Role of 18F-FDG PET/CT in Head and Neck Squamous Cell Carcinoma: Current Evidence and Innovative Applications
Abstract
Simple Summary
Abstract
1. Introduction
2. Methodological Aspects and Pitfalls in Imaging Interpretation
| Blood Sampling | Serum Glucose Level | Prescription |
| Basal | ≤200 mg/dL | 18F-FDG injection |
| 200–300 mg/dL | Invite the patient to hydrate and walk for at least 30 min and recheck serum glucose levels | |
| >300 mg/dL | Reschedule | |
| After hydration and walking | ≤200 mg/dL | 18F-FDG injection |
| ↓ but still >200 mg/dL | Decision of rescheduling or injecting 18F-FDG made by nuclear medicine physician | |
| Further ↑ | Reschedule | |
| ↓ : decrease; ↑ : increase. | ||
3. Pre-Treatment Staging
3.1. Primary Tumor Assessment
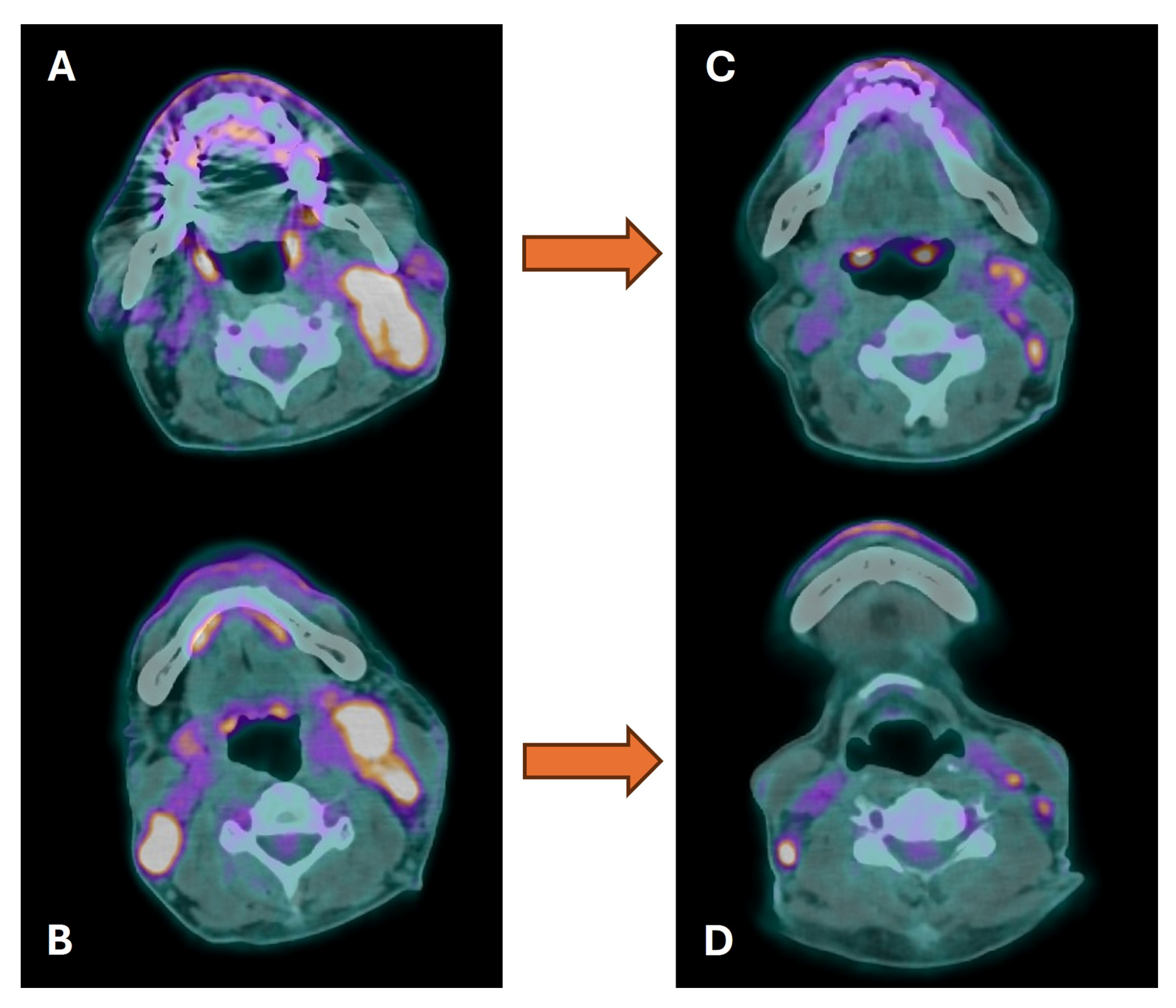
3.2. Cervical Lymph Node Assessment
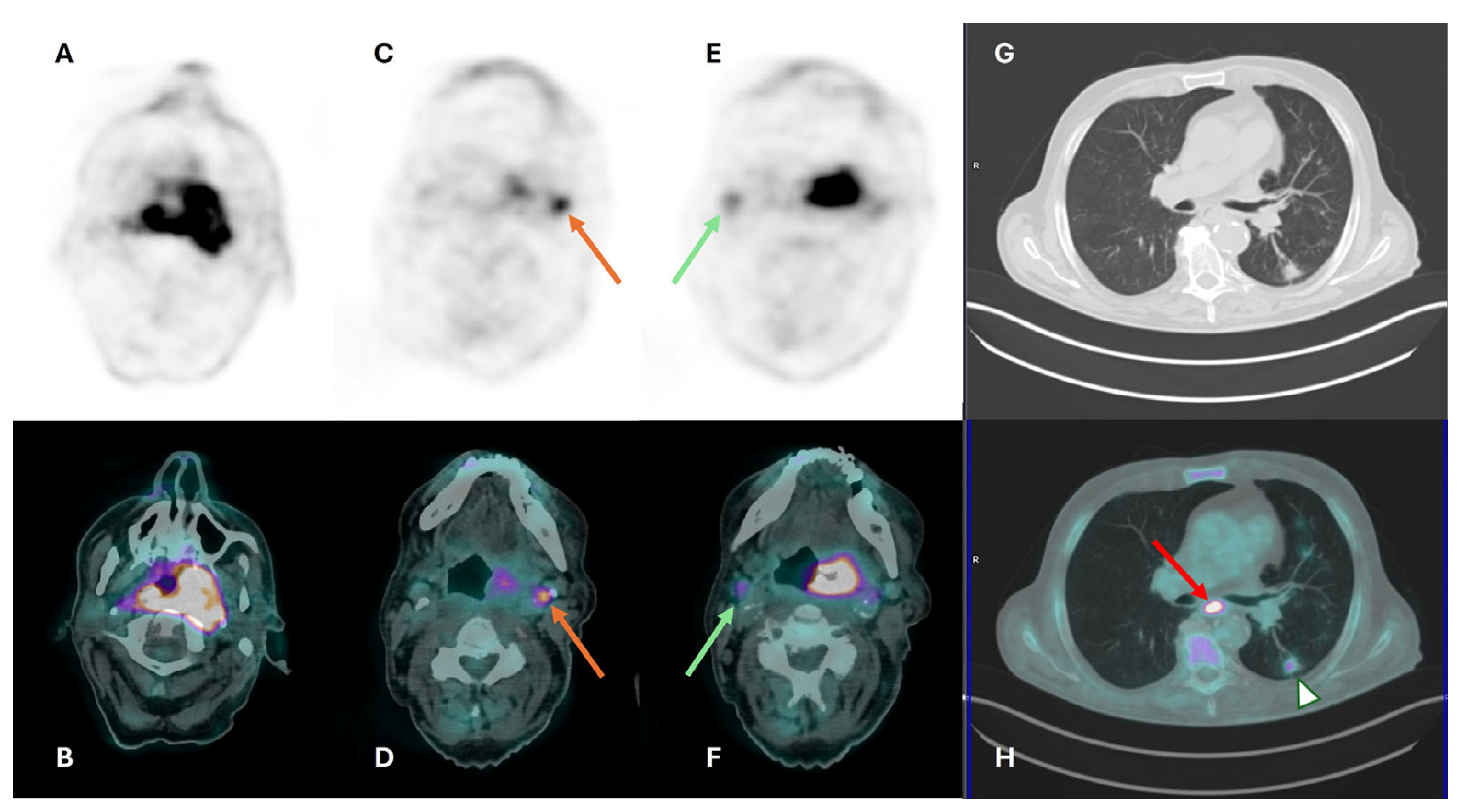
3.3. Distant Metastasis Assessment
3.4. Second Primary Tumor Assessment
3.5. Prognostic Significance of Pre-Treatment PET/CT
3.6. Proper Clinical Assessment and the Issue of HPV Involvement
- the high rate of cystic/necrotic neck metastasis with a typical low 18F-FDG uptake [68];
- the low rate of second primary tumors (mainly lung and esophagus) [75];
- the slow response with a longer persistence of increased SUV, particularly in neck nodes, even in case of complete response after chemoradiotherapy [14].
