Parenchyma-Sparing Bronchial Sleeve Resection in Low-Grade Malignant Diseases
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
2.2. Patient Selection
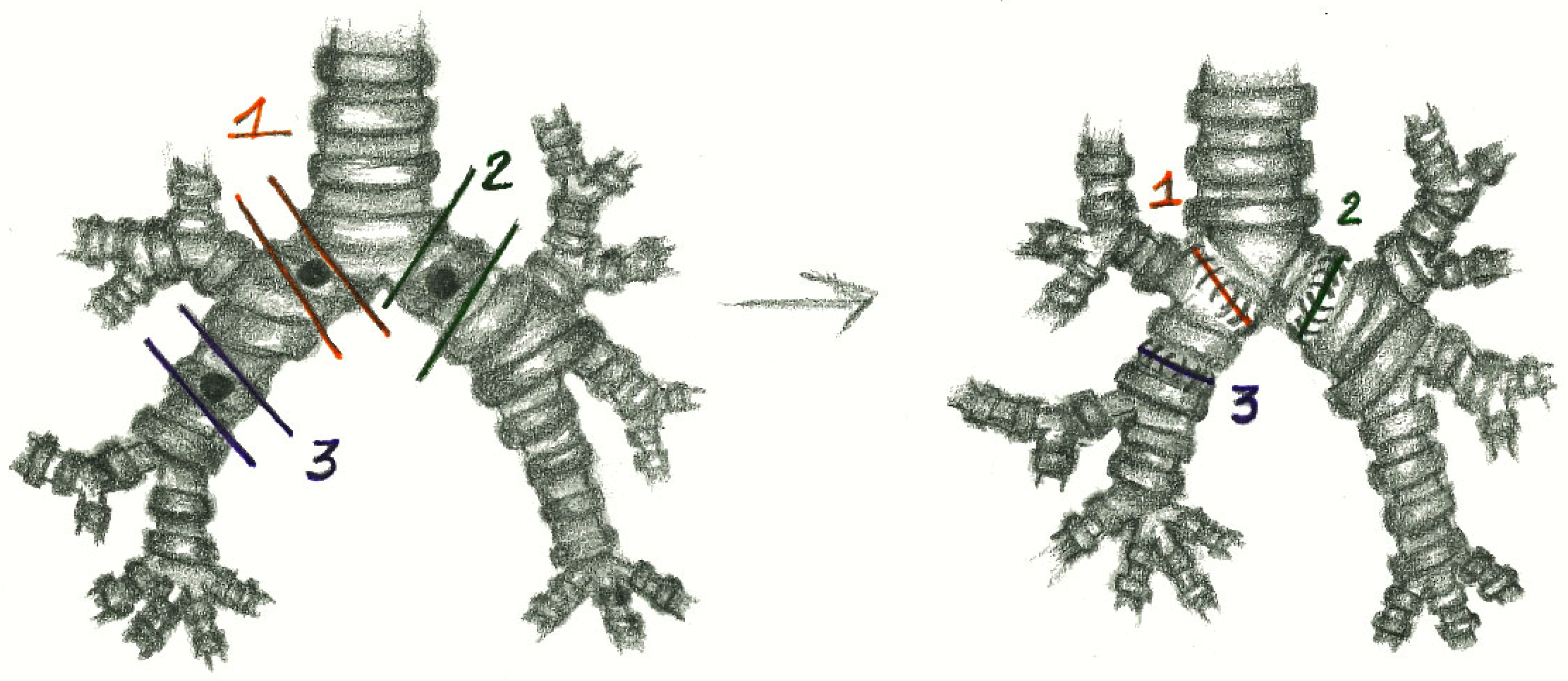
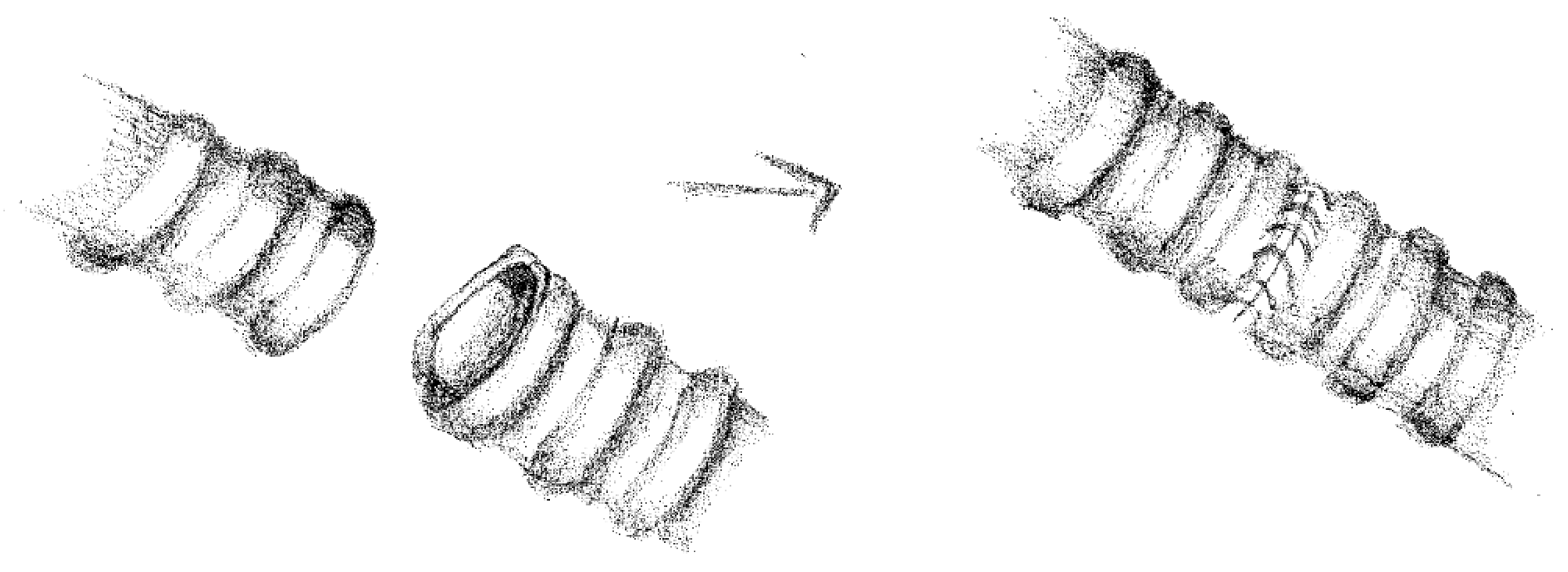
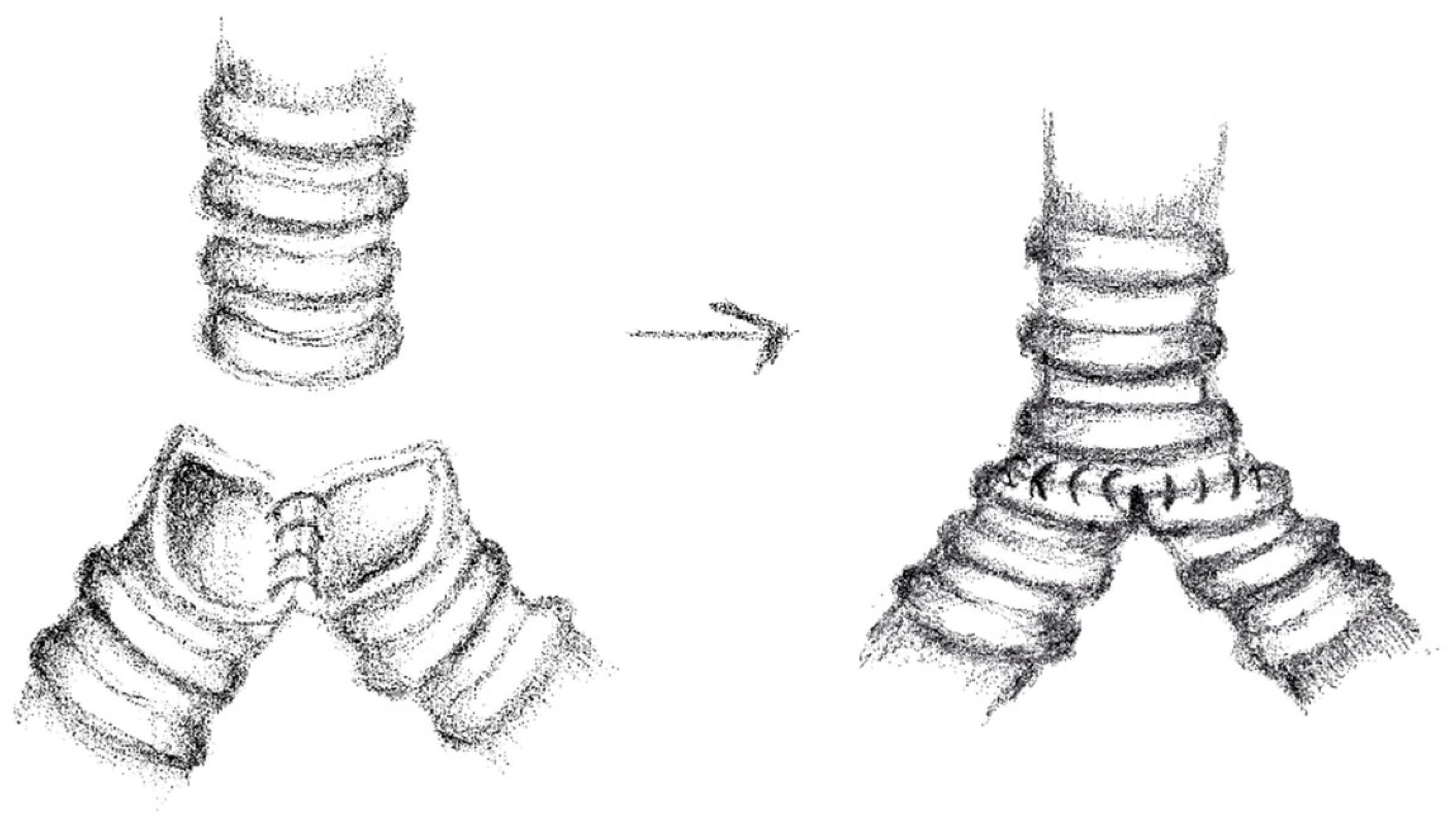
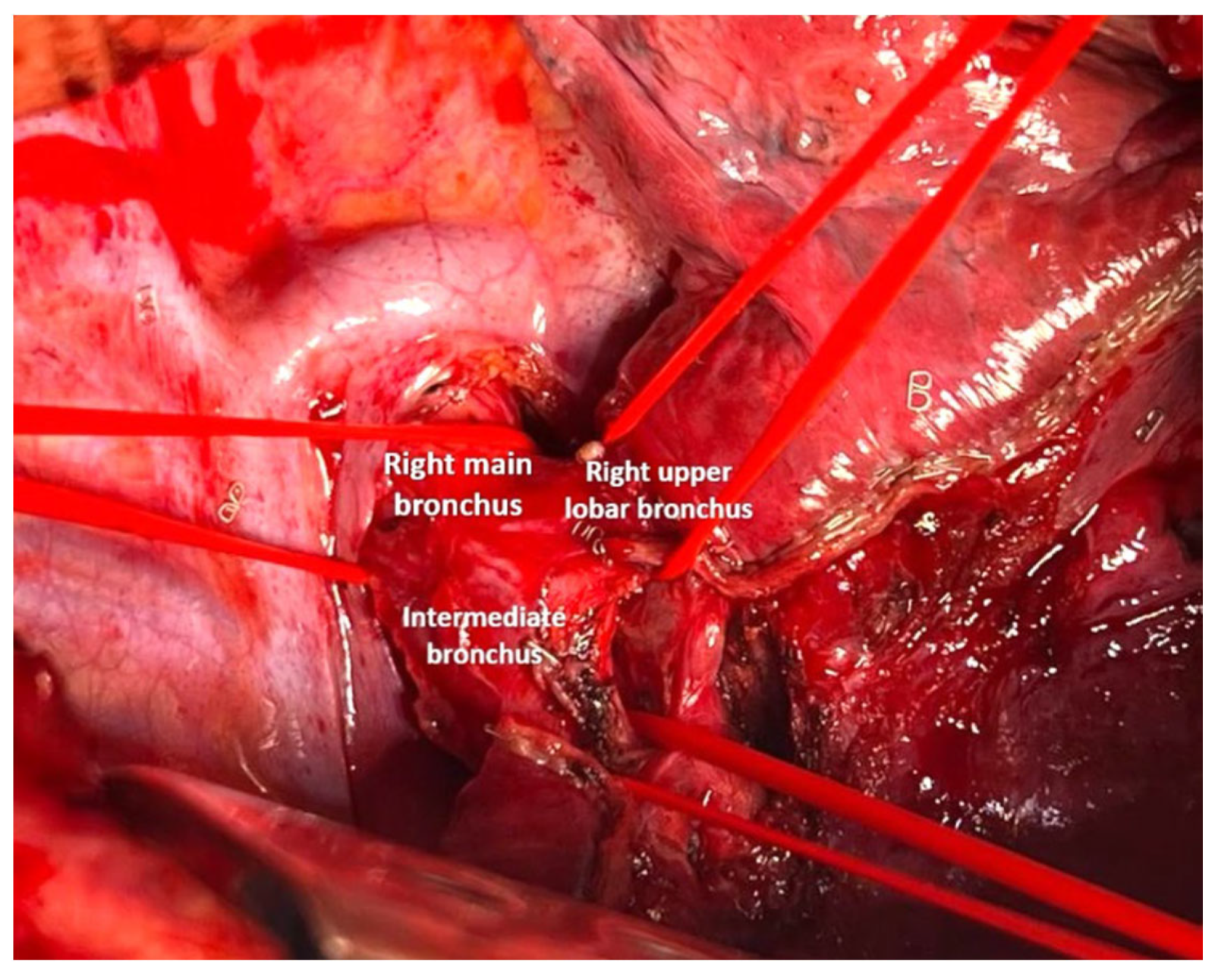
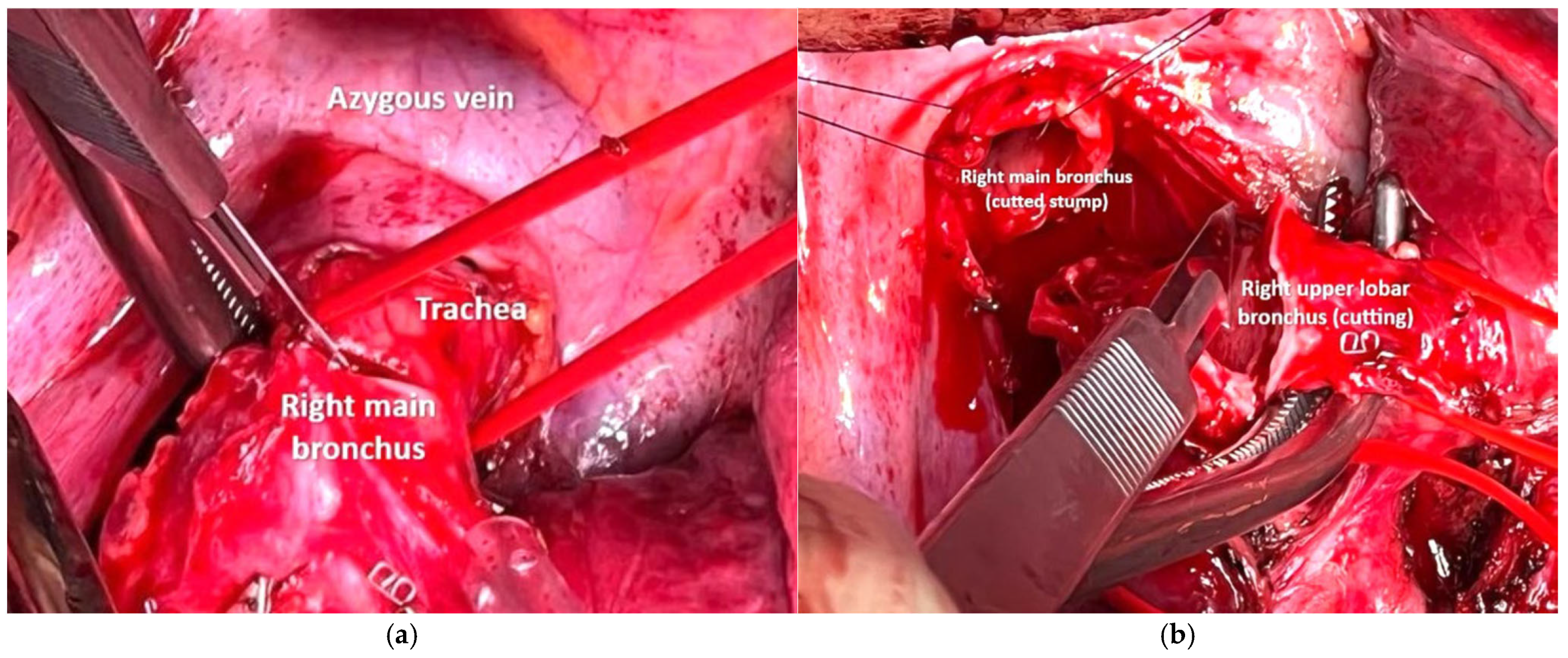
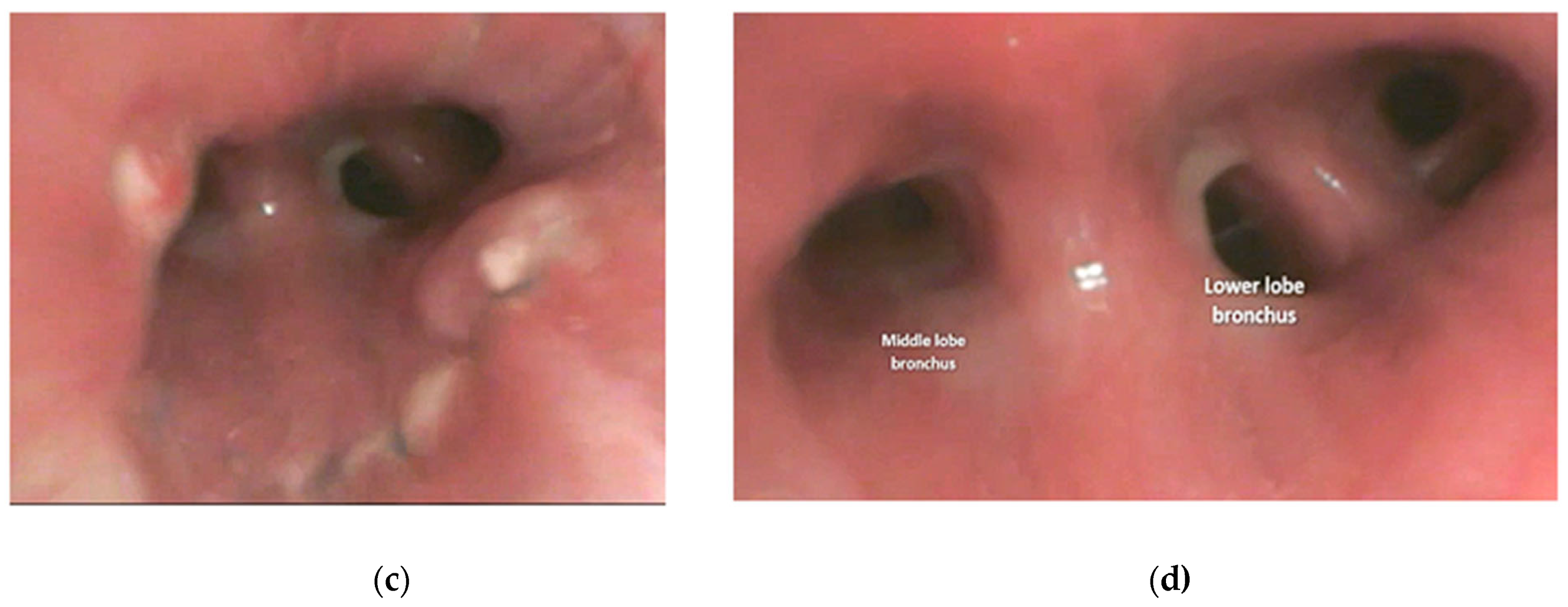
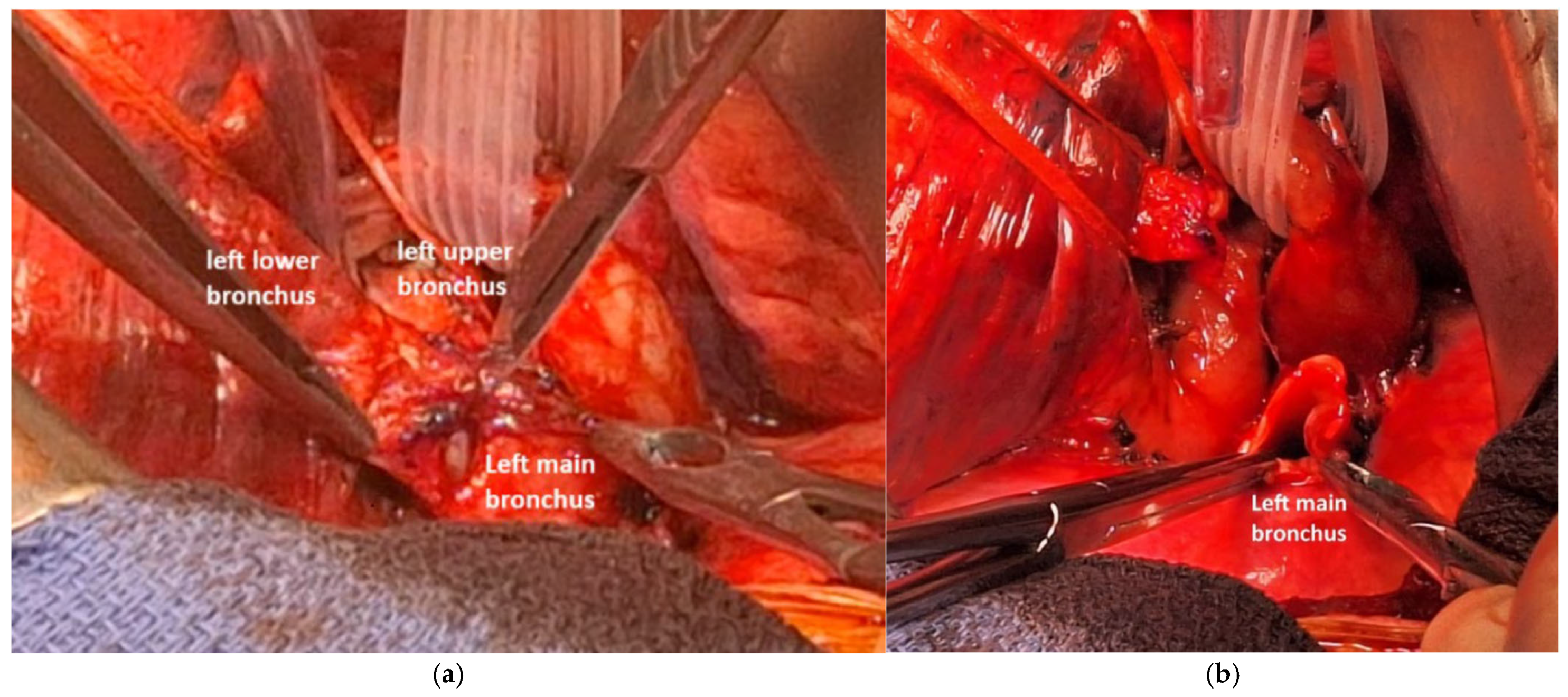
2.3. Anesthesia and Surgical Steps
2.4. Perioperative Management
2.5. Follow-Up
3. Results
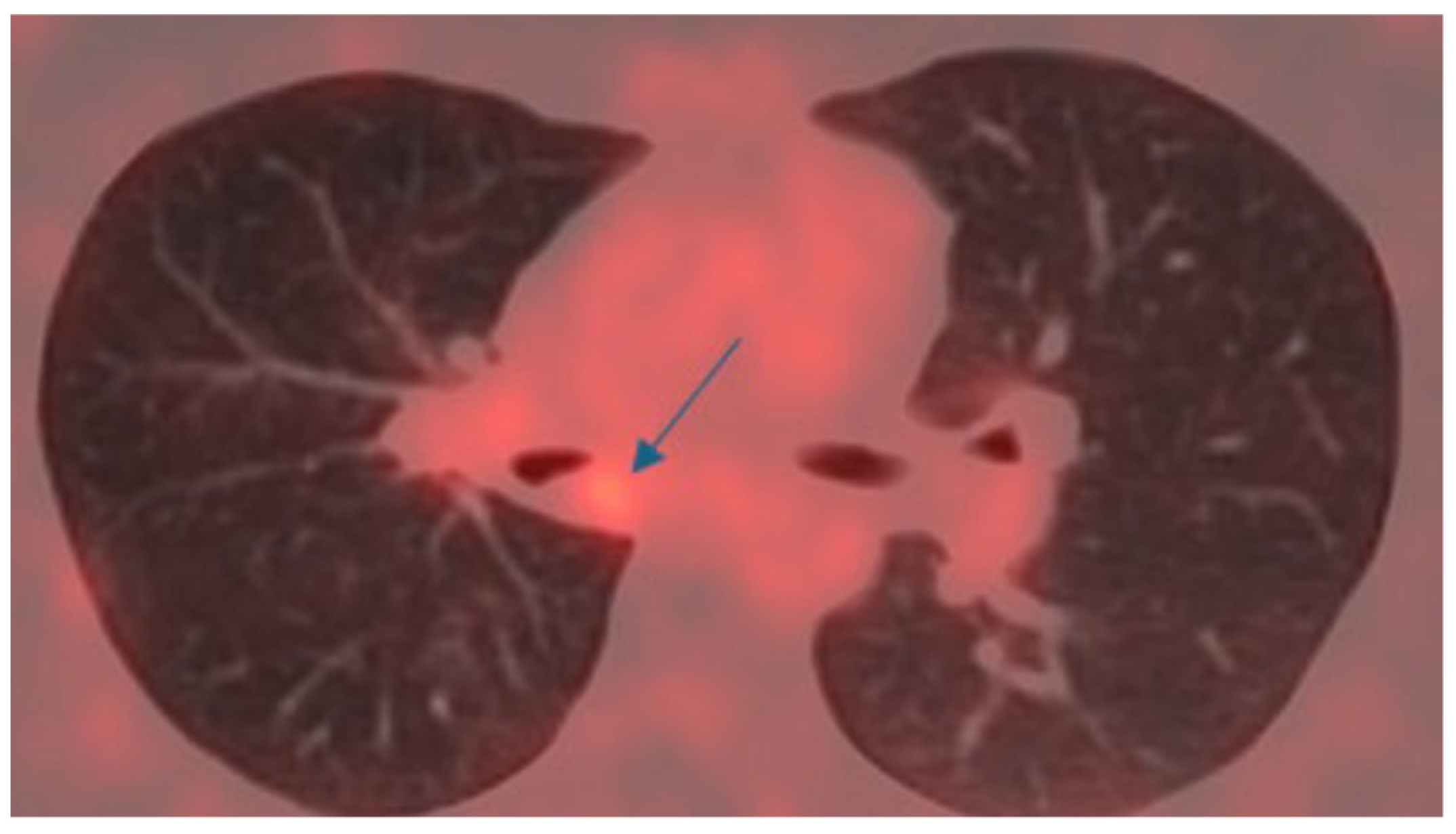
3.1. Patient’s Clinical Features, Diagnosis and Preoperative Treatment
3.2. Surgical Indications and Type of Resection
3.3. Postoperative Outcome
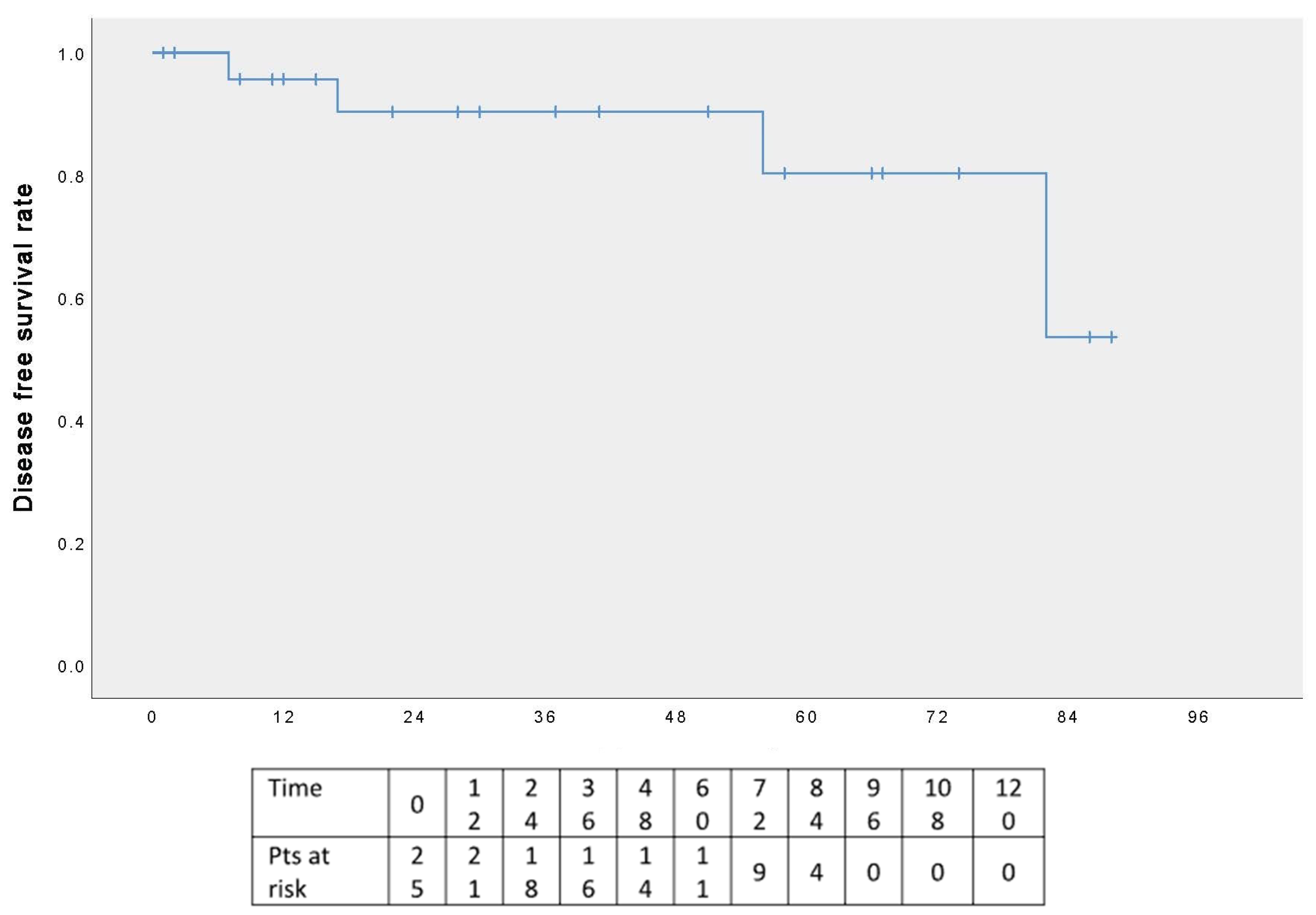
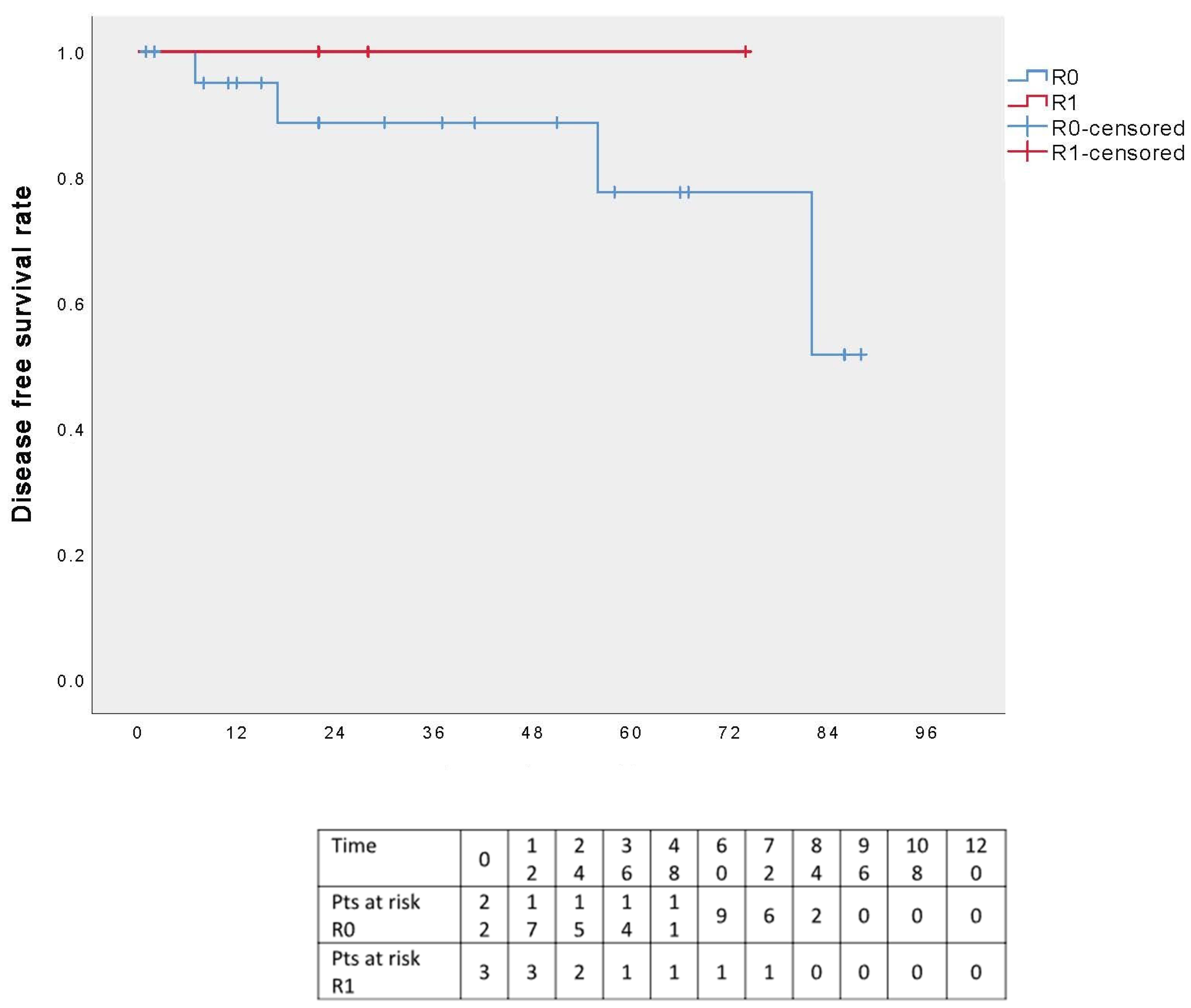
3.4. Follow-Up Results
4. Discussion
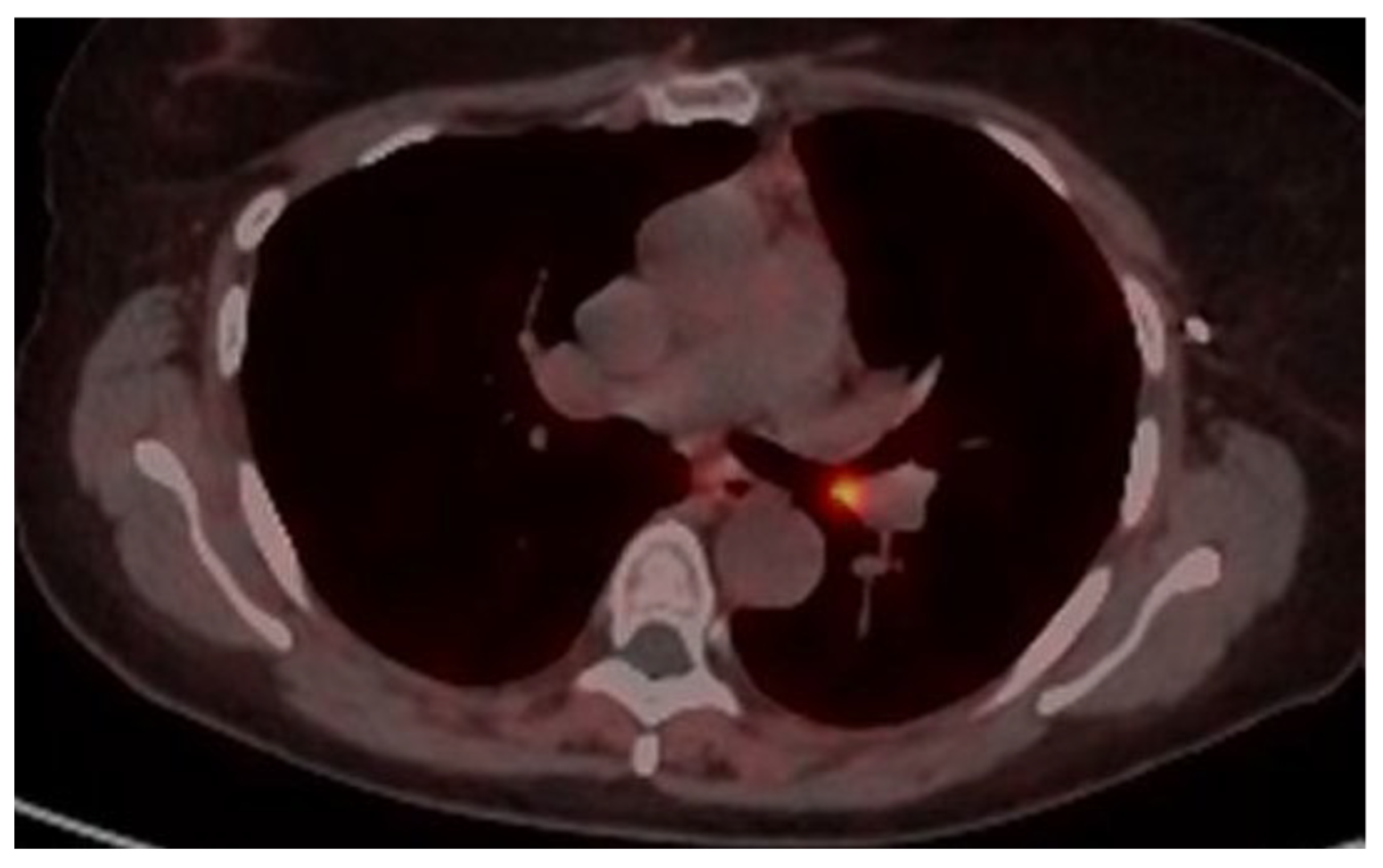
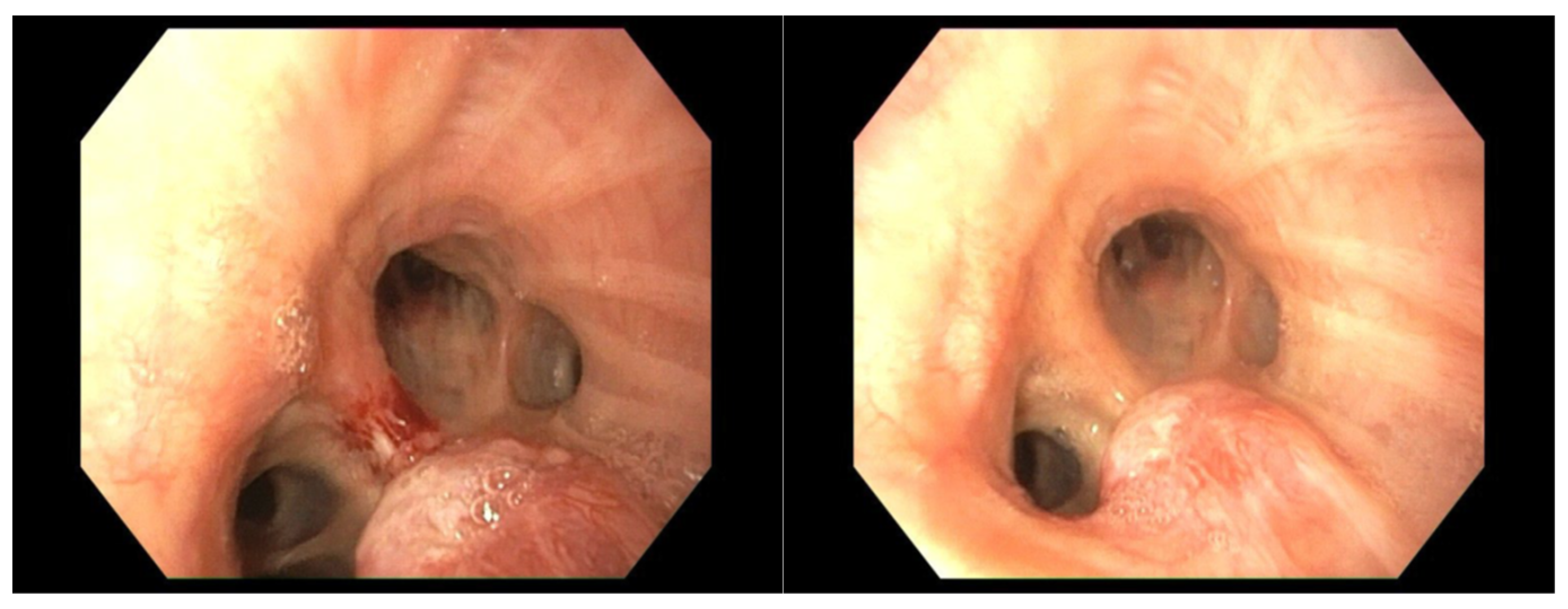
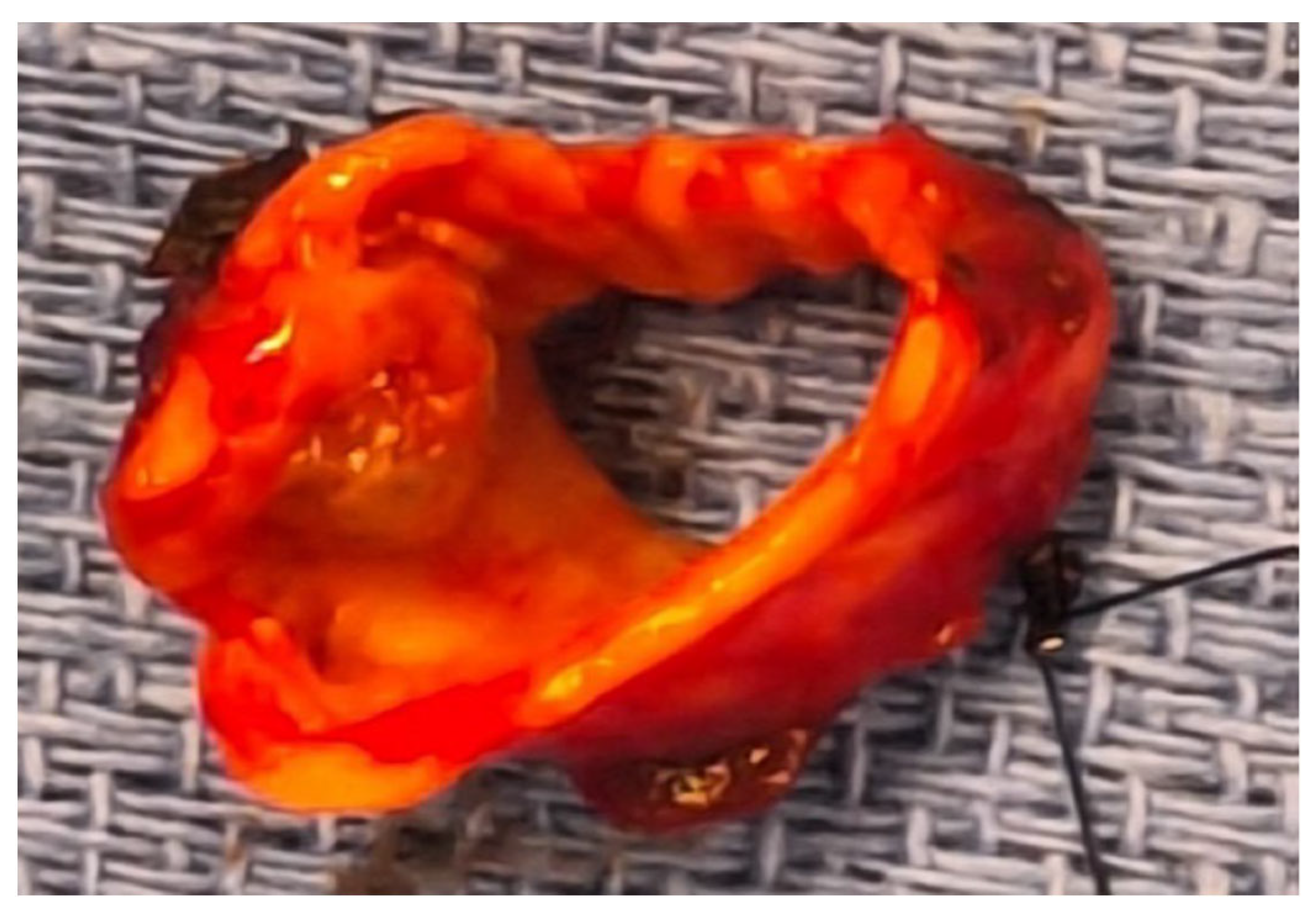
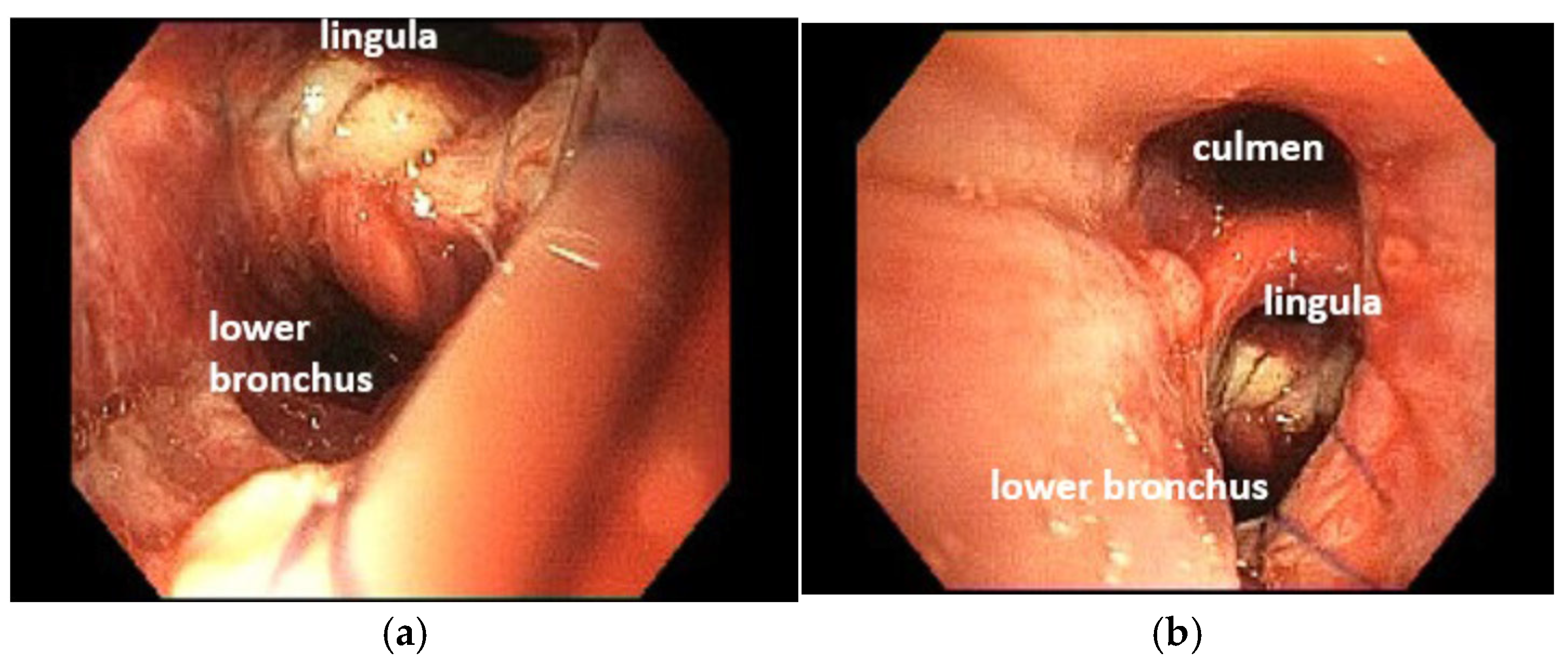
4.1. Case 1
4.2. Case 2
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Price-Thomas, C. Conservative resection of the bronchial tree. J. R. Coll. Surg. Edinb. 1956, 1, 169–186. [Google Scholar]
- Paulson, D.L.; Shaw, R.R. Bronchial anastomosis and bronchoplastic procedures in the interest of preservation of lung tissue. J. Thorac. Cardiovasc. Surg. 1955, 29, 238–259. [Google Scholar] [CrossRef]
- Terzi, A.; Lonardoni, A.; Feil, B.; Spilmbergo, I.; Falezza, G.; Calabrò, F. Bronchoplastic procedures for central carcinoid tumors: Clinical experience. Eur. J. Cardiothorac. Surg. 2004, 26, 1196–1199. [Google Scholar] [CrossRef]
- Faber, L.P.; Jensik, R.J.; Kittle, C.F. Results of sleeve lobectomy for bronchogenic carcinoma in 101 patients. Ann. Thorac. Surg. 1984, 37, 279–285. [Google Scholar] [CrossRef]
