Conventional versus Hepatic Arteriography and C-Arm CT-Guided Ablation of Liver Tumors (HepACAGA): A Comparative Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Patients
2.3. Treatment
2.3.1. Conventional Procedure
2.3.2. HepACAGA Procedure
2.4. Imaging Follow-Up
2.5. Study Objectives
2.5.1. Local Tumor Recurrence
2.5.2. Technical Success and Procedure-Related Characteristics
2.5.3. Complications
2.6. Statistical Analysis
3. Results
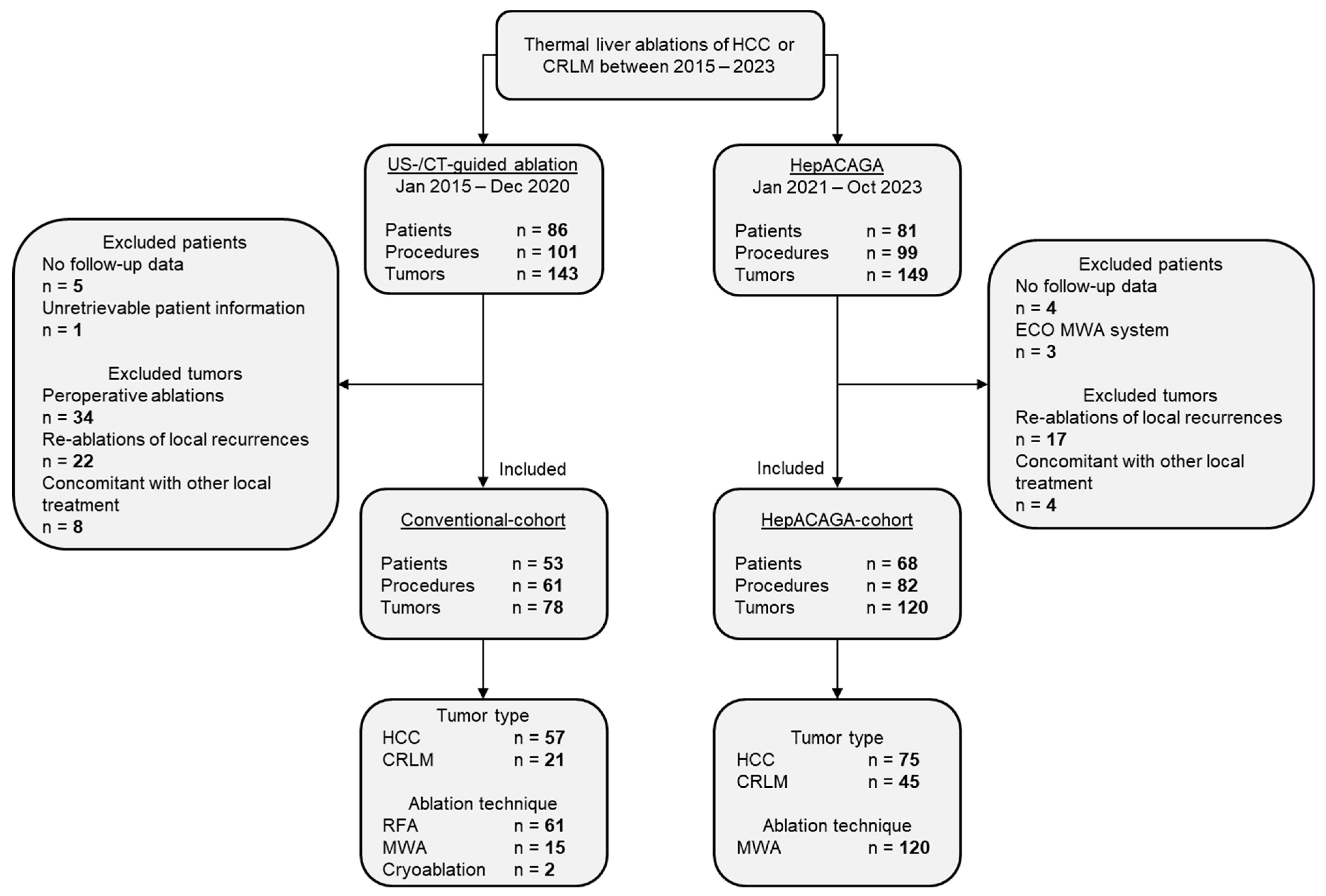
3.1. Patient Inclusion and Characteristics
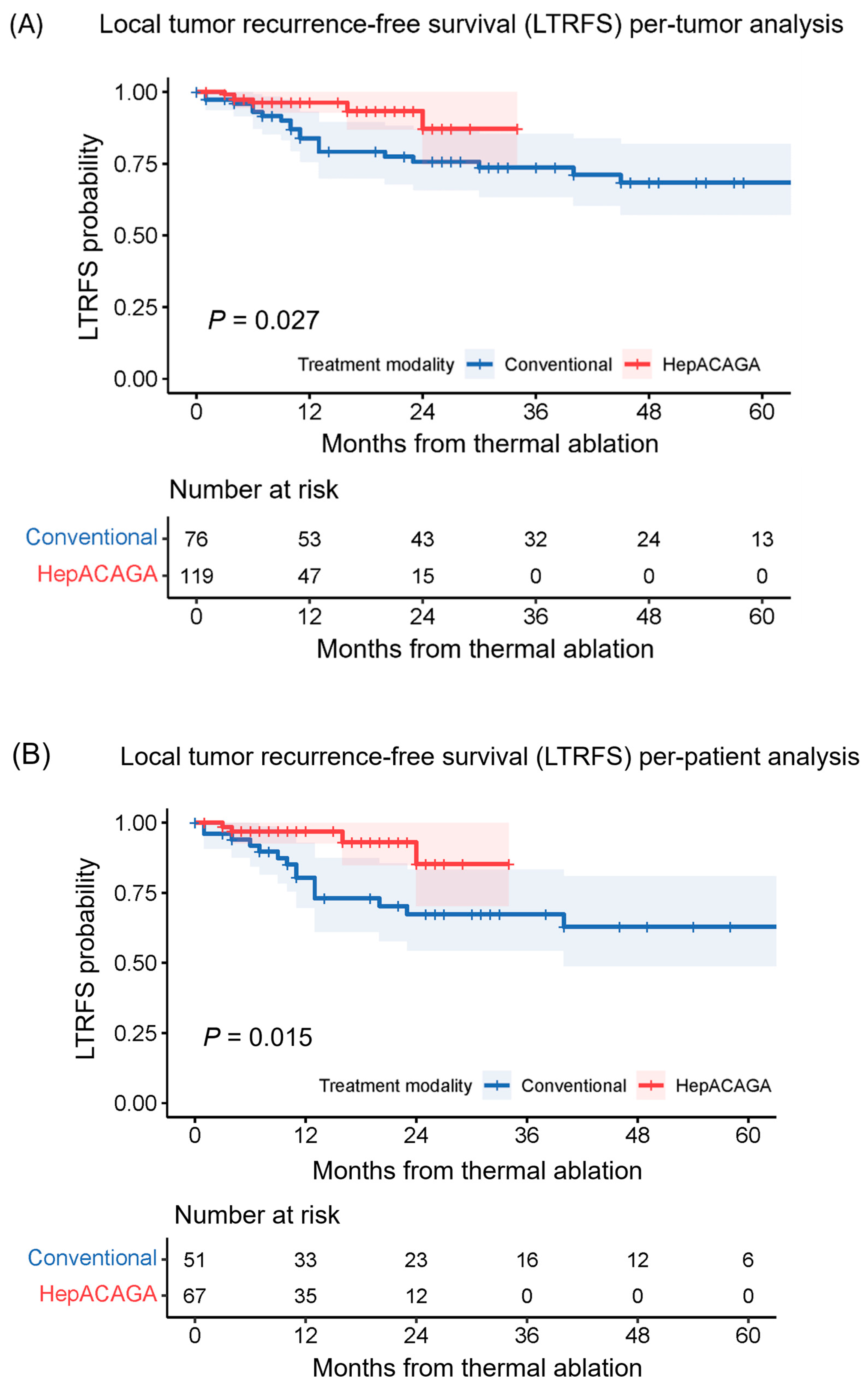
3.2. Local Tumor Recurrence
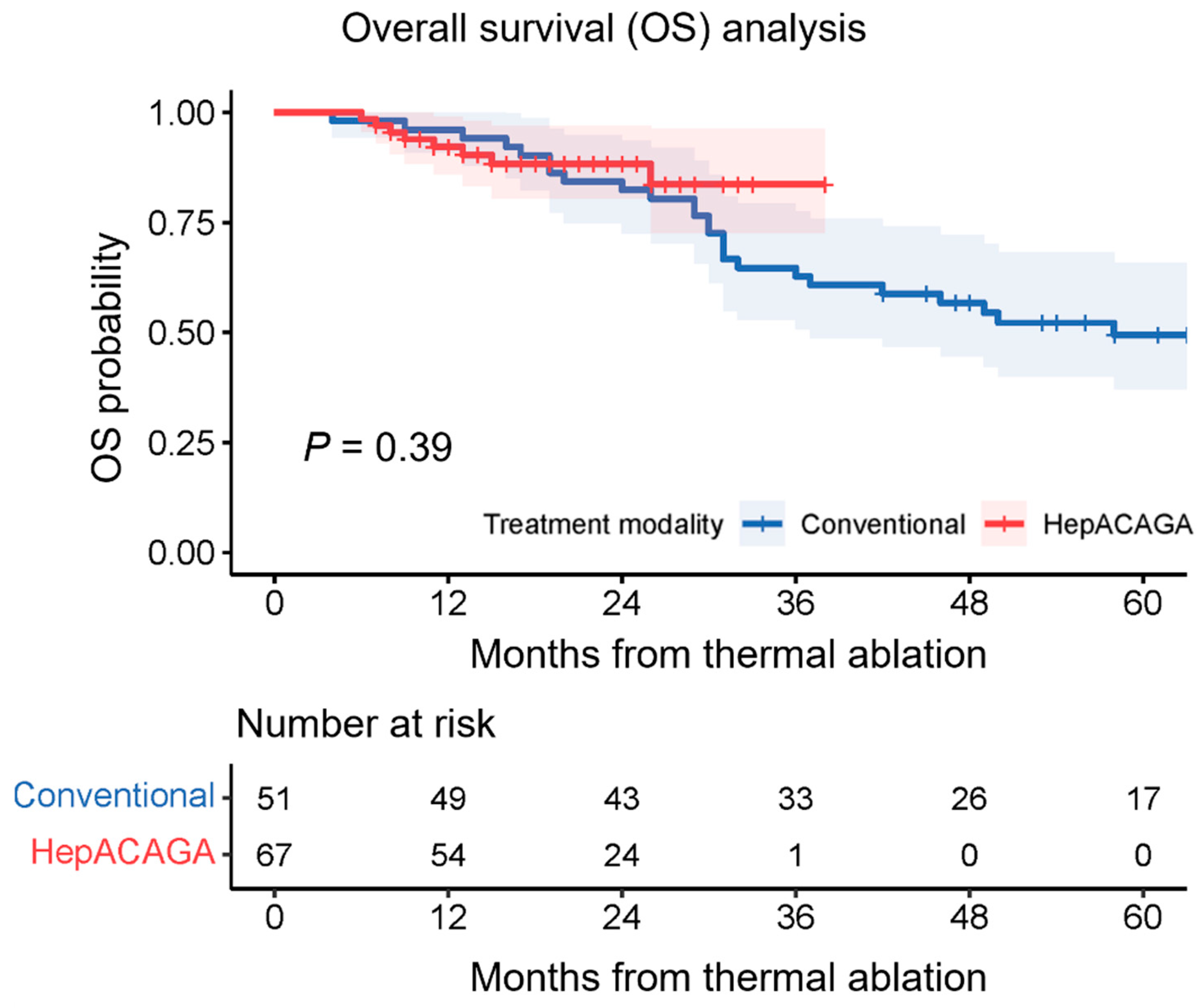
3.3. Overall Survival
3.4. Technical Success
3.5. Procedure-Related Characteristics
3.6. Complications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Chen, Q.; Chhatwal, J. Changing Epidemiology of Hepatocellular Carcinoma and Role of Surveillance. In Hepatocellular Carcinoma: Translational Precision Medicine Approaches; Hoshida, Y., Ed.; Humana Press: Cham, Switzerland, 2019; Chapter 3; ISBN 978-3-030-21539-2. [Google Scholar]
- Engstrand, J.; Nilsson, H.; Strömberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, Z.; Wang, Y.; Wen, X.; Amador, E.H.; Yuan, L.; Ran, X.; Xiong, L.; Ran, Y.; Chen, W.; et al. Colorectal liver metastasis: Molecular mechanism and interventional therapy. Signal Transduct. Target. Ther. 2022, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.-C.; Neumann, U.; Ricke, J.; Sangro, B.; et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv238–iv255. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.J.; Willatt, J.; Majdalany, B.S.; Kielar, A.Z.; Chong, S.; Ruma, J.A.; Pandya, A. Ablation techniques for primary and metastatic liver tumors. World J. Hepatol. 2016, 8, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Hui, T.C.H.; Kwan, J.; Pua, U. Advanced Techniques in the Percutaneous Ablation of Liver Tumours. Diagnostics 2021, 11, 585. [Google Scholar] [CrossRef] [PubMed]
