Fluorescence-Guided Surgery in Pediatric Oncology: Current Practice and Future Directions
Simple Summary
Abstract
1. Introduction
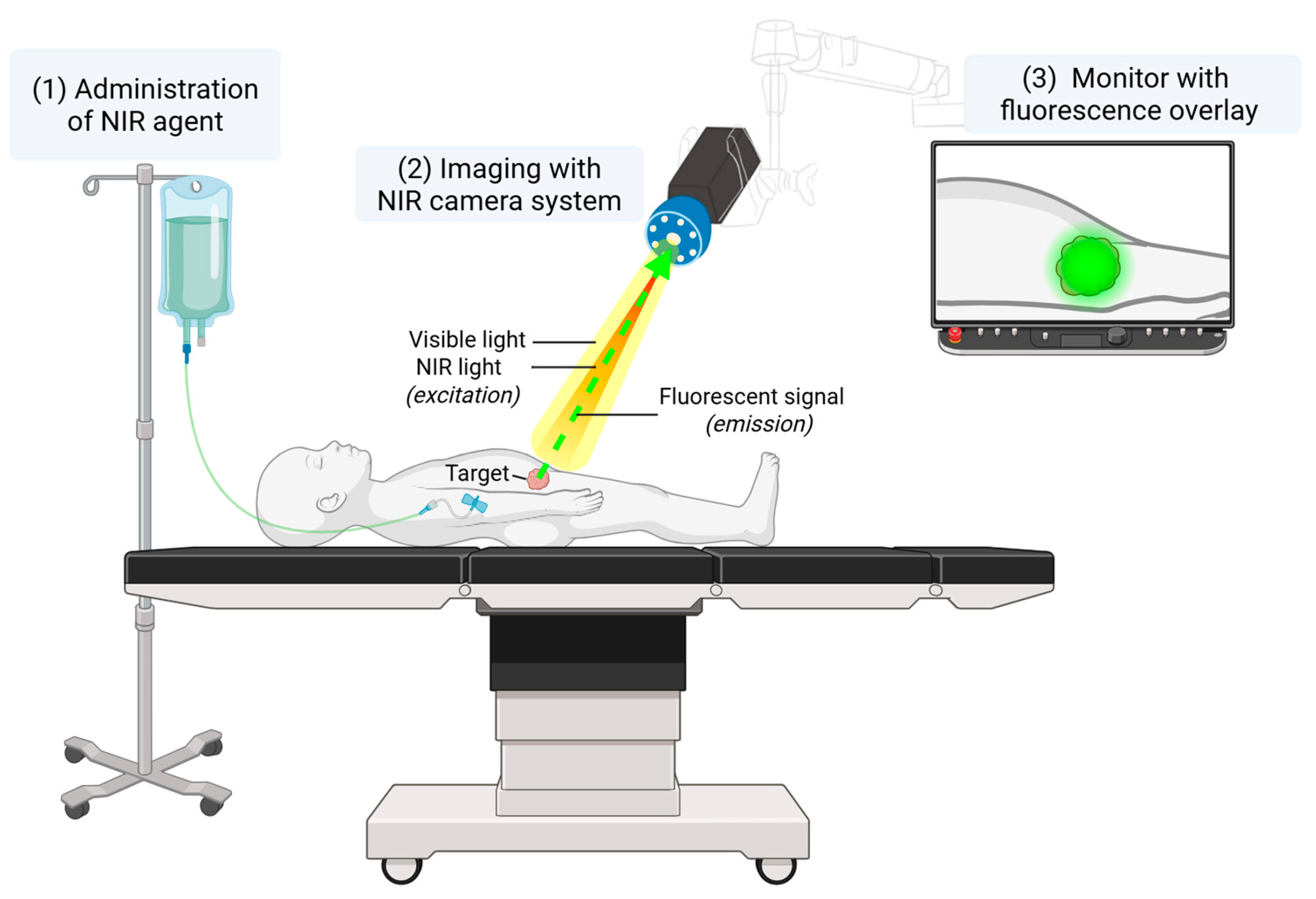
2. Materials and Methods
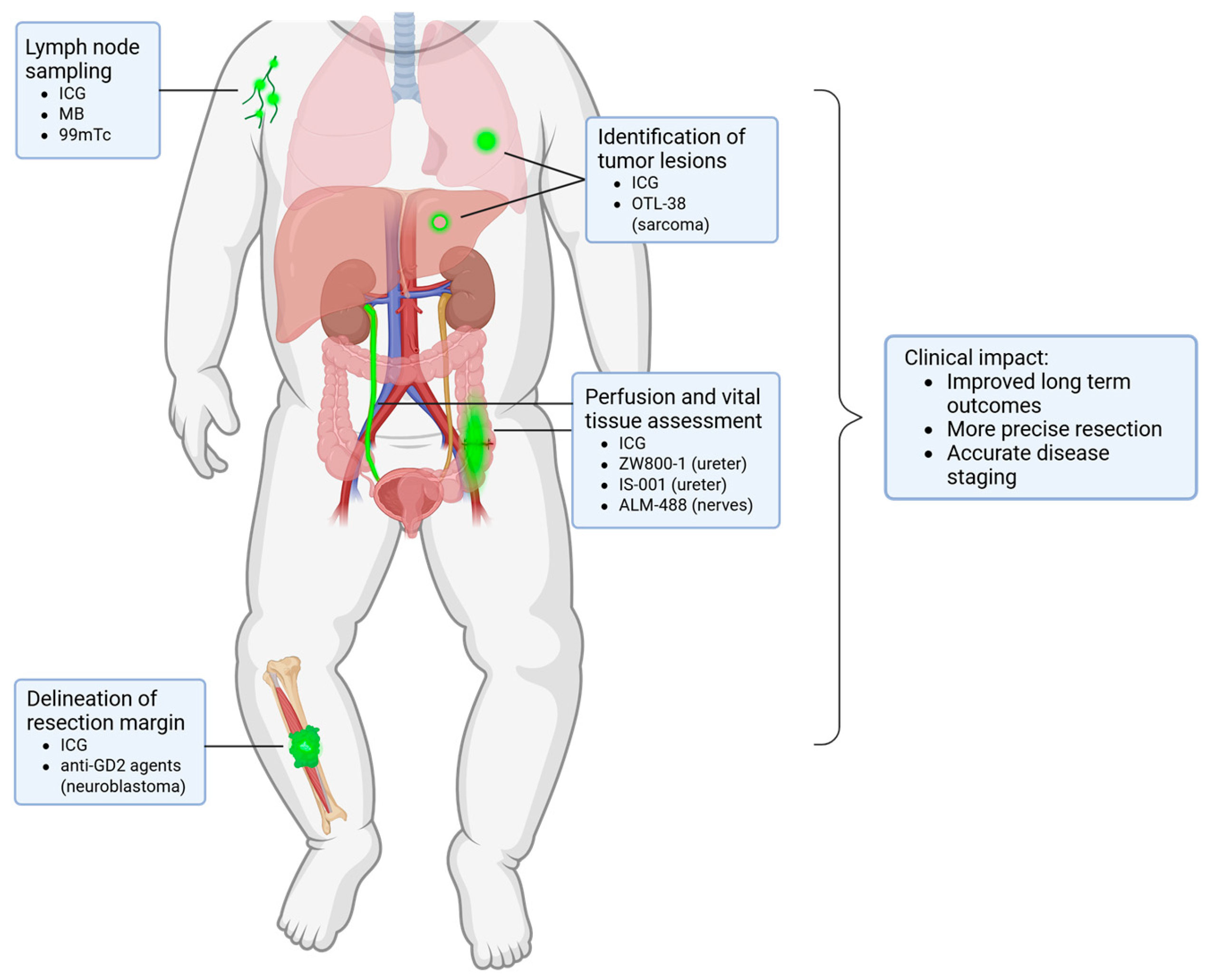
3. Identification of Lymph Nodes
4. Imaging of Vital Structures
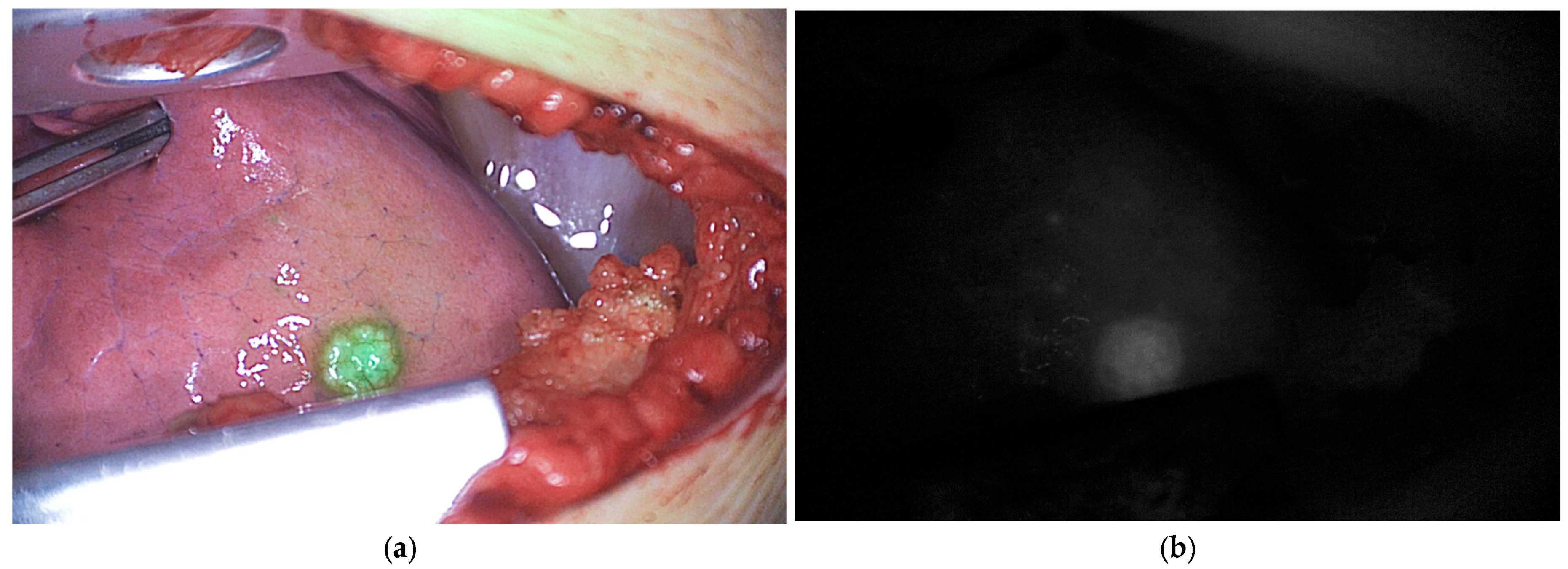
4.1. Non-Specific Tissue Imaging
4.2. Advances in Tissue-Specific Imaging Approaches
5. Visualization of Pediatric Tumors
5.1. Non-Specific Imaging
5.1.1. Hepatoblastoma
5.1.2. Renal Tumors
5.1.3. Abdominal Tumors and Lymphoma
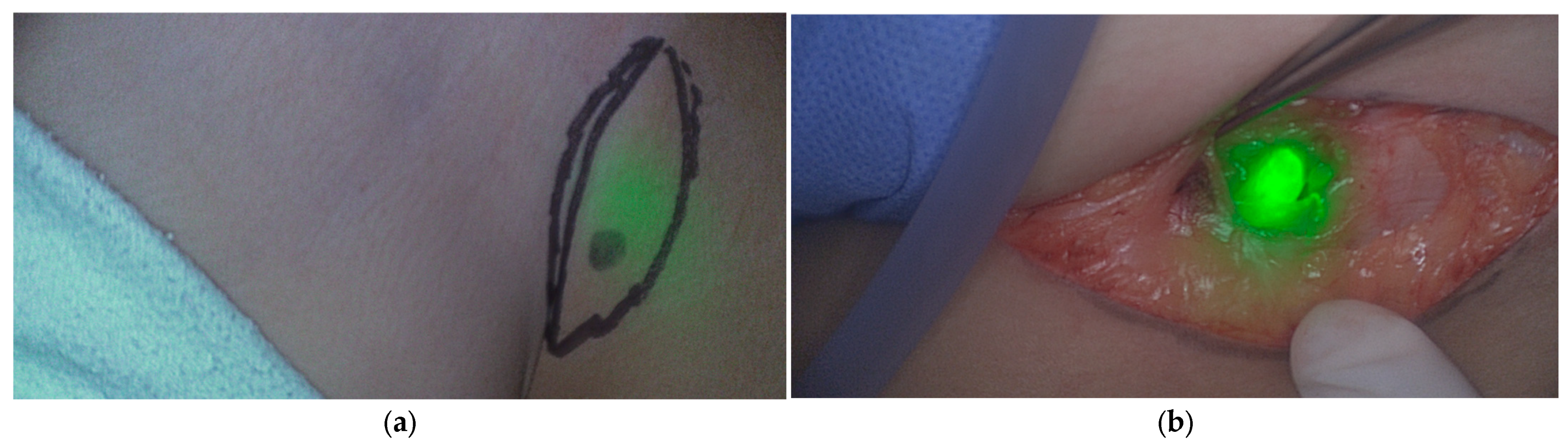
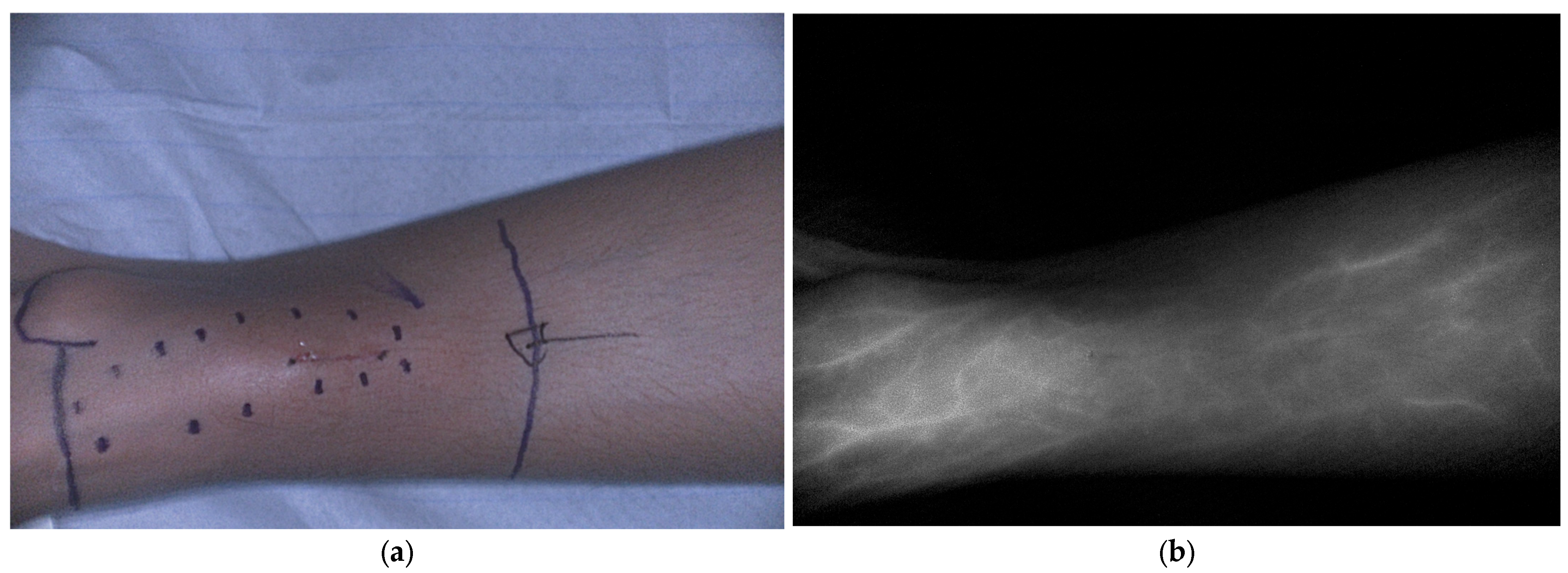
5.1.4. Bone and Soft Tissue Sarcoma
5.1.5. Peripheral Nerve Sheath Tumors
5.1.6. Otolaryngologic Malignancies
5.2. Tissue-Specific Tumor Imaging
5.2.1. Targeting Neuroblastoma with Anti-GD2 Based Imaging Probes
5.2.2. Targeting Pulmonary Metastases of (Osteo)Sarcoma with Pafolacianine
6. Future Directions
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FGS | Fluorescence-guided surgery |
| NIR | Near-infrared |
| ICG | Indocyanine green |
| MB | Methylene blue |
| SNP | Sentinel lymph node procedure |
| EPR | Enhanced permeability and retention |
| NSS | Nephron-sparing surgery |
| CT | Computed Tomography |
| SWIR | Shortwave infrared imaging |
| GD2 | Disialoganglioside |
References
- Davidoff, A.M. Advocating for the Surgical Needs of Children with Cancer. J. Pediatr. Surg. 2022, 57, 959–966. [Google Scholar] [CrossRef]
- von Allmen, D. Pediatric Surgical Oncology: A Brief Overview of Where We Have Been and the Challenges We Face. Semin. Pediatr. Surg. 2019, 28, 150864. [Google Scholar] [CrossRef]
- Birkmeyer, J.D.; Stukel, T.A.; Siewers, A.E.; Goodney, P.P.; Wennberg, D.E.; Lucas, F.L. Surgeon Volume and Operative Mortality in the United States. N. Engl. J. Med. 2003, 349, 2117–2127. [Google Scholar] [CrossRef]
- Latoch, E.; Zubowska, M.; Młynarski, W.; Stachowicz-Stencel, T.; Stefanowicz, J.; Sławińska, D.; Kowalczyk, J.; Skalska-Sadowska, J.; Wachowiak, J.; Badowska, W.; et al. Late Effects of Childhood Cancer Treatment in Long-Term Survivors Diagnosed before the Age of 3 Years—A Multicenter, Nationwide Study. Cancer Epidemiol. 2022, 80, 102209. [Google Scholar] [CrossRef] [PubMed]
- Keereweer, S.; Van Driel, P.B.A.A.; Snoeks, T.J.A.; Kerrebijn, J.D.F.; Baatenburg de Jong, R.J.; Vahrmeijer, A.L.; Sterenborg, H.J.C.M.; Löwik, C.W.G.M. Optical Image-Guided Cancer Surgery: Challenges and Limitations. Clin. Cancer Res. 2013, 19, 3745–3754. [Google Scholar] [CrossRef] [PubMed]
- van Manen, L.; Handgraaf, H.J.M.; Diana, M.; Dijkstra, J.; Ishizawa, T.; Vahrmeijer, A.L.; Mieog, J.S.D. A Practical Guide for the Use of Indocyanine Green and Methylene Blue in Fluorescence-Guided Abdominal Surgery. J. Surg. Oncol. 2018, 118, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.C.H.; Lau, K.C.; Wong, K.K.Y. Fluorescence-Guided Pediatric Surgery: The Past, Present, and Future. J. Pediatr. Surg. Open 2024, 5, 100106. [Google Scholar] [CrossRef]
- Campwala, I.; Vignali, P.D.A.; Seynnaeve, B.K.; Davit, A.J.; Weiss, K.; Malek, M.M. Utility of Indocyanine Green for Sentinel Lymph Node Biopsy in Pediatric Sarcoma and Melanoma. J. Pediatr. Surg. 2024, 59, 1326–1333. [Google Scholar] [CrossRef]
- Johnston, M.E.; Farooqui, Z.A.; Nagarajan, R.; Pressey, J.G.; Turpin, B.; Dasgupta, R. Fluorescent-Guided Surgery and the Use of Indocyanine Green Sentinel Lymph Node Mapping in the Pediatric and Young Adult Oncology Population. Cancer 2023, 129, 3962–3970. [Google Scholar] [CrossRef]
- Jeremiasse, B.; van Scheltinga, C.E.J.T.; Smeele, L.E.; Tolboom, N.; Wijnen, M.H.W.A.; van der Steeg, A.F.W. Sentinel Lymph Node Procedure in Pediatric Patients with Melanoma, Squamous Cell Carcinoma, or Sarcoma Using Near-Infrared Fluorescence Imaging with Indocyanine Green: A Feasibility Trial. Ann. Surg. Oncol. 2023, 30, 2391–2398. [Google Scholar] [CrossRef] [PubMed]