- Wilkins, E.W., Jr.; Grillo, H.C.; Moncure, A.C.; Scannell, J.G. Changing times in surgical management of bronchopulmonary carcinoid tumor. Ann. Thorac. Surg. 1984, 38, 339–344. [Google Scholar] [CrossRef]
- Cerfolio, R.J.; Deschamps, C.; Allen, M.S.; Trastek, V.F.; Pairolero, P.C. Mainstem bronchial sleeve resection with pulmonary preservaton. Ann. Thorac. Surg. 1996, 61, 1458–1462. [Google Scholar] [CrossRef]
- Dell’Amore, A.; Chen, L.; Monaci, N.; Campisi, A.; Wang, Z.; Mammana, M.; Pangoni, A.; Zhao, H.; Schiavon, M.; Yao, F.; et al. Total Lung-sparing Surgery for Tracheobronchial Low-grade Malignancies. Ann. Thorac. Surg. 2021, 112, 450–458. [Google Scholar] [CrossRef]
- Lucchi, M.; Melfi, F.; Ribechini, A.; Dini, P.; Duranti, L.; Fontanini, G.; Mussi, A. Sleeve and wedge parenchyma-sparing bronchial resections in low-grade neoplasms of the bronchial airway. J. Thoracic Surg. 2007, 134, 373–377. [Google Scholar] [CrossRef]
- Bueno, R.; Wain, J.; Wright, C.; Moncure, A.C.; Grillo, H.C.; Mathisen, D.J. Bronchoplasty in the management of low-grade airway neoplasm and benign bronchial stenoses. Ann. Thorac. Surg. 1996, 62, 824–829. [Google Scholar] [CrossRef]
- Nowak, K.; Karenovics, W.; Nicholson, A.G.; Jordan, S.; Dusmet, M. Pure bronchoplastic resections of the bronchus without pulmonary resection for endobronchial carcinoid tumors. Int. Cardiovasc. Surg. 2013, 17, 291–295. [Google Scholar]
- Reuling, E.M.B.P.; Dickhoff, C.; Plaisier, P.W.; Coupé, V.M.H.; Mazairac, A.H.A.; Lely, R.J.; Bonjer, H.J.; Daniels, J.M.A. Endobronchial treatment for bronchial carcinoid: Patient selection and predictors of outcome. Respiration 2018, 95, 220–227. [Google Scholar] [CrossRef]