4. Radiotherapy Planning
5. Treatment-Response Assessment
| Criteria | ||
|---|---|---|
| HOPKINS | 18F-FDG uptake pattern at the primary site and nodes | Response category |
| 1 | focal uptake less than IJV | Complete metabolic response |
| 2 | focal uptake, greater than IJV but less than liver | Likely complete metabolic response |
| 3 | diffuse uptake greater than IJV or liver | Likely inflammatory changes |
| 4 | focal uptake greater than liver | Likely residual tumour |
| 5 | focal and intense uptake | Residual disease |
| NI-RADS | Primary site response | Management recommendations |
| 0 | Incomplete and baseline imaging not available | Assign score after availability of prior scan |
| 1 | No evidence of recurrence | Routine surveillance, CECT |
| 2 | Questionable recurrence:
| Direct visual inspection Short interval follow-up PET/CECT Short interval follow-up or biopsy if clinically indicated |
| 3 | High suspicion of recurrence: new discrete nodule or mass, 18F-FDG avid | Biopsy if clinically needed |
| 4 | Known recurrence, biopsy proven | Clinical management |
| Node response | ||
| 1 | No evidence of nodal disease recurrence | Routine surveillance |
| 2 | Questionable nodal recurrence or residual nodal disease:
| Surveillance Biopsy or short-interval follow-up |
| 3 | High suspicion of recurrence (new, enlarging, FDG avid) | Biopsy if clinically needed |
| 4 | Known recurrence, biopsy proven | Clinical management |
6. Long-Term Follow-Up (≥6 Months to 5 Years Post-Treatment)
7. Cost-Effectiveness Analysis
8. PET Radiopharmaceuticals Other Than 18F-FDG
9. The Role of PET/MRI
10. Application of Radiomics and Machine Learning
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Machiels, J.P.; René Leemans, C.; Golusinski, W.; Grau, C.; Licitra, L.; Gregoire, V.; on behalf of the EHNS Executive Board, ESMO Guidelines Committee and ESTRO Executive Board. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1462–1475. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Head and Neck Cancers (Version 3.2024). Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed on 25 March 2024).
- Arboleda, L.P.A.; de Carvalho, G.B.; Santos-Silva, A.R.; Fernandes, G.A.; Vartanian, J.G.; Conway, D.I.; Virani, S.; Brennan, P.; Kowalski, L.P.; Curado, M.P. Squamous Cell Carcinoma of the Oral Cavity, Oropharynx, and Larynx: A Scoping Review of Treatment Guidelines Worldwide. Cancers 2023, 15, 4405. [Google Scholar] [CrossRef] [PubMed]
- Eyassu, E.; Young, M. Nuclear Medicine PET/CT Head and Neck Cancer Assessment, Protocols, and Interpretation. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Richter, S.; Qiu, B.; Ghering, M.; Kunath, C.; Constantinescu, G.; Luths, C.; Pamporaki, C.; Bechmann, N.; Meuter, L.; Kwapiszewska, A.; et al. Head/neck paragangliomas: Focus on tumor location, mutational status and plasma methoxytyramine. Endocr. Relat. Cancer 2022, 29, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Fleischman, G.M.; Thorp, B.D.; Difurio, M.; Hackman, T.G. Accuracy of Ultrasonography-Guided Fine-Needle Aspiration in Detecting Persistent Nodal Disease After Chemoradiotherapy. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 377–382. [Google Scholar] [CrossRef]
- Kouketsu, A.; Miyashita, H.; Kojima, I.; Sakamoto, M.; Murata, T.; Mori, S.; Nogami, S.; Yamauchi, K.; Nagai, H.; Kumamoto, H.; et al. Comparison of different diagnostic imaging techniques for the detection of bone invasion in oral cancers. Oral Oncol. 2021, 120, 105453. [Google Scholar] [CrossRef]
- Tantiwongkosi, B.; Yu, F.; Kanard, A.; Miller, F.R. Role of 18F-FDG PET/CT in pre and post treatment evaluation in head and neck carcinoma. World J. Radiol. 2014, 6, 177–191. [Google Scholar] [CrossRef]
- Liao, L.J.; Lo, W.C.; Hsu, W.L.; Wang, C.T.; Lai, M.S. Detection of cervical lymph node metastasis in head and neck cancer patients with clinically N0 neck-a meta-analysis comparing different imaging modalities. BMC Cancer 2012, 12, 236. [Google Scholar] [CrossRef]
- Castaldi, P.; Leccisotti, L.; Bussu, F.; Miccichè, F.; Rufini, V. Role of 18F-FDG PET-CT in head and neck squamous cell carcinoma. Acta Otorhinolaryngol. Ital. 2013, 33, 1–8. [Google Scholar]
- Sanli, Y.; Zukotynski, K.; Mittra, E.; Chen, D.L.; Nadel, H.; Niederkohr, R.D.; Subramaniam, R.M. Update 2018: 18F-FDG PET/CT and PET/MRI in Head and Neck Cancer. Clin. Nucl. Med. 2018, 43, e439–e452. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L. PET-CT for Staging and Detection of Recurrence of Head and Neck Cancer. Semin. Nucl. Med. 2021, 51, 13–25. [Google Scholar] [CrossRef]
- Subramaniam, R.M. Quarter Century Positron Emission Tomography/Computed Tomography Transformation of Oncology: Head and Neck Cancer. PET Clin. 2024, 19, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, H.; Wong, W.L.; McConkey, C.C.; Rahman, J.K.; Robinson, M.; Hartley, A.G.; Nutting, C.; Powell, N.; Al-Booz, H.; Robinson, M.; et al. PET-NECK Trial Management Group. PET-CT Surveillance versus Neck Dissection in Advanced Head and Neck Cancer. N. Engl. J. Med. 2016, 374, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Creff, G.; Devillers, A.; Depeursinge, A.; Palard-Novello, X.; Acosta, O.; Jegoux, F.; Castelli, J. Evaluation of the Prognostic Value of FDG PET/CT Parameters for Patients with Surgically Treated Head and Neck Cancer: A Systematic Review. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Maghami, E.; Ismaila, N.; Alvarez, A.; Chernock, R.; Duvvuri, U.; Geiger, J.; Gross, N.; Haughey, B.; Paul, D.; Rodriguez, C.; et al. Diagnosis and Management of Squamous Cell Carcinoma of Unknown Primary in the Head and Neck: ASCO Guideline. J. Clin. Oncol. 2020, 38, 2570–2596. [Google Scholar] [CrossRef] [PubMed]
- Huasong, H.; Shurui, S.; Shi, G.; Bin, J. Performance of 18F-FDG-PET/CT as a next step in the search of occult primary tumors for patients with head and neck squamous cell carcinoma of unknown primary: A systematic review and meta-analysis. Clin. Transl. Imaging 2021, 9, 361–371. [Google Scholar] [CrossRef]
- Helsen, N.; Van den Wyngaert, T.; Carp, L.; Stroobants, S. FDG-PET/CT for treatment response assessment in head and neck squamous cell carcinoma: A systematic review and meta-analysis of diagnostic performance. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Marcus, C.; Subramaniam, R.M. Role of Non-FDG-PET/CT in Head and Neck Cancer. Semin. Nucl. Med. 2021, 51, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Welz, S.; Paulsen, F.; Pfannenberg, C.; Reimold, M.; Reischl, G.; Nikolaou, K.; La Fougère, C.; Alber, M.; Belka, C.; Zips, D.; et al. Dose escalation to hypoxic subvolumes in head and neck cancer: A randomized phase II study using dynamic [18F]FMISO PET/CT. Radiother. Oncol. 2022, 171, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Promteangtrong, C.; Siripongsatian, D.; Jantarato, A.; Kunawudhi, A.; Kiatkittikul, P.; Yaset, S.; Boonkawin, N.; Chotipanich, C. Head-to-Head Comparison of 68Ga-FAPI-46 and 18F-FDG PET/CT for Evaluation of Head and Neck Squamous Cell Carcinoma: A Single-Center Exploratory Study. J. Nucl. Med. 2022, 63, 1155–1161. [Google Scholar] [CrossRef]
- Lindholm, P.; Leskinen-Kallio, S.; Grénman, R.; Lehikoinen, P.; Någren, K.; Teräs, M.; Ruotsalainen, U.; Joensuu, H. Evaluation of response to radiotherapy in head and neck cancer by positron emission tomography and [11C]methionine. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 787–794. [Google Scholar] [CrossRef]
- Wedman, J.; Pruim, J.; van der Putten, L.; Hoekstra, O.S.; de Bree, R.; van Dijk, B.A.C.; van der Laan, B.F.A.M. Is C-11 Methionine PET an alternative to 18-F FDG-PET for identifying recurrent laryngeal cancer after radiotherapy? Clin. Otolaryngol. 2019, 44, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Baxa, J.; Ferda, J.; Ferdova, E.; Vojtisek, R.; Topolcan, O.; Finek, J. Hybrid Imaging PET/CT with Application of 18F-Fluorothymidine in Patients with Head and Neck Carcinoma Undergoing Radiotherapy. Anticancer Res. 2018, 38, 4153–4157. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Chen, C.C.; Taieb, D.; Patronas, N.J.; Millo, C.M.; Adams, K.T.; Nambuba, J.; Herscovitch, P.; Sadowski, S.M.; Fojo, A.T.; et al. 68Ga-DOTATATE PET/CT in the localization of head and neck paragangliomas compared with other functional imaging modalities and CT/MRI. J. Nucl. Med. 2016, 57, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Surti, S.; Viswanath, V.; Daube-Witherspoom, M.E.; Conti, M.; Casey, M.E.; Karp, J.S. Benefit of improved performance with state-of-the art digital PET/CT for lesion detection in oncology. J. Nucl. Med. 2020, 61, 1684–1690. [Google Scholar] [CrossRef]
