Mechanisms Underlying the Development of Murine T-Cell Lymphoblastic Lymphoma/Leukemia Induced by Total-Body Irradiation
Abstract
Simple Summary
Abstract
1. Introduction
2. Genetic Alterations of Murine Thymic Lymphoma Induced by FX Treatment
3. DNA Methylation of FX-Induced Thymic Lymphomas in Mice
4. Dynamic Changes in the Production of Reactive Oxygen Species in the Thymus Following FX Treatment
5. Detection of Pre-Lymphoma Cells within the Thymus of FX-Treated Mice
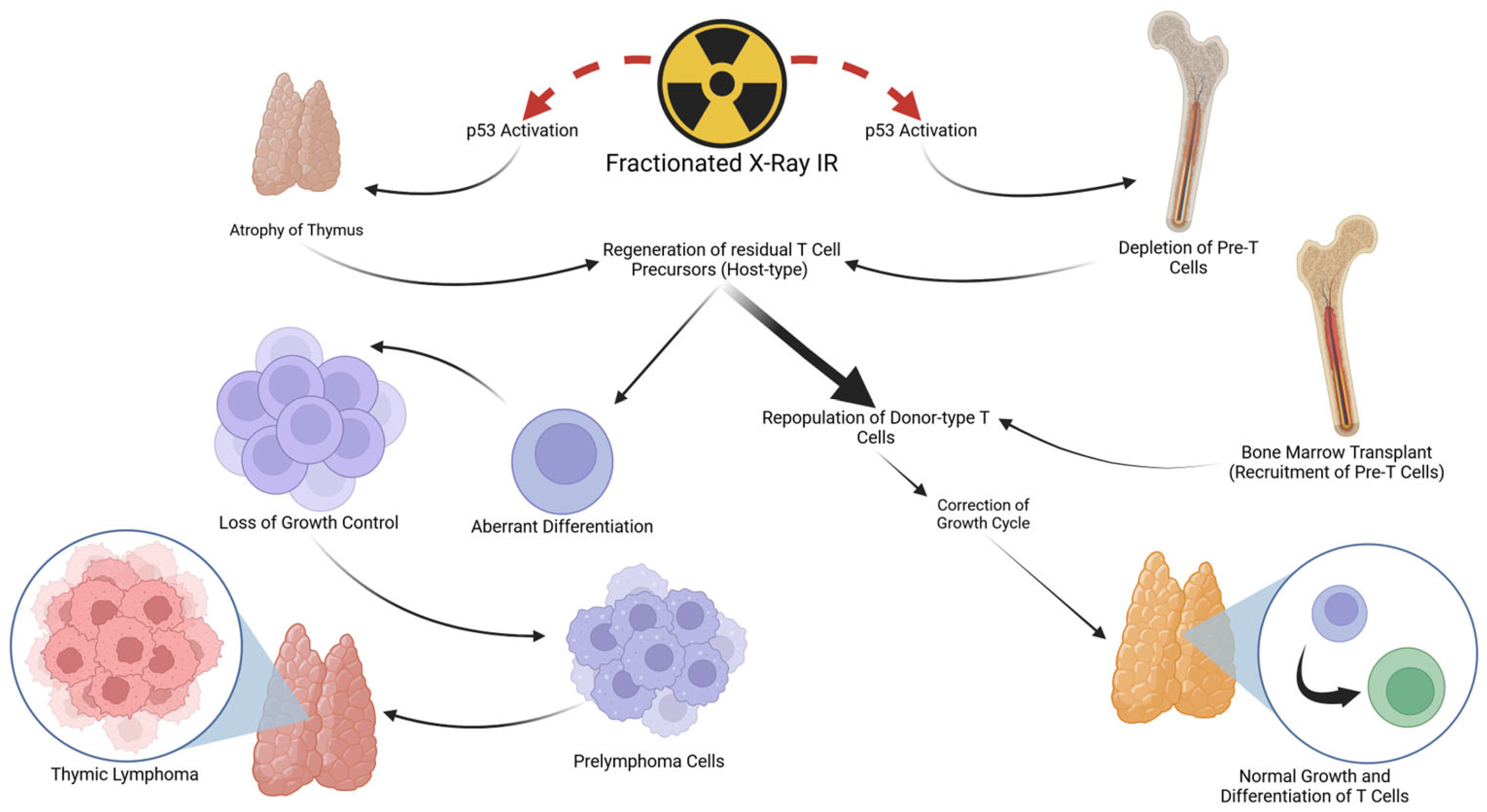
6. FX Treatment Induces Thymic Lymphoma in Mice through Non-Cell-Autonomous Mechanisms
7. Suppressing the Development of Thymic Lymphomas in FX-Treated Mice by Transplantation of Bone Marrow Cells from Unirradiated Donors
8. Contributions of Thymus Stromal Cells to Tissue Regeneration in Response to TBI
9. Possible Cellular Mechanism of Thymic Lymphomagenesis Induced by FX Treatment
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- UNSCEAR. UNSCEAR 2006 report. In Epidemiological Studies of Radiation and Cancer; United Nations: New York, NY, USA, 2008; pp. 13–322. [Google Scholar]
- Shuryak, I.; Sachs, R.K.; Hlatky, L.; Little, M.P.; Hahnfeldt, P.; Brenner, D.J. Radiation-induced leukemia at doses relevant to radiation therapy: Modeling mechanisms and estimating risks. J. Natl. Cancer Inst. 2006, 98, 1794–1806. [Google Scholar] [CrossRef]
- Genetic susceptibility to cancer. ICRP publication 79. Approved by the Commission in May 1997. International Commission on Radiological Protection. Ann. ICRP 1998, 28, 1–157. [Google Scholar] [PubMed]
- Smith, S.M.; Le Beau, M.M.; Huo, D.; Karrison, T.; Sobecks, R.M.; Anastasi, J.; Vardiman, J.W.; Rowley, J.D.; Larson, R.A. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: The University of Chicago series. Blood 2003, 102, 43–52. [Google Scholar] [CrossRef]
- Radivoyevitch, T.; Sachs, R.K.; Gale, R.P.; Molenaar, R.J.; Brenner, D.J.; Hill, B.T.; Kalaycio, M.E.; Carraway, H.E.; Mukherjee, S.; Sekeres, M.A.; et al. Defining AML and MDS second cancer risk dynamics after diagnoses of first cancers treated or not with radiation. Leukemia 2016, 30, 285–294. [Google Scholar] [CrossRef]
- Richardson, D.; Sugiyama, H.; Nishi, N.; Sakata, R.; Shimizu, Y.; Grant, E.J.; Soda, M.; Hsu, W.L.; Suyama, A.; Kodama, K.; et al. Ionizing radiation and leukemia mortality among Japanese Atomic Bomb Survivors, 1950–2000. Radiat. Res. 2009, 172, 368–382. [Google Scholar] [CrossRef]
- Hsu, W.L.; Preston, D.L.; Soda, M.; Sugiyama, H.; Funamoto, S.; Kodama, K.; Kimura, A.; Kamada, N.; Dohy, H.; Tomonaga, M.; et al. The incidence of leukemia, lymphoma and multiple myeloma among atomic bomb survivors: 1950–2001. Radiat. Res. 2013, 179, 361–382. [Google Scholar] [CrossRef]
- Iwanaga, M.; Hsu, W.L.; Soda, M.; Takasaki, Y.; Tawara, M.; Joh, T.; Amenomori, T.; Yamamura, M.; Yoshida, Y.; Koba, T.; et al. Risk of myelodysplastic syndromes in people exposed to ionizing radiation: A retrospective cohort study of Nagasaki atomic bomb survivors. J. Clin. Oncol. 2011, 29, 428–434. [Google Scholar] [CrossRef]
- Ozasa, K. Epidemiological research on radiation-induced cancer in atomic bomb survivors. J. Radiat. Res. 2016, 57 (Suppl. S1), i112–i117. [Google Scholar] [CrossRef]