- Pio, L.; Richard, C.; Zaghloul, T.; Murphy, A.J.; Davidoff, A.M.; Abdelhafeez, A.H. Sentinel Lymph Node Mapping with Indocyanine Green Fluorescence (ICG) for Pediatric and Adolescent Tumors: A Prospective Observational Study. Sci. Rep. 2024, 14, 30135. [Google Scholar] [CrossRef]
- Mansfield, S.A.; Murphy, A.J.; Talbot, L.; Prajapati, H.; Maller, V.; Pappo, A.; Singhal, S.; Krasin, M.J.; Davidoff, A.M.; Abdelhafeez, A. Alternative Approaches to Retroperitoneal Lymph Node Dissection for Paratesticular Rhabdomyosarcoma. J. Pediatr. Surg. 2020, 55, 2677–2681. [Google Scholar] [CrossRef]
- Pio, L.; Zaghloul, T.; Abdelhafeez, A.H. Indocyanine Green Fluorescence-Guided Lymphadenectomy with Single Site Retroperitoneoscopy in Children. J. Pediatr. Urol. 2023, 19, 491–492. [Google Scholar] [CrossRef]
- Pachl, M.J. Fluorescent Guided Lymph Node Harvest in Laparoscopic Wilms Nephroureterectomy. Urology 2021, 158, 189–192. [Google Scholar] [CrossRef]
- Abdelhafeez, A.H.; Davidoff, A.M.; Murphy, A.J.; Arul, G.S.; Pachl, M.J. Fluorescence-Guided Lymph Node Sampling Is Feasible during up-Front or Delayed Nephrectomy for Wilms Tumor. J. Pediatr. Surg. 2022, 57, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.; Heidekrueger, P.I.; Geis, S.; Von Kunow, F.; Taeger, C.; Strauss, C.; Wendl, C.; Brebant, V.; Broer, P.N.; Prantl, L.; et al. A Novel Indication for Indocyanine Green (ICG): Intraoperative Monitoring of Limb and Sciatic Nerve Perfusion during Rotationplasty for Sarcoma Patients. Clin. Hemorheol. Microcirc. 2018, 70, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.; Del Conte, F.; Cerulo, M.; Gargiulo, F.; Izzo, S.; Esposito, G.; Spagnuolo, M.I.; Escolino, M. Clinical Application and Technical Standardization of Indocyanine Green (ICG) Fluorescence Imaging in Pediatric Minimally Invasive Surgery. Pediatr. Surg. Int. 2019, 35, 1043–1050. [Google Scholar] [CrossRef]
- Esposito, C.; Settimi, A.; Del Conte, F.; Cerulo, M.; Coppola, V.; Farina, A.; Crocetto, F.; Ricciardi, E.; Esposito, G.; Escolino, M. Image-Guided Pediatric Surgery Using Indocyanine Green (ICG) Fluorescence in Laparoscopic and Robotic Surgery. Front. Pediatr. 2020, 8, 314. [Google Scholar] [CrossRef]
- Esposito, C.; Masieri, L.; Cerulo, M.; Castagnetti, M.; Del Conte, F.; Di Mento, C.; Esposito, G.; Tedesco, F.; Carulli, R.; Continisio, L.; et al. Indocyanine Green (ICG) Fluorescence Technology in Pediatric Robotic Surgery. J. Rob. Surg. 2024, 18, 209. [Google Scholar] [CrossRef] [PubMed]
- Ciro, E.; Vincenzo, C.; Mariapina, C.; Fulvia, D.C.; Vincenzo, B.; Giorgia, E.; Roberto, C.; Lepore, B.; Castagnetti, M.; Califano, G.; et al. Review of a 25-Year Experience in the Management of Ovarian Masses in Neonates, Children and Adolescents: From Laparoscopy to Robotics and Indocyanine Green Fluorescence Technology. Children 2022, 9, 1219. [Google Scholar] [CrossRef]
- Esposito, C.; Blanc, T.; Di Mento, C.; Ballouhey, Q.; Fourcade, L.; Mendoza-Sagaon, M.; Chiodi, A.; Cardone, R.; Escolino, M. Robotic-Assisted Surgery for Gynecological Indications in Children and Adolescents: European Multicenter Report. J. Rob. Surg. 2024, 18, 20. [Google Scholar] [CrossRef]
- Cho, Y.J.; Namgoong, J.-M.; Kwon, H.H.; Kwon, Y.J.; Kim, D.Y.; Kim, S.C. The Advantages of Indocyanine Green Fluorescence Imaging in Detecting and Treating Pediatric Hepatoblastoma: A Preliminary Experience. Front. Pediatr. 2021, 9, 635394. [Google Scholar] [CrossRef]
- Feng, J.; Qin, H.; Yang, W.; Cheng, H.; Xu, J.; Han, J.; Mou, J.; Wang, H.; Ni, X. Tumor-Background Ratio Is an Effective Method to Identify Tumors and False-Positive Nodules in Indocyanine-Green Navigation Surgery for Pediatric Liver Cancer. Front. Pediatr. 2022, 10, 875688. [Google Scholar] [CrossRef]
- Kitagawa, N.; Shinkai, M.; Mochizuki, K.; Usui, H.; Miyagi, H.; Nakamura, K.; Tanaka, M.; Tanaka, Y.; Kusano, M.; Ohtsubo, S. Navigation Using Indocyanine Green Fluorescence Imaging for Hepatoblastoma Pulmonary Metastases Surgery. Pediatr. Surg. Int. 2015, 31, 407–411. [Google Scholar] [CrossRef]
