Neoadjuvant Therapy or Upfront Surgery for Pancreatic Cancer—To Whom, When, and How?
Simple Summary
Abstract
1. Introduction
2. Evaluation of the Role of Neoadjuvant, Adjuvant, and Surgical Treatment of R-PDAC and BR-PDAC over the Years in Light of Clinical Studies
2.1. Historical Standard of Care in PDAC Treatment
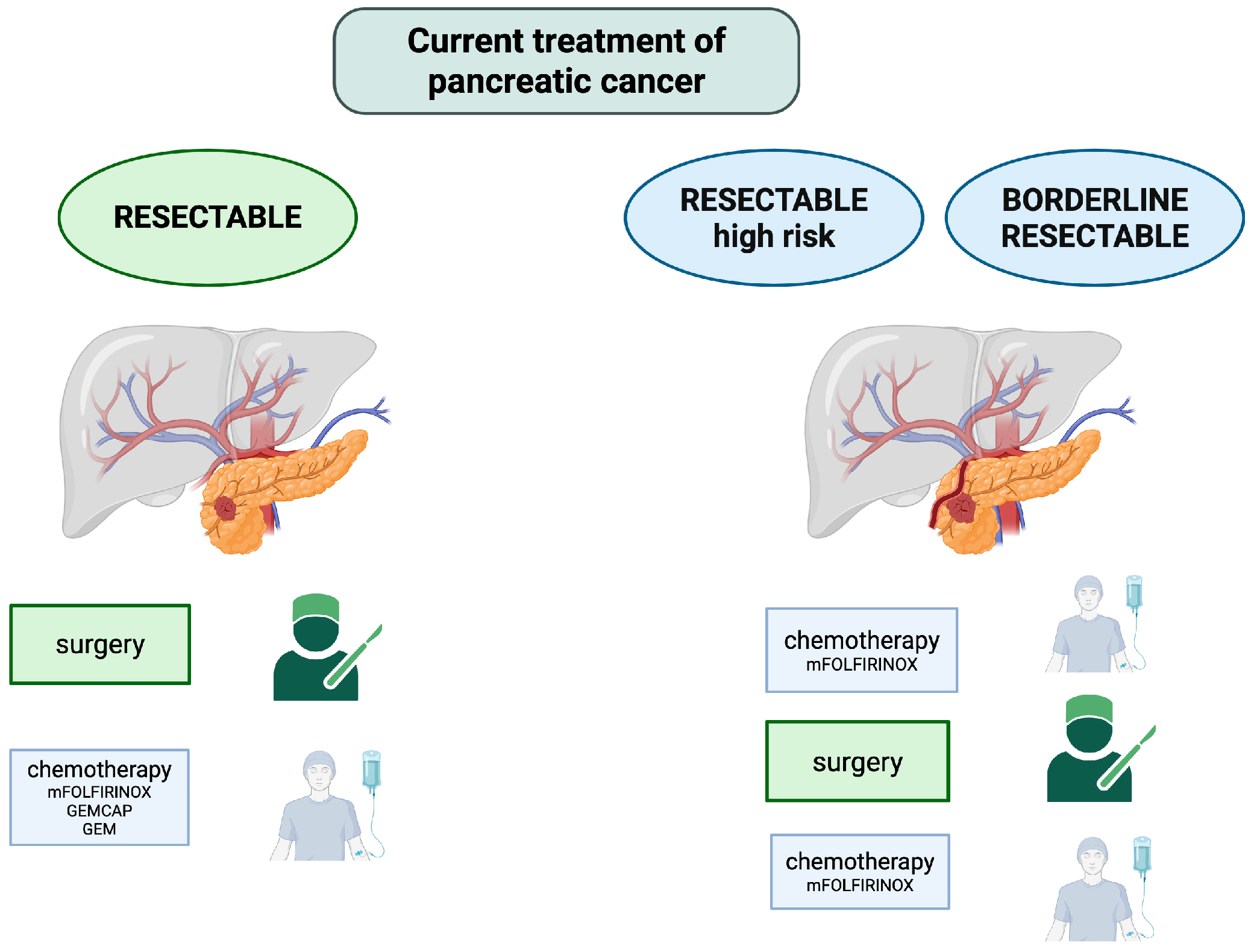
2.2. Actual Standard of Care and Challenges in PDAC Treatment
2.3. Clinical Trial Results—Looking for the Best Therapeutic Option
2.4. Meta-Analyses of Clinical Trials
3. Current Recommendations for Neoadjuvant Therapy and Upfront Surgery in Pancreatic Cancer
4. Neoadjuvant Immunotherapy
5. Neoadjuvant Targeted Therapies
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PDAC | pancreatic ductal adenocarcinoma cancer |
| NAT | neoadjuvant treatment |
| PC | pancreatic cancer |
| R-PDAC | resectable pancreatic ductal adenocarcinoma |
| BR-PDAC | borderline resectable pancreatic ductal adenocarcinoma |
| R0 | resection margin 0 (no cancer cells seen microscopically at the primary tumor site) |
| LA-PDAC | locally advanced pancreatic ductal adenocarcinoma |
| RCTs | randomized controlled trials |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| 5-FU+LV | 5 fluorouracil plus leucovorin |
| GEM | gemcitabine |
| RES | recurrence-free survival |
| CRT | chemoradiotherapy |
| Mos | median overall survival |
| HR | hazard ratio |
| CI | confidence interval |
| mDFS | median disease-free survival |
| NR | not reported |
| ITT | intention-to-treat |
| MDCT | multidetector computed tomography |
| SMA | superior mesenteric artery |
| MRI | magnetic resonance imaging |
| CT | computed tomography |
| NCCN | National Comprehensive Cancer Network |
| CHA | common hepatic artery |
| SMV | superior mesenteric vein (SMV) |
| PV | portal vein |
| NICE | National Institute for Health and Care Excellence |
| ESMO | European Society for Medical Oncology |
| ICIs | immune checkpoint inhibitors |
| SBRT | SIB simultaneous integrated boost |
| Ct-DNA | circulating tumor DNA |
| maxVAF | maximum somatic variant allele frequency |
| GVAX | tumor vaccines |
| EGFR | epidermal growth factor receptor |
| IGF-1 | insulin growth factor |
| VEGF | vascular endothelial growth factor |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Li, T.; Lin, C.; Wang, W. Global, regional, and national burden of pancreatic cancer from 1990 to 2021, its attributable risk factors, and projections to 2050: A systematic analysis of the global burden of disease study 2021. BMC Cancer 2025, 25, 189. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- Janssen, Q.P.; O’Reilly, E.M.; van Eijck, C.H.J.; Groot Koerkamp, B. Neoadjuvant Treatment in Patients With Resectable and Borderline Resectable Pancreatic Cancer. Front. Oncol. 2020, 10, 41. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 439–457. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Song, L.N.; Zhao, R.; Tian, Y.; Wang, Z.Q. Serum exosomal hsa-let-7f-5p: A potential diagnostic biomarker for metastatic pancreatic cancer detection. World J. Gastroenterol. 2025, 31, 109500. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Dunn, J.A.; Stocken, D.D.; Almond, J.; Link, K.; Beger, H.; Bassi, C.; Falconi, M.; Pederzoli, P.; Dervenis, C.; et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: A randomised controlled trial. Lancet 2001, 358, 1576–1585. [Google Scholar] [CrossRef]