- Brutinel, W.M.; Cortese, D.A.; McDougall, J.C.; Grillo, R.G.; Bergstralh, E.J. A two-year experience with neodymium-YAG laser in endobronchial obstruction. Chest 1987, 91, 159–165. [Google Scholar] [CrossRef]
- Shah, H.; Garbe, L.; Nussbaum, E. Benign tumors of the traheobronchial tree. Endoscopic characteristics and role of laser resection. Chest 1995, 107, 1744–1751. [Google Scholar] [CrossRef]
- Detterbeck, F.C. Management of carcinoid tumors. Ann. Thorac. Surg. 2010, 89, 998–1005. [Google Scholar] [CrossRef]
- Goswami, D.; Kashyap, L.; Batra, R.K.; Bhagat, C. Central bronchial carcinoid: Management of a case and anesthetic perspectives. Saudi J. Anaesth. 2016, 10, 104–106. [Google Scholar] [CrossRef]
- Corbetta, L. (Ed.) Hot Topics in Pneumologia Interventistica: Volume 2/A Cura di Lorenzo Corbetta; Strumenti per la didattica e la ricerca, 200; Firenze University Press: Firenze, Italy, 2018. [Google Scholar]
- Escalon, J.; Detterbeck, F. Carcinoid Tumors. In General Thoracic Surgery, 7th ed.; Shields, T., LoCicero, J.I., Reed, C., Feins, R., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2009; pp. 1539–1554. [Google Scholar]
- Daniels, C.E.; Lowe, V.J.; Aubry, M.C.; Allen, M.S.; Jett, J.R. The utility of fluorodeoxyglucose positron emission tomography in the evaluation of carcinoid tumors presenting as pulmonary nodules. Chest 2007, 131, 255–260. [Google Scholar] [CrossRef]
- Venkitaraman, B.; Karunanithi, S.; Kumar, A.; Khilnani, G.C.; Kumar, R. Role of 68Ga 720 DOTATOC PET/CT in initial evaluation of patients with suspected bronchopulmonary carcinoid. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 856–864. [Google Scholar] [CrossRef]
- Reidy-Lagunes, D.L.; Gollub, M.J.; Saltz, L.B. Addition of octreotide functional imaging to cross-sectional computed tomography or magnetic resonance imaging for the detection of neuroendocrine tumors: Added value or an anachronism? J. Clin. Oncol. 2011, 29, e74–e75. [Google Scholar] [CrossRef]
