Current and Emerging Fluorescence-Guided Techniques in Glioma to Enhance Resection
Simple Summary
Abstract
1. Introduction
2. 5-Aminolevulinic Acid (5-ALA)
2.1. Mechanism
2.2. Limitation
2.3. Clinical Applications
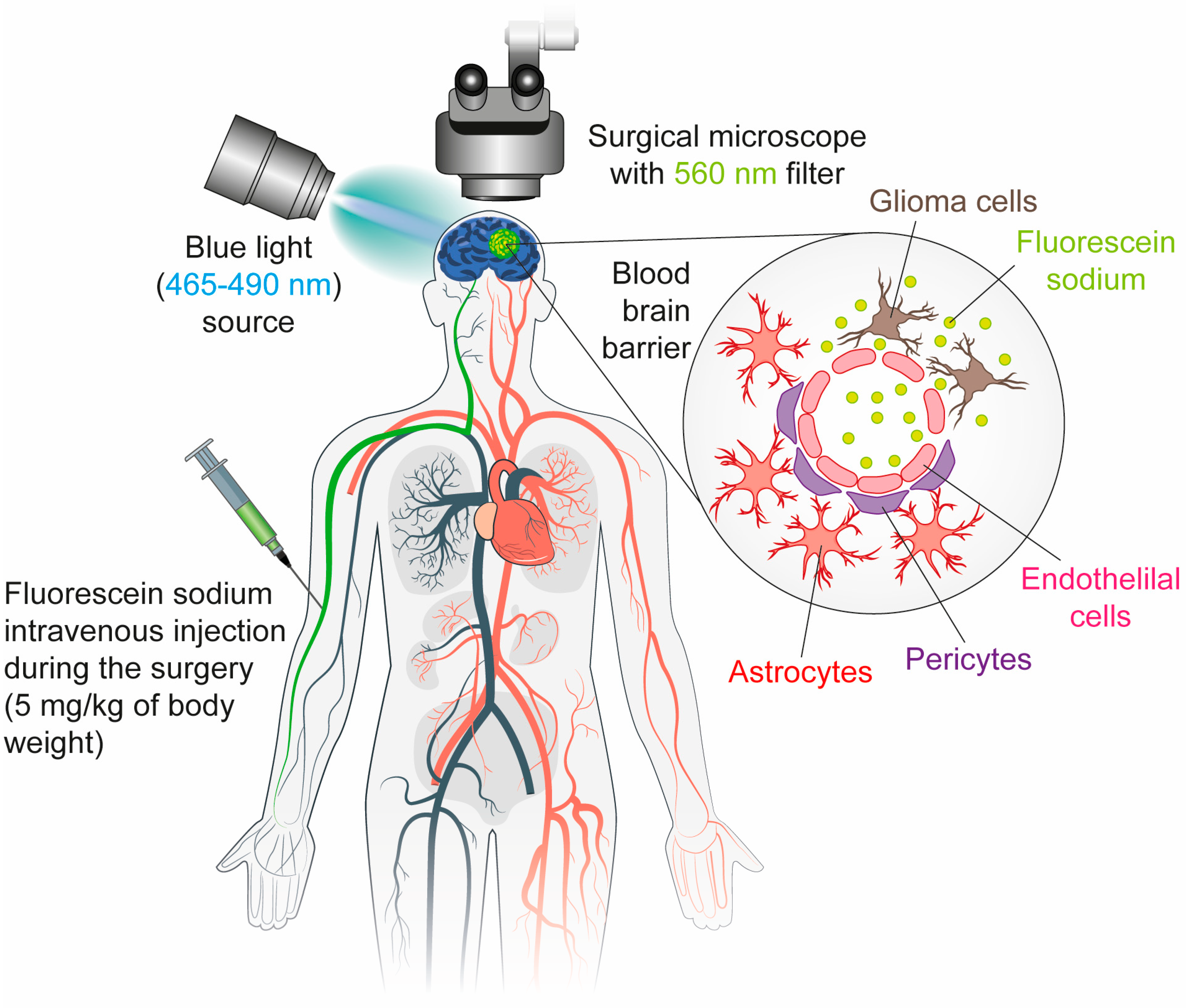
3. Fluorescein Sodium (FS)
3.1. Mechanism
3.2. Limitation
3.3. Clinical Applications
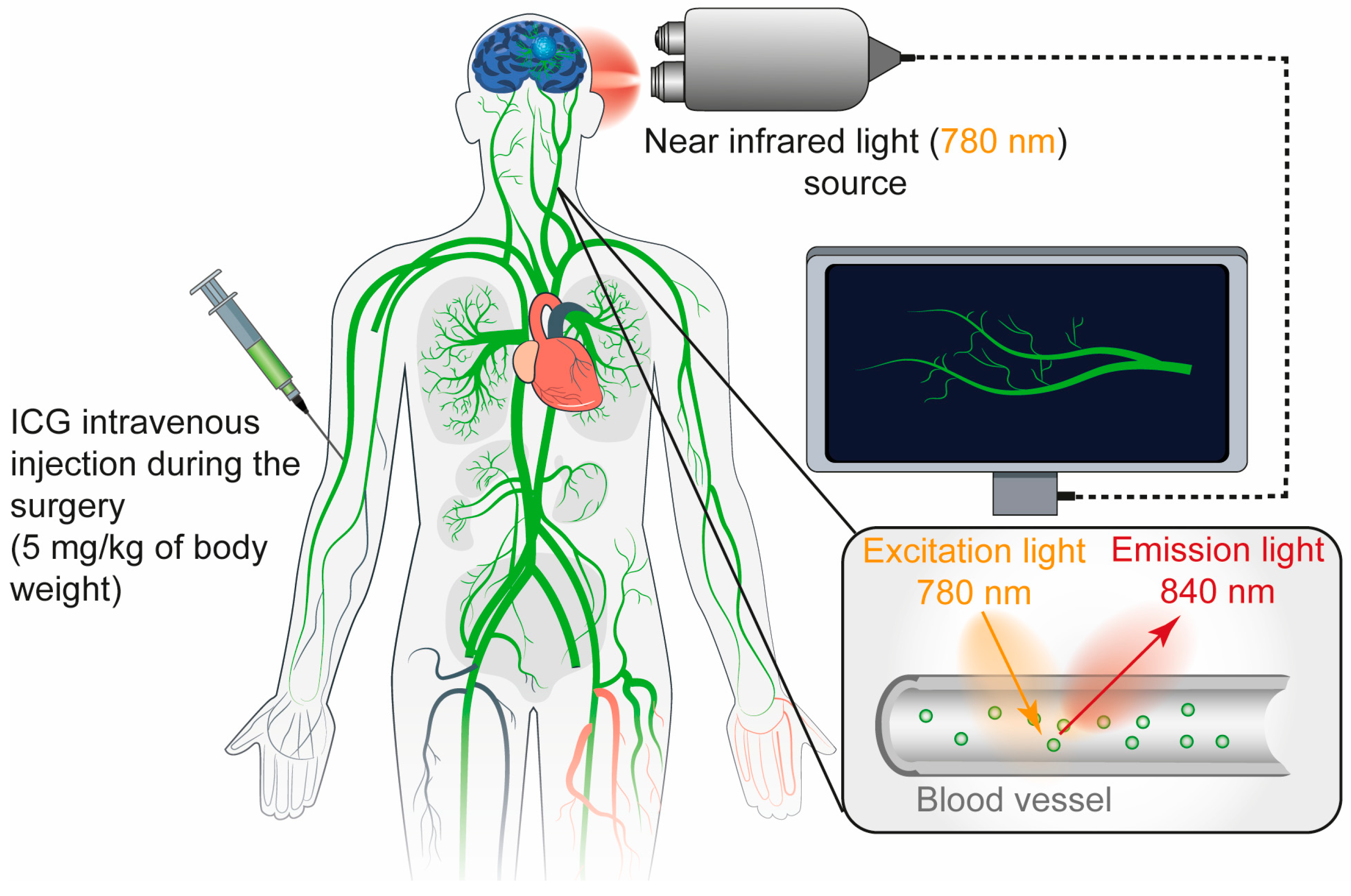
4. Indocyanine Green (ICG)
4.1. Mechanism
4.2. Limitation
5. Antibody-Based Probes for Fluorescence Imaging
5.1. Cetuximab-IRDye 800
5.2. Bevacizumab-IRDye800CW
5.3. MCT4-ICG-NIR-II
5.4. Miltuximab-NIR
6. Peptide-Based Probes for Fluorescence Imaging
7. Conclusions
| Agent | 5-ALA | FS | ICG-NIR |
|---|---|---|---|
| Mechanism | Bind to PpIX in tumor | Bind to BBB disruption | Bind to plasma protein |
| Excitation/Emission | 410–420/635 nm | 465–490/520–530 nm | NIR I: 780–805/820–835 nm NIR II: 900–1100/1000–1700 nm |
| Depth Penetration | Shallow | Shallow | Deeper (via NIR) |
| Limitations | Phototoxicity, equipment required | False positives, non-specific | False positives, non-specific |
| Cost | High | Low | Moderate |
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GBM | glioblastoma |
| 5-ALA | 5-aminolevulinic acid |
| SF | sodium fluorescein |
| ICG | indocyanine green |
| NIR | near-infrared imaging |
| FGS | fluorescence-guided surgery |
| BBB | blood–brain barrier |
| PpIX | protoporphyrin IX |
| EGFR | epidermal growth factor receptor |
| VEGFα | vascular endothelial growth factor α |
| CTX | chlorotoxin |
| PFS | progression-free survival |
| GTR | gross total resection |
| OS | overall survival |
| CE | contrast-enhancing |
| NCE | non-contrast-enhancing |
| KSNO | Korean Society for Neuro-Oncology |
| NCCN | National Comprehensive Cancer Network |
References
- Nguyen, T.T.T.; Greene, L.A.; Mnatsakanyan, H.; Badr, C.E. Revolutionizing Brain Tumor Care: Emerging Technologies and Strategies. Biomedicines 2024, 12, 1376. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, S.; Cui, X.; Wang, Q.; Yang, E.; Tong, F.; Hong, B.; Xiao, M.; Xin, L.; Xu, C.; et al. A novel compound EPIC-0412 reverses temozolomide resistance via inhibiting DNA repair/MGMT in glioblastoma. Neuro-Oncology 2023, 25, 857–870. [Google Scholar] [CrossRef]
- Jiapaer, S.; Furuta, T.; Tanaka, S.; Kitabayashi, T.; Nakada, M. Potential Strategies Overcoming the Temozolomide Resistance for Glioblastoma. Neurol. Med.-Chir. 2018, 58, 405–421. [Google Scholar] [CrossRef]
- Salvato, I.; Marchini, A. Immunotherapeutic Strategies for the Treatment of Glioblastoma: Current Challenges and Future Perspectives. Cancers 2024, 16, 1276. [Google Scholar] [CrossRef] [PubMed]
- Yasinjan, F.; Xing, Y.; Geng, H.; Guo, R.; Yang, L.; Liu, Z.; Wang, H. Immunotherapy: A promising approach for glioma treatment. Front. Immunol. 2023, 14, 1255611. [Google Scholar] [CrossRef] [PubMed]
- Bausart, M.; Preat, V.; Malfanti, A. Immunotherapy for glioblastoma: The promise of combination strategies. J. Exp. Clin. Cancer Res. 2022, 41, 35. [Google Scholar] [CrossRef]
- Lowe, S.; Bhat, K.P.; Olar, A. Current clinical management of patients with glioblastoma. Cancer Rep. 2019, 2, e1216. [Google Scholar] [CrossRef]
- Watson, S.S.; Duc, B.; Kang, Z.; de Tonnac, A.; Eling, N.; Font, L.; Whitmarsh, T.; Massara, M.; iMAXT Consortium; Bodenmiller, B.; et al. Microenvironmental reorganization in brain tumors following radiotherapy and recurrence revealed by hyperplexed immunofluorescence imaging. Nat. Commun. 2024, 15, 3226. [Google Scholar] [CrossRef]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the Extent of Resection with Survival in Glioblastoma: A Systematic Review and Meta-analysis. JAMA Oncol. 2016, 2, 1460–1469. [Google Scholar] [CrossRef]
- Sanai, N.; Polley, M.Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef]
- Jenkinson, M.D.; Barone, D.G.; Hart, M.G.; Bryant, A.; Lawrie, T.A.; Watts, C. Intraoperative imaging technology to maximise extent of resection for glioma. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef]
- Bettag, C.; Schatlo, B.; Abboud, T.; Behme, D.; Bock, C.; von der Brelie, C.; Rohde, V.; Mielke, D. Endoscope-enhanced fluorescence-guided microsurgery increases survival in patients with glioblastoma. Acta Neurochir. 2023, 165, 4221–4226. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, L.Z.; Shi, W.; Demonte, F.; Lang, F.F.; McCutcheon, E.I.; Hassenbushch, J.S.; Holland, E.; et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Monroy-Sosa, A.; Chakravarthi, S.S.; Cortes-Contreras, A.P.; Hernandez-Varela, M.; Andres-Arrieta, V.; Epping, A.; Rovin, R.A. The Evolution of Cerebral Language Localization: Historical Analysis and Current Trends. World Neurosurg. 2021, 145, 89–97. [Google Scholar] [CrossRef]
