From Intensification to Optimization: Balancing Efficacy, Safety, and Costs in High-Risk Localized Soft Tissue Sarcomas
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Population
2.2.1. uhpRT Cohort
2.2.2. SU2C-SARC032 RCT Cohort
2.2.3. Comparison of Inclusion Criteria
2.3. Treatment Protocols
- Control Arm: Patients received conventional normofractionated radiotherapy (50 Gy in 25 fractions over 5 weeks) followed by surgery without systemic therapy.
- Experimental Arm: Patients received conventional normofractionated radiotherapy combined with systemic therapy (pembrolizumab), administered over a median of 14 cycles (maximum 17), spanning approximately 3–11 months (Table 2).
2.4. Outcome Measures
2.4.1. uhpRT Study Endpoints
2.4.2. SU2C-SARC032 Trial Endpoints
2.4.3. Alignment and Deviations
2.5. Statistical Analysis
2.5.1. Cohort Analysis
2.5.2. Comparison with SU2C-SARC032 Trial
2.5.3. Adjustments and Limitations
3. Results
3.1. Patient Characteristics and Treatment Protocols
3.1.1. Baseline Characteristics
3.1.2. Treatment Protocols
3.2. Survival Outcomes
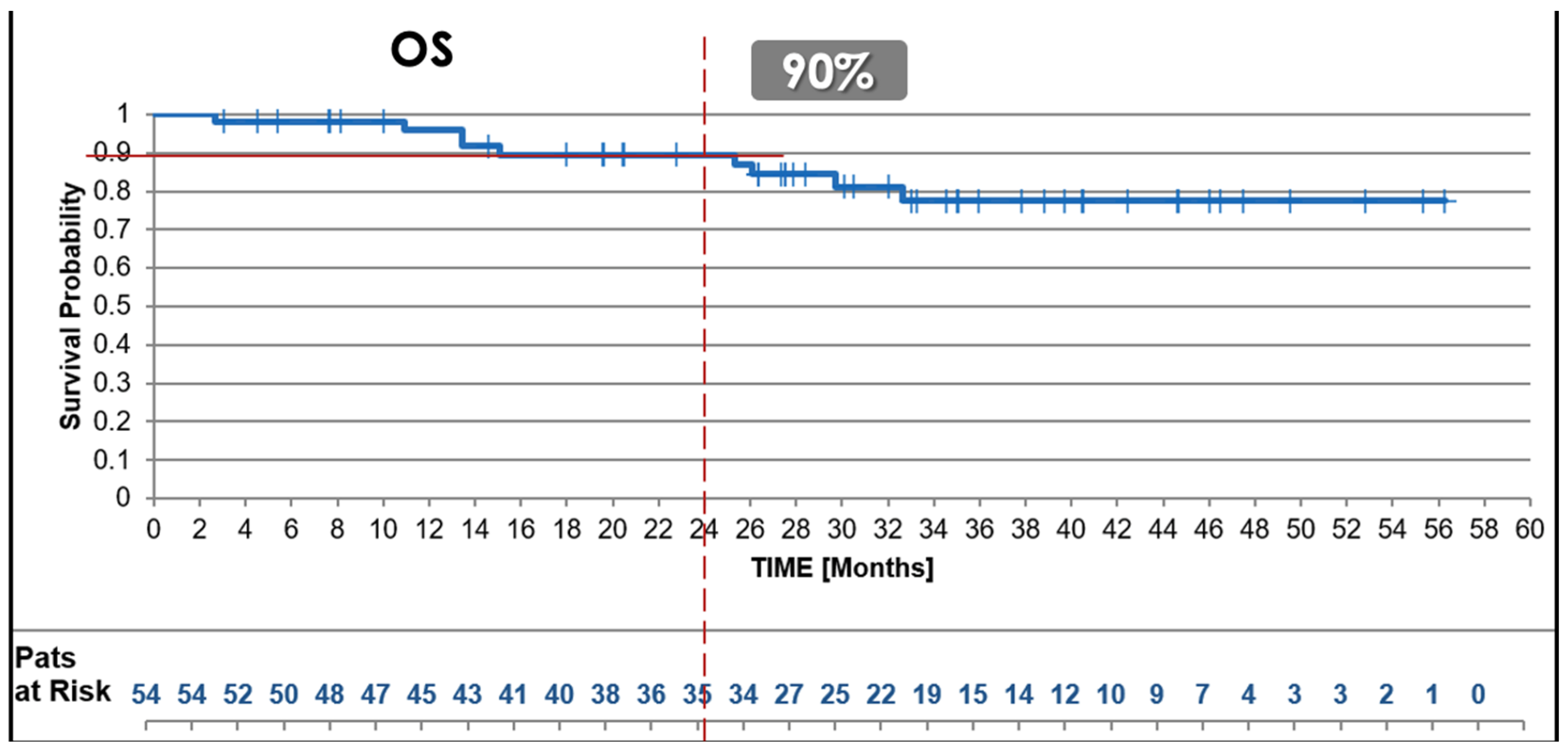
3.2.1. Overall Survival (OS)
3.2.2. Disease-Free Survival (DFS)
3.2.3. Local Disease-Free Survival (LDFS)
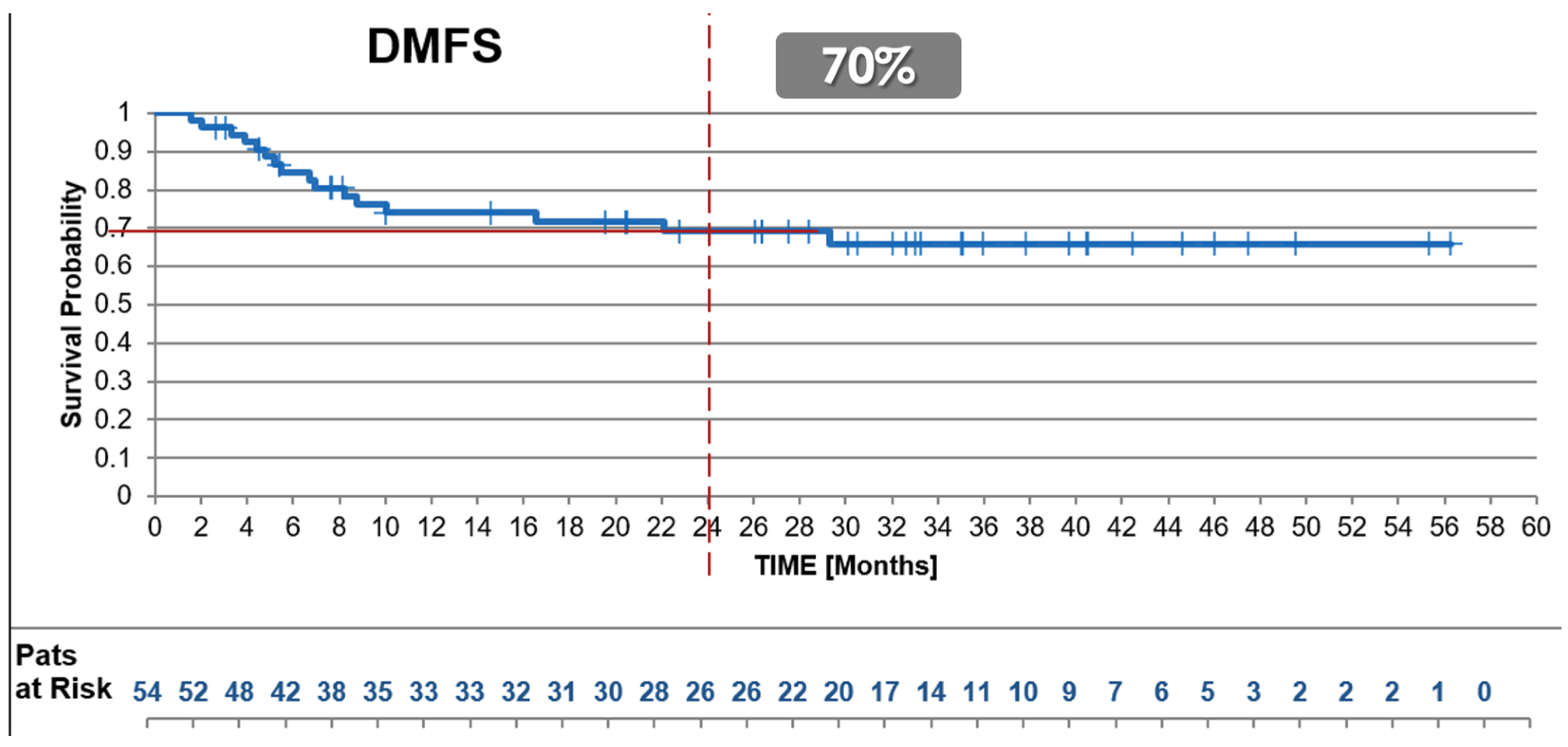
3.2.4. Distant Disease-Free Survival (DDFS)
3.3. Safety and Adverse Events
3.3.1. Radiation-Related Early Side Effects
3.3.2. Wound Complications
3.3.3. Late-Term Effects
3.3.4. Overall Adverse Events
3.4. Subgroup Analysis
3.4.1. Tumor Grade
3.4.2. Patient Age
3.4.3. Tumor Volume
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antonescu, C.; Blay, J. WHO Classification of Tumours: Soft Tissue and Bone Tumours, 5th ed.; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Kollár, A.; Rothermundt, C.; Klenke, F.; Bode, B.; Baumhoer, D.; Arndt, V.; Feller, A. Incidence, mortality, and survival trends of soft tissue and bone sarcoma in Switzerland between 1996 and 2015. Cancer Epidemiol. 2019, 63, 101596. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft tissue and visceral sarcomas: ESMO–EURACAN–GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef]
- Blay, J.Y.; Hindi, N.; Bollard, J.; Aguiar, S.; Angel, M.; Araya, B.; Badilla, R.; Bernabeu, D.; Campos, F.; Caro-Sánchez, C.H.S.; et al. SELNET clinical practice guidelines for soft tissue sarcoma and GIST. Cancer Treat. Rev. 2022, 102, 102312. [Google Scholar] [CrossRef]
- Blank, A.T.; Larson, B.M.; Shaw, S.; Wakefield, C.J.; King, T.; Jones, K.B.; Randall, R.L. National Comprehensive Cancer Network guidelines compliance of a sarcoma service: A retrospective review. World J. Clin. Oncol. 2020, 11, 389–396. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Davis, A.M.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Kandel, R.; Goddard, K.; Sadura, A.; et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet 2002, 359, 2235–2241. [Google Scholar] [CrossRef]