- Quaedvlieg, P.F.; Visser, O.; Lamers, C.B.; Janssen-Heijen, M.L.; Taal, B.G. Epidemiology and survival in patients with carcinoid disease in The Netherlands. An epidemiological study with 2391 patients. Ann. Oncol. 2001, 12, 1295–1300. [Google Scholar] [CrossRef]
- Ferguson, M.K.; Landrenau, R.; Hazelrigg, S.R.; Altorki, N.K.; Naunheim Zwishenberger Kent, M.; Yim, A.P. Long-term outcome after resection for bronchial carcinoid tumors. Eur. J. Cardiothoracic 2000, 18, 156–161. [Google Scholar] [CrossRef]
- Fink, G.; Krelbaum, T.; Yellin, A.; Bendayan, D.; Saute, M.; Glazer, M.; Kramer, M.R. Pulmonary carcinoid: Presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. Chest 2001, 119, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, R.J. New technique for one-lung anesthesia using endobronchial blocker. J. Thorac. Cardiovasc. Surg. 1981, 82, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.R.; Grillo, H.C.; Mathisen, D.J. Main bronchial sleeve resection with pulmonary conservation. Ann. Thorac. Surg. 1991, 52, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, T.; Nenekidis, I.; Stadler, J.E.; Schwarz, S.; Benazzo, A.; Jaksch, P.; Hoetzenecker, K.; Klepetko, W. Single running suture technique is associated with low rate of bronchial complications after lung transplantation. Vienna Lung Transplant. Progr. J. Thorac. Cardiovasc. Surg. 2020, 160, 1099–1108.e3. [Google Scholar] [CrossRef]
- Maurizi, G.; D’Andrilli, A.; Venuta, F.; Rendina, E.A. Reconstruction of the bronchus and pulmonary artery. J. Thorac. Dis. 2016, 8, S168–S180. [Google Scholar]
- Maurizi, G.; D’Andrilli, A.; Venuta, F.; Rendina, E.A. Bronchial and arterial sleeve resection for centrally-located lung cancers. J. Thorac. Dis. 2016, 8 (Suppl. S11), S872–S881. [Google Scholar] [CrossRef]
