Local Treatment of Colorectal Liver Metastases in the Presence of Extrahepatic Disease: Survival Outcomes from the Amsterdam Colorectal Liver Met Registry (AmCORE)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
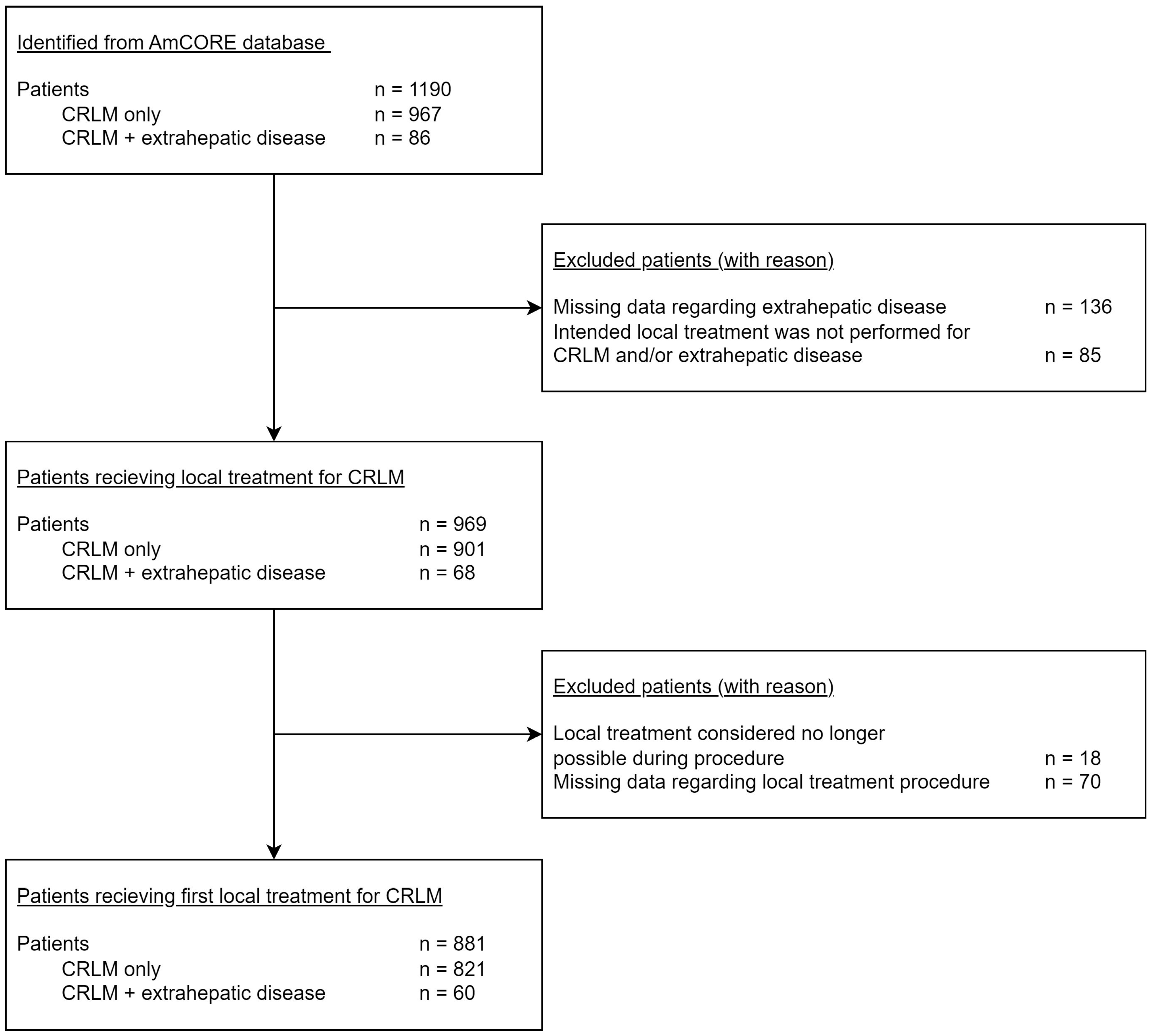
2.1. Data Collection and Patient Selection
2.2. Diagnostic Work-up and Follow-up
2.3. Statistical Analysis
3. Results
3.1. Patient and Disease Characteristics
