Beneficial Use of the Combination of Gemcitabine and Dacarbazine in Advanced Soft Tissue Sarcomas: Real-World Data
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Objectives
2.1.1. Treatment
2.1.2. Prognostic Markers
2.2. Statistical Method
3. Results
3.1. Baseline Characteristics of the Population
3.2. Effectiveness of the Combination
3.2.1. Response Rates
3.2.2. Survival Data
3.3. Combination Safety Profile
3.4. Prognostic and Predictive Factors of Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Dose Level | Gemcitabine Dose | Dacarbazine Dose |
|---|---|---|
| Level-0 | 1800 mg/m2 | 500 mg/m2 |
| Level-1 | 1500 mg/m2 | 400 mg/m2 |
| Level-2 | 1200 mg/m2 | 350 mg/m2 |
| Variables | p Value (Kaplan–Meier) † |
|---|---|
| Age of relapse | 0.161 |
| Sex | 0.263 |
| Histology—SFT | 0.476 |
| Histology—sarcoma NOS | 0.058 |
| Histology—LMS | 0.005 |
| L-sarcomas (LPS and LMS) | 0.012 |
| Complex karyotype | 0.035 |
| Translocated subtype | 0.002 |
| Histological grade at diagnosis | 0.171 |
| Ki67 at diagnosis | 0.018 |
| Relapse localization (locoregional, lung, hepatic, multisystemic, others) | 0.045 |
| Exclusive lung relapse | 0.029 |
| Number of previous lines used | 0.489 |
| Presence of ORR | 0.000 |
| Leucocytes in 1st GD (≤5400, >5400–≤7100, >7100–≤9400, >9400) | 0.442 |
| Neutrophils in 1st GD (≤3400, >3400–≤4600, >4600–≤6550, >6550) | 0.788 |
| Lymphocytes in 1st GD (≤900, >900–≤1300, >1300–≤1800, >1800) | 0.217 |
| Platelets in 1st GD (≤200,000, >200,000–≤257,000, >257,000–≤359,000, >359,000) | 0.992 |
| NLR in 1st GD (≤2.5, >2.5) | 0.166 |
| PLR in 1st GD (≤190, >190) | 0.716 |
| Albumin in 1st GD (<3.3, ≥3.3) | 0.391 |
| Albumin in last GD (<3.3, ≥3.3) | 0.452 |
| RDW in 1st GD (≤15, >15) | 0.327 |
| RDW in last GD (≤16, >16–≤17.30, >17.30–≤19.20, >19.20) | 0.249 |
| LDH in 1st GD (≤157, >157–≤190.5, >190.5–≤250, >250) | 0.736 |
| Variables | p Value (Kaplan–Meier) † |
|---|---|
| Age of relapse | 0.470 |
| Sex | 0.052 |
| Histology—SFT | 0.245 |
| Histology—sarcoma NOS | 0.000 |
| Histology—LMS | 0.013 |
| L-Sarcomas (LMS and LPS) | 0.011 |
| Complex karyotype | 0.694 |
| Translocated subtype | 0.672 |
| Histological grade at diagnosis | 0.600 |
| Ki67 at diagnosis | 0.944 |
| Exclusive lung relapse | 0.776 |
| Relapse localization (locoregional, lung, hepatic, multisystemic, others) | 0.635 |
