Unusual Manifestations of Primary Pancreatic Neoplasia
Abstract
Simple Summary
Abstract
1. Introduction
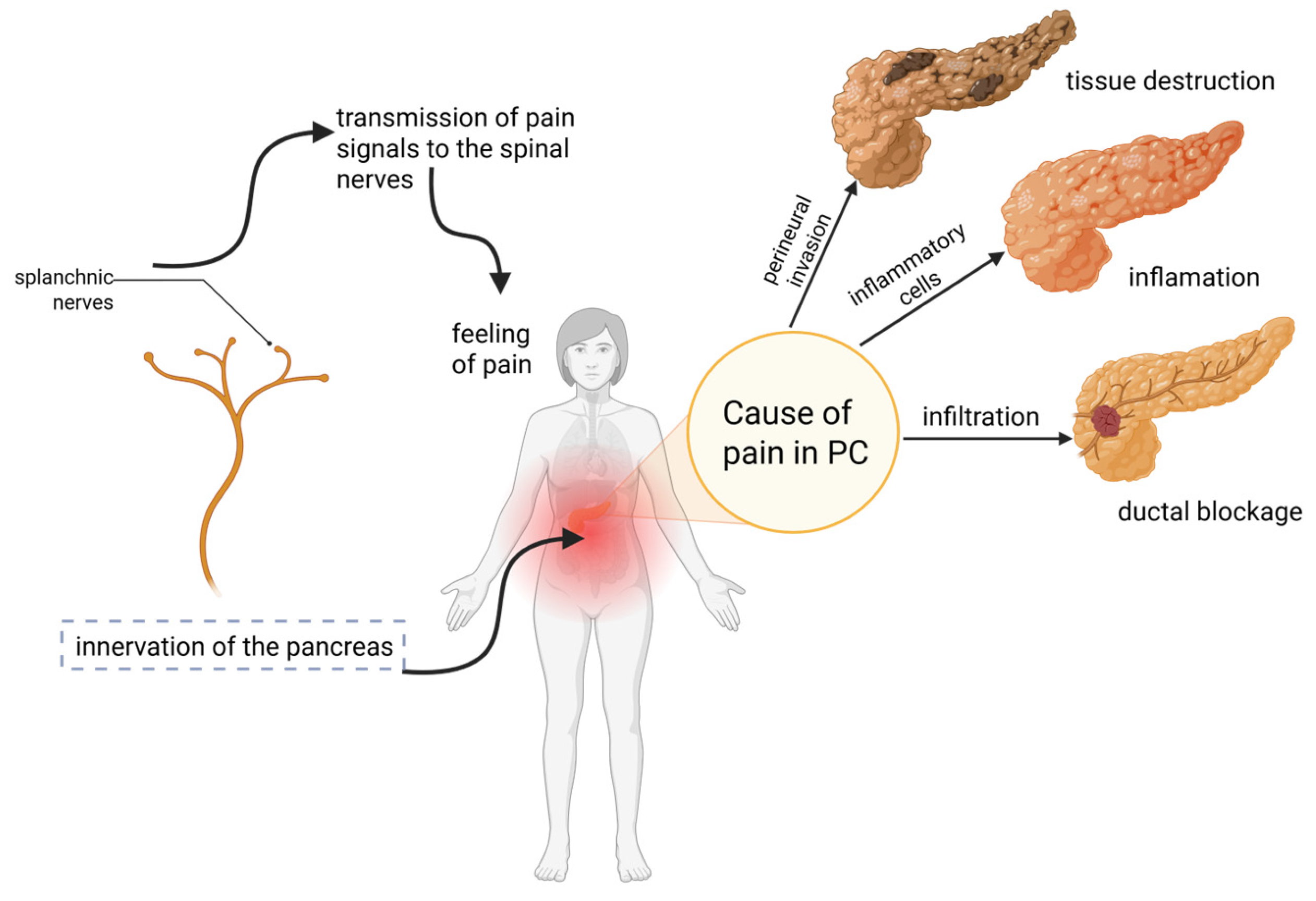
2. Pain in Pancreatic Cancer
2.1. Underlying Biological Mechanism of Pain in Pancreatic Cancer
2.2. Influence of Pain on Prognosis and Treatment
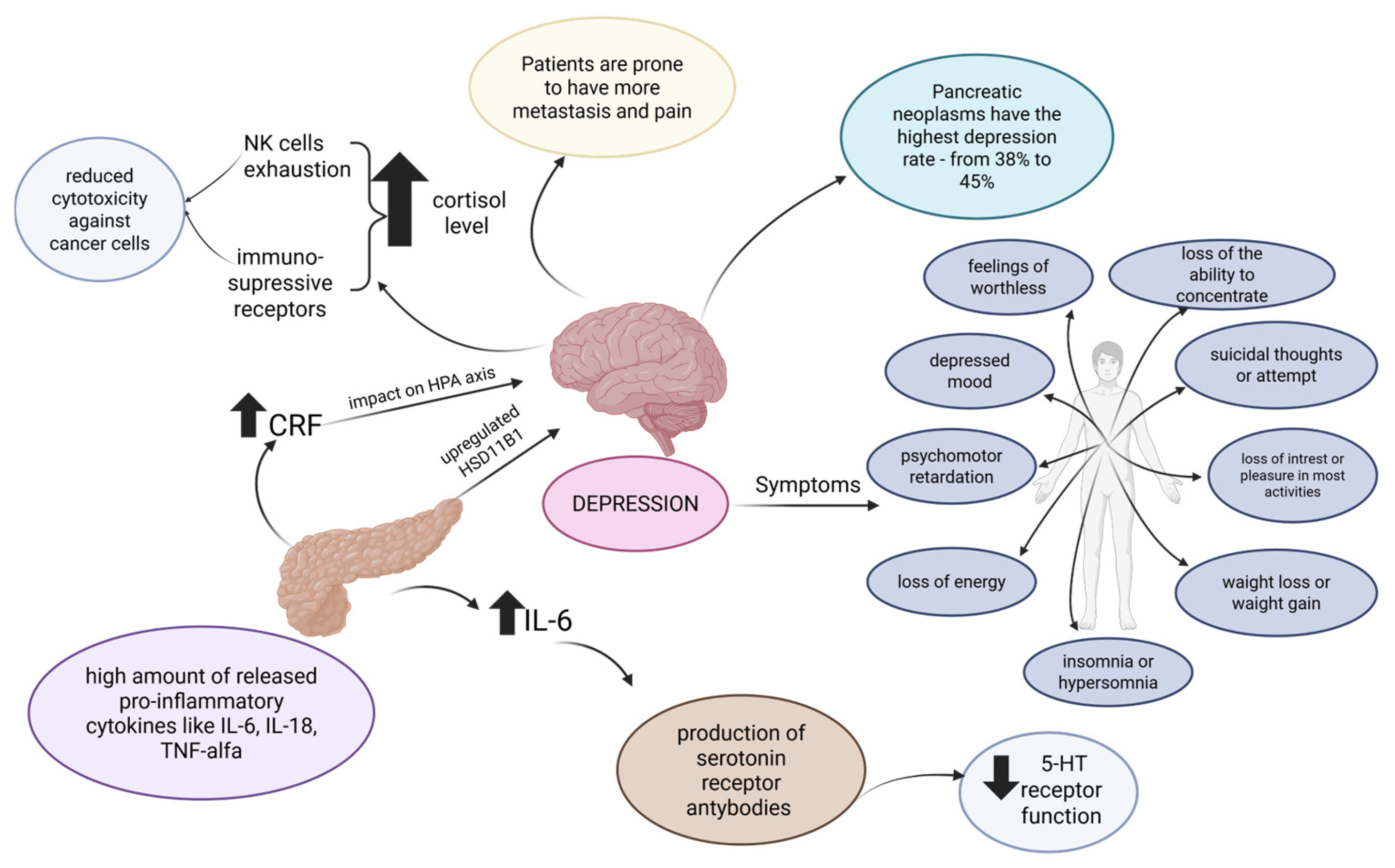
3. Depression in Pancreatic Cancer
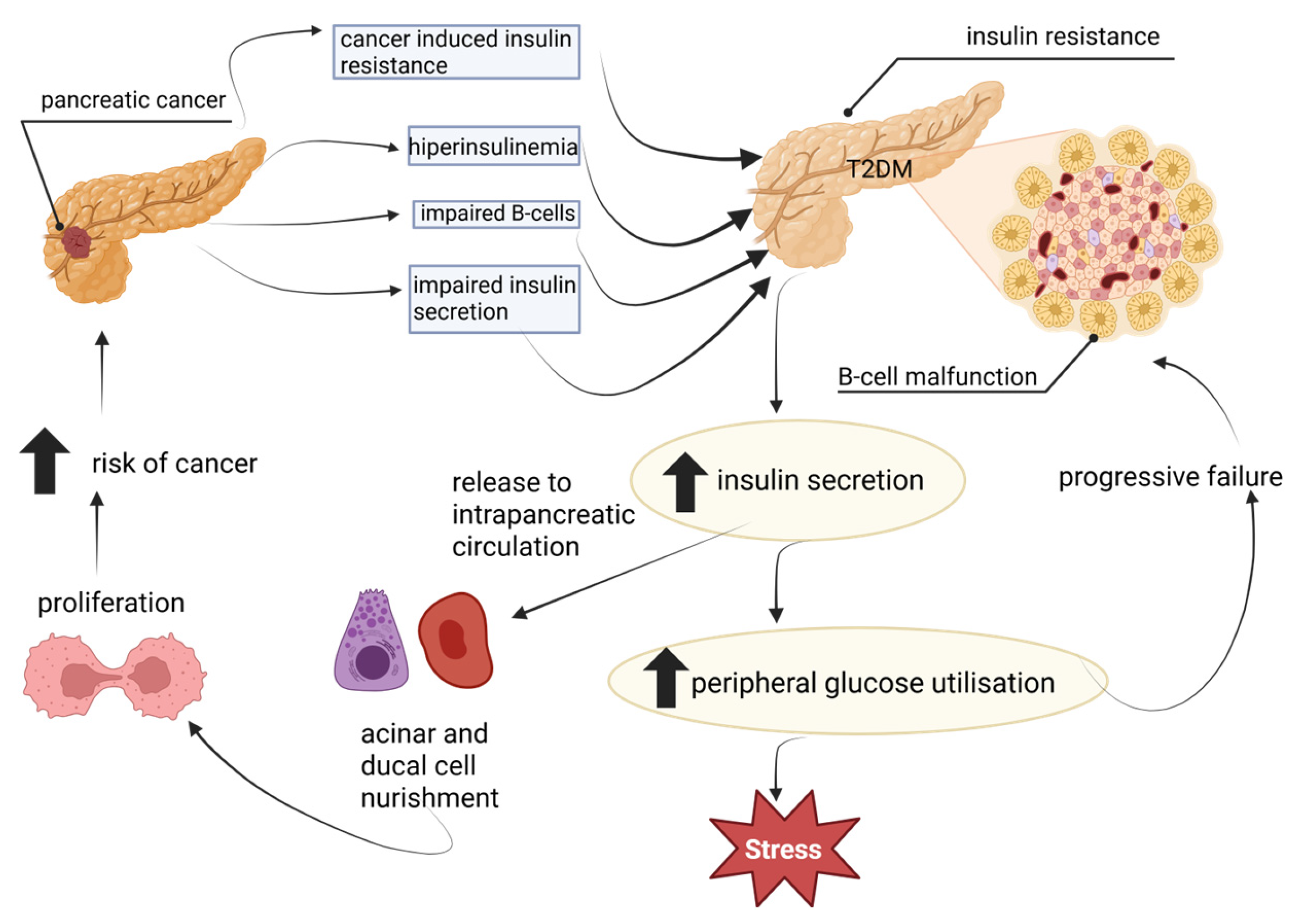
4. Diabetes in Pancreatic Cancer
4.1. Epidemiology and Characteristics
- Type 1 DM (T1DM) (autoimmune β-cell destruction);
- Type 2 DM (T2DM) (non-autoimmune progressive loss of adequate β-cell insulin secretion);
- Specific types of DM due to other causes (this type includes diseases of the exocrine pancreas);
- Gestational DM [67].