- Lake, C.M.; Bondoc, A.J.; Dasgupta, R.; Jenkins, T.M.; Towbin, A.J.; Smith, E.A.; Alonso, M.H.; Geller, J.I.; Tiao, G.M. Indocyanine Green Is a Sensitive Adjunct in the Identification and Surgical Management of Local and Metastatic Hepatoblastoma. Cancer Med. 2021, 10, 4322–4343. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Wu, Y.; Su, J.; Chen, L.; Liao, M.; Zhao, Z.; Lu, Z.; Xiong, X.; Jin, S.; Deng, X. Deploying Indocyanine Green Fluorescence-Guided Navigation System in Precise Laparoscopic Resection of Pediatric Hepatoblastoma. Cancers 2022, 14, 6057. [Google Scholar] [CrossRef]
- Shen, Y.; Zheng, M.; Li, J.; Tan, T.; Yang, J.; Pan, J.; Hu, C.; Zou, Y.; Yang, T. Clinical Application of Indocyanine Green Fluorescence Imaging in the Resection of Hepatoblastoma: A Single Institution’s Experiences. Front. Surg. 2022, 9, 932721. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, R.S.; Patel, K.R.; Yang, T.; Nguyen, H.N.; Masand, P.; Vasudevan, S.A. Pathologic Correlation with near Infrared-Indocyanine Green Guided Surgery for Pediatric Liver Cancer. J. Pediatr. Surg. 2022, 57, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Ohno, M.; Fujino, A.; Kanamori, Y.; Irie, R.; Yoshioka, T.; Miyazaki, O.; Uchida, H.; Fukuda, A.; Sakamoto, S.; et al. Fluorescence-Guided Surgery for Hepatoblastoma with Indocyanine Green. Cancers 2019, 11, 1215. [Google Scholar] [CrossRef]
- Liu, S.; Feng, J.; Ren, Q.; Qin, H.; Yang, W.; Cheng, H.; Yao, X.; Xu, J.; Han, J.; Chang, S.; et al. Evaluating the Clinical Efficacy and Limitations of Indocyanine Green Fluorescence-Guided Surgery in Childhood Hepatoblastoma: A Retrospective Study. Photodiagn. Photodyn. Ther. 2023, 44, 103790. [Google Scholar] [CrossRef]
- Shen, Q.; Liu, X.; Pan, S.; Li, T.; Zhou, J. Effectiveness of Indocyanine Green Fluorescence Imaging in Resection of Hepatoblastoma. Pediatr. Surg. Int. 2023, 39, 181. [Google Scholar] [CrossRef]
- Souzaki, R.; Kawakubo, N.; Matsuura, T.; Yoshimaru, K.; Koga, Y.; Takemoto, J.; Shibui, Y.; Kohashi, K.; Hayashida, M.; Oda, Y.; et al. Navigation Surgery Using Indocyanine Green Fluorescent Imaging for Hepatoblastoma Patients. Pediatr. Surg. Int. 2019, 35, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Tanaka, M.; Kitagawa, N.; Nozawa, K.; Shinkai, M.; Goto, H.; Tanaka, Y. Clinicopathological Study of Surgery for Pulmonary Metastases of Hepatoblastoma with Indocyanine Green Fluorescent Imaging. Pediatr. Blood Cancer 2022, 69, e29488. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafeez, A.H.; Murphy, A.J.; Brennan, R.; Santiago, T.C.; Lu, Z.; Krasin, M.J.; Bissler, J.J.; Gleason, J.M.; Davidoff, A.M. Indocyanine Green-Guided Nephron-Sparing Surgery for Pediatric Renal Tumors. J. Pediatr. Surg. 2022, 57, 174–178. [Google Scholar] [CrossRef]
- Feng, J.; Yang, W.; Qin, H.; Xu, J.; Liu, S.; Han, J.; Li, N.; He, L.; Wang, H. Clinical Application of Indocyanine Green Fluorescence Imaging Navigation for Pediatric Renal Cancer. Front. Pediatr. 2023, 11, 1108997. [Google Scholar] [CrossRef]
- Harris, A.C.; Choudhury, S.; Pachl, M. Early Results of Minimally Invasive Fluorescent Guided Pediatric Oncology Surgery with Delivery of Indocyanine Green during Induction of Anesthesia. Photodiagn. Photodyn. Ther. 2023, 42, 103639. [Google Scholar] [CrossRef] [PubMed]
- Brookes, M.J.; Chan, C.D.; Crowley, T.P.; Ragbir, M.; Ghosh, K.M.; Beckingsale, T.; Rankin, K.S. Intraoperative Near-Infrared Fluorescence Guided Surgery Using Indocyanine Green (ICG) May Aid the Surgical Removal of Benign Bone and Soft Tissue Tumours. Surg. Oncol. 2024, 55, 102091. [Google Scholar] [CrossRef]
- Wang, H.; Ji, T.; Qu, H.; Yan, T.; Li, D.; Yang, R.; Tang, X.; Guo, W. Indocyanine Green Fluorescence Imaging May Detect Tumour Residuals during Surgery for Bone and Soft-Tissue Tumours. Bone Jt. J. 2023, 105-B, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Asayama, B.; Sato, K.; Fukui, T.; Okuma, M.; Nakagaki, Y.; Nakagaki, Y.; Osato, T.; Nakamura, H. Skull Bone Tumor Resection with Intraoperative Indocyanine Green Fluorescence Imaging: A Series of Four Surgical Cases. Interdiscip. Neurosurg. 2017, 9, 8–13. [Google Scholar] [CrossRef]
