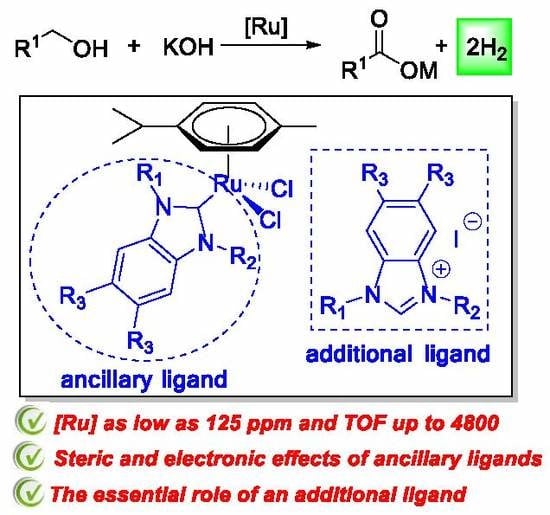
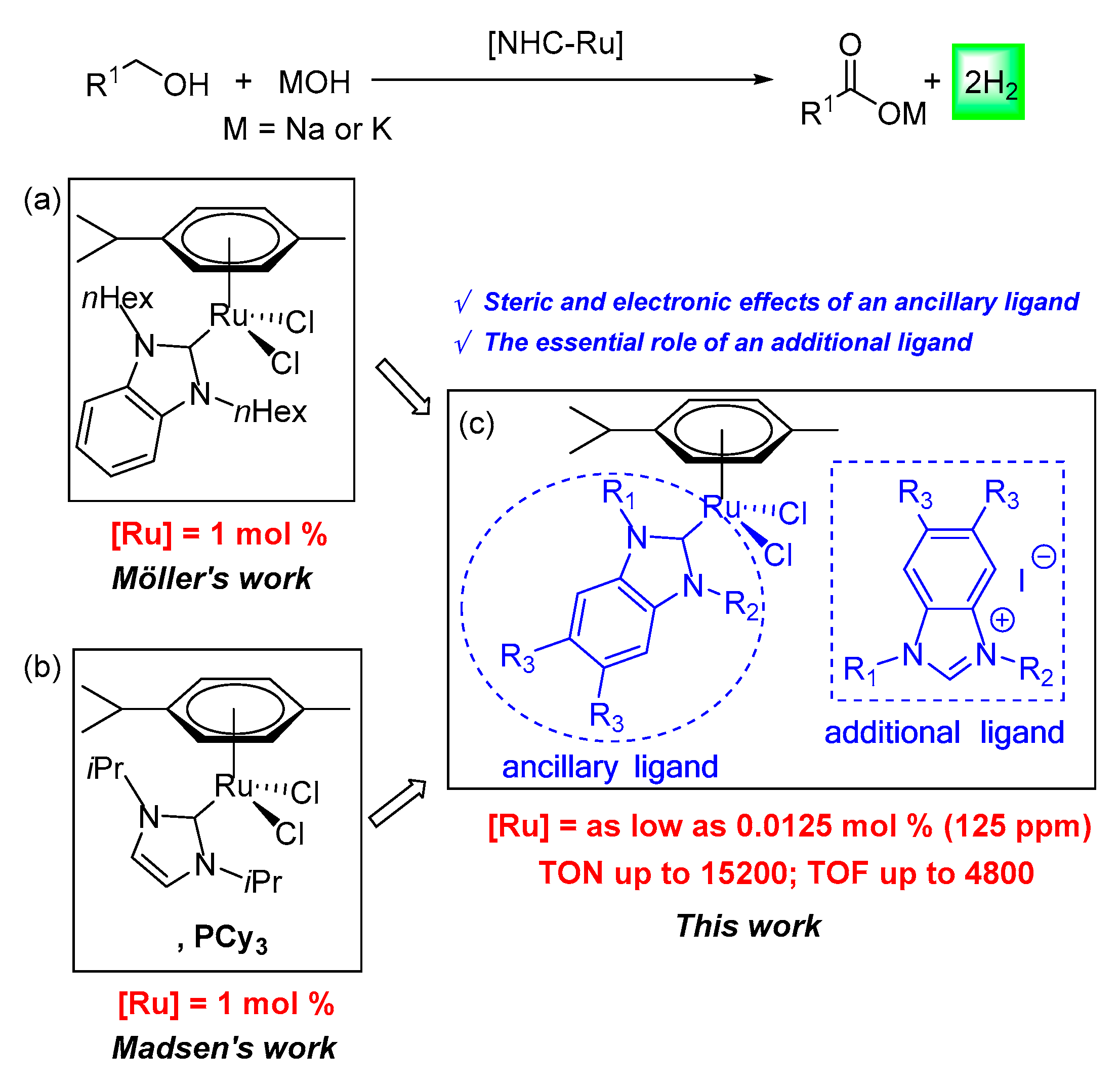
Highly Efficient N-Heterocyclic Carbene/Ruthenium Catalytic Systems for the Acceptorless Dehydrogenation of Alcohols to Carboxylic Acids: Effects of Ancillary and Additional Ligands
Abstract
:1. Introduction
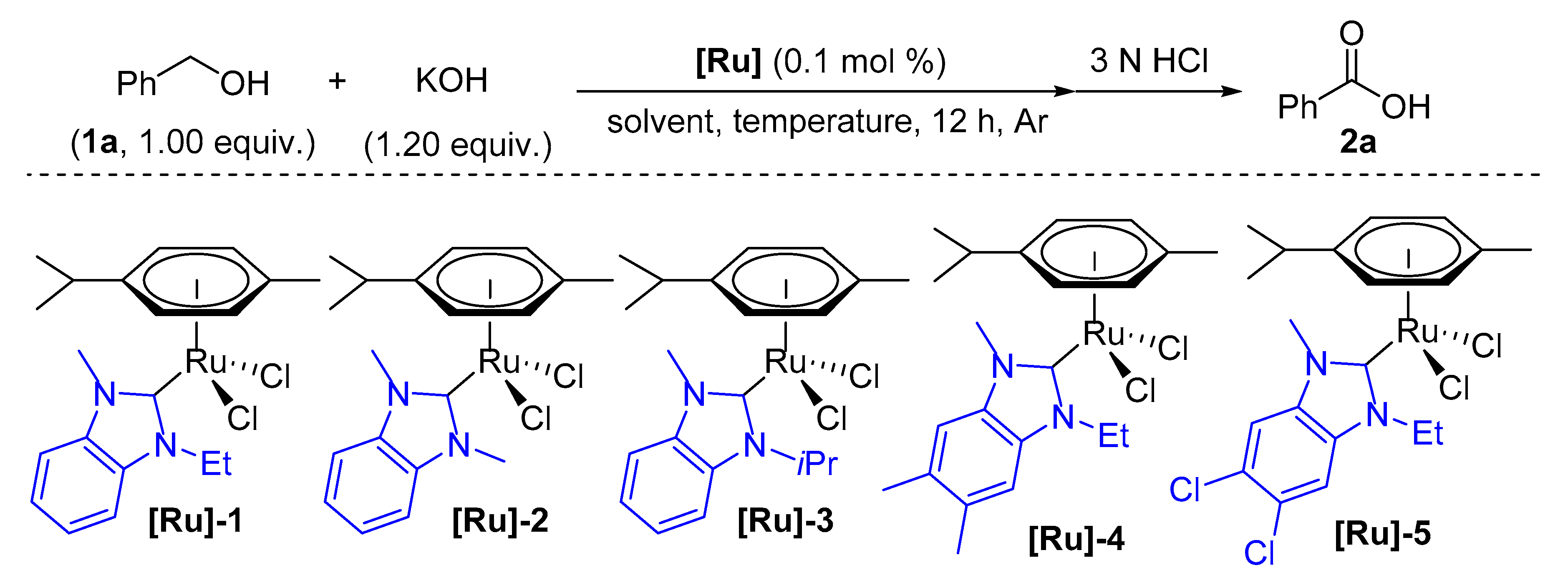
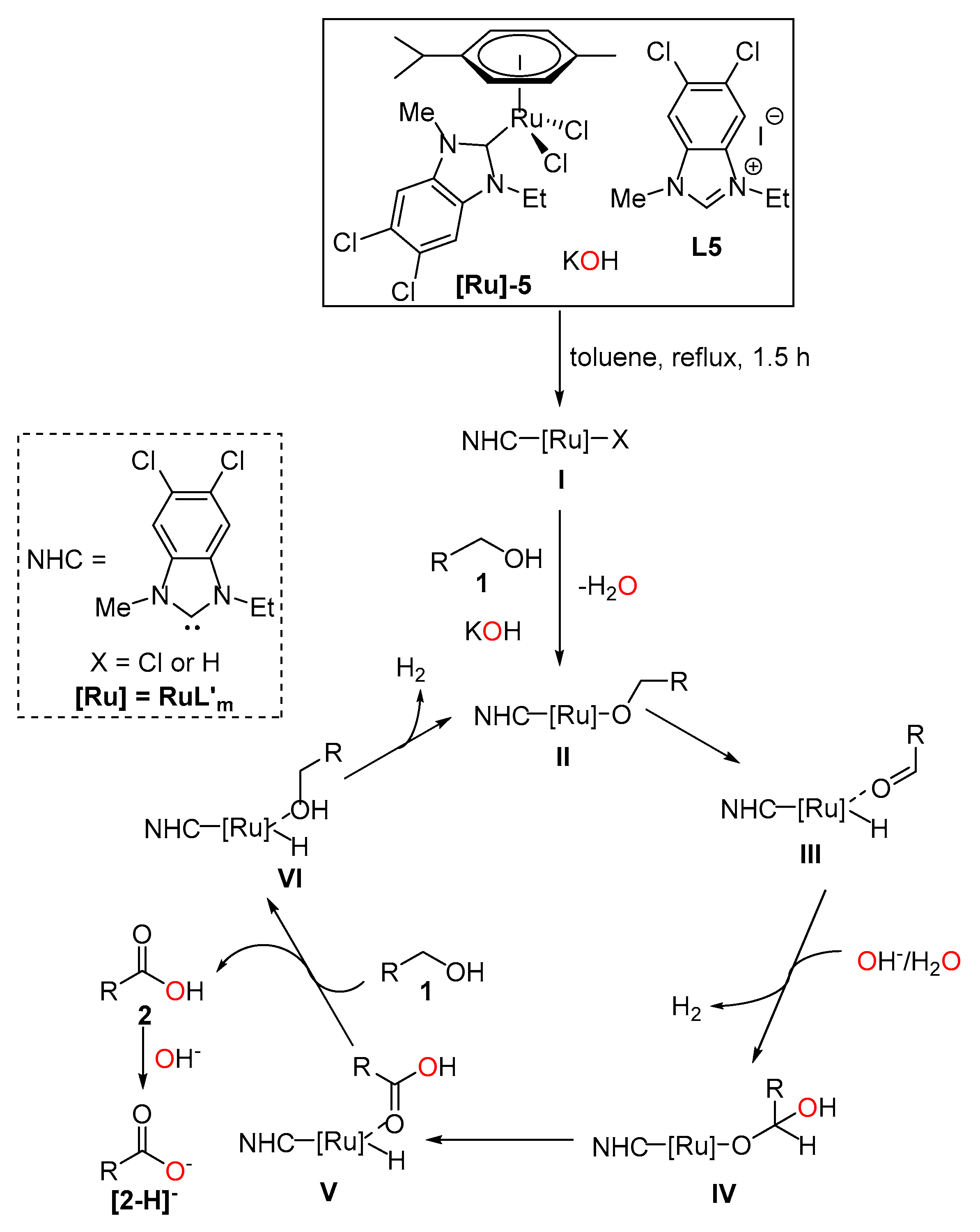
2. Results and Discussion
3. Experimental Section
3.1. General Considerations
3.2. General Procedure for the Synthesis of L5
3.3. General Procedure for the Synthesis of [Ru]-3–[Ru]-5
3.4. General Procedure for the NHC/Ru-Catalyzed Acceptorless Dehydrogenation of Alcohols to Acids
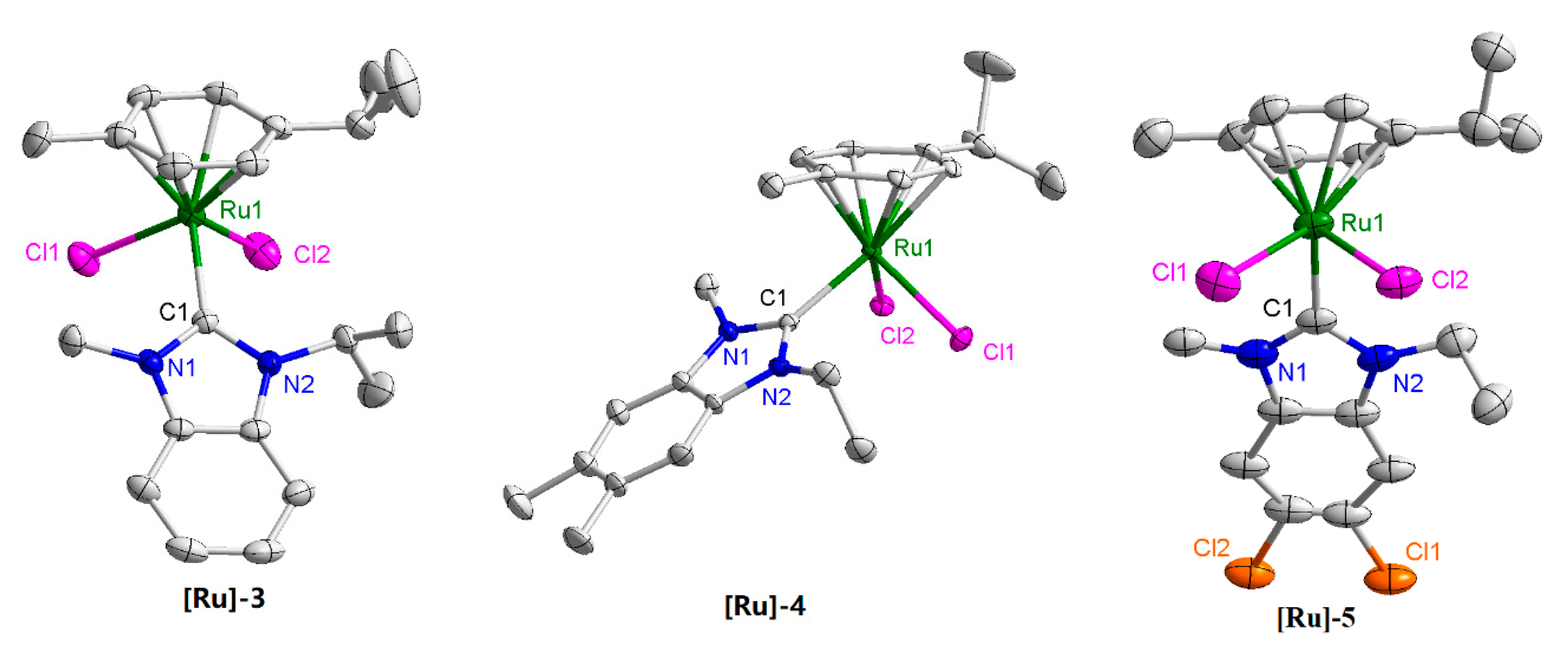
3.5. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ryland, B.L.; Stahl, S.S. Practical aerobic oxidations of alcohols and amines with homogeneous copper/TEMPO and related catalyst systems. Angew. Chem. Int. Ed. 2014, 53, 8824–8838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Moteki, T.; Gokhale, A.A.; Flaherty, D.W.; Toste, F.D. Production of fuels and chemicals from biomass: Condensation reactions and beyond. Chem 2016, 1, 32–58. [Google Scholar] [CrossRef] [Green Version]
- Tojo, G.; Fernández, M. Oxidation of Primary Alcohols to Carboxylic Acids; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Mazitschek, R.; Muelbaier, M.; Giannis, A. IBX-mediated oxidation of primary alcohols and aldehydes to form carboxylic acids. Angew. Chem. Int. Ed. 2002, 41, 4059–4061. [Google Scholar] [CrossRef]
- Tohma, H.; Kita, Y. Hypervalent iodine reagents for the oxidation of alcohols and their application to complex molecule synthesis. Adv. Synth. Catal. 2004, 346, 111–124. [Google Scholar] [CrossRef]
- Patel, S.; Mishra, B.K. Chromium (VI) oxidants having quaternary ammonium ions: Studies on synthetic applications and oxidation kinetics. Tetrahedron 2007, 63, 4367–4406. [Google Scholar] [CrossRef]
- ten Brink, G.J.; Arends, I.W.; Sheldon, R.A. Green, catalytic oxidation of alcohols in water. Science 2000, 287, 1636–1639. [Google Scholar] [CrossRef]
- Yamada, Y.M.A.; Arakawa, T.; Hocke, H.; Uozumi, Y. A nanoplatinum catalyst for aerobic oxidation of alcohols in water. Angew. Chem. Int. Ed. 2007, 46, 704–706. [Google Scholar] [CrossRef]
