Optimising Cancer Medicine in Clinical Practices: Are Neoadjuvant and Adjuvant Immunotherapies Affordable for Cancer Patients in Low- and Middle-Income Countries?
Simple Summary
Abstract
1. Introduction
2. Current Landscape of Licensed Anticancer Drugs
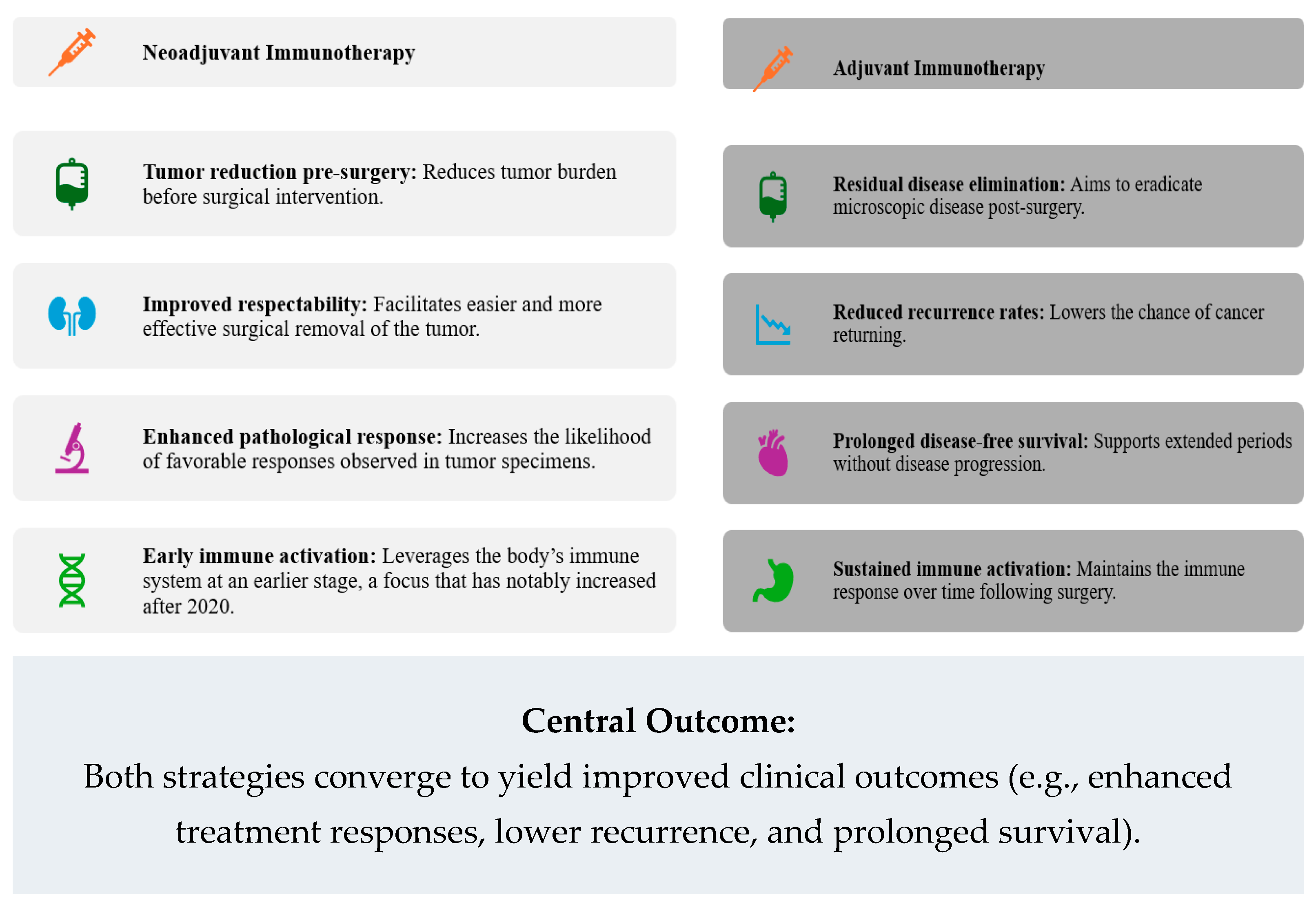
3. Clinical Significance of Neoadjuvant and Adjuvant Immunotherapies
4. Cost and Affordability Challenges
5. Strategies to Improve Accessibility and Affordability
6. Recommendations
6.1. Implement Targeted Policy Interventions
- Advocate for negotiations and global or regional pooled procurement mechanisms to lower the cost of high-priced immunotherapeutic agents.