- Puijk, R.S.; Ruarus, A.H.; Scheffer, H.J.; Vroomen, L.G.P.H.; van Tilborg, A.A.J.M.; de Vries, J.J.J.; Berger, F.H.; van den Tol, P.M.P.; Meijerink, M.R. Percutaneous Liver Tumour Ablation: Image Guidance, Endpoint Assessment, and Quality Control. Can. Assoc. Radiol. J. 2018, 69, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H. Current role of ultrasound in the diagnosis of hepatocellular carcinoma. J. Med. Ultrason. 2020, 47, 239–255. [Google Scholar] [CrossRef]
- Mansur, A.; Garg, T.; Shrigiriwar, A.; Etezadi, V.; Georgiades, C.; Habibollahi, P.; Huber, T.C.; Camacho, J.C.; Nour, S.G.; Sag, A.A.; et al. Image-Guided Percutaneous Ablation for Primary and Metastatic Tumors. Diagnostics 2022, 12, 1300. [Google Scholar] [CrossRef]
- van Tilborg, A.A.J.M.; Scheffer, H.J.; Nielsen, K.; van Waesberghe, J.H.T.M.; Comans, E.F.; van Kuijk, C.; van den Tol, P.M.; Meijerink, M.R. Transcatheter CT arterial portography and CT hepatic arteriography for liver tumor visualization during percutaneous ablation. J. Vasc. Interv. Radiol. 2014, 25, 1101–1111.e4. [Google Scholar] [CrossRef] [PubMed]
- van Tilborg, A.A.J.M.; Scheffer, H.J.; van der Meijs, B.B.; van Werkum, M.H.; Melenhorst, M.C.A.M.; van den Tol, P.M.; Meijerink, M.R. Transcatheter CT hepatic arteriography-guided percutaneous ablation to treat ablation site recurrences of colorectal liver metastases: The incomplete ring sign. J. Vasc. Interv. Radiol. 2015, 26, 583–587.e1. [Google Scholar] [CrossRef] [PubMed]