- Zope, B.N.; Hibbitts, D.D.; Neurock, M.; Davis, R.J. Reactivity of the gold/water interface during selective oxidation catalysis. Science 2010, 330, 74–78. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, C.; Yuan, J.; Lei, A. Transition-metal-free aerobic oxidation of primary alcohols to carboxylic acids. New J. Chem. 2013, 37, 1700–1703. [Google Scholar] [CrossRef]
- Han, L.; Xing, P.; Jiang, B. Selective aerobic oxidation of alcohols to aldehydes, carboxylic Acids, and imines catalyzed by a Ag-NHC complex. Org. Lett. 2014, 16, 3428–3431. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, J.; Ma, S. Iron catalysis for room-temperature aerobic oxidation of alcohols to carboxylic acids. J. Am. Chem. Soc. 2016, 138, 8344–8347. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Shin, H.Y.; Kim, D.W.; Yang, J.W. Metal-free chemoselective oxidative dehomologation or direct oxidation of alcohols: Implication for biomass conversion. ChemSusChem 2016, 9, 241–245. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Mannel, D.S.; Root, T.W.; Stahl, S.S. Aerobic oxidation of diverse primary alcohols to carboxylic acids with a heterogeneous Pd–Bi–Te/C (PBT/C) catalyst. Org. Process Res. Dev. 2017, 21, 1388–1393. [Google Scholar] [CrossRef]
- Hazra, S.; Deb, M.; Elias, A.J. Iodine catalyzed oxidation of alcohols and aldehydes to carboxylic acids in water: A metal-free route to the synthesis of furandicarboxylic acid and terephthalic acid. Green Chem. 2017, 19, 5548–5552. [Google Scholar] [CrossRef]
- Liu, K.J.; Jiang, S.; Lu, L.H.; Tang, L.L.; Tang, S.S.; Tang, H.S.; Tang, Z.; He, W.M.; Xu, X. Bis (methoxypropyl) ether-promoted oxidation of aromatic alcohols into aromatic carboxylic acids and aromatic ketones with O2 under metal- and base-free conditions. Green Chem. 2018, 20, 3038–3043. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, X.; Li, Y. A new approach for the aerobic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid without using transition metal catalysts. J. Energy Chem. 2018, 27, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Zweifel, T.; Naubron, J.V.; Grützmacher, H. Catalyzed dehydrogenative coupling of primary alcohols with water, methanol, or amines. Angew. Chem. Int. Ed. 2009, 48, 559–563. [Google Scholar] [CrossRef]