- Surti, S.; Karp, J.S. Update on latest advances in time-of-flight PET. Phys. Med. 2020, 80, 251–258. [Google Scholar] [CrossRef]
- Van den Wyngaert, T.; De Schepper, S.; Carp, L. Quality Assessment in FDG-PET/CT Imaging of Head-and-Neck Cancer: One Home Run Is Better Than Two Doubles. Front. Oncol. 2020, 10, 1458. [Google Scholar] [CrossRef]
- López-Mora, D.A.; Carrió, I.; Flotats, A. Digital PET vs Analog PET: Clinical Implications? Semin. Nucl. Med. 2022, 52, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Cherry, S.R.; Jones, T.; Karp, J.S.; Qi, J.; Moses, W.W.; Badawi, R.D. Total-body PET: Maximizing sensitivity to create new opportunities for clinical research and patient care. J. Nucl. Med. 2018, 59, 3–12. [Google Scholar] [CrossRef]
- Badawi, R.D.; Shi, H.; Hu, P.; Chen, S.; Xu, T.; Price, P.M.; Ding, Y.; Spencer, B.A.; Nardo, L.; Liu, W.; et al. First human imaging studies with the EXPLORER total-body PET scanner. J. Nucl. Med. 2019, 60, 299–303. [Google Scholar] [CrossRef]
- Slart, R.H.J.A.; Tsoumpas, C.; Glaudemans, A.W.J.M.; Noordzij, W.; Willemsen, A.T.M.; Borra, R.J.H.; Dierckx, R.A.J.O.; Lammertsma, A.A. Long axial field of view PET scanners: A road map to implementation and new possibilities. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4236–4245. [Google Scholar] [CrossRef]
- Roya, M.; Mostafapour, S.; Mohr, P.; Providência, L.; Li, Z.; van Snick, J.H.; Brouwers, A.H.; Noordzij, W.; Willemsen, A.T.M.; Dierckx, R.A.J.O.; et al. Current and Future Use of Long Axial Field-of-View Positron Emission Tomography/Computed Tomography Scanners in Clinical Oncology. Cancers 2023, 15, 5173. [Google Scholar] [CrossRef]
- Mei, R.; Pyka, T.; Sari, H.; Fanti, S.; Afshar-Oromieh, A.; Giger, R.; Caobelli, F.; Rominger, A.; Alberts, I. The clinical acceptability of short versus long duration acquisitions for head and neck cancer using long-axial field-of-view PET/CT: A retrospective evaluation. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Fischbein, N.J.; Baugnon, K.L.; Policeni, B.A.; Raghavan, P. Contemporary Imaging and Reporting Strategies for Head and Neck Cancer: MRI, FDG PET/MRI, NI-RADS, and Carcinoma of Unknown Primary-AJR Expert Panel Narrative Review. AJR Am. J. Roentgenol. 2023, 220, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Philip, M.M.; Welch, A.; McKiddie, F.; Nath, M. A systematic review and meta-analysis of predictive and prognostic models for outcome prediction using positron emission tomography radiomics in head and neck squamous cell carcinoma patients. Cancer Med. 2023, 12, 16181–16194. [Google Scholar] [CrossRef] [PubMed]
- Illimoottil, M.; Ginat, D. Recent Advances in Deep Learning and Medical Imaging for Head and Neck Cancer Treatment: MRI, CT, and PET Scans. Cancers 2023, 15, 3267. [Google Scholar] [CrossRef] [PubMed]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. European Association of Nuclear Medicine (EANM). FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Suzuki, A.; Nagashima, T.; Lee, J.; Horiuchi, C.; Tsukuda, M.; Inoue, T. Staging primary head and neck cancers with 18F-FDG PET/CT: Is intravenous contrast administration really necessary? Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1417–1424. [Google Scholar] [CrossRef]
- Morbelli, S.; Conzi, R.; Campus, C.; Cittadini, G.; Bossert, I.; Massollo, M.; Fornarini, G.; Calamia, I.; Marini, C.; Fiz, F.; et al. Contrast-enhanced [18 F] fluorodeoxyglucose-positron emission tomography/computed tomography in clinical oncology: Tumor-, site-, and question-based comparison with standard positron emission tomography/computed tomography. Cancer Imaging 2014, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Beyer, T.; Antoch, G.; Müller, S.; Egelhof, T.; Freudenberg, L.S.; Debatin, J.; Bockisch, A. Acquisition protocol considerations for combined PET/CT imaging. J. Nucl. Med. 2004, 45 (Suppl. 1), 25S–35S. [Google Scholar]
- Yamamoto, Y.; Wong, T.Z.; Turkington, T.G.; Hawk, T.C.; Coleman, R.E. Head and neck cancer: Dedicated FDG PET/CT protocol for detection-phantom and initial clinical studies. Radiology 2007, 244, 263–272. [Google Scholar] [CrossRef]
- Guzmán Pérez-Carrillo, G.J.; Ivanidze, J. PET/CT and PET/MR Imaging of the Post-treatment Head and Neck: Traps and Tips. Neuroimaging Clin. N. Am. 2022, 32, 111–132. [Google Scholar] [CrossRef] [PubMed]
- Mihailovic, J.; Killeen, R.P.; Duignan, J.A. PET/CT Variants and Pitfalls in Head and Neck Cancers Including Thyroid Cancer. Semin. Nucl. Med. 2021, 51, 419–440. [Google Scholar] [CrossRef] [PubMed]
- Rassekh, C.H.; Cost, J.L.; Hogg, J.P.; Hurst, M.K.; Marano, G.D.; Ducatman, B.S. Positron emission tomography in Warthin’s tumor mimicking malignancy impacts the evaluation of head and neck patients. Am. J. Otolaryngol. 2015, 36, 259–263. [Google Scholar] [CrossRef]
- Lee, H.; Lazor, J.W.; Assadsangabi, R.; Shah, J. An Imager’s Guide to Perineural Tumor Spread in Head and Neck Cancers: Radiologic Footprints on 18F-FDG PET, with CT and MRI Correlates. J. Nucl. Med. 2019, 60, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.; Moore, W.; Sumer, B.; Khan, S.; Sher, D.; Subramaniam, R.M. Clinical Practice in PET/CT for the Management of Head and Neck Squamous Cell Cancer. AJR Am. J. Roentgenol. 2017, 209, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, Y.; Kitajima, K.; Ishihara, T.; Sasaki, R.; Otsuki, N.; Nibu, K.; Minamikawa, T.; Kiyota, N.; Sugimura, K. FDG-PET/contrast-enhanced CT as a post-treatment tool in head and neck squamous cell carcinoma: Comparison with FDG-PET/non-contrast-enhanced CT and contrast-enhanced CT. Eur. Radiol. 2016, 26, 1018–1030. [Google Scholar] [CrossRef]
- Barai, S.; Ora, M.; Gambhir, S.; Singh, A. Does Intravenous Contrast Improve the Diagnostic Yield of Fluorodeoxyglucose Positron-emission Tomography/Computed Tomography in Patients with Head-and-neck Malignancy. Indian J. Nucl. Med. 2020, 35, 13–16. [Google Scholar] [CrossRef]
- Johansen, J.; Buus, S.; Loft, A.; Keiding, S.; Overgaard, M.; Hansen, H.S.; Grau, C.; Bundgaard, T.; Kirkegaard, J.; Overgaard, J. Prospective study of 18FDG-PET in the detection and management of patients with lymph node metastases to the neck from an unknown primary tumor. Results from the DAHANCA-13 study. Head Neck 2008, 30, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Xue, J.; Hu, M.; Zheng, J.; Wang, X. Clinical value of 18F-FDG PET-CT in detecting primary tumor for patients with carcinoma of unknown primary. Cancer Epidemiol. 2012, 3, 470–475. [Google Scholar] [CrossRef]
- Barbosa, M.; Duarte, H.; Breda, E.; Monteiro, E. PET/CT in the management of metastatic cervical lymphadenopathy from unknown primary site: A seven years retrospective study. Rev. Laryngol. Otol. Rhinol. 2013, 134, 89–94. [Google Scholar]
- Deonarine, P.; Han, S.; Poon, F.W.; de Wet, C. The role of 18F-fluoro-2-deoxyglucose positron emission tomography/computed tomography in the management of patients with carcinoma of unknown primary. Scott. Med. J. 2013, 5, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; Mahajan, A.; Vaish, R.; Rane, S.; Shukla, S.; D’Cruz, A.K. Imaging of Neck Nodes in Head and Neck Cancers—A Comprehensive Update. Clin. Oncol. 2023, 35, 429–445. [Google Scholar] [CrossRef]
- Gourin, C.G.; Conger, B.T.; Porubsky, E.S.; Sheils, W.C.; Bilodeau, P.A.; Coleman, T.A. The effect of occult nodal metastases on survival and regional control in patients with head and neck squamous cell carcinoma. Laryngoscope 2008, 118, e1191–e1194. [Google Scholar] [CrossRef]
- Strohl, M.P.; Ha, P.K.; Flavell, R.R.; Yom, S.S. PET/CT in Surgical Planning for Head and Neck Cancer. Semin. Nucl. Med. 2021, 51, 50–58. [Google Scholar] [CrossRef]