| Number of previous lines used | 0.874 |
| Presence of ORR | 0.0021 |
| Leucocytes in 1st GD (≤5400, >5400–≤7100, >7100–≤9400, >9400) | 0.183 |
| Neutrophils in 1st GD (≤3400, >3400–≤4600, >4600–≤6550, >6550) | 0.193 |
| Lymphocytes in 1st GD (<1000, ≥1000) | 0.039 |
| Platelets in 1st GD (≤200,000, >200,000–≤257,000, >257,000–≤359,000, >359,000) | 0.256 |
| NLR in 1st GD (≤2.5, >2.5) | 0.056 |
| PLR in 1st GD (≤190, >190) | 0.000 |
| RDW in 1st GD (≤15 >15) | 0.098 |
| RDW in last GD (≤16, <16–≤17.30, <17.30–≤19.20, >19.20) | 0.001 |
| Albumin in 1st GD (<3.3, ≥3.3) | 0.001 |
| Albumin in last GD (<3.3, ≥3.3) | 0.001 |
| LDH in 1st GD (≤157, >157–≤190.5, >190.5–≤250, >250) | 0.606 |
References
- Sbaraglia, M.; Bellan, E.; Tos, A.P.D. The 2020 WHO Classification of Soft Tissue Tumours: News and perspectives. Pathologica 2021, 113, 70–84. [Google Scholar] [CrossRef]
- Zhao, B.; Tan, Y.; Bell, D.J.; Marley, S.E.; Guo, P.; Mann, H.; Scott, M.L.; Schwartz, L.H.; Ghiorghiu, D.C. Exploring intra- and inter-reader variability in uni-dimensional, bi-dimensional, and volumetric measurements of solid tumors on CT scans reconstructed at different slice intervals. Eur. J. Radiol. 2013, 82, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Toro, J.R.; Travis, L.B.; Wu, H.J.; Zhu, K.; Fletcher, C.D.M.; Devesa, S.S. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: An analysis of 26,758 cases. Int. J. Cancer 2006, 119, 2922–2930. [Google Scholar] [CrossRef]
- Judson, I.; Verweij, J.; Gelderblom, H.; Hartmann, J.T.; Schöffski, P.; Blay, J.-Y.; van der Graaf, W.T.; Marreaudet, S.; Sufliarsky, J.; Fisher, C.; et al. Doxorubicin alone versus intensified dox-orubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol. 2014, 15, 415–423. [Google Scholar] [CrossRef]
- Penel, N.; Bui, B.N.; Bay, J.-O.; Cupissol, D.; Ray-Coquard, I.; Piperno-Neumann, S.; Kerbrat, P.; Fournier, C.; Taieb, S.; Jimenez, M.; et al. Phase II Trial of Weekly Paclitaxel for Unresectable Angiosarcoma: The ANGIOTAX Study. J. Clin. Oncol. 2008, 26, 5269–5274. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; Antonescu, C.R.; Bjerkehagen, B.; Bovee, J.V.; Boye, K.; Chacón, M.; Dei Tos, A.P.; Desai, J.; Fletcher, J.A.; Gelderblom, H.; et al. Diagnosis and management of tro-pomyosin receptor kinase (TRK) fusion sarcomas: Expert recommendations from the World Sarcoma Network. Ann. Oncol. 