD) of newly formed and heated BMOs before and after treatments in 0.5 mM Cr(NO3)3/100 mM MES (pH 6.0) for 24 h under aerobic condition. Newly formed BMOs (a) before and (b) after the treatments, and heated BMOs (c) before and (d) after the treatments.
3. Materials and Methods
3.1. Formation of Fungal BMOs by A. strictum KR21-2
Acremonium strictum KR21-2, which is capable of enzymatically oxidizing Mn(II) to BMO [51,52], was grown in HAY liquid medium (20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid (HEPES) buffer (pH 7.0) with 1 mM MnSO4) as reported previously [27,30,39,41].
3.2. Cr(III)-Oxidizing Ability of Newly Formed and Heated BMOs at pH 6.0
After incubating A. strictum KR21-2 in 1 mM Mn2+-supplemented HAY medium (pH 7.0) for 72 h, the solid phase (biooxide plus fungal mycelia; hereafter designated “newly formed BMO”) was harvested, washed with 100 mM 2-morpholinoethanesulfonic acid (MES) buffer (pH 6.0) three times, and treated with 0.1–0.5 mM Cr(NO3)3 in 100 mM MES (pH 6.0) for 24 h. To monitor the abilities of Cr(III) oxidation and sequestration of newly formed BMO under air-equilibrated (aerobic) and N2 gas-purged (anaerobic) conditions as reported previously [30,39,41]. Supernatants were sampled at 0, 2, 4, 8, and 24 h, separated by centrifugation (11,000× g for 2 min), and preserved in 2% HNO3. Total Cr(III) and Cr(VI) concentrations were analyzed by a Varian 730-ES inductively coupled plasma-atomic emission spectrometer (ICP-AES) (Agilent Inc., Santa Clara, CA, USA). The Cr(VI) concentrations were determined by colorimetric method according to Urone [58], and Bartlett and James [59]. Newly formed BMOs were enzymatically inactivated by being heated at 85 °C for 1 h in a liquid culture medium using a water bath (Thermo Minder Mini-80, Taitec Corp., Koshigaya, Japan), allowed to cool to room temperature before use (hereafter designated “heated BMO”; [27]), and then used for Cr(III) oxidation experiments with 0.1–0.5 mM Cr(NO3)3 in 100 mM MES (pH 6.0) under aerobic conditions.
Repeated treatment experiments were also carried out with renewal of the bathing solutions (0.1 or 0.5 mM Cr(NO3)3 solution with 100 mM MES) every 24 h under aerobic and anaerobic conditions.
3.3. X-ray Absorption Near-Edge Structure (XANES) Spectroscopy
Chromium K-edge XANES spectra were collected at the beamline 12C at the Photon Factory, KEK (Tsukuba, Japan). Newly formed (enzymatically active) and heated (enzymatically inactive) BMOs were treated with 0.5 mM Cr(NO3)3 in 100 mM MES (pH = 6.0) for under aerobic condition (24 h), harvested and lyophilized. As references for Cr(VI) and Cr(III) species, aqueous solutions of K2Cr2O7 and Cr(NO3)3·9H2O, were measured respectively. The storage ring was operated at 2.5 GeV with a typical beam current of 450 mA. The incident X-ray beam was obtained by monochromatization of the broad band synchrotron radiation from the storage ring with a Si (111) double-crystal monochromator. The beam size of the incident X-ray was focused into an area 1 × 1 mm2 at the sample position. The spectra of the reference solutions and the BMO samples were collected in transmission mode using ionization chambers.
3.4. Powder X-ray Diffraction (XRD)
The XRD measurements BMOs were carried out after single treatment experiments with 0.5 mM Cr(NO3)3 in 100 mM MES (pH = 6.0) for newly formed (enzymatically active) and heated (enzymatically inactive) BMOs under aerobic condition (24 h). Treated BMOs were harvested, and lyophilized. A RINT-2500 diffractometer (Rigaku Corp., Tokyo, Japan) was used with CuKα radiation at 26 mA and 40 kV. The scanning range was from 5° to 70° of 2θ and scanning rate was 1.0° min−1 with a step interval of 0.02°. The diffractograms were smoothed by the 10-point moving average method to display broad peaks more clearly.