- Murer, K.; Huber, G.F.; Haile, S.R.; Stoeckli, S.J. Comparison of morbidity between sentinel node biopsy and elective neck dissection for treatment of the N0 neck in patients with oral squamous cell carcinoma. Head Neck 2011, 33, 1260–1264. [Google Scholar] [CrossRef]
- Vaish, R.; Mittal, N.; Mahajan, A.; Rane, S.U.; Agrawal, A.; D’Cruz, A.K. Sentinel node biopsy in node negative early oral cancers: Solution to the conundrum! Oral Oncol. 2022, 134, 106070. [Google Scholar] [CrossRef] [PubMed]
- Stoeckli, S.J.; Pfaltz, M.; Ross, G.L.; Steinert, H.C.; MacDonald, D.G.; Wittekind, C.; Soutar, D.S. The second international conference on sentinel node biopsy in mucosal head and neck cancer. Ann. Surg. Oncol. 2005, 12, 919–924. [Google Scholar] [CrossRef]
- Mallo Magariños, M.; Suárez Ajuria, M.; Marichalar Mendía, X.; Álvarez-Calderón Iglesias, Ó.; Chamorro Petronacci, C.M.; García García, A.; Pérez Sayáns, M. Diagnostic yield of sentinel lymph node biopsy in oral squamous cell carcinoma T1/T2-N0: Systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2021, 50, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Garrel, R.; Poissonnet, G.; Moyà Plana, A.; Fakhry, N.; Dolivet, G.; Lallemant, B.; Sarini, J.; Vergez, S.; Guelfucci, B.; Choussy, O.; et al. Equivalence Randomized Trial to Compare Treatment on the Basis of Sentinel Node Biopsy Versus Neck Node Dissection in Operable T1-T2N0. Oral and Oropharyngeal Cancer. J. Clin. Oncol. 2020, 38, 4010–4018. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Tsukahara, K.; Yoshimoto, S.; Miura, K.; Yokoyama, J.; Hirano, S.; Uemura, H.; Sugasawa, M.; Yoshizaki, T.; Homma, A.; et al. HNCMM Research Group. Neck Dissections Based on Sentinel Lymph Node Navigation Versus Elective Neck Dissections in Early Oral Cancers: A Randomized, Multicenter, and Noninferiority Trial. J. Clin. Oncol. 2021, 39, 2025–2036. [Google Scholar] [CrossRef]
- Kyzas, P.A.; Evangelou, E.; Denaxa-Kyza, D.; Ioannidis, J.P. 18F-Fluorodeoxyglucose positron emission tomography to evaluate cervical node metastases in patients with head and neck squamous cell carcinoma: A meta-analysis. J. Natl. Cancer Inst. 2008, 100, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Park, J.T.; Roh, J.L.; Kim, J.S.; Lee, J.H.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. 18F FDG PET/CT versus CT/MR Imaging and the Prognostic Value of Contralateral Neck Metastases in Patients with Head and Neck Squamous Cell Carcinoma. Radiology 2016, 279, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Tauzin, M.; Rabalais, A.; Hagan, J.L.; Wood, C.G.; Ferris, R.L.; Walvekar, R.R. PET-CT staging of the neck in cancers of the oropharynx: Patterns of regional and retropharyngeal nodal metastasis. Laryngoscope 2010, 120 (Suppl. 4), S186. [Google Scholar] [CrossRef] [PubMed]
- Bussu, F.; Tagliaferri, L.; Crescio, C.; Rizzo, D.; Gallus, R.; Parrilla, C.; Fionda, B.; Lancellotta, V.; Mattiucci, G.C.; Galli, J. New standards for the management of nose vestibule malignancies. Acta Otolaryngol. 2023, 143, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Bussu, F.; Tagliaferri, L.; Corbisiero, M.F.; Lotto, C.; Pellini, R.; Guarino, P.; Mercante, G.; Galuppi, A.; Cariti, F.; Almadori, G.; et al. Management of nasal vestibule carcinomas: Recommendations by the Oncological Committee of the Italian Society of Otorhinolaryngology—Head and Neck Surgery. Acta Otorhinolaryngol. Ital. 2024, 44, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Haerle, S.K.; Strobel, K.; Ahmad, N.; Soltermann, A.; Schmid, D.T.; Stoeckli, S.J. Contrast-enhanced 18F-FDG-PET/CT for the assessment of necrotic lymph node metastases. Head Neck 2011, 33, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.L.; Park, J.P.; Kim, J.S.; Lee, J.H.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. 18F fluorodeoxyglucose PET/CT in head and neck squamous cell carcinoma with negative neck palpation findings: A prospective study. Radiology 2014, 271, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Lowe, V.J.; Duan, F.; Subramaniam, R.M.; Sicks, J.D.; Romanoff, J.; Bartel, T.; Yu, J.Q.M.; Nussenbaum, B.; Richmon, J.; Arnold, C.D.; et al. Multicenter Trial of [18F]fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Staging of Head and Neck Cancer and Negative Predictive Value and Surgical Impact in the N0 Neck: Results From ACRIN 6685. J. Clin. Oncol. 2019, 37, 1704–1712. [Google Scholar] [CrossRef] [PubMed]
- Stack, B.C., Jr.; Duan, F.; Subramaniam, R.M.; Romanoff, J.; Sicks, J.D.; Bartel, T.; Chen, C.; Lowe, V.J. FDG-PET/CT and Pathology in Newly Diagnosed Head and Neck Cancer: ACRIN 6685 Trial, FDG-PET/CT cN0. Otolaryngol. Head Neck Surg. 2021, 164, 1230–1239. [Google Scholar] [CrossRef]
- Price, P.M.; Badawi, R.D.; Cherry, S.R.; Jones, T. Ultra staging to unmask the prescribing of adjuvant therapy in cancer patients: The future opportunity to image micrometastases using total-body 18F-FDG PET scanning. J. Nucl. Med. 2014, 55, 696–697. [Google Scholar] [CrossRef]
- Barrett, T.F.; Gill, C.M.; Miles, B.A.; Iloreta, A.M.C.; Bakst, R.L.; Fowkes, M.; Brastianos, P.K.; Bederson, J.B.; Shrivastava, R.K. Brain metastasis from squamous cell carcinoma of the head and neck: A review of the literature in the genomic era. Neurosurg. Focus 2018, 44, E11. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zeng, M.; Ju, X.; Lin, A.; Zhou, C.; Shen, J.; Liu, Z.; Tang, B.; Cheng, Q.; Wang, Y.; et al. Correlation between second and first primary cancer: Systematic review and meta-analysis of 9 million cancer patients. Br. J. Surg. 2024, 111, znad377. [Google Scholar] [CrossRef]
- Peck, B.W.; Dahlstrom, K.R.; Gan, S.J.; Caywood, W.; Li, G.; Wei, Q.; Zafereo, M.E.; Sturgis, E.M. Low risk of second primary malignancies among never smokers with human papillomavirus-associated index oropharyngeal cancers. Head Neck 2013, 35, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Ishimori, T.; Patel, P.V.; Wahl, R.L. Detection of unexpected additional primary malignancies with PET/CT. J. Nucl. Med. 2005, 46, 752–757. [Google Scholar] [PubMed]
- Haerle, S.K.; Strobel, K.; Hany, T.F.; Sidler, D.; Stoeckli, S.J. 18F-FDG-PET/CT versus panendoscopy for the detection of synchronous second primary tumors in patients with head and neck squamous cell carcinoma. Head Neck 2010, 32, 319–325. [Google Scholar] [CrossRef]
- Balgobind, S.; Cheung, V.K.Y.; Luk, P.; Low, T.H.; Wykes, J.; Wu, R.; Lee, J.; Ch’ng, S.; Palme, C.E.; Clark, J.R.; et al. Prognostic and predictive biomarkers in head and neck cancer: Something old, something new, something borrowed, something blue and a sixpence in your shoe. Pathology 2024, 56, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Kwee, T.C.; Basu, S.; Saboury, B.; Ambrosini, V.; Torigian, D.A.; Alavi, A. A new dimension of FDG-PET interpretation: Assessment of tumor biology. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1158–1170. [Google Scholar] [CrossRef] [PubMed]
- Bussu, F.; Ragin, C.; Boscolo-Rizzo, P.; Rizzo, D.; Gallus, R.; Delogu, G.; Morbini, P.; Tommasino, M. HPV as a marker for molecular characterization in head and neck oncology: Looking for a standardization of clinical use and of detection method(s) in clinical practice. Head Neck 2019, 41, 1104–1111. [Google Scholar] [CrossRef]
- Bussu, F.; Sali, M.; Gallus, R.; Petrone, G.; Zannoni, G.F.; Autorino, R.; Dinapoli, N.; Santangelo, R.; Vellone, V.G.; Graziani, C.; et al. Human papillomavirus (HPV) infection in squamous cell carcinomas arising from the oropharynx: Detection of HPV DNA and p16 immunohistochemistry as diagnostic and prognostic indicators—A pilot study. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Bussu, F.; Muresu, N.; Crescio, C.; Gallus, R.; Rizzo, D.; Cossu, A.; Sechi, I.; Fedeli, M.; Cossu, A.; Delogu, G.; et al. Low Prevalence of HPV Related Oropharyngeal Carcinogenesis in Northern Sardinia. Cancers 2022, 14, 4205. [Google Scholar] [CrossRef]
- Gallus, R.; Nauta, I.H.; Marklund, L.; Rizzo, D.; Crescio, C.; Mureddu, L.; Tropiano, P.; Delogu, G.; Bussu, F. Accuracy of p16 IHC in Classifying HPV-Driven OPSCC in Different Populations. Cancers 2023, 15, 656. [Google Scholar] [CrossRef]