- van der Lei, S.; Opperman, J.; Dijkstra, M.; Kors, N.; Boon, R.; van den Bemd, B.A.T.; Timmer, F.E.F.; Nota, I.M.G.C.; van den Bergh, J.E.; de Vries, J.J.J.; et al. The Added Diagnostic Value of Transcatheter CT Hepatic Arteriography for Intraprocedural Detection of Previously Unknown Colorectal Liver Metastases During Percutaneous Ablation and Impact on the Definitive Treatment Plan. Cardiovasc. Interv. Radiol. 2023, 46, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.L.J.; Bruijnen, R.C.G.; Tetteroo, P.; Vonken, E.J.P.A.; Meijerink, M.R.; Hagendoorn, J.; de Bruijne, J.; Prevoo, W. Hepatic Arteriography and C-Arm CT-Guided Ablation (HepACAGA) to Improve Tumor Visualization, Navigation and Margin Confirmation in Percutaneous Liver Tumor Ablation. Cardiovasc. Interv. Radiol. 2023, 46, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Puijk, R.S.; Ahmed, M.; Adam, A.; Arai, Y.; Arellano, R.; de Baère, T.; Bale, R.; Bellera, C.; Binkert, C.A.; Brace, C.L.; et al. Consensus Guidelines for the Definition of Time-to-Event End Points in Image-guided Tumor Ablation: Results of the SIO and DATECAN Initiative. Radiology 2021, 301, 533–540. [Google Scholar] [CrossRef]
- The American Association of Physicists in Medicine, Report No. 096—The Measurement, Reporting, and Management of Radiation Dose in CT (2008). College Park, MD, USA, 2008. Available online: https://www.aapm.org/pubs/reports/rpt_96.pdf (accessed on 21 February 2024).
- Tyan, Y.S.; Li, Y.Y.; Ku, M.C.; Huang, H.H.; Chen, T.R. The effective dose assessment of C-arm CT in hepatic arterial embolisation therapy. Br. J. Radiol. 2013, 86, 20120551. [Google Scholar] [CrossRef] [PubMed]
- N. Cancer Institute, Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 25 January 2024).
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria, 2019. Available online: http://www.R-project.org/ (accessed on 10 January 2024).
- Meijerink, M.R. COLLISION trial interim results: Surgery vs. MWA. In Proceedings of the CIRSE 2021 Summit, Virtual, 25–28 September 2021. [Google Scholar]
