The Fanconi Anemia Pathway Inhibits mTOR Signaling and Prevents Accelerated Translation in Head and Neck Cancer Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
2.2. Metabolomic Analysis
2.2.1. Cell Collection and Metabolite Extraction
2.2.2. Nuclear Magnetic Resonance (NMR) Spectroscopy and Data Analysis
2.2.3. Mass Spectrometry (MS) and Data Analysis
2.3. Translation and Proliferation Assays
2.4. Immunofluorescence (IF)
2.5. BCA and Western Blot Analysis
2.6. CellTiter-Fluor Viability Assay
2.7. Statstical Analysis
3. Results
3.1. FA Pathway Loss in HNSCC Cells Increases Total Protein and Amino Acid Levels
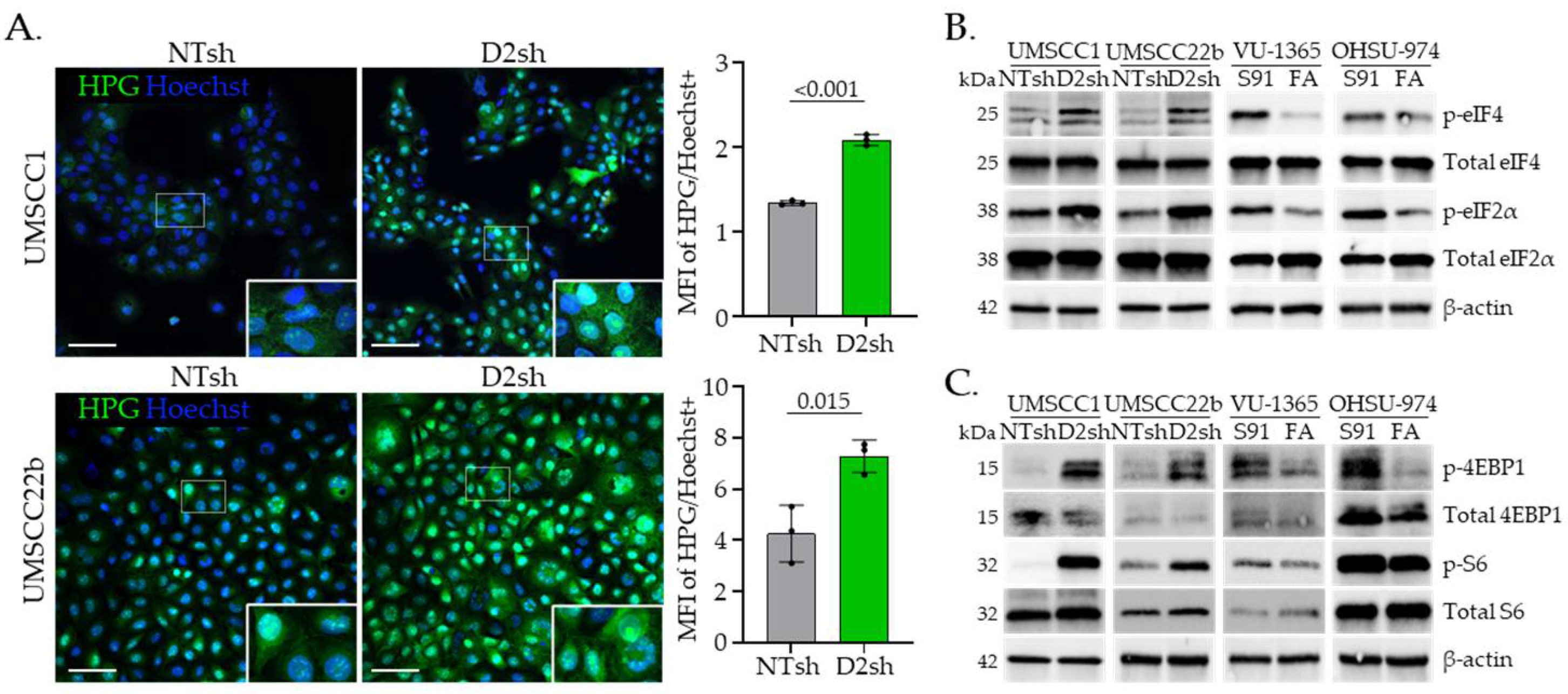
3.2. FA Pathway Loss Increases Translation and Activates mTOR Signaling in HNSCC Cells
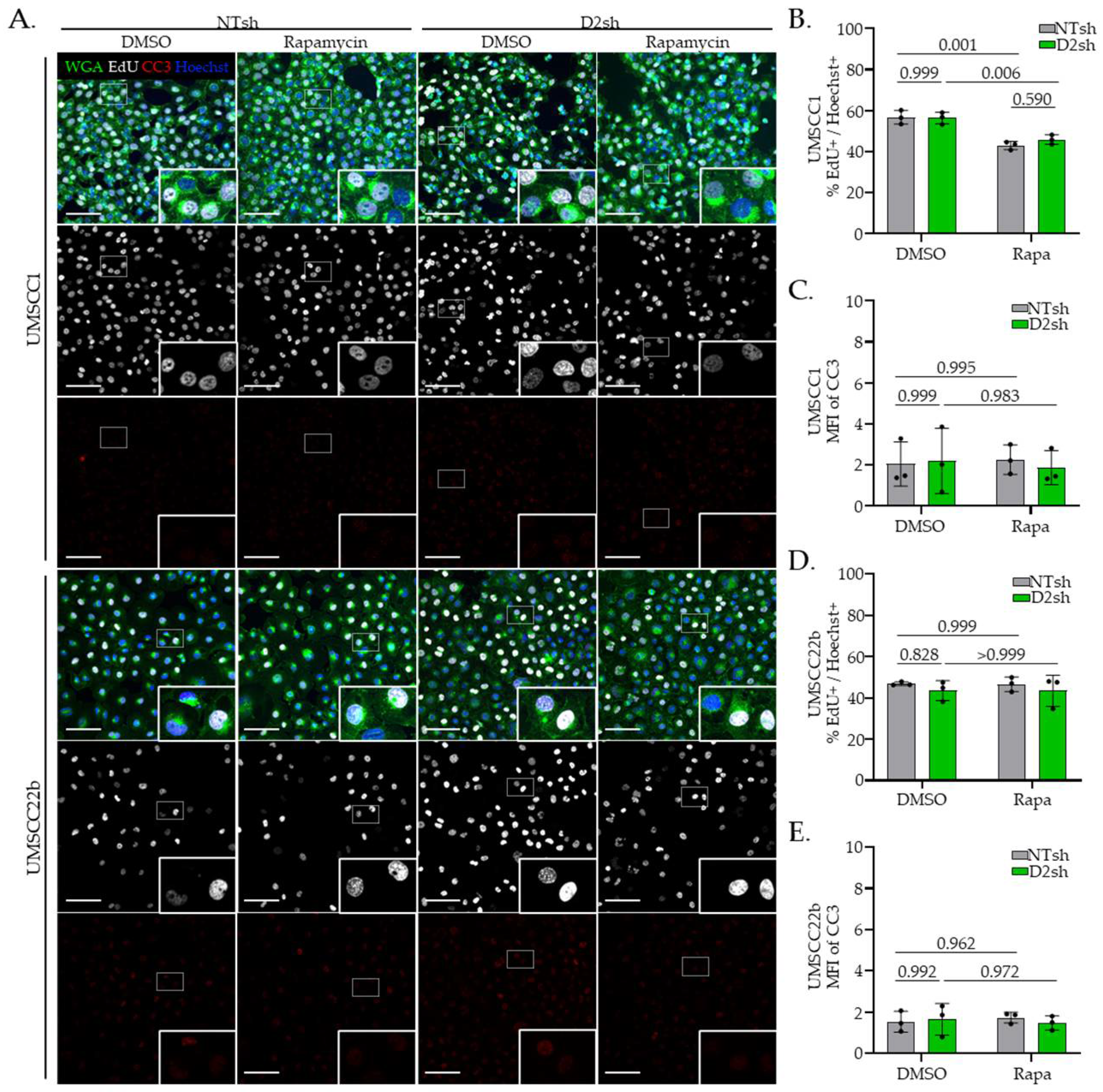
3.3. Rapamycin Suppresses Protein Translation in FA− Deficient HNSCC Cells
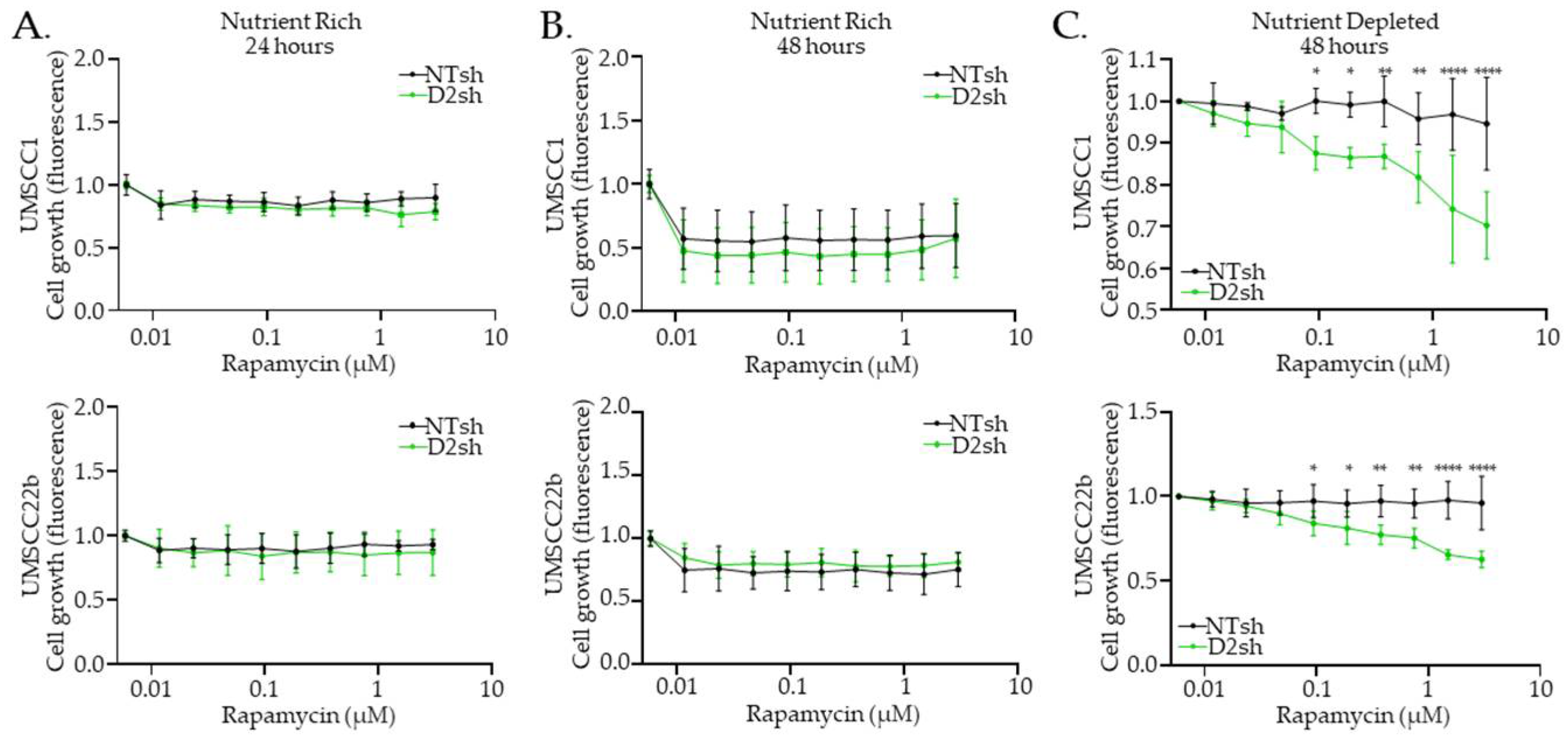
3.4. Rapamycin Inhibits FA− HNS Cell Growth Under Nutrient-Depleted Conditions
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| 4E-BP1 | 4E binding protein 1 |
| AML | Acute myeloid leukemia |
| ANOVA | Analysis of variance |
| BCA | Bicinchoninic acid |
| BSA | Bovine serum albumin |
| CC3 | Cleaved caspase-3 |
| D2sh | Short-hairpin RNA-treated cells targeted for FANCD2 |
| DDR | DNA damage response |
| EdU | 5-ethynyl-2′-deoxyuridine; a nucleotide analog used to quantify cellular proliferation |
| eIF4/2 | Eukaryotic initiation factor-4 or -2 |
| EMT | Epithelial-to-mesenchymal transition |
| ESI | Electrospray ionization |
| FA | Fanconi anemia; a genetic disease and DNA repair pathway |
| FA+ | Fanconi anemia pathway-proficient; indicates cells have a functional FA pathway |
| FA− | Fanconi anemia pathway-deficient; indicates cells have a nonfunctional FA pathway |
| GSD | Global spectra deconvolution |
| HMDB | Human Metabolome Database |
| HNSCC | Head and neck squamous cell carcinoma |
| HPG | L-homopropargylglycine; a methionine analog used to quantify cellular translation |
| HR | Homologous recombination |
| HSQC | Heteronuclear single quantum correlation |
| ICL | DNA interstrand crosslinks |
| ID2 kDa | The FANCI/FANCD2 intermediate complex in the Fanconi anemia pathway kilodalton |
| mTOR | Mechanistic target of rapamycin |
| MS | Mass spectrometry |
| NIKS | Normal immortalized keratinocytes |
| NMR | Nuclear magnetic resonance |
| NOKS | Normal oral mucosal keratinocytes |
| NTsh | Non-targeting short-hairpin RNA-treated cells |
| PBS | Phosphate-buffered saline |
| PFA | Paraformaldehyde |
| PPARγ | Peroxisome proliferator-activated receptor-γ |
| RIPA | Radioimmunoprecipitation assay |
| SCC | Squamous cell carcinoma |
| TBST | Tris-buffered saline with Tween 20 |
| TSP | Trimethylsilyl propionic acid-d4 sodium salt |
| UHPLC | Ultra-high performance liquid chromatography |
| WGA | Wheat germ agglutinin |
References
- Niraj, J.; Färkkilä, A.; D’Andrea, A.D. The Fanconi Anemia Pathway in Cancer. Annu. Rev. Cancer Biol. 2019, 3, 457–478. [Google Scholar] [CrossRef]
- Harrison, B.A.; Mizrahi-Powell, E.; Pappas, J.; Thomas, K.; Vasishta, S.; Hebbar, S.; Shukla, A.; Nayak, S.S.; Truong, T.K.; Woroch, A.; et al. Deficiency of the Fanconi anemia core complex protein FAAP100 results in severe Fanconi anemia. J. Clin. Investig. 2025, 135, e185126. [Google Scholar] [CrossRef]