- Haas, R.L.; Gronchi, A.; van de Sande, M.A.J.; Baldini, E.H.; Gelderblom, H.; Messiou, C.; Wardelmann, E.; Le Cesne, A. Perioperative Management of Extremity Soft Tissue Sarcomas. J. Clin. Oncol. 2018, 36, 118–124. [Google Scholar] [CrossRef]
- Braik, D.; Lemieux, C.; Wilson, B.E.; Salawu, A.; Abdul Razak, A.R. Clinical benefit and fragility evaluation of systemic therapy trials for advanced soft tissue sarcoma. Cancer 2025, 131, e35564. [Google Scholar] [CrossRef]
- Le Cesne, A.; Ouali, M.; Leahy, M.G.; Santoro, A.; Hoekstra, H.J.; Hohenberger, P.; Van Coevorden, F.; Rutkowski, P.; Van Hoesel, R.; Verweij, J.; et al. Doxorubicin-based adjuvant chemotherapy in soft tissue sarcoma: Pooled analysis of two STBSG-EORTC phase III clinical trials. Ann. Oncol. 2014, 25, 2425–2432. [Google Scholar] [CrossRef]
- Gronchi, A.; Palmerini, E.; Quagliuolo, V.; Martin Broto, J.; Lopez Pousa, A.; Grignani, G.; Brunello, A.; Blay, J.-Y.; Tendero, O.; Diaz Beveridge, R.; et al. Neoadjuvant Chemotherapy in High-Risk Soft Tissue Sarcomas: Final Results of a Randomized Trial From Italian (ISG), Spanish (GEIS), French (FSG), and Polish (PSG) Sarcoma Groups. J. Clin. Oncol. (JCO) 2020, 38, 2178–2186. [Google Scholar] [CrossRef]
- Gyawali, B.; Booth, C.M. Cancer treatments should benefit patients: A common-sense revolution in oncology. Nat. Med. 2022, 28, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Soon, J.A.; Franchini, F.; IJzerman, M.J.; McArthur, G.A. Leveraging the potential for deintensification in cancer care. Nat. Cancer 2024, 5, 1597–1599. [Google Scholar] [CrossRef] [PubMed]
- Roohani, S.; Handtke, J.; Hummedah, K.; Albertsmeier, M.; Andreou, D.; Apostolidis, L.; Augustin, M.; Bauer, S.; Billner, M.; Bosch, F.; et al. The sarcoma ring trial: A case-based analysis of inter-center agreement across 21 German-speaking sarcoma centers. J. Cancer Res. Clin. Oncol. 2025, 151, 30. [Google Scholar] [CrossRef] [PubMed]
- Roland, C.L.; van Houdt, W.; Gronchi, A. The Landmark Series: Multimodality Treatment of Extremity Sarcoma. Ann. Surg. Oncol. 2020, 27, 3672–3682. [Google Scholar] [CrossRef]
- Wilson, R.; Reinke, D.; van Oortmerssen, G.; Gonzato, O.; Ott, G.; Raut, C.P.; Guadagnolo, B.A.; Haas, R.L.M.; Trent, J.; Jones, R.; et al. What Is a Sarcoma ‘Specialist Center’? Multidisciplinary Research Finds an Answer. Cancers 2024, 16, 1857. [Google Scholar] [CrossRef]