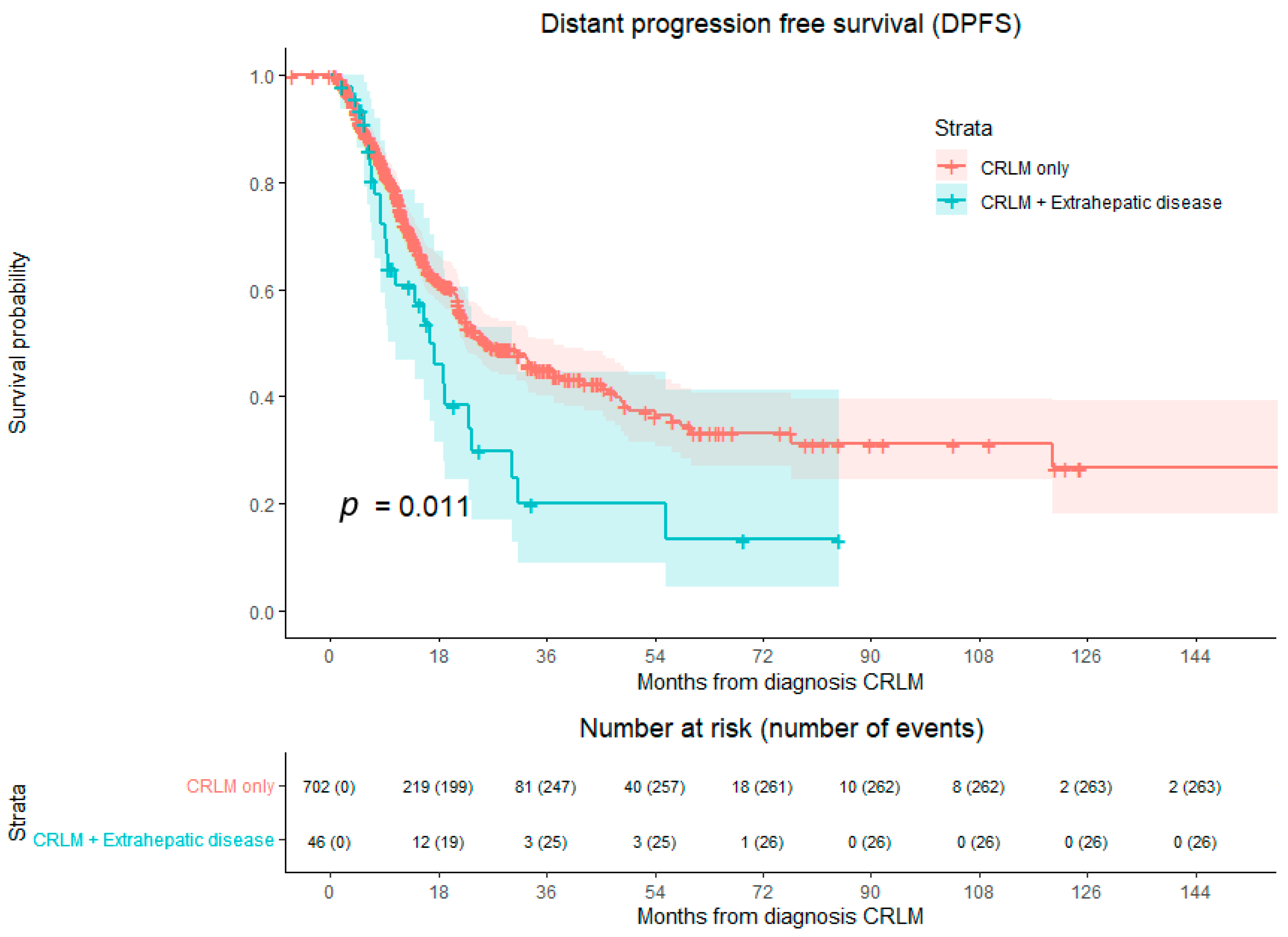
3.2. Overall Survival and Disease-Free Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Agency for Research on Cancer; WHO. WHO Estimated Age-Standardized Incidence Rates (World) in 2022. All Cancers, Both Sexes, All Ages. Nog Completteren. 2020. Available online: https://gco.iarc.fr/today/online-analysis-map (accessed on 11 November 2023).