- Weisel, R.D.; Cooper, J.D.; Delarue, N.C.; Theman, T.E.; Todd, T.R.; Pearson, F.G. Sleeve lobectomy for carcinoma of the lung. Thorac. Cardiovasc. Surg. 1979, 78, 839–849. [Google Scholar] [CrossRef]
- Bayram, A.S.; Erol, M.M.; Salci, H.; Ozyiğit, O.; Görgül, S.; Gebitekin, C. Basic interrupted versus continuous suturing techniques in bronchial anastomosis following sleeve lobectomy in dogs. Eur. J. Cardiothorac. Surg. 2007, 32, 852–854. [Google Scholar] [CrossRef]
- Sayan, M.; Tastepe, A.I. Bronchial sleeve resections. Turk. Gogus Kalp Damar Cerrahisi Derg. 2023, 31 (Suppl. S1), S21–S28. [Google Scholar] [CrossRef]
- Rendina, E.A.; Venuta, F.; Ciriaco, P.; Ricci, C. Bronchovascular sleeve resection: Technique, perioperative management, prevention, and treatment of complications. J. Thorac. Cardiovasc. Surg. 1993, 106, 73–79. [Google Scholar] [CrossRef]
- Tsuchiya, R. Bronchoplastic techniques. In Pearson’s Thoracic and Esophageal Surgery, 2nd ed.; Patterson, G.A., Deslauriers, J., Lerut, A., Eds.; Churchill Livingstone: Philadelphia, PA, USA, 2002; p. 1005. [Google Scholar]
- Yildizeli, B.; Fadel, E.; Mussot, S.; Fabre, D.; Chataigner, O.; Dartevelle, P.G. Morbidity, mortality, and long-term survival after sleeve lobectomy for non-small cell lung cancer. Eur. J. Cardiothorac. Surg. 2007, 31, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Kutlu, C.A.; Goldstraw, P. Tracheobronchial sleeve resection with the use of a continuous anastomosis:results of one hundred consecutive cases. J. Thorac. Cardiovasc. Surg. 1999, 117, 11127. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, M.; Potaris, K.; Sakellaridis, T.; Chamalakis, G. Sleeve lobectomy for patients with non-small-cell lung cancer: A simplified approach. Eur. J. Cardiothorac. Surg. 2009, 36, 1045–1051. [Google Scholar] [CrossRef]