- Puijk, R.S.; Ruarus, A.H.; Vroomen, L.G.P.H.; van Tilborg, A.A.J.M.; Scheffer, H.J.; Nielsen, K.; de Jong, M.C.; de Vries, J.J.J.; Zonderhuis, B.M.; Eker, H.H.; et al. Colorectal liver metastases: Surgery versus thermal ablation (COLLISION)—A phase III single-blind prospective randomized controlled trial. BMC Cancer 2018, 18, 821. [Google Scholar] [CrossRef] [PubMed]
- Pillai, K.; Akhter, J.; Chua, T.C.; Shehata, M.; Alzahrani, N.; Al-Alem, I.; Morris, D.L. Heat sink effect on tumor ablation characteristics as observed in monopolar radiofrequency, bipolar radiofrequency, and microwave, using ex vivo calf liver model. Medicine 2015, 94, e580. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Abd Elmoety, A.A.; Rostom, Y.A.M.; Shater, M.S.; Lashen, S.A. Percutaneous radiofrequency versus microwave ablation for management of hepatocellular carcinoma: A randomized controlled trial. J. Gastrointest. Oncol. 2019, 10, 562–571. [Google Scholar] [CrossRef]
- Nielsen, K.; van Tilborg, A.A.J.M.; Meijerink, M.R.; Macintosh, M.O.; Zonderhuis, B.M.; de Lange, E.S.M.; Comans, E.F.I.; Meijer, S.; van den Tol, M.P. Incidence and treatment of local site recurrences following RFA of colorectal liver metastases. World J. Surg. 2013, 37, 1340–1347. [Google Scholar] [CrossRef]
- Tanis, E.; Nordlinger, B.; Mauer, M.; Sorbye, H.; van Coevorden, F.; Gruenberger, T.; Schlag, P.M.; Punt, C.J.A.; Ledermann, J.; Ruers, T.J.M. Local recurrence rates after radiofrequency ablation or resection of colorectal liver metastases. Analysis of the European Organisation for Research and Treatment of Cancer #40004 and #40983. Eur. J. Cancer 2014, 50, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Puijk, R.S.; Dijkstra, M.; van der Lei, S.; Schulz, H.H.; Vos, D.J.W.; Timmer, F.E.F.; Geboers, B.; Scheffer, H.J.; de