4. Conclusions
The results presented here demonstrate that under aerobic conditions, enzymatically active BMOs efficiently mediate oxidation of Cr(III) to Cr(VI) without releasing reduced Mn(II) at pH 6.0. Thus, enzymatically active BMOs may aerobically leach (remove) Cr from the Cr(III)-contaminated sites, through catalytic oxidation to more labile Cr(VI). In contrast, Cr(III) oxidation readily ceases under anaerobic conditions due to the surface passivation of BMOs, whereas sequestration of Cr(III) (most probably as polynuclear Cr(III) species) progresses with extraordinary sequestration capacity of Cr(III) (up to 120% mol of Cr relative to solid Mn). This shows the potential applicability of BMOs as an efficient sorbent for Cr(III) from wastewater at a circumneutral pH. Interestingly, anaerobically sequestered Cr(III) can subsequently be released by switching to aerobic conditions, through the catalytic (enzymatic) Mn oxidation cycles by BMOs. These findings provide supporting evidence for the potential applications of BMOs towards the recovery of Cr species from industrial wastewater (anaerobic Cr sequestration) and environmental remediation of Cr-contaminated sites (aerobic oxidative Cr leaching).
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/1/44/s1, Abbreviations, Figure S1: Release of Mn(II) during single treatments of BMOs in Cr(NO3)3. Figure S2: Stability of dissolved 0.5 mM Cr(NO3)3, and sorption of Cr(VI) on BMOs, Figure S3: Repeated treatments of heated BMOs in aerobic Cr(NO3)3, Table S1: Summary of single treatment experiments of BMOs in Cr(NO3)3, Table S2: Summary of repeated treatments of BMOs in Cr(NO3)3.
Author Contributions
Conceptualization, R.S. and Y.T.; methodology, Y.T., N.M. and K.T.; Characterization, R.S., H.N., N.M. and K.T.; investigation, R.S. and H.N.; writing—original draft preparation, R.S. and Y.T.; writing—review and editing, R.S. and Y.T.; visualization, R.S. and Y.T.; supervision, Y.T.; funding acquisition, Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by The Salt Science Research Foundation, No. 1907 (Y.T.), and the Japan Society for the Promotion of Science KAKENHI, No. 16K00615 (Y.T.).
Acknowledgments
The authors would like to thank The Salt Science Research Foundation, and the Japan Society for the Promotion of Science KAKENHI for the research funding and Editage for providing editorial assistance. Chromium K-edge XANES measurement was performed with the approval of the Photon Factory, KEK (Proposal No. 2018G111).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zayed, A.M.; Terry, N. Chromium in the environment: Factors affecting biological remediation. Plant Soil 2003, 249, 139–156. [Google Scholar] [CrossRef]
- Oliveira, H. Chromium as an environmental pollutant: Insights on induced plant toxicity. J. Bot. 2012, 2012, 375843. [Google Scholar] [CrossRef]
- Sun, H.; Brocato, J.; Costa, M. Oral chlomium exposure and toxicity. Curr. Environ. Health Rep. 2015, 2, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.; Yousafzai, A.M. Chromium toxicity in fish: A review article. J. Entomol. Zool. Stud. 2017, 5, 1483–1488. [Google Scholar]