- Campbell, S.R.; Wooley, J.R.; Nystrom, L.M. Modern Multidisciplinary Management of Soft Tissue Sarcoma of the Extremity and Trunk. JCO Oncol. Pract. 2024, 20, 907–914. [Google Scholar] [CrossRef]
- Mowery, Y.M.; Ballman, K.V.; Hong, A.M.; Schuetze, S.M.; Wagner, A.J.; Monga, V.; Heise, R.S.; Attia, S.; Choy, E.; Burgess, M.A.; et al. Safety and efficacy of pembrolizumab, radiation therapy, and surgery versus radiation therapy and surgery for stage III soft tissue sarcoma of the extremity (SU2C-SARC032): An open-label, randomised clinical trial. Lancet 2024, 404, 2053–2064. [Google Scholar] [CrossRef]
- Haddox, C.L.; Nathenson, M.J.; Mazzola, E.; Lin, J.-R.; Baginska, J.; Nau, A.; Weirather, J.L.; Choy, E.; Marino-Enriquez, A.; Morgan, J.A.; et al. Phase II Study of Eribulin plus Pembrolizumab in Metastatic Soft-tissue Sarcomas: Clinical Outcomes and Biological Correlates. Clin. Cancer Res. 2024, 30, 1281–1292. [Google Scholar] [CrossRef]
- Popat, S.; Curioni-Fontecedro, A.; Dafni, U.; Shah, R.; O’Brien, M.; Pope, A.; Fisher, P.; Spicer, J.; Roy, A.; Gilligan, D.; et al. A multicentre randomised phase III trial comparing pembrolizumab vs. single-agent chemotherapy for advanced pre-treated malignant pleural mesothelioma: The European Thoracic Oncology Platform (ETOP 9-15) PROMISE-meso trial. Ann. Oncol. 2020, 31, 1734–1745. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- Heesen, P.; Schelling, G.; Birbaumer, M.; Jager, R.; Bode, B.; Studer, G.; Fuchs, B.; Swiss Sarcoma, N. Real-World-Time Data and RCT Synergy: Advancing Personalized Medicine and Sarcoma Care through Digital Innovation. Cancers 2024, 16, 2516. [Google Scholar] [CrossRef] [PubMed]
- Heesen, P.; Di Lonardo, M.; Ciobanu-Caraus, O.; Schelling, G.; Zwahlen, D.; Bode-Lesniewska, B.; Glanzmann, C.; Studer, G.; Fuchs, B.; Swiss Sarcoma, N. Ultrahypofractionated Versus Normofractionated Preoperative Radiotherapy for Soft Tissue Sarcoma: A Multicenter, Prospective Real-World-Time Phase 2 Clinical Trial. Cancers 2024, 16, 4063. [Google Scholar] [CrossRef] [PubMed]
- Mattmann, A.; Glanzmann, C.; Fuchs, B.; Bode, B.; Studer, G.; Swiss Sarcoma, N. Preoperative Ultrahypofractionated Radiation Therapy for Soft Tissue Sarcomas: Low Rate of Wound Complications. Adv. Radiat. Oncol. 2024, 9, 101562. [Google Scholar] [CrossRef] [PubMed]
- Kosela-Paterczyk, H.; Szumera-Cieckiewicz, A.; Szacht, M.; Haas, R.; Morysinski, T.; Dziewirski, W.; Prochorec-Sobieszek, M.; Rutkowski, P. Efficacy of neoadjuvant hypofractionated radiotherapy in patients with locally advanced myxoid liposarcoma. Eur. J. Surg. Oncol. 2016, 42, 891–898. [Google Scholar] [CrossRef]