- Trincado, M.; Grützmacher, H.; Vizza, F.; Bianchini, C. Domino rhodium/palladium-catalyzed dehydrogenation reactions of alcohols to acids by hydrogen transfer to inactivated alkenes. Chem. Eur. J. 2010, 16, 2751–2757. [Google Scholar] [CrossRef]
- Gianetti, T.L.; Annen, S.P.; Santiso-Quinones, G.; Reiher, M.; Driess, M.; Grützmacher, H. Nitrous oxide as a hydrogen acceptor for the dehydrogenative coupling of alcohols. Angew. Chem. Int. Ed. 2016, 55, 1854–1858. [Google Scholar] [CrossRef]
- Balaraman, E.; Khaskin, E.; Leitus, G.; Milstein, D. Catalytic transformation of alcohols to carboxylic acid salts and H-2 using water as the oxygen atom source. Nat. Chem. 2013, 5, 122–125. [Google Scholar] [CrossRef]
- Choi, J.H.; Heim, L.E.; Ahrens, M.; Prechtl, M.H.G. Selective conversion of alcohols in water to carboxylic acids by in situ generated ruthenium trans dihydrido carbonyl PNP complexes. Dalton Trans. 2014, 43, 17248–17254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malineni, J.; Keul, H.; Möller, M. A green and sustainable phosphine-free NHC-ruthenium catalyst for selective oxidation of alcohols to carboxylic acids in water. Dalton Trans. 2015, 44, 17409–17414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Nielsen, M.; Li, B.; Dixneuf, P.H.; Junge, H.; Beller, M. Ruthenium-catalyzed hydrogen generation from glycerol and selective synthesis of lactic acid. Green Chem. 2015, 17, 193–198. [Google Scholar] [CrossRef]
- Zhang, L.; Nguyen, D.H.; Raffa, G.; Trivelli, X.; Capet, F.; Desset, S.; Paul, S.; Dumeignil, F.; Gauvin, R.M. Catalytic conversion of alcohols into carboxylic acid salts in water: Scope, recycling, and mechanistic insights. ChemSusChem 2016, 9, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Santilli, C.; Makarov, I.S.; Fristrup, P.; Madsen, R. Dehydrogenative synthesis of carboxylic acids from primary alcohols and hydroxide catalyzed by a ruthenium N-heterocyclic carbene complex. J. Org. Chem. 2016, 81, 9931–9938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, P.; Ben-David, Y.; Milstein, D. General synthesis of amino acid salts from amino alcohols and basic water liberating H-2. J. Am. Chem. Soc. 2016, 138, 6143–6146. [Google Scholar] [CrossRef] [PubMed]