- Thureau, S.; Briens, A.; Decazes, P.; Castelli, J.; Barateau, A.; Garcia, R.; Thariat, J.; de Crevoisier, R. PET and MRI guided adaptive radiotherapy: Rational, feasibility and benefit. Cancer Radiother. 2020, 24, 635–644. [Google Scholar] [CrossRef]
- van den Bosch, S.; Vogel, W.V.; Raaijmakers, C.P.; Dijkema, T.; Terhaard, C.H.J.; Al-Mamgani, A.; Kaanders, J.H.A.M. Implications of improved diagnostic imaging of small nodal metastases in head and neck cancer: Radiotherapy target volume transformation and dose de-escalation. Radiother. Oncol. 2018, 128, 472–478. [Google Scholar] [CrossRef]
- van den Bosch, S.; Dijkema, T.; Kunze-Busch, M.C.; Terhaard, C.H.; Raaijmakers, C.P.; Doornaert, P.A.; Hoebers, F.J.; Vergeer, M.R.; Kreike, B.; Wijers, O.B.; et al. Uniform FDG-PET guided GRAdient Dose prEscription to reduce late Radiation Toxicity (UPGRADE-RT): Study protocol for a randomized clinical trial with dose reduction to the elective neck in head and neck squamous cell carcinoma. BMC Cancer 2017, 17, 208. [Google Scholar] [CrossRef]
- Manca, G.; Vanzi, E.; Rubello, D.; Giammarile, F.; Grassetto, G.; Wong, K.K.; Perkins, A.C.; Colletti, P.M.; Volterrani, D. 18F-FDG PET/CT quantification in head and neck squamous cell cancer: Principles, technical issues and clinical applications. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1360–1375. [Google Scholar] [CrossRef]
- Daisne, J.F.; Duprez, T.; Weynand, B.; Lonneux, M.; Hamoir, M.; Reychler, H.; Grégoire, V. Tumour volume in pharyngolaryngeal squamous cell carcinoma: Comparison at CT, MR imaging, and FDG PET and validation with surgical specimen. Radiology 2004, 233, 93–100. [Google Scholar] [CrossRef]
- Chatterjee, S.; Frew, J.; Mott, J.; McCallum, H.; Stevenson, P.; Maxwell, R.; Wilsdon, J.; Kelly, C. Variation in Radiotherapy Target Volume Definition, Dose to Organs at Risk and Clinical Target Volumes using Anatomic (Computed Tomography) versus Combined Anatomic and Molecular Imaging (Positron Emission Tomography/Computed Tomography): Intensity-modulated Radiotherapy Delivered using a Tomotherapy Hi Art Machine: Final Results of the VortigERN Study. Clin. Oncol. 2012, 24, e173–e179. [Google Scholar]
- Geets, X.; Tomsej, M.; Lee, J.A.; Duprez, T.; Coche, E.; Cosnard, G.; Lonneux, M.; Grégoire, V. Adaptive biological image-guided IMRT with anatomic and functional imaging in pharyngo-laryngeal tumors: Impact on target volume delineation and dose distribution using helical tomotherapy. Radiother. Oncol. 2007, 85, 105–115. [Google Scholar] [CrossRef]
- Guido, A.; Fuccio, L.; Rombi, B.; Castellucci, P.; Cecconi, A.; Bunkheila, F.; Fuccio, C.; Spezi, E.; Angelini, A.L.; Barbieri, E. Combined 18F-FDG-PET/CT Imaging in Radiotherapy Target Delineation for Head-and-Neck Cancer. Int. J. Radiat. Oncol. 2009, 73, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Lapa, C.; Nestle, U.; Albert, N.L.; Baues, C.; Beer, A.; Buck, A.; Budach, V.; Bütof, R.; Combs, S.E.; Derlin, T.; et al. Arbeitsgemeinschaft Nuklearmedizin und Strahlentherapie der DEGRO und DGN. Value of PET imaging for radiation therapy. Strahlenther. Onkol. 2021, 197, 1–23, Erratum in: Strahlenther. Onkol. 2022, 198, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.P.; le Maître, A.; Maillard, E.; Bourhis, J.; MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother. Oncol. 2009, 92, 4–14. [Google Scholar] [CrossRef]
- Studer, G.; Luetolf, U.M.; Glanzmann, C. Locoregional failure analysis in head-and-neck cancer patients treated with IMRT. Strahlenther. Onkol. 2007, 183, 417–423, discussion 424–425. [Google Scholar] [CrossRef]
- Horiot, J.C.; Bontemps, P.; van den Bogaert, W.; Le Fur, R.; van den Weijngaert, D.; Bolla, M.; Bernier, J.; Lusinchi, A.; Stuschke, M.; Lopez-Torrecilla, J.; et al. Accelerated fractionation (AF) compared to conventional fractionation (CF) improves loco-regional control in the radiotherapy of advanced head and neck cancers: Results of the EORTC 22851 randomized trial. Radiother. Oncol. 1997, 44, 111–121. [Google Scholar] [CrossRef]
- Servagi-Vernat, S.; Differding, S.; Sterpin, E.; Hanin, F.X.; Labar, D.; Bol, A.; Lee, J.A.; Grégoire, V. Hypoxia-guided adaptive radiation dose escalation in head and neck carcinoma: A planning study. Acta Oncol. 2015, 54, 1008–1016. [Google Scholar] [CrossRef]
- Saksø, M.; Mortensen, L.S.; Primdahl, H.; Johansen, J.; Kallehauge, J.; Hansen, C.R.; Overgaard, J. Influence of FAZA PET hypoxia and HPV-status for the outcome of head and neck squamous cell carcinoma (HNSCC) treated with radiotherapy: Long-term results from the DAHANCA 24 trial (NCT01017224). Radiother. Oncol. 2020, 151, 126–133. [Google Scholar]
- Bouleftour, W.; Rowinski, E.; Louati, S.; Sotton, S.; Wozny, A.S.; Moreno-Acosta, P.; Mery, B.; Rodriguez-Lafrasse, C.; Magne, N.A. Review of the Role of Hypoxia in Radioresistance in Cancer Therapy. Med. Sci. Monit. 2021, 27, e934116. [Google Scholar] [CrossRef]
- Michaelidou, A.; Adjogatse, D.; Suh, Y.; Pike, L.; Thomas, C.; Woodley, O.; Rackely, T.; Palaniappan, N.; Jayaprakasam, V.; Sanchez-Nieto, B.; et al. 18F-FDG-PET in guided dose-painting with intensity modulated radiotherapy in oropharyngeal tumours: A phase I study (FiGaRO). Radiother. Oncol. 2021, 155, 261–268. [Google Scholar] [CrossRef]
- de Leeuw, A.L.M.P.; Giralt, J.; Tao, Y.; Benavente, S.; France Nguyen, T.V.; Hoebers, F.J.P.; Hoeben, A.; Terhaard, C.H.J.; Wai Lee, L.; Friesland, S.; et al. A multicentric randomized controlled phase III trial of adaptive and 18F-FDG-PET-guided dose-redistribution in locally advanced head and neck squamous cell carcinoma (ARTFORCE). Radiother. Oncol. 2024, 196, 110281. [Google Scholar] [CrossRef]
- Dolezel, M.; Slavik, M.; Blazek, T.; Kazda, T.; Koranda, P.; Veverkova, L.; Burkon, P.; Cvek, J. FMISO-Based Adaptive Radiotherapy in Head and Neck Cancer. J. Pers. Med. 2022, 12, 1245. [Google Scholar] [CrossRef]
- Flaus, A.; Nevesny, S.; Guy, J.B.; Sotton, S.; Magné, N.; Prévot, N. Positron emission tomography for radiotherapy planning in head and neck cancer: What impact? Nucl. Med. Commun. 2021, 42, 234–243. [Google Scholar] [CrossRef]
- Trada, Y.; Keall, P.; Jameson, M.; Moses, D.; Lin, P.; Chlap, P.; Holloway, L.; Min, M.; Forstner, D.; Fowler, A.; et al. Changes in serial multiparametric MRI and FDG-PET/CT functional imaging during radiation therapy can predict treatment response in patients with head and neck cancer. Eur. Radiol. 2023, 33, 8788–8799. [Google Scholar] [CrossRef]
- De Bruycker, A.; De Neve, W.; Daisne, J.F.; Vercauteren, T.; De Gersem, W.; Olteanu, L.; Berwouts, D.; Deheneffe, S.; Madani, I.; Goethals, I.; et al. Disease control and late toxicity in Adaptive Dose Painting by Numbers vs. non-adaptive radiotherapy for head and neck cancer: A randomized controlled phase II trial. Int. J. Radiat. Oncol. Biol. Phys. 2024. [Google Scholar] [CrossRef]
- Heineman, T.E.; Kuan, E.C.; St John, M.A. When should surveillance imaging be performed after treatment for head and neck cancer? Laryngoscope 2017, 127, 533–534. [Google Scholar] [CrossRef]
- Helsen, N.; Van den Wyngaert, T.; Carp, L.; De Bree, R.; VanderVeken, O.M.; De Geeter, F.; Maes, A.; Cambier, J.P.; Spaepen, K.; Martens, M.; et al. Quantification of 18F-fluorodeoxyglucose uptake to detect residual nodal disease in locally advanced head and neck squamous cell carcinoma after chemoradiotherapy: Results from the ECLYPS study. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1075–1082. [Google Scholar] [CrossRef]
- Cheung, P.K.; Chin, R.Y.; Eslick, G.D. Detecting Residual/Recurrent Head Neck Squamous Cell Carcinomas Using PET or PET/CT: Systematic Review and Meta-analysis. Otolaryngol. Head Neck Surg. 2016, 154, 421–432. [Google Scholar] [CrossRef]
- Bar-Ad, V.; Mishra, M.; Ohri, N.; Intenzo, C. Positron emission tomography for neck evaluation following definitive treatment with chemoradiotherapy for locoregionally advanced head and neck squamous cell carcinoma. Rev. Recent Clin. Trials 2012, 7, 36–41. [Google Scholar] [CrossRef]