- Kalbasi, A.; Kamrava, M.; Chu, F.I.; Telesca, D.; Van Dams, R.; Yang, Y.; Ruan, D.; Nelson, S.D.; Dry, S.M.; Hernandez, J.; et al. A Phase II Trial of 5-Day Neoadjuvant Radiotherapy for Patients with High-Risk Primary Soft Tissue Sarcoma. Clin. Cancer Res. 2020, 26, 1829–1836. [Google Scholar] [CrossRef]
- Guadagnolo, B.A.; Bassett, R.L.; Mitra, D.; Farooqi, A.; Hempel, C.; Dorber, C.; Willis, T.; Wang, W.-L.; Ratan, R.; Somaiah, N.; et al. Hypofractionated, 3-week, preoperative radiotherapy for patients with soft tissue sarcomas (HYPORT-STS): A single-centre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2022, 23, 1547–1557. [Google Scholar] [CrossRef]
- Pointer, K.B.; Pitroda, S.P.; Weichselbaum, R.R. Radiotherapy and immunotherapy: Open questions and future strategies. Trends Cancer 2022, 8, 9–20. [Google Scholar] [CrossRef]
- Schaue, D.; Ratikan, J.A.; Iwamoto, K.S.; McBride, W.H. Maximizing tumor immunity with fractionated radiation. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1306–1310. [Google Scholar] [CrossRef]
- Formenti, S.C.; Demaria, S. Combining radiotherapy and cancer immunotherapy: A paradigm shift. J. Natl. Cancer Inst. 2013, 105, 256–265. [Google Scholar] [CrossRef]
- Golden, E.B.; Apetoh, L. Radiotherapy and immunogenic cell death. Semin. Radiat. Oncol. 2015, 25, 11–17. [Google Scholar] [CrossRef]
- Dovedi, S.J.; Cheadle, E.J.; Popple, A.L.; Poon, E.; Morrow, M.; Stewart, R.; Yusko, E.C.; Sanders, C.M.; Vignali, M.; Emerson, R.O.; et al. Fractionated Radiation Therapy Stimulates Antitumor Immunity Mediated by Both Resident and Infiltrating Polyclonal T-cell Populations when Combined with PD-1 Blockade. Clin. Cancer Res. 2017, 23, 5514–5526. [Google Scholar] [CrossRef] [PubMed]
- Demaria, S.; Ng, B.; Devitt, M.L.; Babb, J.S.; Kawashima, N.; Liebes, L.; Formenti, S.C. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 862–870. [Google Scholar] [CrossRef]
- Fahey, C.C.; Rathmell, W.K. Clinical Trials-Real-World Data to Build a Future for Our Patients. J. Clin. Oncol. 2024, 42, 2117–2120. [Google Scholar] [CrossRef]
- Le Cesne, A.; Martin-Broto, J.; Reichardt, P.; Picci, P.; Blay, J.Y. Current treatment patterns in advanced soft tissue sarcoma: Real-world evidence of over 5,000 European patients. Cancer Chemother. Rev. 2020, 15, 21–30. [Google Scholar]
- Solà-Morales, O.; Sigurðardóttir, K.; Akehurst, R.; Murphy, L.A.; Mestre-Ferrandiz, J.; Cunningham, D.; de Pouvourville, G. Data Governance for Real-World Data Management: A Proposal for a Checklist to Support Decision Making. Value Health 2023, 26, 32–42. [Google Scholar] [CrossRef]
- von Konow, A.; Ghanei, I.; Styring, E.; Vult von Steyern, F. Late Local Recurrence and Metastasis in Soft Tissue Sarcoma of the Extremities and Trunk Wall: Better Outcome After Treatment of Late Events Compared with Early. Ann. Surg. Oncol. 2021, 28, 7891–7902. [Google Scholar] [CrossRef]