- Oettle, H.; Neuhaus, P.; Hochhaus, A.; Hartmann, J.T.; Gellert, K.; Ridwelski, K.; Niedergethmann, M.; Zülke, C.; Fahlke, J.; Arning, M.B.; et al. Adjuvant Chemotherapy With Gemcitabine and Long-term Outcomes Among Patients With Resected Pancreatic Cancer: The CONKO-001 Randomized Trial. JAMA 2013, 310, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Sarfaty, E.; Khajoueinejad, N.; Zewde, M.G.; Yu, A.T.; Cohen, N.A. Surgical management of pancreatic ductal adenocarcinoma: A narrative review. Transl. Gastroenterol. Hepatol. 2023, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Kwaśniewska, D.; Fudalej, M.; Nurzyński, P.; Badowska-Kozakiewicz, A.; Czerw, A.; Cipora, E.; Sygit, K.; Bandurska, E.; Deptała, A. How A Patient with Resectable or Borderline Resectable Pancreatic Cancer should Be Treated-A Comprehensive Review. Cancers 2023, 15, 4275. [Google Scholar] [CrossRef]
- Isaji, S.; Mizuno, S.; Windsor, J.A.; Bassi, C.; Fernández-del Castillo, C.; Hackert, T.; Hayasaki, A.; Katz, M.H.G.; Kim, S.-W.; Kishiwada, M.; et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018, 18, 2–11. [Google Scholar] [CrossRef]
- Al-Hawary, M.M.; Francis, I.R.; Chari, S.T.; Fishman, E.K.; Hough, D.M.; Lu, D.S.; Macari, M.; Megibow, A.J.; Miller, F.H.; Mortele, K.J.; et al. Pancreatic ductal adenocarcinoma radiology reporting template: Consensus statement of the society of abdominal radiology and the american pancreatic association. Gastroenterology 2014, 146, 291–304.e291. [Google Scholar] [CrossRef]
- Latenstein, A.E.J.; van Roessel, S.; van der Geest, L.G.M.; Bonsing, B.A.; Dejong, C.H.C.; Groot Koerkamp, B.; de Hingh, I.H.J.T.; Homs, M.Y.V.; Klaase, J.M.; Lemmens, V.; et al. Conditional Survival After Resection for Pancreatic Cancer: A Population-Based Study and Prediction Model. Ann. Surg. Oncol. 2020, 27, 2516–2524. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Stocken, D.D.; Friess, H.; Bassi, C.; Dunn, J.A.; Hickey, H.; Beger, H.; Fernandez-Cruz, L.; Dervenis, C.; Lacaine, F.; et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N. Engl. J. Med. 2004, 350, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Kosuge, T.; Matsuyama, Y.; Yamamoto, J.; Nakao, A.; Egawa, S.; Doi, R.; Monden, M.; Hatori, T.; Tanaka, M.; et al. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br. J. Cancer 2009, 101, 908–915. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Stocken, D.D.; Bassi, C.; Ghaneh, P.; Cunningham, D.; Goldstein, D.; Padbury, R.; Moore, M.J.; Gallinger, S.; Mariette, C.; et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: A randomized controlled trial. Jama 2010, 304, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Castan, F.; Lopez, A.; Turpin, A.; Ben Abdelghani, M.; Wei, A.C.; Mitry, E.; Biagi, J.J.; Evesque, L.; Artru, P.; et al. Five-Year Outcomes of FOLFIRINOX vs Gemcitabine as Adjuvant Therapy for Pancreatic Cancer: A Randomized Clinical Trial. JAMA Oncol. 2022, 8, 1571–1578. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef] [PubMed]
- Brown, Z.J.; Heh, V.; Labiner, H.E.; Brock, G.N.; Ejaz, A.; Dillhoff, M.; Tsung, A.; Pawlik, T.M.; Cloyd, J.M. Surgical resection rates after neoadjuvant therapy for localized pancreatic ductal adenocarcinoma: Meta-analysis. Br. J. Surg. 2022, 110, 34–42. [Google Scholar] [CrossRef]
- Hamad, A.; Crossnohere, N.; Ejaz, A.; Tsung, A.; Pawlik, T.M.; Sarna, A.; Santry, H.; Wills, C.; Cloyd, J.M. Patient Preferences for Neoadjuvant Therapy in Pancreatic Ductal Adenocarcinoma. Pancreas 2022, 51, 657–662. [Google Scholar] [CrossRef]
- Halbrook, C.J.; Lyssiotis, C.A.; Pasca di Magliano, M.; Maitra, A. Pancreatic cancer: Advances and challenges. Cell 2023, 186, 1729–1754. [Google Scholar] [CrossRef]
- Di Carlo, A.; Gunder, M.; Doria, C. Surgical Management of Pancreatic Adenocarcinoma. In Hepato-Pancreato-Biliary Malignancies: Diagnosis and Treatment in the 21st Century; Doria, C., Rogart, J.N., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–12. [Google Scholar]
