Positron Emission Tomography Radiotracers for Identification of Site of Recurrence in Prostate Cancer After Primary Treatment Failure
Simple Summary
Abstract
1. Introduction
2. PET Radiotracers
2.1. 18F-FDG PET
2.2. 18F-NaF PET
2.3. 18F/11C-Choline PET
2.4. 18F-Fluciclovine (FACBC) PET
2.5. PSMA Radioligands
2.5.1. 68Ga-PSMA-11
2.5.2. 64Cu-DOTAGA-PSMA
2.5.3. 64Cu-SAR-bisPSMA
2.5.4. 18F-DCFPyL
2.5.5. 18F-PSMA-1007
2.5.6. 18F-rhPSMA-7.3
2.6. Bombesin
2.7. PSMA Radioligand Therapy
3. Hybrid Imaging and Future Research
3.1. PET/MRI PSMA Imaging
3.2. Future Directions and AI
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Ketchandji, M.; Kuo, Y.F.; Shahinian, V.B.; Goodwin, J.S. Cause of death in older men after the diagnosis of prostate cancer. J. Am. Geriatr. Soc. 2009, 57, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Paller, C.J.; Antonarakis, E.S. Management of biochemically recurrent prostate cancer after local therapy: Evolving standards of care and new directions. Clin. Adv. Hematol. Oncol. 2013, 11, 14–23. [Google Scholar]
- Ravery, V. The significance of recurrent PSA after radical prostatectomy: Benign versus malignant sources. Semin. Urol. Oncol. 1999, 17, 127–129. [Google Scholar]
- Pound, C.R.; Partin, A.W.; Eisenberger, M.A.; Chan, D.W.; Pearson, J.D.; Walsh, P.C. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999, 281, 1591–1597. [Google Scholar] [CrossRef]
- Partin, A.W.; Pearson, J.D.; Landis, P.K.; Carter, H.B.; Pound, C.R.; Clemens, J.Q.; Epstein, J.I.; Walsh, P.C. Evaluation of serum prostate-specific antigen velocity after radical prostatectomy to distinguish local recurrence from distant metastases. Urology 1994, 43, 649–659. [Google Scholar] [CrossRef]
- Cookson, M.S.; Aus, G.; Burnett, A.L.; Canby-Hagino, E.D.; D’Amico, A.V.; Dmochowski, R.R.; Eton, D.T.; Forman, J.D.; Goldenberg, S.L.; Hernandez, J.; et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: The American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J. Urol. 2007, 177, 540–545. [Google Scholar]
- Hinnen, K.A.; Monninkhof, E.M.; Battermann, J.J.; van Roermund, J.G.; Frank, S.J.; van Vulpen, M. Prostate specific antigen bounce is related to overall survival in prostate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 883–888. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Available online: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp (accessed on 27 February 2016).
- Taneja, S.S. Imaging in the diagnosis and management of prostate cancer. Rev. Urol. 2004, 6, 101–113. [Google Scholar]
- Hofer, C.; Laubenbacher, C.; Block, T.; Breul, J.; Hartung, R.; Schwaiger, M. Fluorine-18-fluorodeoxyglucose positron emission tomography is useless for the detection of local recurrence after radical prostatectomy. Eur. Urol. 1999, 36, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Chandler, J.D.; Williams, E.D.; Slavin, J.L.; Best, J.D.; Rogers, S. Expression and localization of GLUT1 and GLUT12 in prostate carcinoma. Cancer 2003, 97, 2035–2042. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Lakkis, C.L.; Laucirica, R.; Epner, D.E. Regulation of prostate cancer cell division by glucose. J. Cell. Physiol. 1999, 180, 431–438. [Google Scholar] [CrossRef]
- Liu, I.J.; Zafar, M.B.; Lai, Y.H.; Segall, G.M.; Terris, M.K. Fluorodeoxyglucose positron emission tomography studies in diagnosis and staging of clinically organ-confined prostate cancer. Urology 2001, 57, 108–111. [Google Scholar] [CrossRef]
- Jadvar, H. Is There Use for FDG-PET in Prostate Cancer? Semin. Nucl. Med. 2016, 46, 502–506. [Google Scholar] [CrossRef]
- Chang, C.H.; Wu, H.C.; Tsai, J.J.; Shen, Y.Y.; Changlai, S.P.; Kao, A. Detecting metastatic pelvic lymph nodes by 18F-2-deoxyglucose positron emission tomography in patients with prostate-specific antigen relapse after treatment for localized prostate cancer. Urol. Int. 2003, 70, 311–315. [Google Scholar] [CrossRef]
- Yu, C.Y.; Desai, B.; Ji, L.; Groshen, S.; Jadvar, H. Comparative performance of PET tracers in biochemical recurrence of prostate cancer: A critical analysis of literature. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 580–601. [Google Scholar]
- Schoder, H.; Herrmann, K.; Gonen, M.; Hricak, H.; Eberhard, S.; Scardino, P.; Scher, H.I.; Larson, S.M. 2-[18F]fluoro-2-deoxyglucose positron emission tomography for the detection of disease in patients with prostate-specific antigen relapse after radical prostatectomy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 4761–4769. [Google Scholar] [CrossRef]
- Castellucci, P.; Jadvar, H. PET/CT in prostate cancer: Non-choline radiopharmaceuticals. Q. J. Nucl. Med. Mol. Imaging Off. Publ. Ital. Assoc. Nucl. Med. 2012, 56, 367–374. [Google Scholar]
- Kao, P.F.; Chou, Y.H.; Lai, C.W. Diffuse FDG uptake in acute prostatitis. Clin. Nucl. Med. 2008, 33, 308–310. [Google Scholar] [CrossRef]
- Mease, R.C.; Foss, C.A.; Pomper, M.G. PET imaging in prostate cancer: Focus on prostate-specific membrane antigen. Curr. Top. Med. Chem. 2013, 13, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, Y.; Shi, Y.; Zhu, Y.; Xu, L.; Huang, G.; Liu, J. Diagnostic value of 18F-FDG PET/CT in patients with biochemical recurrent prostate cancer and negative 68Ga-PSMA PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2970–2977. [Google Scholar] [CrossRef]
- Jadvar, H.; Desai, B.; Ji, L.; Conti, P.S.; Dorff, T.B.; Groshen, S.G.; Gross, M.E.; Pinski, J.K.; Quinn, D.I. Prospective evaluation of 18F-NaF and 18F-FDG PET/CT in detection of occult metastatic disease in biochemical recurrence of prostate cancer. Clin. Nucl. Med. 2012, 37, 637–643. [Google Scholar] [CrossRef] [PubMed]
- von Eyben, F.E.; Kairemo, K. Acquisition with 11C-choline and 18F-fluorocholine PET/CT for patients with biochemical recurrence of prostate cancer: A systematic review and meta-analysis. Ann. Nucl. Med. 2016, 30, 385–392. [Google Scholar] [CrossRef]
- CMS.gov. NaF-18 PET for Bone Metastasis. 2004. Available online: https://www.cms.gov/medicare/coverage/evidence/bone-metastasis (accessed on 10 September 2024).
- Yoon, J.; Ballas, L.; Desai, B.; Jadvar, H. Prostate-Specific Antigen and Prostate-Specific Antigen Kinetics in Predicting 18F-Sodium Fluoride Positron Emission Tomography-Computed Tomography Positivity for First Bone Metastases in Patients with Biochemical Recurrence after Radical Prostatectomy. World J. Nucl. Med. 2017, 16, 229–236. [Google Scholar]
- Jadvar, H. Molecular imaging of prostate cancer: PET radiotracers. AJR Am. J. Roentgenol. 2012, 199, 278–291. [Google Scholar] [CrossRef]
- Poulsen, M.H.; Petersen, H.; Hoilund-Carlsen, P.F.; Jakobsen, J.S.; Gerke, O.; Karstoft, J.; Steffansen, S.I.; Walter, S. Spine metastases in prostate cancer: Comparison of technetium-99m-MDP whole-body bone scintigraphy, [18F]choline positron emission tomography (PET)/computed tomography (CT) and [18 F]NaF PET/CT. BJU Int. 2014, 114, 818–823. [Google Scholar] [CrossRef]
- Madsen, C.; Ostergren, P.; Haarmark, C. The Value of 68Ga-PSMA PET/CT Following Equivocal 18F-NaF PET/CT in Prostate Cancer Patients. Diagnostics 2020, 10, 352. [Google Scholar] [CrossRef]
- Agrawal, A.; Natarajan, A.; Mithun, S.; Bakshi, G.; Joshi, A.; Murthy, V.; Menon, S.; Purandare, N.; Shah, S.; Puranik, A.; et al. Bone metastases in prostate cancer—Gallium-68-labeled prostate-specific membrane antigen or Fluorine 18 sodium fluoride PET/computed tomography—The better tracer? Nucl. Med. Commun. 2022, 43, 1225–1232. [Google Scholar] [CrossRef]
- DeGrado, T.R.; Baldwin, S.W.; Wang, S.; Orr, M.D.; Liao, R.P.; Friedman, H.S.; Reiman, R.; Price, D.T.; Coleman, R.E. Synthesis and evaluation of 18F-labeled choline analogs as oncologic PET tracers. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2001, 42, 1805–1814. [Google Scholar]
- Hara, T.; Kosaka, N.; Shinoura, N.; Kondo, T. PET imaging of brain tumor with [methyl-11C]choline. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1997, 38, 842–847. [Google Scholar]
- Hara, T.; Kosaka, N.; Kishi, H. PET imaging of prostate cancer using carbon-11-choline. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1998, 39, 990–995. [Google Scholar]
- Leiblich, A.; Stevens, D.; Sooriakumaran, P. The Utility of Molecular Imaging in Prostate Cancer. Curr. Urol. Rep. 2016, 17, 26. [Google Scholar] [CrossRef]
- Imaging Technology News. Illinois Hospital Offering C-11 Choline PET Imaging for Prostate Cancer. 2016. Available online: https://www.itnonline.com/content/illinois-hospital-offering-c-11-choline-pet-imaging-prostate-cancer (accessed on 1 December 2020).