- (1)
- T2DM [69].
- (2)
- PDAC-associated type 3c diabetes mellitus (T3cDM)—a state of hyperglycemia as a result of pancreatic dysfunction, which can be detected in the early stage of PDAC, even if the tumor is not yet visible in imaging studies [70].
4.2. Underlying Biological Mechanism
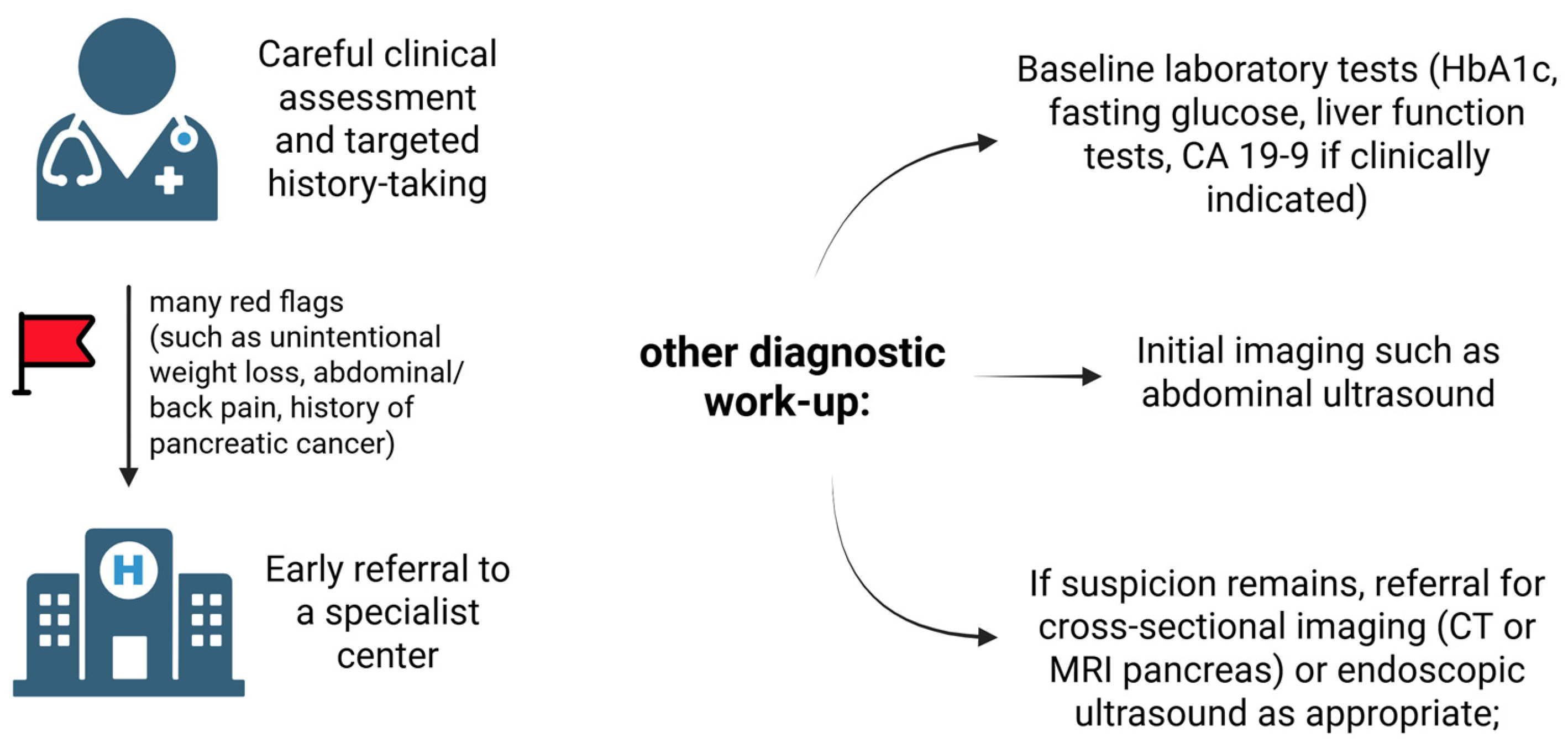
4.3. Available Solutions for Screening
4.4. Influence on Prognosis and Treatment
5. Paraneoplastic Syndromes Preceding the Diagnosis of Pancreatic Cancer
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| PC | Pancreatic cancer |
| PDAC | Pancreatic ductal adenocarcinoma |
| OS | Overall survival |
| NOD | New-onset diabetes mellitus |
| PNI | Perineural invasion |
| NGF | Nerve growth factor |
| HIFU | High-intensity focused ultrasound |
| CBR | Clinical benefit rate |
| CPN | Celiac plexus neurolysis |
| SLIT2 | Slit guidance ligand 2 |
| FUS | Focused ultrasound |
| MDD | Major depressive disorder |
| CRF | Corticotrophin-releasing factor |
| HSD11B1 | 11-hydroxysteroid dehydrogenase type-1 |
| 5-HT | 5-hydroxytryptamine |
| QoL | Quality of life |
| TCAs | Tricyclic antidepressants |
| HCC | Hepatocellular carcinoma |
| GR | Glucocorticoid receptor |
| SSRIs | Selective serotonin reuptake inhibitors |
| SNRI | Serotonin–norepinephrine reuptake inhibitor |
| DM | Diabetes mellitus |
| T1DM | Type 1 DM |
| T2DM | Type 2 DM |
| T3cDM | Type 3c diabetes mellitus |
| TME | Tumor microenvironment |
| YAP | Hip-po-Yes-associated protein |
| ROS | Reactive oxygen species |
| EGFs | Epidermal growth factors |
| TGF-β | Transforming growth factor β |
| EMT | Epithelial-to-mesenchymal transition |
| PFK | Phosphofructokinase |
| RNR | Ribonucleotide reductase |
| IAPP | Islet amyloid polypeptide |
| OR | Odds ratio |
| DDP-4 | Dipeptidyl Peptidase 4 |
| SDF-1α | Stromal cell-derived factor-1α |
| GLP-1 RAs | Glucagon-like peptide-1 receptor agonists |
| SGLT-2 | Sodium-glucose co-transporter 2 |
| PaCDM | PC-associated diabetes mellitus |
| CA19-9 | Carbohydrate antigen 19-9 |
| CT | Computed tomography |
| MR | Mortality rate |
| AI | Artificial intelligence |
| PFPAS | Palmar fasciitis and polyarthritis syndrome |
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Taherian, M.; Wang, H.; Wang, H. Pancreatic Ductal Adenocarcinoma: Molecular Pathology and Predictive Biomarkers. Cells 2022, 11, 3068. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Singhi, A.D.; Koay, E.J.; Chari, S.T.; Maitra, A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 2019, 156, 2024–2040. [Google Scholar] [CrossRef]
- Jensen, M.H.; Cichosz, S.L.; Hejlesen, O.; Henriksen, S.D.; Drewes, A.M.; Olesen, S.S. Risk of pancreatic cancer in people with new-onset diabetes: A Danish nationwide population-based cohort study. Pancreatology 2023, 23, 642–649. [Google Scholar] [CrossRef]
- Westermann, A.; Matrisian, L.M.; Rahib, L. The need for improvement in the management of fatigue, depression and pain in pancreatic cancer. J. Clin. Oncol. 2019, 37, 429. [Google Scholar] [CrossRef]
- Wu, M.; Zhu, A.; Shen, L. Pancreatic cancer-related pain: Mechanism and management. J. Pancreatol. 2023, 6, 202–209. [Google Scholar] [CrossRef]
- McNearney, T.A.; Digbeu, B.D.E.; Baillargeon, J.G.; Ladnier, D.; Rahib, L.; Matrisian, L.M. Pre-Diagnosis Pain in Patients With Pancreatic Cancer Signals the Need for Aggressive Symptom Management. Oncologist 2023, 28, e1185–e1197. [Google Scholar] [CrossRef]
- Coveler, A.L.; Mizrahi, J.; Eastman, B.; Apisarnthanarax, S.J.; Dalal, S.; McNearney, T.; Pant, S. Pancreas Cancer-Associated Pain Management. Oncologist 2021, 26, e971–e982. [Google Scholar] [CrossRef]
- de Oliveira, R.; dos Reis, M.P.; Prado, W.A. The effects of early or late neurolytic sympathetic plexus block on the management of abdominal or pelvic cancer pain. Pain 2004, 110, 400–408. [Google Scholar] [CrossRef]
- Giri, S.S.; Tripathi, A.S.; Erkekoğlu, P.; Zaki, M.E.A. Molecular pathway of pancreatic cancer-associated neuropathic pain. J. Biochem. Mol. Toxicol. 2024, 38, e23638. [Google Scholar] [CrossRef]
- Li, Z.J.; Cho, C.H. Neurotransmitters, more than meets the eye--neurotransmitters and their perspectives in cancer development and therapy. Eur. J. Pharmacol. 2011, 667, 17–22. [Google Scholar] [CrossRef]
- Watkins, L.R.; Maier, S.F. Immune regulation of central nervous system functions: From sickness responses to pathological pain. J. Intern. Med. 2005, 257, 139–155. [Google Scholar] [CrossRef]
- Zochodne, D.W. Diabetes mellitus and the peripheral nervous system: Manifestations and mechanisms. Muscle Nerve 2007, 36, 144–166. [Google Scholar] [CrossRef]
- Hirth, M.; Gandla, J.; Höper, C.; Gaida, M.M.; Agarwal, N.; Simonetti, M.; Demir, A.; Xie, Y.; Weiss, C.; Michalski, C.W.; et al. CXCL10 and CCL21 Promote Migration of Pancreatic Cancer Cells Toward Sensory Neurons and Neural Remodeling in Tumors in Mice, Associated With Pain in Patients. Gastroenterology 2020, 159, 665–681.e613. [Google Scholar] [CrossRef] [PubMed]
- Damm, M.; Weniger, M.; Kölsch, A.K.; Lampert, C.; Ceyhan, G.O.; Beer, S.; Schorn, S.; Moir, J.; Michl, P.; Rosendahl, J. The quality of pain management in pancreatic cancer: A prospective multi-center study. Pancreatology 2020, 20, 1511–1518. [Google Scholar] [CrossRef]
- Carvajal, G. Pancreatic Cancer Related Pain: Review of Pathophysiology and Intrathecal Drug Delivery Systems for Pain Management. Pain. Physician 2021, 24, E583–E594. [Google Scholar] [PubMed]
- Ceyhan, G.O.; Bergmann, F.; Kadihasanoglu, M.; Altintas, B.; Demir, I.E.; Hinz, U.; Müller, M.W.; Giese, T.; Büchler, M.W.; Giese, N.A.; et al. Pancreatic neuropathy and neuropathic pain--a comprehensive pathomorphological study of 546 cases. Gastroenterology 2009, 136, 177–186.e171. [Google Scholar] [CrossRef] [PubMed]
- Kelsen, D.P.; Portenoy, R.; Thaler, H.; Tao, Y.; Brennan, M. Pain as a predictor of outcome in patients with operable pancreatic carcinoma. Surgery 1997, 122, 53–59. [Google Scholar] [CrossRef]
- Grahm, A.L.; Andrén-Sandberg, A. Prospective evaluation of pain in exocrine pancreatic cancer. Digestion 1997, 58, 542–549. [Google Scholar] [CrossRef]
- Tao, S.F.; Gu, W.H.; Gu, J.C.; Zhu, M.L.; Wang, Q.; Zheng, L.Z. A Retrospective Case Series Of High-Intensity Focused Ultrasound (HIFU) In Combination With Gemcitabine And Oxaliplatin (Gemox) On Treating Elderly Middle And Advanced Pancreatic Cancer. Onco Targets Ther. 2019, 12, 9735–9745. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.; Hirpara, D.H.; Raphael, M.; Karanicolas, P.J. Focused ultrasound and concurrent chemotherapy for the treatment of advanced pancreatic cancer: A systematic review. J. Surg. Oncol. 2024, 130, 1617–1623. [Google Scholar] [CrossRef]
- Puli, S.R.; Reddy, J.B.; Bechtold, M.L.; Antillon, M.R.; Brugge, W.R. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: A meta-analysis and systematic review. Dig. Dis. Sci. 2009, 54, 2330–2337. [Google Scholar] [CrossRef]
- Barnes, A.F.; Yeo, T.P.; Leiby, B.; Kay, A.; Winter, J.M. Pancreatic Cancer-Associated Depression: A Case Report and Review of the Literature. Pancreas 2018, 47, 1065–1077. [Google Scholar] [CrossRef]
- Krebber, A.M.; Buffart, L.M.; Kleijn, G.; Riepma, I.C.; de Bree, R.; Leemans, C.R.; Becker, A.; Brug, J.; van Straten, A.; Cuijpers, P.; et al. Prevalence of depression in cancer patients: A meta-analysis of diagnostic interviews and self-report instruments. Psychooncology 2014, 23, 121–130. [Google Scholar] [CrossRef]
- Wang, Y.H.; Li, J.Q.; Shi, J.F.; Que, J.Y.; Liu, J.J.; Lappin, J.M.; Leung, J.; Ravindran, A.V.; Chen, W.Q.; Qiao, Y.L.; et al. Depression and anxiety in relation to cancer incidence and mortality: A systematic review and meta-analysis of cohort studies. Mol. Psychiatry 2020, 25, 1487–1499. [Google Scholar] [CrossRef]