- Abdelhafeez, A.H.; Mothi, S.S.; Pio, L.; Mori, M.; Santiago, T.C.; McCarville, M.B.; Kaste, S.C.; Pappo, A.S.; Talbot, L.J.; Murphy, A.J.; et al. Feasibility of Indocyanine Green-Guided Localization of Pulmonary Nodules in Children with Solid Tumors. Pediatr. Blood Cancer 2023, 70, e30437. [Google Scholar] [CrossRef]
- Jeremiasse, B.; Hulsker, C.C.C.; van den Bosch, C.H.; Buser, M.A.D.; van der Ven, C.P.; Bökkerink, G.M.J.; Wijnen, M.H.W.A.; Van der Steeg, A.F.W. Fluorescence Guided Surgery Using Indocyanine Green for Pulmonary Osteosarcoma Metastasectomy in Pediatric Patients: A Feasibility Study. EJC Paediatr. Oncol. 2023, 2, 100019. [Google Scholar] [CrossRef]
- Vetrano, I.G.; Acerbi, F.; Falco, J.; Devigili, G.; Rinaldo, S.; Messina, G.; Prada, F.; D’Ammando, A.; Nazzi, V. Fluorescein-Guided Removal of Peripheral Nerve Sheath Tumors: A Preliminary Analysis of 20 Cases. J. Neurosurg. 2021, 134, 260–269. [Google Scholar] [CrossRef]
- Richard, C.; White, S.; Williams, R.; Zaghloul, T.; Helmig, S.; Sheyn, A.; Abramson, Z.; Abdelhafeez, H. Indocyanine Green near Infrared-Guided Surgery in Children, Adolescents, and Young Adults with Otolaryngologic Malignancies. Auris Nasus Larynx 2023, 50, 576–585. [Google Scholar] [CrossRef]
- Wellens, L.M.; Deken, M.M.; Sier, C.F.M.; Johnson, H.R.; de la Jara Ortiz, F.; Bhairosingh, S.S.; Houvast, R.D.; Kholosy, W.M.; Baart, V.M.; Pieters, A.M.M.J.; et al. Anti-GD2-IRDye800CW as a Targeted Probe for Fluorescence-Guided Surgery in Neuroblastoma. Sci. Rep. 2020, 10, 17667. [Google Scholar] [CrossRef]
- Privitera, L.; Waterhouse, D.J.; Preziosi, A.; Paraboschi, I.; Ogunlade, O.; Da Pieve, C.; Barisa, M.; Ogunbiyi, O.; Weitsman, G.; Hutchinson, J.C.; et al. Shortwave Infrared Imaging Enables High-Contrast Fluorescence-Guided Surgery in Neuroblastoma. Cancer Res. 2023, 83, 2077–2089. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, L.T.; Sever, R.E.; Gilbert, R.; Guerrero, D.; Vincze, S.R.; Menendez, D.M.; Birikorang, P.A.; Rodgers, M.R.; Jaswal, A.P.; Vanover, A.C.; et al. Dual-Labeled Anti-GD2 Targeted Probe for Intraoperative Molecular Imaging of Neuroblastoma. J. Transl. Med. 2024, 22, 940. [Google Scholar] [CrossRef]
- Lehane, A.; Polites, S.F.; Dodd, A.; Goldstein, S.D.; Lautz, T.B. Let It Glow: Intraoperative Visualization of Pulmonary Metastases Using Pafolacianine, a Next-generation Fluorescent Agent, for Young Adults Undergoing Pulmonary Metastasectomy. Pediatr. Blood Cancer 2024, 71, e31293. [Google Scholar] [CrossRef]
- van den Berg, N.S.; Brouwer, O.R.; Schaafsma, B.E.; Mathéron, H.M.; Klop, W.M.C.; Balm, A.J.M.; van Tinteren, H.; Nieweg, O.E.; van Leeuwen, F.W.B.; Valdés Olmos, R.A. Multimodal Surgical Guidance during Sentinel Node Biopsy for Melanoma: Combined Gamma Tracing and Fluorescence Imaging of the Sentinel Node through Use of the Hybrid Tracer Indocyanine Green-(99m)Tc-Nanocolloid. Radiology 2015, 275, 521–529. [Google Scholar] [CrossRef]
- Mertes, P.M.; Malinovsky, J.M.; Mouton-Faivre, C.; Bonnet-Boyer, M.C.; Benhaijoub, A.; Lavaud, F.; Valfrey, J.; O’Brien, J.; Pirat, P.; Lalourcey, L.; et al. Anaphylaxis to Dyes during the Perioperative Period: Reports of 14 Clinical Cases. J. Allergy Clin. Immunol. 2008, 122, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, V.M.; Brown, A.C.; Szocik, J.F.; Pass, H.A.; Moline, S.; De, S.K.; Domino, E.F. Allergic Reactions to Isosulfan Blue during Sentinel Node Biopsy--a Common Event. Surgery 2001, 130, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.B.; Reed, M.S.; Cao, X.; García, H.A.; Ochoa, M.I.; Jiang, S.; Hasan, T.; Doyley, M.M.; Pogue, B.W. Combined Dual-Channel Fluorescence Depth Sensing of Indocyanine Green and Protoporphyrin IX Kinetics in Subcutaneous Murine Tumors. JBO 2024, 30, S13709. [Google Scholar] [CrossRef] [PubMed]
- Pitsinis, V.; Kanitkar, R.; Vinci, A.; Choong, W.L.; Benson, J. Results of a Prospective Randomized Multicenter Study Comparing Indocyanine Green (ICG) Fluorescence Combined with a Standard Tracer Versus ICG Alone for Sentinel Lymph Node Biopsy in Early Breast Cancer: The INFLUENCE Trial. Ann. Surg. Oncol. 2024, 31, 8848–8855. [Google Scholar] [CrossRef]