- IKNL. Rapport ‘Uitgezaaide Kanker in Beeld’ 2018. Available online: https://iknl.nl/uitgezaaide-kanker (accessed on 8 August 2023).
- Engstrand, J.; Nilsson, H.; Stromberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef] [PubMed]
- NCCN (National Comprehensive Cancer Network) Guidelines—Colorectal Cancer 2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed on 6 February 2024).
- Scheele, J.; Stangl, R.; Altendorf-Hofmann, A. Hepatic metastases from colorectal carcinoma: Impact of surgical resection on the natural history. Br. J. Surg. 1990, 77, 1241–1246. [Google Scholar] [CrossRef]
- Stangl, R.; Altendorf-Hofmann, A.; Charnley, R.M.; Scheele, J. Factors influencing the natural history of colorectal liver metastases. Lancet 1994, 343, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liao, F.; Huang, Y.; Jiang, C.; Liu, S.; He, W.; Kong, P.; Zhang, B.; Xia, L. Longterm effects of palliative local treatment of incurable metastatic lesions in colorectal cancer patients. Oncotarget 2016, 7, 21034–21045. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cremolini, C.; Loupakis, F.; Antoniotti, C.; Lupi, C.; Sensi, E.; Lonardi, S.; Mezi, S.; Tomasello, G.; Ronzoni, M.; Zaniboni, A.; et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015, 16, 1306–1315. [Google Scholar] [CrossRef]
- Cremolini, C.; Marmorino, F.; Loupakis, F.; Masi, G.; Antoniotti, C.; Salvatore, L.; Schirripa, M.; Boni, L.; Zagonel, V.; Lonardi, S.; et al. TRIBE-2: A phase III, randomized, open-label, strategy trial in unresectable metastatic colorectal cancer patients by the GONO group. BMC Cancer 2017, 17, 408. [Google Scholar] [CrossRef]
- Adam, R.; Delvart, V.; Pascal, G.; Valeanu, A.; Castaing, D.; Azoulay, D.; Giacchetti, S.; Paule, B.; Kunstlinger, F.; Ghémard, O.; et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: A model to predict long-term survival. Ann. Surg. 2004, 240, 644–657, discussion 657–658. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, M.; Nieuwenhuizen, S.; Puijk, R.S.; Timmer, F.E.F.; Geboers, B.; Schouten, E.A.C.; Opperman, J.; Scheffer, H.J.; de Vries, J.J.J.; Swijnenburg, R.-J.; et al. Thermal Ablation Compared to Partial Hepatectomy for Recurrent Colorectal Liver Metastases: An Amsterdam Colorectal Liver Met Registry (AmCORE) Based Study. Cancers 2021, 13, 2769. [Google Scholar] [CrossRef]
- Abdalla, E.K.; Vauthey, J.N.; Ellis, L.M.; Ellis, V.; Pollock, R.; Broglio, K.R.; Hess, K.; Curley, S.A. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann. Surg. 2004, 239, 818–825, discussion 825–827. [Google Scholar] [CrossRef]
- Gleisner, A.L.; Choti, M.A.; Assumpcao, L.; Nathan, H.; Schulick, R.D.; Pawlik, T.M. Colorectal liver metastases: Recurrence and survival following hepatic resection, radiofrequency ablation, and combined resection-radiofrequency ablation. Arch. Surg. 2008, 143, 1204–1212. [Google Scholar] [CrossRef]