- Yaskin, J.C. Nervous Symptoms As Earliest Manifestations of Carcinoma of the Pancreas. J. Am. Med. Assoc. 1931, 96, 1664–1668. [Google Scholar] [CrossRef]
- Clark, K.L.; Loscalzo, M.; Trask, P.C.; Zabora, J.; Philip, E.J. Psychological distress in patients with pancreatic cancer—An understudied group. Psycho-Oncology 2010, 19, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Michoglou, K.; Ravinthiranathan, A.; San Ti, S.; Dolly, S.; Thillai, K. Pancreatic cancer and depression. World J. Clin. Cases 2023, 11, 2631–2636. [Google Scholar] [CrossRef] [PubMed]
- Fras, I.; Litin, E.M.; Bartholomew, L.G. Mental Symptoms as an Aid in the Early Diagnosis of Carcinoma of the Pancreas. Gastroenterology 1968, 55, 191–198. [Google Scholar] [CrossRef]
- Seoud, T.; Syed, A.; Carleton, N.; Rossi, C.; Kenner, B.; Quershi, H.; Anand, M.; Thakkar, P.; Thakkar, S. Depression Before and After a Diagnosis of Pancreatic Cancer: Results From a National, Population-Based Study. Pancreas 2020, 49, 1117–1122. [Google Scholar] [CrossRef]
- FRAS, I.; LITIN, E.M.; PEARSON, J.S. Comparison of Psychiatric Symptoms in Carcinoma of the Pancreas with Those in Some Other Intra-abdominal Neoplasms. Am. J. Psychiatry 1967, 123, 1553–1562. [Google Scholar] [CrossRef]
- Massie, M.J.; Gagnon, P.; Holland, J.C. Depression and suicide in patients with cancer. J. Pain Symptom Manag. 1994, 9, 325–340. [Google Scholar] [CrossRef]
- Mayr, M.; Schmid, R.M. Pancreatic cancer and depression: Myth and truth. BMC Cancer 2010, 10, 569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, A.; Bo, W.; Zhang, M.; Wang, H.; Feng, X.; Wu, Y. Upregulation of HSD11B1 promotes cortisol production and inhibits NK cell activation in pancreatic adenocarcinoma. Mol. Immunol. 2024, 175, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Jarrin Jara, M.D.; Gautam, A.S.; Peesapati, V.S.R.; Sadik, M.; Khan, S. The Role of Interleukin-6 and Inflammatory Cytokines in Pancreatic Cancer-Associated Depression. Cureus 2020, 12, e9969. [Google Scholar] [CrossRef]
- Musselman, D.L.; Miller, A.H.; Porter, M.R.; Manatunga, A.; Gao, F.; Penna, S.; Pearce, B.D.; Landry, J.; Glover, S.; McDaniel, J.S.; et al. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: Preliminary findings. Am. J. Psychiatry 2001, 158, 1252–1257. [Google Scholar] [CrossRef]
- Brown, J.H.; Paraskevas, F. Cancer and depression: Cancer presenting with depressive illness: An autoimmune disease? Br. J. Psychiatry 1982, 141, 227–232. [Google Scholar] [CrossRef]
- Hue, J.J.; Graor, H.J.; Zarei, M.; Katayama, E.S.; Ji, K.; Hajihassani, O.; Loftus, A.W.; Vaziri-Gohar, A.; Winter, J.M. IDO1 Is a Therapeutic Target for Pancreatic Cancer-Associated Depression. Mol. Cancer Ther. 2022, 21, 1810–1822. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.R. Depression in cancer patients: Pathogenesis, implications and treatment (Review). Oncol. Lett. 2015, 9, 1509–1514. [Google Scholar] [CrossRef]
- O’Connor, E.A.; Whitlock, E.P.; Gaynes, B.; Beil, T.L. U.S. Preventive Services Task Force Evidence Syntheses, Formerly Systematic Evidence Reviews. In Screening for Depression in Adults and Older Adults in Primary Care: An Updated Systematic Review; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2009. [Google Scholar]
- Lu, D.; Andersson, T.M.; Fall, K.; Hultman, C.M.; Czene, K.; Valdimarsdóttir, U.; Fang, F. Clinical Diagnosis of Mental Disorders Immediately Before and After Cancer Diagnosis: A Nationwide Matched Cohort Study in Sweden. JAMA Oncol. 2016, 2, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.H.; Xu, Y.; Herzog, K.; Saldia, A.; DeFilippis, E.M.; Li, P.; Allen, P.J.; O’Reilly, E.M.; Kurtz, R.C. Weight Loss, Diabetes, Fatigue, and Depression Preceding Pancreatic Cancer. Pancreas 2016, 45, 986–991. [Google Scholar] [CrossRef]
- Ferreira, M.; Moreira, H.; Esperto, H.; Carvalho, A. Depression preceding the diagnosis of pancreatic cancer. BMJ Case Rep. 2021, 14, e231585. [Google Scholar] [CrossRef]
- Cipora, E.; Czerw, A.; Partyka, O.; Pajewska, M.; Badowska-Kozakiewicz, A.; Fudalej, M.; Sygit, K.; Kaczmarski, M.; Krzych-Fałta, E.; Jurczak, A.; et al. Quality of Life in Patients with Pancreatic Cancer—A Literature Review. Int. J. Environ. Res. Public Health 2023, 20, 4895. [Google Scholar] [CrossRef]
- Jia, L.; Jiang, S.M.; Shang, Y.Y.; Huang, Y.X.; Li, Y.J.; Xie, D.R.; Huang, K.H.; Zhi, F.C. Investigation of the incidence of pancreatic cancer-related depression and its relationship with the quality of life of patients. Digestion 2010, 82, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Sheibani-Rad, S.; Velanovich, V. Effects of depression on the survival of pancreatic adenocarcinoma. Pancreas 2006, 32, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.A.; Benarroch-Gampel, J.; Sheffield, K.M.; Han, Y.; Kuo, Y.F.; Riall, T.S. The effect of depression on stage at diagnosis, treatment, and survival in pancreatic adenocarcinoma. Surgery 2012, 152, 403–413. [Google Scholar] [CrossRef]
- Satin, J.R.; Linden, W.; Phillips, M.J. Depression as a predictor of disease progression and mortality in cancer patients: A meta-analysis. Cancer 2009, 115, 5349–5361. [Google Scholar] [CrossRef]
- Davis, N.E.; Hue, J.J.; Kyasaram, R.K.; Elshami, M.; Graor, H.J.; Zarei, M.; Ji, K.; Katayama, E.S.; Hajihassani, O.; Loftus, A.W.; et al. Prodromal depression and anxiety are associated with worse treatment compliance and survival among patients with pancreatic cancer. Psychooncology 2022, 31, 1390–1398. [Google Scholar] [CrossRef]
- Ciaramella, A.; Poli, P. Assessment of depression among cancer patients: The role of pain, cancer type and treatment. Psychooncology 2001, 10, 156–165. [Google Scholar] [CrossRef]
- Spiegel, D.; Sands, S.; Koopman, C. Pain and depression in patients with cancer. Cancer 1994, 74, 2570–2578. [Google Scholar] [CrossRef]
- Li, P.; Hu, Y.; Scelo, G.; Myrskylä, M.; Martikainen, P. Pre-existing psychological disorders, diabetes, and pancreatic cancer: A population-based study of 38,952 Finns. Cancer Epidemiol. 2023, 82, 102307. [Google Scholar] [CrossRef]
- Makrilia, N.; Indeck, B.; Syrigos, K.; Saif, M.W. Depression and pancreatic cancer: A poorly understood link. Jop 2009, 10, 69–76. [Google Scholar]
- Chu, A.; Wadhwa, R. Selective Serotonin Reuptake Inhibitors; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Karrouri, R.; Hammani, Z.; Benjelloun, R.; Otheman, Y. Major depressive disorder: Validated treatments and future challenges. World J. Clin. Cases 2021, 9, 9350–9367. [Google Scholar] [CrossRef]
- Maekawa, R.M.; Guerra, L.T.L.; Bouso, J.C.; Hallak, J.E.C.; dos Santos, R.G. Adverse Effects and Safety of Antidepressants and Psychedelics for Depression in Cancer: A Systematic Review of Randomized Controlled Trials. Psychoactives 2025, 4, 6. [Google Scholar] [CrossRef]
- Chen, W.T.; Hsu, F.T.; Liu, Y.C.; Chen, C.H.; Hsu, L.C.; Lin, S.S. Fluoxetine Induces Apoptosis through Extrinsic/Intrinsic Pathways and Inhibits ERK/NF-κB-Modulated Anti-Apoptotic and Invasive Potential in Hepatocellular Carcinoma Cells In Vitro. Int. J. Mol. Sci. 2019, 20, 757. [Google Scholar] [CrossRef]
- Di Rosso, M.E.; Sterle, H.A.; Cremaschi, G.A.; Genaro, A.M. Beneficial Effect of Fluoxetine and Sertraline on Chronic Stress-Induced Tumor Growth and Cell Dissemination in a Mouse Model of Lymphoma: Crucial Role of Antitumor Immunity. Front. Immunol. 2018, 9, 1341. [Google Scholar] [CrossRef]
- Jia, L.; Shang, Y.Y.; Li, Y.Y. Effect of antidepressants on body weight, ethology and tumor growth of human pancreatic carcinoma xenografts in nude mice. World J. Gastroenterol. 2008, 14, 4377–4382. [Google Scholar] [CrossRef] [PubMed]