2020, 31, 1506–1517. Available online: https://www.sciencedirect.com/science/article/pii/S0923753420422975 (accessed on 20 November 2023). [CrossRef]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion–Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Pautier, P.; Italiano, A.; Piperno-Neumann, S.; Chevreau, C.; Penel, N.; Firmin, N.; Boudou-Rouquette, P.; Bertucci, F.; Balleyguier, C.; Lebrun-Ly, V.; et al. Doxorubicin alone versus doxorubicin with trabectedin followed by trabectedin alone as first-line therapy for metastatic or unresectable leiomyosarcoma (LMS-04): A randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 2022, 23, 1044–1054. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Soft Tissue Sarcoma. Version 2. 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf (accessed on 10 November 2023).
- Gronchi, A.; Miah, A.B.; Dei Tos, A.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft tissue and visceral sarcomas: ESMO–EURACAN–GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef]
- Le Cesne, A.; Blay, J.; Judson, I.; Van Oosterom, A.; Verweij, J.; Radford, J.; Lorigan, P.; Rodenhuis, S.; Ray-Coquard, I.; Bonvalot, S.; et al. Phase II Study of ET-743 in Advanced Soft Tissue Sarcomas: A European Organisation for the Research and Treatment of Cancer (EORTC) Soft Tissue and Bone Sarcoma Group Trial. J. Clin. Oncol. 2005, 23, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Sleijfer, S.; Ray-Coquard, I.; Papai, Z.; Le Cesne, A.; Scurr, M.; Schöffski, P.; Collin, F.; Pandite, L.; Marreaud, S.; De Brauwer, A.; et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: A phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043). J. Clin. Oncol. 2009, 27, 3126–3132. [Google Scholar]
- Van Der Graaf, W.T.; Blay, J.Y.; Chawla, S.P.; Kim, D.W.; Bui-Nguyen, B.; Casali, P.G.; Schöffski, P.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef]
- Attia, S.; Bolejack, V.; Ganjoo, K.N.; George, S.; Agulnik, M.; Rushing, D.; Loggers, E.T.; Livingston, M.B.; Wright, J.; Chawla, S.P.; et al. A phase II trial of regorafenib in patients with advanced Ewing sarcoma and related tumors of soft tissue and bone: SARC024 trial results. Cancer Med. 2023, 12, 1532–1539. [Google Scholar] [CrossRef]