- Bond, M.J.G.; Bolhuis, K.; Loosveld, O.J.L.; de Groot, J.W.B.; Droogendijk, H.; Helgason, H.H.; Hendriks, M.P.; Klaase, J.M.; Kazemier, G.; Liem, M.S.L.; et al. First-line systemic treatment strategies in patients with initially unresectable colorectal cancer liver metastases (CAIRO5): An open-label, multicentre, randomised, controlled, phase 3 study from the Dutch Colorectal Cancer Group. Lancet Oncol. 2023, 24, 757–771. [Google Scholar] [CrossRef] [PubMed]
- Puijk, R.S.; Dijkstra, M.; van den Bemd, B.A.T.; Ruarus, A.H.; Nieuwenhuizen, S.; Geboers, B.; Timmer, F.E.F.; Schouten, E.A.C.; de Vries, J.J.J.; van der Meijs, B.B.; et al. Improved Outcomes of Thermal Ablation for Colorectal Liver Metastases: A 10-Year Analysis from the Prospective Amsterdam CORE Registry (AmCORE). Cardiovasc. Interv. Radiol. 2022, 45, 1074–1089. [Google Scholar] [CrossRef] [PubMed]
- Hugen, N.; van de Velde, C.J.H.; de Wilt, J.H.W.; Nagtegaal, I.D. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann. Oncol. 2014, 25, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Liberale, G.; Vernerey, D.; Pocard, M.; Ducreux, M.; Boige, V.; Malka, D.; Pignon, J.-P.; Lasser, P. Hepatic and extrahepatic colorectal metastases: When resectable, their localization does not matter, but their total number has a prognostic effect. Ann. Surg. Oncol. 2005, 12, 900–909. [Google Scholar] [CrossRef] [PubMed]
- de Haas, R.J.; Wicherts, D.A.; Adam, R. Resection of colorectal liver metastases with extrahepatic disease. Dig. Surg. 2008, 25, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef]
- Giacchetti, S.; Itzhaki, M.; Gruia, G.; Adam, R.; Zidani, R.; Kunstlinger, F.; Brienza, S.; Alafaci, E.; Bertheault-Cvitkovic, F.; Jasmin, C.; et al. Long-term survival of patients with unresectable colorectal cancer liver metastases following infusional chemotherapy with 5-fluorouracil, leucovorin, oxaliplatin and surgery. Ann. Oncol. 1999, 10, 663–669. [Google Scholar] [CrossRef]
- Schlijper, R.C.; Grutters, J.P.; Houben, R.; Dingemans, A.M.; Wildberger, J.E.; Van Raemdonck, D.; Van Cutsem, E.; Haustermans, K.; Lammering, G.; Lambin, P.; et al. What to choose as radical local treatment for lung metastases from colo-rectal cancer: Surgery or radiofrequency ablation? Cancer Treat. Rev. 2014, 40, 60–67. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Puijk, R.S.; Ahmed, M.; Adam, A.; Arai, Y.; Arellano, R.; de Baere, T.; Bale, R.; Bellera, C.; Binkert, C.A.; Brace, C.L.; et al. Consensus Guidelines for the Definition of Time-to-Event End Points in Image-guided Tumor Ablation: Results of the SIO and DATECAN Initiative. Radiology 2021, 301, 533–540. [Google Scholar] [CrossRef] [PubMed]
- NICE guideline—Colorectal Cancer 2020. Available online: https://www.nice.org.uk/guidance/ng151 (accessed on 6 February 2024).
- Richtlijnen Database—Colorectaal Carcinoom (CRC)—Lokale Therapie Peritoneale Metastasen 2022. Available online: https://richtlijnendatabase.nl/richtlijn/colorectaal_carcinoom_crc/gemetastaseerd_colorectaalcarcinoom_crc/lokale_therapie_peritoneale_metastasen.html (accessed on 6 February 2024).