Vries, J.J.J.; Smits, M.L.J.; et al. The Added Value of Transcatheter CT Hepatic Angiography (CTHA) Image Guidance in Percutaneous Thermal Liver Ablation: An Experts’ Opinion Pictorial Essay. Cancers 2024, 16, 1193. [Google Scholar] [CrossRef] [PubMed]
| Conventional Ablation | HepACAGA | p Value | ||
|---|---|---|---|---|
| Patient-related characteristics | n = 53 | n = 68 | ||
| Sex, n (%) | Male | 36 (68) | 51 (75) | 0.390 * |
| Female | 17 (32) | 17 (25) | ||
| Age (years), median (range) | 64 (38–86) | 68 (16–87) | 0.102 † | |
| BMI (kg/m2), median (range) | 25 (17–34) | 27 (16–42) | 0.301 † | |
| ASA, n (%) | 1 | 1 (2) | 4 (6) | 0.270 * |
| 2 | 27 (51) | 22 (32) | ||
| 3 | 21 (40) | 35 (51) | ||
| 4 | 1 (2) | 3 (4) | ||
| Unknown | 3 (6) | 4 (6) | ||
| Tumor-related characteristics | n = 78 | n = 120 | ||
| Tumor type, n (%) | HCC | 57 (73) | 75 (63) | 0.123 * |
| CRLM | 21 (27) | 45 (37) | ||
| Diameter (mm), median (range) | 15 (5–32) | 15 (2–46) | 0.303 † | |
| Size (mm), n (%) | 1–10 | 14 (18) | 33 (28) | 0.021 * |
| 11–20 | 41 (53) | 58 (48) | ||
| 21–30 | 22 (28) | 19 (16) | ||
| >31 | 1 (1) | 10 (8) | ||
| Procedure-related characteristics | n = 78 | n = 120 | ||
| Image guidance for needle placement, n (%) | Percutaneous US | 54 (69) | – | |
| CT fluoroscopy | 24 (31) | – | ||
| C-arm CT | – | 120 (100) | ||
| Ablation modality, n (%) | RFA | 61 (78) | – | |
| MWA | 15 (19) | 120 (100) | ||
| Cryoablation | 2 (3) | – |
| Conventional Ablation | HepACAGA | p Value | ||
|---|---|---|---|---|
| Follow-up analysis | n = 51 | n = 67 | ||
| Follow-up (months), median (range) | 20 (1–72) | 12 (1–34) | <0.001 † | |
| Follow-up imaging modality, n (%) | MRI only | 21 (41) | 39 (58) | 0.184 * |
| CT only | 5 (10) | 2 (3) | ||
| MRI + CT | 21 (41) | 23 (34) | ||