- Picchio, M.; Briganti, A.; Fanti, S.; Heidenreich, A.; Krause, B.J.; Messa, C.; Montorsi, F.; Reske, S.N.; Thalmann, G.N. The role of choline positron emission tomography/computed tomography in the management of patients with prostate-specific antigen progression after radical treatment of prostate cancer. Eur. Urol. 2011, 59, 51–60. [Google Scholar] [CrossRef]
- Evangelista, L.; Zattoni, F.; Rossi, E.; Karnes, R.J.; Lowe, V. Early detection of prostate cancer relapse by biochemistry and diagnostic imaging. Q. J. Nucl. Med. Mol. Imaging Off. Publ. Ital. Assoc. Nucl. Med. 2015, 59, 359–373. [Google Scholar]
- Iagaru, A.; Mittra, E.; Dick, D.W.; Gambhir, S.S. Prospective evaluation of (99m)Tc MDP scintigraphy, 18F NaF PET/CT, and 18F FDG PET/CT for detection of skeletal metastases. Mol. Imaging Biol. 2012, 14, 252–259. [Google Scholar] [CrossRef]
- Fanti, S.; Minozzi, S.; Castellucci, P.; Balduzzi, S.; Herrmann, K.; Krause, B.J.; Oyen, W.; Chiti, A. PET/CT with 11C-choline for evaluation of prostate cancer patients with biochemical recurrence: Meta-analysis and critical review of available data. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 55–69. [Google Scholar] [CrossRef]
- Deconinck, S.; Tosco, L.; Merckx, L.; Gheysens, O.; Deroose, C.M.; Baldewijns, M.; Weynand, B.; Laere, K.V.; Oyen, R.; Poppel, H.V.; et al. Anatomical mapping of lymph nodes in patients receiving salvage lymphadenectomy based on a positive 11C-choline positron emission tomography/computed tomography scan. Cent. Eur. J. Urol. 2019, 72, 232–239. [Google Scholar]
- Guo, Y.; Wang, L.; Hu, J.; Feng, D.; Xu, L. Diagnostic performance of choline PET/CT for the detection of bone metastasis in prostate cancer: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0203400. [Google Scholar] [CrossRef]
- de Leiris, N.; Leenhardt, J.; Boussat, B.; Montemagno, C.; Seiller, A.; Phan Sy, O.; Roux, J.; Laramas, M.; Verry, C.; Iriart, C.; et al. Does whole-body bone SPECT/CT provide additional diagnostic information over [18F]-FCH PET/CT for the detection of bone metastases in the setting of prostate cancer biochemical recurrence? Cancer Imaging 2020, 20, 58. [Google Scholar] [CrossRef]
- Nanni, C.; Schiavina, R.; Brunocilla, E.; Boschi, S.; Borghesi, M.; Zanoni, L.; Pettinato, C.; Martorana, G.; Fanti, S. 18F-Fluciclovine PET/CT for the Detection of Prostate Cancer Relapse: A Comparison to 11C-Choline PET/CT. Clin. Nucl. Med. 2015, 40, e386–e391. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves New Diagnostic Imaging Agent to Detect Recurrent Prostate Cancer. 2016. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-diagnostic-imaging-agent-detect-recurrent-prostate-cancer (accessed on 24 April 2024).
- Turkbey, B.; Mena, E.; Shih, J.; Pinto, P.A.; Merino, M.J.; Lindenberg, M.L.; Bernardo, M.; McKinney, Y.L.; Adler, S.; Owenius, R.; et al. Localized prostate cancer detection with 18F FACBC PET/CT: Comparison with MR imaging and histopathologic analysis. Radiology 2014, 270, 849–856. [Google Scholar] [CrossRef] [PubMed]
- von Eyben, F.E.; Kiljunen, T.; Kangasmaki, A.; Kairemo, K.; von Eyben, R.; Joensuu, T. Radiotherapy Boost for the Dominant Intraprostatic Cancer Lesion-A Systematic Review and Meta-Analysis. Clin. Genitourin. Cancer 2016, 14, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Segawa, A.; Nagamori, S.; Kanai, Y.; Masawa, N.; Oyama, T. L-type amino acid transporter 1 expression is highly correlated with Gleason score in prostate cancer. Mol. Clin. Oncol. 2013, 1, 274–280. [Google Scholar] [CrossRef]
- Kairemo, K.; Rasulova, N.; Partanen, K.; Joensuu, T. Preliminary clinical experience of trans-1-Amino-3-18F-fluorocyclobutanecarboxylic Acid (anti-18F-FACBC) PET/CT imaging in prostate cancer patients. BioMed Res. Int. 2014, 2014, 305182. [Google Scholar] [CrossRef]
- Nanni, C.; Zanoni, L.; Pultrone, C.; Schiavina, R.; Brunocilla, E.; Lodi, F.; Malizia, C.; Ferrari, M.; Rigatti, P.; Fonti, C.; et al. 18F-FACBC (anti1-amino-3-18F-fluorocyclobutane-1-carboxylic acid) versus 11C-choline PET/CT in prostate cancer relapse: Results of a prospective trial. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1601–1610. [Google Scholar] [CrossRef]
- Schuster, D.M.; Nieh, P.T.; Jani, A.B.; Amzat, R.; Bowman, F.D.; Halkar, R.K.; Master, V.A.; Nye, J.A.; Odewole, O.A.; Osunkoya, A.O.; et al. Anti-3-[18F]FACBC positron emission tomography-computerized tomography and 111In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: Results of a prospective clinical trial. J. Urol. 2014, 191, 1446–1453. [Google Scholar] [CrossRef]
- Virarkar, M.K.; Gruschkus, S.K.; Ravizzini, G.C.; Vulasala, S.S.R.; Javadi, S.; Bhosale, P. Assessing the effectiveness of MRI, 18F-fluciclovine PET, SUV(max), and PSA in detecting local recurrence of prostate cancer after prostatectomy. Pol. J. Radiol. 2024, 89, e196–e203. [Google Scholar] [CrossRef]
- Andriole, G.L.; Kostakoglu, L.; Chau, A.; Duan, F.; Mahmood, U.; Mankoff, D.A.; Schuster, D.M.; Siegel, B.A.; Group, L.S. The Impact of Positron Emission Tomography with 18F-Fluciclovine on the Treatment of Biochemical Recurrence of Prostate Cancer: Results from the LOCATE Trial. J. Urol. 2019, 201, 322–331. [Google Scholar] [CrossRef]
- Abiodun-Ojo, O.A.; Jani, A.B.; Akintayo, A.A.; Akin-Akintayo, O.O.; Odewole, O.A.; Tade, F.I.; Joshi, S.S.; Master, V.A.; Fielder, B.; Halkar, R.K.; et al. Salvage Radiotherapy Management Decisions in Postprostatectomy Patients with Recurrent Prostate Cancer Based on 18F-Fluciclovine PET/CT Guidance. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2021, 62, 1089–1096. [Google Scholar]
- Jani, A.B.; Schreibmann, E.; Goyal, S.; Halkar, R.; Hershatter, B.; Rossi, P.J.; Shelton, J.W.; Patel, P.R.; Xu, K.M.; Goodman, M.; et al. 18F-fluciclovine-PET/CT imaging versus conventional imaging alone to guide postprostatectomy salvage radiotherapy for prostate cancer (EMPIRE-1): A single centre, open-label, phase 2/3 randomised controlled trial. Lancet 2021, 397, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Lawal, I.O.; Jani, A.B.; Adediran, O.A.; Goyal, S.; Abiodun-Ojo, O.A.; Dhere, V.R.; Marcus, C.V.; Joshi, S.S.; Master, V.A.; Patel, P.R.; et al. Differences in Failure-Free Survival After Salvage Radiotherapy Guided by Conventional Imaging Versus 18F-Fluciclovine PET/CT in Postprostatectomy Patients: A Post Hoc Substratification Analysis of the EMPIRE-1 Trial. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2023, 64, 586–591. [Google Scholar]
- Gusman, M.; Aminsharifi, J.A.; Peacock, J.G.; Anderson, S.B.; Clemenshaw, M.N.; Banks, K.P. Review of 18F-Fluciclovine PET for Detection of Recurrent Prostate Cancer. Radiographics 2019, 39, 822–841. [Google Scholar] [CrossRef]
- Savir-Baruch, B.; Odewole, O.; Alaei Taleghani, P.; Master, V.; Nieh, P.; Halkar, R.; Jani, A.; Goodman, M.; Yu, W.; Schster, D. Anti-3-[F18] FACBC uptake pattern in the prostate affects positive predictive value and is associated with the presence of brachytherapy seeds. J. Nucl. Med. 2013, 54, 346. [Google Scholar]
- Mesters, J.R.; Barinka, C.; Li, W.; Tsukamoto, T.; Majer, P.; Slusher, B.S.; Konvalinka, J.; Hilgenfeld, R. Structure of glutamate carboxypeptidase II, a drug target in neuronal damage and prostate cancer. EMBO J. 2006, 25, 1375–1384. [Google Scholar] [CrossRef]
- Pianou, N.K.; Stavrou, P.Z.; Vlontzou, E.; Rondogianni, P.; Exarhos, D.N.; Datseris, I.E. More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT. Hell. J. Nucl. Med. 2019, 22, 6–9. [Google Scholar]
- Deb, N.; Goris, M.; Trisler, K.; Fowler, S.; Saal, J.; Ning, S.; Becker, M.; Marquez, C.; Knox, S. Treatment of hormone-refractory prostate cancer with 90Y-CYT-356 monoclonal antibody. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1996, 2, 1289–1297. [Google Scholar]