- Baldini, E.H.; Guadagnolo, B.A.; Salerno, K.E.; Chung, P.; Bishop, A.J.; Kalbasi, A.; Miah, A.; Bedi, M.; Harris, J.P.; Petersen, I.; et al. Hypofractionated Preoperative Radiation Should Not Yet Be Used as Standard of Care for Extremity and Truncal Soft Tissue Sarcoma. J. Clin. Oncol. (JCO) 2024, 42, 4240–4245. [Google Scholar] [CrossRef]
- Gyawali, B.; Eisenhauer, E.A.; van der Graaf, W.; Booth, C.M.; Cherny, N.I.; Goodman, A.M.; Koven, R.; Pe, M.L.; Marini, B.L.; Mohyuddin, G.R.; et al. Common Sense Oncology principles for the design, analysis, and reporting of phase 3 randomised clinical trials. Lancet Oncol. 2025, 26, e80–e89. [Google Scholar] [CrossRef]
- Studer, G.; GLanzmann, C.; Maduz, F.; Bode, B.; Fuchs, B. Preoperative IMRT for soft-tissue sarcoma of the extremities and trunk: Low rate of wound complications. Curr. Orthopaeidc Pract. 2018, 29, 466–470. [Google Scholar] [CrossRef]
- Bernheim, S.M.; Rudolph, N.; Quinton, J.K.; Driessen, J.; Rawal, P.; Fowler, E. Elevating Quality, Outcomes, and Patient Experience Through Value-Based Care: CMS Innovation Center’s Quality Pathway. NEJM Catal. 2024, 5, e240132. [Google Scholar] [CrossRef]
- Fuchs, B.; Heesen, P.; SwissSarcomaNetwork. From Data Integration to Precision Medicine: A Value-Based Healthcare Approach for Sarcoma Care. J. Clin. Med. 2024, 13, 6500. [Google Scholar] [CrossRef] [PubMed]
- Saraswathula, A.; Merck, S.J.; Bai, G.; Weston, C.M.; Skinner, E.A.; Taylor, A.; Kachalia, A.; Demski, R.; Wu, A.W.; Berry, S.A. The Volume and Cost of Quality Metric Reporting. JAMA 2023, 329, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Abernethy, A. Time for real-world health data to become routine. Nat. Med. 2023, 29, 1317. [Google Scholar] [CrossRef] [PubMed]
- Concato, J.; Corrigan-Curay, J. Real-World Evidence—Where Are We Now? N. Engl. J. Med. 2022, 386, 1680–1682. [Google Scholar] [CrossRef]
- Ramsey, S.D.; Onar-Thomas, A.; Wheeler, S.B. Real-World Database Studies in Oncology: A Call for Standards. J. Clin. Oncol. 2024, 42, 977–980. [Google Scholar] [CrossRef]
- Wilson, B.E.; Booth, C.M. Real-world data: Bridging the gap between clinical trials and practice. EClinicalMedicine 2024, 78, 102915. [Google Scholar] [CrossRef]
- Booth, C.M.; Karim, S.; Mackillop, W.J. Real-world data: Towards achieving the achievable in cancer care. Nat. Rev. Clin. Oncol. 2019, 16, 312–325. [Google Scholar] [CrossRef]
- Gyorki, D.E.; Roland, C.L. ASO Author Reflections: Standardization in the Management of Retroperitoneal Sarcoma Through International Collaboration. Ann. Surg. Oncol. 2021, 28, 7889–7890. [Google Scholar] [CrossRef]