- Dahl, E.W.; Louis-Goff, T.; Szymczak, N.K. Second sphere ligand modifications enable a recyclable catalyst for oxidant-free alcohol oxidation to carboxylates. Chem. Commun. 2017, 53, 2287–2289. [Google Scholar] [CrossRef]
- Dai, Z.; Luo, Q.; Meng, X.; Li, R.; Zhang, J.; Peng, T. Ru (II) complexes bearing 2, 6-bis (benzimidazole-2-yl) pyridine ligands: A new class of catalysts for efficient dehydrogenation of primary alcohols to carboxylic acids and H2 in the alcohol/CsOH system. J. Organomet. Chem. 2017, 830, 11–18. [Google Scholar] [CrossRef]
- Sarbajna, A.; Dutta, I.; Daw, P.; Dinda, S.; Rahaman, S.M.W.; Sarkar, A.; Bera, J.K. Catalytic conversion of alcohols to carboxylic acid salts and hydrogen with alkaline water. ACS Catal. 2017, 7, 2786–2790. [Google Scholar] [CrossRef]
- Singh, A.; Singh, S.K.; Saini, A.K.; Mobin, S.M.; Mathur, P. Facile oxidation of alcohols to carboxylic acids in basic water medium by employing ruthenium picolinate cluster as an efficient catalyst. Appl. Organomet. Chem. 2018, 32, e4574. [Google Scholar] [CrossRef]
- Gong, D.; Hu, B.; Chen, D. Bidentate Ru (ii)-NC complexes as catalysts for the dehydrogenative reaction from primary alcohols to carboxylic acids. Dalton Trans. 2019, 48, 8826–8834. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Tang, X.S.; Yang, Z.Q.; Yu, B.Y.; Wang, H.J.; Sang, W.; Yuan, Y.; Chen, C.; Verpoort, F. Highly active bidentate N-heterocyclic carbene/ruthenium complexes performing dehydrogenative coupling of alcohols and hydroxides in open air. Chem. Commun. 2019, 55, 8591–8594. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Jian, L.; Li, C.; Zhang, C.C.; Fu, H.Y.; Zheng, X.L.; Chen, H.; Li, R.X. Dehydrogenation of Alcohols to Carboxylic Acid Catalyzed by in Situ-Generated Facial Ruthenium-CPP Complex. J. Org. Chem. 2019, 84, 9151–9160. [Google Scholar] [CrossRef] [PubMed]