- Cervantes, A.; Adam, R.; Rosello, S.; Arnold, D.; Normanno, N.; Taieb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Gorgec, B.; Hansen, I.; Kemmerich, G.; Syversveen, T.; Abu Hilal, M.; Belt, E.J.T.; Bisschops, R.H.C.; Bollen, T.L.; Bosscha, K.; Burgmans, M.C.; et al. Clinical added value of MRI to CT in patients scheduled for local therapy of colorectal liver metastases (CAMINO): Study protocol for an international multicentre prospective diagnostic accuracy study. BMC Cancer 2021, 21, 1116. [Google Scholar] [CrossRef] [PubMed]
- Gorgec, B.; Hansen, I.S.; Kemmerich, G.; Syversveen, T.; Abu Hilal, M.; Belt, E.J.T.; Bosscha, K.; Burgmans, M.C.; Cappendijk, V.C.; D’Hondt, M.; et al. MRI in addition to CT in patients scheduled for local therapy of colorectal liver metastases (CAMINO): An international, multicentre, prospective, diagnostic accuracy trial. Lancet Oncol. 2023, 25, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Hadoop, A. IBM SPSS Statistics for Windows, Version 28.0 ed.; IBM Corp.: Armonk, NY, USA, 2021. [Google Scholar]
- R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing, R for Windows Version 4.0.3 ed.; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Siriwardena, A.K.; Serrablo, A.; Fretland, A.A.; Wigmore, S.J.; Ramia-Angel, J.M.; Malik, H.Z.; Stättner, S.; Søreide, K.; Zmora, O.; Meijerink, M.; et al. Multisocietal European consensus on the terminology, diagnosis, and management of patients with synchronous colorectal cancer and liver metastases: An E-AHPBA consensus in partnership with ESSO, ESCP, ESGAR, and CIRSE. Br. J. Surg. 2023, 110, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Chua, T.C.; Saxena, A.; Liauw, W.; Chu, F.; Morris, D.L. Hepatectomy and resection of concomitant extrahepatic disease for colorectal liver metastases—A systematic review. Eur. J. Cancer 2012, 48, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Carpizo, D.R.; Are, C.; Jarnagin, W.; Dematteo, R.; Fong, Y.; Gonen, M.; Blumgart, L.; D’angelica, M. Liver resection for metastatic colorectal cancer in patients with concurrent extrahepatic disease: Results in 127 patients treated at a single center. Ann. Surg. Oncol. 2009, 16, 2138–2146. [Google Scholar] [CrossRef] [PubMed]
- Byam, J.; Reuter, N.P.; Woodall, C.E.; Scoggins, C.R.; McMasters, K.M.; Martin, R.C. Should hepatic metastatic colorectal cancer patients with extrahepatic disease undergo liver resection/ablation? Ann. Surg. Oncol. 2009, 16, 3064–3069. [Google Scholar] [CrossRef]
- Gillams, A.R.; Lees, W.R. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur. Radiol. 2009, 19, 1206–1213. [Google Scholar] [CrossRef]
- Gootjes, E.C.; van der Stok, E.P.; Buffart, T.E.; Bakkerus, L.; Labots, M.; Zonderhuis, B.M.; Tuynman, J.B.; Meijerink, M.R.; van de Ven, P.M.; Haasbeek, C.J.; et al. Safety and Feasibility of Additional Tumor Debulking to First-Line Palliative Combination Chemotherapy for Patients with Multiorgan Metastatic Colorectal Cancer. Oncologist 2020, 25, e1195–e1201. [Google Scholar] [CrossRef]
- Pfannschmidt, J.; Dienemann, H.; Hoffmann, H. Surgical resection of pulmonary metastases from colorectal cancer: A systematic review of published series. Ann. Thorac. Surg. 2007, 84, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Pfannschmidt, J.; Hoffmann, H.; Dienemann, H. Reported outcome factors for pulmonary resection in metastatic colorectal cancer. J. Thorac. Oncol. 2010, 5 (Suppl. S2), S172–S178. [Google Scholar] [CrossRef] [PubMed]