- Expand cancer-specific insurance schemes and introduce targeted subsidies to reduce out-of-pocket expenses, ensuring that patients in LMICs can access these therapies without financial hardship.
- Develop transparent pricing and reimbursement policies that balance innovation with equitable access, guided by international organisations (e.g., the WHO).
6.2. Strengthen Health Infrastructure and Capacity
- Invest in developing robust diagnostic facilities, including cost-effective biomarker testing (e.g., simplified PD-L1 assays or blood-based diagnostics) to accurately identify eligible patients.
- Increase training for oncologists, laboratory technicians, and support staff to ensure proper patient selection, monitoring, and management of immunotherapy-related adverse events.
- Improve logistics, including reliable drug supply chains and storage facilities, to support consistent availability of immunotherapeutic agents.
6.3. Promote Local Production and Technology Transfer
- Adopt partnerships among governments, local industries, and international pharmaceutical companies to transfer technology and set up local production facilities, potentially reducing costs and dependency on imports.
6.4. Expand Collaborative Research and Clinical Trials
- Initiate and support clinical trials within LMIC populations to generate context-specific evidence on immunotherapy efficacy, safety, and cost-effectiveness.
- Develop international collaborations that bring together researchers from HICs and LMICs, ensuring that clinical guidelines are tailored to regional epidemiological and socioeconomic conditions.
6.5. Incorporate Policy Guidance on Resource-Appropriate Care
- Urge policymakers and guideline developers in LMICs to integrate economic criteria into national cancer control programs, prioritising interventions demonstrated to be both effective and cost-saving.
- Drawing on the EAGLE-FM example [37], recommend that regional health authorities consider formal health technology assessments before adopting high-cost immunotherapies or extensive surgeries.
7. Future Directions
8. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Marken, V.; Timmons, J.A.; Pedersen, B.K. The spectrum of benefit from checkpoint blockade in hypermutated tumors. N. Engl. J. Med. 2021, 384, 1168–1170. [Google Scholar]
- Powles, T.; Eder, J.P.; Fine, G.D.; Braiteh, F.S.; Loriot, Y.; Cruz, C.; Bellmunt, J.; Burris, H.A.; Petrylak, D.P.; Teng, S.L.; et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014, 515, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair–Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef]
- Forde, P.M.; Chaft, J.E.; Smith, K.N.; Anagnostou, V.; Cottrell, T.R.; Hellmann, M.D.; Zahurak, M.; Yang, S.C.; Jones, D.R.; Broderick, S.; et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N. Engl. J. Med. 2018, 378, 1976–1986. [Google Scholar] [CrossRef]
- Ocran Mattila, P.; Ahmad, R.; Hasan, S.S.; Babar, Z.U.D. Availability, affordability, access, and pricing of anti-cancer medicines in low- and middle-income countries: A systematic review of literature. Front. Public Health 2021, 9, 628744. [Google Scholar] [CrossRef]
- Cardone, C.; Arnold, D. The cancer treatment gap in lower- to middle-income countries. Oncology 2023, 101, 2–4. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Long, G.V.; Saw, R.P.; Lo, S.; Nieweg, O.E.; Shannon, K.F.; Gonzalez, M.; Guminski, A.; Lee, J.H.; Lee, H.; Ferguson, P.M.; et al. Neoadjuvant dabrafenib combined with trametinib for resectable, stage IIIB–C, BRAFV600 mutation-positive melanoma (NeoCombi): A single-arm, open-label, single-centre, phase 2 trial. Lancet Oncol. 2019, 20, 961–971. [Google Scholar] [CrossRef]