- Smogorzewska, A.; Matsuoka, S.; Vinciguerra, P.; McDonald, E.R.; Hurov, K.E.; Luo, J.; Ballif, B.A.; Gygi, S.P.; Hofmann, K.; D’Andrea, A.D.; et al. Identification of the FANCI protein, a monoubiquitinated FANCD2 paralog required for DNA repair. Cell 2007, 129, 289–301. [Google Scholar] [CrossRef]
- Tan, W.; van Twest, S.; Leis, A.; Bythell-Douglas, R.; Murphy, V.J.; Sharp, M.; Parker, M.W.; Crismani, W.; Deans, A.J. Monoubiquitination by the human Fanconi anemia core complex clamps FANCI:FANCD2 on DNA in filamentous arrays. Elife 2020, 9, e54128. [Google Scholar] [CrossRef]
- Timmers, C.; Taniguchi, T.; Hejna, J.; Reifsteck, C.; Lucas, L.; Bruun, D.; Thayer, M.; Cox, B.; Olson, S.; D’Andrea, A.D.; et al. Positional cloning of a novel Fanconi anemia gene, FANCD2. Mol. Cell 2001, 7, 241–248. [Google Scholar] [CrossRef]
- Kim, H.; D’Andrea, A.D. Regulation of DNA cross-link repair by the Fanconi anemia/BRCA pathway. Genes Dev. 2012, 26, 1393–1408. [Google Scholar] [CrossRef]
- Long, D.T.; Räschle, M.; Joukov, V.; Walter, J.C. Mechanism of RAD51-dependent DNA interstrand cross-link repair. Science 2011, 333, 84–87. [Google Scholar] [CrossRef]
- Nakanishi, K.; Yang, Y.G.; Pierce, A.J.; Taniguchi, T.; Digweed, M.; D’Andrea, A.D.; Wang, Z.Q.; Jasin, M. Human Fanconi anemia monoubiquitination pathway promotes homologous DNA repair. Proc. Natl. Acad. Sci. USA 2005, 102, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Pace, P.; Mosedale, G.; Hodskinson, M.R.; Rosado, I.V.; Sivasubramaniam, M.; Patel, K.J. Ku70 corrupts DNA repair in the absence of the Fanconi anemia pathway. Science 2010, 329, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Adamo, A.; Collis, S.J.; Adelman, C.A.; Silva, N.; Horejsi, Z.; Ward, J.D.; Martinez-Perez, E.; Boulton, S.J.; La Volpe, A. Preventing nonhomologous end joining suppresses DNA repair defects of Fanconi anemia. Mol. Cell 2010, 39, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Dan, C.; Pei, H.; Zhang, B.; Ran, D.; Du, C. Fanconi anemia pathway and its relationship with cancer. Genome Instab. Dis. 2021, 2, 175–183. [Google Scholar] [CrossRef]
- Webster, A.L.H.; Sanders, M.A.; Patel, K.; Dietrich, R.; Noonan, R.J.; Lach, F.P.; White, R.R.; Goldfarb, A.; Hadi, K.; Edwards, M.M.; et al. Genomic signature of Fanconi anaemia DNA repair pathway deficiency in cancer. Nature 2022, 612, 495–502. [Google Scholar] [CrossRef]
- Hoover, A.; Turcotte, L.M.; Phelan, R.; Barbus, C.; Rayannavar, A.; Miller, B.S.; Reardon, E.E.; Theis-Mahon, N.; MacMillan, M.L. Longitudinal clinical manifestations of Fanconi anemia: A systematized review. Blood Rev. 2024, 68, 101225. [Google Scholar] [CrossRef]
- Kutler, D.I.; Auerbach, A.D.; Satagopan, J.; Giampietro, P.F.; Batish, S.D.; Huvos, A.G.; Goberdhan, A.; Shah, J.P.; Singh, B. High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, P.S.; Greene, M.H.; Alter, B.P. Cancer incidence in persons with Fanconi anemia. Blood 2003, 101, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, P.S.; Alter, B.P.; Ebell, W. Cancer risks in Fanconi anemia: Findings from the German Fanconi Anemia Registry. Haematologica 2008, 93, 511–517. [Google Scholar] [CrossRef]