- Rea, F.; Marulli, G.; Schiavon, M.; Zuin, A.; Hamad, A.M.; Rizzardi, G.; Perissinotto, E.; Sartori, F. A quarter of a century experience with sleeve lobectomy for non-small cell lung cancer. Eur. J. Cardiothorac. Surg. 2008, 34, 488–492. [Google Scholar] [CrossRef]
- Todd, T.R.; Cooper, J.D.; Weissberg, D.; Delarue, N.C.; Pearson, F.G. Bronchial carcinoid tumors: Twenty years’ experience. J. Thorac. Cardiovasc. Surg. 1980, 79, 532–536. [Google Scholar] [CrossRef]
- Mackley, H.B.; Videtic, G.M. Primary carcinoid tumors of the lung: A role for radiotherapy. Oncology 2006, 20, 1537–1543. [Google Scholar]
- Cardillo, G.; Sera, F.; Di Martino, M.; Graziano, P.; Giunti, R.; Carbone, L.; Facciolo, F.; Martelli, M. Bronchial carcinoid tumors: Nodal status and long-term survival after resection. Ann. Thorac. Surg. 2004, 77, 1781–1785. [Google Scholar] [CrossRef]
- Schreurs, A.J.; Westermann, C.J.; van den Bosh, J.M.; Vanderschueren, R.G.; De La Rivière, A.B.; Knaepen, P.J. A twenty-five-year follow-up of ninety-three resected typical carcinoid tumors of the lung. J. Thorac. Cardiovasc. Surg. 1992, 104, 1470–1475. [Google Scholar] [CrossRef]
- Wegner, R.E.; Abel, S.; Hasan, S.; Horne, Z.D.; Colonias, A.; Weksler, B.; Verma, V. The role of adjuvant therapy for atypical bronchopulmonary carcinoids. Lung Cancer. 2019, 131, 90–94. [Google Scholar] [CrossRef]
- Thomas, C.F., Jr.; Tazelaar, H.D.; Jett, J.R. Typical and atypical pulmonary carcinoids: Outcome inpatients presenting with regional lymph node involvement. Chest 2001, 119, 1143–1150. [Google Scholar] [CrossRef]