- Jenei, K.; Aziz, Z.; Booth, C.; Cappello, B.; Ceppi, F.; de Vries, E.G.; Fojo, A.; Gyawali, B.; Ilbawi, A.; Lombe, D.; et al. Cancer medicines on the WHO Model List of Essential Medicines: Processes, challenges, and a way forward. Lancet Glob. Health 2022, 10, e1860–e1866. [Google Scholar] [CrossRef]
- Amaria, R.N.; Prieto, P.A.; Tetzlaff, M.T.; Reuben, A.; Andrews, M.C.; Ross, M.I.; Glitza, I.C.; Cormier, J.; Hwu, W.J.; Tawbi, H.A.; et al. Neoadjuvant plus adjuvant dabrafenib and trametinib versus standard of care in patients with high-risk, surgically resectable melanoma: A single-centre, open-label, randomised, phase 2 trial. Lancet Oncol. 2018, 19, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Provencio, M.; Nadal, E.; Insa, A.; García-Campelo, M.R.; Casal-Rubio, J.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; Carpeño, J.D.C.; et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef]
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
- Liu, J.; Kang, Y.; Li, L.; Zang, L.; Luo, L.; Zhu, F.; Zhu, M.; Zhang, H.; Xu, Q. Efficacy and safety of immunotherapy in cervical cancer: A review of advances in immune-related therapy for cervical cancer. Holist. Integr. Oncol. 2025, 4, 15. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Xu, M.; Cao, C.; Wu, P.; Huang, X.; Ma, D. Advances in cervical cancer: Current insights and future directions. Cancer Commun. 2024, 45, 77–109. [Google Scholar] [CrossRef]
- Lien, A.C.; Johnson, G.S.; Guan, T.; Burns, C.P.; Parker, J.M.; Dong, L.; Wakefield, M.R.; Fang, Y. The past, present, and future of cervical cancer vaccines. Vaccines 2025, 13, 201. [Google Scholar] [CrossRef] [PubMed]
- Dey, T.; Agrawal, S. Immunotherapy in cervical cancer: An innovative approach for better treatment outcomes. Explor. Target. Anti Tumor Ther. 2025, 6, 1002296. [Google Scholar] [CrossRef]
- Vogler, S. Can we achieve affordable cancer medicine prices? Developing a pathway for change. Expert Rev. pharmacoecon. Outcomes Res. 2021, 21, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Leighl, N.B.; Nirmalakumar, S.; Ezeife, D.A.; Gyawali, B. An Arm and a Leg: The Rising Cost of Cancer Drugs and Impact on Access. In American Society of Clinical Oncology Educational Book; ASCO Publications: Alexandria, VA, USA, 2021; Volume 41, pp. 1–12. [Google Scholar]
- Mahoney, K.M.; Rennert, P.D.; Freeman, G.J. Combination cancer immunotherapy and new immunomodulatory targets. Nat. Rev. Drug Discov. 2015, 14, 561–584. [Google Scholar] [CrossRef]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef]
- Tran, G.; Zafar, S.Y. Financial toxicity and implications for cancer care in the era of molecular and immune therapies. Ann. Transl. Med. 2018, 6, 166. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Knight, R.; Roughead, E.E.; Brooks, G.; Mant, A. Policy options for pharmaceutical pricing and purchasing: Issues for low- and middle-income countries. Health Policy Plan. 2015, 30, 267–280. [Google Scholar] [CrossRef]
- Chalkidou, K.; Glassman, A.; Marten, R.; Vega, J.; Teerawattananon, Y.; Tritasavit, N.; Gyansa-Lutterodt, M.; Seiter, A.; Kieny, M.P.; Hofman, K.; et al. Priority-setting for achieving universal health coverage. Bull. World Health Organ. 2016, 94, 462–467. [Google Scholar] [CrossRef]
- Moon, S.; Jambert, E.; Childs, M.; von Schoen-Angerer, T. A win-win solution? A critical analysis of tiered pricing to improve access to medicines in developing countries. Glob. Health 2011, 7, 39. [Google Scholar] [CrossRef]