- Saltzman, A.F.; Smith, D.E.; Gao, D.; Ghosh, D.; Amini, A.; Aldrink, J.H.; Dasgupta, R.; Gow, K.W.; Glick, R.D.; Ehrlich, P.F.; et al. How Many Lymph Nodes Are Enough? Assessing the Adequacy of Lymph Node Yield for Staging in Favorable Histology Wilms Tumor. J. Pediatr. Surg. 2019, 54, 2331–2335. [Google Scholar] [CrossRef]
- Vujanić, G.M.; Gessler, M.; Ooms, A.H.A.G.; Collini, P.; Coulomb-l’Hermine, A.; D’Hooghe, E.; de Krijger, R.R.; Perotti, D.; Pritchard-Jones, K.; Vokuhl, C.; et al. The UMBRELLA SIOP–RTSG 2016 Wilms Tumour Pathology and Molecular Biology Protocol. Nat. Rev. Urol. 2018, 15, 693–701. [Google Scholar] [CrossRef]
- Dome, J.S.; Fernandez, C.V.; Mullen, E.A.; Kalapurakal, J.A.; Geller, J.I.; Huff, V.; Gratias, E.J.; Dix, D.B.; Ehrlich, P.F.; Khanna, G.; et al. Children’s Oncology Group’s 2013 Blueprint for Research: Renal Tumors. Pediatr. Blood Cancer 2013, 60, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Walterhouse, D.O.; Barkauskas, D.A.; Hall, D.; Ferrari, A.; De Salvo, G.L.; Koscielniak, E.; Stevens, M.C.G.; Martelli, H.; Seitz, G.; Rodeberg, D.A.; et al. Demographic and Treatment Variables Influencing Outcome for Localized Paratesticular Rhabdomyosarcoma: Results from a Pooled Analysis of North American and European Cooperative Groups. J. Clin. Oncol. 2018, 36, 3466–3476. [Google Scholar] [CrossRef]
- Geller, J.I.; Hong, A.L.; Vallance, K.L.; Evageliou, N.; Aldrink, J.H.; Cost, N.G.; Treece, A.L.; Renfro, L.A.; Mullen, E.A. COG Renal Tumor Committee Children’s Oncology Group’s 2023 Blueprint for Research: Renal Tumors. Pediatr. Blood Cancer 2023, 70, e30586. [Google Scholar] [CrossRef]
- Bou-Samra, P.; Muhammad, N.; Chang, A.; Karsalia, R.; Azari, F.; Kennedy, G.; Stummer, W.; Tanyi, J.; Martin, L.; Vahrmeijer, A.; et al. Intraoperative Molecular Imaging: 3rd Biennial Clinical Trials Update. JBO 2023, 28, 050901. [Google Scholar] [CrossRef]
- Wang, L.G.; Gibbs, S.L. Improving Precision Surgery: A Review of Current Intraoperative Nerve Tissue Fluorescence Imaging. Curr. Opin. Chem. Biol. 2023, 76, 102361. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Orosco, R.K.; Bouvet, M.; Richmon, J.D.; Berman, B.J.; Crawford, K.L.; Hom, M.; Nguyen, Q.T.; Rosenthal, E.L. Intraoperative Nerve-Specific Fluorescence Visualization in Head and Neck Surgery: A Phase 1 Trial. Nat. Commun. 2025, 16, 6060. [Google Scholar] [CrossRef]
- Montaño, A.R.; Masillati, A.; Szafran, D.A.; Shams, N.A.; Hubbell, G.E.; Barth, C.W.; Gibbs, S.L.; Wang, L.G. Matrix-Designed Bright near-Infrared Fluorophores for Precision Peripheral Nerve Imaging. Biomaterials 2025, 319, 123190. [Google Scholar] [CrossRef]
- Dyer, R.B.; Chen, M.Y.; Zagoria, R.J.; Regan, J.D.; Hood, C.G.; Kavanagh, P.V. Complications of Ureteral Stent Placement. RadioGraphics 2002, 22, 1005–1022. [Google Scholar] [CrossRef]
- Lange, D.; Bidnur, S.; Hoag, N.; Chew, B.H. Ureteral Stent-Associated Complications—Where We Are and Where We Are Going. Nat. Rev. Urol. 2015, 12, 17–25. [Google Scholar] [CrossRef]
- de Valk, K.S.; Handgraaf, H.J.; Deken, M.M.; Sibinga Mulder, B.G.; Valentijn, A.R.; Terwisscha van Scheltinga, A.G.; Kuil, J.; van Esdonk, M.J.; Vuijk, J.; Bevers, R.F.; et al. A Zwitterionic Near-Infrared Fluorophore for Real-Time Ureter Identification during Laparoscopic Abdominopelvic Surgery. Nat. Commun. 2019, 10, 3118. [Google Scholar] [CrossRef]
- Farnam, R.W.; Richard GArms, I.I.I.; Klaassen, A.H.; Sorger, J.M. Intraoperative Ureter Visualization Using a Near-Infrared Imaging Agent. J. Biomed. Opt. 2019, 24, 066004. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafeez, A.; Talbot, L.; Murphy, A.J.; Davidoff, A.M. Indocyanine Green-Guided Pediatric Tumor Resection: Approach, Utility, and Challenges. Front. Pediatr. 2021, 9, 689612. [Google Scholar] [CrossRef]
- Busweiler, L.A.D.; Wijnen, M.H.W.A.; Wilde, J.C.H.; Sieders, E.; Terwisscha van Scheltinga, S.E.J.; van Heurn, L.W.E.; Ziros, J.; Bakx, R.; Heij, H.A. Surgical Treatment of Childhood Hepatoblastoma in the Netherlands (1990–2013). Pediatr. Surg. Int. 2017, 33, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Erginel, B.; Gun Soysal, F.; Keskin, E.; Kebudi, R.; Celik, A.; Salman, T. Pulmonary Metastasectomy in Pediatric Patients. World J. Surg. Oncol. 2016, 14, 27. [Google Scholar] [CrossRef]