- Chang, E.F.; Clark, A.; Smith, J.S.; Polley, M.Y.; Chang, S.M.; Barbaro, N.M.; Parsa, A.T.; McDermott, M.W.; Berger, M.S. Functional mapping-guided resection of low-grade gliomas in eloquent areas of the brain: Improvement of long-term survival. Clinical article. J. Neurosurg. 2011, 114, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Hayhurst, C.; Byrne, P.; Eldridge, P.R.; Mallucci, C.L. Application of electromagnetic technology to neuronavigation: A revolution in image-guided neurosurgery. J. Neurosurg. 2009, 111, 1179–1184. [Google Scholar] [CrossRef]
- Roder, C.; Stummer, W.; Coburger, J.; Scherer, M.; Haas, P.; von der Brelie, C.; Kamp, M.A.; Lohr, M.; Hamisch, C.A.; Skardelly, M.; et al. Intraoperative MRI-Guided Resection Is Not Superior to 5-Aminolevulinic Acid Guidance in Newly Diagnosed Glioblastoma: A Prospective Controlled Multicenter Clinical Trial. J. Clin. Oncol. 2023, 41, 5512–5523. [Google Scholar] [CrossRef]
- Sweeney, J.F.; Rosoklija, G.; Sheldon, B.L.; Bondoc, M.; Bandlamuri, S.; Adamo, M.A. Comparison of sodium fluorescein and intraoperative ultrasonography in brain tumor resection. J. Clin. Neurosci. 2022, 106, 141–144. [Google Scholar] [CrossRef]
- Quick-Weller, J.; Lescher, S.; Forster, M.T.; Konczalla, J.; Seifert, V.; Senft, C. Combination of 5-ALA and iMRI in re-resection of recurrent glioblastoma. Br. J. Neurosurg. 2016, 30, 313–317. [Google Scholar] [CrossRef]
- Li, Z.; Song, Y.; Farrukh Hameed, N.U.; Yuan, S.; Wu, S.; Gong, X.; Zhuang, D.; Lu, J.; Zhu, F.; Qiu, T.; et al. Effect of high-field iMRI guided resection in cerebral glioma surgery: A randomized clinical trial. Eur. J. Cancer 2024, 199, 113528. [Google Scholar] [CrossRef]
- Mansour, H.M.; Shah, S.; Aguilar, T.M.; Abdul-Muqsith, M.; Gonzales-Portillo, G.S.; Mehta, A.I. Enhancing Glioblastoma Resection with NIR Fluorescence Imaging: A Systematic Review. Cancers 2024, 16, 3984. [Google Scholar] [CrossRef]
- Mazurek, M.; Lehman, N.; Stoma, F.; Czaja, G.; Banach, A.; Jarosz, B.; Rola, R. How 5-ALA enlightens neurosurgery—Results of a single centre study on high-grade gliomas. Ann. Agric. Environ. Med. 2025, 32, 133–141. [Google Scholar] [CrossRef]
- Neira, J.A.; Ung, T.H.; Sims, J.S.; Malone, H.R.; Chow, D.S.; Samanamud, J.L.; Zanazzi, G.J.; Guo, X.; Bowden, S.G.; Zhao, B.; et al. Aggressive resection at the infiltrative margins of glioblastoma facilitated by intraoperative fluorescein guidance. J. Neurosurg. 2017, 127, 111–122. [Google Scholar] [CrossRef]
- Schupper, A.J.; Hadjipanayis, C.G. Novel approaches to targeting gliomas at the leading/cutting edge. J. Neurosurg. 2023, 139, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Muther, M.; Spille, D. Beyond fluorescence-guided resection: 5-ALA-based glioblastoma therapies. Acta. Neurochir. 2024, 166, 163. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, Y.; Wang, Y.; Yu, J.; Zhang, C. Utility and Safety of 5-ALA Guided Surgery in Pediatric Brain Tumors: A Systematic Review. Cancers 2024, 16, 3677. [Google Scholar] [CrossRef] [PubMed]
- Traylor, J.I.; Pernik, M.N.; Sternisha, A.C.; McBrayer, S.K.; Abdullah, K.G. Molecular and Metabolic Mechanisms Underlying Selective 5-Aminolevulinic Acid-Induced Fluorescence in Gliomas. Cancers 2021, 13, 580. [Google Scholar] [CrossRef]
- Ahmed, N.; Hossain, M.N.; Ahmed, R.; Abbasi, M.M.; Azab, M.A.; Alam, M.; Nusrat Khan, N.; Kazi, M.R.; Ghafoor, N.; Siraj, N.; et al. Safety, efficacy, and side effects of sodium fluorescein-aided resection of glioblastoma: A quasi-experimental study. Ann. Med. Surg. 2024, 86, 6521–6530. [Google Scholar] [CrossRef]
- Reinhart, M.B.; Huntington, C.R.; Blair, L.J.; Heniford, B.T.; Augenstein, V.A. Indocyanine Green: Historical Context, Current Applications, and Future Considerations. Surg. Innov. 2016, 23, 166–175. [Google Scholar] [CrossRef]
- Kim, E.H.; Cho, J.M.; Chang, J.H.; Kim, S.H.; Lee, K.S. Application of intraoperative indocyanine green videoangiography to brain tumor surgery. Acta Neurochir. 2011, 153, 1487–1495; discussion 1494–1495. [Google Scholar] [CrossRef]
- Della Puppa, A.; Rustemi, O.; Gioffre, G.; Rolma, G.; Grandis, M.; Munari, M.; Scienza, R. Application of indocyanine green video angiography in parasagittal meningioma surgery. Neurosurg. Focus 2014, 36, E13. [Google Scholar] [CrossRef]