- Babula, P.; Adam, V.; Opatrilova, R.; Zehnalek, J.; Havel, L.; Kizek, R. Uncommon heavy metals, metalloids and their plant toxicity: A review. Environ. Chem. Lett. 2008, 6, 189–213. [Google Scholar] [CrossRef]
- Diwan, H.; Ahmad, A.; Iqbal, M. Characterization of chromium toxicity in food crops and their role in phytoremediation. J. Bioremed. Biodegrad. 2012, 3, 1000159. [Google Scholar] [CrossRef]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant systems: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef]
- Bartlett, R.J. Chromium cycling in soils and water: Links, gaps, and methods. Environ. Health Perspect. 1991, 92, 17–24. [Google Scholar] [CrossRef]
- Adriano, D.C.; Wenzel, W.W.; Vangronsveld, J.; Bolan, N.S. Role of assisted natural remediation in environmental cleanup. Geoderma 2004, 122, 121–142. [Google Scholar] [CrossRef]
- Tebo, B.M.; Bargar, J.R.; Clement, B.G.; Dick, G.J.; Murray, K.J.; Parker, D.; Verity, R.; Webb, S.M. Biogenic manganese oxides: Properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci. 2004, 32, 287–328. [Google Scholar] [CrossRef]
- Miyata, N.; Tani, Y.; Sakata, M.; Iwahori, K. Microbial manganese oxide formation and interaction with toxic metal ions. J. Biosci. Bioeng. 2007, 104, 1–8. [Google Scholar] [CrossRef]
- Tani, Y.; Miyata, N.; Iwahori, K.; Soma, M.; Tokuda, S.; Seyama, H.; Theng, B.K.G. Biogeochemistry of manganese oxide coatings on pebble surfaces in the Kikukawa River System, Shizuoka, Japan. Appl. Geochem. 2003, 18, 1541–1554. [Google Scholar] [CrossRef]
- Furuta, S.; Ikegaya, H.; Hashimoto, H.; Ichise, S.; Kohno, T.; Miyata, N.; Takada, J. Formation of filamentous Mn oxide particles by the alphaproteobacterium Bosea sp. strain BIWAKO-01. Geomicrobiol. J. 2015, 32, 666–676. [Google Scholar] [CrossRef]
- Cahyani, V.R.; Murase, J.; Ishibashi, E.; Asakuwa, S.; Kimura, M. Phylogenetic positions of Mn2+-oxidizing bacteria and fungi isolated from Mn nodules in rice field subsoils. Biol. Fertil. Soils 2009, 45, 337–346. [Google Scholar] [CrossRef]
- Miller, A.Z.; Dionisio, A.; Braga, M.A.S.; Hernandez-Marine, M.; Afonso, M.J.; Muralha, V.S.F.; Herrera, L.K.; Raabe, J.; Fernandez-Cortes, A.; Cuezva, S.; et al. Biogenic Mn oxide minerals coating in a subsurface granite environment. Chem. Geol. 2012, 322–323, 181–191. [Google Scholar] [CrossRef]
- Ivarsson, M.; Broman, C.; Gustafsson, H.; Holm, N.G. Biogenic Mn-oxide in subseafloor basalts. PLoS ONE 2015, 10, e0128863. [Google Scholar] [CrossRef]
- Murray, K.J.; Mozafarzadeh, M.L.; Tebo, B.M. Cr(III) oxidation and Cr toxicity in cultures of the manganese(II)-oxidizing Pseudomonas putida strain GB-1. Geomicrobiol. J. 2005, 22, 151–159. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, B.; Xu, H.; Kornishi, H. Chromium(III) oxidation coupled with microbially mediated Mn(II) oxidation. Geomicrobiol. J. 2005, 22, 161–170. [Google Scholar] [CrossRef]