- van Dorp, M.; Wolfhagen, N.; Torensma, B.; Dickhoff, C.; Kazemier, G.; Heineman, D.J.; Schreurs, W.H. Pulmonary Metastasectomy and Repeat Metastasectomy for Colorectal Pulmonary Metastases: Outcomes from the Dutch Lung Cancer Audit for Surgery. BJS Open 2023, 7, zrad009. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-K.; Hsieh, M.-L.; Chen, S.-Y.; Liu, C.-Y.; Lin, P.-H.; Kan, H.-C.; Pang, S.-T.; Yu, K.-J. Clinical Benefits of Indocyanine Green Fluorescence in Robot-Assisted Partial Nephrectomy. Cancers 2022, 14, 3032. [Google Scholar] [CrossRef]
- Montanic, S.; Terdoslavich, M.; Rajcevic, U.; De Leo, L.; Bonin, S.; Serbec, V.C.; Passamonti, S. Development and Characterization of a Novel mAb against Bilitranslocase—A New Biomarker of Renal Carcinoma. Radiol. Oncol. 2013, 47, 128–137. [Google Scholar] [CrossRef]
- Zajac, J.; Liu, A.; Hassan, S.; Gibson, A. Mechanisms of Delayed Indocyanine Green Fluorescence and Applications to Clinical Disease Processes. Surgery 2024, 176, 386–395. [Google Scholar] [CrossRef]
- Klenke, F.M.; Wenger, D.E.; Inwards, C.Y.; Rose, P.S.; Sim, F.H. Giant Cell Tumor of Bone: Risk Factors for Recurrence. Clin. Orthop. Relat. Res. 2011, 469, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Danis, R.P.; Wolverton, S.; Steffens, T. Phototoxicity from Systemic Sodium Fluorescein. Retina 2000, 20, 370–373. [Google Scholar] [CrossRef]
- Goldstein, S.D.; Heaton, T.E.; Bondoc, A.; Dasgupta, R.; Abdelhafeez, A.; Davidoff, A.M.; Lautz, T.B. Evolving Applications of Fluorescence Guided Surgery in Pediatric Surgical Oncology: A Practical Guide for Surgeons. J. Pediatr. Surg. 2021, 56, 215–223. [Google Scholar] [CrossRef]
- Paraboschi, I.; De Coppi, P.; Stoyanov, D.; Anderson, J.; Giuliani, S. Fluorescence Imaging in Pediatric Surgery: State-of-the-Art and Future Perspectives. J. Pediatr. Surg. 2021, 56, 655–662. [Google Scholar] [CrossRef]
- Debie, P.; Devoogdt, N.; Hernot, S. Targeted Nanobody-Based Molecular Tracers for Nuclear Imaging and Image-Guided Surgery. Antibodies 2019, 8, 12. [Google Scholar] [CrossRef]
- Schouw, H.M.; Huisman, L.A.; Janssen, Y.F.; Slart, R.H.J.A.; Borra, R.J.H.; Willemsen, A.T.M.; Brouwers, A.H.; van Dijl, J.M.; Dierckx, R.A.; van Dam, G.M.; et al. Targeted Optical Fluorescence Imaging: A Meta-Narrative Review and Future Perspectives. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4272–4292. [Google Scholar] [CrossRef] [PubMed]
- Dumba, M.; Jawad, N.; McHugh, K. Neuroblastoma and Nephroblastoma: A Radiological Review. Cancer Imaging 2015, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Zwaveling, S.; Tytgat, G.A.M.; van der Zee, D.C.; Wijnen, M.H.W.A.; Heij, H.A. Is Complete Surgical Resection of Stage 4 Neuroblastoma a Prerequisite for Optimal Survival or May >95% Tumour Resection Suffice? Pediatr. Surg. Int. 2012, 28, 953–959. [Google Scholar] [CrossRef]
- Von Allmen, D.; Davidoff, A.M.; London, W.B.; Van Ryn, C.; Haas-Kogan, D.A.; Kreissman, S.G.; Khanna, G.; Rosen, N.; Park, J.R.; La Quaglia, M.P. Impact of Extent of Resection on Local Control and Survival in Patients From the COG A3973 Study With High-Risk Neuroblastoma. J. Clin. Oncol. 2017, 35, 208–216. [Google Scholar] [CrossRef]
- Jain, A.; Peters, N.J.; Samujh, R.; Trehan, A.; Malik, M.A.; Madan, R.; Dogra, S.; Solanki, S.; Singh, J.; Kanojia, R.P.; et al. Outcome Analysis of Surgical Complications in Pediatric Solid Tumors: A Retrospective Clinical Study. Pediatr. Surg. Int. 2025, 41, 201. [Google Scholar] [CrossRef]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 Antibody with GM-CSF, Interleukin-2, and Isotretinoin for Neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334. [Google Scholar] [CrossRef]
- Nazha, B.; Inal, C.; Owonikoko, T.K. Disialoganglioside GD2 Expression in Solid Tumors and Role as a Target for Cancer Therapy. Front. Oncol. 2020, 10, 1000. [Google Scholar] [CrossRef]