- Golcher, H.; Brunner, T.B.; Witzigmann, H.; Marti, L.; Bechstein, W.O.; Bruns, C.; Jungnickel, H.; Schreiber, S.; Grabenbauer, G.G.; Meyer, T.; et al. Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer: Results of the first prospective randomized phase II trial. Strahlenther. Onkol. 2015, 191, 7–16. [Google Scholar] [CrossRef]
- Casadei, R.; Di Marco, M.; Ricci, C.; Santini, D.; Serra, C.; Calculli, L.; D’Ambra, M.; Guido, A.; Morselli-Labate, A.M.; Minni, F. Neoadjuvant Chemoradiotherapy and Surgery Versus Surgery Alone in Resectable Pancreatic Cancer: A Single-Center Prospective, Randomized, Controlled Trial Which Failed to Achieve Accrual Targets. J. Gastrointest. Surg. 2015, 19, 1802–1812. [Google Scholar] [CrossRef]
- Jang, J.Y.; Han, Y.; Lee, H.; Kim, S.W.; Kwon, W.; Lee, K.H.; Oh, D.Y.; Chie, E.K.; Lee, J.M.; Heo, J.S.; et al. Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Ann. Surg. 2018, 268, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Motoi, F.; Kosuge, T.; Ueno, H.; Yamaue, H.; Satoi, S.; Sho, M.; Honda, G.; Matsumoto, I.; Wada, K.; Furuse, J.; et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn. J. Clin. Oncol. 2019, 49, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Versteijne, E.; van Dam, J.L.; Suker, M.; Janssen, Q.P.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; et al. Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J. Clin. Oncol. 2022, 40, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Janssen, Q.P.; van Dam, J.L.; Bonsing, B.A.; Bos, H.; Bosscha, K.P.; Coene, P.; van Eijck, C.H.J.; de Hingh, I.; Karsten, T.M.; van der Kolk, M.B.; et al. Total neoadjuvant FOLFIRINOX versus neoadjuvant gemcitabine-based chemoradiotherapy and adjuvant gemcitabine for resectable and borderline resectable pancreatic cancer (PREOPANC-2 trial): Study protocol for a nationwide multicenter randomized controlled trial. BMC Cancer 2021, 21, 300. [Google Scholar] [CrossRef]
- Groot Koerkamp, B.; Janssen, Q.P.; van Dam, J.L.; Bonsing, B.A.; Bos, H.; Bosscha, K.P.; Haberkorn, B.C.M.; de Hingh, I.H.J.T.; Karsten, T.M.; Van der Kolk, M.B.; et al. LBA83 Neoadjuvant chemotherapy with FOLFIRINOX versus neoadjuvant gemcitabine-based chemoradiotherapy for borderline resectable and resectable pancreatic cancer (PREOPANC-2): A multicenter randomized controlled trial. Ann. Oncol. 2023, 34, S1323. [Google Scholar] [CrossRef]
- Labori, K.J.; Bratlie, S.O.; Andersson, B.; Angelsen, J.H.; Biörserud, C.; Björnsson, B.; Bringeland, E.A.; Elander, N.; Garresori, H.; Grønbech, J.E.; et al. Neoadjuvant FOLFIRINOX versus upfront surgery for resectable pancreatic head cancer (NORPACT-1): A multicentre, randomised, phase 2 trial. Lancet Gastroenterol. Hepatol. 2024, 9, 205–217. [Google Scholar] [CrossRef]
- Sohal, D.P.S.; Duong, M.; Ahmad, S.A.; Gandhi, N.S.; Beg, M.S.; Wang-Gillam, A.; Wade, J.L., 3rd; Chiorean, E.G.; Guthrie, K.A.; Lowy, A.M.; et al. Efficacy of Perioperative Chemotherapy for Resectable Pancreatic Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 421–427. [Google Scholar] [CrossRef]
- Seufferlein, T.; Uhl, W.; Kornmann, M.; Algül, H.; Friess, H.; König, A.; Ghadimi, M.; Gallmeier, E.; Bartsch, D.K.; Lutz, M.P.; et al. Perioperative or only adjuvant gemcitabine plus nab-paclitaxel for resectable pancreatic cancer (NEONAX)-a randomized phase II trial of the AIO pancreatic cancer group. Ann. Oncol. 2023, 34, 91–100. [Google Scholar] [CrossRef]