- Castaldi, P.; Rufini, V.; Bussu, F.; Miccichè, F.; Dinapoli, N.; Autorino, R.; Lago, M.; De Corso, E.; Almadori, G.; Galli, J.; et al. Can “early” and “late” 18F-FDG PET-CT be used as prognostic factors for the clinical outcome of patients with locally advanced head and neck cancer treated with radio-chemotherapy? Radiother. Oncol. 2012, 103, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Mogilner, J.; Siplovich, L.; Bar-Ziv, J.; Mares, A.J. Surgical management of the cleft sternum. J. Pediatr. Surg. 1988, 23, 889–891. [Google Scholar] [CrossRef] [PubMed]
- Marcus, C.; Ciarallo, A.; Tahari, A.K.; Mena, E.; Koch, W.; Wahl, R.L.; Kiess, A.P.; Kang, H.; Subramaniam, R.M. Head and neck PET/CT: Therapy response interpretation criteria (Hopkins Criteria)-interreader reliability, accuracy, and survival outcomes. J. Nucl. Med. 2014, 55, 1411–1416. [Google Scholar] [CrossRef]
- Aiken, A.H.; Farley, A.; Baugnon, K.L.; Corey, A.; El-Deiry, M.; Duszak, R.; Beitler, J.; Hudgins, P.A. Implementation of a Novel Surveillance Template for Head and Neck Cancer: Neck Imaging Reporting and Data System (NI-RADS). J. Am. Coll. Radiol. 2016, 13, 743–746. [Google Scholar] [CrossRef]
- Zhong, J.; Sundersingh, M.; Dyker, K.; Currie, S.; Vaidyanathan, S.; Prestwich, R.; Scarsbrook, A. Post-treatment FDG PET-CT in head and neck carcinoma: Comparative analysis of 4 qualitative interpretative criteria in a large patient cohort. Sci. Rep. 2020, 10, 4086. [Google Scholar] [CrossRef]
- Garibaldi, C.; Ronchi, S.; Cremonesi, M.; Gilardi, L.; Travaini, L.; Ferrari, M.; Alterio, D.; Kaanders, J.H.A.M.; Ciardo, D.; Orecchia, R.; et al. Interim 18F-FDG PET/CT During Chemoradiation Therapy in the Management of Head and Neck Cancer Patients: A Systematic Review. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 555–573. [Google Scholar] [CrossRef]
- Hentschel, M.; Appold, S.; Schreiber, A.; Abolmaali, N.; Abramyuk, A.; Dörr, W.; Kotzerke, J.; Baumann, M.; Zöphel, K. Early FDG PET at 10 or 20 Gy under chemoradiotherapy is prognostic for locoregional control and overall survival in patients with head and neck cancer. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1203–1211. [Google Scholar] [CrossRef]
- Chen, S.W.M.; Hsieh, T.C.; Yen, K.Y.; Yang, S.N.; Wang, Y.C.; Chien, C.R.; Liang, J.A.; Kao, C.H. Interim FDG PET/CT for predicting the outcome in patients with head and neck cancer. Laryngoscope 2014, 124, 2732–2738. [Google Scholar] [CrossRef]
- Dunsky, K.A.; Wehrmann, D.J.; Osman, M.M.; Thornberry, B.M.; Varvares, M.A. PET-CT and the detection of the asymptomatic recurrence or second primary lesions in the treated head and neck cancer patient. Laryngoscope 2013, 123, 2161–2164. [Google Scholar] [CrossRef]
- Leeman, J.E.; Li, J.G.; Pei, X.; Venigalla, P.; Zumsteg, Z.S.; Katsoulakis, E.; Lupovitch, E.; McBride, S.M.; Tsai, C.J.; Boyle, J.O.; et al. Patterns of Treatment Failure and Postrecurrence Outcomes among Patients with Locally Advanced Head and Neck Squamous Cell Carcinoma After Chemoradiotherapy Using Modern Radiation Techniques. JAMA Oncol. 2017, 3, 1487–1494. [Google Scholar] [CrossRef]
- Pryor, D.I.; Porceddu, S.V.; Scuffham, P.A.; Whitty, J.A.; Thomas, P.A.; Burmeister, B.H. Economic analysis of FDG-PET-guided management of the neck after primary chemoradiotherapy for node-positive head and neck squamous cell carcinoma. Head Neck 2013, 35, 1287–1294. [Google Scholar] [CrossRef]
- Hollenbeak, C.S.; Lowe, V.J.; Stack, B.C., Jr. The cost-effectiveness of fluorodeoxyglucose 18-F positron emission tomography in the N0 neck. Cancer 2001, 92, 2341–2348. [Google Scholar] [CrossRef]
- Sher, D.J.; Tishler, R.B.; Annino, D.; Punglia, R.S. Cost-effectiveness of CT and PET-CT for determining the need for adjuvant neck dissection in locally advanced head and neck cancer. Ann. Oncol. 2010, 21, 1072–1077. [Google Scholar] [CrossRef]
- Rabalais, A.; Walvekar, R.R.; Johnson, J.T.; Smith, K.J. A cost-effectiveness analysis of positron emission tomography-computed tomography surveillance versus up-frontneck dissection for management of the neck for N2 disease after chemoradiotherapy. Laryngoscope 2012, 122, 311–314. [Google Scholar] [CrossRef]
- Smith, A.F.; Hall, P.S.; Hulme, C.T.; Dunn, J.A.; McConkey, C.C.; Rahman, J.K.; McCabe, C.; Mehanna, H. Cost-effectiveness analysis of PET-CT-guided management for locally advanced head and neck cancer. Eur. J. Cancer 2017, 85, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Hodolič, M.; Fettich, J.; Kairemo, K. Hypoxia PET Tracers in EBRT Dose Planning in Head and Neck Cancer. Curr. Radiopharm. 2015, 8, 32–37. [Google Scholar] [CrossRef]
- Bertout, J.A.; Patel, S.A.; Simon, M.C. The impact of O2 availability on human cancer. Nat. Rev. Cancer 2008, 8, 967–975. [Google Scholar] [CrossRef]
- Pigorsch, S.U.; Wilkens, J.J.; Kampfer, S.; Kehl, V.; Hapfelmeier, A.; Schläger, C.; Bier, H.; Schwaiger, M.; Combs, S.E. Do selective radiation dose escalation and tumour hypoxia status impact the loco-regional tumour control after radio-chemotherapy of head & neck tumours? The ESCALOX protocol. Radiat. Oncol. 2017, 12, 45. [Google Scholar]
- Lopes, S.; Ferreira, S.; Caetano, M. PET/CT in the Evaluation of Hypoxia for Radiotherapy Planning in Head and Neck Tumors: Systematic Literature Review. J. Nucl. Med. Technol. 2021, 49, 107–113. [Google Scholar] [CrossRef]
- Welz, S.; Mönnich, D.; Pfannenberg, C.; Nikolaou, K.; Reimold, M.; La Fougère, C.; Reischl, G.; Mauz, P.S.; Paulsen, F.; Alber, M.; et al. Prognostic value of dynamic hypoxia PET in head and neck cancer: Results from a planned interim analysis of a randomized phase II hypoxia-image guided dose escalation trial. Radiother. Oncol. 2017, 124, 526–532. [Google Scholar] [CrossRef]
- Zschaeck, S.; Haase, R.; Abolmaali, N.; Perrin, R.; Stützer, K.; Appold, S.; Steinbach, J.; Kotzerke, J.; Zips, D.; Richter, C.; et al. Spatial distribution of FMISO in head and neck squamous cell carcinomas during radio-chemotherapy and its correlation to pattern of failure. Acta Oncol. 2015, 54, 1355–1363. [Google Scholar] [CrossRef]
- Boeke, S.; Thorwarth, D.; Mönnich, D.; Pfannenberg, C.; Reischl, G.; La Fougère, C.; Nikolaou, K.; Mauz, P.S.; Paulsen, F.; Zips, D.; et al. Geometric analysis of loco-regional recurrences in relation to pre-treatment hypoxia in patients with head and neck cancer. Acta Oncol. 2017, 56, 1571–1576. [Google Scholar] [CrossRef]
- Wiedenmann, N.E.; Bucher, S.; Hentschel, M.; Mix, M.; Vach, W.; Bittner, M.I.; Nestle, U.; Pfeiffer, J.; Weber, W.A.; Grosu, A.L. Serial [18F]-fluoromisonidazole PET during radiochemotherapy for locally advanced head and neck cancer and its correlation with outcome. Radiother. Oncol. 2015, 117, 113–117. [Google Scholar] [CrossRef]
- Thorwarth, D.; Eschmann, S.M.; Holzner, F.; Paulsen, F.; Alber, M. Combined uptake of [18F]FDG and [18F]FMISO correlates with radiation therapy outcome in head-and-neck cancer patients. Radiother. Oncol. 2006, 80, 151–156. [Google Scholar] [CrossRef]
- Chen, H.; Pang, Y.; Wu, J.; Zhao, L.; Hao, B.; Wu, J.; Wei, J.; Wu, S.; Zhao, L.; Luo, Z.; et al. Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1820–1832. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Miceli, A.; Racca, M.; Bauckneht, M.; Morbelli, S.; Albano, D.; Dondi, F.; Bertagna, F.; Galizia, D.; Muoio, B.; et al. Diagnostic Accuracy of [68Ga]Ga Labeled Fibroblast-Activation Protein Inhibitors in Detecting Head and Neck Cancer Lesions Using Positron Emission Tomography: A Systematic Review and a Meta-Analysis. Pharmaceuticals 2023, 16, 1664. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Pang, Y.; Zheng, H.; Han, C.; Gu, J.; Sun, L.; Wu, H.; Wu, S.; Lin, Q.; Chen, H. Clinical utility of [68Ga]Ga-labeled fibroblast activation protein inhibitor (FAPI) positron emission tomography/computed tomography for primary staging and recurrence detection in nasopharyngeal carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3606–3617. [Google Scholar] [CrossRef] [PubMed]
- Linz, C.; Brands, R.C.; Kertels, O.; Dierks, A.; Brumberg, J.; Gerhard-Hartmann, E.; Hartmann, S.; Schirbel, A.; Serfling, S.; Zhi, Y.; et al. Targeting fibroblast activation protein in newly diagnosed squamous cell carcinoma of the oral cavity—Initial experience and comparison to [18F]FDG PET/CT and MRI. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3951–3960. [Google Scholar] [CrossRef] [PubMed]