- Murray, K.J.; Tebo, B.M. Cr(III) is indirectly oxidized by the Mn(II)-oxidizing bacterium Bacillus sp. strain SG-1. Environ. Sci. Technol. 2007, 41, 528–533. [Google Scholar] [CrossRef]
- He, J.Z.; Meng, Y.T.; Zheng, Y.M.; Zhang, L.M. Cr(III) oxidation coupled with Mn(II) bacterial oxidation in the environment. J. Soils Sediments 2010, 10, 767–773. [Google Scholar] [CrossRef][Green Version]
- Tang, Y.; Webb, S.M.; Estes, E.R.; Hansel, C.M. Chromium(III) oxidation by biogenic manganese oxides with varying structural ripening. Environ. Sci. Process. Impacts 2014, 16, 2127–2136. [Google Scholar] [CrossRef]
- Fendorf, S.E.; Fendorf, M.; Spaeks, D.L.; Gronsky, R. Inhibitory mechanisms of Cr(III) oxidation by d-MnO2. J. Colloid Interface Sci. 1992, 153, 37–54. [Google Scholar] [CrossRef]
- Fendorf, S.E.; Zasoski, R.J. Chromium(III) oxidation by d-MnO2. 1. Characterization. Environ. Sci. Technol. 1992, 26, 79–85. [Google Scholar] [CrossRef]
- Stepniewska, Z.; Bucior, K.; Bennicelli, R.P. The effect of MnO2 on sorption and oxidation of Cr(III) by soils. Geoderma 2004, 122, 291–296. [Google Scholar] [CrossRef]
- Landrot, G.; Ginder-Vogel, M.; Livi, K.; Fitts, J.P.; Sparks, D.L. Chromium(III) oxidation by three poorly-crystalline manganese(IV) oxides. 1. Chromium(III)-oxidizing capacity. Environ. Sci. Technol. 2012, 46, 11594–11600. [Google Scholar] [CrossRef]
- Landrot, G.; Ginder-Vogel, M.; Livi, K.; Fitts, J.P.; Sparks, D.L. Chromium(III) oxidation by three poorly-crystalline manganese(IV) oxides. 2. Solid phase analyses. Environ. Sci. Technol. 2012, 46, 11601–11609. [Google Scholar] [CrossRef]
- Chang, J.; Tani, Y.; Naitou, H.; Miyata, M.; Seyama, H. Fungal Mn oxides supporting Mn(II) oxidase activity as effective Mn(II) sequestering materials. Environ. Technol. 2013, 34, 2781–2787. [Google Scholar] [CrossRef]
- Tani, Y.; Miyata, N.; Ohashi, M.; Ohnuki, T.; Seyama, H.; Iwahori, K.; Soma, M. Interaction of inorganic arsenic with biogenic manganese oxide produced by a Mn-oxidizing fungus, strain KR21-2. Environ. Sci. Technol. 2004, 38, 6618–6624. [Google Scholar] [CrossRef]
- Watanabe, J.; Tani, Y.; Chang, J.; Miyata, N.; Naitou, H.; Seyama, H. As(III) oxidation kinetics of biogenic manganese oxides formed by Acremonium strictum strain KR21-2. Chem. Geol. 2013, 347, 227–232. [Google Scholar] [CrossRef]
- Chang, J.; Tani, Y.; Naitou, H.; Miyata, N.; Seyama, H.; Tanaka, K. Cobalt(II) sequestration on fungal biogenic manganese oxide enhanced by manganese(II) oxidase activity. Appl. Geochem. 2013, 37, 170–178. [Google Scholar] [CrossRef]
- Villalobos, M.; Bargar, J.; Sposito, G. Trace metal retention on biogenic manganese oxide nanoparticles. Elements 2005, 1, 223–226. [Google Scholar] [CrossRef]
- Grangeon, S.; Lanson, B.; Miyata, N.; Tani, Y.; Manceau, A. Structure of nanocrystalline phyllomanganates produced by freshwater fungi. Am. Mineral. 2010, 95, 1608–1616. [Google Scholar] [CrossRef]