- Ravizzini, G.; Turkbey, B.; Kurdziel, K.; Choyke, P.L. New horizons in prostate cancer imaging. Eur. J. Radiol. 2009, 70, 212–226. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Eder, M.; Kopka, K.; Mier, W.; Hadaschik, B.A.; Haberkorn, U.; Dimitrakopoulou-Strauss, A. 68Ga-PSMA-11 dynamic PET/CT imaging in biochemical relapse of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1288–1299. [Google Scholar] [CrossRef]
- Lin, M.; Paolillo, V.; Ta, R.T.; Damasco, J.; Rojo, R.D.; Carl, J.C.; Melancon, M.P.; Ravizzini, G.C.; Le, D.B.; Santos, E.B. Fully automated preparation of 68Ga-PSMA-11 at curie level quantity using cyclotron-produced 68Ga for clinical applications. Appl. Radiat. Isot. 2020, 155, 108936. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Hetzheim, H.; Kratochwil, C.; Benesova, M.; Eder, M.; Neels, O.C.; Eisenhut, M.; Kubler, W.; Holland-Letz, T.; Giesel, F.L.; et al. The Theranostic PSMA Ligand PSMA-617 in the Diagnosis of Prostate Cancer by PET/CT: Biodistribution in Humans, Radiation Dosimetry, and First Evaluation of Tumor Lesions. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2015, 56, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Bluemel, C.; Weineisen, M.; Schottelius, M.; Wester, H.J.; Czernin, J.; Eberlein, U.; Beykan, S.; Lapa, C.; Riedmiller, H.; et al. Biodistribution and radiation dosimetry for a probe targeting prostate-specific membrane antigen for imaging and therapy. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2015, 56, 855–861. [Google Scholar] [CrossRef]
- Hawkey, N.M.; Sartor, A.O.; Morris, M.J.; Armstrong, A.J. Prostate-specific membrane antigen-targeted theranostics: Past, present, and future approaches. Clin. Adv. Hematol. Oncol. 2022, 20, 227–238. [Google Scholar] [PubMed]
- Sachpekidis, C.; Kopka, K.; Eder, M.; Hadaschik, B.A.; Freitag, M.T.; Pan, L.; Haberkorn, U.; Dimitrakopoulou-Strauss, A. 68Ga-PSMA-11 Dynamic PET/CT Imaging in Primary Prostate Cancer. Clin. Nucl. Med. 2016, 41, e473–e479. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Hadaschik, B.A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Haufe, S.; et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 486–495. [Google Scholar] [CrossRef]
- Rauscher, I.; Maurer, T.; Fendler, W.P.; Sommer, W.H.; Schwaiger, M.; Eiber, M. 68Ga-PSMA ligand PET/CT in patients with prostate cancer: How we review and report. Cancer Imaging 2016, 16, 14. [Google Scholar] [CrossRef]
- Hofman, M.S.; Hicks, R.J.; Maurer, T.; Eiber, M. Prostate-specific Membrane Antigen PET: Clinical Utility in Prostate Cancer, Normal Patterns, Pearls, and Pitfalls. Radiographics 2018, 38, 200–217. [Google Scholar] [CrossRef]
- Maurer, T.; Gschwend, J.E.; Rauscher, I.; Souvatzoglou, M.; Haller, B.; Weirich, G.; Wester, H.J.; Heck, M.; Kubler, H.; Beer, A.J.; et al. Diagnostic Efficacy of 68Gallium-PSMA Positron Emission Tomography Compared to Conventional Imaging for Lymph Node Staging of 130 Consecutive Patients with Intermediate to High Risk Prostate Cancer. J. Urol. 2016, 195, 1436–1443. [Google Scholar] [CrossRef]
- Ceci, F.; Uprimny, C.; Nilica, B.; Geraldo, L.; Kendler, D.; Kroiss, A.; Bektic, J.; Horninger, W.; Lukas, P.; Decristoforo, C.; et al. 68Ga-PSMA PET/CT for restaging recurrent prostate cancer: Which factors are associated with PET/CT detection rate? Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1284–1294. [Google Scholar] [CrossRef]
- Perera, M.; Papa, N.; Christidis, D.; Wetherell, D.; Hofman, M.S.; Murphy, D.G.; Bolton, D.; Lawrentschuk, N. Sensitivity, Specificity, and Predictors of Positive 68Ga-Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2016, 70, 926–937. [Google Scholar] [CrossRef]
- Burgard, C.; Hoffmann, M.A.; Frei, M.; Buchholz, H.G.; Khreish, F.; Marlowe, R.J.; Schreckenberger, M.; Ezziddin, S.; Rosar, F. Detection Efficacy of 68Ga-PSMA-11 PET/CT in Biochemical Recurrence of Prostate Cancer with Very Low PSA Levels: A 7-Year, Two-Center “Real-World” Experience. Cancers 2023, 15, 1376. [Google Scholar] [CrossRef] [PubMed]
- Savir-Baruch, B.; Zanoni, L.; Schuster, D.M. Imaging of Prostate Cancer Using Fluciclovine. PET Clin. 2017, 12, 145–157. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. FDA Approves First PSMA-Targeted PET Imaging Drug for Men with Prostate Cancer. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-psma-targeted-pet-imaging-drug-men-prostate-cancer (accessed on 1 December 2020).
- Calais, J.; Ceci, F.; Eiber, M.; Hope, T.A.; Hofman, M.S.; Rischpler, C.; Bach-Gansmo, T.; Nanni, C.; Savir-Baruch, B.; Elashoff, D.; et al. 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: A prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019, 20, 1286–1294. [Google Scholar] [CrossRef]
- Singh, A.; Kulkarni, K.; Klette, I.; Baum, R. Clinical application of Cu-64 PSMA PET/CT in Theranostics of prostate cancer. J. Nucl. Med. 2016, 57 (Suppl. S2), 1538. [Google Scholar]
- Mirzaei, S.; Lipp, R.; Zandieh, S.; Leisser, A. Single-Center Comparison of [64Cu]-DOTAGA-PSMA and [18F]-PSMA PET-CT for Imaging Prostate Cancer. Curr. Oncol. 2021, 28, 4167–4173. [Google Scholar] [CrossRef]
- Carlos Dos Santos, J.; Beijer, B.; Bauder-Wust, U.; Schafer, M.; Leotta, K.; Eder, M.; Benesova, M.; Kleist, C.; Giesel, F.; Kratochwil, C.; et al. Development of Novel PSMA Ligands for Imaging and Therapy with Copper Isotopes. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2020, 61, 70–79. [Google Scholar] [CrossRef]
- Sevcenco, S.; Klingler, H.C.; Eredics, K.; Friedl, A.; Schneeweiss, J.; Knoll, P.; Kunit, T.; Lusuardi, L.; Mirzaei, S. Application of Cu-64 NODAGA-PSMA PET in Prostate Cancer. Adv. Ther. 2018, 35, 779–784. [Google Scholar] [CrossRef]
- Lee, C.H.; Lim, I.; Woo, S.K.; Kim, K.I.; Lee, K.C.; Song, K.; Choi, C.W.; Lim, S.M. The Feasibility of 64Cu-PSMA I&T PET for Prostate Cancer. Cancer Biother. Radiopharm. 2022, 37, 417–423. [Google Scholar]
- Zurita, A.J.; Ravizzini, G.C.; Nordquist, L.; Osman, M.; Friedman, N.C.; Mehr, S.; Hope, T.A.; Denner, D.R.; Bartalotta, A.; Mors-ing, A.; et al. Prospective, multicenter, first in human phase 1/2 study of 64Cu PSMA I&T investigating dosimetry, safety and efficacy in men with biochemical recurrent metastatic prostate cancer. In Proceedings of the SNMMI Mid-Winter, Orlando, FL, USA, 1–3 February 2024. [Google Scholar]
- Curium, N.R. Curium Enrolls First Prostate Cancer Patients in Its Phase 3 SOLAR Trials. 2024. Available online: https://www.curiumpharma.com/2024/04/23/patients-phase-3-solar/ (accessed on 24 April 2024).
- Zia, N.A.; Cullinane, C.; Van Zuylekom, J.K.; Waldeck, K.; McInnes, L.E.; Buncic, G.; Haskali, M.B.; Roselt, P.D.; Hicks, R.J.; Donnelly, P.S. A Bivalent Inhibitor of Prostate Specific Membrane Antigen Radiolabeled with Copper-64 with High Tumor Uptake and Retention. Angew. Chem. Int. Ed. Engl. 2019, 58, 14991–14994. [Google Scholar] [CrossRef]
- McInnes, L.E.; Cullinane, C.; Roselt, P.D.; Jackson, S.; Blyth, B.J.; van Dam, E.M.; Zia, N.A.; Harris, M.J.; Hicks, R.J.; Donnelly, P.S. Therapeutic Efficacy of a Bivalent Inhibitor of Prostate-Specific Membrane Antigen Labeled with 67Cu. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2021, 62, 829–832. [Google Scholar]
- Lengyelova, E.; Wong, V.; Lenzo, N.; Parker, M.; Emmett, L. Cu-SAR-bisPSMA (PROPELLER) positron emission tomography (PET) imaging in patients with confirmed prostate cancer. J. Clin. Oncol. 2023, 41, 5039. [Google Scholar] [CrossRef]
- Pharmaceuticals, C. PROPELLER Trial Results—SAR-bisPSMA Safe, Well Tolerated and Efficacious in the Detection of Pros-tate Cancer. 2024. Available online: https://www.claritypharmaceuticals.com/news/propeller_results/ (accessed on 23 October 2024).