- Velleuer, E.; Dietrich, R. Fanconi anemia: Young patients at high risk for squamous cell carcinoma. Mol. Cell Pediatr. 2014, 1, 9. [Google Scholar] [CrossRef]
- Beddok, A.; Krieger, S.; Castera, L.; Stoppa-Lyonnet, D.; Thariat, J. Management of Fanconi Anemia patients with head and neck carcinoma: Diagnosis and treatment adaptation. Oral Oncol. 2020, 108, 104816. [Google Scholar] [CrossRef]
- Kutler, D.I.; Patel, K.R.; Auerbach, A.D.; Kennedy, J.; Lach, F.P.; Sanborn, E.; Cohen, M.A.; Kuhel, W.I.; Smogorzewska, A. Natural history and management of Fanconi anemia patients with head and neck cancer: A 10-year follow-up. Laryngoscope 2016, 126, 870–879. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.; Wu, Z. Fanconi anemia pathway defects in inherited and sporadic cancers. Transl. Pediatr. 2014, 3, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Romick-Rosendale, L.E.; Lui, V.W.Y.; Grandis, J.R.; Wells, S.I. The Fanconi anemia pathway: Repairing the link between DNA damage and squamous cell carcinoma. Mutat. Res. 2013, 743–744, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Stecklein, S.R.; Jensen, R.A. Identifying and exploiting defects in the Fanconi anemia/BRCA pathway in oncology. Transl. Res. 2012, 160, 178–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Palovcak, A.; Li, F.; Zafar, A.; Yuan, F.; Zhang, Y. Fanconi anemia pathway as a prospective target for cancer intervention. Cell Biosci. 2020, 10, 39. [Google Scholar] [CrossRef]
- Romick-Rosendale, L.E.; Hoskins, E.E.; Privette Vinnedge, L.M.; Foglesong, G.D.; Brusadelli, M.G.; Potter, S.S.; Komurov, K.; Brugmann, S.A.; Lambert, P.F.; Kimple, R.J.; et al. Defects in the Fanconi Anemia Pathway in Head and Neck Cancer Cells Stimulate Tumor Cell Invasion through DNA-PK and Rac1 Signaling. Clin. Cancer Res. 2016, 22, 2062–2073. [Google Scholar] [CrossRef]
- Kottemann, M.C.; Smogorzewska, A. Fanconi anaemia and the repair of Watson and Crick DNA crosslinks. Nature 2013, 493, 356–363. [Google Scholar] [CrossRef]
- Wang, S.; Ding, B.; Cui, M.; Yan, W.; Xia, Q.; Meng, D.; Shen, S.; Xie, S.; Jin, H.; Zhang, X. Fanconi Anemia Pathway Genes Advance Cervical Cancer. Front. Cell Dev. Biol. 2021, 9, 734794. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; He, X.; Huang, C.; Li, J.; Dong, Z.; Liu, K. Protein translation: Biological processes and therapeutic strategies for human diseases. Signal Transduct. Target. Ther. 2024, 9, 44. [Google Scholar]
- Gulhati, P.; Bowen, K.A.; Liu, J.; Stevens, P.D.; Rychahou, P.G.; Chen, M.; Lee, E.Y.; Weiss, H.L.; O’Connor, K.L.; Gao, T.; et al. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011, 71, 3246–3256. [Google Scholar] [CrossRef]
- Lamouille, S.; Connolly, E.; Smyth, J.W.; Akhurst, R.J.; Derynck, R. TGF-β-induced activation of mTOR complex 2 drives epithelial-mesenchymal transition and cell invasion. J. Cell Sci. 2012, 125, 1259–1273. [Google Scholar] [CrossRef]
- Szwed, A.; Kim, E.; Jacinto, E. Regulation and metabolic functions of mTORC1 and mTORC2. Physiol. Rev. 2021, 101, 1371–1426. [Google Scholar] [CrossRef]