- Raabe, A.; Beck, J.; Gerlach, R.; Zimmermann, M.; Seifert, V. Near-infrared Indocyanine Green Video Angiography: A New Method for Intraoperative Assessment of Vascular Flow. Neurosurgery 2003, 52, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Picart, T.; Berhouma, M.; Dumot, C.; Pallud, J.; Metellus, P.; Armoiry, X.; Guyotat, J. Optimization of high-grade glioma resection using 5-ALA fluorescence-guided surgery: A literature review and practical recommendations from the neuro-oncology club of the French society of neurosurgery. Neurochirurgie 2019, 65, 164–177. [Google Scholar] [CrossRef]
- Stepp, H.; Stummer, W. 5-ALA in the management of malignant glioma. Lasers Surg. Med. 2018, 50, 399–419. [Google Scholar] [CrossRef]
- Singh, D.K.; Khan, K.A.; Singh, A.K.; Kaif, M.; Yadav, K.; Kumar Singh, R.; Ahmad, F. Fluorescein sodium fluorescence: Role in stereotactic brain biopsy. Br. J. Neurosurg. 2023, 37, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Li, D.; Ren, Y.; Qu, C.; Shi, X.; Liu, R.; Liu, H.; Tian, J.; Hu, Z.; Sun, T.; et al. A phosphorescent probe for in vivo imaging in the second near-infrared window. Nat. Biomed. Eng. 2022, 6, 629–639. [Google Scholar] [CrossRef]
- Chirizzi, C.; Pellegatta, S.; Gori, A.; Falco, J.; Rubiu, E.; Acerbi, F.; Bombelli, F.B. Next-generation agents for fluorescence-guided glioblastoma surgery. Bioeng. Transl. Med. 2024, 9, e10608. [Google Scholar] [CrossRef] [PubMed]
- Cosco, E.D.; Spearman, A.L.; Ramakrishnan, S.; Lingg, J.G.P.; Saccomano, M.; Pengshung, M.; Arus, B.A.; Wong, K.C.Y.; Glasl, S.; Ntziachristos, V.; et al. Shortwave infrared polymethine fluorophores matched to excitation lasers enable non-invasive, multicolour in vivo imaging in real time. Nat. Chem. 2020, 12, 1123–1130. [Google Scholar] [CrossRef]
- Lee, J.Y.K.; Pierce, J.T.; Thawani, J.P.; Zeh, R.; Nie, S.; Martinez-Lage, M.; Singhal, S. Near-infrared fluorescent image-guided surgery for intracranial meningioma. J. Neurosurg. 2018, 128, 380–390. [Google Scholar] [CrossRef]
- Picart, T.; Pallud, J.; Berthiller, J.; Dumot, C.; Berhouma, M.; Ducray, F.; Armoiry, X.; Margier, J.; Guerre, P.; Varlet, P.; et al. Use of 5-ALA fluorescence-guided surgery versus white-light conventional microsurgery for the resection of newly diagnosed glioblastomas (RESECT study): A French multicenter randomized phase III study. J. Neurosurg. 2024, 140, 987–1000. [Google Scholar] [CrossRef]
- Su, X.; Huang, Q.F.; Chen, H.L.; Chen, J. Fluorescence-guided resection of high-grade gliomas: A systematic review and meta-analysis. Photodiagn. Photodyn. Ther. 2014, 11, 451–458. [Google Scholar] [CrossRef]
- Hadjipanayis, C.G.; Stummer, W. 5-ALA and FDA approval for glioma surgery. J. Neurooncol. 2019, 141, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Lakomkin, N.; Hadjipanayis, C.G. Fluorescence-guided surgery for high-grade gliomas. J. Surg. Oncol. 2018, 118, 356–361. [Google Scholar] [CrossRef]
- Schupper, A.J.; Rao, M.; Mohammadi, N.; Baron, R.; Lee, J.Y.K.; Acerbi, F.; Hadjipanayis, C.G. Fluorescence-Guided Surgery: A Review on Timing and Use in Brain Tumor Surgery. Front. Neurol. 2021, 12, 682151. [Google Scholar] [CrossRef]
- Mortezaei, A.; Dmytriw, A.A. Letter to the Editor. Is it time for a radial-first approach to diagnostic cerebral angiography? J. Neurosurg. 2024, 140, 1811–1812. [Google Scholar] [CrossRef]
- Howley, R.; Chandratre, S.; Chen, B. 5-Aminolevulinic Acid as a Theranostic Agent for Tumor Fluorescence Imaging and Photodynamic Therapy. Bioengineering 2023, 10, 496. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Chen, C.C. 5-aminolevulinic enhanced brain lesions mimic glioblastoma: A case report and literature review. Medicine 2024, 103, e34518. [Google Scholar] [CrossRef] [PubMed]
- Chandratre, S.; Merenich, D.; Myers, K.; Chen, B. 5-Aminolevulinic acid-mediated photodynamic therapy in combination with kinase inhibitor lapatinib enhances glioblastoma cell death. Apoptosis 2024, 29, 1978–1987. [Google Scholar] [CrossRef]
- Ajioka, R.S.; Phillips, J.D.; Kushner, J.P. Biosynthesis of heme in mammals. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2006, 1763, 723–736. [Google Scholar] [CrossRef]
- Peciu-Florianu, I.; Vannod-Michel, Q.; Vauleon, E.; Bonneterre, M.E.; Reyns, N. Long term follow-up of patients with newly diagnosed glioblastoma treated by intraoperative photodynamic therapy: An update from the INDYGO trial (NCT03048240). J. Neuroonc. 2024, 168, 495–505. [Google Scholar] [CrossRef]