- Schwarz, L.; Bachet, J.B.; Meurisse, A.; Bouché, O.; Assenat, E.; Piessen, G.; Hammel, P.; Regenet, N.; Taieb, J.; Turrini, O.; et al. Neoadjuvant FOLF(IRIN)OX Chemotherapy for Resectable Pancreatic Adenocarcinoma: A Multicenter Randomized Noncomparative Phase II Trial (PANACHE01 FRENCH08 PRODIGE48 study). J. Clin. Oncol. 2025, 43, 1984–1996. [Google Scholar] [CrossRef]
- Katz, M.H.G.; Shi, Q.; Meyers, J.; Herman, J.M.; Chuong, M.; Wolpin, B.M.; Ahmad, S.; Marsh, R.; Schwartz, L.; Behr, S.; et al. Efficacy of Preoperative mFOLFIRINOX vs mFOLFIRINOX Plus Hypofractionated Radiotherapy for Borderline Resectable Adenocarcinoma of the Pancreas: The A021501 Phase 2 Randomized Clinical Trial. JAMA Oncol. 2022, 8, 1263–1270. [Google Scholar] [CrossRef]
- Reni, M.; Macchini, M.; Orsi, G.; Procaccio, L.; Malleo, G.; Balzano, G.; Rapposelli, I.G.; Bencardino, K.B.; Scartozzi, M.; Carconi, C.; et al. Results of a randomized phase III trial of pre-operative chemotherapy with mFOLFIRINOX or PAXG regimen for stage I-III pancreatic ductal adenocarcinoma. J. Clin. Oncol. 2025, 43, LBA4004. [Google Scholar] [CrossRef]
- Jones, J.B.; Blecker, S.; Shah, N.R. Meta-analysis 101: What you want to know in the era of comparative effectiveness. Am. Health Drug Benefits 2008, 1, 38–43. [Google Scholar]
- Versteijne, E.; Vogel, J.A.; Besselink, M.G.; Busch, O.R.C.; Wilmink, J.W.; Daams, J.G.; van Eijck, C.H.J.; Groot Koerkamp, B.; Rasch, C.R.N.; van Tienhoven, G. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br. J. Surg. 2018, 105, 946–958. [Google Scholar] [CrossRef]
- Aliseda, D.; Martí-Cruchaga, P.; Zozaya, G.; Blanco, N.; Ponz, M.; Chopitea, A.; Rodríguez, J.; Castañón, E.; Pardo, F.; Rotellar, F. Neoadjuvant therapy versus upfront surgery in resectable pancreatic cancer: Reconstructed patient-level meta-analysis of randomized clinical trials. BJS Open 2024, 8, zrae087. [Google Scholar] [CrossRef] [PubMed]
- Uson Junior, P.L.S.; Dias, E.S.D.; de Castro, N.M.; da Silva Victor, E.; Rother, E.T.; Araújo, S.E.A.; Borad, M.J.; Moura, F. Does neoadjuvant treatment in resectable pancreatic cancer improve overall survival? A systematic review and meta-analysis of randomized controlled trials. ESMO Open 2023, 8, 100771. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, L.D.; Gittens, J.; Brunning, C.; Jackson, R.; Schmid, M.C.; Mielgo, A.; Palmer, D.; Halloran, C.M.; Ghaneh, P. Neoadjuvant treatment versus upfront surgery in borderline resectable and resectable pancreatic ductal adenocarcinoma: Meta-analysis. BJS Open 2025, 9, zrae172. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.H.Y.; Zhao, Y.; Tan, H.L.; Chua, D.W.; Ng, K.Y.Y.; Lee, S.Y.; Lee, J.J.X.; Tai, D.; Goh, B.K.P.; Koh, Y.X. Clinical Outcomes of Neoadjuvant Therapy Versus Upfront Surgery in Resectable Pancreatic Cancer: Systematic Review and Meta-analysis of Latest Randomized Controlled Trials. Ann. Surg. Oncol. 2025, 32, 4094–4107. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Heh, V.; Pawlik, T.M.; Ejaz, A.; Dillhoff, M.; Tsung, A.; Williams, T.; Abushahin, L.; Bridges, J.F.P.; Santry, H. Neoadjuvant Therapy for Resectable and Borderline Resectable Pancreatic Cancer: A Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2020, 9, 1129. [Google Scholar] [CrossRef]
- Jang, J.K.; Byun, J.H.; Choi, S.J.; Kim, J.H.; Lee, S.S.; Kim, H.J.; Yoo, C.; Kim, K.P.; Hong, S.M.; Seo, D.W.; et al. Survival Outcomes According to NCCN Criteria for Resection Following Neoadjuvant Therapy for Patients with Localized Pancreatic Cancer. Ann. Surg. Oncol. 2025, 32, 1321–1330. [Google Scholar] [CrossRef]
- Network, N.C.C. Pancreatic Adenocarcinoma (Version 2.2025). Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (accessed on 20 February 2025).