- Sawama, Y.; Morita, K.; Yamada, T.; Nagata, S.; Yabe, Y.; Monguchi, Y.; Sajiki, H. Rhodium-on-carbon catalyzed hydrogen scavenger- and oxidant-free dehydrogenation of alcohols in aqueous media. Green Chem. 2014, 16, 3439–3443. [Google Scholar] [CrossRef]
- Sawama, Y.; Morita, K.; Asai, S.; Kozawa, M.; Tadokoro, S.; Nakajima, J.; Monguchi, Y.; Sajiki, H. Palladium on carbon-catalyzed aqueous transformation of primary alcohols to carboxylic acids based on dehydrogenation under mildly reduced pressure. Adv. Synth. Catal. 2015, 357, 1205–1210. [Google Scholar] [CrossRef]
- Fujita, K.I.; Tamura, R.; Tanaka, Y.; Yoshida, M.; Onoda, M.; Yamaguchi, R. Dehydrogenative oxidation of alcohols in aqueous media catalyzed by a water-soluble dicationic iridium complex bearing a functional N-heterocyclic carbene ligand without using base. ACS Catal. 2017, 7, 7226–7230. [Google Scholar] [CrossRef]
- Cherepakhin, V.; Williams, T.J. Iridium catalysts for acceptorless dehydrogenation of alcohols to carboxylic acids: Scope and mechanism. ACS Catal. 2018, 8, 3754–3763. [Google Scholar] [CrossRef]
- Fujita, K.I. Development and application of new iridium catalysts for efficient dehydrogenative reactions of organic molecules. Bull. Chem. Soc. Jpn. 2019, 92, 344–351. [Google Scholar] [CrossRef] [Green Version]
- Ghalehshahi, H.G.; Madsen, R. Silver-catalyzed dehydrogenative synthesis of carboxylic acids from primary alcohols. Chem. Eur. J. 2017, 23, 11920–11926. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Morin, Y.; Zhang, L.; Trivelli, X.; Capet, F.; Paul, S.; Desset, S.; Dumeignil, F.; Gauvin, R.M. Oxidative transformations of biosourced alcohols catalyzed by earth-abundant transition metals. ChemCatChem 2017, 9, 2652–2660. [Google Scholar] [CrossRef]
- Shao, Z.; Wang, Y.; Liu, Y.; Wang, Q.; Fu, X.; Liu, Q. A general and efficient Mn-catalyzed acceptorless dehydrogenative coupling of alcohols with hydroxides into carboxylates. Org. Chem. Front. 2018, 5, 1248–1256. [Google Scholar] [CrossRef]
- Monda, F.; Madsen, R. Zinc oxide-catalyzed dehydrogenation of primary alcohols into carboxylic acids. Chem. Eur. J. 2018, 24, 17832–17837. [Google Scholar] [CrossRef] [PubMed]