- Hasebe, M.; Yoshikawa, K.; Ohashi, S.; Toubaru, S.; Kawaguchi, K.; Sato, J.; Mizoe, J.; Tsujii, H. A study on the prognostic evaluation of carbon ion radiotherapy for head and neck adenocarcinoma with C-11 methionine PET. Mol. Imaging Biol. 2010, 12, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Toubaru, S.; Yoshikawa, K.; Ohashi, S.; Tanimoto, K.; Hasegawa, A.; Kawaguchi, K.; Saga, T.; Kamada, T. Accuracy of methionine-PET in predicting the efficacy of heavy-particle therapy on primary adenoid cystic carcinomas of the head and neck. Radiat. Oncol. 2013, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Hasebe, M.; Yoshikawa, K.; Nishii, R.; Kawaguchi, K.; Kamada, T.; Hamada, Y. Usefulness of 11C-methionine-PET for predicting the efficacy of carbon ion radiation therapy for head and neck mucosal malignant melanoma. Int. J. Oral Maxillofac. Surg. 2017, 46, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Pauleit, D.; Zimmermann, A.; Stoffels, G.; Bauer, D.; Risse, J.; Flüss, M.O.; Hamacher, K.; Coenen, H.H.; Langen, K.J. 18F-FET PET compared with 18F-FDG PET and CT in patients with head and neck cancer. J. Nucl. Med. 2006, 47, 256–261. [Google Scholar] [PubMed]
- Haerle, S.K.; Fischer, D.R.; Schmid, D.T.; Ahmad, N.; Huber, G.F.; Buck, A. 18F-FET PET/CT in advanced head and neck squamous cell carcinoma: An intra-individual comparison with 18F-FDG PET/CT. Mol. Imaging Biol. 2011, 13, 1036–1042. [Google Scholar] [CrossRef]
- Balogova, S.; Périé, S.; Kerrou, K.; Grahek, D.; Montravers, F.; Angelard, B.; Susini, B.; El Chater, P.; St Guily, J.L.; Talbot, J.N. Prospective comparison of FDG and FET PET/CT in patients with head and neck squamous cell carcinoma. Mol. Imaging Biol. 2008, 10, 364–373. [Google Scholar] [CrossRef]
- Cegla, P.; Kazmierska, J.; Gwozdz, S.; Czepczynski, R.; Malicki, J.; Cholewinski, W. Assessment of biological parameters in head and neck cancer based on in vivo distribution of 18F-FDG-FLT-FMISO-PET/CT images. Tumori J. 2020, 106, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Hoshikawa, H.; Yamamoto, Y.; Mori, T.; Kishino, T.; Fukumura, T.; Samukawa, Y.; Mori, N.; Nishiyama, Y. Predictive value of SUV-based parameters derived from pre-treatment 18F-FLT PET/CT for short-term outcome with head and neck cancers. Ann. Nucl. Med. 2014, 28, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Schaefferkoetter, J.D.; Carlson, E.R.; Heidel, R.E. Can 3′-Deoxy-3′-(18F) Fluorothymidine Outperform 2-Deoxy-2-(18F) Fluoro-D-Glucose Positron Emission Tomography/Computed Tomography in the Diagnosis of Cervical Lymphadenopathy in Patients with Oral/Head and Neck Cancer? J. Oral. Maxillofac. Surg. 2015, 73, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.P.; Chin, B.B.; Fishbein, L.; Moritani, T.; Montoya, S.P.; Ellika, S.; Newlands, S. Head and Neck Paragangliomas: An Update on the Molecular Classification, State-of-the-Art Imaging, and Management Recommendations. Radiol. Imaging Cancer 2022, 4, e210088. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Blanchet, E.M.; Adams, K.; Chen, C.C.; Millo, C.M.; Herscovitch, P.; Taieb, D.; Kebebew, E.; Lehnert, H.; Fojo, A.T.; et al. Superiority of [68Ga]-DOTATATE PET/CT to other functional imaging modalities in the localization of SDHB-associated metastatic pheochromocytoma and paraganglioma. Clin. Cancer Res. 2015, 21, 3888–3895. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Thakar, A.; Suman, K.C.S.; Dhull, V.S.; Singh, H.; Naswa, N.; Reddy, R.M.; Karunanithi, S.; Kumar, R.; Kumar, R.; et al. 68Ga-DOTANOC PET/CT for baseline evaluation of patients with head and neck paraganglioma. J. Nucl. Med. 2013, 54, 841–847. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Neuroendocrine and Adrenal Tumors. NCCN Version 1. 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf (accessed on 25 March 2024).
- Taïeb, D.; Hicks, R.J.; Hindié, E.; Guillet, B.A.; Avram, A.; Ghedini, P.; Timmers, H.J.; Scott, A.T.; Elojeimy, S.; Rubello, D.; et al. European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2112–2137. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Suh, C.H.; Woo, S.; Kim, Y.J.; Lee, J.J. Performance of 68Ga-DOTA-Conjugated Somatostatin Receptor-Targeting Peptide PET in Detection of Pheochromocytoma and Paraganglioma: A Systematic Review and Metaanalysis. J. Nucl. Med. 2019, 60, 369–376. [Google Scholar] [CrossRef]
- Sekine, T.; Barbosa, F.G.; Delso, G.; Burger, I.A.; Stolzmann, P.; Ter Voert, E.E.; Huber, G.F.; Kollias, S.S.; von Schulthess, G.K.; Veit-Haibach, P.; et al. Local resectability assessment of head and neck cancer: Positron emission tomography/MRI versus positron emission tomography/CT. Head Neck 2017, 39, 1550–1558. [Google Scholar] [CrossRef]
- Huang, S.H.; Chien, C.Y.; Lin, W.C.; Fang, F.M.; Wang, P.W.; Lui, C.C.; Huang, Y.C.; Hung, B.T.; Tu, M.C.; Chang, C.C. A comparative study of fused FDG PET/MRI, PET/CT, MRI, and CT imaging for assessing surrounding tissue invasion of advanced buccal squamous cell carcinoma. Clin. Nucl. Med. 2011, 36, 518–525. [Google Scholar] [CrossRef]
- Covello, M.; Cavaliere, C.; Aiello, M.; Cianelli, M.S.; Mesolella, M.; Iorio, B.; Rossi, A.; Nicolai, E. Simultaneous PET/MRI head–neck cancer imaging: Preliminary clinical experience and multiparametric evaluation. Eur. J. Radiol. 2015, 84, 1269–1276. [Google Scholar] [PubMed]
- Kuhn, F.; Huellner, M.; von Schulthess, G.; Veit-Haibach, P. Comparison of contrast enhanced PET/MRI and contrast enhanced PET/CT in patients with head and neck cancer. J. Nucl. Med. 2013, 54 (Suppl. 2), 515. [Google Scholar]
- Cavaliere, C.; Romeo, V.; Aiello, M.; Mesolella, M.; Iorio, B.; Barbuto, L.; Cantone, E.; Nicolai, E.; Covello, M. Multiparametric evaluation by simultaneous PET-MRI examination in patients with histologically proven laryngeal cancer. Eur. J. Radiol. 2017, 88, 47–55. [Google Scholar] [CrossRef]
- Kuhn, F.P.; Hullner, M.; Mader, C.E.; Kastrinidis, N.; Huber, G.F.; von Schulthess, G.K.; Kollias, S.; Veit-Haibach, P. Contrast-enhanced PET/MR imaging versus contrast-enhanced PET/CT in head and neck cancer: How much MR information is needed? J. Nucl. Med. 2014, 55, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Huellner, M.W. PET/MR in Head and Neck Cancer—An Update. Semin. Nucl. Med. 2021, 51, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Pizzuto, D.A.; Husmann, L.; Stolzmann, P.; Meerwein, C.; Orita, E.; von Schulthess, G.K.; Huellner, M.W. Fluoro-deoxy-glucose uptake in the mylohyoid muscle: A common misconception. Nucl. Med. Commun. 2020, 41, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Haerle, S.K.; Hany, T.F.; Ahmad, N.; Burger, I.; Huber, G.F.; Schmid, D.T. Physiologic [18F]fluorodeoxyglucose uptake of floor of mouth muscles in PET/CT imaging: A problem of body position during FDG uptake? Cancer Imaging 2013, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Monti, S.; Cavaliere, C.; Covello, M.; Nicolai, E.; Salvatore, M.; Aiello, M. An evaluation of the benefits of simultaneous acquisition on PET/MR coregistration in head/neck imaging. J. Healthc. Eng. 2017, 2017, 2634389. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.C.; Yeh, C.H.; Yen, T.C.; Ng, S.H.; Chang, J.T.; Lin, C.Y.; Yen-Ming, T.; Fan, K.H.; Huang, B.S.; Hsu, C.L.; et al. Clinical utility of simultaneous whole-body 18F-FDG PET/MRI as a single-step imaging modality in the staging of primary nasopharyngeal carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1297–1308. [Google Scholar] [CrossRef]
- Cheng, Y.; Bai, L.; Shang, J.; Tang, Y.; Ling, X.; Guo, B.; Gong, J.; Wang, L.; Xu, H. Preliminary clinical results for PET/MR compared with PET/CT in patients with nasopharyngeal carcinoma. Oncol. Rep. 2020, 43, 177–187. [Google Scholar] [CrossRef]
- Huang, C.; Song, T.; Mukherji, S.K.; Zhang, L.; Lu, J.; Chen, X.; Xian, J. Comparative study between integrated positron emission tomography/magnetic resonance and positron emission tomography/computed tomography in the T and N staging of hypopharyngeal cancer: An initial result. J. Comput. Assist. Tomogr. 2020, 44, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Fang, Y.; Yu, B.; Xu, Y.; Qiang, M.; Tao, C.; Huang, S.; Chen, X. Use of 18F-FDG PET/MRI as an Initial Staging Procedure for Nasopharyngeal Carcinoma. J. Magn. Reson. Imaging 2024, 59, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Partovi, S.; Kohan, A.; Vercher-Conejero, J.L.; Rubbert, C.; Margevicius, S.; Schluchter, M.D.; Gaeta, C.; Faulhaber, P.; Robbin, M.R. Qualitative and quantitative performance of 18F-FDG-PET/MRI versus 18F-FDG-PET/CT in patients with head and neck cancer. AJNR Am. J. Neuroradiol. 2014, 35, 1970–1975. [Google Scholar] [CrossRef] [PubMed]