- Anacker, C.; Zunszain, P.A.; Carvalho, L.A.; Pariante, C.M. The glucocorticoid receptor: Pivot of depression and of antidepressant treatment? Psychoneuroendocrinology 2011, 36, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Kosakowska, E.; Cencelewicz-Lesikow, A.; Pałucki, J.; Kunkiel, M.; Jagiełło-Gruszfeld, A. Long-term complete remission of pancreatic cancer after first-line chemotherapy with gemcitabine and nab-paclitaxel in a patient with depressive disorder. Oncol. Clin. Pract. 2020, 16, 83–86. [Google Scholar] [CrossRef]
- O’Reilly, D.; Fou, L.; Hasler, E.; Hawkins, J.; O’Connell, S.; Pelone, F.; Callaway, M.; Campbell, F.; Capel, M.; Charnley, R.; et al. Diagnosis and management of pancreatic cancer in adults: A summary of guidelines from the UK National Institute for Health and Care Excellence. Pancreatology 2018, 18, 962–970. [Google Scholar] [CrossRef]
- Woo, S.M.; Song, M.K.; Lee, M.; Joo, J.; Kim, D.H.; Kim, J.H.; Han, S.S.; Park, S.J.; Kim, T.H.; Lee, W.J. Effect of Early Management on Pain and Depression in Patients with Pancreatobiliary Cancer: A Randomized Clinical Trial. Cancers 2019, 11, 79. [Google Scholar] [CrossRef]
- Adenis, A.; Da Silva, A.; Ben Abdelghani, M.; Bourgeois, V.; Bogart, E.; Turpin, A.; Evin, A.; Proux, A.; Galais, M.P.; Jaraudias, C.; et al. Early palliative care and overall survival in patients with metastatic upper gastrointestinal cancers (EPIC): A multicentre, open-label, randomised controlled phase 3 trial. EClinicalMedicine 2024, 74, 102470. [Google Scholar] [CrossRef] [PubMed]
- Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47, S20–S42. [Google Scholar] [CrossRef] [PubMed]
- Zendehdel, K.; Nyrén, O.; Ostenson, C.G.; Adami, H.O.; Ekbom, A.; Ye, W. Cancer incidence in patients with type 1 diabetes mellitus: A population-based cohort study in Sweden. J. Natl. Cancer Inst. 2003, 95, 1797–1800. [Google Scholar] [CrossRef]
- Bures, J.; Kohoutova, D.; Skrha, J.; Bunganic, B.; Ngo, O.; Suchanek, S.; Skrha, P.; Zavoral, M. Diabetes Mellitus in Pancreatic Cancer: A Distinct Approach to Older Subjects with New-Onset Diabetes Mellitus. Cancers 2023, 15, 3669. [Google Scholar] [CrossRef]
- Hart, P.A.; Bellin, M.D.; Andersen, D.K.; Bradley, D.; Cruz-Monserrate, Z.; Forsmark, C.E.; Goodarzi, M.O.; Habtezion, A.; Korc, M.; Kudva, Y.C.; et al. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol. Hepatol. 2016, 1, 226–237. [Google Scholar] [CrossRef]
- Klein, A.P. Pancreatic cancer epidemiology: Understanding the role of lifestyle and inherited risk factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [Google Scholar] [CrossRef]
- Popovic, K.; Smolović, B.; Martinović, M.; Vučković, L. The Relationship between Diabetes Mellitus and Pancreatic Cancer-Diabetes Mellitus as a Red Flag for Pancreatic Cancer. Cancer Epidemiol. Biomark. Prev. 2023, 32, 298–305. [Google Scholar] [CrossRef]
- Pereira, S.P.; Oldfield, L.; Ney, A.; Hart, P.A.; Keane, M.G.; Pandol, S.J.; Li, D.; Greenhalf, W.; Jeon, C.Y.; Koay, E.J.; et al. Early detection of pancreatic cancer. Lancet Gastroenterol. Hepatol. 2020, 5, 698–710. [Google Scholar] [CrossRef]
- Frič, P.; Šedo, A.; Škrha, J.; Bušek, P.; Laclav, M.; Škrha, P.; Zavoral, M. Early detection of sporadic pancreatic cancer: Time for change. Eur. J. Gastroenterol. Hepatol. 2017, 29, 885–891. [Google Scholar] [CrossRef]
- Stoffel, E.M.; Brand, R.E.; Goggins, M. Pancreatic Cancer: Changing Epidemiology and New Approaches to Risk Assessment, Early Detection, and Prevention. Gastroenterology 2023, 164, 752–765. [Google Scholar] [CrossRef]
- Pannala, R.; Leirness, J.B.; Bamlet, W.R.; Basu, A.; Petersen, G.M.; Chari, S.T. Prevalence and Clinical Profile of Pancreatic Cancer—Associated Diabetes Mellitus. Gastroenterology 2008, 134, 981–987. [Google Scholar] [CrossRef]
- Mellenthin, C.; Balaban, V.D.; Dugic, A.; Cullati, S. Risk Factors for Pancreatic Cancer in Patients with New-Onset Diabetes: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 4684. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Chae, W.; Sung, M.J.; Keum, J.; Jo, J.H.; Chung, M.J.; Park, J.Y.; Park, S.W.; Song, S.Y.; Park, E.C.; et al. Difference of Risk of Pancreatic Cancer in New-Onset Diabetes and Long-standing Diabetes: A Population-based Cohort Study. J. Clin. Endocrinol. Metab. 2023, 108, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bian, X.; Wei, S.; He, M.; Yang, Y. The relationship between pancreatic cancer and type 2 diabetes: Cause and consequence. Cancer Manag. Res. 2019, 11, 8257–8268. [Google Scholar] [CrossRef]
- Sapoor, S.; Nageh, M.; Shalma, N.M.; Sharaf, R.; Haroun, N.; Salama, E.; Pratama Umar, T.; Sharma, S.; Sayad, R. Bidirectional relationship between pancreatic cancer and diabetes mellitus: A comprehensive literature review. Ann. Med. Surg. 2024, 86, 3522–3529. [Google Scholar] [CrossRef]
- Ruze, R.; Song, J.; Yin, X.; Chen, Y.; Xu, R.; Wang, C.; Zhao, Y. Mechanisms of obesity- and diabetes mellitus-related pancreatic carcinogenesis: A comprehensive and systematic review. Signal Transduct. Target. Ther. 2023, 8, 139. [Google Scholar] [CrossRef]
- Wang, F.; Huang, L.; Zhang, J.; Fan, J.; Wu, H.; Xu, J. Dyslipidemia in Chinese Pancreatic Cancer Patients: A Two-Center Retrospective Study. J. Cancer 2021, 12, 5338–5344. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Sachdev, E.; Robbins, L.A.; Lin, E.; Hendifar, A.E.; Mita, M.M. Statins and pancreatic cancer. Oncol. Lett. 2017, 13, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Azizzadeh, B.; Majidinia, M.; Gheysarzadeh, A. The reciprocal effects of autophagy and the Warburg effect in pancreatic ductal adenocarcinoma: An in vitro study. Med. Oncol. 2025, 42, 86. [Google Scholar] [CrossRef]
- Zhang, A.M.Y.; Chu, K.H.; Daly, B.F.; Ruiter, T.; Dou, Y.; Yang, J.C.C.; de Winter, T.J.J.; Chhuor, J.; Wang, S.; Flibotte, S.; et al. Effects of hyperinsulinemia on pancreatic cancer development and the immune microenvironment revealed through single-cell transcriptomics. Cancer Metab. 2022, 10, 5. [Google Scholar] [CrossRef]
- Trajkovic-Arsic, M.; Kalideris, E.; Siveke, J.T. The role of insulin and IGF system in pancreatic cancer. J. Mol. Endocrinol. 2013, 50, R67–R74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.M.Y.; Xia, Y.H.; Lin, J.S.H.; Chu, K.H.; Wang, W.C.K.; Ruiter, T.J.J.; Yang, J.C.C.; Chen, N.; Chhuor, J.; Patil, S.; et al. Hyperinsulinemia acts via acinar insulin receptors to initiate pancreatic cancer by increasing digestive enzyme production and inflammation. Cell Metab. 2023, 35, 2119–2135.e2115. [Google Scholar] [CrossRef] [PubMed]
- Bak, J.F. Insulin receptor function and glycogen synthase activity in human skeletal muscle. Physiology and pathophysiology. Dan. Med. Bull. 1994, 41, 179–192. [Google Scholar]
- Zhou, W.; Lim, A.; Edderkaoui, M.; Osipov, A.; Wu, H.; Wang, Q.; Pandol, S. Role of YAP Signaling in Regulation of Programmed Cell Death and Drug Resistance in Cancer. Int. J. Biol. Sci. 2024, 20, 15–28. [Google Scholar] [CrossRef]
- Hao, F.; Xu, Q.; Zhao, Y.; Stevens, J.V.; Young, S.H.; Sinnett-Smith, J.; Rozengurt, E. Insulin Receptor and GPCR Crosstalk Stimulates YAP via PI3K and PKD in Pancreatic Cancer Cells. Mol. Cancer Res. 2017, 15, 929–941. [Google Scholar] [CrossRef]
- Chang, S.C.; Yang, W.V. Hyperglycemia, tumorigenesis, and chronic inflammation. Crit. Rev. Oncol. Hematol. 2016, 108, 146–153. [Google Scholar] [CrossRef]
- Quoc Lam, B.; Shrivastava, S.K.; Shrivastava, A.; Shankar, S.; Srivastava, R.K. The Impact of obesity and diabetes mellitus on pancreatic cancer: Molecular mechanisms and clinical perspectives. J. Cell Mol. Med. 2020, 24, 7706–7716. [Google Scholar] [CrossRef]