- Chen, J.S.; Young, J.S.; Berger, M.S. Current and Future Applications of 5-Aminolevulinic Acid in Neurosurgical Oncology. Cancers 2025, 17, 1332. [Google Scholar] [CrossRef]
- Herta, J.; Cho, A.; Roetzer-Pejrimovsky, T.; Hoftberger, R.; Marik, W.; Kronreif, G.; Peilnsteiner, T.; Rossler, K.; Wolfsberger, S. Optimizing maximum resection of glioblastoma: Raman spectroscopy versus 5-aminolevulinic acid. J. Neurosurg. 2023, 139, 334–343. [Google Scholar] [CrossRef]
- Gautheron, A.; Bernstock, J.D.; Picart, T.; Guyotat, J.; Valdes, P.A.; Montcel, B. 5-ALA induced PpIX fluorescence spectroscopy in neurosurgery: A review. Front. Neurosci. 2024, 18, 1310282. [Google Scholar] [CrossRef]
- Walke, A.; Black, D.; Valdes, P.A.; Stummer, W.; Konig, S.; Suero-Molina, E. Challenges in, and recommendations for, hyperspectral imaging in ex vivo malignant glioma biopsy measurements. Sci. Rep. 2023, 13, 3829. [Google Scholar] [CrossRef]
- Woo, P.Y.M.; Law, M.; Gai, X.; Ng, B.C.F.; Ko, N.M.W.; Wong, H.T.; Chan, K.Y. Novel Wavelength-Specific Blue Light-Emitting Headlamp for 5-Aminolevulinic Acid Fluorescence-Guided Resection of Glioblastoma. World Neurosurg. 2019, 131, 220–226. [Google Scholar] [CrossRef]
- Valerio, J.E.; de Jesus Aguirre Vera, G.; Zumaeta, J.; Rea, N.S.; Fernandez Gomez, M.P.; Mantilla-Farfan, P.; Valente, L.; Alvarez-Pinzon, A.M. Comparative Analysis of 5-ALA and Fluorescent Techniques in High-Grade Glioma Treatment. Biomedicines 2025, 13, 1161. [Google Scholar] [CrossRef] [PubMed]
- Keles, E.G.; Anderson, B.; Berger, S.M. The Effect of Extent of Resection on Time to Tumor Progression and Survival in Patients With Glioblastoma Multiforme of the Cerebral Hemisphere. Surg. Neurol. 1999, 52, 371–379. [Google Scholar] [CrossRef]
- Sanai, N.; Berger, M.S. Glioma Extent of Resection and Its Impact on Patient Outcome. Neurosurgery 2008, 62, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J.; Group, A.L.-G.S. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Cozzens, J.W.; Lokaitis, B.C.; Moore, B.E.; Amin, D.V.; Espinosa, J.A.; MacGregor, M.; Michael, A.P.; Jones, B.A. A Phase 1 Dose-Escalation Study of Oral 5-Aminolevulinic Acid in Adult Patients Undergoing Resection of a Newly Diagnosed or Recurrent High-Grade Glioma. Neurosurgery 2017, 81, 46–55. [Google Scholar] [CrossRef]
- Aldave, G.; Tejada, S.; Pay, E.; Marigil, M.; Bejarano, B.; Idoate, M.A.; Diez-Valle, R. Prognostic value of residual fluorescent tissue in glioblastoma patients after gross total resection in 5-aminolevulinic Acid-guided surgery. Neurosurgery 2013, 72, 915–920; discussion 920–911. [Google Scholar] [CrossRef]
- Roder, C.; Bisdas, S.; Ebner, F.H.; Honegger, J.; Naegele, T.; Ernemann, U.; Tatagiba, M. Maximizing the extent of resection and survival benefit of patients in glioblastoma surgery: High-field iMRI versus conventional and 5-ALA-assisted surgery. Eur. J. Surg. Oncol. 2014, 40, 297–304. [Google Scholar] [CrossRef]
- Nduom, E.K.; Yang, C.; Merrill, M.J.; Zhuang, Z.; Lonser, R.R. Characterization of the blood–brain barrier of metastatic and primary malignant neoplasms. J. Neurosurg. 2013, 119, 427–433. [Google Scholar] [CrossRef]
- Smith, E.J.; Gohil, K.; Thompson, C.M.; Naik, A.; Hassaneen, W. Fluorescein-Guided Resection of High Grade Gliomas: A Meta-Analysis. World Neurosurg. 2021, 155, 181–188. [Google Scholar] [CrossRef]
- Rey-Dios, R.; Hattab, E.M.; Cohen-Gadol, A.A. Use of intraoperative fluorescein sodium fluorescence to improve the accuracy of tissue diagnosis during stereotactic needle biopsy of high-grade gliomas. Acta Neurochir. 2014, 156, 1071–1075; discussion 1075. [Google Scholar] [CrossRef]
- Hohne, J.; Schebesch, K.M.; de Laurentis, C.; Akcakaya, M.O.; Pedersen, C.B.; Brawanski, A.; Poulsen, F.R.; Kiris, T.; Cavallo, C.; Broggi, M.; et al. Fluorescein Sodium in the Surgical Treatment of Recurrent Glioblastoma Multiforme. World Neurosurg. 2019, 125, e158–e164. [Google Scholar] [CrossRef] [PubMed]
- Lovato, R.M.; Vitorino Araujo, J.L.; Esteves Veiga, J.C. Low-Cost Device for Fluorescein-Guided Surgery in Malignant Brain Tumor. World Neurosurg. 2017, 104, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Bongetta, D.; Zoia, C.; Pugliese, R.; Adinolfi, D.; Silvani, V.; Gaetani, P. Low-Cost Fluorescein Detection System for High-Grade Glioma Surgery. World Neurosurg. 2016, 88, 54–58. [Google Scholar] [CrossRef]