- Vidarsdottir, H.; Moller, P.H.; Jonasson, J.G.; Pfannschmidt, J.; Gudbjartsson, T. Indications and surgical outcome following pulmonary metastasectomy: A nationwide study. Thorac. Cardiovasc. Surg. 2012, 60, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Meimarakis, G.; Angele, M.; Conrad, C.; Schauer, R.; Weidenhagen, R.; Crispin, A.; Giessen, C.; Preissler, G.; Wiedemann, M.; Jauch, K.-W.; et al. Combined resection of colorectal hepatic-pulmonary metastases shows improved outcome over chemotherapy alone. Langenbecks Arch. Surg. 2013, 398, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.; Robert, J.H.; Halkic, N.; Mentha, G.; Roth, A.; Perneger, T.; Ris, H.B.; Gervaz, P. Survival after lung metastasectomy in colorectal cancer patients with previously resected liver metastases. World J. Surg. 2012, 36, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Limmer, S.; Oevermann, E.; Killaitis, C.; Kujath, P.; Hoffmann, M.; Bruch, H.P. Sequential surgical resection of hepatic and pulmonary metastases from colorectal cancer. Langenbeck’s Arch. Surg. 2010, 395, 1129–1138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shady, W.; Petre, E.N.; Gonen, M.; Erinjeri, J.P.; Brown, K.T.; Covey, A.M.; Alago, W.; Durack, J.C.; Maybody, M.; Brody, L.A.; et al. Percutaneous Radiofrequency Ablation of Colorectal Cancer Liver Metastases: Factors Affecting Outcomes—A 10-year Experience at a Single Center. Radiology 2016, 278, 601–611. [Google Scholar] [CrossRef]
- Fiorentino, F.; Hunt, I.; Teoh, K.; Treasure, T.; Utley, M. Pulmonary metastasectomy in colorectal cancer: A systematic review and quantitative synthesis. J. R. Soc. Med. 2010, 103, 60–66. [Google Scholar] [CrossRef]
- Treasure, T.; Fallowfield, L.; Lees, B.; Farewell, V. Pulmonary metastasectomy in colorectal cancer: The PulMiCC trial. Thorax 2012, 67, 185–187. [Google Scholar] [CrossRef]
- Treasure, T.; Macbeth, F. Percutaneous Image Guided Thermal Ablation (IGTA) therapies are to be included in the interventional arm of the Pulmonary Metastasectomy in Colorectal Cancer (PulMiCC) trial to test if survival and quality of life are better than with intention to treat without intervention. Eur. J. Surg. Oncol. 2016, 42, 435–436. [Google Scholar]
- Treasure, T.; Russell, C.; Macbeth, F. Re-launch of PulMiCC trial to discover the true effect of pulmonary metastasectomy on survival in advanced colorectal cancer. BMJ 2015, 351, h6045. [Google Scholar] [CrossRef] [PubMed]
- Nguyenhuy, M.; Xu, Y.; Maingard, J.; Barnett, S.; Kok, H.K.; Brooks, M.; Jhamb, A.; Asadi, H.; Knight, S. A Systematic Review and Meta-analysis of Patient Survival and Disease Recurrence following Percutaneous Ablation of Pulmonary Metastasis. Cardiovasc. Interv. Radiol. 2022, 45, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- De Baere, T.; Woodrum, D.; Tselikas, L.; Abtin, F.; Littrup, P.; Deschamps, F.; Suh, R.; Aoun, H.D.; Callstrom, M. The ECLIPSE Study: Efficacy of Cryoablation on Metastatic Lung Tumors with a 5-Year Follow-up. J. Thorac. Oncol. 2021, 16, 840–849. [Google Scholar] [CrossRef] [PubMed]