- Mir, O.; Brodowicz, T.; Italiano, A.; Wallet, J.; Blay, J.-Y.; Bertucci, F.; Chevreau, C.; Piperno-Neumann, S.; Bompas, E.; Salas, S.; et al. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016, 17, 1732–1742. [Google Scholar] [CrossRef]
- Seddon, B.; Strauss, S.J.; Whelan, J.; Leahy, M.; Woll, P.J.; Cowie, F.; Rothermundt, C.; Wood, Z.; Benson, C.; Ali, N.; et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): A randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1397–1410. [Google Scholar] [CrossRef]
- MMaki, R.G.; Wathen, J.K.; Patel, S.R.; Priebat, D.A.; Okuno, S.H.; Samuels, B.; Fanucchi, M.; Harmon, D.C.; Schuetze, S.M.; Reinke, D.; et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas. J. Clin. Oncol. 2007, 25, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Pautier, P.; Floquet, A.; Penel, N.; Piperno-Neumann, S.; Isambert, N.; Rey, A.; Bompas, E.; Cioffi, A.; Delcambre, C.; Cupissol, D.; et al. Randomized Multicenter and Stratified Phase II Study of Gemcitabine Alone Versus Gemcitabine and Docetaxel in Patients with Metastatic or Relapsed Leiomyosarcomas: A Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) French Sarcoma Group Study (TAXOGEM study). Oncologist 2012, 17, 1213–1220. [Google Scholar] [CrossRef]
- Buesa, J.M.; Losa, R.; Fernández, A.; Sierra, M.; Esteban, E.; Díaz, Á.; López-Pousa, A.; Fra, J. Phase I clinical trial of fixed-dose rate infusional gemcitabine and dacarbazine in the treatment of advanced soft tissue sarcoma, with assessment of gemcitabine tri-phosphate accumulation. Cancer 2004, 101, 2261–2269. [Google Scholar] [CrossRef]
- Losa, R.; Fra, J.; Pousa, A.L.; Sierra, M.; Goitía, A.; Uña, E.; Nadal, R.; Del Muro, J.G.; Gion, M.; Maurel, J.; et al. Phase II study with the combination of gemcitabine and DTIC in patients with advanced soft tissue sarcomas. Cancer Chemother. Pharmacol. 2007, 59, 251–259. [Google Scholar] [CrossRef]
- García-del-Muro, X.; López-Pousa, A.; Maurel, J.; Martín, J.; Martínez-Trufero, J.; Casado, A.; Gómez-España, A.; Fra, J.; Cruz, J.; Poveda, A.; et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sar-coma: A Spanish group for research on sarcomas study. J. Clin. Oncol. 2011, 29, 2528–2533. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0; U.S. Department of Health and Human Services: Washington, DC, USA, 2017.