- Sasaki, S. Influence of the age of mice at exposure to radiation on life-shortening and carcinogenesis. J. Radiat. Res. 1991, 32 (Suppl. S2), 73–85. [Google Scholar] [CrossRef]
- Kaplan, H.S.; Brown, M.B. A quantitative dose-response study of lymphoid-tumor development in irradiated C 57 black mice. J. Natl. Cancer Inst. 1952, 13, 185–208. [Google Scholar]
- Kaplan, H.S.; Brown, M.B.; Paull, J. Influence of bone-marrow injections on involution and neoplasia of mouse thymus after systemic irradiation. J. Natl. Cancer Inst. 1953, 14, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, H.S.; Brown, M.B.; Paull, J. Influence of postirradiation thymectomy and of thymic implants on lymphoid tumor incidence in C57BL mice. Cancer Res. 1953, 13, 677–680. [Google Scholar] [PubMed]
- Kaplan, H.S.; Carnes, W.H.; Brown, M.B.; Hirsch, B.B. Indirect induction of lymphomas in irradiated mice. I. Tumor incidence and morphology in mice bearing nonirradiated thymic grafts. Cancer Res. 1956, 16, 422–425. [Google Scholar] [PubMed]
- Kaplan, H.S.; Hirsch, B.B.; Brown, M.B. Indirect induction of lymphomas in irradiated mice. IV. Genetic evidence of the origin of the tumor cells from the thymic grafts. Cancer Res. 1956, 16, 434–436. [Google Scholar] [PubMed]
- Kaplan, H.S.; Brown, M.B. Further observations on inhibition of lymphoid tumor development by shielding and partial-body irradiation of mice. J. Natl. Cancer Inst. 1951, 12, 427–436. [Google Scholar] [PubMed]
- Kaplan, H.S.; Brown, M.B. Effect of Peripheral Shielding on Lymphoid Tissue Response to Irradiation in C 57 Black Mice. Science 1952, 116, 195–196. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Castle, K.D.; Moding, E.J.; Blum, J.M.; Williams, N.; Luo, L.; Ma, Y.; Borst, L.B.; Kim, Y.; Kirsch, D.G. Acute DNA damage activates the tumour suppressor p53 to promote radiation-induced lymphoma. Nat. Commun. 2015, 6, 8477. [Google Scholar] [CrossRef] [PubMed]
- Hasapis, S.; Caraballo, I.; Lee, C.L. Transplantation of Unirradiated Bone Marrow Cells after Total-Body Irradiation Prevents the Development of Thymic Lymphoma in Mice through Niche Competition. Radiat. Res. 2021, 195, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Brock, K.D.; Hasapis, S.; Zhang, D.; Sibley, A.B.; Qin, X.; Gresham, J.S.; Caraballo, I.; Luo, L.; Daniel, A.R.; et al. Whole-Exome Sequencing of Radiation-Induced Thymic Lymphoma in Mouse Models Identifies Notch1 Activation as a Driver of p53 Wild-Type Lymphoma. Cancer Res. 2021, 81, 3777–3790. [Google Scholar] [CrossRef] [PubMed]
- Hasapis, S.; Caraballo, I.; Sears, T.J.; Brock, K.D.; Cart, J.B.; Moding, E.J.; Lee, C.L. Characterizing the role of Phlda3 in the development of acute toxicity and malignant transformation of hematopoietic cells induced by total-body irradiation in mice. Sci. Rep. 2023, 13, 12916. [Google Scholar] [CrossRef]
- Lieberman, M.; Kaplan, H.S. Leukemogenic activity of filtrates from radiation-induced lymphoid tumors of mice. Science 1959, 130, 387–388. [Google Scholar] [CrossRef]
- Haran-Ghera, N. Leukemogenic activity of centrifugates from irradiated mouse thymus and bone marrow. Int. J. Cancer 1966, 1, 81–87. [Google Scholar] [CrossRef]