- De Baere, T.; Auperin, A.; Deschamps, F.; Chevallier, P.; Gaubert, Y.; Boige, V.; Fonck, M.; Escudier, B.; Palussiére, J. Radiofrequency ablation is a valid treatment option for lung metastases: Experience in 566 patients with 1037 metastases. Ann. Oncol. 2015, 26, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Mathai, V.K.; Aung, S.Y.; Wong, V.; Dunn, C.; Shapiro, J.; Jalali, A.; Wong, R.; Lee, M.; Tie, J.; Ananda, S.; et al. Treatment and Outcomes of Oligometastatic Colorectal Cancer Limited to Lymph Node Metastases. Clin. Color. Cancer 2021, 20, e233–e239. [Google Scholar] [CrossRef] [PubMed]
- Osterlund, P.; Salminen, T.; Soveri, L.M.; Kallio, R.; Kellokumpu, I.; Lamminmaki, A.; Halonen, P.; Ristamäki, R.; Lantto, E.; Uutela, A.; et al. Repeated centralized multidisciplinary team assessment of resectability, clinical behavior, and outcomes in 1086 Finnish metastatic colorectal cancer patients (RAXO): A nationwide prospective intervention study. Lancet Reg. Health Eur. 2021, 3, 100049. [Google Scholar] [CrossRef]
- Tree, A.C.; Khoo, V.S.; Eeles, R.A.; Ahmed, M.; Dearnaley, D.P.; Hawkins, M.A.; Huddart, R.A.; Nutting, C.M.; Ostler, P.J.; van As, N.J. Stereotactic body radiotherapy for oligometastases. Lancet Oncol. 2013, 14, e28–e37. [Google Scholar] [CrossRef]
- Polderdijk, M.C.E.; Brouwer, M.; Haverkamp, L.; Ziesemer, K.A.; Tenhagen, M.; Boerma, D.; Kok, N.F.M.; Versteeg, K.S.; Sommeijer, D.W.; Tanis, P.J.; et al. Outcomes of Combined Peritoneal and Local Treatment for Patients with Peritoneal and Limited Liver Metastases of Colorectal Origin: A Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2022, 29, 1952–1962. [Google Scholar] [CrossRef]



| Total n = 881 | CRLM Only n = 821 | CRLM and Extrahepatic n = 60 | p-Value | ||
|---|---|---|---|---|---|
| Patient-related characteristics | |||||
| Gender | Male | 66.0 | 66.9 | 54.2 | |
| Female | 34.0 | 33.1 | 45.8 | 0.063 a | |
| Age (years) | Mean (SD) | 65.6 (11.3) | 65.8 (11.3) | 64.0 (10.7) | 0.261 b |
| ASA physical status | 1 | 6.0 | 6.3 | 2.0 | |
| 2 | 70.3 | 70.3 | 70.6 | ||
| 3 | 23.3 | 23.0 | 27.5 | ||
| 4 | 0.4 | 0.4 | 0.0 | 0.549 c | |
| Comorbidities | None | 48.1 | 48.1 | 48.1 | |
| Minimal | 38.6 | 38.1 | 44.2 | ||
| Major | 13.3 | 13.7 | 7.7 | 0.407 c | |
| BMI (kg/cm2) | Mean (SD) | 26.1 (4.4) | 26.1 (4.4) | 26.0 (4.6) | 0.964 b |
| Disease-related characteristics | |||||
| Primary tumor location | Rectum | 33.8 | 24.3 | 34.5 | |
| Right-sided colon | 24.1 | 33.8 | 22.4 | ||
| Left-sided colon | 42.0 | 41.9 | 43.1 | 0.950 c | |
| Molecular profile | RASwt/mut/unknown | 9.6/7.3/83.1 | 9.7/6.6/83.7 | 8.3/16.7/75.0 | 0.050 c |
| BRAFwt/mut/unknown | 14.0/1.1/84.9 | 13.8/1.0/85.3 | 16.7/3.3/80.0 | 0.208 c | |
| MSS/MSI/unknown | 25.7/0.5/73.9 | 25.6/0.6/73.9 | 26.7/0.0/73.3 | 0.581 c | |
| Diagnosis of CRLM | Synchronous d | 46.9 | 47.2 | 42.9 | |
| Early metachronous e | 22.6 | 23.0 | 17.9 | ||
| Late metachronous f | 30.5 | 29.8 | 39.3 | 0.306 c | |
| Treatment-related characteristics | |||||
| Type of local treatment | Resection | 34.6 | 33.6 | 48.3 | |
| TA | 30.1 | 30.6 | 23.3 | ||
| Resection and TA | 26.7 | 27.3 | 18.3 | ||
| IRE | 3.6 | 3.7 | 3.3 | ||
| SABR | 5.0 | 4.9 | 6.7 | 0.163 c | |
| RC n = 20 | Left-Sided CR n = 25 | Right-Sided CR n = 13 | p-Value | ||
|---|---|---|---|---|---|
| Extrahepatic disease | Lung | 12 (60) | 8 (32) | 5 (39) | |
| Non-regional lymph node(s) | 1 (5) | 2 (8) | 2 (15) | ||
| Peritoneum | 0 | 7 (28) | 2 (15) | ||
| Multiple | 4 (20) | 2 (8) | 3 (23) | ||