- Nelson, Y.M.; Lion, L.W.; Ghiorse, W.C.; Shuler, M.L. Production of biogenic Mn oxides by Leprothrix discophora SS-1 in a chemically defined growth medium and evaluation of their Pb adsorption characteristics. Appl. Environ. Microbiol. 1999, 65, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Nelson, Y.M.; Lion, L.W.; Shuler, M.L.; Ghiorse, W.C. Effect of oxide formation mechanisms on lead adsorption by biogenic manganese (hydr)oxides, iron (hydr)oxides, and their mixtures. Environ. Sci. Technol. 2002, 36, 421–425. [Google Scholar] [CrossRef]
- Villalobos, M.; Bargar, J.; Sposito, G. Mechanism of Pb(II) sorption on a biogenic manganese oxide. Environ. Sci. Technol. 2005, 39, 569–576. [Google Scholar] [CrossRef]
- Toner, B.; Manceau, A.; Webb, S.M.; Sposito, G. Zinc sorption to biogenic hexagonal birnessite particles within a hydrated bacterial biofilm. Geochim. Cosmochim. Acta 2006, 70, 27–43. [Google Scholar] [CrossRef]
- Wang, W.M.; Shao, Z.Z.; Liu, Y.J.; Wang, G.J. Removal of multi-heavy metals using biogenic manganese oxides generated by a deep-sea sedimentary bacterium Brachybacterium sp. strain Mn32. Microbiology 2009, 155, 1989–1996. [Google Scholar] [CrossRef]
- Yu, Q.; Sasaki, K.; Tanaka, K.; Ohnuki, T.; Hirajima, T. Zinc sorption during bio-oxidation and precipitation of manganese modifies the layer stacking of biogenic birnessite. Geomicrobiol. J. 2013, 30, 829–839. [Google Scholar] [CrossRef]
- Chang, J.; Tani, Y.; Naitou, H.; Miyata, M.; Tojo, F.; Seyama, H. Zn(II) sequestration by fungal biogenic manganese oxide through enzymatic and abiotic processes. Chem. Geol. 2014, 383, 155–163. [Google Scholar] [CrossRef]
- Meng, Y.T.; Zheng, Y.M.; Zhang, L.M.; He, J.Z. Biogenic Mn oxides for effective adsorption of Cd from aquatic environment. Environ. Pollut. 2009, 157, 2577–2583. [Google Scholar] [CrossRef]
- Chang, J.; Tani, Y.; Naitou, H.; Miyata, N.; Seyama, H.; Tanaka, K. Sequestration of Cd(II) and Ni(II) ions on fungal manganese oxides associated with Mn(II) oxidase activity. Appl. Geochem. 2014, 47, 198–208. [Google Scholar] [CrossRef]
- Zhu, M.Q.; Ginder-Vogel, M.; Sparks, D.L. Ni(II) sorption on biogenic Mn-oxides with varying Mn octahedral layer structure. Environ. Sci. Technol. 2010, 44, 4472–4478. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Kaseyama, T.; Hirajima, T. Selective sorption of Co(II) over Ni(II) using biogenic manganese oxides. Mater. Trans. 2009, 50, 2643–2648. [Google Scholar] [CrossRef]
- Yu, Q.; Ohnuki, T.; Tanaka, K.; Kozai, N.; Yamasaki, S.; Sakamoto, F.; Tani, Y. Fungal-promoted transformation of lanthanides during the biooxidation of divalent manganese. Geochim. Cosmochim. Acta. 2016, 174, 1–12. [Google Scholar] [CrossRef]
- Zheng, H.; Tani, Y.; Naitou, H.; Miyata, N.; Tojo, F.; Seyama, H. Sequestration of La3+ by fungal manganese oxides and the effect of Mn(II) oxidase activity. J. Environ. Chem. Eng. 2017, 5, 735–743. [Google Scholar] [CrossRef]
- Stunzi, H.; Rotzinger, F.P.; Marty, W. Early stages of the hydrolysis of chromium(III) in aqueous solution. 2. Kinetics and mechanism of the interconversion between two tetrametric species. Inorg. Chem. 1984, 23, 2160–2164. [Google Scholar] [CrossRef]