- Gorin, M.A.; Lengyelova, E.; Nordquist, L.; Morrish, G.; Gervasio, O.; Miller, R.M.; Shore, N.D. CLARIFY: Positron emission tomography using Cu-SAR-bisPSMA in patients with high-risk prostate cancer prior to radical prostatectomy (a phase 3 di-agnostic study). J. Clin. Oncol. 2024, 42, TPS5129. [Google Scholar] [CrossRef]
- Johnson, G.; Lengyelova, E.; Nordquist, L.; Prasad, V.; Anderson, M.; Gervasio, O.; Parker, M.; Miller, R.M.; Sartor, A.O.; Tagawa, S.T. SECuRE: A dose escalation/expansion study to assess the anti-tumor efficacy of 67Cu-SAR-bisPSMA in patients with metastatic castrate resistant prostate cancer. J. Clin. Oncol. 2024, 42, TPS5116. [Google Scholar] [CrossRef]
- Giesel, F.L.; Hadaschik, B.; Cardinale, J.; Radtke, J.; Vinsensia, M.; Lehnert, W.; Kesch, C.; Tolstov, Y.; Singer, S.; Grabe, N.; et al. F-18 labelled PSMA-1007: Biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 678–688. [Google Scholar] [CrossRef]
- Mease, R.C.; Dusich, C.L.; Foss, C.A.; Ravert, H.T.; Dannals, R.F.; Seidel, J.; Prideaux, A.; Fox, J.J.; Sgouros, G.; Kozikowski, A.P.; et al. N-[N-[(S)-1,3-Dicarboxypropyl]carbamoyl]-4-[18F]fluorobenzyl-L-cysteine, [18F]DCFBC: A new imaging probe for prostate cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 3036–3043. [Google Scholar] [CrossRef]
- Dietlein, M.; Kobe, C.; Kuhnert, G.; Stockter, S.; Fischer, T.; Schomacker, K.; Schmidt, M.; Dietlein, F.; Zlatopolskiy, B.D.; Krapf, P.; et al. Comparison of [18F]DCFPyL and [ 68Ga]Ga-PSMA-HBED-CC for PSMA-PET Imaging in Patients with Relapsed Prostate Cancer. Mol. Imaging Biol. 2015, 17, 575–584. [Google Scholar] [CrossRef]
- Pienta, K.J.; Gorin, M.A.; Rowe, S.P.; Carroll, P.R.; Pouliot, F.; Probst, S.; Saperstein, L.; Preston, M.A.; Alva, A.S.; Patnaik, A.; et al. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18F-DCFPyL in Prostate Cancer Patients (OSPREY). J. Urol. 2021, 206, 52–61. [Google Scholar] [CrossRef]
- Morris, M.J.; Rowe, S.P.; Gorin, M.A.; Saperstein, L.; Pouliot, F.; Josephson, D.; Wong, J.Y.C.; Pantel, A.R.; Cho, S.Y.; Gage, K.L.; et al. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 3674–3682. [Google Scholar] [CrossRef]
- Keam, S.J. Piflufolastat F 18: Diagnostic First Approval. Mol. Diagn. Ther. 2021, 25, 647–656. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network Guidelines. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 24 January 2024).
- Ulaner, G.A.; Thomsen, B.; Bassett, J.; Torrey, R.; Cox, C.; Lin, K.; Patel, T.; Techasith, T.; Mauguen, A.; Rowe, S.P.; et al. 18F-DCFPyL PET/CT for Initially Diagnosed and Biochemically Recurrent Prostate Cancer: Prospective Trial with Pathologic Confirmation. Radiology 2022, 305, 419–428. [Google Scholar] [CrossRef]
- Rowe, S.P.; Macura, K.J.; Mena, E.; Blackford, A.L.; Nadal, R.; Antonarakis, E.S.; Eisenberger, M.; Carducci, M.; Fan, H.; Dannals, R.F.; et al. PSMA-Based [18F]DCFPyL PET/CT Is Superior to Conventional Imaging for Lesion Detection in Patients with Metastatic Prostate Cancer. Mol. Imaging Biol. 2016, 18, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Sachpekidis, C.; Afshar-Oromieh, A.; Kopka, K.; Strauss, D.S.; Pan, L.; Haberkorn, U.; Dimitrakopoulou-Strauss, A. 18F-PSMA-1007 multiparametric, dynamic PET/CT in biochemical relapse and progression of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 592–602. [Google Scholar] [CrossRef]
- Pattison, D.A.; Debowski, M.; Gulhane, B.; Arnfield, E.G.; Pelecanos, A.M.; Garcia, P.L.; Latter, M.J.; Lin, C.Y.; Roberts, M.J.; Ramsay, S.C.; et al. Prospective intra-individual blinded comparison of [18F]PSMA-1007 and [68 Ga]Ga-PSMA-11 PET/CT imaging in patients with confirmed prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 763–776. [Google Scholar] [CrossRef]
- Rauscher, I.; Kronke, M.; Konig, M.; Gafita, A.; Maurer, T.; Horn, T.; Schiller, K.; Weber, W.; Eiber, M. Matched-Pair Comparison of 68Ga-PSMA-11 PET/CT and 18F-PSMA-1007 PET/CT: Frequency of Pitfalls and Detection Efficacy in Biochemical Recurrence After Radical Prostatectomy. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2020, 61, 51–57. [Google Scholar]
- Huang, S.; Ong, S.; McKenzie, D.; Mirabelli, A.; Chen, D.C.; Chengodu, T.; Murphy, D.G.; Hofman, M.S.; Lawrentschuk, N.; Perera, M. Comparison of 18F-based PSMA radiotracers with [68Ga]Ga-PSMA-11 in PET/CT imaging of prostate cancer-a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2023, 27, 654–664. [Google Scholar] [CrossRef]
- Seifert, R.; Telli, T.; Opitz, M.; Barbato, F.; Berliner, C.; Nader, M.; Umutlu, L.; Stuschke, M.; Hadaschik, B.; Herrmann, K.; et al. Unspecific 18F-PSMA-1007 Bone Uptake Evaluated Through PSMA-11 PET, Bone Scanning, and MRI Triple Validation in Patients with Biochemical Recurrence of Prostate Cancer. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2023, 64, 738–743. [Google Scholar]
- Kroenke, M.; Schweiger, L.; Horn, T.; Haller, B.; Schwamborn, K.; Wurzer, A.; Maurer, T.; Wester, H.J.; Eiber, M.; Rauscher, I. Validation of 18F-rhPSMA-7 and 18F-rhPSMA-7.3 PET Imaging Results with Histopathology from Salvage Surgery in Patients with Biochemical Recurrence of Prostate Cancer. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2022, 63, 1809–1814. [Google Scholar]
- Tolvanen, T.; Kalliokoski, K.; Malaspina, S.; Kuisma, A.; Lahdenpohja, S.; Postema, E.J.; Miller, M.P.; Scheinin, M. Safety, Biodistribution, and Radiation Dosimetry of 18F-rhPSMA-7.3 in Healthy Adult Volunteers. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2021, 62, 679–684. [Google Scholar]
- Jani, A.B.; Ravizzini, G.C.; Gartrell, B.A.; Siegel, B.A.; Twardowski, P.; Saltzstein, D.; Fleming, M.T.; Chau, A.; Davis, P.; Chapin, B.F.; et al. Diagnostic Performance and Safety of 18F-rhPSMA-7.3 Positron Emission Tomography in Men with Suspected Prostate Cancer Recurrence: Results From a Phase 3, Prospective, Multicenter Study (SPOTLIGHT). Reply. J. Urol. 2023, 210, 411–412. [Google Scholar] [CrossRef]
- Jani, A.B.; Ravizzini, G.C.; Gartrell, B.A.; Siegel, B.A.; Twardowski, P.; Saltzstein, D.; Fleming, M.T.; Chau, A.; Davis, P.; Chapin, B.F.; et al. Diagnostic Performance and Safety of 18F-rhPSMA-7.3 Positron Emission Tomography in Men with Suspected Prostate Cancer Recurrence: Results From a Phase 3, Prospective, Multicenter Study (SPOTLIGHT). J. Urol. 2023, 210, 299–311. [Google Scholar] [CrossRef]
- Chantadisai, M.; Buschner, G.; Kronke, M.; Rauscher, I.; Langbein, T.; Nekolla, S.G.; Schiller, K.; Heck, M.M.; Maurer, T.; Wurzer, A.; et al. Positive Predictive Value and Correct Detection Rate of 18F-rhPSMA-7 PET in Biochemically Recurrent Prostate Cancer Validated by Composite Reference Standard. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2021, 62, 968–974. [Google Scholar]
- FDA Approval. POSLUMA. 216023s000lbl PDF. 2023. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216023s000lbl.pdf (accessed on 4 December 2024).