- Kaplan, H.S. The Role of Radiation on Experimental Leukemogenesis. Natl. Cancer Inst. Monogr. 1964, 14, 207–220. [Google Scholar] [PubMed]
- Kaplan, H.S. On the natural history of the murine leukemias: Presidential address. Cancer Res. 1967, 27, 1325–1340. [Google Scholar]
- Okumoto, M.; Nishikawa, R.; Iwai, M.; Iwai, Y.; Takamori, Y.; Niwa, O.; Yokoro, K. Lack of evidence for the involvement of type-C and type-B retroviruses in radiation leukemogenesis of NFS mice. Radiat. Res. 1990, 121, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Muto, M.; Sado, T.; Hayata, I.; Nagasawa, F.; Kamisaku, H.; Kubo, E. Reconfirmation of indirect induction of radiogenic lymphomas using thymectomized, irradiated B10 mice grafted with neonatal thymuses from Thy 1 congenic donors. Cancer Res. 1983, 43, 3822–3827. [Google Scholar] [PubMed]
- Sado, T.; Kamisaku, H.; Kubo, E. Bone marrow-thymus interactions during thymic lymphomagenesis induced by fractionated radiation exposure in B10 mice: Analysis using bone marrow transplantation between Thy 1 congenic mice. J. Radiat. Res. 1991, 32 (Suppl. S2), 168–180. [Google Scholar] [CrossRef]
- Muto, M.; Kubo, E.; Sado, T. Development of prelymphoma cells committed to thymic lymphomas during radiation-induced thymic lymphomagenesis in B10 mice. Cancer Res. 1987, 47, 3469–3472. [Google Scholar]
- Muto, M.; Kubo, E.; Kamisaku, H.; Sado, T. Phenotypic characterization of thymic prelymphoma cells of B10 mice treated with split-dose irradiation. J. Immunol. 1990, 144, 849–853. [Google Scholar] [CrossRef]
- Muto, M.; Kubo, E.; Sado, T.; Yamagishi, H. Characterization of thymic prelymphoma cells that develop during radiation-induced lymphomagenesis in B10 mice. J. Radiat. Res. 1991, 32 (Suppl. S2), 156–167. [Google Scholar] [CrossRef][Green Version]
- Maisin, J.R.; Wambersie, A.; Gerber, G.B.; Mattelin, G.; Lambiet-Collier, M.; Gueulette, J. The effects of a fractionated gamma irradiation on life shortening and disease incidence in BALB/c mice. Radiat. Res. 1983, 94, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, R.L.; Storer, J.B. Influence of gamma irradiation on the development of neoplastic disease in mice. I. Reticular tissue tumors. Radiat. Res. 1979, 80, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Dange, P.; Sarma, H.; Pandey, B.N.; Mishra, K.P. Radiation-induced incidence of thymic lymphoma in mice and its prevention by antioxidants. J. Environ. Pathol. Toxicol. Oncol. 2007, 26, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Kamisaku, H.; Aizawa, S.; Kitagawa, M.; Ikarashi, Y.; Sado, T. Limiting dilution analysis of T-cell progenitors in the bone marrow of thymic lymphoma-susceptible B10 and -resistant C3H mice after fractionated whole-body X-irradiation. Int. J. Radiat. Biol. 1997, 72, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Kamisaku, H.; Aizawa, S.; Tanaka, K.; Watanabe, K.; Sado, T. Different cellular basis for the resistance of C3H and STS strain mice to the development of thymic lymphomas following fractionated whole-body irradiation: Analysis using radiation bone marrow chimeras. Int. J. Radiat. Biol. 2000, 76, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kubo, E.; Sado, T.; Muto, M. Cytogenetic analysis of thymocytes during early stages after irradiation in mice with different susceptibilities to radiation-induced lymphomagenesis. J. Radiat. Res. 1996, 37, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Okumoto, M.; Nishikawa, R.; Imai, S.; Hilgers, J. Resistance of STS/A mice to lymphoma induction by X-irradiation. J. Radiat. Res. 1989, 30, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Kominami, R.; Niwa, O. Radiation carcinogenesis in mouse thymic lymphomas. Cancer Sci. 2006, 97, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, M.; Yamamoto, T.; Kohara, Y.; Katsuragi, Y.; Mishima, Y.; Aoyagi, Y.; Kominami, R. Mtf-1 lymphoma-susceptibility locus affects retention of large thymocytes with high ROS levels in mice after gamma-irradiation. Biochem. Biophys. Res. Commun. 2007, 354, 209–215. [Google Scholar] [CrossRef]
- Saito, Y.; Ochiai, Y.; Kodama, Y.; Tamura, Y.; Togashi, T.; Kosugi-Okano, H.; Miyazawa, T.; Wakabayashi, Y.; Hatakeyama, K.; Wakana, S.; et al. Genetic loci controlling susceptibility to gamma-ray-induced thymic lymphoma. Oncogene 2001, 20, 5243–5247. [Google Scholar] [CrossRef]
- Tamura, Y.; Maruyama, M.; Mishima, Y.; Fujisawa, H.; Obata, M.; Kodama, Y.; Yoshikai, Y.; Aoyagi, Y.; Niwa, O.; Schaffner, W.; et al. Predisposition to mouse thymic lymphomas in response to ionizing radiation depends on variant alleles encoding metal-responsive transcription factor-1 (Mtf-1). Oncogene 2005, 24, 399–406. [Google Scholar] [CrossRef]
- Yoshida, M.A.; Nakata, A.; Akiyama, M.; Kakinuma, S.; Sado, T.; Nishimura, M.; Shimada, Y. Distinct structural abnormalities of chromosomes 11 and 12 associated with loss of heterozygosity in X-ray-induced mouse thymic lymphomas. Cancer Genet. Cytogenet. 2007, 179, 1–10. [Google Scholar] [CrossRef]
- Takabatake, T.; Kakinuma, S.; Hirouchi, T.; Nakamura, M.M.; Fujikawa, K.; Nishimura, M.; Oghiso, Y.; Shimada, Y.; Tanaka, K. Analysis of changes in DNA copy number in radiation-induced thymic lymphomas of susceptible C57BL/6, resistant C3H and hybrid F1 Mice. Radiat. Res. 2008, 169, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Dofuku, R.; Biedler, J.L.; Spengler, B.A.; Old, L.J. Trisomy of chromosome 15 in spontaneous leukemia of AKR mice. Proc. Natl. Acad. Sci. USA 1975, 72, 1515–1517. [Google Scholar] [CrossRef] [PubMed]
- Herbst, E.W.; Gropp, A.; Tietgen, C. Chromosome rearrangements involved in the origin of trisomy 15 in spontaneous leukemia of AKR mice. Int. J. Cancer 1981, 28, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Klein, G. Lymphoma development in mice and humans: Diversity of initiation is followed by convergent cytogenetic evolution. Proc. Natl. Acad. Sci. USA 1979, 76, 2442–2446. [Google Scholar] [CrossRef]
- Spira, J.; Wiener, F.; Klein, G. Robertsonian translocation studies on the significance of trisomy 15 in murine T-cell leukemia. Cancer Genet. Cytogenet. 1983, 9, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Babonits, M.; Wiener, F.; Klein, G. Further studies on chromosome 15 trisomy in murine T-cell lymphomas: Mapping of the relevant chromosome segment. Int. J. Cancer 1988, 41, 738–743. [Google Scholar] [CrossRef]