- O’reilly, D.; Fou, L.; Hasler, E.; Hawkins, J.; O’connell, S.; Pelone, F.; Callaway, M.; Campbell, F.; Capel, M.; Charnley, R. Diagnosis and management of pancreatic cancer in adults: A summary of guidelines from the UK National Institute for Health and Care Excellence. Pancreatology 2018, 18, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Pfeiffer, P.; Vilgrain, V.; Lamarca, A.; Seufferlein, T.; O’Reilly, E.M.; Hackert, T.; Golan, T.; Prager, G.; Haustermans, K.; et al. Pancreatic cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up☆. Ann. Oncol. 2023, 34, 987–1002. [Google Scholar] [CrossRef]
- Bear, A.S.; Vonderheide, R.H.; O’Hara, M.H. Challenges and Opportunities for Pancreatic Cancer Immunotherapy. Cancer Cell 2020, 38, 788–802. [Google Scholar] [CrossRef]
- Robert, C. Is earlier better for melanoma checkpoint blockade? Nat. Med. 2018, 24, 1645–1648. [Google Scholar] [CrossRef]
- Springfeld, C.; Bailey, P.; Hackert, T.; Neoptolemos, J.P. Perioperative immunotherapy for pancreatic cancer is on its way. Hepatobiliary Surg. Nutr. 2021, 10, 534–537. [Google Scholar] [CrossRef]
- Pęczek, P.; Gajda, M.; Rutkowski, K.; Fudalej, M.; Deptała, A.; Badowska-Kozakiewicz, A.M. Cancer-associated inflammation: Pathophysiology and clinical significance. J. Cancer Res. Clin. Oncol. 2023, 149, 2657–2672. [Google Scholar] [CrossRef]
- Du, J.; Lu, C.; Mao, L.; Zhu, Y.; Kong, W.; Shen, S.; Tang, M.; Bao, S.; Cheng, H.; Li, G.; et al. PD-1 blockade plus chemoradiotherapy as preoperative therapy for patients with BRPC/LAPC: A biomolecular exploratory, phase II trial. Cell Rep. Med. 2023, 4, 100972. [Google Scholar] [CrossRef] [PubMed]
- Heumann, T.; Judkins, C.; Li, K.; Lim, S.J.; Hoare, J.; Parkinson, R.; Cao, H.; Zhang, T.; Gai, J.; Celiker, B.; et al. A platform trial of neoadjuvant and adjuvant antitumor vaccination alone or in combination with PD-1 antagonist and CD137 agonist antibodies in patients with resectable pancreatic adenocarcinoma. Nat. Commun. 2023, 14, 3650. [Google Scholar] [CrossRef] [PubMed]
- Byrne, K.T.; Betts, C.B.; Mick, R.; Sivagnanam, S.; Bajor, D.L.; Laheru, D.A.; Chiorean, E.G.; O’Hara, M.H.; Liudahl, S.M.; Newcomb, C.; et al. Neoadjuvant Selicrelumab, an Agonist CD40 Antibody, Induces Changes in the Tumor Microenvironment in Patients with Resectable Pancreatic Cancer. Clin. Cancer Res. 2021, 27, 4574–4586. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, C.; Bai, X.; Gao, S.; Shen, Y.; Zhang, M.; Wu, J.; Que, R.; Li, X.; Liang, T.; et al. Randomized phase II trial of neoadjuvant chemotherapy with modified FOLFIRINOX versus modified FOLFIRINOX and PD-1 antibody for borderline resectable and locally advanced pancreatic cancer (the CISPD-4 study). J. Clin. Oncol. 2022, 40, 562. [Google Scholar] [CrossRef]
- Grant, T.J.; Hua, K.; Singh, A. Chapter Six—Molecular Pathogenesis of Pancreatic Cancer. In Progress in Molecular Biology and Translational Science; Pruitt, K., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 144, pp. 241–275. [Google Scholar]
- Chippalkatti, R.; Parisi, B.; Kouzi, F.; Laurini, C.; Ben Fredj, N.; Abankwa, D.K. RAS isoform specific activities are disrupted by disease associated mutations during cell differentiation. Eur. J. Cell Biol. 2024, 103, 151425. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef]