- Manoharan, R.; Parkinson, J. Sodium Fluorescein in Brain Tumor Surgery: Assessing Relative Fluorescence Intensity at Tumor Margins. Asian J. Neurosurg. 2020, 15, 88–93. [Google Scholar] [CrossRef]
- Stockhammer, F. What does fluorescence depict in glioma surgery? Acta Neurochir. 2013, 155, 1479–1480. [Google Scholar] [CrossRef] [PubMed]
- Schebesch, K.M.; Proescholdt, M.; Hohne, J.; Hohenberger, C.; Hansen, E.; Riemenschneider, M.J.; Ullrich, W.; Doenitz, C.; Schlaier, J.; Lange, M.; et al. Sodium fluorescein-guided resection under the YELLOW 560 nm surgical microscope filter in malignant brain tumor surgery—A feasibility study. Acta Neurochir. 2013, 155, 693–699. [Google Scholar] [CrossRef]
- Stummer, W. Fluorescein in brain metastasis and glioma surgery. Acta Neurochir. 2015, 157, 2199–2200. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, M.; Wang, X.; Tan, J.; Qin, C.; Liu, Q. Fluorescein-guided surgery in high-grade gliomas: Focusing on the eloquent and deep-seated areas. J. Cancer Res. Clin. Oncol. 2024, 150, 274. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.W.; Valdes, P.A.; Harris, B.T.; Hartov, A.; Fan, X.; Ji, S.; Leblond, F.; Tosteson, T.D.; Wilson, B.C.; Paulsen, K.D. Glioblastoma multiforme treatment with clinical trials for surgical resection (aminolevulinic acid). Neurosurg. Clin. N. Am. 2012, 23, 371–377. [Google Scholar] [CrossRef]
- Valdes, P.A.; Jacobs, V.; Harris, B.T.; Wilson, B.C.; Leblond, F.; Paulsen, K.D.; Roberts, D.W. Quantitative fluorescence using 5-aminolevulinic acid-induced protoporphyrin IX biomarker as a surgical adjunct in low-grade glioma surgery. J. Neurosurg. 2015, 123, 771–780. [Google Scholar] [CrossRef]
- Elliott, J.T.; Dsouza, A.V.; Davis, S.C.; Olson, J.D.; Paulsen, K.D.; Roberts, D.W.; Pogue, B.W. Review of fluorescence guided surgery visualization and overlay techniques. Biomed. Opt. Express 2015, 6, 3765–3782. [Google Scholar] [CrossRef]
- Pop, C.F.; Veys, I.; Bormans, A.; Larsimont, D.; Liberale, G. Fluorescence imaging for real-time detection of breast cancer tumors using IV injection of indocyanine green with non-conventional imaging: A systematic review of preclinical and clinical studies of perioperative imaging technologies. Breast Cancer Res. Treat. 2024, 204, 429–442. [Google Scholar] [CrossRef]
- Kosaka, N.; Ogawa, M.; Choyke, P.L.; Kobayashi, H. Clinical implications of near-infrared fluorescence imaging in cancer. Future Oncol. 2009, 5, 1501–1511. [Google Scholar] [CrossRef]
- Kamp, M.A.; Slotty, P.; Turowski, B.; Etminan, N.; Steiger, H.J.; Hanggi, D.; Stummer, W. Microscope-integrated quantitative analysis of intraoperative indocyanine green fluorescence angiography for blood flow assessment: First experience in 30 patients. Neurosurgery 2012, 70, 65–73; discussion 73–64. [Google Scholar] [CrossRef]
- Lee, J.Y.K.; Pierce, J.T.; Zeh, R.; Cho, S.S.; Salinas, R.; Nie, S.; Singhal, S. Intraoperative Near-Infrared Optical Contrast Can Localize Brain Metastases. World Neurosurg. 2017, 106, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Zeh, R.; Sheikh, S.; Xia, L.; Pierce, J.; Newton, A.; Predina, J.; Cho, S.; Nasrallah, M.; Singhal, S.; Dorsey, J.; et al. The second window ICG technique demonstrates a broad plateau period for near infrared fluorescence tumor contrast in glioblastoma. PLoS ONE 2017, 12, e0182034. [Google Scholar] [CrossRef]
- Padalkar, M.V.; Pleshko, N. Wavelength-dependent penetration depth of near infrared radiation into cartilage. Analyst 2015, 140, 2093–2100. [Google Scholar] [CrossRef]
- Frangioni, J.V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef]
- Cho, S.S.; Salinas, R.; Lee, J.Y.K. Indocyanine-Green for Fluorescence-Guided Surgery of Brain Tumors: Evidence, Techniques, and Practical Experience. Front. Surg. 2019, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Fang, C.; Li, B.; Zhang, Z.; Cao, C.; Cai, M.; Su, S.; Sun, X.; Shi, X.; Li, C.; et al. First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat. Biomed. Eng. 2020, 4, 259–271. [Google Scholar] [CrossRef]
- Dai, Z.Y.; Shen, C.; Mi, X.Q.; Pu, Q. The primary application of indocyanine green fluorescence imaging in surgical oncology. Front. Surg. 2023, 10, 1077492. [Google Scholar] [CrossRef] [PubMed]
- Raabe, A.; Nakaji, P.; Beck, J.; Kim, J.L.; Hsu, P.K.F.; Kamerman, D.J.; Seifert, V.; Spetzler, F.R. Prospective evaluation of surgical microscope–integrated intraoperative near-infrared indocyanine green videoangiography during aneurysm surgery. J. Neurosurg. 2005, 103, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Zha, M.; Zheng, H.; Gao, D.; Sheng, Z. Recent Advances in Indocyanine Green-Based Probes for Second Near-Infrared Fluorescence Imaging and Therapy. Research 2025, 8, 0583. [Google Scholar] [CrossRef]