- Dowling, R.J.; Topisirovic, I.; Alain, T.; Bidinosti, M.; Fonseca, B.D.; Petroulakis, E.; Wang, X.; Larsson, O.; Selvaraj, A.; Liu, Y.; et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science 2010, 328, 1172–1176. [Google Scholar] [CrossRef]
- Khaleghpour, K.; Pyronnet, S.; Gingras, A.C.; Sonenberg, N. Translational homeostasis: Eukaryotic translation initiation factor 4E control of 4E-binding protein 1 and p70 S6 kinase activities. Mol. Cell Biol. 1999, 19, 4302–4310. [Google Scholar] [CrossRef]
- Kimple, R.J.; Harari, P.M.; Torres, A.D.; Yang, R.Z.; Soriano, B.J.; Yu, M.; Armstrong, E.A.; Blitzer, G.C.; Smith, M.A.; Lorenz, L.D.; et al. Development and characterization of HPV-positive and HPV-negative head and neck squamous cell carcinoma tumorgrafts. Clin. Cancer Res. 2013, 19, 855–864. [Google Scholar] [CrossRef]
- Hoskins, E.E.; Morris, T.A.; Higginbotham, J.M.; Spardy, N.; Cha, E.; Kelly, P.; Williams, D.A.; Wikenheiser-Brokamp, K.A.; Duensing, S.; Wells, S.I. Fanconi anemia deficiency stimulates HPV-associated hyperplastic growth in organotypic epithelial raft culture. Oncogene 2009, 28, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Flores, E.R.; Allen-Hoffmann, B.L.; Lee, D.; Sattler, C.A.; Lambert, P.F. Establishment of the human papillomavirus type 16 (HPV-16) life cycle in an immortalized human foreskin keratinocyte cell line. Virology 1999, 262, 344–354. [Google Scholar] [CrossRef][Green Version]
- Zhao, X.; Brusadelli, M.G.; Sauter, S.; Butsch Kovacic, M.; Zhang, W.; Romick-Rosendale, L.E.; Lambert, P.F.; Setchell, K.D.R.; Wells, S.I. Lipidomic Profiling Links the Fanconi Anemia Pathway to Glycosphingolipid Metabolism in Head and Neck Cancer Cells. Clin. Cancer Res. 2018, 24, 2700–2709. [Google Scholar] [CrossRef]
- Fan, T.W.; Lorkiewicz, P.K.; Sellers, K.; Moseley, H.N.; Higashi, R.M.; Lane, A.N. Stable isotope-resolved metabolomics and applications for drug development. Pharmacol. Ther. 2012, 133, 366–391. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Ulrich, E.L.; Akutsu, H.; Doreleijers, J.F.; Harano, Y.; Ioannidis, Y.E.; Lin, J.; Livny, M.; Mading, S.; Maziuk, D.; Miller, Z.; et al. BioMagResBank. Nucleic Acids Res. 2007, 36, D402–D408. [Google Scholar] [CrossRef]
- Fan, T.W.M.; Lane, A.N. Structure-based profiling of metabolites and isotopomers by NMR. Prog. Nucl. Magn. Reson. Spectrosc. 2008, 52, 69–117. [Google Scholar] [CrossRef]
- Fan, T.W.M. Metabolite profiling by one- and two-dimensional NMR analysis of complex mixtures. Prog. Nucl. Magn. Reson. Spectrosc. 1996, 28, 161–219. [Google Scholar] [CrossRef]
- Adusumilli, R.; Mallick, P. Data Conversion with ProteoWizard msConvert. Methods Mol. Biol. 2017, 1550, 339–368. [Google Scholar] [CrossRef]
- Agrawal, S.; Kumar, S.; Sehgal, R.; George, S.; Gupta, R.; Poddar, S.; Jha, A.; Pathak, S. El-MAVEN: A Fast, Robust, and User-Friendly Mass Spectrometry Data Processing Engine for Metabolomics. Methods Mol. Biol. 2019, 1978, 301–321. [Google Scholar] [CrossRef]
- Clasquin, M.F.; Melamud, E.; Rabinowitz, J.D. LC-MS data processing with MAVEN: A metabolomic analysis and visualization engine. Curr. Protoc. Bioinform. 2012, Chapter 14, Unit14.11. [Google Scholar] [CrossRef]