- Nandy, D.; Mukhopadhyay, D. Growth factor mediated signaling in pancreatic pathogenesis. Cancers 2011, 3, 841–871. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Q.; Castaneda, C.; Cook, S. Targeted Therapies in Pancreatic Cancer: A New Era of Precision Medicine. Biomedicines 2024, 12, 2175. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Z.; Wang, Y. Advances in Targeted Therapy and Immunotherapy for Pancreatic Cancer. Adv. Biol. 2021, 5, e1900236. [Google Scholar] [CrossRef]

| Stage | Arterial Involvement | Venous Involvement |
|---|---|---|
| Resectable | No contact with the following:
| (≤180°) contact without contour irregularity |
| Borderline resectable | Head/uncinate process
| >180° contact with contour irregularity or thrombosis, but resection and reconstruction are possible |
| Unresectable | Head/uncinate process
| >180° contact or with contour irregularity or thrombosis, and resection and reconstruction are not possible |
| Trial/ Author | Phase | R/BR | Study Design | n | mOS | HR (95% CI) | p- Value | mDFS | HR (95% CI) | p- Value | R0 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ESPAC-1 [14] | III | R | Observation CRT | 69 63 | 17.9 15.9 | 1.28 (0.99–1.66) | 0.05 | 15.2 10.7 | 1.32 (1.00–1.74) | 0.04 | NR |
| Chemotherapy observation | 75 72 | 20.1 15.5 | 0.71(055–0.92) | 0.009 | 15.3 9.4 | 0.73 (0.55–0.96) | 0.02 | ||||
| CONKO-001 [8] | III | R | GEM | 179 | 22.8 | 0.76 (0.61–0.95) | 0.01 | 13.4 | 0.55 (0.44–0.69) | 0.001 | 80% |
| Observation | 175 | 20.2 | 6.9 | ||||||||
| JSAP-02 [15] | III | R | GEM | 58 | 22.3 | 0.77 (0.51–1.14) | 0.19 | 11.4 | 0.60 (0.40–0.89) | 0.01 | NR |
| Observation | 60 | 18.4 | 5.0 | ||||||||
| ESPAC-3 [16] | III | R | 5-FU | 551 | 23.0 | 0.94 (0.81–1.08) | 0.39 | 14.3 | 0.96 (0.86–1.07) | 0.53 | NR |
| GEM | 537 | 23.6 | 14.1 |
| Trial/Author | Phase | Treatment | n | mOS (Months) | HR (95% CI) | p-Value | mDFS (Months) | HR (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| PRODIGE24 | III | mFOLFIRINOX | 493 | 53.5 | 0.68 (0.54–0.85) | 0.001 | 21.4 | 0.66 (0.54–0.82) | 0.001 |
| GEM | 35.5 | 12.8 | |||||||
| ESPAC-4 | III | GEM | 730 | 25.5 | 0.82 (0.68–0.98) | 0.032 | NR | NR | NR |
| GEM-CAP | 28.0 | NR |
| Trial/ Author | Phase | R/ BR | Treatment Arm | n | mOS Months | HR (95% CI) | p- Value | mDFS Months | HR (95% CI) | p- Value | R0 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Golcher et al. [23] | II | R | GEM/CIS+RTH/ surgery | 73 | 17.4 | NR | 0.96 | NR | NR | NR | 52% |
| Upfront surgery/GEM | 14.4 | 48% | |||||||||
| Casadei et al. [24] | II | R | GEM+RTH | 38 | NR | NR | 0.174 | NR | NR | NR | NR |
| Upfront surgery/GEM | NR | ||||||||||
| Jang et al. [25] | II/III | BR | GEM+RTH/surgery | 110 | 21 | 1.495 (0.66–3.360 | 0.028 | NR | NR | NR | 52% |
| Surgery/GEM+RTH | 12 | 26% | |||||||||
| Prep-02/JSAP-05 [26] | II | R/ RB | GEM+S-1/surgery | 364 | 36.7 | 0.72 (0.55–0.94) | 0.015 | 14.3 | 0.77 (0.61–0.98) | 0.030 | NR |
| Surgery/S-1 | 26.6 | 11.3 | |||||||||
| PREOPANC [27] | III | R/ BR | GEM+RTH/ Surgery/GEM | 246 | 15.7 | 0.73 (0.58–0.96) | 0.025 | 8.1 | 0.70 (0.53–0.92) | 0.009 | 71% |
| Surgery/GEM | 14.3 | 7.7 | 40% | ||||||||
| PREOPANC-2 [28,29] | III | R/ BR | FOLFIRINOX/ SURGERY | 368 | 21.9 | 0.87 (0.68–1.12) | 0.28 | NR | NR | NR | 77% |
| GEM+RTH/ surgery | 21.3 | 75% | |||||||||
| NORPACT-1 [30] | II | R | FOLFIRINOX/ surgery/adjuvant chemotherapy | 140 | 25.1 | 1.52 (1.00–2.33) | 0.05 | NR | NR | NR | 82% |