- Croteau, N.J.; Heaton, T.E. Pulmonary Metastasectomy in Pediatric Solid Tumors. Children 2019, 6, 6. [Google Scholar] [CrossRef]
- Heaton, T.E.; Davidoff, A.M. Surgical Treatment of Pulmonary Metastases in Pediatric Solid Tumors. Semin. Pediatr. Surg. 2016, 25, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Tanyi, J.L.; Randall, L.M.; Chambers, S.K.; Butler, K.A.; Winer, I.S.; Langstraat, C.L.; Han, E.S.; Vahrmeijer, A.L.; Chon, H.S.; Morgan, M.A.; et al. A Phase III Study of Pafolacianine Injection (OTL38) for Intraoperative Imaging of Folate Receptor-Positive Ovarian Cancer (Study 006). J. Clin. Oncol. 2023, 41, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Sarkaria, I.S.; Martin, L.W.; Rice, D.C.; Blackmon, S.H.; Slade, H.B.; Singhal, S. ELUCIDATE Study Group Pafolacianine for Intraoperative Molecular Imaging of Cancer in the Lung: The ELUCIDATE Trial. J. Thorac. Cardiovasc. Surg. 2023, 166, e468–e478. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Kolb, E.A.; Qin, J.; Chou, A.; Sowers, R.; Hoang, B.; Healey, J.H.; Huvos, A.G.; Meyers, P.A.; Gorlick, R. The Folate Receptor Alpha Is Frequently Overexpressed in Osteosarcoma Samples and Plays a Role in the Uptake of the Physiologic Substrate 5-Methyltetrahydrofolate. Clin. Cancer Res. 2007, 13, 2557–2567. [Google Scholar] [CrossRef]
- Maeda, H.; Fang, J.; Inutsuka, T.; Kitamoto, Y. Vascular Permeability Enhancement in Solid Tumor: Various Factors, Mechanisms Involved and Its Implications. Int. Immunopharmacol. 2003, 3, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Tummers, Q.R.J.G.; Hoogstins, C.E.S.; Peters, A.A.W.; de Kroon, C.D.; Trimbos, J.B.M.Z.; van de Velde, C.J.H.; Frangioni, J.V.; Vahrmeijer, A.L.; Gaarenstroom, K.N. The Value of Intraoperative Near-Infrared Fluorescence Imaging Based on Enhanced Permeability and Retention of Indocyanine Green: Feasibility and False-Positives in Ovarian Cancer. PLoS ONE 2015, 10, e0129766. [Google Scholar] [CrossRef]
- Dirheimer, L.; Cortese, S.; Dolivet, G.; Merlin, J.L.; Marchal, F.; Mastronicola, R.; Bezdetnaya, L. Fluorescence Imaging-Assessed Surgical Margin Detection in Head and Neck Oncology by Passive and Active Targeting. Mol. Diagn. Ther. 2025, 29, 465–481. [Google Scholar] [CrossRef]
- Cai, Y.; Chai, T.; Nguyen, W.; Liu, J.; Xiao, E.; Ran, X.; Ran, Y.; Du, D.; Chen, W.; Chen, X. Phototherapy in Cancer Treatment: Strategies and Challenges. Signal Transduct. Target. Ther. 2025, 10, 115. [Google Scholar] [CrossRef]
- Mazur, A.; Koziorowska, K.; Dynarowicz, K.; Aebisher, D.; Bartusik-Aebisher, D. Photodynamic Therapy for Treatment of Disease in Children—A Review of the Literature. Children 2022, 9, 695. [Google Scholar] [CrossRef]
- Mieog, J.S.D.; Achterberg, F.B.; Zlitni, A.; Hutteman, M.; Burggraaf, J.; Swijnenburg, R.-J.; Gioux, S.; Vahrmeijer, A.L. Fundamentals and Developments in Fluorescence-Guided Cancer Surgery. Nat. Rev. Clin. Oncol. 2022, 19, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Kattner, P.; Strobel, H.; Khoshnevis, N.; Grunert, M.; Bartholomae, S.; Pruss, M.; Fitzel, R.; Halatsch, M.-E.; Schilberg, K.; Siegelin, M.D.; et al. Compare and Contrast: Pediatric Cancer versus Adult Malignancies. Cancer Metastasis Rev. 2019, 38, 673–682. [Google Scholar] [CrossRef]
- Giuliani, S.; Paraboschi, I.; McNair, A.; Smith, M.; Rankin, K.S.; Elson, D.S.; Paleri, V.; Leff, D.; Stasiuk, G.; Anderson, J. Monoclonal Antibodies for Targeted Fluorescence-Guided Surgery: A Review of Applicability across Multiple Solid Tumors. Cancers 2024, 16, 1045. [Google Scholar] [CrossRef] [PubMed]
- Jeremiasse, B.; Rijs, Z.; Angoelal, K.R.; Hiemcke-Jiwa, L.S.; de Boed, E.A.; Kuppen, P.J.K.; Sier, C.F.M.; van Driel, P.B.A.A.; van de Sande, M.A.J.; Wijnen, M.H.W.A.; et al. Evaluation of Potential Targets for Fluorescence-Guided Surgery in Pediatric Ewing Sarcoma: A Preclinical Proof-of-Concept Study. Cancers 2023, 15, 3896. [Google Scholar] [CrossRef]