- Melamud, E.; Vastag, L.; Rabinowitz, J.D. Metabolomic analysis and visualization engine for LC-MS data. Anal. Chem. 2010, 82, 9818–9826. [Google Scholar] [CrossRef]
- Smith, R.C.L.; Kanellos, G.; Vlahov, N.; Alexandrou, C.; Willis, A.E.; Knight, J.R.P.; Sansom, O.J. Translation initiation in cancer at a glance. J. Cell Sci. 2021, 134, jcs248476. [Google Scholar] [CrossRef] [PubMed]
- Mounir, Z.; Krishnamoorthy, J.L.; Robertson, G.P.; Scheuner, D.; Kaufman, R.J.; Georgescu, M.M.; Koromilas, A.E. Tumor suppression by PTEN requires the activation of the PKR-eIF2alpha phosphorylation pathway. Sci. Signal. 2009, 2, ra85. [Google Scholar] [CrossRef] [PubMed]
- Truitt, M.L.; Conn, C.S.; Shi, Z.; Pang, X.; Tokuyasu, T.; Coady, A.M.; Seo, Y.; Barna, M.; Ruggero, D. Differential Requirements for eIF4E Dose in Normal Development and Cancer. Cell 2015, 162, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Mamane, Y.; Petroulakis, E.; LeBacquer, O.; Sonenberg, N. mTOR, translation initiation and cancer. Oncogene 2006, 25, 6416–6422. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR Signaling in Growth Control and Disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Hua, H.; Kong, Q.; Zhang, H.; Wang, J.; Luo, T.; Jiang, Y. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 2019, 12, 71. [Google Scholar] [CrossRef]
- Chandrasekharappa, S.C.; Chinn, S.B.; Donovan, F.X.; Chowdhury, N.I.; Kamat, A.; Adeyemo, A.A.; Thomas, J.W.; Vemulapalli, M.; Hussey, C.S.; Reid, H.H.; et al. Assessing the spectrum of germline variation in Fanconi anemia genes among patients with head and neck carcinoma before age 50. Cancer 2017, 123, 3943–3954. [Google Scholar] [CrossRef]
- Sumpter, R.; Levine, B. Emerging functions of the Fanconi anemia pathway at a glance. J. Cell Sci. 2017, 130, 2657–2662. [Google Scholar] [CrossRef]
- Schwab, R.A.; Nieminuszczy, J.; Shah, F.; Langton, J.; Lopez Martinez, D.; Liang, C.C.; Cohn, M.A.; Gibbons, R.J.; Deans, A.J.; Niedzwiedz, W. The Fanconi Anemia Pathway Maintains Genome Stability by Coordinating Replication and Transcription. Mol. Cell 2015, 60, 351–361. [Google Scholar] [CrossRef]
- Sumpter, R.; Sirasanagandla, S.; Fernández, Á.; Wei, Y.; Dong, X.; Franco, L.; Zou, Z.; Marchal, C.; Lee, M.Y.; Clapp, D.W.; et al. Fanconi Anemia Proteins Function in Mitophagy and Immunity. Cell 2016, 165, 867–881. [Google Scholar] [CrossRef]
- Chihanga, T.; Vicente-Muñoz, S.; Ruiz-Torres, S.; Pal, B.; Sertorio, M.; Andreassen, P.R.; Khoury, R.; Mehta, P.; Davies, S.M.; Lane, A.N.; et al. Head and Neck Cancer Susceptibility and Metabolism in Fanconi Anemia. Cancers 2022, 14, 2040. [Google Scholar] [CrossRef]
- Reuter, T.Y.; Medhurst, A.L.; Waisfisz, Q.; Zhi, Y.; Herterich, S.; Hoehn, H.; Gross, H.J.; Joenje, H.; Hoatlin, M.E.; Mathew, C.G.; et al. Yeast two-hybrid screens imply involvement of Fanconi anemia proteins in transcription regulation, cell signaling, oxidative metabolism, and cellular transport. Exp. Cell Res. 2003, 289, 211–221. [Google Scholar] [CrossRef]