| Surgery/FOLFIRINOX | 38.5 | 89% | |||||||||
| SWOG S1505 [31] | II | R | FOLFIRINOX/surgery/FOLFIRINOX | 102 | 22.4 | 0.97 (0.76–1.24) | 0.82 | 10.9 | 0.87 (0.66–1.15) | 0.87 | 85% |
| NP/surgery/NP | 23.6 | 14.2 | 85% | ||||||||
| NEONAX [32] | II | R | NP/surgery | 127 | 25.2 | (19.0–29.7) | 0.028 | 11.5 | (8.8–14.5) | NR | 88% |
| Surgery/NP | 16.7 | (11.6–22.2) | 5.9 | (3.6–11.5) | 67% | ||||||
| ALLIANCE A021501 [34] | II | BR | FOLFIRINOX/ surgery | 126 | 29.8 | 1.88 (1.16–3.04) | 0.009 | 15.0 | 1.28 (0.88–1.87) | NR | 57% |
| FOLFIRINOX+SBRT/surgery | 17.1 | 11.5 | 33% | ||||||||
| CASSANDRA [35] | III | R/ BR | PAXG/surgery mFOLFIRINOX | 261 | 37.3 | 0.7 (0.47–1.04) | 0.07 | 17.3 | 0.64 (0.46–0.89) | 0.008 | 74% |
| 26.0 | 10.4 | 51% |
| Author | Trials (n) | Number of Patients | R/ BR | Treatment Arm | mOS | HR (95% CI) | p- Value | mDFS | HR (95% CI) | p- Value | R0 | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Versteijne et al. [37] | 38 | 3484 | R/ RB | NAT | 18.8 | 0.73 | NR | NR | NR | NR | 87% | NAT may improve OS and R0 resection in BR-PDAC |
| Upfront surgery | 14.8 | 67% | ||||||||||
| Aliseda et al. [38] | 5 | 625 | R | NAT | 25.9 | 0.88 (0.72–1.08) | 0.223 | NR | NR | NR | NR | No significant OS benefit from NAT in R-PDAC |
| Upfront surgery | 23.8 | |||||||||||
| Uson Junior et al. [39] | 6 | 805 | R | NAT | NR | 0.76 (0.52–1.11) | NR | NR | 0.71 | NR | ↑20% with NAT | No OS/DFS improvement; improved R0 rate |
| Upfront surgery | ||||||||||||
| Dickerson et al. [40] | 9 | 1194 | R/ BR | NAT | NR | 0.73 (0.55–0.98) | NR | NR | NR | NR | NR | No significant OS benefit in R-PDAC |
| Upfront surgery | ||||||||||||
| Chan et al. [41] | 8 | 982 | R | NAT | NR | 0.81 (0.65–1.01) | 0.06 | 12.7 | 0.66 (0.47–0.92) | 0.01 | 72% | DFS, R0, and N0 improved; no significant OS difference. |
| Upfront surgery | 6.3 | 60% | ||||||||||
| Cloyd et al. [42] | 6 | 850 | R/ BR | NAT | 25.4 | 0.73 (0.61–0.86) | 0.001 | NR | NR | NR | 60% | OS, R0 benefit in R-PDAC and BR-PDAC |
| Upfront surgery | 19.4 | 40% |
| Trial | Phase | R/BR | Treatment | n | Primary Outcome | Secondary Outcomes |
|---|---|---|---|---|---|---|
| NCT04927780 | III | R | FOLFIRINOX/surgery /FOLFIRINOX | 378 | OS | PFS, number of cycles received, dose intensity, resection rate, quality of life, AE, and surgical complications. |
| Surgery/FOLFIRINOX | ||||||
| NCT04340141 | III | R | FOLFIRINOX/surgery /FOLFIRINOX | 352 | OS | DFS, resection rate, AE, and quality of life. |
| Surgery/FOLFIRINOX | ||||||
| NCT02172976 | II/III | R | FOLFIRINOX/surgery/ FOLFIRINOX | 40 | OS | PFS, peri-operative morbidity and mortality, R0 RR, tolerability, and feasibility of neoadjuvant FOLFIRINOX |
| NCT01314027 | III | R | GEM/oxaliplatin | 38 | PFS | - |
| Upfront surgery | ||||||
| NCT02676349 | II | BR | mFOLFIRINOX+ CAP-based CRT/surgery | 90 | R0 RR | - |
| mFOLFIRINOX/surgery | ||||||
| NCT02717091 | II | BR | FOLFIRINOX | 50 | R0 RR | - |
| NP/GEM |
| Author | Phase | n | R/BR/LA | Treatment Arm | Outcomes | |
|---|---|---|---|---|---|---|
| Du et al. [51] | II | 29 | RB/ LA | tislelizumab +GEM/NP +SBRT-SIB | ORR: 60% R0: 90% 12-Month OS Rate: 72% 12-Month PFS Rate: 64% | |
| Heumann et al. [52] | Ib/II | 40 | R | Arm A: GVAX+cyclofosphamide | mOS: Arm A: 23.59 m Arm B: 27.01 m Arm C: 35.55 m | mDFS: Arm A: 13.90 m Arm B: 14.98 m Arm C: 33.51 m |
| Arm B: GVAX+cyclofosphamide + nivolumab | ||||||