| MRI + 18FFDG-PET | 4 (8) | 3 (4) | ||
| New liver tumors (other than ablated), n (%) | 19 (37) | 20 (30) | 0.397 * | |
| New or progressive extrahepatic tumors, n (%) | 13 (25) | 14 (21) | 0.556 * | |
| LTR per patient, n (%) | 14 (27) | 4 (6) | 0.002 ‡ | |
| n = 76 | n = 119 | |||
| LTR per tumor, n (%) | 20 (26) | 6 (5) | <0.001 ‡ | |
| LTR subanalysis HCC | n = 33 | n = 38 | ||
| LTR per patient, n (%) | 9 (22) | 2 (5) | 0.019 ‡ | |
| n = 55 | n = 74 | |||
| LTR per tumor, n (%) | 14 (25) | 2 (3) | <0.001 ‡ | |
| LTR subanalysis CRLM | n = 18 | n = 29 | ||
| LTR per patient, n (%) | 5 (27) | 2 (7) | 0.089 ‡ | |
| n = 21 | n = 45 | |||
| LTR per tumor, n (%) | 6 (29) | 4 (8) | 0.062 ‡ | |
| LTR subanalysis MWA | n = 12 | n = 67 | ||
| LTR per patient, n (%) | 4 (33) | 4 (6) | 0.016 ‡ | |
| n = 15 | n = 119 | |||
| LTR per tumor, n (%) | 5 (33) | 6 (5) | 0.003 ‡ |
| Conventional Ablation | HepACAGA | p Value | ||
|---|---|---|---|---|
| Technical Success | n = 61 | n = 82 | ||
| Technically successful procedures, n (%) | 59 (97) | 81 (99) | 0.575 ‡ | |
| Complications | ||||
| Complicated procedures, n (%) | 9 (15) | 3 (4) | 0.041 ‡ | |
| Complications (CTCAE v5), n (%) | Grade 2 | 3 (5) | 1 (1) | 0.641 * |
| Grade 3 | 5 (8) | 1 (1) | ||
| Grade 4 | 1 (2) | 1 (1) | ||
| Type of complication, n (%) | Pneumothorax | 5 (8) | – | |
| Perihepatic hematoma | 2 (3) | 1 (1) | ||
| Subcapsular hematoma | 1 (2) | – | ||
| Active bleeding (embolization required) | 1 (2) | 1 (1) | ||
| Abscess | 1 (2) | 1 (1) | ||
| Time parameters | ||||
| In-room time (min), median (range) | 106 (61–162) | 135 (73–236) | <0.001 † | |
| Procedure duration (min), median (range) | 60 (24–119) | 86 (43–179) | <0.001 † | |
| Per-lesion procedure time (min), median (range) | 45 (24–119) | 59 (16–137) | <0.001 † | |