- Tsuji, H.; Ishii-Ohba, H.; Ukai, H.; Katsube, T.; Ogiu, T. Radiation-induced deletions in the 5′ end region of Notch1 lead to the formation of truncated proteins and are involved in the development of mouse thymic lymphomas. Carcinogenesis 2003, 24, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Ishii-Ohba, H.; Katsube, T.; Ukai, H.; Aizawa, S.; Doi, M.; Hioki, K.; Ogiu, T. Involvement of illegitimate V(D)J recombination or microhomology-mediated nonhomologous end-joining in the formation of intragenic deletions of the Notch1 gene in mouse thymic lymphomas. Cancer Res. 2004, 64, 8882–8890. [Google Scholar] [CrossRef]
- Tsuji, H.; Ishii-Ohba, H.; Shiomi, T.; Shiomi, N.; Katsube, T.; Mori, M.; Nenoi, M.; Ohno, M.; Yoshimura, D.; Oka, S.; et al. Nature of nontargeted radiation effects observed during fractionated irradiation-induced thymic lymphomagenesis in mice. J. Radiat. Res. 2013, 54, 453–466. [Google Scholar] [CrossRef][Green Version]
- Shimada, Y.; Nishimura, M.; Kakinuma, S.; Okumoto, M.; Shiroishi, T.; Clifton, K.H.; Wakana, S. Radiation-associated loss of heterozygosity at the Znfn1a1 (Ikaros) locus on chromosome 11 in murine thymic lymphomas. Radiat. Res. 2000, 154, 293–300. [Google Scholar] [CrossRef]
- Hirano, S.; Kakinuma, S.; Amasaki, Y.; Nishimura, M.; Imaoka, T.; Fujimoto, S.; Hino, O.; Shimada, Y. Ikaros is a critical target during simultaneous exposure to X-rays and N-ethyl-N-nitrosourea in mouse T-cell lymphomagenesis. Int. J. Cancer 2013, 132, 259–268. [Google Scholar] [CrossRef]
- Ohi, H.; Mishima, Y.; Kamimura, K.; Maruyama, M.; Sasai, K.; Kominami, R. Multi-step lymphomagenesis deduced from DNA changes in thymic lymphomas and atrophic thymuses at various times after gamma-irradiation. Oncogene 2007, 26, 5280–5289. [Google Scholar] [CrossRef]
- Lana, T.; de Lorenzo, P.; Bresolin, S.; Bronzini, I.; den Boer, M.L.; Cave, H.; Fronkova, E.; Stanulla, M.; Zaliova, M.; Harrison, C.J.; et al. Refinement of IKZF1 status in pediatric Philadelphia-positive acute lymphoblastic leukemia. Leukemia 2015, 29, 2107–2110. [Google Scholar] [CrossRef]
- Liu, Y.; Easton, J.; Shao, Y.; Maciaszek, J.; Wang, Z.; Wilkinson, M.R.; McCastlain, K.; Edmonson, M.; Pounds, S.B.; Shi, L.; et al. The genomic landscape of pediatric and young adult T-lineage acute lymphoblastic leukemia. Nat. Genet. 2017, 49, 1211–1218. [Google Scholar] [CrossRef]
- Koturbash, I.; Pogribny, I.; Kovalchuk, O. Stable loss of global DNA methylation in the radiation-target tissue--a possible mechanism contributing to radiation carcinogenesis? Biochem. Biophys. Res. Commun. 2005, 337, 526–533. [Google Scholar] [CrossRef]
- Herranz, M.; Martin-Caballero, J.; Fraga, M.F.; Ruiz-Cabello, J.; Flores, J.M.; Desco, M.; Marquez, V.; Esteller, M. The novel DNA methylation inhibitor zebularine is effective against the development of murine T-cell lymphoma. Blood 2006, 107, 1174–1177. [Google Scholar] [CrossRef]
- Malumbres, M.; Perez de Castro, I.; Santos, J.; Melendez, B.; Mangues, R.; Serrano, M.; Pellicer, A.; Fernandez-Piqueras, J. Inactivation of the cyclin-dependent kinase inhibitor p15INK4b by deletion and de novo methylation with independence of p16INK4a alterations in murine primary T-cell lymphomas. Oncogene 1997, 14, 1361–1370. [Google Scholar] [CrossRef][Green Version]