- Jiang, M.; Ma, S.; Hua, Z.; Zhao, Z.; Gao, S. Prognostic Value of Pretreated Blood Inflammatory Markers in Patients with Bone Sarcoma: A Meta-Analysis. Dis. Markers 2021, 2021, 8839512. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, H.; Yin, F.; Guo, P.; Yang, X.; Liu, J.; Han, Y.; Ren, Z. Systemic Inflammatory Markers for Predicting Overall Survival in Patients with Osteosarcoma: A Systematic Review and Meta-Analysis. Mediat. Inflamm. 2021, 2021, 3456629. [Google Scholar] [CrossRef] [PubMed]
- Loong, H.H.-F.; Wong, C.K.H.; Wei, Y.; Kwan, S.S.C.; Zhang, Y.; Tse, T.; Lau, Y.-M.; Leung, L.K.; Tang, G.C. Prevalence and prognostic impact of comorbidities and peripheral blood indices in sarcomas. ESMO Open 2020, 5, e001035. [Google Scholar] [CrossRef]
- Von Hoff, D.D. There are no bad anticancer agents, only bad clinical trial designs--twenty-first Richard and Hinda Rosenthal Foundation Award Lecture. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1998, 4, 1079–1086. [Google Scholar]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Demetri, G.D.; Chawla, S.P.; von Mehren, M.; Ritch, P.; Baker, L.H.; Blay, J.Y.; Hande, K.R.; Keohan, M.L.; Samuels, B.L.; Schuetze, S.; et al. Efficacy and Safety of Trabectedin in Patients With Advanced or Metastatic Liposarcoma or Leiomyosarcoma After Failure of Prior Anthracyclines and Ifosfamide: Results of a Randomized Phase II Study of Two Different Schedules. J. Clin. Oncol. 2009, 27, 4188–4196. [Google Scholar] [CrossRef]
- Schöffski, P.; Chawla, S.; Maki, R.G.; Italiano, A.; Gelderblom, H.; Choy, E.; Grignani, G.; Camargo, V.; Bauer, S.; Rha, S.Y.; et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: A randomised, open-label, multicentre, phase 3 trial. Lancet 2016, 387, 1629–1637. [Google Scholar] [CrossRef]
- Cousin, S.; Blay, J.Y.; Bertucci, F.; Isambert, N.; Italiano, A.; Bompas, E.; Ray-Coquard, I.; Perrot, D.; Chaix, M.; Bui-Nguyen, B.; et al. Correlation between overall survival and growth modulation index in pre-treated sarcoma patients: A study from the French Sarcoma Group. Ann. Oncol. 2013, 24, 2681–2685. [Google Scholar] [CrossRef]
- Penel, N.; Demetri, G.D.; Blay, J.Y.; Cousin, S.; Maki, R.G.; Chawla, S.P.; Judson, I.; von Mehren, M.; Schöffski, P.; Verweij, J.; et al. Growth modulation index as metric of clinical benefit assessment among advanced soft tissue sarcoma patients receiving trabectedin as a salvage therapy. Ann. Oncol. 2013, 24, 537–542. [Google Scholar] [CrossRef]