- Anastasi, A.; Erspamer, V.; Bucci, M. Isolation and structure of bombesin and alytesin, 2 analogous active peptides from the skin of the European amphibians Bombina and Alytes. Experientia 1971, 27, 166–167. [Google Scholar] [CrossRef] [PubMed]
- Beer, M.; Montani, M.; Gerhardt, J.; Wild, P.J.; Hany, T.F.; Hermanns, T.; Muntener, M.; Kristiansen, G. Profiling gastrin-releasing peptide receptor in prostate tissues: Clinical implications and molecular correlates. Prostate 2012, 72, 318–325. [Google Scholar] [CrossRef]
- Jensen, R.T.; Battey, J.F.; Spindel, E.R.; Benya, R.V. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: Nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol. Rev. 2008, 60, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Halmos, G.; Schally, A.V.; Wang, X.; Martinez, M. Presence of receptors for bombesin/gastrin-releasing peptide and mRNA for three receptor subtypes in human prostate cancers. Prostate 2000, 42, 295–303. [Google Scholar] [CrossRef]
- Reubi, J.C.; Wenger, S.; Schmuckli-Maurer, J.; Schaer, J.C.; Gugger, M. Bombesin receptor subtypes in human cancers: Detection with the universal radioligand 125I-[D-TYR6, beta-ALA11, PHE13, NLE14] bombesin(6-14). Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2002, 8, 1139–1146. [Google Scholar]
- Markwalder, R.; Reubi, J.C. Gastrin-releasing peptide receptors in the human prostate: Relation to neoplastic transformation. Cancer Res. 1999, 59, 1152–1159. [Google Scholar]
- Minamimoto, R.; Hancock, S.; Schneider, B.; Chin, F.T.; Jamali, M.; Loening, A.; Vasanawala, S.; Gambhir, S.S.; Iagaru, A. Pilot Comparison of 68Ga-RM2 PET and 68Ga-PSMA-11 PET in Patients with Biochemically Recurrent Prostate Cancer. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2016, 57, 557–562. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X.; Liu, H.; Bu, L.; Ma, X.; Cheng, K.; Li, J.; Tian, M.; Zhang, H.; Cheng, Z. A comparative study of radiolabeled bombesin analogs for the PET imaging of prostate cancer. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2013, 54, 2132–2138. [Google Scholar] [CrossRef]
- Kahkonen, E.; Jambor, I.; Kemppainen, J.; Lehtio, K.; Gronroos, T.J.; Kuisma, A.; Luoto, P.; Sipila, H.J.; Tolvanen, T.; Alanen, K.; et al. In vivo imaging of prostate cancer using [68Ga]-labeled bombesin analog BAY86-7548. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 5434–5443. [Google Scholar] [CrossRef]
- Ceci, F.; Castellucci, P.; Polverari, G.; Iagaru, A. Clinical application of Fluciclovine PET, choline PET and gastrin-releasing polypeptide receptor (bombesin) targeting PET in prostate cancer. Curr. Opin. Urol. 2020, 30, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Pharmaceuticals, C. SAR-Bombesin: A Pan-Cancer Theranostic. 2024. Available online: https://www.claritypharmaceuticals.com/pipeline/SAR-BBN/ (accessed on 24 April 2024).
- Nordquist, L.T.; Lengyelova, E.; Iagaru, A.; Mancini, B.R.; Song, H.; Osman, M.; Josephson, D.; Almaguel, F.; Saltzstein, D.; Par-ker, M.; et al. SABRE: Assessment of safety and efficacy of 64Cu-SAR-BBN in patients with PSMA-negative biochemical recurrent prostate cancer. J. Clin. Oncol. 2024, 42, TPS346. [Google Scholar] [CrossRef]