- Tummers, W.S.; Warram, J.M.; vn den Berg, N.S.; Miller, S.E.; Swijnenburg, R.-J.; Vahrmeijer, A.L.; Rosenthal, E.L. Recommendations for Reporting on Emerging Optical Imaging Agents to Promote Clinical Approval. Theranostics 2018, 8, 5336–5347. [Google Scholar] [CrossRef]
| Clinical Application | Clinical/ Preclinical | Population | Disease | Purpose | Imaging Agents | Citations |
|---|---|---|---|---|---|---|
| Non-Specific Imaging | ||||||
| Lymphatic mapping, SNP | Clinical | Pediatric and young adults | Melanoma, myoepithelial neoplasm, squamous cell carcinoma and sarcoma | SNLB | ICG + 99 mTc or MB + 99 mTc 4 mg intraoperative ICG + 99 mTc + MB 1.25 mg intraoperative ICG + 99 mTc 0.25–5 mg intraoperative | [8,9,10,11] |
| Clinical | Pediatric and (young) adults | Wilm’s tumor, (synovial) sarcoma, melanoma, squamous cell carcinoma, paratesticular rhabdomyosarcoma, renal tumors, myoepithelial neoplasm | Nodal sampling | ICG 5–10 mg intraoperative | [12,13,14,15] | |
| Vital structures | Clinical | Pediatric | Sarcoma | Visualization of blood vessels and perisvascular system of nerves | ICG 0.1 mg/kg intraoperative | [16] |
| Clinical | Pediatric | Abdominal masses and lymphoma | Vascular anatomy of mass, plane of resection during mesenteric division and perfusion assessment of bowel or organs | ICG 0.2–0.5 mg/kg intraoperative | [17,18,19,20,21] | |
| Tumor imaging | Clinical | Pediatric | Hepatoblastoma | Identification of primary residual, and metastatic lesions | ICG 0.1–0.5 mg/kg, 24–96 h before surgery | [22,23,24,25,26,27,28,29,30,31,32,33] |
| Clinical | Pediatric | Wilms’ tumor and renal cell carcinoma | Nephron-sparing surgery and identification of pulmonary (metastatic) lesions | ICG 1.5 mg/kg 24 h before surgery 2.5/5 mg/kg intraoperative | [34,35,36] | |
| Clinical | Pediatric | Abdominal tumors and lymphoma | Ovarian-sparing surgery, resection margins and vascularity of the abdominal mass | ICG 0.2–0.5 mg/kg intraoperative | [17,18,19,20,21] | |
| Clinical | Pediatric and young adults | Bone and soft tissue sarcoma | Guiding resection margins, identification of residual, and metastatic lesions | ICG 0.5–2.5 mg/kg 24 h before surgery | [36,37,38,39,40,41] | |
| Clinical | Young adults | Peripheral nerve sheath tumors | Localizing residual tumor tissue | Fluorescein 1 mg/kg after intubation | [42] | |
| Clinical | Pediatric | Otolaryngologic malignancies | Tumor extension | ICG 1.5 mg/kg 24 h before surgery | [43] | |
| Tissue-specific imaging | ||||||
| Tumor imaging | Preclinical | Mice models | Neuroblastoma | Tumor (margin) identification | Anti-GD2-IRDye800CW * Anti-GD2-IR800 & anti-GD2-IR12 * DPTA-aGD2-IR800 * | [44,45,46] |
| Metastases imaging | Clinical | Young adults | Pulmonary metastases of osteosarcoma and Ewing sarcoma | Tumor identification | Pafolacianine (Cytalux, OTL-38) 0.025 mg/kg 3–8 h before surgery | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Simons, D.C.; van Schalkwijk, L.H.M.; van de Sande, M.A.J.; Vahrmeijer, A.L.; Wijnen, M.H.W.A.; van der Steeg, A.F.W.; Tummers, W.S.F.J. Fluorescence-Guided Surgery in Pediatric Oncology: Current Practice and Future Directions. Cancers 2026, 18, 149. https://doi.org/10.3390/cancers18010149
Simons DC, van Schalkwijk LHM, van de Sande MAJ, Vahrmeijer AL, Wijnen MHWA, van der Steeg AFW, Tummers WSFJ. Fluorescence-Guided Surgery in Pediatric Oncology: Current Practice and Future Directions. Cancers. 2026; 18(1):149. https://doi.org/10.3390/cancers18010149
Chicago/Turabian StyleSimons, Dominique C., Lorenz H. M. van Schalkwijk, Michiel A. J. van de Sande, Alexander L. Vahrmeijer, Marc H. W. A. Wijnen, Alida F. W. van der Steeg, and Willemieke S. F. J. Tummers. 2026. "Fluorescence-Guided Surgery in Pediatric Oncology: Current Practice and Future Directions" Cancers 18, no. 1: 149. https://doi.org/10.3390/cancers18010149
APA StyleSimons, D. C., van Schalkwijk, L. H. M., van de Sande, M. A. J., Vahrmeijer, A. L., Wijnen, M. H. W. A., van der Steeg, A. F. W., & Tummers, W. S. F. J. (2026). Fluorescence-Guided Surgery in Pediatric Oncology: Current Practice and Future Directions. Cancers, 18(1), 149. https://doi.org/10.3390/cancers18010149