- Langevin, F.; Crossan, G.P.; Rosado, I.V.; Arends, M.J.; Patel, K.J. Fancd2 counteracts the toxic effects of naturally produced aldehydes in mice. Nature 2011, 475, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, X.; Lin, Q.; Chowdhury, F.; Mazumder, M.H.; Du, W. FANCD2 and HES1 suppress inflammation-induced PPARɣ to prevent haematopoietic stem cell exhaustion. Br. J. Haematol. 2021, 192, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Sancak, Y.; Sabatini, D.M. Rag proteins regulate amino-acid-induced mTORC1 signalling. Biochem. Soc. Trans. 2009, 37, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Takahara, T.; Amemiya, Y.; Sugiyama, R.; Maki, M.; Shibata, H. Amino acid-dependent control of mTORC1 signaling: A variety of regulatory modes. J. Biomed. Sci. 2020, 27, 87. [Google Scholar] [CrossRef]
- Jewell, J.L.; Kim, Y.C.; Russell, R.C.; Yu, F.X.; Park, H.W.; Plouffe, S.W.; Tagliabracci, V.S.; Guan, K.L. Metabolism. Differential regulation of mTORC1 by leucine and glutamine. Science 2015, 347, 194–198. [Google Scholar] [CrossRef]
- Gulhati, P.; Cai, Q.; Li, J.; Liu, J.; Rychahou, P.G.; Qiu, S.; Lee, E.Y.; Silva, S.R.; Bowen, K.A.; Gao, T.; et al. Targeted inhibition of mammalian target of rapamycin signaling inhibits tumorigenesis of colorectal cancer. Clin. Cancer Res. 2009, 15, 7207–7216. [Google Scholar] [CrossRef]
- Cheng, K.Y.; Hao, M. Mammalian Target of Rapamycin (mTOR) Regulates Transforming Growth Factor-β1 (TGF-β1)-Induced Epithelial-Mesenchymal Transition via Decreased Pyruvate Kinase M2 (PKM2) Expression in Cervical Cancer Cells. Med. Sci. Monit. 2017, 23, 2017–2028. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.L.; Ding, R.; Sharma, V.K.; Chon, W.J.; Lagman, M.; Suthanthiran, M. Rapamycin is an effective inhibitor of human renal cancer metastasis. Kidney Int. 2003, 63, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Gremke, N.; Polo, P.; Dort, A.; Schneikert, J.; Elmshäuser, S.; Brehm, C.; Klingmüller, U.; Schmitt, A.; Reinhardt, H.C.; Timofeev, O.; et al. mTOR-mediated cancer drug resistance suppresses autophagy and generates a druggable metabolic vulnerability. Nat. Commun. 2020, 11, 4684. [Google Scholar] [CrossRef]
- Si, Y.; Chu, H.; Zhu, W.; Xiao, T.; Shen, X.; Fu, Y.; Xu, R.; Jiang, H. Concentration-dependent effects of rapamycin on proliferation, migration and apoptosis of endothelial cells in human venous malformation. Exp. Ther. Med. 2018, 16, 4595–4601. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Chatterjee, A.; Kogan, D.; Patel, D.; Foster, D.A. 5-Aminoimidazole-4-carboxamide-1-β-4-ribofuranoside (AICAR) enhances the efficacy of rapamycin in human cancer cells. Cell Cycle 2015, 14, 3331–3339. [Google Scholar] [CrossRef]
- Yellen, P.; Saqcena, M.; Salloum, D.; Feng, J.; Preda, A.; Xu, L.; Rodrik-Outmezguine, V.; Foster, D.A. High-dose rapamycin induces apoptosis in human cancer cells by dissociating mTOR complex 1 and suppressing phosphorylation of 4E-BP1. Cell Cycle 2011, 10, 3948–3956. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. Regulation of mTORC1 and its impact on gene expression at a glance. J. Cell Sci. 2013, 126, 1713–1719. [Google Scholar] [CrossRef]
- Schreiber, K.H.; Ortiz, D.; Academia, E.C.; Anies, A.C.; Liao, C.Y.; Kennedy, B.K. Rapamycin-mediated mTORC2 inhibition is determined by the relative expression of FK506-binding proteins. Aging Cell 2015, 14, 265–273. [Google Scholar] [CrossRef]