| Other | 3 (15) | 6 (24) | 1 (8) | 0.161 a |
| Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Extrahepatic disease at first diagnosis of CRLM | No Yes | Reference 1.477 (1.029–2.121) | 0.035 | Reference 1.512 (1.011–2.260) | 0.044 |
| Patient-related characteristics | |||||
| Gender | Male | Reference | 0.221 | ||
| Female | 0.861 (0.677–1.095) | ||||
| Age | |||||
| ASA physical status | 1 | Reference | <0.001 | Reference | <0.001 |
| 2 | 0.920 (0.559–1.513) | 1.058 (0.601–1.864) | |||
| 3 | 1.498 (0.884–2.539) | 1.649 (0.907–3.000) | |||
| 4 | 9.958 (1.278–77.610) | 18.836 (2.372–149.565) | |||
| Comorbidities | None | Reference | 0.821 | ||
| Minimal | 1.015 (0.707–1.457) | ||||
| Major | 0.801 (0.396–1.621) | ||||
| BMI | 1.001 (0.974–1.029) | 0.916 | |||
| Disease-related characteristics | |||||
| Primary tumor location | Rectum | Reference | 0.021 | Reference | 0.068 |
| Right-sided colon | 0.793 (0.612–1.029) | 0.862 (0.642–1.157) | |||
| Left-sided colon | 1.187 (0.883–1.595) | 1.276 (0.909–1.791) | |||
| Diagnosis of CRLM | Synchronous a | Reference | <0.001 | Reference | <0.001 |
| Early metachronous b | 1.711 (1.294–2.264) | 1.752 (1.307–2.351) | |||
| Late metachronous c | 1.015 (0.765–1.349) | 0.908 (0.666–1.239) | |||
| Treatment-related characteristics | |||||
| Type of local treatment | Resection | Reference | <0.001 | Reference | 0.056 |
| TA | 1.166 (0.868–1.567) | 1.272 (0.924–1.751) | |||
| Resection and TA | 1.061 (0.789–1.427) | 1.035 (0.748–1.432) | |||
| IRE | 1.466 (0.803–2.675) | 1.560 (0.847–2.873) | |||
| SABR | 2.597 (1.758–3.837) | 2.307 (1.215–4.383) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulz, H.H.; Dijkstra, M.; van der Lei, S.; Vos, D.J.W.; Timmer, F.E.F.; Puijk, R.S.; Scheffer, H.J.; van den Tol, M.P.; Lissenberg-Witte, B.I.; Buffart, T.E.; et al. Local Treatment of Colorectal Liver Metastases in the Presence of Extrahepatic Disease: Survival Outcomes from the Amsterdam Colorectal Liver Met Registry (AmCORE). Cancers 2024, 16, 1098. https://doi.org/10.3390/cancers16061098
Schulz HH, Dijkstra M, van der Lei S, Vos DJW, Timmer FEF, Puijk RS, Scheffer HJ, van den Tol MP, Lissenberg-Witte BI, Buffart TE, et al. Local Treatment of Colorectal Liver Metastases in the Presence of Extrahepatic Disease: Survival Outcomes from the Amsterdam Colorectal Liver Met Registry (AmCORE). Cancers. 2024; 16(6):1098. https://doi.org/10.3390/cancers16061098
Chicago/Turabian StyleSchulz, Hannah H., Madelon Dijkstra, Susan van der Lei, Danielle J. W. Vos, Florentine E. F. Timmer, Robbert S. Puijk, Hester J. Scheffer, M. Petrousjka van den Tol, Birgit I. Lissenberg-Witte, Tineke E. Buffart, and et al. 2024. "Local Treatment of Colorectal Liver Metastases in the Presence of Extrahepatic Disease: Survival Outcomes from the Amsterdam Colorectal Liver Met Registry (AmCORE)" Cancers 16, no. 6: 1098. https://doi.org/10.3390/cancers16061098
APA StyleSchulz, H. H., Dijkstra, M., van der Lei, S., Vos, D. J. W., Timmer, F. E. F., Puijk, R. S., Scheffer, H. J., van den Tol, M. P., Lissenberg-Witte, B. I., Buffart, T. E., Versteeg, K. S., Swijnenburg, R.-J., & Meijerink, M. R. (2024). Local Treatment of Colorectal Liver Metastases in the Presence of Extrahepatic Disease: Survival Outcomes from the Amsterdam Colorectal Liver Met Registry (AmCORE). Cancers, 16(6), 1098. https://doi.org/10.3390/cancers16061098