- van Kalsbeek, R.J.; Hudson, M.M.; Mulder, R.L.; Ehrhardt, M.; Green, D.M.; Mulrooney, D.A.; Hakkert, J.; den Hartogh, J.; Nijenhuis, A.; van Santen, H.M.; et al. A joint international consensus statement for measuring quality of survival for patients with childhood cancer. Nat. Med. 2023, 29, 1340–1348. [Google Scholar] [CrossRef]
- Dei Tos, A.P.; Webster, F.; Agaimy, A.; Bovee, J.; Dickson, B.; Doyle, L.; Dry, S.; Gronchi, A.; Hameed, M.; Hemmings, C.; et al. Datasets for reporting of soft-tissue sarcoma: Recommendations from the International Collaboration on Cancer Reporting (ICCR). Histopathology 2023, 82, 745–754. [Google Scholar] [CrossRef]
- Li, B.T.; Daly, B.; Gospodarowicz, M.; Bertagnolli, M.M.; Brawley, O.W.; Chabner, B.A.; Fashoyin-Aje, L.; de Claro, R.A.; Franklin, E.; Mills, J.; et al. Reimagining patient-centric cancer clinical trials: A multi-stakeholder international coalition. Nat. Med. 2022, 28, 620–626. [Google Scholar] [CrossRef]
- MacLean, C.H.; Antao, V.C.; Fontana, M.A.; Sandhu, H.S.; McLawhorn, A.S. PROMs: Opportunities, Challenges, and Unfinished Business. NEJM Catal. 2021, 2, e210280. [Google Scholar] [CrossRef]


| Criteria | uhpRT RWD Cohort [23] | SU2C-SARC032 RCT |
|---|---|---|
| Stage | Stage III (T2 N0 M0) | Stage III (T2 N0 M0) * |
| Tumor Grade | Grade 2/3 | Grade 2/3 |
| Age restrictions | None | Specific thresholds for fitness |
| Comorbidities | No exclusions | Excluded significant comorbidities |
| Tumor Locations | Includes retroperitoneal or trunk wall | Excluded retroperitoneal |
| Prior Surgeries | Included unplanned surgeries or recurred disease | Excluded prior unplanned surgeries and recurred disease |
| Systemic Therapy History | No exclusions | Excluded prior systemic therapy |
| Residual Disease | included | excluded |
| Organ Function | No exclusions | Excluded patients with inadequate organ function |
| Component | uhpRT RWD Cohort | SU2C-SARC032 RCT Control Arm | SU2C-SARC032 RCT Test Arm |
|---|---|---|---|
| Radiotherapy | 25 Gy in 5 fractions (1 week) | 50 Gy in 25 fractions (5 weeks) | 50 Gy in 25 fractions (5 weeks) |
| Interval (RT-Surgery) | Median 14 days | Median 34 days | Median 36 days |
| Surgery | Limb-salvage only (100%) | Limb-salvage only (100%) | Limb-salvage only (100%) |
| Systemic Therapy | none | none | Pembrolizumab: median 14 cycles (max 17) |
| Toral treatment Duration | <1 month | 2.5 months | 3–11 months |
| Parameter | uhpRT RWD Cohort [23] | SU2C-SARC032 RCT Control Arm | SU2C-SARC032 RCT Test Arm |
|---|---|---|---|
| Recruitment | 04.2020–11.2024 (4.5 y) | 11.2017–11.2023 (6 y) | 11.2017–11.2023 (6 y) |