- Li, J.; Kim, S.G.; Blenis, J. Rapamycin: One drug, many effects. Cell Metab. 2014, 19, 373–379. [Google Scholar] [CrossRef]
- Dang, C.V.; Semenza, G.L. Oncogenic alterations of metabolism. Trends Biochem. Sci. 1999, 24, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Moscat, J.; Richardson, A.; Diaz-Meco, M.T. Nutrient stress revamps cancer cell metabolism. Cell Res. 2015, 25, 537–538. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vernieri, C.; Fucà, G.; Ligorio, F.; Huber, V.; Vingiani, A.; Iannelli, F.; Raimondi, A.; Rinchai, D.; Frigè, G.; Belfiore, A.; et al. Fasting-Mimicking Diet Is Safe and Reshapes Metabolism and Antitumor Immunity in Patients with Cancer. Cancer Discov. 2022, 12, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Naughton, K.J.; Song, X.; Childress, A.R.; Skaggs, E.M.; Byrd, A.L.; Gosser, C.M.; Esoe, D.P.; DuCote, T.J.; Plaugher, D.R.; Lukyanchuk, A.; et al. Methionine Restriction Reduces Lung Cancer Progression and Increases Chemotherapy Response. bioRxiv 2024. [Google Scholar] [CrossRef]
- Müller-Richter, U.; Betz, C.; Hartmann, S.; Brands, R.C. Nutrition management for head and neck cancer patients improves clinical outcome and survival. Nutr. Res. 2017, 48, 1–8. [Google Scholar] [CrossRef]
- Luan, C.-W.; Tsai, Y.-T.; Yang, H.-Y.; Chen, K.-Y.; Chen, P.-H.; Chou, H.-H. Pretreatment prognostic nutritional index as a prognostic marker in head and neck cancer: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 17117. [Google Scholar] [CrossRef]
- Saroul, N.; Puechmaille, M.; Lambert, C.; Hassan, A.S.; Biau, J.; Lapeyre, M.; Mom, T.; Bernadach, M.; Gilain, L. Prognosis in Head and Neck Cancer: Importance of Nutritional and Biological Inflammatory Status. Otolaryngol. Head Neck Surg. 2022, 166, 118–127. [Google Scholar] [CrossRef]
- Velleuer, E.; Carlberg, C. A Nutrigenomic View on the Premature-Aging Disease Fanconi Anemia. Nutrients 2024, 16, 2271. [Google Scholar] [CrossRef]
- Li, Y.; Liu, F.; Wang, Y.; Li, D.; Guo, F.; Xu, L.; Zeng, Z.; Zhong, X.; Qian, K. Rapamycin-induced autophagy sensitizes A549 cells to radiation associated with DNA damage repair inhibition. Thorac. Cancer 2016, 7, 379–386. [Google Scholar] [CrossRef]
- Chen, H.; Ma, Z.; Vanderwaal, R.P.; Feng, Z.; Gonzalez-Suarez, I.; Wang, S.; Zhang, J.; Roti Roti, J.L.; Gonzalo, S. The mTOR inhibitor rapamycin suppresses DNA double-strand break repair. Radiat. Res. 2011, 175, 214–224. [Google Scholar] [CrossRef]
- Kovuru, N.; Mochizuki-Kashio, M.; Menna, T.; Jeffrey, G.; Hong, Y.; me Yoon, Y.; Zhang, Z.; Kurre, P. Deregulated protein homeostasis constrains fetal hematopoietic stem cell pool expansion in Fanconi anemia. Nat. Commun. 2024, 15, 1852. [Google Scholar] [CrossRef] [PubMed]
- Nalepa, G.; Clapp, D.W. Fanconi anaemia and cancer: An intricate relationship. Nat. Rev. Cancer 2018, 18, 168–185. [Google Scholar] [CrossRef]
- Garcia-Higuera, I.; Taniguchi, T.; Ganesan, S.; Meyn, M.S.; Timmers, C.; Hejna, J.; Grompe, M.; D’Andrea, A.D. Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Mol. Cell 2001, 7, 249–262. [Google Scholar] [CrossRef]