- Malumbres, M.; Perez de Castro, I.; Santos, J.; Fernandez Piqueras, J.; Pellicer, A. Hypermethylation of the cell cycle inhibitor p15INK4b 3′-untranslated region interferes with its transcriptional regulation in primary lymphomas. Oncogene 1999, 18, 385–396. [Google Scholar] [CrossRef]
- Santos, J.; Herranz, M.; Fernandez, M.; Vaquero, C.; Lopez, P.; Fernandez-Piqueras, J. Evidence of a possible epigenetic inactivation mechanism operating on a region of mouse chromosome 19 in gamma-radiation-induced thymic lymphomas. Oncogene 2001, 20, 2186–2189. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Liu, Y.; Liu, Y.; Zhang, C.; Yuan, B.; Zhang, L.; Sun, S. Increased p16 DNA methylation in mouse thymic lymphoma induced by irradiation. PLoS ONE 2014, 9, e93850. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Takabatake, T.; Kakinuma, S.; Amasaki, Y.; Nishimura, M.; Imaoka, T.; Yamauchi, K.; Shang, Y.; Miyoshi-Imamura, T.; Nogawa, H.; et al. Complicated biallelic inactivation of Pten in radiation-induced mouse thymic lymphomas. Mutat. Res. 2010, 686, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhou, C.; Zhang, J.; Gao, F.; Li, B.; Chuai, Y.; Liu, C.; Cai, J. Hydrogen protects mice from radiation induced thymic lymphoma in BALB/c mice. Int. J. Biol. Sci. 2011, 7, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, H.S.; Brown, M.B.; Hirsch, B.B.; Carnes, W.H. Indirect induction of lymphomas in irradiated mice. II. Factor of irradiation of the host. Cancer Res. 1956, 16, 426–428. [Google Scholar] [PubMed]
- Hacein-Bey-Abina, S.; Garrigue, A.; Wang, G.P.; Soulier, J.; Lim, A.; Morillon, E.; Clappier, E.; Caccavelli, L.; Delabesse, E.; Beldjord, K.; et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Investig. 2008, 118, 3132–3142. [Google Scholar] [CrossRef] [PubMed]
- Howe, S.J.; Mansour, M.R.; Schwarzwaelder, K.; Bartholomae, C.; Hubank, M.; Kempski, H.; Brugman, M.H.; Pike-Overzet, K.; Chatters, S.J.; de Ridder, D.; et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J. Clin. Investig. 2008, 118, 3143–3150. [Google Scholar] [CrossRef] [PubMed]
- Ginn, S.L.; Hallwirth, C.V.; Liao, S.H.Y.; Teber, E.T.; Arthur, J.W.; Wu, J.; Lee, H.C.; Tay, S.S.; Hu, M.; Reddel, R.R.; et al. Limiting Thymic Precursor Supply Increases the Risk of Lymphoid Malignancy in Murine X-Linked Severe Combined Immunodeficiency. Molecular therapy. Nucleic Acids 2017, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Potworowski, E.F.; Gagnon, F.; Beauchemin, C.; St Pierre, Y. Dendritic cells prevent radiation-induced thymic lymphoma. Leukemia 1996, 10, 1639–1647. [Google Scholar] [PubMed]
- Ardavin, C.; Wu, L.; Li, C.L.; Shortman, K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature 1993, 362, 761–763. [Google Scholar] [CrossRef]
- Katsura, Y. Redefinition of lymphoid progenitors. Nat. Rev. Immunol. 2002, 2, 127–132. [Google Scholar] [CrossRef]