- Gallagher, E.J.; LeRoith, D. Hyperinsulinaemia in cancer. Nat. Rev. Cancer 2020, 20, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Permert, J.; Larsson, J.; Westermark, G.T.; Herrington, M.K.; Christmanson, L.; Pour, P.M.; Westermark, P.; Adrian, T.E. Islet amyloid polypeptide in patients with pancreatic cancer and diabetes. N. Engl. J. Med. 1994, 330, 313–318. [Google Scholar] [CrossRef]
- Taylor, A.J.; Panzhinskiy, E.; Orban, P.C.; Lynn, F.C.; Schaeffer, D.F.; Johnson, J.D.; Kopp, J.L.; Verchere, C.B. Islet amyloid polypeptide does not suppress pancreatic cancer. Mol. Metab. 2023, 68, 101667. [Google Scholar] [CrossRef]
- Basso, D.; Greco, E.; Fogar, P.; Pucci, P.; Flagiello, A.; Baldo, G.; Giunco, S.; Valerio, A.; Navaglia, F.; Zambon, C.F.; et al. Pancreatic cancer-derived S-100A8 N-terminal peptide: A diabetes cause? Clin. Chim. Acta 2006, 372, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Kenner, B.J.; Chari, S.T.; Cleeter, D.F.; Go, V.L. Early detection of sporadic pancreatic cancer: Strategic map for innovation--a white paper. Pancreas 2015, 44, 686–692. [Google Scholar] [CrossRef]
- Force, U.S.P.S.T.; Owens, D.K.; Davidson, K.W.; Krist, A.H.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Curry, S.J.; Doubeni, C.A.; Epling, J.W.; et al. Screening for Pancreatic Cancer: US Preventive Services Task Force Reaffirmation Recommendation Statement. JAMA 2019, 322, 438–444. [Google Scholar] [CrossRef]
- Henrikson, N.B.; Aiello Bowles, E.J.; Blasi, P.R.; Morrison, C.C.; Nguyen, M.; Pillarisetty, V.G.; Lin, J.S. Screening for Pancreatic Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2019, 322, 445–454. [Google Scholar] [CrossRef]
- Sawhney, M.S.; Calderwood, A.H.; Thosani, N.C.; Rebbeck, T.R.; Wani, S.; Canto, M.I.; Fishman, D.S.; Golan, T.; Hidalgo, M.; Kwon, R.S.; et al. ASGE guideline on screening for pancreatic cancer in individuals with genetic susceptibility: Summary and recommendations. Gastrointest. Endosc. 2022, 95, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Smyrk, T.C.; Levy, M.J.; Topazian, M.A.; Chari, S.T. Fasting Blood Glucose Levels Provide Estimate of Duration and Progression of Pancreatic Cancer Before Diagnosis. Gastroenterology 2018, 155, 490–500.e492. [Google Scholar] [CrossRef]
- Andersen, D.K.; Korc, M.; Petersen, G.M.; Eibl, G.; Li, D.; Rickels, M.R.; Chari, S.T.; Abbruzzese, J.L. Diabetes, Pancreatogenic Diabetes, and Pancreatic Cancer. Diabetes 2017, 66, 1103–1110. [Google Scholar] [CrossRef]
- Huang, B.Z.; Pandol, S.J.; Jeon, C.Y.; Chari, S.T.; Sugar, C.A.; Chao, C.R.; Zhang, Z.F.; Wu, B.U.; Setiawan, V.W. New-Onset Diabetes, Longitudinal Trends in Metabolic Markers, and Risk of Pancreatic Cancer in a Heterogeneous Population. Clin. Gastroenterol. Hepatol. 2020, 18, 1812–1821.e1817. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Washington, L. The seemingly innocuous presentation of metastatic pancreatic tail cancer: A case report. J. Med. Case Rep. 2019, 13, 178. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kandlakunta, H.; Nagpal, S.J.S.; Feng, Z.; Hoos, W.; Petersen, G.M.; Chari, S.T. Model to Determine Risk of Pancreatic Cancer in Patients With New-Onset Diabetes. Gastroenterology 2018, 155, 730–739.e733. [Google Scholar] [CrossRef]
- Khan, S.; Safarudin, R.F.; Kupec, J.T. Validation of the ENDPAC model: Identifying new-onset diabetics at risk of pancreatic cancer. Pancreatology 2021, 21, 550–555. [Google Scholar] [CrossRef]
- Boursi, B.; Patalon, T.; Webb, M.; Margalit, O.; Beller, T.; Yang, Y.X.; Chodick, G. Validation of the Enriching New-Onset Diabetes for Pancreatic Cancer Model: A Retrospective Cohort Study Using Real-World Data. Pancreas 2022, 51, 196–199. [Google Scholar] [CrossRef]
- Cichosz, S.L.; Jensen, M.H.; Hejlesen, O.; Henriksen, S.D.; Drewes, A.M.; Olesen, S.S. Prediction of pancreatic cancer risk in patients with new-onset diabetes using a machine learning approach based on routine biochemical parameters. Comput. Methods Programs Biomed. 2024, 244, 107965. [Google Scholar] [CrossRef] [PubMed]
- Takai, E.; Totoki, Y.; Nakamura, H.; Morizane, C.; Nara, S.; Hama, N.; Suzuki, M.; Furukawa, E.; Kato, M.; Hayashi, H.; et al. Clinical utility of circulating tumor DNA for molecular assessment in pancreatic cancer. Sci. Rep. 2015, 5, 18425. [Google Scholar] [CrossRef]
- Nakamura, K.; Zhu, Z.; Roy, S.; Jun, E.; Han, H.; Munoz, R.M.; Nishiwada, S.; Sharma, G.; Cridebring, D.; Zenhausern, F.; et al. An Exosome-based Transcriptomic Signature for Noninvasive, Early Detection of Patients With Pancreatic Ductal Adenocarcinoma: A Multicenter Cohort Study. Gastroenterology 2022, 163, 1252–1266.e1252. [Google Scholar] [CrossRef]
- Schwartz, N.R.M.; Matrisian, L.M.; Shrader, E.E.; Feng, Z.; Chari, S.; Roth, J.A. Potential Cost-Effectiveness of Risk-Based Pancreatic Cancer Screening in Patients With New-Onset Diabetes. J. Natl. Compr. Canc Netw. 2021, 20, 451–459. [Google Scholar] [CrossRef]
- Ali, S.; Coory, M.; Donovan, P.; Na, R.; Pandeya, N.; Pearson, S.A.; Spilsbury, K.; Tuesley, K.; Jordan, S.J.; Neale, R.E. Predicting the risk of pancreatic cancer in women with new-onset diabetes mellitus. J. Gastroenterol. Hepatol. 2024, 39, 1057–1064. [Google Scholar] [CrossRef]
- Radon, T.P.; Massat, N.J.; Jones, R.; Alrawashdeh, W.; Dumartin, L.; Ennis, D.; Duffy, S.W.; Kocher, H.M.; Pereira, S.P.; Guarner posthumous, L.; et al. Identification of a Three-Biomarker Panel in Urine for Early Detection of Pancreatic Adenocarcinoma. Clin. Cancer Res. 2015, 21, 3512–3521. [Google Scholar] [CrossRef]
- Škrha, J.; Bušek, P.; Uhrová, J.; Hrabal, P.; Kmochová, K.; Laclav, M.; Bunganič, B.; Frič, P. Lower plasma levels of glucose-dependent insulinotropic peptide (GIP) and pancreatic polypeptide (PP) in patients with ductal adenocarcinoma of the pancreas and their relation to the presence of impaired glucoregulation and weight loss. Pancreatology 2017, 17, 89–94. [Google Scholar] [CrossRef]
- Honda, K.; Kobayashi, M.; Okusaka, T.; Rinaudo, J.A.; Huang, Y.; Marsh, T.; Sanada, M.; Sasajima, Y.; Nakamori, S.; Shimahara, M.; et al. Plasma biomarker for detection of early stage pancreatic cancer and risk factors for pancreatic malignancy using antibodies for apolipoprotein-AII isoforms. Sci. Rep. 2015, 5, 15921. [Google Scholar] [CrossRef]
- Wolrab, D.; Jirásko, R.; Cífková, E.; Höring, M.; Mei, D.; Chocholoušková, M.; Peterka, O.; Idkowiak, J.; Hrnčiarová, T.; Kuchař, L.; et al. Lipidomic profiling of human serum enables detection of pancreatic cancer. Nat. Commun. 2022, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Bosch, N.; Barranco, L.E.; Orozco, C.A.; Moreno, M.; Visa, L.; Iglesias, M.; Oldfield, L.; Neoptolemos, J.P.; Greenhalf, W.; Earl, J.; et al. Increased plasma levels of galectin-1 in pancreatic cancer: Potential use as biomarker. Oncotarget 2018, 9, 32984–32996. [Google Scholar] [CrossRef] [PubMed]
- Papapanagiotou, A.; Sgourakis, G.; Karkoulias, K.; Raptis, D.; Parkin, E.; Brotzakis, P.; Panchal, S.; Papavassiliou, A.G. Osteonectin as a screening marker for pancreatic cancer: A prospective study. J. Int. Med. Res. 2018, 46, 2769–2779. [Google Scholar] [CrossRef]
- Balasenthil, S.; Liu, S.; Dai, J.; Bamlet, W.R.; Petersen, G.; Chari, S.T.; Maitra, A.; Chen, N.; Sen, S.; McNeill Killary, A. Blood-based Migration Signature Biomarker Panel Discriminates Early Stage New Onset Diabetes related Pancreatic Ductal Adenocarcinoma from Type 2 Diabetes. Clin. Chim. Acta 2023, 551, 117567. [Google Scholar] [CrossRef]
- Škrha, P.; Hořínek, A.; Pazourková, E.; Hajer, J.; Frič, P.; Škrha, J.; Anděl, M. Serum microRNA-196 and microRNA-200 in pancreatic ductal adenocarcinoma of patients with diabetes mellitus. Pancreatology 2016, 16, 839–843. [Google Scholar] [CrossRef]