- Pourmasoumi, P.; Moradi, A.; Bayat, M. BRCA1/2 Mutations and Breast/Ovarian Cancer Risk: A New Insights Review. Reprod. Sci. 2024, 31, 3624–3634. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Nandi, S. DNA damage response and cancer therapeutics through the lens of the Fanconi Anemia DNA repair pathway. Cell Commun. Signal. 2017, 15, 41. [Google Scholar] [CrossRef]
- Abuetabh, Y.; Wu, H.H.; Chai, C.; Al Yousef, H.; Persad, S.; Sergi, C.M.; Leng, R. DNA damage response revisited: The p53 family and its regulators provide endless cancer therapy opportunities. Exp. Mol. Med. 2022, 54, 1658–1669. [Google Scholar] [CrossRef]
- Ceccaldi, R.; Parmar, K.; Mouly, E.; Delord, M.; Kim, J.M.; Regairaz, M.; Pla, M.; Vasquez, N.; Zhang, Q.S.; Pondarre, C.; et al. Bone marrow failure in Fanconi anemia is triggered by an exacerbated p53/p21 DNA damage response that impairs hematopoietic stem and progenitor cells. Cell Stem Cell 2012, 11, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Tombari, C.; Zannini, A.; Bertolio, R.; Pedretti, S.; Audano, M.; Triboli, L.; Cancila, V.; Vacca, D.; Caputo, M.; Donzelli, S.; et al. Mutant p53 sustains serine-glycine synthesis and essential amino acids intake promoting breast cancer growth. Nat. Commun. 2023, 14, 6777. [Google Scholar] [CrossRef]
- Nolan, M.; Knudson, K.; Holz, M.K.; Chaudhury, I. Fanconi anemia and mTOR pathways functionally interact during stalled replication fork recovery. FEBS Lett. 2021, 595, 595–603. [Google Scholar] [CrossRef]
- Guo, F. Mtor-Fanconi Anemia DNA Damage Repair Pathway in Cancer. J. Oncobiomark. 2014, 2, 5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guo, F.; Li, J.; Du, W.; Zhang, S.; O’Connor, M.; Thomas, G.; Kozma, S.; Zingarelli, B.; Pang, Q.; Zheng, Y. mTOR regulates DNA damage response through NF-κB-mediated FANCD2 pathway in hematopoietic cells. Leukemia 2013, 27, 2040–2046. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruffolo, B.; Vicente-Muñoz, S.; Mehta, K.Y.; Rivera-Cruz, C.M.; Zhao, X.; Romick, L.; Setchell, K.D.R.; Lane, A.; Wells, S.I. The Fanconi Anemia Pathway Inhibits mTOR Signaling and Prevents Accelerated Translation in Head and Neck Cancer Cells. Cancers 2025, 17, 2583. https://doi.org/10.3390/cancers17152583
Ruffolo B, Vicente-Muñoz S, Mehta KY, Rivera-Cruz CM, Zhao X, Romick L, Setchell KDR, Lane A, Wells SI. The Fanconi Anemia Pathway Inhibits mTOR Signaling and Prevents Accelerated Translation in Head and Neck Cancer Cells. Cancers. 2025; 17(15):2583. https://doi.org/10.3390/cancers17152583
Chicago/Turabian StyleRuffolo, Bianca, Sara Vicente-Muñoz, Khyati Y. Mehta, Cosette M. Rivera-Cruz, Xueheng Zhao, Lindsey Romick, Kenneth D. R. Setchell, Adam Lane, and Susanne I. Wells. 2025. "The Fanconi Anemia Pathway Inhibits mTOR Signaling and Prevents Accelerated Translation in Head and Neck Cancer Cells" Cancers 17, no. 15: 2583. https://doi.org/10.3390/cancers17152583
APA StyleRuffolo, B., Vicente-Muñoz, S., Mehta, K. Y., Rivera-Cruz, C. M., Zhao, X., Romick, L., Setchell, K. D. R., Lane, A., & Wells, S. I. (2025). The Fanconi Anemia Pathway Inhibits mTOR Signaling and Prevents Accelerated Translation in Head and Neck Cancer Cells. Cancers, 17(15), 2583. https://doi.org/10.3390/cancers17152583