- Mahumud, R.A.; Janda, M.; Soyer, H.P.; Fernández-Peñas, P.; Mar, V.J.; Morton, R.L. Assessing the value of precision medicine health technologies to detect and manage melanoma. Med. J. Aust. 2022, 217, 275–278. [Google Scholar] [CrossRef]
- Favre-Bulle, A.; Bencina, G.; Zhang, S.; Jiang, R.; Andritschke, D.; Bhadhuri, A. Cost-effectiveness of pembrolizumab as an adjuvant treatment for patients with resected stage IIB or IIC melanoma in Switzerland. J. Med. Econ. 2023, 26, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Pompilio, G.; Campanella, P.; Integlia, D. Cost-effectiveness and budget impact of new therapies for metastatic melanoma. Eur. J. Public Health 2017, 27 (Suppl. S3), 461. [Google Scholar] [CrossRef]
- Sirohi, B.; Chalkidou, K.; Pramesh, C.S.; Anderson, B.O.; Loeher, P.; El Dewachi, O.; Shamieh, O.; Shrikhande, S.V.; Venkataramanan, R.; Parham, G.; et al. Developing institutions for cancer care in low-income and middle-income countries: From cancer units to comprehensive cancer centres. Lancet Oncol. 2018, 19, e395–e406. [Google Scholar] [CrossRef]
- Peppercorn, J.M.; Smith, T.J.; Helft, P.R.; DeBono, D.J.; Berry, S.R.; Wollins, D.S.; Hayes, D.M.; Von Roenn, J.H.; Schnipper, L.E. American society of clinical oncology statement: Toward individualized care for patients with advanced cancer. J. Clin. Oncol. 2011, 29, 755–760. [Google Scholar] [CrossRef]
- WHO. WHO report on cancer: Setting priorities, investing wisely and providing care for all. In Die Gynäkologie; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Mahumud, R.A.; Law, C.K.; Ospino, D.A.; de Wilt, J.H.; van Leeuwen, B.L.; Allan, C.; de Lima Vazquez, V.; Jones, R.P.; Howle, J.; Spillane, A.J.; et al. Economic evaluation of inguinal versus Ilio inguinal lymphadenectomy for patients with stage III metastatic melanoma to groin lymph nodes: Evidence from the EAGLE FM randomized trial. Ann. Surg. Oncol. 2025, 32, 4211–4222. [Google Scholar] [CrossRef]
| Summary Results as of Last Build on 28 November 2024 | Number | Percentage |
|---|---|---|
| Number of licensed anticancer drugs | 321 | |
| FDA-approved anticancer drugs | 290 | 90.34% |
| EMA-approved anticancer drugs | 215 | 66.98% |
| Euro nationally approved anticancer drugs | 50 | 15.58% |
| Non-FDA/EMA/European | 11 | 3.43% |
| Drugs included in WHO EML | 54 | 16.82% |
| Single API drugs | 317 | 98.75% |
| Combination (>1 API) drugs | 4 | 1.25% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahumud, R.A. Optimising Cancer Medicine in Clinical Practices: Are Neoadjuvant and Adjuvant Immunotherapies Affordable for Cancer Patients in Low- and Middle-Income Countries? Cancers 2025, 17, 1722. https://doi.org/10.3390/cancers17101722
Mahumud RA. Optimising Cancer Medicine in Clinical Practices: Are Neoadjuvant and Adjuvant Immunotherapies Affordable for Cancer Patients in Low- and Middle-Income Countries? Cancers. 2025; 17(10):1722. https://doi.org/10.3390/cancers17101722
Chicago/Turabian StyleMahumud, Rashidul Alam. 2025. "Optimising Cancer Medicine in Clinical Practices: Are Neoadjuvant and Adjuvant Immunotherapies Affordable for Cancer Patients in Low- and Middle-Income Countries?" Cancers 17, no. 10: 1722. https://doi.org/10.3390/cancers17101722
APA StyleMahumud, R. A. (2025). Optimising Cancer Medicine in Clinical Practices: Are Neoadjuvant and Adjuvant Immunotherapies Affordable for Cancer Patients in Low- and Middle-Income Countries? Cancers, 17(10), 1722. https://doi.org/10.3390/cancers17101722