- Rea, F.; Rizzardi, G.; Zuin, A.; Marulli, G.; Nicotra, S.; Bulf, R.; Schiavon, M.; Sartori, F. Outcome and surgical strategy in bronchial carcinoid tumors:single institution experience with 252 patients. Eur. J. Cardiothorac. Surg. 2007, 31, 186–191. [Google Scholar] [CrossRef] [PubMed]
- McCaughan, B.C.; Martini, N.; Bains, M.S. Bronchial carcinoids. Review of 124 cases. J Thorac. Cardiovasc. Surg. 1985, 89, 8–17. [Google Scholar]
- Lou, F.; Sarkaria, I.; Pietanza, C.; Travis, W.; Roh, M.S.; Sica, G.; Healy, D.; Rusch, V.; Huang, J. Recurrence of pulmonary carcinoid tumors after resection: Implications for postoperative surveillance. Ann. Thorac. Surg. 2013, 96, 1156–1162. [Google Scholar] [CrossRef]
- Garcia-Yuste, M.; Matilla, J.M.; Cueto, A.; Paniagua, J.M.R.; Ramos, G.; Canizares, M.A.; Muguruza, I. Typical and atypical carcinoid tumours: Analysis ofthe experience of the Spanish Multi-centric Study of Neuroendocrine Tumours. Eur. J. Cardiothorac. Surg. 2007, 31, 192–197. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology, Version 2; NCCN: Plymouth, PA, USA, 2025. [Google Scholar]










| Variables | Values N = 25 | |
|---|---|---|
| Sex | Male | 10 (40%) |
| Female | 15 (60%) | |
| Age, y | Median | 50 |
| Minimum, maximum | 26–77 | |
| Comorbidities | Arterial hypertension | 5 (20%) |
| COPD | 4 (16%) | |
| Obesity | 2 (8%) | |
| Heart diseases | 3 (12%) | |
| Clinical presentation | Cough | 10 (40%) |
| Recurrent respiratory infections | 8 (32%) | |
| Dyspnoea | 6 (24%) | |
| Chest pain | 3 (12%) | |
| Haemoptysis | 3 (12%) | |
| More than 1 symptom | 10 (40%) | |
| Asymptomatic | 5 (20%) | |
| Laser treatment | 7 (28%) | |
| Variables | Results n (%) or n |
|---|---|
| Median length of resected bronchus (cm) | 2.1 (range 1.2–2.9) |
| Median operative time (min) | 220 (range 120–330) |
| OR extubation (pts) | 25 (100%) |