- Miller, S.E.; Tummers, W.S.; Teraphongphom, N.; van den Berg, N.S.; Hasan, A.; Ertsey, R.D.; Nagpal, S.; Recht, L.D.; Plowey, E.D.; Vogel, H.; et al. First-in-human intraoperative near-infrared fluorescence imaging of glioblastoma using cetuximab-IRDye800. J. Neurooncol. 2018, 139, 135–143. [Google Scholar] [CrossRef]
- Hernot, S.; van Manen, L.; Debie, P.; Mieog, J.S.D.; Vahrmeijer, A.L. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. 2019, 20, e354–e367. [Google Scholar] [CrossRef]
- Hatanpaa, K.J.; Burma, S.; Zhao, D.; Habib, A.A. Epidermal growth factor receptor in glioma: Signal transduction, neuropathology, imaging, and radioresistance. Neoplasia 2010, 12, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Heimberger, B.A.; Hlatky, R.; Suki, D.; Yang, D.; Weinberg, J.; Gilbert, M.; Sawaya, R.; Aldape, K. Prognostic Effect of Epidermal Growth Factor Receptor and EGFRvIII in Glioblastoma Multiforme Patients. Clin. Cancer Res. 2005, 11, 1462–1466. [Google Scholar] [CrossRef]
- Combs, S.E.; Heeger, S.; Haselmann, R.; Edler, L.; Debus, J.; Schulz-Ertner, D. Treatment of primary glioblastoma multiforme with cetuximab, radiotherapy and temozolomide (GERT)–phase I/II trial: Study protocol. BMC Cancer 2006, 6, 133. [Google Scholar] [CrossRef]
- Baumann, M.; Krause, M. Targeting the epidermal growth factor receptor in radiotherapy: Radiobiological mechanisms, preclinical and clinical results. Radiother. Oncol. 2004, 72, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Warram, J.M.; de Boer, E.; Korb, M.; Hartman, Y.; Kovar, J.; Markert, J.M.; Gillespie, G.Y.; Rosenthal, E.L. Fluorescence-guided resection of experimental malignant glioma using cetuximab-IRDye 800CW. Br. J. Neurosurg. 2015, 29, 850–858. [Google Scholar] [CrossRef]
- Kim, E.S.; Khuri, F.R.; Herbst, R.S. Epidermal growth factor receptor biology (IMC-C225). Curr. Opin. Oncol. 2001, 13, 506–513. [Google Scholar] [CrossRef]
- Ogasawara, C.; Philbrick, B.D.; Adamson, D.C. Meningioma: A Review of Epidemiology, Pathology, Diagnosis, Treatment, and Future Directions. Biomedicines 2021, 9, 319. [Google Scholar] [CrossRef]
- Huntoon, K.; Toland, A.M.S.; Dahiya, S. Meningioma: A Review of Clinicopathological and Molecular Aspects. Front. Oncol. 2020, 10, 579599. [Google Scholar] [CrossRef]
- Butta, S.; Pal, M.; Ghosh, S.; Gupta, M.K. The role of vascular endothelial growth factor in predicting the tumor dynamics of meningiomas. Int. J. Res. Med. Sci. 2021, 9, 3397. [Google Scholar] [CrossRef]
- Winter, R.C.; Antunes, A.C.M.; de Oliveira, F.H. The relationship between vascular endothelial growth factor and histological grade in intracranial meningioma. Surg. Neurol. Int. 2020, 11, 328. [Google Scholar] [CrossRef]
- Josserand, V.; Bernard, C.; Michy, T.; Guidetti, M.; Vollaire, J.; Coll, J.L.; Hurbin, A. Tumor-Specific Imaging with Angiostamp800 or Bevacizumab-IRDye 800CW Improves Fluorescence-Guided Surgery over Indocyanine Green in Peritoneal Carcinomatosis. Biomedicines 2022, 10, 1059. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, B.M.; Cordia, Q.C.F.; Nonnekens, J.; Meersma, G.J.; Donthu, V.S.; Nagengast, W.B.; Kruijff, S.; den Dunnen, W.F.A.; Kruyt, F.A.E.; Groen, R.J.M. Bevacizumab-IRDye800CW for tumor detection in fluorescence-guided meningioma surgery (LUMINA trial): A single-center phase I study. J. Neurosurg. 2024, 141, 1655–1666. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, J.W.; Lee, J.H.; Kim, Y.S. Expression of lactate/H(+) symporters MCT1 and MCT4 and their chaperone CD147 predicts tumor progression in clear cell renal cell carcinoma: Immunohistochemical and The Cancer Genome Atlas data analyses. Hum. Pathol. 2015, 46, 104–112. [Google Scholar] [CrossRef]
- Pinheiro, C.; Longatto-Filho, A.; Azevedo-Silva, J.; Casal, M.; Schmitt, F.C.; Baltazar, F. Role of monocarboxylate transporters in human cancers: State of the art. J. Bioenerg. Biomembr. 2012, 44, 127–139. [Google Scholar] [CrossRef]
- Zhao, H.; Li, C.; Shi, X.; Zhang, J.; Jia, X.; Hu, Z.; Gao, Y.; Tian, J. Near-infrared II fluorescence-guided glioblastoma surgery targeting monocarboxylate transporter 4 combined with photothermal therapy. eBioMedicine 2024, 106, 105243. [Google Scholar] [CrossRef] [PubMed]