| Interval Center Study Setup | Single institution | 20 international institutions | 20 international institutions |
| Prospective RWD | Prospective RCT | Prospective RCT | |
| N of patients | 54 | 63 | 64 |
| Follow-up, median (months) | 30 | 43 | 43 |
| Sex | |||
| Female | 21 (39%) | 24 (38%) | 23 (36%) |
| Male | 33 (61%) | 39 (62%) | 41 (64%) |
| Age | 63 (38–86) | 60 (26–84) | 59 (35–87) |
| Ethnicity (white) | 100% | 95% | 80% |
| Stage 1 | III | III | III |
| TNM | |||
| T2-4 N0 M0 | 100% | 100% | 100% |
| rT2-4 N0 M0 | 6 (11%) | 0 | 0 |
| Grade | |||
| Grade 2 | 13 (24%) | 20 (32%) | 22 (34%) |
| Grade 3 | 41 (76%) | 43 (68%) | 42 (66%) |
| Histologic Subtype | |||
| Undiff. pleomorphic Sarcoma (UPS) | 15 (28%) | 48 (76%) | 53 (83%) |
| Dedifferentiated Liposarcoma (DDLS) | 12 (22%) | 4 (6%) | 4 (6%) |
| Myxofibrosarcoma (MFS) | 12 (22%) | 6 (10%) | 7 (11%) |
| Undiff./Unclassified Sarcoma (NOS) | 0 | 5 (8%) | 0 |
| Myxoid Liposarcoma (MLS) | 5 (9%) | 0 | 0 |
| Leiomyosarcoma (LMS) | 4 (7.5%) | 0 | 0 |
| Others | 3 (5.5%) | 0 | 0 |
| Tumor Volume, median cc (range) | 161 (18–3084) | NA | NA |
| Tumor Size (median cm, range) | 10 (7–18) | 10 (7–13) | 11 (8–14) |
| Not available | 5 (whoops) | NA | NA |
| Tumor Location | |||
| Lower limb | 25 (46%) | 38 (60%) | 41 (64%) |
| Lower limb girdle | 6 (11%) | 6 (10%) | 3 (5%) |
| Upper limb | 7 (13%) | 9 (14%) | 10 (16%) |
| Upper limb girdle | 5 (9%) | 10 (16%) | 10 (16%) |
| Retroperitoneal | 5 (9%) | 0 | 0 |
| Trunk wall | 5 (9%) | 0 | 0 |
| others | 1 (2%) | 0 | 0 |
| Previously treated | |||
| Unpanned excision (whoops) | 5 (9%) | 0 | 0 |
| Surgery for initial manifestation | 6 (11%) | 0 | 0 |
| Previous adj. Radiation Therapy | 3 (5.5%) | 0 | 0 |
| Radiation Therapy | |||
| (Ultra-)hypofractionation | 54 (100%) | 2 (3%) | 1 (2%) |
| normofractionation | 0 | 61 (97%) | 63 (98%) |
| Systemic Therapy | |||
| Preoperative Chemotherapy | 1 (2%) | 0 | 0 |
| Postoperative Chemotherapy | 0 | 0 | 0 |
| Pembrolizumab | 0 | 0 | 100% |
| (PD-1-ihibitor, max 17, 3-weekly) | (median 14 cycles) | ||
| Surgery | |||
| No surgery | 0 | 1 | 2 |
| Limb salvage | 43/43 (100%) | NA | NA |
| Resection status (R0/1/2) | 49 (91%)/5 (9%)/0 | NA | NA |
| amputation | 0 | NA | NA |
| Parameter | RWD Cohort | SU2C-SARC032 Control Arm | SU2C-SARC032 Test Arm | p-Value RCT Arms |
|---|---|---|---|---|
| Local Disease-free Survival @ 2y, all | 94% | 100% | 97% | |
| G2 (n) | 100% (13) | NA | NA | X |
| G3 (n) | 92% (41) | NA | NA | X |
| Female (n = 21)/male (n = 33) | 94%/93% | NA | NA | X |
| Age ≤ 65 y/>65 y (n = 29/25) | 92%/95% | NA | NA | X |
| Distant Disease-free Survival @ 2y, all | 70% | 52% | 67% | |