- Bauri, S.; Donthireddy, S.N.R.; Illam, P.M.; Rit, A. Effect of ancillary ligand in cyclometalated Ru (II)-NHC-catalyzed transfer hydrogenation of unsaturated compounds. Inorg. Chem. 2018, 57, 14582–14593. [Google Scholar] [CrossRef] [PubMed]
- Huynh, H.V.; Han, Y.; Jothibasu, R.; Yang, J.A. 13C NMR spectroscopic determination of ligand donor strengths using N-heterocyclic carbene complexes of palladium (II). Organometallics 2009, 28, 5395–5404. [Google Scholar] [CrossRef]
- Chen, C.; Kim, M.H.; Hong, S.H. N-heterocyclic carbene-based ruthenium-catalyzed direct amidation of aldehydes with amines. Org. Chem. Front. 2015, 2, 241–247. [Google Scholar] [CrossRef]
- Kaufhold, S.; Petermann, L.; Staehle, R.; Rau, S. Transition metal complexes with N-heterocyclic carbene ligands: From organometallic hydrogenation reactions toward water splitting. Coord. Chem. Rev. 2015, 304, 73–87. [Google Scholar] [CrossRef]
- Xie, X.; Huynh, H.V. Cyclometallated ruthenium (ii) complexes with ditopic thienyl NHC-ligands: Syntheses and alkyne annulations. Org. Chem. Front. 2015, 2, 1598–1603. [Google Scholar] [CrossRef]
- Peris, E. Smart N-heterocyclic carbene ligands in catalysis. Chem. Rev. 2017, 118, 9988–10031. [Google Scholar] [CrossRef]
- Huynh, H.V. Electronic properties of N-heterocyclic carbenes and their experimental determination. Chem. Rev. 2018, 118, 9457–9492. [Google Scholar] [CrossRef]
- Wang, W.Q.; Yuan, Y.; Miao, Y.; Yu, B.Y.; Wang, H.J.; Wang, Z.Q.; Sang, W.; Chen, C.; Verpoort, F. Well-defined N-heterocyclic carbene/ruthenium complexes for the alcohol amidation with amines: The dual role of cesium carbonate and improved activities applying an added ligand. Appl. Organomet. Chem. 2019. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, C.; Ghosh, S.C.; Li, Y.; Hong, S.H. Well-defined N-heterocyclic carbene based ruthenium catalysts for direct amide synthesis from alcohols and amines. Organometallics 2010, 29, 1374–1378. [Google Scholar] [CrossRef]
- Kim, K.; Kang, B.; Hong, S.H. N-Heterocyclic carbene-based well-defined ruthenium hydride complexes for direct amide synthesis from alcohols and amines under base-free conditions. Tetrahedron 2015, 71, 4565–4569. [Google Scholar] [CrossRef]