- Martínez-Trufero, J.; De Sande-González, L.M.; Luna, P.; Martin-Broto, J.; Álvarez, R.; Marquina, G.; Diaz-Beveridge, R.; Poveda, A.; Cano, J.M.; Cruz-Jurado, J.; et al. A Growth Modulation Index-Based GEISTRA Score as a New Prognostic Tool for Trabectedin Efficacy in Patients with Advanced Soft Tissue Sarcomas: A Spanish Group for Sarcoma Research (GEIS) Retrospective Study. Cancers 2021, 13, 792. [Google Scholar] [CrossRef] [PubMed]
- Singer, S.; Antonescu, C.R. DeVita, Hellman, and Rosenberg’s Cancer: Principles & Practice of Oncology, 11th ed.; DeVita, V.T., Hellmann, M.D., Rosenberg, S.A., Eds.; LippincottWilliamsand WikinsPress: Philadelphia, PA, USA, 2019. [Google Scholar]
- Kawai, A.; Araki, N.; Sugiura, H.; Ueda, T.; Yonemoto, T.; Takahashi, M.; Morioka, H.; Hiraga, H.; Hiruma, T.; Kunisada, T.; et al. Trabectedin monotherapy after standard chemotherapy versus best supportive care in patients with advanced, translocation-related sarcoma: A randomised, open-label, phase 2 study. Lancet Oncol. 2015, 16, 406–416. [Google Scholar] [CrossRef]
- Blay, J.-Y.; Leahy, M.G.; Nguyen, B.B.; Patel, S.R.; Hohenberger, P.; Santoro, A.; Staddon, A.P.; Penel, N.; Piperno-Neumann, S.; Hendifar, A.; et al. Randomised phase III trial of trabectedin versus doxorubicin-based chemotherapy as first-line therapy in translocation-related sarcomas. Eur. J. Cancer 2014, 50, 1137–1147. [Google Scholar] [CrossRef]
- Le Cesne, A.; Cresta, S.; Maki, R.G.; Blay, J.Y.; Verweij, J.; Poveda, A.; Casali, P.G.; Balaña, C.; Schöffski, P.; Grosso, F.; et al. A retrospective analysis of antitumour activity with trabectedin in translocation-related sarcomas. Eur. J. Cancer 2012, 48, 3036–3044. [Google Scholar] [CrossRef]
- Fang, E.; Wang, X.; Feng, J.; Zhao, X. The Prognostic Role of Glasgow Prognostic Score and C-reactive Protein to Albumin Ratio for Sarcoma: A System Review and Meta-Analysis. Dis. Markers 2020, 2020, 8736509. [Google Scholar] [CrossRef]
- Matsuyama, Y.; Nakamura, T.; Yoshida, K.; Nakamura, K.; Hagi, T.; Asanuma, K.; Sudo, A. Role of the Prognostic Nutritional Index in Patients With Soft-tissue Sarcoma. In Vivo 2021, 35, 2349–2355. [Google Scholar] [CrossRef]
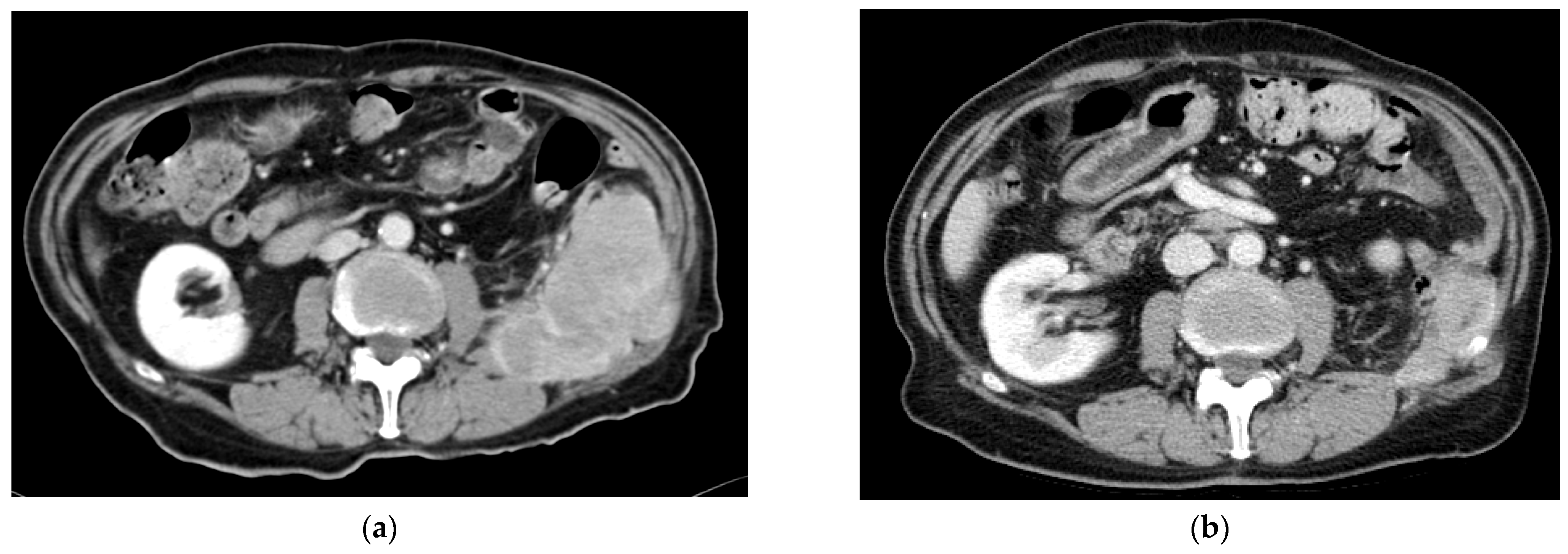