- Kawamoto, H.; Katsura, Y. A new paradigm for hematopoietic cell lineages: Revision of the classical concept of the myeloid-lymphoid dichotomy. Trends Immunol. 2009, 30, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.W.; Bell, J.J.; Zlotoff, D.A.; Bhandoola, A. Untangling the T branch of the hematopoiesis tree. Curr. Opin. Immunol. 2009, 21, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, E.V.; Hosokawa, H.; Ungerback, J. Mechanisms of Action of Hematopoietic Transcription Factor PU.1 in Initiation of T-Cell Development. Front. Immunol. 2019, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Luc, S.; Luis, T.C.; Boukarabila, H.; Macaulay, I.C.; Buza-Vidas, N.; Bouriez-Jones, T.; Lutteropp, M.; Woll, P.S.; Loughran, S.J.; Mead, A.J.; et al. The earliest thymic T cell progenitors sustain B cell and myeloid lineage potential. Nat. Immunol. 2012, 13, 412–419. [Google Scholar] [CrossRef]
- Manz, M.G.; Traver, D.; Miyamoto, T.; Weissman, I.L.; Akashi, K. Dendritic cell potentials of early lymphoid and myeloid progenitors. Blood 2001, 97, 3333–3341. [Google Scholar] [CrossRef] [PubMed]
- Guyden, J.C.; Pezzano, M. Thymic nurse cells: A microenvironment for thymocyte development and selection. Int. Rev. Cytol. 2003, 223, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pezzano, M.; Philp, D.; Reid, V.; Guyden, J. Thymic nurse cells exclusively bind and internalize CD4+CD8+ thymocytes. Cell Immunol. 1992, 140, 495–506. [Google Scholar] [CrossRef]
- Defresne, M.P.; Rongy, A.M.; Greimers, R.; Boniver, J. Cellular aspects of radiation leukemogenesis in C57 BL/Ka mice: Alterations to thymic microenvironment and lymphopoiesis. Leuk. Res. 1986, 10, 783–789. [Google Scholar] [CrossRef]
- Defresne, M.P.; Greimers, R.; Lenaerts, P.; Boniver, J. Effects of marrow grafting on preleukemia cells and thymic nurse cells in C57BL/Ka mice after a leukemogenic split-dose irradiation. J. Natl. Cancer Inst. 1986, 77, 1079–1085. [Google Scholar]
- Pierce, G.B.; Speers, W.C. Tumors as caricatures of the process of tissue renewal: Prospects for therapy by directing differentiation. Cancer Res. 1988, 48, 1996–2004. [Google Scholar] [PubMed]
- Sell, S.; Pierce, G.B. Maturation arrest of stem cell differentiation is a common pathway for the cellular origin of teratocarcinomas and epithelial cancers. Lab. Investig. 1994, 70, 6–22. [Google Scholar]
- Sell, S. Leukemia: Stem cells, maturation arrest, and differentiation therapy. Stem. Cell Rev. 2005, 1, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Dukor, P.; Miller, J.F.; House, W.; Allman, V. Regeneration of thymus grafts. I. Histological and cytological aspects. Transplantation 1965, 3, 639–668. [Google Scholar] [CrossRef]
- Behjati, S.; Gilbertson, R.J.; Pfister, S.M. Maturation Block in Childhood Cancer. Cancer Discov. 2021, 11, 542–544. [Google Scholar] [CrossRef]
- Martins, V.C.; Busch, K.; Juraeva, D.; Blum, C.; Ludwig, C.; Rasche, V.; Lasitschka, F.; Mastitsky, S.E.; Brors, B.; Hielscher, T.; et al. Cell competition is a tumour suppressor mechanism in the thymus. Nature 2014, 509, 465–470. [Google Scholar] [CrossRef]
- Paiva, R.A.; Sousa, A.G.G.; Ramos, C.V.; Avila, M.; Lilue, J.; Paixao, T.; Martins, V.C. Self-renewal of double-negative 3 early thymocytes enables thymus autonomy but compromises the beta-selection checkpoint. Cell Rep. 2021, 35, 108967. [Google Scholar] [CrossRef]