- Cheng, H.; Xiong, M.Q.; Cheng, C.X.; Wang, H.J.; Lu, Q.; Liu, H.F.; Yao, F.B.; Chen, C.; Verpoort, F. Insitu generated ruthenium catalyst systems bearing diverse N-heterocyclic carbene precursors for atom-economic amide synthesis from alcohols and amines. Chem. Asian J. 2018, 13, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Miao, Y.; De Winter, K.; Wang, H.J.; Demeyere, P.; Yuan, Y.; Verpoort, F. Ruthenium-based catalytic systems incorporating a labile cyclooctadiene ligand with N-heterocyclic carbene precursors for the atom-economic alcohol amidation using amines. Molecules 2018, 23, 2413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.J.; Wang, H.J.; Yang, Z.Q.; Tang, X.S.; Yuan, Y.; Su, W.; Chen, C.; Verpoort, F. Efficient and phosphine-free bidentate N-heterocyclic carbene/ruthenium catalytic systems for the dehydrogenative amidation of alcohols and amines. Org. Chem. Front. 2019, 6, 563–570. [Google Scholar] [CrossRef]
- Astakhov, A.V.; Khazipov, O.V.; Degtyareva, E.S.; Khrustalev, V.N.; Chernyshev, V.M.; Ananikov, V.P. Facile hydrolysis of nickel (II) complexes with N-heterocyclic carbene ligands. Organometallics 2015, 34, 5759–5766. [Google Scholar] [CrossRef]
- Cheng, H.; Xiong, M.Q.; Zhang, N.; Wang, H.J.; Miao, Y.; Su, W.; Yuan, Y.; Chen, C.; Verpoort, F. Efficient N-heterocyclic carbene/ruthenium catalytic systems for the alcohol amidation with amines: Involvement of poly-carbene complexes? ChemCatChem 2018, 10, 4338–4345. [Google Scholar] [CrossRef]
- Oehninger, L.; Stefanopoulou, M.; Alborzinia, H.; Schur, J.; Ludewig, S.; Namikawa, K.; Muñoz-Castro, A.; Köster, R.W.; Baumann, K.; Wölfl, S.; et al. Evaluation of arene ruthenium (ii) N-heterocyclic carbene complexes as organometallics interacting with thiol and selenol containing biomolecules. Dalton Trans. 2013, 42, 1657–1666. [Google Scholar] [CrossRef]

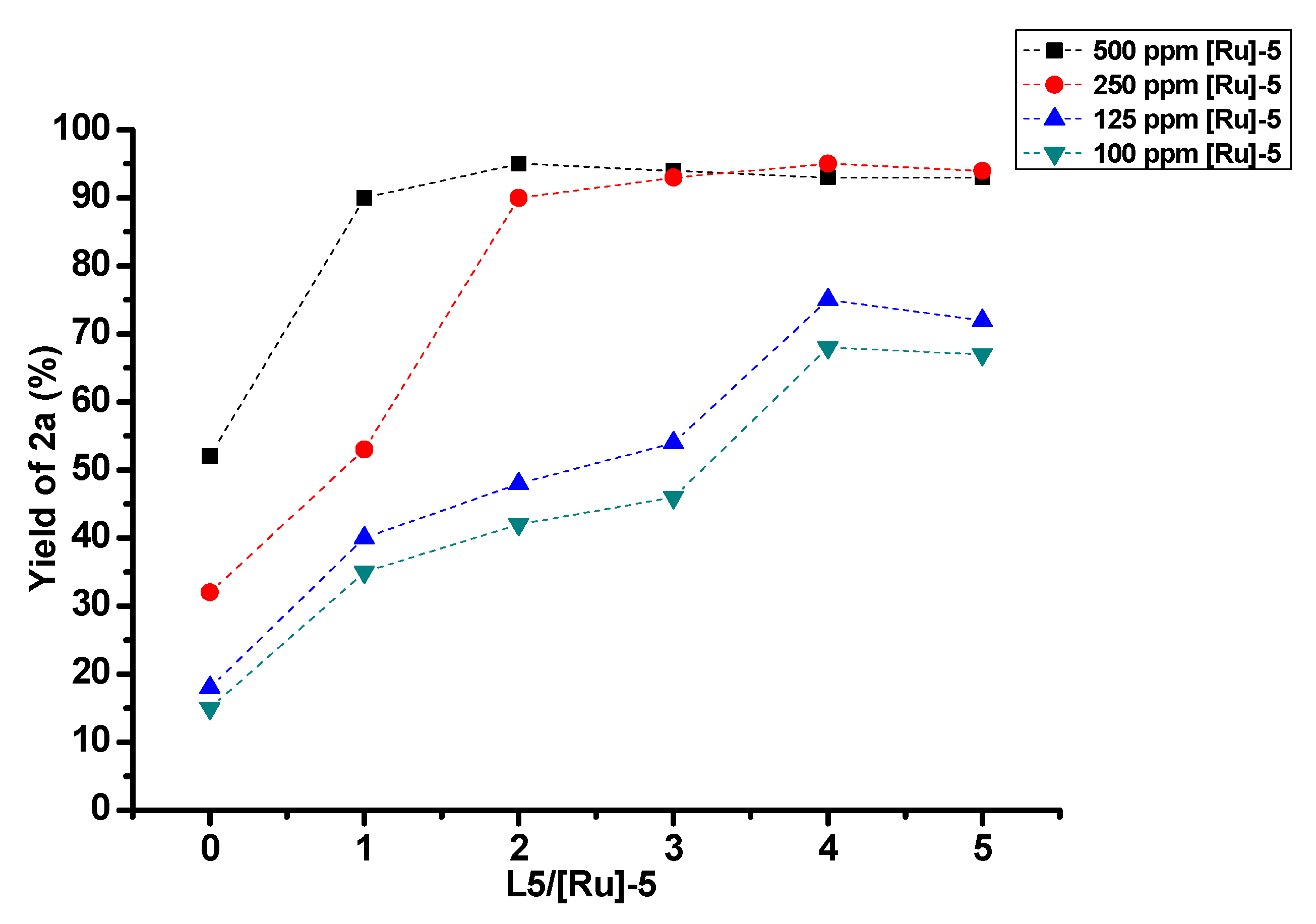
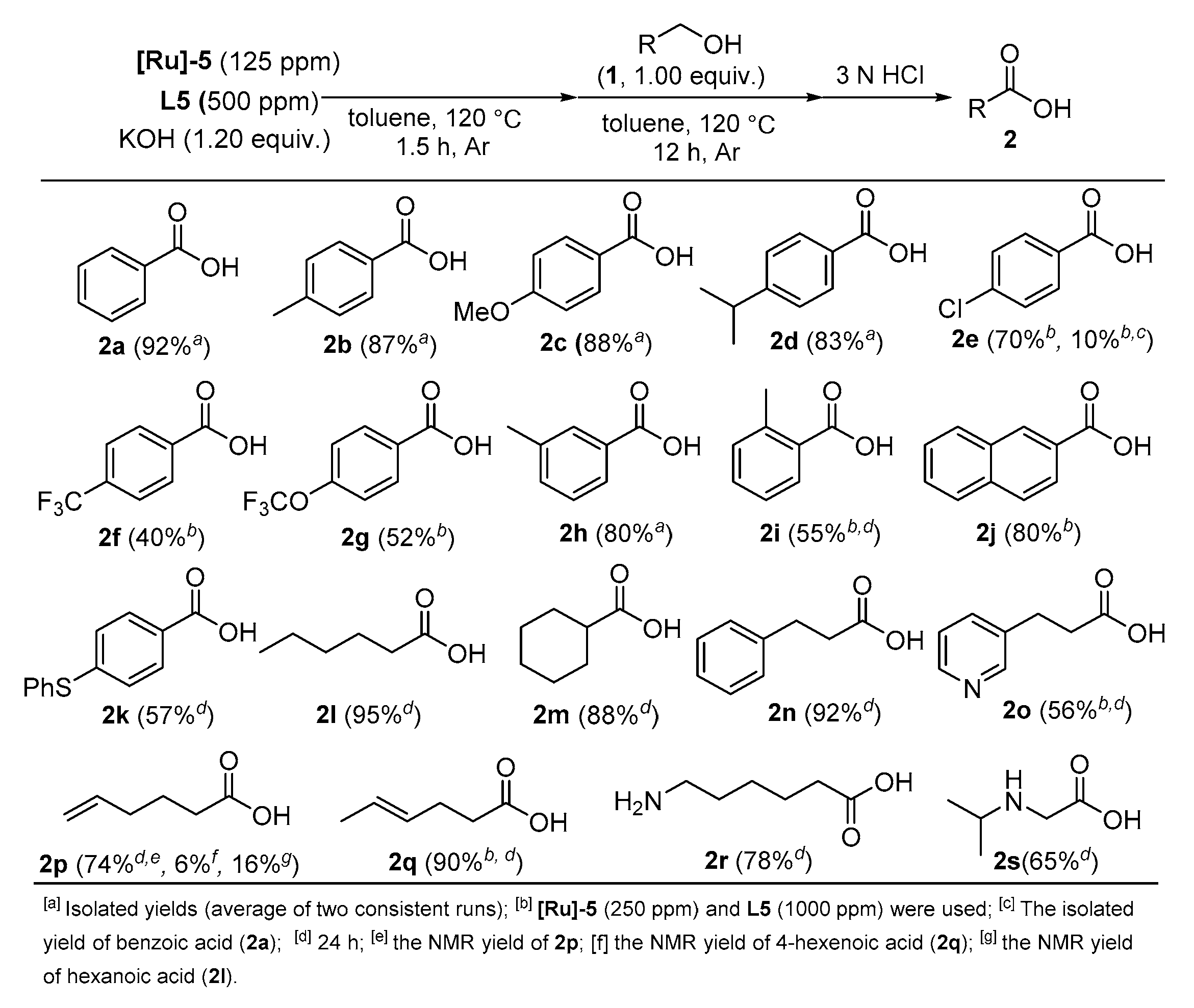

| Entry | x | y | m | n | Yield (%) b | TONs for 2a | TOFs for 2a | |
|---|---|---|---|---|---|---|---|---|
| 2a | Unreacted 1a | |||||||
| 1 | 0.0125 | 0 | 0.5 | 12 | 18 | 80 | 1440 | 120 |