- Jin, F.; Yang, L.; Wang, W.; Yuan, N.; Zhan, S.; Yang, P.; Chen, X.; Ma, T.; Wang, Y. A novel class of tsRNA signatures as biomarkers for diagnosis and prognosis of pancreatic cancer. Mol. Cancer 2021, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.A.; Kudva, Y.C.; Yadav, D.; Andersen, D.K.; Li, Y.; Toledo, F.G.S.; Wang, F.; Bellin, M.D.; Bradley, D.; Brand, R.E.; et al. A Reduced Pancreatic Polypeptide Response is Associated With New-onset Pancreatogenic Diabetes Versus Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2023, 108, e120–e128. [Google Scholar] [CrossRef]
- Hu, Y.; Zeng, N.; Ge, Y.; Wang, D.; Qin, X.; Zhang, W.; Jiang, F.; Liu, Y. Identification of the Shared Gene Signatures and Biological Mechanism in Type 2 Diabetes and Pancreatic Cancer. Front. Endocrinol. 2022, 13, 847760. [Google Scholar] [CrossRef] [PubMed]
- Marin, A.M.; Sanchuki, H.B.S.; Namur, G.N.; Uno, M.; Zanette, D.L.; Aoki, M.N. Circulating Cell-Free Nucleic Acids as Biomarkers for Diagnosis and Prognosis of Pancreatic Cancer. Biomedicines 2023, 11, 1069. [Google Scholar] [CrossRef]
- Samir, S.; El-Ashry, M.; Soliman, W.; Hassan, M. Urinary biomarkers analysis as a diagnostic tool for early detection of pancreatic adenocarcinoma: Molecular quantification approach. Comput. Biol. Chem. 2024, 112, 108171. [Google Scholar] [CrossRef]
- Hayasaki, A.; Murata, Y.; Usui, M.; Hibi, T.; Fujii, T.; Iizawa, Y.; Kato, H.; Tanemura, A.; Azumi, Y.; Kuriyama, N.; et al. Clinical Significance of Plasma Apolipoprotein-AII Isoforms as a Marker of Pancreatic Exocrine Disorder for Patients with Pancreatic Adenocarcinoma Undergoing Chemoradiotherapy, Paying Attention to Pancreatic Morphological Changes. Biomed. Res. Int. 2019, 2019, 5738614. [Google Scholar] [CrossRef]
- Majumder, S.; Taylor, W.R.; Foote, P.H.; Berger, C.K.; Wu, C.W.; Mahoney, D.W.; Bamlet, W.R.; Burger, K.N.; Postier, N.; de la Fuente, J.; et al. High Detection Rates of Pancreatic Cancer Across Stages by Plasma Assay of Novel Methylated DNA Markers and CA19-9. Clin. Cancer Res. 2021, 27, 2523–2532. [Google Scholar] [CrossRef]
- Grossberg, A.J.; Chu, L.C.; Deig, C.R.; Fishman, E.K.; Hwang, W.L.; Maitra, A.; Marks, D.L.; Mehta, A.; Nabavizadeh, N.; Simeone, D.M.; et al. Multidisciplinary standards of care and recent progress in pancreatic ductal adenocarcinoma. CA Cancer J. Clin. 2020, 70, 375–403. [Google Scholar] [CrossRef]
- Cao, K.; Xia, Y.; Yao, J.; Han, X.; Lambert, L.; Zhang, T.; Tang, W.; Jin, G.; Jiang, H.; Fang, X.; et al. Large-scale pancreatic cancer detection via non-contrast CT and deep learning. Nat. Med. 2023, 29, 3033–3043. [Google Scholar] [CrossRef]
- Takikawa, T.; Kikuta, K.; Kume, K.; Hamada, S.; Miura, S.; Yoshida, N.; Hongo, S.; Tanaka, Y.; Matsumoto, R.; Sano, T.; et al. New-Onset or Exacerbation of Diabetes Mellitus Is a Clue to the Early Diagnosis of Pancreatic Cancer. Tohoku J. Exp. Med. 2020, 252, 353–364. [Google Scholar] [CrossRef]
- Badowska-Kozakiewicz, A.; Fudalej, M.; Kwaśniewska, D.; Durlik, M.; Nasierowska-Guttmejer, A.; Mormul, A.; Włoszek, E.; Czerw, A.; Banaś, T.; Deptała, A. Diabetes Mellitus and Pancreatic Ductal Adenocarcinoma-Prevalence, Clinicopathological Variables, and Clinical Outcomes. Cancers 2022, 14, 2840. [Google Scholar] [CrossRef] [PubMed]
- Toriola, A.T.; Stolzenberg-Solomon, R.; Dalidowitz, L.; Linehan, D.; Colditz, G. Diabetes and pancreatic cancer survival: A prospective cohort-based study. Br. J. Cancer 2014, 111, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Xu, W.; Zhang, M. Association between type 2 diabetes and 5-year overall survival in early-stage pancreatic cancer: A retrospective cohort study. PeerJ 2022, 10, e14538. [Google Scholar] [CrossRef]
- Li, D.; Mao, Y.; Chang, P.; Liu, C.; Hassan, M.M.; Yeung, S.J.; Abbruzzese, J.L. Impacts of new-onset and long-term diabetes on clinical outcome of pancreatic cancer. Am. J. Cancer Res. 2015, 5, 3260–3269. [Google Scholar]
- Deo, K.B.; Kulkarni, A.A.; Kumar-M, P.; Krishnamurthy, G.; Shenvi, S.; Rana, S.S.; Kapoor, R.; Gupta, R. Impact of diabetes mellitus on morbidity and survival after pancreaticoduodenectomy for malignancy. Ann. Hepatobiliary Pancreat. Surg. 2021, 25, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Hank, T.; Sandini, M.; Qadan, M.; Weniger, M.; Ciprani, D.; Li, A.; Ferrone, C.R.; Warshaw, A.L.; Lillemoe, K.D.; Fernández-Del Castillo, C. Diabetes mellitus is associated with unfavorable pathologic features, increased postoperative mortality, and worse long-term survival in resected pancreatic cancer. Pancreatology 2020, 20, 125–131. [Google Scholar] [CrossRef]
- Szymczak-Pajor, I.; Drzewoski, J.; Świderska, E.; Strycharz, J.; Gabryanczyk, A.; Kasznicki, J.; Bogdańska, M.; Śliwińska, A. Metformin Induces Apoptosis in Human Pancreatic Cancer (PC) Cells Accompanied by Changes in the Levels of Histone Acetyltransferases (Particularly, p300/CBP-Associated Factor (PCAF) Protein Levels). Pharmaceuticals 2023, 16, 115. [Google Scholar] [CrossRef]
- Kim, J.; Bae, Y.J.; Kang, H.T. Metformin Use May Increase Risk of Pancreatic Cancer in Diabetic Women: An Analysis of the Korean National Health Insurance Service-National Health Screening Cohort Database. Korean J. Fam. Med. 2022, 43, 327–333. [Google Scholar] [CrossRef]
- van Eijck, C.W.F.; Vadgama, D.; van Eijck, C.H.J.; Wilmink, J.W. Metformin boosts antitumor immunity and improves prognosis in upfront resected pancreatic cancer: An observational study. J. Natl. Cancer Inst. 2024, 116, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, T.; Liu, Z.; Gou, S.; Wang, C. The effect of metformin on survival of patients with pancreatic cancer: A meta-analysis. Sci. Rep. 2017, 7, 5825. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Sun, X.; Li, F.; Wang, X.; Li, C.; Li, H.; Yu, X.; Cao, F. Survival Benefit of Metformin Adjuvant Treatment For Pancreatic Cancer Patients: A Systematic Review and Meta-Analysis. Cell Physiol. Biochem. 2018, 49, 837–847. [Google Scholar] [CrossRef]
- Nowicka, Z.; Matyjek, A.; Płoszka, K.; Łaszczych, M.; Fendler, W. Metanalyses on metformin’s role in pancreatic cancer suffer from severe bias and low data quality—An umbrella review. Pancreatology 2023, 23, 192–200. [Google Scholar] [CrossRef]
- Wei, M.; Liu, Y.; Bi, Y.; Zhang, Z.J. Metformin and pancreatic cancer survival: Real effect or immortal time bias? Int. J. Cancer 2019, 145, 1822–1828. [Google Scholar] [CrossRef]
- Lee, D.; Liew, M.S.; Fourlanos, S.; Choi, J. Metformin use and pancreatic ductal adenocarcinoma outcomes: A narrative review. ANZ J. Surg. 2025, 95, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Kounatidis, D.; Vallianou, N.G.; Karampela, I.; Rebelos, E.; Kouveletsou, M.; Dalopoulos, V.; Koufopoulos, P.; Diakoumopoulou, E.; Tentolouris, N.; Dalamaga, M. Anti-Diabetic Therapies and Cancer: From Bench to Bedside. Biomolecules 2024, 14, 1479. [Google Scholar] [CrossRef] [PubMed]
- Szymczak-Pajor, I.; Fleszar, K.; Kasznicki, J.; Gralewska, P.; Śliwińska, A. A potential role of calpains in sulfonylureas (SUs)-mediated death of human pancreatic cancer cells (1.2B4). Toxicol. In Vitro 2021, 73, 105128. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, M.; Wang, M.; Xu, W.; Duan, X.; Han, X.; Ren, J. Pioglitazone, a peroxisome proliferator-activated receptor γ agonist, induces cell death and inhibits the proliferation of hypoxic HepG2 cells by promoting excessive production of reactive oxygen species. Oncol. Lett. 2024, 27, 160. [Google Scholar] [CrossRef]
- Ohta, T.; Elnemr, A.; Yamamoto, M.; Ninomiya, I.; Fushida, S.; Nishimura, G.; Fujimura, T.; Kitagawa, H.; Kayahara, M.; Shimizu, K.; et al. Thiazolidinedione, a peroxisome proliferator-activated receptor-gamma ligand, modulates the E-cadherin/beta-catenin system in a human pancreatic cancer cell line, BxPC-3. Int. J. Oncol. 2002, 21, 37–42. [Google Scholar] [PubMed]