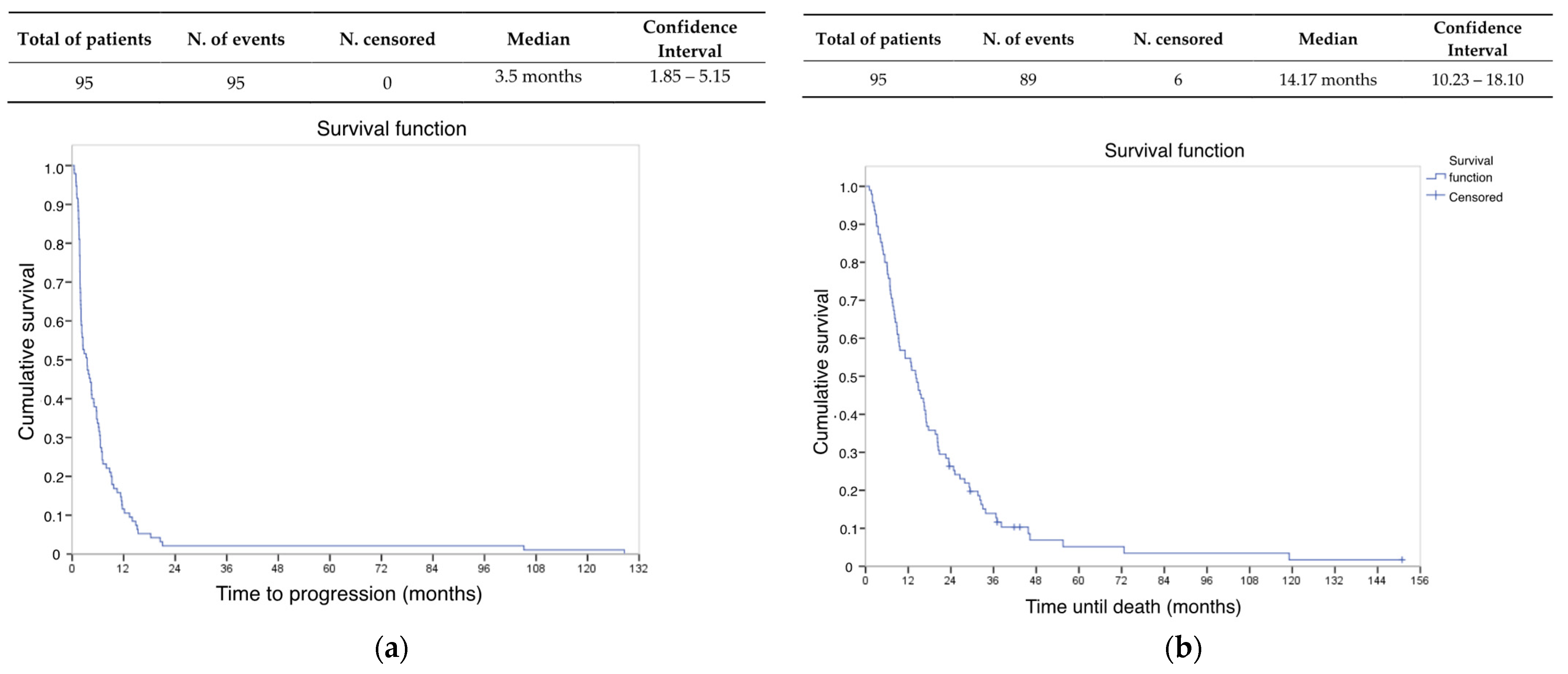
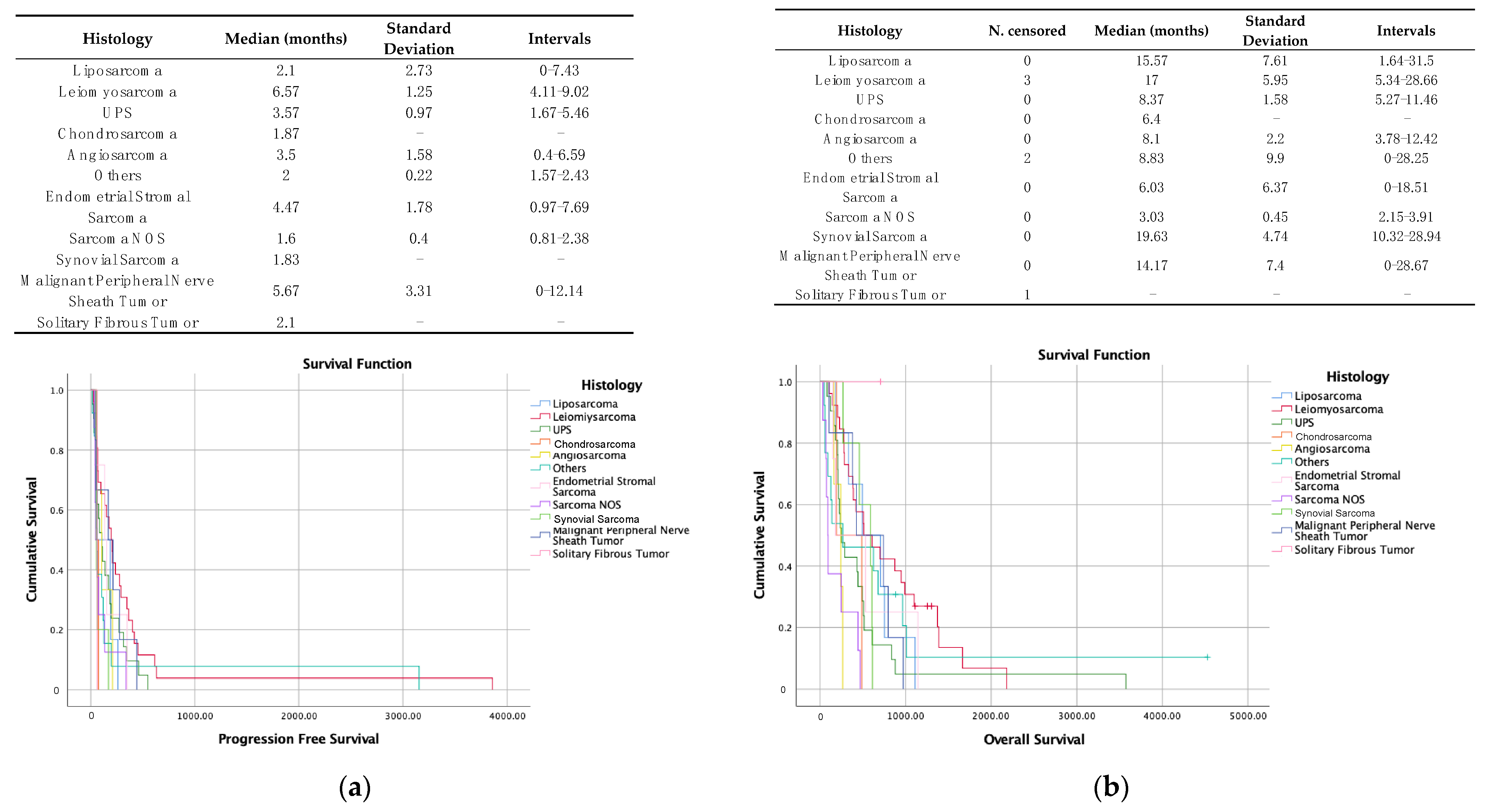
| Baseline Characteristics | n = 95 |
|---|---|
| Mean age | At diagnosis: 55 years old At unresectable disease: 59 years old |
| Sex | Men 49.5% (n = 47) Women 50.5% (n = 48) |
| Histological subtypes | LMS 27.4% (n = 26) UPS 22% (n = 21) NOS sarcoma 8% (n = 8) LPS 6% (n = 6) SS 4% (n = 5) Others 30.5% (n = 29) |
| Complex karyotype | 78.7% (n = 74) |
| Primary tumor location | Trunk and limbs 43% (n = 41) Retroperitoneum 15.8% (n = 15) Gynecologic 15.8% (n = 15) Viscera 11.6% (n = 11) Others 13.7% (n = 13) |
| Stage of palliative disease | Multisystemic 24.7% (n = 33) Lung 29.5% (n = 28) Locoregional non-surgical 26% (n = 24) Hepatic 2% (n = 2) Other localizations 5% (n = 5) |
| Previously used chemotherapy agents | Anthracycline combination or monotherapy 85% (n = 83) Ifosfamide 7.3% (n = 7) Combination of taxane 4.2% (n = 4) Trabectedine 1% (n = 1) |
| Treatment after progression to GD | Trabectedine 36.8% (n = 35) TKI 16.8% (n = 16) Others 2% None 29.5% (n = 28) |
| Toxicity | Any Grade | Grades 3–4 |
|---|---|---|
| Anemia | 64.2% (n = 61) | 1% (n = 1) |
| Thrombopenia | 27.4% (n = 26) | 3.2% (n = 3) |
| Neutropenia | 8.4% (n = 8) | 5.3% (n = 5) |
| Febrile Neutropenia | 0% | 0% |
| Total | 75.8% (n = 72) | 9.5% (n = 9) |
| Covariate | HR | CI (HR) | Significance |
|---|---|---|---|
| Translocated histological subtype | 1.87 | 1.74–4.89 | 0.036 |
| ORR | 2.71 | 1.60–4.6 | 0.000 |
| Covariate | HR | CI (HR) | Significance |
|---|---|---|---|
| Lymphocyte at beginning of treatment | 1.84 | 1.08–3.13 | 0.026 |
| Albumin at beginning of treatment | 0.52 | 0.33–0.81 | 0.004 |
| ORR | 3.52 | 1.99–6.23 | 0.000 |
| PLR previous to start of treatment | 1.77 | 1.03–3.02 | 0.0038 |
| Variable | Our Study | JM Buesa [20] | R. Losa [21] | X. Garcial-Del-Muro [22] |
|---|---|---|---|---|
| N° of patients | 95 | 22 | 26 | 59 vs. 54 |
| PFS | 3.5 months | At 6 months 29% free of progression | At 6 months 28% free of progression | 4.2 months (vs. 2 months with gem.) |
| OS (months) | 14.2 | - | 8.63 months | 16.8 months (vs. 8.2 months with gem.) |
| ORR | 22% | 26% | 4% | |
| SD | 30.5% | 31.6% | 47.8% | ORR + SD 49% (vs. 25% with gem.) |