| 2 | 0.0125 | 0.05 | 0.5 | 12 | 75 | 23 | 6000 | 500 |
| 3 | 0.0125 | 0.05 | 0 | 12 | 18 | 78 | 1440 | 120 |
| 4 | 0.0125 | 0.05 | 1.0 | 12 | 86 | 11 | 6880 | 573 |
| 5 | 0.0125 | 0.05 | 1.5 | 12 | 94 | 3 | 7520 | 627 |
| 6 | 0.0125 | 0.05 | 2.0 | 12 | 92 | 5 | 7360 | 613 |
| 7 | 0.0125 | 0.05 | 2.5 | 12 | 89 | 8 | 7120 | 593 |
| 8 c | 0.0125 | 0.05 | 1.5 | 12 | 85 | 13 | 6800 | 567 |
| 9 d | 0.0125 | 0.05 | 1.5 | 12 | 91 | 8 | 7280 | 607 |
| 10 | 0 | 0.05 | 1.5 | 12 | trace | 91 | - | - |
| 11 | 0 | 0 | 1.5 | 12 | trace | 92 | - | - |
| 12 | 0.0025 | 0.01 | 1.5 | 1 | 12 | 87 | 4800 | 4800 |
| 13 | 0.0025 | 0.01 | 1.5 | 36 | 38 | 59 | 15,200 | 422 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.-Q.; Cheng, H.; Yuan, Y.; He, Y.-Q.; Wang, H.-J.; Wang, Z.-Q.; Sang, W.; Chen, C.; Verpoort, F. Highly Efficient N-Heterocyclic Carbene/Ruthenium Catalytic Systems for the Acceptorless Dehydrogenation of Alcohols to Carboxylic Acids: Effects of Ancillary and Additional Ligands. Catalysts 2020, 10, 10. https://doi.org/10.3390/catal10010010
Wang W-Q, Cheng H, Yuan Y, He Y-Q, Wang H-J, Wang Z-Q, Sang W, Chen C, Verpoort F. Highly Efficient N-Heterocyclic Carbene/Ruthenium Catalytic Systems for the Acceptorless Dehydrogenation of Alcohols to Carboxylic Acids: Effects of Ancillary and Additional Ligands. Catalysts. 2020; 10(1):10. https://doi.org/10.3390/catal10010010
Chicago/Turabian StyleWang, Wan-Qiang, Hua Cheng, Ye Yuan, Yu-Qing He, Hua-Jing Wang, Zhi-Qin Wang, Wei Sang, Cheng Chen, and Francis Verpoort. 2020. "Highly Efficient N-Heterocyclic Carbene/Ruthenium Catalytic Systems for the Acceptorless Dehydrogenation of Alcohols to Carboxylic Acids: Effects of Ancillary and Additional Ligands" Catalysts 10, no. 1: 10. https://doi.org/10.3390/catal10010010
APA StyleWang, W.-Q., Cheng, H., Yuan, Y., He, Y.-Q., Wang, H.-J., Wang, Z.-Q., Sang, W., Chen, C., & Verpoort, F. (2020). Highly Efficient N-Heterocyclic Carbene/Ruthenium Catalytic Systems for the Acceptorless Dehydrogenation of Alcohols to Carboxylic Acids: Effects of Ancillary and Additional Ligands. Catalysts, 10(1), 10. https://doi.org/10.3390/catal10010010