- Truong, Q.; Justiniano, I.O.; Nocon, A.L.; Soon, J.T.; Wissmueller, S.; Campbell, D.H.; Walsh, B.J. Glypican-1 as a Biomarker for Prostate Cancer: Isolation and Characterization. J. Cancer 2016, 7, 1002–1009. [Google Scholar] [CrossRef]
- Su, G.; Meyer, K.; Nandini, C.D.; Qiao, D.; Salamat, S.; Friedl, A. Glypican-1 is frequently overexpressed in human gliomas and enhances FGF-2 signaling in glioma cells. Am. J. Pathol. 2006, 168, 2014–2026. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Sugiyama, K.; Hama, S.; Yamasaki, F.; Takayasu, T.; Nosaka, R.; Onishi, S.; Muragaki, Y.; Kawamata, T.; Kurisu, K. High Expression of Glypican-1 Predicts Dissemination and Poor Prognosis in Glioblastomas. World Neurosurg. 2017, 105, 282–288. [Google Scholar] [CrossRef]
- Hara, H.; Takahashi, T.; Serada, S.; Fujimoto, M.; Ohkawara, T.; Nakatsuka, R.; Harada, E.; Nishigaki, T.; Takahashi, Y.; Nojima, S.; et al. Overexpression of glypican-1 implicates poor prognosis and their chemoresistance in oesophageal squamous cell carcinoma. Br. J. Cancer 2016, 115, 66–75. [Google Scholar] [CrossRef]
- Aikawa, T.; Whipple, C.A.; Lopez, M.E.; Gunn, J.; Young, A.; Lander, A.D.; Korc, M. Glypican-1 modulates the angiogenic and metastatic potential of human and mouse cancer cells. J. Clin. Investig. 2008, 118, 89–99. [Google Scholar] [CrossRef]
- Polikarpov, D.M.; Campbell, D.H.; McRobb, L.S.; Wu, J.; Lund, M.E.; Lu, Y.; Deyev, S.M.; Davidson, A.S.; Walsh, B.J.; Zvyagin, A.V.; et al. Near-Infrared Molecular Imaging of Glioblastoma by Miltuximab((R))-IRDye800CW as a Potential Tool for Fluorescence-Guided Surgery. Cancers 2020, 12, 984. [Google Scholar] [CrossRef] [PubMed]
- Dardevet, L.; Rani, D.; Aziz, T.A.; Bazin, I.; Sabatier, J.M.; Fadl, M.; Brambilla, E.; De Waard, M. Chlorotoxin: A helpful natural scorpion peptide to diagnose glioma and fight tumor invasion. Toxins 2015, 7, 1079–1101. [Google Scholar] [CrossRef]
- Farkas, S.; Cioca, D.; Muranyi, J.; Hornyak, P.; Brunyanszki, A.; Szeker, P.; Boros, E.; Horvath, P.; Hujber, Z.; Racz, G.Z.; et al. Chlorotoxin binds to both matrix metalloproteinase 2 and neuropilin 1. J. Biol. Chem. 2023, 299, 104998. [Google Scholar] [CrossRef]
- Boltman, T.; Meyer, M.; Ekpo, O. Diagnostic and Therapeutic Approaches for Glioblastoma and Neuroblastoma Cancers Using Chlorotoxin Nanoparticles. Cancers 2023, 15, 3388. [Google Scholar] [CrossRef]
- Cohen, G.; Burks, S.R.; Frank, J.A. Chlorotoxin-A Multimodal Imaging Platform for Targeting Glioma Tumors. Toxins 2018, 10, 496. [Google Scholar] [CrossRef]
- Patil, C.G.; Walker, D.G.; Miller, D.M.; Butte, P.; Morrison, B.; Kittle, D.S.; Hansen, S.J.; Nufer, K.L.; Byrnes-Blake, K.A.; Yamada, M.; et al. Phase 1 Safety, Pharmacokinetics, and Fluorescence Imaging Study of Tozuleristide (BLZ-100) in Adults With Newly Diagnosed or Recurrent Gliomas. Neurosurgery 2019, 85, E641–E649. [Google Scholar] [CrossRef]
- Horbinski, C.; Nabors, L.B.; Portnow, J.; Baehring, J.; Bhatia, A.; Bloch, O.; Brem, S.; Butowski, N.; Cannon, D.M.; Chao, S.; et al. NCCN Guidelines(R) Insights: Central Nervous System Cancers, Version 2.2022. J. Natl. Compr. Cancer Netw. 2023, 21, 12–20. [Google Scholar] [CrossRef]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.Z.; Kim, C.Y.; Lim, D.H. The Overview of Practical Guidelines for Gliomas by KSNO, NCCN, and EANO. Brain Tumor Res. Treat. 2022, 10, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, S.; Paraboschi, I.; McNair, A.; Smith, M.; Rankin, K.S.; Elson, D.S.; Paleri, V.; Leff, D.; Stasiuk, G.; Anderson, J. Monoclonal Antibodies for Targeted Fluorescence-Guided Surgery: A Review of Applicability across Multiple Solid Tumors. Cancers 2024, 16, 1045. [Google Scholar] [CrossRef]
- Kubben, P.L.; ter Meulen, K.J.; Schijns, O.E.; ter Laak-Poort, M.P.; van Overbeeke, J.J.; van Santbrink, H. Intraoperative MRI-guided resection of glioblastoma multiforme: A systematic review. Lancet Oncol. 2011, 12, 1062–1070. [Google Scholar] [CrossRef]
- Legnani, F.G.; Franzini, A.; Mattei, L.; Saladino, A.; Casali, C.; Prada, F.; Perin, A.; Cojazzi, V.; Saini, M.; Kronreif, G.; et al. Image-Guided Biopsy of Intracranial Lesions with a Small Robotic Device (iSYS1): A Prospective, Exploratory Pilot Study. Oper. Oper. Neurosurg. 2019, 17, 403–412. [Google Scholar] [CrossRef]
- Maddahi, Y.; Zareinia, K.; Gan, L.S.; Sutherland, C.; Lama, S.; Sutherland, G.R. Treatment of Glioma Using neuroArm Surgical System. BioMed Res. Int. 2016, 2016, 9734512. [Google Scholar] [CrossRef]