- Nordquist, L.T.; Lengyelova, E.; Almaguel, F.; Mancini, B.R.; Song, H.; Armstrong, A.J.; Zurita, A.J.; Anderson, M.; Parker, M.; Miller, R.M.; et al. COMBAT: A study of 64Cu-SAR-BBN and 67Cu-SAR-BBN for identification and treatment of GRPr-expressing metastatic castrate-resistant prostate cancer. J. Clin. Oncol. 2024, 42, TPS247. [Google Scholar] [CrossRef]
- Baum, R.P.; Kulkarni, H.R.; Schuchardt, C.; Singh, A.; Wirtz, M.; Wiessalla, S.; Schottelius, M.; Mueller, D.; Klette, I.; Wester, H.J. 177Lu-Labeled Prostate-Specific Membrane Antigen Radioligand Therapy of Metastatic Castration-Resistant Prostate Cancer: Safety and Efficacy. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2016, 57, 1006–1013. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Eder, M.; Afshar-Oromieh, A.; Benesova, M.; Mier, W.; Kopka, K.; Haberkorn, U. [177Lu]Lutetium-labelled PSMA ligand-induced remission in a patient with metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 987–988. [Google Scholar] [CrossRef]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schafers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2017, 58, 85–90. [Google Scholar]
- Eiber, M.; Herrmann, K. From NETTER to PETTER: PSMA-Targeted Radioligand Therapy. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2017, 58, 9–10. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. FDA Approves Pluvicto for Metastatic Castration-Resistant Prostate Cancer. 2022. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pluvicto-metastatic-castration-resistant-prostate-cancer (accessed on 4 December 2024).
- Targeted Oncology. FDA Expands Indication for Lutetium-177 PSMA-617 in PSMA-Positive mCRPC. 2025. Available online: https://www.targetedonc.com/view/fda-expands-indication-for-lutetium-177-psma-617-in-psma-positive-mcrpc (accessed on 1 April 2025).
- Morris, M.J.; Castellano, D.; Herrmann, K.; de Bono, J.S.; Shore, N.D.; Chi, K.N.; Crosby, M.; Piulats, J.M.; Flechon, A.; Wei, X.X.; et al. 177Lu-PSMA-617 versus a change of androgen receptor pathway inhibitor therapy for taxane-naive patients with progressive metastatic castration-resistant prostate cancer (PSMAfore): A phase 3, randomised, controlled trial. Lancet 2024, 404, 1227–1239. [Google Scholar] [CrossRef]
- Ma, J.; Li, L.; Liao, T.; Gong, W.; Zhang, C. Efficacy and Safety of 225Ac-PSMA-617-Targeted Alpha Therapy in Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 796657. [Google Scholar]
- Beer, A.J.; Eiber, M.; Souvatzoglou, M.; Schwaiger, M.; Krause, B.J. Radionuclide and hybrid imaging of recurrent prostate cancer. Lancet Oncol. 2011, 12, 181–191. [Google Scholar] [CrossRef]
- Suokonautio, B.; Kouvonen, A.; Nordquist, H. Role identities of emergency medical services personnel and their associations with intention to leave the profession. BMC Emerg. Med. 2024, 24, 96. [Google Scholar] [CrossRef]
- Kranzbuhler, B.; Nagel, H.; Becker, A.S.; Muller, J.; Huellner, M.; Stolzmann, P.; Muehlematter, U.; Guckenberger, M.; Kaufmann, P.A.; Eberli, D.; et al. Clinical performance of 68Ga-PSMA-11 PET/MRI for the detection of recurrent prostate cancer following radical prostatectomy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Haberkorn, U.; Schlemmer, H.P.; Fenchel, M.; Eder, M.; Eisenhut, M.; Hadaschik, B.A.; Kopp-Schneider, A.; Rothke, M. Comparison of PET/CT and PET/MRI hybrid systems using a 68Ga-labelled PSMA ligand for the diagnosis of recurrent prostate cancer: Initial experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Elschot, M.; Selnaes, K.M.; Sandsmark, E.; Kruger-Stokke, B.; Storkersen, O.; Tessem, M.B.; Moestue, S.A.; Bertilsson, H.; Bathen, T.F. A PET/MRI study towards finding the optimal [18F]Fluciclovine PET protocol for detection and characterisation of primary prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 695–703. [Google Scholar] [CrossRef]