| Arm C: GVAX+cyclofosphamide + nivolumab + urelumab | ||||||
| Byrne et al. [53] | I/II | 16 | R | Arm A: selicrelumab | mOS: 95% CI (18.0–28.8 m) Arm A: 23.4 m Arm B: not reached | mDFS: 95% CI (0.4–19.2 m) Arm A: 9.8 m Arm B: not reached |
| Arm B: selicrelumab+GEM+NP | ||||||
| Trial | Phase | R/RB/LA | Treatment | Primary Endpoints |
|---|---|---|---|---|
| NCT03983057 | II | BR/LA | mFOLFIRINOX/surgery mFOLFIRINOX + PD-1 antibody/surgery | PFS, TTP |
| NCT05132504 | II | R | mFOLFIRINOX + Pembrolizumab/surgery | Safety |
| upfront surgery/mFOLFIRINOX + Pembrolizumab | ||||
| NCT06094140 | II | R/BR | mFOLFIRINOX+Durwalumab/surgery | Safety |
| NCT06060405 | II | R | Durwalumab+Oleclumab/surgery | Change in CD8+ T cells within tumor, |
| NCT00727441 | II | R/BR | GVAX GVAX, cyclophosphamid i.v. GVAX, cyclophosphamid p.o | Safety, immune response |
| NCT03727880 | II | R | Chth NAT + pembrolizumab + defactinib | Change in CD8+ T cells within tumor, pCR |
| Chth NAT + pembrolizumab i.v | ||||
| NCT03979066 | II | R | Atezolizumab + PEGPH20 | Change in CD8+ T cells within tumor |
| Atezolizumab i.v. | ||||
| NCT03970252 | II | BR | FOLFIRINOX+Nivolumab | Evaluation of development of clinically relevant pancreatic fistula in the post-operative period Evaluation pCR |
| NCT02305186 | Ib/II | R/BR | CRT (capecitabine) + Pembrolizumab | Number of Tumor Infiltrating Lymphocytes (TILs) per high powered field (hpf) in pancreatic tissue (resected tissue) Safety: Incidence of Dose-Limiting Toxicities (DLTs) |
| CRT | ||||
| NCT04940286 | II | R/BR | GEM+NP+Durvalumab+Oleclumab/Surgery/adjuvant | Major pathological response rate (=<5% viable tumor cells) Incidence of adverse events |
| Trial | Phase | R/BR/LA | Indication Group | Treatment | Primary Endpoints |
|---|---|---|---|---|---|
| NCT04858334 | II | R/BR | BRACA1/2 or PALB2, post platinum-based NAT chemotherapy, and resection | olaparib | RFS |
| NCT04005690 | I | R/BR/LA | MEK inhibitor PARP inhibitor PLK1 inhibitor WEE1/Cell-Cycle Checkpoint Inhibition Anti-CTLA-4 antibody | cobimetinib olaparib/saruparib onvansertib azenosertib tremelimumab | Proportion of pharmacodynamic feasibility |
| NCT05546411 | I/II | R/BR/LA | TGF-beta inhibitor | NIS793 | MPR |
| NTC04117087 | I | R/BR/LA/metastatic | MMR-p | KRAS peptide vaccine +nivolumab+ipilimumab | AE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwaśniewska, D.; Fudalej, M.; Badowska-Kozakiewicz, A.M.; Czerw, A.; Deptała, A. Neoadjuvant Therapy or Upfront Surgery for Pancreatic Cancer—To Whom, When, and How? Cancers 2025, 17, 2584. https://doi.org/10.3390/cancers17152584
Kwaśniewska D, Fudalej M, Badowska-Kozakiewicz AM, Czerw A, Deptała A. Neoadjuvant Therapy or Upfront Surgery for Pancreatic Cancer—To Whom, When, and How? Cancers. 2025; 17(15):2584. https://doi.org/10.3390/cancers17152584
Chicago/Turabian StyleKwaśniewska, Daria, Marta Fudalej, Anna Maria Badowska-Kozakiewicz, Aleksandra Czerw, and Andrzej Deptała. 2025. "Neoadjuvant Therapy or Upfront Surgery for Pancreatic Cancer—To Whom, When, and How?" Cancers 17, no. 15: 2584. https://doi.org/10.3390/cancers17152584
APA StyleKwaśniewska, D., Fudalej, M., Badowska-Kozakiewicz, A. M., Czerw, A., & Deptała, A. (2025). Neoadjuvant Therapy or Upfront Surgery for Pancreatic Cancer—To Whom, When, and How? Cancers, 17(15), 2584. https://doi.org/10.3390/cancers17152584