- Stunzi, H.; Marty, W. Early stages of the hydrolysis of chromium(III) in aqueous solution. 1. Characterization of a tetrameric species. Inorg. Chem. 1983, 22, 2145–2150. [Google Scholar] [CrossRef]
- Saleh, F.Y.; Mbamalu, G.E.; Brungardt, C.E. Ion chromatography-photodiode arra UV-visible detection of Cr(III) hydrolytic polymerization products in pure and natural waters. Anal. Chem. 1996, 68, 740–745. [Google Scholar] [CrossRef]
- Rao, L.; Zhang, Z.; Friese, J.I.; Ritherdon, B.; Clark, S.B.; Hess, N.J.; Rai, D. Oligomerization of chromium(III) and its impact on the oxidation of chromium(III) by hydrogen peroxide in alkaline solutions. J. Chem. Soc. Dalton Trans. 2002, 267–274. [Google Scholar] [CrossRef]
- Hu, L.; Cai, Y.; Jiang, G. Occurrence and speciation of polymeric chromium(III), monomeric chromium(III) and chromium(VI) in environment samples. Chemosphere 2016, 156, 14–20. [Google Scholar] [CrossRef]
- Miyata, N.; Tani, Y.; Iwahori, K.; Soma, M. Enzymatic formation of manganese oxides by an Acremonium-like hyphomycete fungus, strain KR21-2. FEMS Microbiol. Ecol. 2004, 47, 101–109. [Google Scholar] [CrossRef]
- Miyata, N.; Tani, Y.; Maruo, K.; Tsuno, H.; Sakata, M.; Iwahori, K. Manganese(IV) oxide production by Acremonium sp. strain KR21-2 and extracellular Mn(II) oxidase activity. Appl. Environ. Microbiol. 2006, 72, 6467–6473. [Google Scholar] [CrossRef] [PubMed]
- Inthorn, D.; Tani, Y.; Chang, J.; Naitou, H.; Miyata, N. Magnetically modified fungal Mn oxides with high sequestration efficiency for simultaneously removing multiple heavy metal ions from wastewater. J. Environ. Chem. Eng. 2014, 2, 1635–1641. [Google Scholar] [CrossRef]
- Zheng, H.; Tani, Y.; Naitou, H.; Miyata, N.; Tojo, F. Oxidative Ce3+ sequestration on fungal manganese oxides with an associated Mn(II) oxidase activity. Appl. Geochem. 2016, 71, 110–122. [Google Scholar] [CrossRef]
- Elyahyaoui, A.; Ellouzi, K.; Zabadi, H.A.; Razzouki, B.; Bouhlassa, S.; Azzaoui, K.; Mejdoubi, E.M.; Hamed, O.; Jodeh, S.; Lamhamdi, A. Adsorption of chromium(VI) on calcium phosphate: Mechanisms and stability constants of surface complexes. Appl. Sci. 2017, 7, 222. [Google Scholar] [CrossRef]
- Grossl, P.R.; Eick, M.; Spark, D.L.; Goldberg, S.; Ainsworth, C.C. Arsenate and chromate retention mechanisms on goethite. 2. Kinetic evaluation using a pressure-jump relaxation technique. Environ. Sci. Technol. 1997, 31, 321–326. [Google Scholar] [CrossRef]
- Ohta, A.; Kagi, H.; Tsuno, H.; Nomura, M.; Okai, T. Speciation study of Cr(VI/III) reacting with humic substances and determination of local structure of Cr binding humic substances using XAFS spectroscopy. Geochem. J. 2012, 46, 409–420. [Google Scholar] [CrossRef]
- Urone, P.E. Stability of colorimetric reagent for chromium, S-diphenylcarbazides, in various solvents. Anal. Chem. 1955, 27, 1354–1355. [Google Scholar] [CrossRef]
- Bartlett, R.J.; James, J.M. Behavior of chromium in soil III. Oxidation. J. Environ. Qual. 1979, 8, 31–35. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).