- Ramos, C.V.; Ballesteros-Arias, L.; Silva, J.G.; Paiva, R.A.; Nogueira, M.F.; Carneiro, J.; Gjini, E.; Martins, V.C. Cell Competition, the Kinetics of Thymopoiesis, and Thymus Cellularity Are Regulated by Double-Negative 2 to 3 Early Thymocytes. Cell Rep. 2020, 32, 107910. [Google Scholar] [CrossRef]
- Boniver, J.; Humblet, C.; Defresne, M.P. Tumor necrosis factor and interferon gamma inhibit the development of radiation-induced thymic lymphomas in C57BL/Ka mice. Leukemia 1989, 3, 611–613. [Google Scholar]
- Boniver, J.; Humblet, C.; Delvenne, P.; Deman, J.; Rongy, A.M.; Greimers, R.; Defresne, M.P. TNF-alpha and radiation-induced thymic lymphomas. Leukemia 1992, 6 (Suppl. S3), 83S–84S. [Google Scholar]
- Humblet, C.; Greimers, R.; Delvenne, P.; Deman, J.; Boniver, J.; Defresne, M.P. Prevention of murine radiogenic thymic lymphomas by tumor necrosis factor or by marrow grafting. J. Natl. Cancer Inst. 1996, 88, 824–831. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Labi, V.; Erlacher, M.; Krumschnabel, G.; Manzl, C.; Tzankov, A.; Pinon, J.; Egle, A.; Villunger, A. Apoptosis of leukocytes triggered by acute DNA damage promotes lymphoma formation. Genes Dev. 2010, 24, 1602–1607. [Google Scholar] [CrossRef]
- Michalak, E.M.; Vandenberg, C.J.; Delbridge, A.R.; Wu, L.; Scott, C.L.; Adams, J.M.; Strasser, A. Apoptosis-promoted tumorigenesis: Gamma-irradiation-induced thymic lymphomagenesis requires Puma-driven leukocyte death. Genes Dev. 2010, 24, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Feng, D.; Korczeniewska, J.; Alper, N.; Hu, G.; Barnes, B.J. Deletion of Irf5 protects hematopoietic stem cells from DNA damage-induced apoptosis and suppresses gamma-irradiation-induced thymic lymphomagenesis. Oncogene 2014, 33, 3288–3297. [Google Scholar] [CrossRef]
- Carr, M.I.; Roderick, J.E.; Gannon, H.S.; Kelliher, M.A.; Jones, S.N. Mdm2 Phosphorylation Regulates Its Stability and Has Contrasting Effects on Oncogene and Radiation-Induced Tumorigenesis. Cell Rep. 2016, 16, 2618–2629. [Google Scholar] [CrossRef]
- UNSCEAR. Genetic and Somatic Effects of Ionizing Radiation; UNSCEAR: New York, NY, USA, 1986. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sado, T.; Cart, J.B.; Lee, C.-L. Mechanisms Underlying the Development of Murine T-Cell Lymphoblastic Lymphoma/Leukemia Induced by Total-Body Irradiation. Cancers 2024, 16, 2224. https://doi.org/10.3390/cancers16122224
Sado T, Cart JB, Lee C-L. Mechanisms Underlying the Development of Murine T-Cell Lymphoblastic Lymphoma/Leukemia Induced by Total-Body Irradiation. Cancers. 2024; 16(12):2224. https://doi.org/10.3390/cancers16122224
Chicago/Turabian StyleSado, Toshihiko, John B. Cart, and Chang-Lung Lee. 2024. "Mechanisms Underlying the Development of Murine T-Cell Lymphoblastic Lymphoma/Leukemia Induced by Total-Body Irradiation" Cancers 16, no. 12: 2224. https://doi.org/10.3390/cancers16122224
APA StyleSado, T., Cart, J. B., & Lee, C.-L. (2024). Mechanisms Underlying the Development of Murine T-Cell Lymphoblastic Lymphoma/Leukemia Induced by Total-Body Irradiation. Cancers, 16(12), 2224. https://doi.org/10.3390/cancers16122224