| Contrast and effective dose | ||||
| Contrast (mL), median (range) | 180 (90–450) | 150 (65–350) | 0.041 † | |
| Per-lesion contrast (mL), median (range) | 140 (45–300) | 100 (29–250) | 0.033 † | |
| Effective dose (mSv), median (range) | 13 (6–43) | 58 (14–203) | <0.001 † | |
| Per-lesion effective dose (mSv), median (range) | 11 (3–43) | 39 (14–126) | 0.031 † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijnen, N.; Bruijnen, R.C.G.; Vonken, E.-J.P.A.; de Jong, H.W.A.M.; de Bruijne, J.; Bol, G.M.; Hagendoorn, J.; Intven, M.P.W.; Smits, M.L.J. Conventional versus Hepatic Arteriography and C-Arm CT-Guided Ablation of Liver Tumors (HepACAGA): A Comparative Analysis. Cancers 2024, 16, 1925. https://doi.org/10.3390/cancers16101925
Wijnen N, Bruijnen RCG, Vonken E-JPA, de Jong HWAM, de Bruijne J, Bol GM, Hagendoorn J, Intven MPW, Smits MLJ. Conventional versus Hepatic Arteriography and C-Arm CT-Guided Ablation of Liver Tumors (HepACAGA): A Comparative Analysis. Cancers. 2024; 16(10):1925. https://doi.org/10.3390/cancers16101925
Chicago/Turabian StyleWijnen, Niek, Rutger C. G. Bruijnen, Evert-Jan P. A. Vonken, Hugo W. A. M. de Jong, Joep de Bruijne, Guus M. Bol, Jeroen Hagendoorn, Martijn P. W. Intven, and Maarten L. J. Smits. 2024. "Conventional versus Hepatic Arteriography and C-Arm CT-Guided Ablation of Liver Tumors (HepACAGA): A Comparative Analysis" Cancers 16, no. 10: 1925. https://doi.org/10.3390/cancers16101925
APA StyleWijnen, N., Bruijnen, R. C. G., Vonken, E.-J. P. A., de Jong, H. W. A. M., de Bruijne, J., Bol, G. M., Hagendoorn, J., Intven, M. P. W., & Smits, M. L. J. (2024). Conventional versus Hepatic Arteriography and C-Arm CT-Guided Ablation of Liver Tumors (HepACAGA): A Comparative Analysis. Cancers, 16(10), 1925. https://doi.org/10.3390/cancers16101925