- Kim, M.K.; Han, K.; Kwon, H.S.; Yoo, S.J. Risk of Pancreatic Cancer and Use of Dipeptidyl Peptidase 4 Inhibitors in Patients with Type 2 Diabetes: A Propensity Score-Matching Analysis. Endocrinol. Metab. 2023, 38, 426–435. [Google Scholar] [CrossRef]
- Li, C.J.; Sun, B.; Fang, Q.H.; Ding, M.; Xing, Y.Z.; Chen, L.M.; Yu, D.M. Saxagliptin Induces β-Cell Proliferation through Increasing Stromal Cell-Derived Factor-1α In Vivo and In Vitro. Front. Endocrinol. 2017, 8, 326. [Google Scholar] [CrossRef]
- Nreu, B.; Dicembrini, I.; Tinti, F.; Mannucci, E.; Monami, M. Pancreatitis and pancreatic cancer in patients with type 2 diabetes treated with glucagon-like peptide-1 receptor agonists: An updated meta-analysis of randomized controlled trials. Minerva Endocrinol. 2023, 48, 206–213. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Li, L.; Kaelber, D.C.; Xu, R. Glucagon-like peptide-1 receptor agonists and pancreatic cancer risk: Target trial emulation using real-world data. J. Natl. Cancer Inst. 2025, 117, 476–485. [Google Scholar] [CrossRef]
- Dankner, R.; Murad, H.; Agay, N.; Olmer, L.; Freedman, L.S. Glucagon-Like Peptide-1 Receptor Agonists and Pancreatic Cancer Risk in Patients With Type 2 Diabetes. JAMA Netw. Open 2024, 7, e2350408. [Google Scholar] [CrossRef]
- Ming-Yan, Y.; Jing, Z.; Shu-Qin, G.; Xiao-Liang, B.; Zhi-Hong, L.; Xue, Z. Liraglutide inhibits the apoptosis of human nucleus pulposus cells induced by high glucose through PI3K/Akt/caspase-3 signaling pathway. Biosci. Rep. 2019, 39, BSR20190109. [Google Scholar] [CrossRef]
- Cencioni, C.; Malatesta, S.; Vigiano Benedetti, V.; Licursi, V.; Perfetto, L.; Conte, F.; Ranieri, D.; Bartolazzi, A.; Kunkl, M.; Tuosto, L.; et al. The GLP-1R agonist semaglutide reshapes pancreatic cancer associated fibroblasts reducing collagen proline hydroxylation and favoring T lymphocyte infiltration. J. Exp. Clin. Cancer Res. 2025, 44, 18. [Google Scholar] [CrossRef]
- Xu, D.; Zhou, Y.; Xie, X.; He, L.; Ding, J.; Pang, S.; Shen, B.; Zhou, C. Inhibitory effects of canagliflozin on pancreatic cancer are mediated via the downregulation of glucose transporter-1 and lactate dehydrogenase A. Int. J. Oncol. 2020, 57, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Flausino, L.E.; Carrasco, A.G.M.; Furuya, T.K.; Tuan, W.J.; Chammas, R. Impact of SGLT2 inhibitors on survival in gastrointestinal cancer patients undergoing chemotherapy and/or radiotherapy: A real-world data retrospective cohort study. BMC Cancer 2025, 25, 542. [Google Scholar] [CrossRef] [PubMed]
- Park, L.K.; Lim, K.H.; Volkman, J.; Abdiannia, M.; Johnston, H.; Nigogosyan, Z.; Siegel, M.J.; McGill, J.B.; McKee, A.M.; Salam, M.; et al. Safety, tolerability, and effectiveness of the sodium-glucose cotransporter 2 inhibitor (SGLT2i) dapagliflozin in combination with standard chemotherapy for patients with advanced, inoperable pancreatic adenocarcinoma: A phase 1b observational study. Cancer Metab. 2023, 11, 6. [Google Scholar] [CrossRef]
- Amri, F.; Belkhayat, C.; Yeznasni, A.; Koulali, H.; Jabi, R.; Zazour, A.; Abda, N.; Bouziane, M.; Ismaili, Z.; Kharrasse, G. Association between pancreatic cancer and diabetes: Insights from a retrospective cohort study. BMC Cancer 2023, 23, 856. [Google Scholar] [CrossRef]
- Ikushima, S.; Ono, R.; Fukuda, K.; Sakayori, M.; Awano, N.; Kondo, K. Trousseau’s syndrome: Cancer-associated thrombosis. Jpn. J. Clin. Oncol. 2016, 46, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chen, S.; Zhang, X.; Lin, X.; Lin, S.; Chen, Q.; Zhang, Z.; Li, J.; Chen, X. Pancreatic panniculitis associated with presumed advanced pancreatic tumor: A case report and literature review. Pathol. Res. Pract. 2025, 275, 156214. [Google Scholar] [CrossRef]
- Pan, W.; Wang, Q.; Yang, M.; Zhang, L.; Wang, J.; Sun, K. Pancreatic carcinoma presented with panniculitis and polyarthritis: A rare case. J. Cancer Res. Ther. 2021, 17, 1751–1754. [Google Scholar] [CrossRef]
- Betrains, A.; Rosseels, W.; Van Mieghem, E.; Vanderschueren, S.; Nijs, J. Clinical characteristics, treatment, and outcome of pancreatitis, panniculitis, and polyarthritis syndrome: A case-based review. Clin. Rheumatol. 2021, 40, 1625–1633. [Google Scholar] [CrossRef]
- Narváez, J.; Bianchi, M.M.; Santo, P.; de la Fuente, D.; Ríos-Rodriguez, V.; Bolao, F.; Narváez, J.A.; Nolla, J.M. Pancreatitis, panniculitis, and polyarthritis. Semin. Arthritis Rheum. 2010, 39, 417–423. [Google Scholar] [CrossRef]
- Po, X.Y. Pancreatitis, panniculitis, and polyarthritis syndrome presentation, diagnosis, and management. J. Surg. Case Rep. 2025, 2025, rjaf305. [Google Scholar] [CrossRef] [PubMed]
- Nadal, R.; McMahan, Z.H.; Antonarakis, E.S. Paraneoplastic palmar fasciitis and polyarthritis syndrome in a patient with advanced prostate cancer. Clin. Genitourin. Cancer 2013, 11, e15–e23. [Google Scholar] [CrossRef]
- Padniewski, J.J.; Nelson, E.; Mian, I.; Laczniak, A.; Ives, S.; Nasr, R. Paraneoplastic myopathy in pancreatic cancer: A case report and literature review. J. Community Hosp. Intern. Med. Perspect. 2021, 11, 847–851. [Google Scholar] [CrossRef]
- Varki, A. Trousseau’s syndrome: Multiple definitions and multiple mechanisms. Blood 2007, 110, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Wahrenbrock, M.; Borsig, L.; Le, D.; Varki, N.; Varki, A. Selectin-mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J. Clin. Investig. 2003, 112, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak, M.; Lewandowski, K.; Wojtukiewicz, M.Z.; Turowiecka, Z.; Kołacz, E.; Lojko, A.; Skrzydlewska, E.; Zawilska, K.; Komarnicki, M. Cancer procoagulant in patients with adenocarcinomas. Blood Coagul. Fibrinolysis 2005, 16, 543–547. [Google Scholar] [CrossRef]
- Klein, A.; Shepshelovich, D.; Spectre, G.; Goldvaser, H.; Raanani, P.; Gafter-Gvili, A. Screening for occult cancer in idiopathic venous thromboembolism—Systemic review and meta-analysis. Eur. J. Intern. Med. 2017, 42, 74–80. [Google Scholar] [CrossRef]
- Zaki, H.A.; Hamdi Alkahlout, B.; Basharat, K.; Elsayed, W.A.E.; Abdelrahim, M.G.; Al-Marri, N.D.R.; Masood, M.; Shaban, E. Low-Molecular-Weight Heparin Versus Warfarin in Adult Cancer Patients as a Precision Medicine for Thrombosis: A Systematic Review and Meta-Analysis. Cureus 2023, 15, e41268. [Google Scholar] [CrossRef]
- Johan, C.; Nelson, M.; Nadia, G.; Jose, M.; Jorge, D. Multiple cerebral infarction due to Trousseau’s syndrome as the first manifestation of pancreatic adenocarcinoma. Acta Neurol. Belg. 2020, 120, 1445–1447. [Google Scholar] [CrossRef] [PubMed]
- García-Romero, D.; Vanaclocha, F. Pancreatic panniculitis. Dermatol. Clin. 2008, 26, 465–470. [Google Scholar] [CrossRef]
- Zundler, S.; Erber, R.; Agaimy, A.; Hartmann, A.; Kiesewetter, F.; Strobel, D.; Neurath, M.F.; Wildner, D. Pancreatic panniculitis in a patient with pancreatic-type acinar cell carcinoma of the liver--case report and review of literature. BMC Cancer 2016, 16, 130. [Google Scholar] [CrossRef]
- Yamashita, Y.; Joshita, S.; Ito, T.; Maruyama, M.; Wada, S.; Umemura, T. A case report of pancreatic panniculitis due to acute pancreatitis with intraductal papillary mucinous neoplasm. BMC Gastroenterol. 2020, 20, 286. [Google Scholar] [CrossRef]
- Zhu, W.F.; Fang, S.; Qiao, J.J. Pancreatic panniculitis as the first presentation of pancreatic ductal adenocarcinoma. Hepatobiliary Pancreat. Dis. Int. 2024, 23, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Manger, B.; Schett, G. Palmar fasciitis and polyarthritis syndrome-systematic literature review of 100 cases. Semin. Arthritis Rheum. 2014, 44, 105–111. [Google Scholar] [CrossRef]