| Toxicities | Anemia 64.2% (G3-4: 1%) Thrombopenia 27.4% (G3-4: 3%) Neutropenia 8.4% (G3-4: 5.3%) | Anemia 83% (G3-4: 5%) Thrombopenia 11% (G3-4: 5%) Neutropenia 100% (G3-4: 44%) | Anemia 92% (G3-4: 33%) Thrombopenia 58% (G3-4: 12%) Neutropenia 73% (G3-4: 46%) | Anemia 82% (G3-4: 4%) Thrombopenia 40% (G3-4: 6%) Neutropenia 76% (G3-4: 48%) |
| Variable | Nº Patients | PFS (Months) | OS (Months) | Response | Toxicities |
|---|---|---|---|---|---|
| Our study | 95 | 3.5 | 14.2 | CR 2.1% PR 20% SD 30.5% | Anemia 64.2% (G3-4: 1%) Thrombopenia 27.4% (G3-4: 3%) Neutropenia 8.4% (G3-4: 5.3%) |
| Gemcitabine [18,19] | 22–49 | 3–4.7 | 11.5 | PR 8–19% ORR 8% | Anemia 85% (G3-4: 1–13%) Neutropenia 59% (G3-4: 21–28%) Thrombopenia 60% (G3-4: 8–35%) |
| Gemcitabine–Docetaxel [18,19] | 24–73 | 5.5–6.2 | 17.9 | PR 16–24% ORR 16% | Anemia 94% (G3-4: 7–10%) Neutropenia 41% (G3-4: 10–16%) Thrombopenia 62% (G3-4: 18–40%) |
| Eribulin [30] | 228 | 2.6 | 13.5 | PR 4% SD 56% | Anemia 30% (G3-4: 16%) Neutropenia 43% (G3-4: 35%) Thrombopenia 6% (G3-4 < 1%) |
| Pazopanib [14] | 246 | 4.6 | 12.5 | PR 6% SD 67% PD 23% | - |
| Trabectedin [29] | 270 | 3.5 | 13.9 | PR 8% SD 26% | Anemia 97% (G3-4: 8%) Thrombopenia 53% (G3-4: 11%) Neutropenia 74% (G3-4: 47%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurruchaga Sotés, I.; Gómez-Mateo, M.C.; Ortega Izquierdo, M.E.; Martínez-Trufero, J. Beneficial Use of the Combination of Gemcitabine and Dacarbazine in Advanced Soft Tissue Sarcomas: Real-World Data. Cancers 2024, 16, 267. https://doi.org/10.3390/cancers16020267
Gurruchaga Sotés I, Gómez-Mateo MC, Ortega Izquierdo ME, Martínez-Trufero J. Beneficial Use of the Combination of Gemcitabine and Dacarbazine in Advanced Soft Tissue Sarcomas: Real-World Data. Cancers. 2024; 16(2):267. https://doi.org/10.3390/cancers16020267
Chicago/Turabian StyleGurruchaga Sotés, Ibon, M. Carmen Gómez-Mateo, María Eugenia Ortega Izquierdo, and Javier Martínez-Trufero. 2024. "Beneficial Use of the Combination of Gemcitabine and Dacarbazine in Advanced Soft Tissue Sarcomas: Real-World Data" Cancers 16, no. 2: 267. https://doi.org/10.3390/cancers16020267
APA StyleGurruchaga Sotés, I., Gómez-Mateo, M. C., Ortega Izquierdo, M. E., & Martínez-Trufero, J. (2024). Beneficial Use of the Combination of Gemcitabine and Dacarbazine in Advanced Soft Tissue Sarcomas: Real-World Data. Cancers, 16(2), 267. https://doi.org/10.3390/cancers16020267