| Histology | Number (%) | pN | R1 | Disease Relapse | Right Main Bronchus | Left Main Bronchus | Intermediate Bronchus | Neo-Interlobar Carina | |
|---|---|---|---|---|---|---|---|---|---|
| TC | 20 | 20 | N0 | 3 | 1 | 5 | 12 | 3 | 5 |
| 1 | N1 | ||||||||
| AC | 3 | 2 | N0 | - | 1 | 1 | - | 2 | - |
| Mucoepidermoid carcinoma | 2 | 2 | N0 | - | 1 | - | 2 | - | - |
| Total | 25 | 24 | N0 | 3 | 3 | 6 | 14 | 5 | 5 |
| 1 | N1 | ||||||||
| ICU monitoring (pts) | 8 (32%) |
| Prolonged intubation | - |
| Tracheotomy | - |
| Median draining time (days) | 5 (range 1–12) |
| Median hospital stays (days) | 8 (range 3–13) |
| Postoperative morbidity | 4 (16%) |
| Atelectasis | 1 |
| Atrial fibrillation | 1 |
| Hemothorax | 1 |
| Anemization | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salimbene, O.; Voltolini, L.; Mercier, O.; Viggiano, D.; Hanna, A.; Gonfiotti, A.; Fadel, E. Parenchyma-Sparing Bronchial Sleeve Resection in Low-Grade Malignant Diseases. Cancers 2025, 17, 2156. https://doi.org/10.3390/cancers17132156
Salimbene O, Voltolini L, Mercier O, Viggiano D, Hanna A, Gonfiotti A, Fadel E. Parenchyma-Sparing Bronchial Sleeve Resection in Low-Grade Malignant Diseases. Cancers. 2025; 17(13):2156. https://doi.org/10.3390/cancers17132156
Chicago/Turabian StyleSalimbene, Ottavia, Luca Voltolini, Olaf Mercier, Domenico Viggiano, Amir Hanna, Alessandro Gonfiotti, and Elie Fadel. 2025. "Parenchyma-Sparing Bronchial Sleeve Resection in Low-Grade Malignant Diseases" Cancers 17, no. 13: 2156. https://doi.org/10.3390/cancers17132156
APA StyleSalimbene, O., Voltolini, L., Mercier, O., Viggiano, D., Hanna, A., Gonfiotti, A., & Fadel, E. (2025). Parenchyma-Sparing Bronchial Sleeve Resection in Low-Grade Malignant Diseases. Cancers, 17(13), 2156. https://doi.org/10.3390/cancers17132156