- Eze, B.; Freijat, M. Understanding Palmar Fasciitis and Polyarthritis Syndrome as a Rheumatologic Paraneoplastic Syndrome: A Case Report. Cureus 2024, 16, e61248. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Fukumoto, T.; Sowa-Osako, J.; Tateishi, C. Idiopathic palmar fasciitis and polyarthritis syndrome. BMJ Case Rep. 2019, 12, e232954. [Google Scholar] [CrossRef]
- Veitch, D.; Tsai, T.; Joshua, F. Palmar fasciitis and polyarthritis syndrome in pancreatic carcinoma. J. Clin. Rheumatol. 2013, 19, 203–205. [Google Scholar] [CrossRef]
- Costantini, A.; Moletta, L.; Pierobon, E.S.; Serafini, S.; Valmasoni, M.; Sperti, C. Paraneoplastic myopathy-related rhabdomyolysis and pancreatic cancer: A case report and review of the literature. World J. Clin. Cases 2023, 11, 6823–6830. [Google Scholar] [CrossRef]
- Fayyaz, B.; Rehman, H.J.; Uqdah, H. Cancer-associated myositis: An elusive entity. J. Community Hosp. Intern. Med. Perspect. 2019, 9, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Rubnitz, Z.; Nevala-Plagemann, C.; Florou, V.; Garrido-Laguna, I. Gemcitabine-induced myositis in a patient with pancreatic cancer. BMJ Case Rep. 2025, 18, e259660. [Google Scholar] [CrossRef]
- Vinci, P.; Panizon, E.; Tosoni, L.M.; Cerrato, C.; Pellicori, F.; Mearelli, F.; Biasinutto, C.; Fiotti, N.; Di Girolamo, F.G.; Biolo, G. Statin-Associated Myopathy: Emphasis on Mechanisms and Targeted Therapy. Int. J. Mol. Sci. 2021, 22, 11687. [Google Scholar] [CrossRef]
- Lundberg, I.E.; Tjärnlund, A.; Bottai, M.; Werth, V.P.; Pilkington, C.; Visser, M.; Alfredsson, L.; Amato, A.A.; Barohn, R.J.; Liang, M.H.; et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann. Rheum. Dis. 2017, 76, 1955–1964. [Google Scholar] [CrossRef]
- Oldroyd, A.G.S.; Lilleker, J.B.; Amin, T.; Aragon, O.; Bechman, K.; Cuthbert, V.; Galloway, J.; Gordon, P.; Gregory, W.J.; Gunawardena, H.; et al. British Society for Rheumatology guideline on management of paediatric, adolescent and adult patients with idiopathic inflammatory myopathy. Rheumatology 2022, 61, 1760–1768. [Google Scholar] [CrossRef] [PubMed]
- Burg, E.; Sharbin, G.; Edeson, M.E.; Hosseini, H.K.; Westman, C.; Hosseini, D.K.; Patel, S.; Weinberger, J.D.; Zhu, H.; Ligresti, R. Transverse myelitis as a rare paraneoplastic manifestation of a pancreatic tail adenocarcinoma: A case report. Medicine 2025, 104, e42133. [Google Scholar] [CrossRef] [PubMed]
| Biomarker | Clinical Use | Performance |
|---|---|---|
| CEA-19 | approved by FDA | low specificity and sensitivity, used to monitor treatment response |
| glucose-dependent insulinotropic peptide | investigational | differential diagnosis with T2DM: lower in pancreatic cancer irrespective of the degree of glucose intolerance as compared to T2DM patients and healthy controls |
| pancreatic polypeptides | investigational | differential diagnosis with T2DM: lower in pancreatic cancer irrespective of the degree of glucose intolerance as compared to T2DM patients and healthy controls |
| tenascin C | investigational | locoregional recurrence-related poor prognosis marker, potential therapeutic target for PDAC |
| antibodies against apolipoprotein-AII isoforms | investigational | not useful for evaluation of clinical effect of CRT for PDAC; useful for assessment of pancreatic exocrine disorder; possible use in screening for the early stage of pancreatic cancer and, what is more, even identifying patients at risk for pancreatic malignancy |
| serum lipid profile | investigational | sensitivity and specificity over 90%, higher than those of CA 19-9, especially at an early stage, and comparable to established diagnostic imaging methods. What is more, selected lipid species indicate potential as a prognostic biomarker |
| circulating levels of galectin-1 | investigational | sensitivity and specificity values similar to those of CA19-9; combination of galectin-1 and CA19-9 significantly improved their individual discriminatory powers; promising biomarker not only for detection but also for prognostics |
| osteonectin | investigational | possible PDAC screening marker that must be validated in prospective studies |
| LYVE-1, REG1A, and TFF1 | clinical trials | triple protein test in urine, detecting early-stage PDAC: sensitivity of 96%, 100%, and 73.33%, respectively, and specificity of 100%, 82%, and 100%, respectively |
| circulating cell-free nucleatic acids: DNA, mRNA, non-coding RNA (miRNA and lncRNA) | clinical trials | high specificity for specific mutations, low sensitivity in early-stage cancers |
| hub gene S100A6 | investigational | immune-related and potential therapeutic target for patients with PC and T2DM |
| Syndrome | Prevalence | Mechanism | Diagnosis and Management | Prognostic Significance |
|---|---|---|---|---|
| Trousseau’s syndrome | venous thromboembolism is 4- to 7-fold higher in patients with cancer than in those without cancer [160] | tissue factor, thrombin, cancer procoagulant, tissue hypoxia, and mucin overproduction by PDAC cells lead to platelet activation and aggregation without involving thrombin | effective prophylaxis and treatment (first line—low-molecular-weight heparin) reduces morbidity and mortality | high risk of thromboembolic events, worse prognosis |
| panniculitis | 0.3–3.0% of pancreatic disorders | pancreatic enzymes and their activity in adipose tissue | imaging studies, skin biopsy, serum pancreatic enzyme levels, and detection of tumor markers, treatment of underlying malignancy, octreotide | could delay treatment of malignancy because of possible misdiagnosis as infection or rheumatologic disease [161] |
| PPP syndrome | PDAC is present in 11.9% of PPP syndrome cases [162,163]; arthritis is present in 35% of cases with PDAC and PPP syndrome [164] | pancreatic enzymes hyperactivity | imaging studies, skin biopsy, serum pancreatic enzyme levels, and detection of tumor markers, treatment of underlying malignancy | late diagnosis of underlying pancreatitis often results in a worse prognosis and inappropriate treatment [165] |
| PFPAS | extremely rare; 48 cases since first report described in the literature [166] | connective tissue growth factors and autoimmune mechanisms induced by the neoplasm | corticosteroids, treatment of underlying malignancy | unknown |
| dermatomyositis and polymyositis | 14 per 100,000 [167] | anti-cancer immune response triggered by similar antigens found in pancreatic tumors and regenerating muscle | muscle biopsy, serum auto-antibody levels, electromyography, treatment with glucocorticoids | dermatomyositis is linked to a higher risk and worse survival rates compared to polymyositis; they both worsen the prognosis |
| transverse myelitis | 1 to 8 individuals per million | autoimmune inflammation | treatment of the underlying malignancy | unknown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Włoszek, E.; Krupa, K.; Fudalej, M.; Miski, H.; Badowska-Kozakiewicz, A.M.; Deptała, A. Unusual Manifestations of Primary Pancreatic Neoplasia. Cancers 2025, 17, 3240. https://doi.org/10.3390/cancers17193240
Włoszek E, Krupa K, Fudalej M, Miski H, Badowska-Kozakiewicz AM, Deptała A. Unusual Manifestations of Primary Pancreatic Neoplasia. Cancers. 2025; 17(19):3240. https://doi.org/10.3390/cancers17193240
Chicago/Turabian StyleWłoszek, Emilia, Kamila Krupa, Marta Fudalej, Hanna Miski, Anna M. Badowska-Kozakiewicz, and Andrzej Deptała. 2025. "Unusual Manifestations of Primary Pancreatic Neoplasia" Cancers 17, no. 19: 3240. https://doi.org/10.3390/cancers17193240
APA StyleWłoszek, E., Krupa, K., Fudalej, M., Miski, H., Badowska-Kozakiewicz, A. M., & Deptała, A. (2025). Unusual Manifestations of Primary Pancreatic Neoplasia. Cancers, 17(19), 3240. https://doi.org/10.3390/cancers17193240