| G2 (n) | 80% (13) | NA | NA | X |
| G3 (n) | 64% (41) | NA | NA | X |
| Female/male (n) | 68% (21)/68% (33) | NA | NA | X |
| Age ≤ 65 y/>65 y (n) | 58% (29)/68% (25) | NA | NA | X |
| Disease-free Survival @ 2y, all | 66% | 52% | 67% | 0.035 |
| G2 (n) | 80% (13) | 74% (20) | 80% (22) | 0.78 |
| G3 (n) | 60% (41) | 41% (43) | 60% (42) | 0.06 |
| Female/male (n) | 63% (21)/61% (33) | NA | NA | X |
| Age ≤ 65 y/>65 y (n) | 54% (29)/72% (25) | NA | NA | X |
| Overall Survival @ 2y, all | 90% | 85% | 88% | |
| G2 (n) | 100% (13) | NA | NA | X |
| G3 (n) | 86% (41) | NA | NA | X |
| Female/male (n) | 94% (21)/86% (33) | NA | NA | X |
| Age ≤ 65 y/>65 y (n) | 88% (29)/91% (25) | NA | NA | X |
| Parameter | RWD Cohort | SU2C-SARC032 Control Arm | SU2C-SARC032 Test Arm | p-Value RCT |
|---|---|---|---|---|
| Treatment Times | ||||
| Radiation Therapy, weeks | 1 | 5 | 5 | X |
| End of Radiation Therapy to Surgery (median days) | 14 | 34 | 36 | X |
| Pembrolizumab, median cycles | 0 | 0 | 14 | X |
| (max 17, 3-weekly) | ||||
| Total Treatment Time, months (TTT) | <1 | 2.5 | 3–11 | X |
| Acute RT Toxicity | ||||
| G0 | 52 (96%) | NA | NA | X |
| G1 | 2 (4%) | NA | NA | X |
| G2 | 0 | NA | NA | X |
| Wound Complication Rate | ||||
| (acc. CAN-NCIC-SR2 trial) | 12% | NA | NA | X |
| Late-term Toxicity | ||||
| G3/4 | 0 | 21 (31%) | 39 (56%) | X |
| At least 1 serious adverse event | 0 | 13 (19%) | 31 (47%) | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuchs, B.; Schelling, G.; Glanzmann, C.; Studer, G.; on behalf of the Swiss Sarcoma Network. From Intensification to Optimization: Balancing Efficacy, Safety, and Costs in High-Risk Localized Soft Tissue Sarcomas. Cancers 2025, 17, 1724. https://doi.org/10.3390/cancers17101724
Fuchs B, Schelling G, Glanzmann C, Studer G, on behalf of the Swiss Sarcoma Network. From Intensification to Optimization: Balancing Efficacy, Safety, and Costs in High-Risk Localized Soft Tissue Sarcomas. Cancers. 2025; 17(10):1724. https://doi.org/10.3390/cancers17101724
Chicago/Turabian StyleFuchs, Bruno, Georg Schelling, Christoph Glanzmann, Gabriela Studer, and on behalf of the Swiss Sarcoma Network. 2025. "From Intensification to Optimization: Balancing Efficacy, Safety, and Costs in High-Risk Localized Soft Tissue Sarcomas" Cancers 17, no. 10: 1724. https://doi.org/10.3390/cancers17101724
APA StyleFuchs, B., Schelling, G., Glanzmann, C., Studer, G., & on behalf of the Swiss Sarcoma Network. (2025). From Intensification to Optimization: Balancing Efficacy, Safety, and Costs in High-Risk Localized Soft Tissue Sarcomas. Cancers, 17(10), 1724. https://doi.org/10.3390/cancers17101724