- Margail, C.; Merlin, C.; Billoux, T.; Wallaert, M.; Otman, H.; Sas, N.; Molnar, I.; Guillemin, F.; Boyer, L.; Guy, L.; et al. Imaging quality of an artificial intelligence denoising algorithm: Validation in 68Ga PSMA-11 PET for patients with biochemical recurrence of prostate cancer. EJNMMI Res. 2023, 13, 50. [Google Scholar] [CrossRef]
- Li, Y.; Imami, M.R.; Zhao, L.; Amindarolzarbi, A.; Mena, E.; Leal, J.; Chen, J.; Gafita, A.; Voter, A.F.; Li, X.; et al. An Automated Deep Learning-Based Framework for Uptake Segmentation and Classification on PSMA PET/CT Imaging of Patients with Prostate Cancer. J. Imaging Inform. Med. 2024, 37, 2206–2215. [Google Scholar] [CrossRef]
- Marturano, F.; Guglielmo, P.; Bettinelli, A.; Zattoni, F.; Novara, G.; Zorz, A.; Sepulcri, M.; Gregianin, M.; Paiusco, M.; Evangelista, L. Role of radiomic analysis of [18F]fluoromethylcholine PET/CT in predicting biochemical recurrence in a cohort of intermediate and high risk prostate cancer patients at initial staging. Eur. Radiol. 2023, 33, 7199–7208. [Google Scholar] [CrossRef]
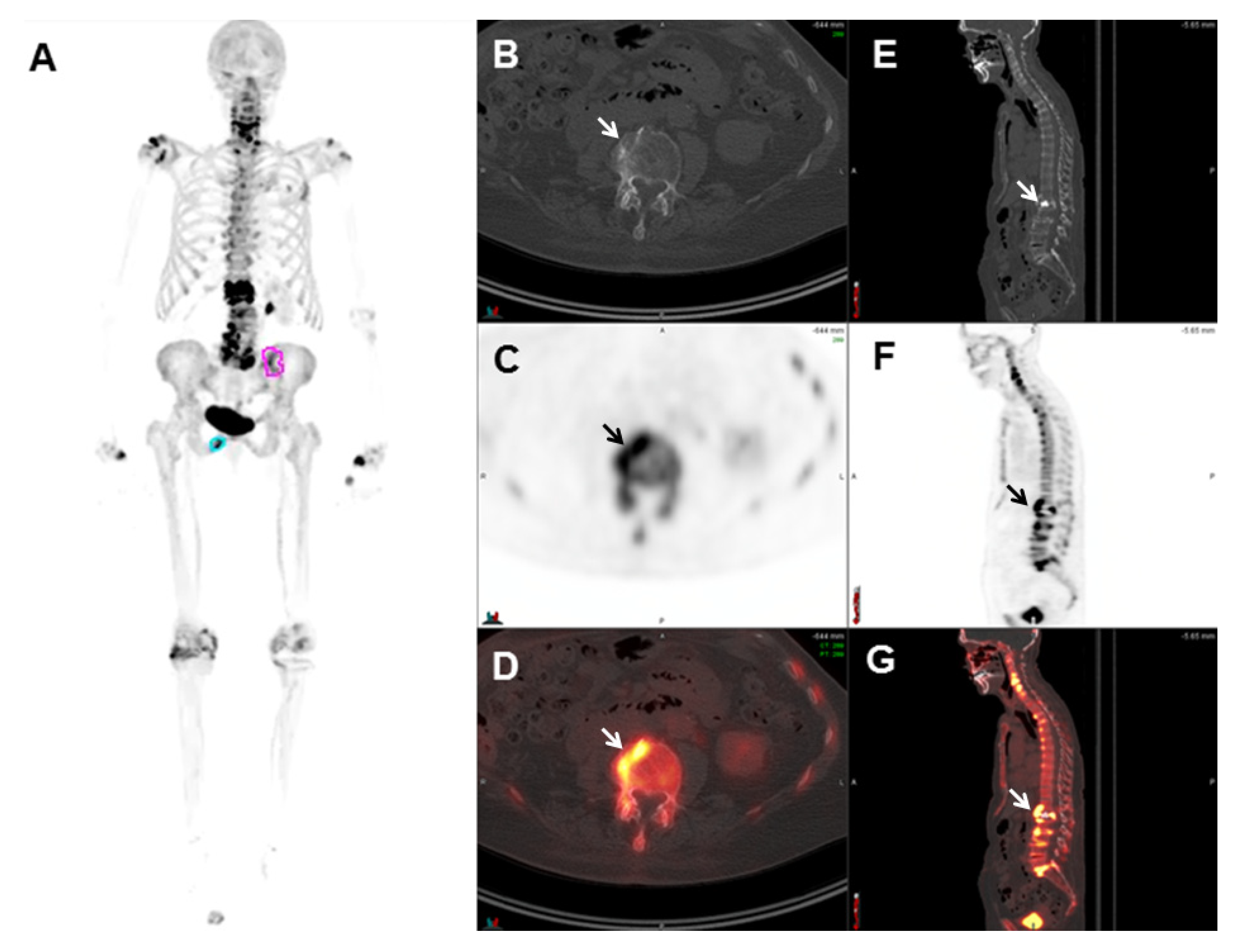
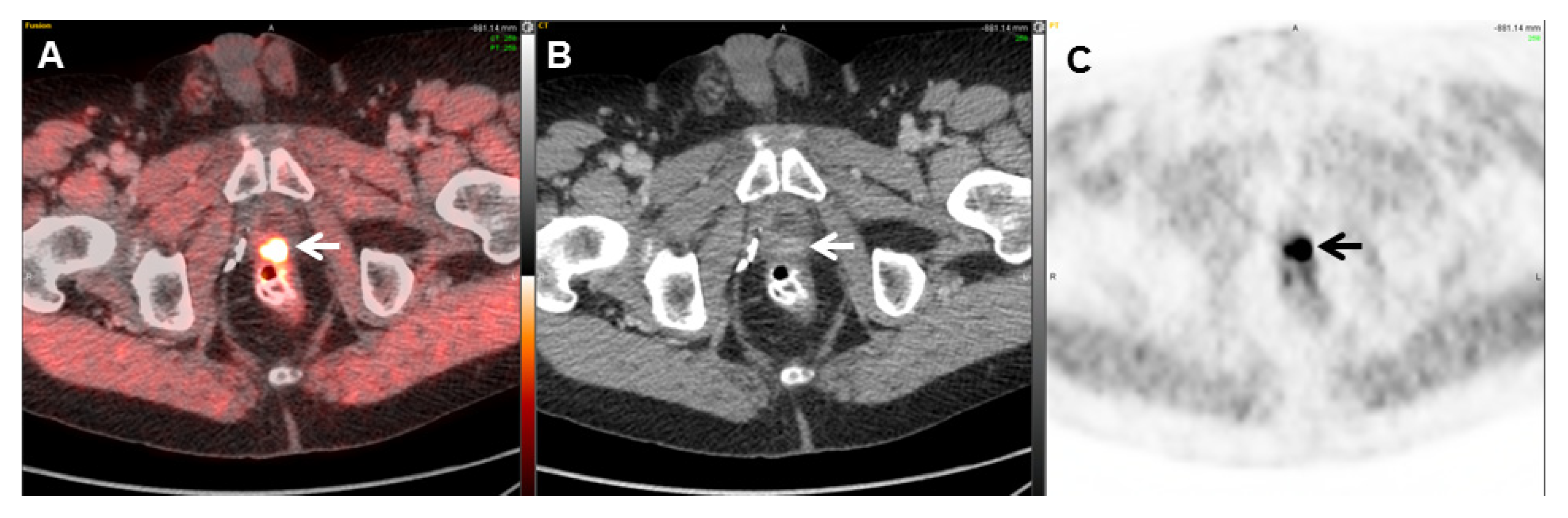
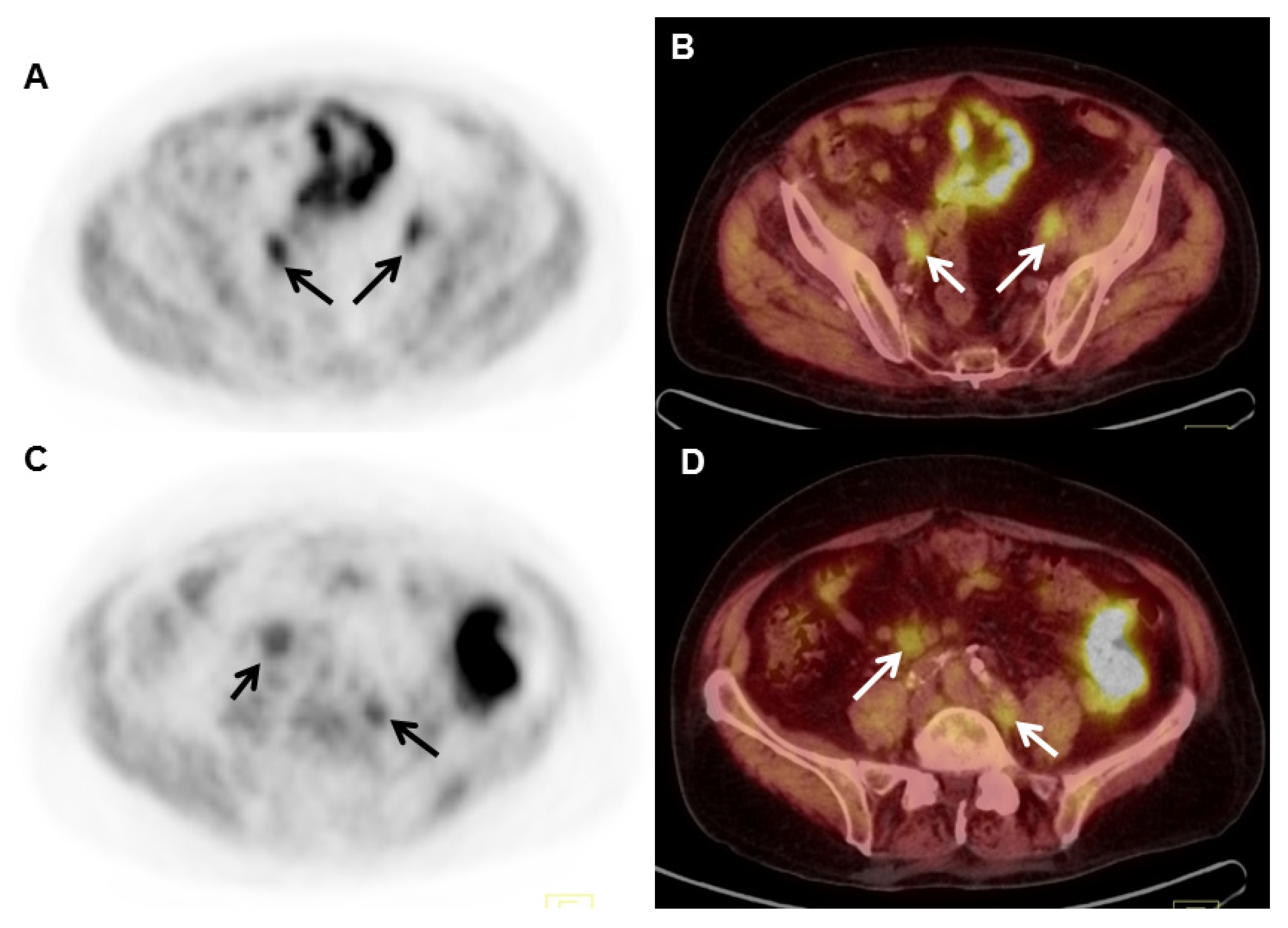
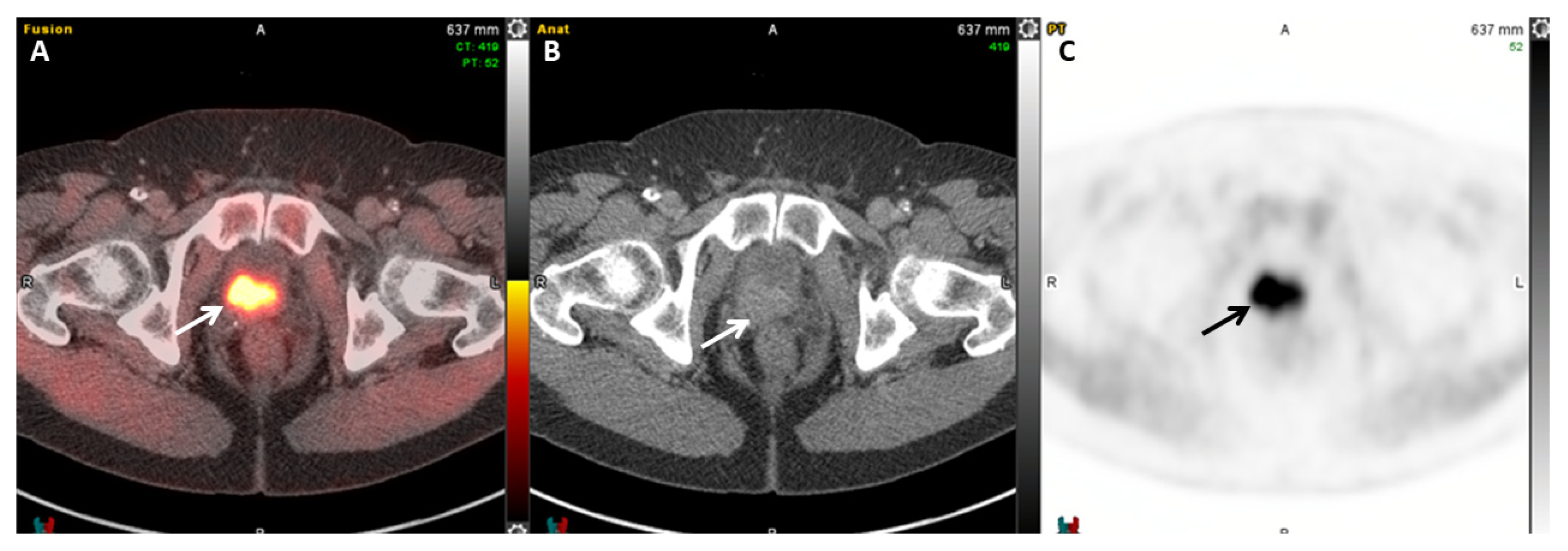
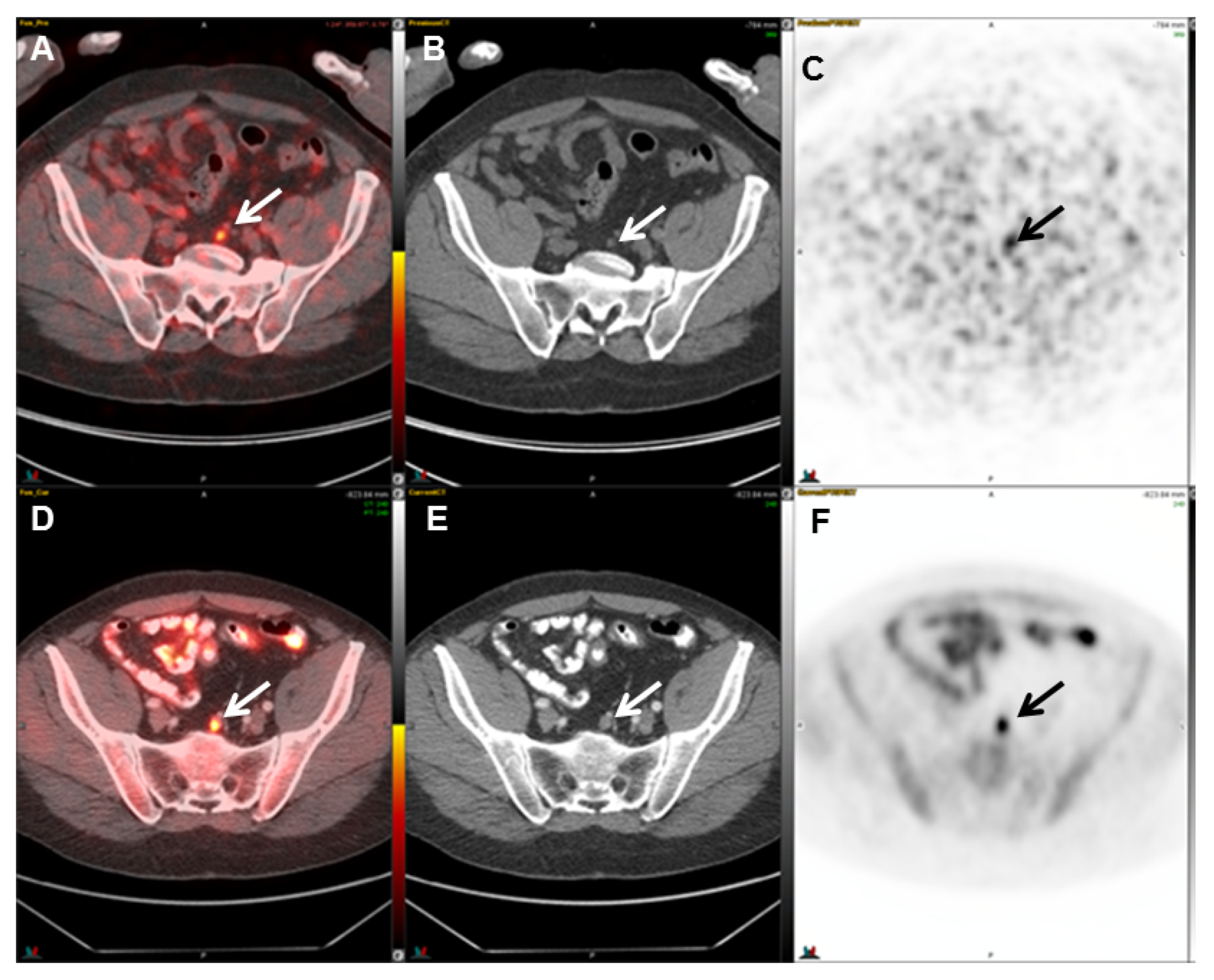
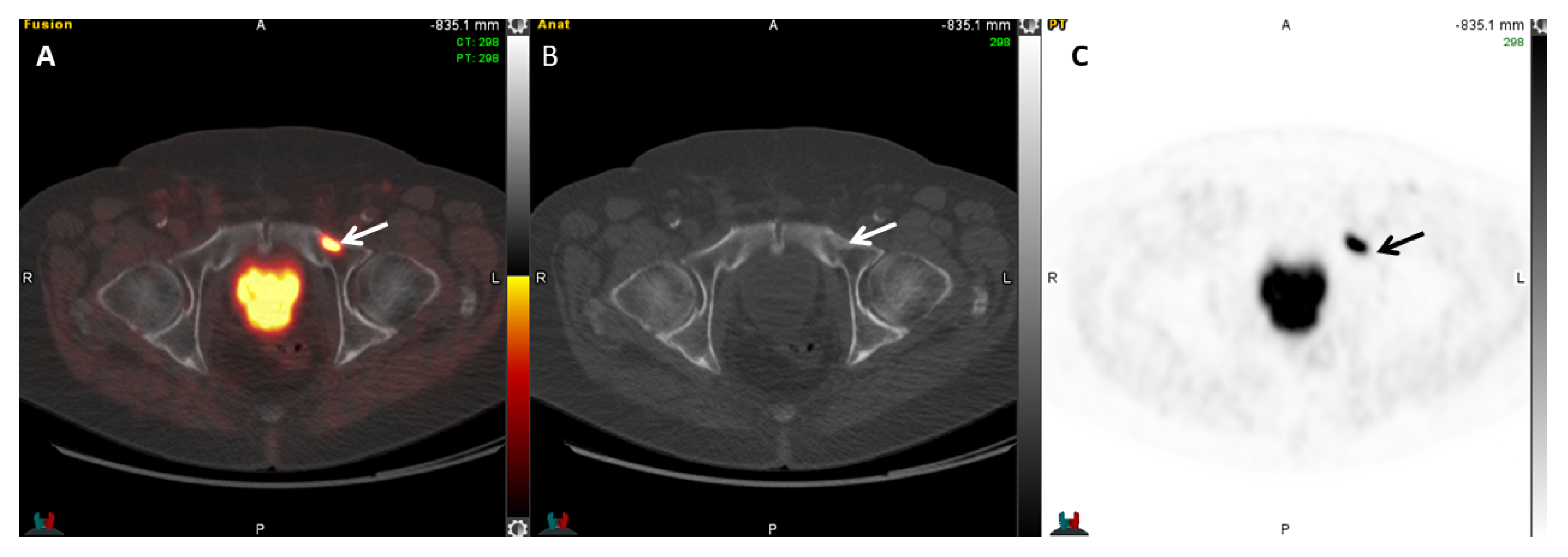
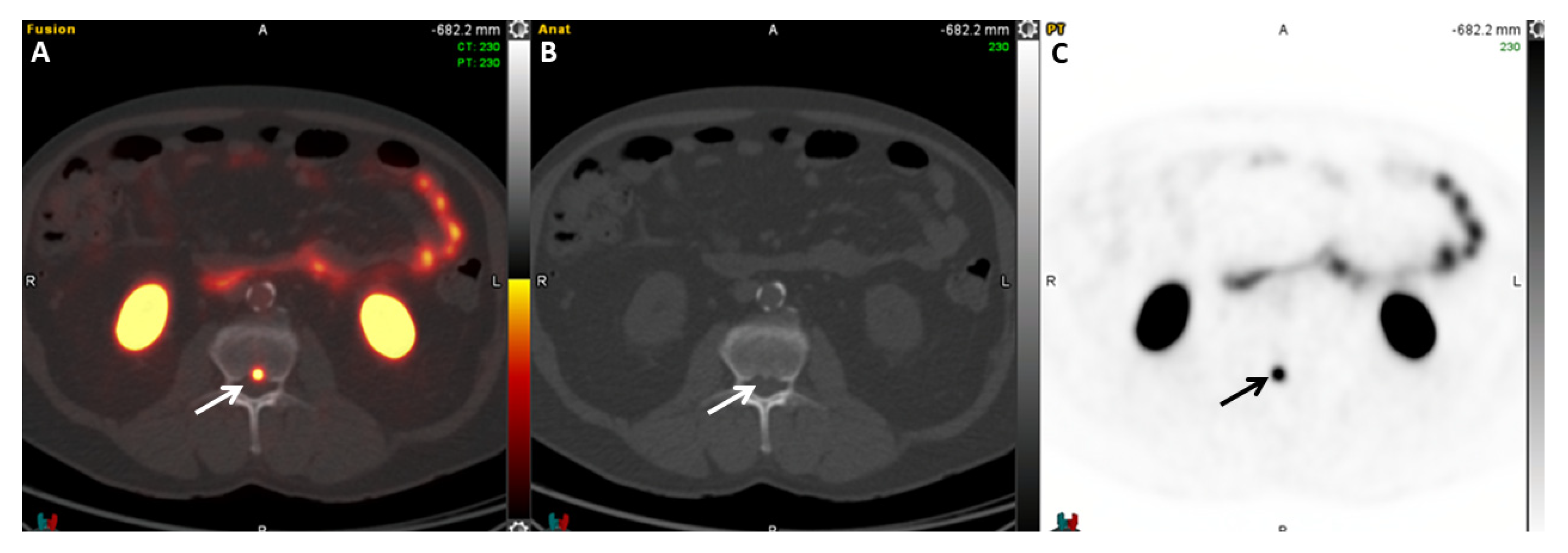
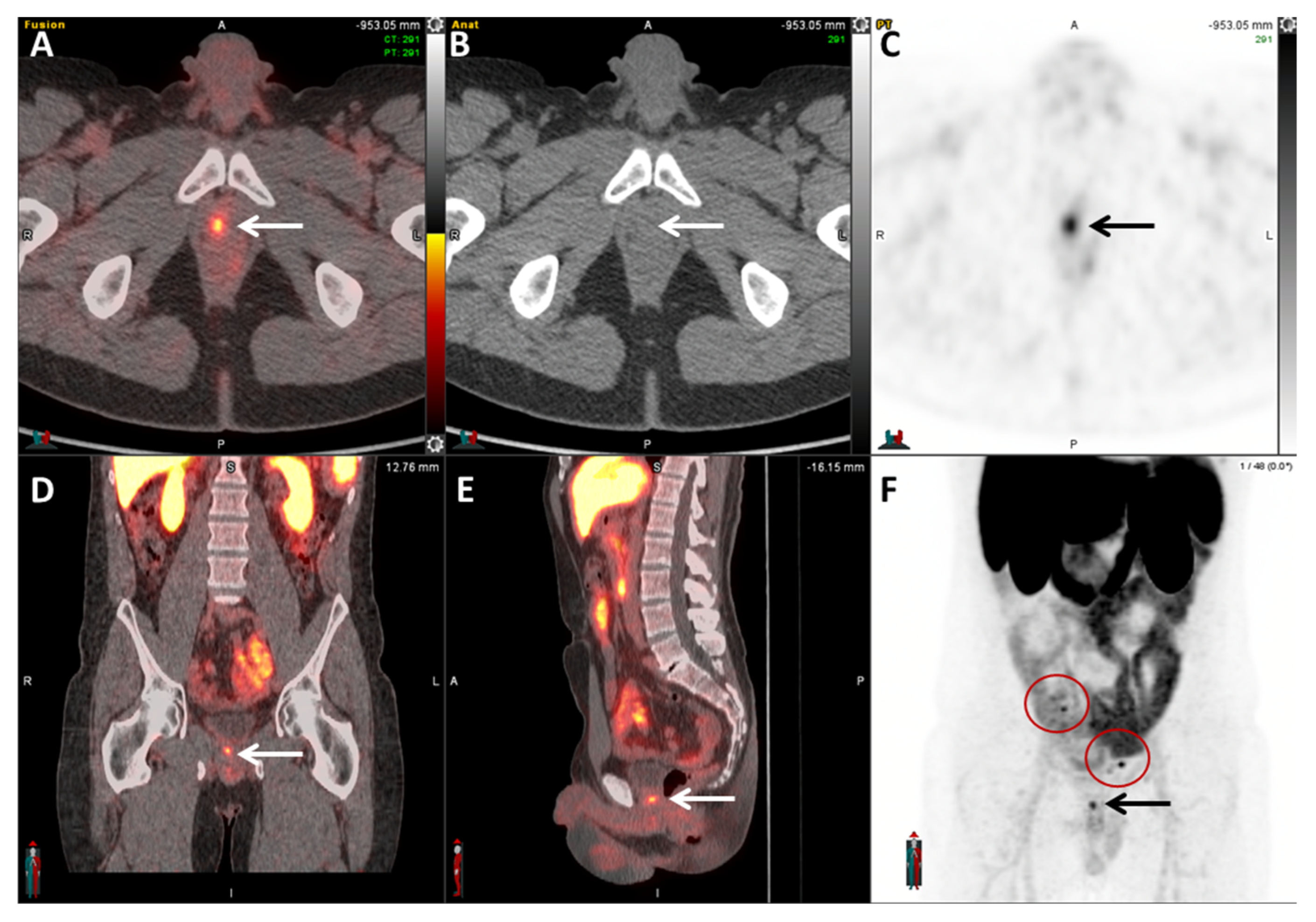
| Radiotracer | Event | Target | Advantage | Disadvantage |
|---|---|---|---|---|
| 18F-FDG | Glycolysis | glut1, glut3, hexokinase | Prognostic, aggressive disease | High urinary elimination, non-specific to prostate cancer |
| 18F-Choline; 11C-Choline | Membrane synthesis | Choline kinase | Rapid tissue uptake | Prone to confounding uptake due to inflammation or prostatic hypertrophy |
| 18F-NaF | Bone formation | Hydroxyapatite | Skeletal disease localization | Limited evaluation of soft-tissue lesions |
| 18F-Fluciclovine | Amino acid transport | l-amino acid transporter | Fast tumor cell uptake and minimal, early urinary excretion | Non-specific uptake related to benign prostatic hyperplasia, infection, and inflammation |
| 68Ga-PSMA-11 | Transmembrane protein | PSMA | TNM staging, post-treatment evaluation | High urinary bladder activity, non-specific uptake in other tumors |
| 64Cu-PSMA (DOTAGA and NODAGA) | Transmembrane protein | PSMA | High theragnostic potential, excellent image quality, radioconjugate stability | Low positron emission/not FDA approved yet |
| 64Cu-PSMA-I&T | Transmembrane protein | PSMA | Uses the same conjugate for imaging and therapy | Not FDA approved yet |
| 18F-DCFBC | Transmembrane protein | PSMA | Adequate tumor-to-muscle ratios | Persistent blood pool activity |
| 18F-DCFPyL | Transmembrane protein | PSMA | Rapid plasma clearance, low accumulation in the liver and muscle tissues | High physiological uptake in the salivary glands, kidneys, and urinary bladder |