- Bailey, D.; Zacharia, B.E. Intraoperative imaging techniques to improve tumor detection in the surgical management of gliomas. Adv. Cancer Res. 2025, 166, 103–135. [Google Scholar] [CrossRef] [PubMed]
- Black, D.; Byrne, D.; Walke, A.; Liu, S.; Di Ieva, A.; Kaneko, S.; Stummer, W.; Salcudean, T.; Suero Molina, E. Towards machine learning-based quantitative hyperspectral image guidance for brain tumor resection. Commun. Med. 2024, 4, 131. [Google Scholar] [CrossRef] [PubMed]
- Elliot, M.; Segaud, S.; Lavrador, J.P.; Vergani, F.; Bhangoo, R.; Ashkan, K.; Xie, Y.; Stasiuk, G.J.; Vercauteren, T.; Shapey, J. Fluorescence Guidance in Glioma Surgery: A Narrative Review of Current Evidence and the Drive Towards Objective Margin Differentiation. Cancers 2025, 17, 2019. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, B.; Brown, C.S.; Colan, J.A.; Zhang, J.Y.; Huq, S.; Rivera, D.; Young, T.; Williams, T.; Subramaniam, V.; Hadjipanayis, C. Fluorescence-Guided Surgery for Gliomas: Past, Present, and Future. Cancers 2025, 17, 1837. [Google Scholar] [CrossRef] [PubMed]

| Clinical Trial Identifier/Therapy | Study Name | Phase/Recruitment Status | Key Findings/Conclusions |
|---|---|---|---|
| NCT01128218/5-ALA | A study of the specificity and sensitivity of 5-ALA fluorescence in malignant brain tumors | Phase 1,2/ Completed | High-dose oral 5-ALA (>40 mg/kg) is safe and effective for intraoperative tumor detection. However, it does not significantly reduce false-negative observations compared to standard dosing [60]. |
| NCT00241670/5-ALA | Fluorescence-guided resection of malignant gliomas with 5-ALA | Phase 3/ Completed | 5-ALA facilitates more complete resection of contrast-enhancing tumors, resulting in improved progression-free survival [59]. |
| NCT00752323/5-ALA | Imaging procedure using ALA in finding residual tumor in grade IV malignant astrocytoma | Phase 2/ Completed | No formal conclusions or outcome data are available. |
| NCT06160492/5-ALA | Phase III clinical trial evaluating the resection efficacy of 5-Aminolevulinic acid hydrochloride (5-ALA HCl) fluorescence-guided microsurgery versus conventional white light microsurgery in patients with malignant glioma (WHO Grade 3/4) | Phase 3/ Recruiting | No formal conclusions or outcome data are available. |
| NCT01811121/5-ALA | Medico-economic evaluation of surgery guided by fluorescence for the optimization of resection of glioblastoma (RESECT) | Unknown Status | 5-ALA–guided fluorescence surgery is a safe, easy-to-use, cost-effective, and time-efficient technique that enhances the extent of tumor resection [40]. |
| NCT03291977/FS | Interest of fluorescein in fluorescence-guided resection of gliomas (FLEGME) | Phase 3/ Completed | The fluorescein-guided technique significantly improved the extent of tumor resection. However, data on progression-free survival, overall survival, and adverse events were not publicly reported. |
| NCT02691923/FS and 5-ALA | Diagnostic performance of fluorescein as an intraoperative brain tumor biomarker | Phase 2/ Recruiting | No formal conclusions or outcome data are available. |
| NCT04597801/FS | Comparison of fluorescein-intra-vital microscopy versus conventional frozen section diagnosis for intraoperative histopathological evaluation (INVIVO) | Phase 2/ Completed | No formal conclusions or outcome data are available. |
| NCT03579602/BLZ-100 | Study of Tozuleristide and the canvas imaging system in pediatric subjects with CNS tumors undergoing surgery | Phase 2, 3/ Completed | No formal conclusions or outcome data are available. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.T.; Mnatsakanyan, H.; Yi, E.; Badr, C.E. Current and Emerging Fluorescence-Guided Techniques in Glioma to Enhance Resection. Cancers 2025, 17, 2702. https://doi.org/10.3390/cancers17162702
Nguyen TTT, Mnatsakanyan H, Yi E, Badr CE. Current and Emerging Fluorescence-Guided Techniques in Glioma to Enhance Resection. Cancers. 2025; 17(16):2702. https://doi.org/10.3390/cancers17162702
Chicago/Turabian StyleNguyen, Trang T. T., Hayk Mnatsakanyan, Eunhee Yi, and Christian E. Badr. 2025. "Current and Emerging Fluorescence-Guided Techniques in Glioma to Enhance Resection" Cancers 17, no. 16: 2702. https://doi.org/10.3390/cancers17162702
APA StyleNguyen, T. T. T., Mnatsakanyan, H., Yi, E., & Badr, C. E. (2025). Current and Emerging Fluorescence-Guided Techniques in Glioma to Enhance Resection. Cancers, 17(16), 2702. https://doi.org/10.3390/cancers17162702