| 18F-PSMA-1007 | Transmembrane protein | PSMA | Predominant hepatobiliary excretory route | False-positive bone lesions |
| 18F-rhPSMA-7.3 | Transmembrane protein | PSMA | Reduced urinary excretion than 18F-DCFPyL and 68Ga-PSMA-11 | False-positive bone lesions |
| 68Ga-RM2 | G protein-coupled receptor | Gastrin-releasing peptide receptor (GRPR) | High accuracy in identifying primary and locally recurrent disease | Slow major-vessel blood pool clearance complicating lymph node identification |
| 177Lu-PSMA-617, 225Ac-PSMA-617 | Transmembrane protein | PSMA | Therapy | Efficacy of treatment dependent on PSMA uptake |
| Radiotracer | Sensitivity | Specificity | Comments |
|---|---|---|---|
| 18F-FDG | + | + | Limited sensitivity; highly nonspecific, given uptake in inflammation. |
| 18F/11C-Choline | +++ | ++++ | Good sensitivity in patients with PSA values > 2.0; specificity improved from 18F-FDG and 18F-NaF. Limited specificity due to mild uptake in inflammation and infection and uptake in non-prostate malignancies and other benign processes. |
| 18F-NaF | ++ | + | Highly sensitive for the detection of bone metastases; however, has no role in the detection of soft-tissue lesions; highly nonspecific, given uptake in benign sclerotic lesions. |
| 18F-Fluciclovine (FACBC) | ++++ | +++ | Increased sensitivity in comparison to 18F/11C-Choline; limited specificity due to mild uptake in inflammation and infection and uptake in non-prostate malignancies and other benign processes. |
| 68Ga/18F/64Cu-PSMA-11 18F-DCFPyL 18F-PSMA-1007 18F-rhPSMA-7.3 | +++++ | +++++ | Increased sensitivity in comparison to 18F-Fluciclovine. False negatives in PSMA non-expressing prostate cancer (approximately 10% of prostate cancers); specificity slightly limited in patients with benign osseous lesions (particularly 18F-rhPSMA-7.3 and 18F-PSMA-1007) and uptake in non-prostate malignancies and other benign processes. |
| 68Ga-DOTA-Bombesin | +++ | ++++ | Data remain limited; however, promising alternative in cases of PSMA-negative disease. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bitar, R.; Zurita, P.; Martiniova, L.; Zurita, A.J.; Ravizzini, G.C. Positron Emission Tomography Radiotracers for Identification of Site of Recurrence in Prostate Cancer After Primary Treatment Failure. Cancers 2025, 17, 1723. https://doi.org/10.3390/cancers17101723
Bitar R, Zurita P, Martiniova L, Zurita AJ, Ravizzini GC. Positron Emission Tomography Radiotracers for Identification of Site of Recurrence in Prostate Cancer After Primary Treatment Failure. Cancers. 2025; 17(10):1723. https://doi.org/10.3390/cancers17101723
Chicago/Turabian StyleBitar, Ryan, Pablo Zurita, Lucia Martiniova, Amado J. Zurita, and Gregory C. Ravizzini. 2025. "Positron Emission Tomography Radiotracers for Identification of Site of Recurrence in Prostate Cancer After Primary Treatment Failure" Cancers 17, no. 10: 1723. https://doi.org/10.3390/cancers17101723
APA StyleBitar, R., Zurita, P., Martiniova, L., Zurita, A. J., & Ravizzini, G. C. (2025). Positron Emission Tomography Radiotracers for Identification of Site of Recurrence in Prostate Cancer After Primary Treatment Failure. Cancers, 17(10), 1723. https://doi.org/10.3390/cancers17101723