- Kubiessa, K.; Purz, S.; Gawlitza, M.; Kühn, A.; Fuchs, J.; Steinhoff, K.G.; Boehm, A.; Sabri, O.; Kluge, R.; Kahn, T.; et al. Initial clinical results of simultaneous 18F-FDG PET/MRI in comparison to 18F-FDG PET/CT in patients with head and neck cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Sekine, T.; de Galiza Barbosa, F.; Kuhn, F.P.; Burger, I.A.; Stolzmann, P.; Huber, G.F.; Kollias, S.S.; von Schulthess, G.K.; Veit-Haibach, P.; Huellner, M.W. PET+MR versus PET/CT in the initial staging of head and neck cancer, using a trimodality PET/CT+MR system. Clin. Imaging 2017, 42, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Platzek, I.; Beuthien-Baumann, B.; Schneider, M.; Gudziol, V.; Kitzler, H.H.; Maus, J.; Schramm, G.; Popp, M.; Laniado, M.; Kotzerke, J.; et al. FDG PET/MR for lymph node staging in head and neck cancer. Eur. J. Radiol. 2014, 83, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Schaarschmidt, B.M.; Heusch, P.; Buchbender, C.; Ruhlmann, M.; Bergmann, C.; Ruhlmann, V.; Schlamann, M.; Antoch, G.; Forsting, M.; Wetter, A. Locoregional tumour evaluation of squamous cell carcinoma in the head and neck area: A comparison between MRI, PET/CT and integrated PET/MRI. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Varoquaux, A.; Rager, O.; Poncet, A.; Delattre, B.M.; Ratib, O.; Becker, C.D.; Dulguerov, P.; Dulguerov, N.; Zaidi, H.; Becker, M. Detection and quantification of focal uptake in head and neck tumours: 18F-FDG PET/MR versus PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 462–475. [Google Scholar] [CrossRef]
- Stolzmann, P.; Veit-Haibach, P.; Chuck, N.; Rossi, C.; Frauenfelder, T.; Alkadhi, H.; von Schulthess, G.; Boss, A. Detection rate, location, and size of pulmonary nodules in trimodality PET/CT-MR: Comparison of low-dose CT and Dixon-based MR imaging. Investig. Radiol. 2013, 48, 241–246. [Google Scholar] [CrossRef]
- Raad, R.A.; Friedman, K.P.; Heacock, L.; Ponzo, F.; Melsaether, A.; Chandarana, H. Outcome of small lung nodules missed on hybrid PET/MRI in patients with primary malignancy. J. Magn. Reson. Imaging 2016, 43, 504–511. [Google Scholar] [CrossRef]
- Chang, S.T.; Nguyen, D.C.; Raptis, C.; Menias, C.O.; Zhou, G.; Wang-Gillam, A.; Linehan, D.C.; Hawkins, W.G.; Strasberg, S.M.; Fields, R.C. Natural history of preoperative subcentimeter pulmonary nodules in patients with resectable pancreatic adenocarcinoma: A retrospective cohort study. Ann. Surg. 2015, 261, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Sekine, T.; Barbosa, F.G.; Sah, B.R.; Mader, C.E.; Delso, G.; Burger, I.A.; Stolzmann, P.; Ter Voert, E.E.; von Schulthess, G.K.; Veit-Haibach, P.; et al. PET/MR Outperforms PET/CT in Suspected Occult Tumors. Clin. Nucl. Med. 2017, 42, e88–e95. [Google Scholar] [CrossRef] [PubMed]
- Ruhlmann, V.; Ruhlmann, M.; Bellendorf, A.; Grueneisen, J.; Sawicki, L.M.; Grafe, H.; Forsting, M.; Bockisch, A.; Umutlu, L. Hybrid imaging for detection of carcinoma of unknown primary: A preliminary comparison trial of whole-body PET/MRI versus PET/CT. Eur. J. Radiol. 2016, 85, 1941–1947. [Google Scholar] [CrossRef] [PubMed]
- Samołyk-Kogaczewska, N.; Sierko, E.; Zuzda, K.; Gugnacki, P.; Szumowski, P.; Mojsak, M.; Burzyńska-Śliwowska, J.; Wojtukiewicz, M.Z.; Szczecina, K.; Jurgilewicz, D.H. PET/MRI-guided GTV delineation during radiotherapy planning in patients with squamous cell carcinoma of the tongue. Strahlenther. Onkol. 2019, 195, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Mullins, B.T.; Falchook, A.D.; Lian, J.; He, K.; Shen, D.; Dance, M.; Lin, W.; Sills, T.M.; Das, S.K.; et al. Evaluation of PET/MRI for Tumor Volume Delineation for Head and Neck Cancer. Front. Oncol. 2017, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Terzidis, E.; Friborg, J.; Vogelius, I.R.; Lelkaitis, G.; von Buchwald, C.; Olin, A.B.; Johannesen, H.H.; Fischer, B.M.; Wessel, I.; Rasmussen, J.H. Tumor volume definitions in head and neck squamous cell carcinoma—Comparing PET/MRI and histopathology. Radiother. Oncol. 2023, 180, 109484. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, J.; Schaarschmidt, B.M.; Sauerwein, W.; Deuschl, C.; Arweiler-Harbeck, D.; Holtmann, L.; Stebner, V.; Umutlu, L.; Antoch, G.; Ruhlmann, V. 18F-FDG PET/MRI vs MRI in patients with recurrent adenoid cystic carcinoma. Head Neck 2019, 41, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Varoquaux, A.D.; Combescure, C.; Rager, O.; Pusztaszeri, M.; Burkhardt, K.; Delattre, B.M.A.; Dulguerov, P.; Dulguerov, N.; Katirtzidou, E.; et al. Local recurrence of squamous cell carcinoma of the head and neck after radio(chemo)therapy: Diagnostic performance of FDG-PET/MRI with diffusion-weighted sequences. Eur. Radiol. 2018, 28, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.A.; Hullner, M.; Kuhn, F.; Huber, G.; Meerwein, C.; Kollias, S.; von Schulthess, G.; Veit-Haibach, P. Use of diffusion-weighted imaging (DWI) in PET/MRI for head and neck cancer evaluation. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2212–2221. [Google Scholar] [CrossRef]
- Queiroz, M.A.; Huellner, M.W. PET/MR in cancers of the head and neck. Semin. Nucl. Med. 2015, 45, 248–265. [Google Scholar] [CrossRef]
- Castelli, J.; De Bari, B.; Depeursinge, A.; Simon, A.; Devillers, A.; Roman Jimenez, G.; Prior, J.; Ozsahin, M.; de Crevoisier, R.; Bourhis, J. Overview of the predictive value of quantitative 18 FDG PET in head and neck cancer treated with chemoradiotherapy. Crit. Rev. Oncol. Hematol. 2016, 108, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Guo, Y.; Jin, Q. Radiomics and Its Feature Selection: A Review. Symmetry 2023, 15, 1834. [Google Scholar] [CrossRef]
- Avanzo, M.; Stancanello, J.; El Naqa, I. Beyond imaging: The promise of radiomics. Phys. Medica 2017, 38, 122–139. [Google Scholar] [CrossRef] [PubMed]
- Alkhadar, H.; Macluskey, M.; White, S.; Ellis, I.; Gardner, A. Comparison of machine learning algorithms for the prediction of five-year survival in oral squamous cell carcinoma. J. Oral Pathol. Med. 2021, 50, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Guezennec, C.; Robin, P.; Orlhac, F.; Bourhis, D.; Delcroix, O.; Gobel, Y.; Rousset, J.; Schick, U.; Salaün, P.Y.; Abgral, R. Prognostic value of textural indices extracted from pretherapeutic 18-F-FDG-PET/CT in head and neck squamous cell carcinoma. Head Neck 2019, 41, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Feliciani, G.; Fioroni, F.; Grassi, E.; Bertolini, M.; Rosca, A.; Timon, G.; Galaverni, M.; Iotti, C.; Versari, A.; Iori, M.; et al. Radiomic Profiling of Head and Neck Cancer: 18F-FDG PET Texture Analysis as Predictor of Patient Survival. Contrast Media Mol. Imaging 2018, 2018, 3574310. [Google Scholar] [CrossRef] [PubMed]
- Bogowicz, M.; Leijenaar, R.T.H.; Tanadini-Lang, S.; Riesterer, O.; Pruschy, M.; Studer, G.; Unkelbach, J.; Guckenberger, M.; Konukoglu, E.; Lambin, P. Post-radiochemotherapy PET radiomics in head and neck cancer—The influence of radiomics implementation on the reproducibility of local control tumor models. Radiother. Oncol. 2017, 125, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Bogowicz, M.; Riesterer, O.; Stark, L.S.; Studer, G.; Unkelbach, J.; Guckenberger, M.; Tanadini-Lang, S. Comparison of PET and CT radiomics for prediction of local tumor control in head and neck squamous cell carcinoma. Acta Oncol. 2017, 56, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Tian, Y.; Lu, X.; Chen, G.; Lv, X. Prognostic value of 18F-FDG PET radiomics and sarcopenia in patients with oral squamous cell carcinoma. Med. Phys. 2024. [Google Scholar] [CrossRef]
- Choi, J.H.; Choi, J.Y.; Woo, S.K.; Moon, J.E.; Lim, C.H.; Park, S.B.; Seo, S.; Ahn, Y.C.; Ahn, M.J.; Moon, S.H.; et al. Prognostic Value of Radiomic Analysis Using Pre- and Post-Treatment 18F-FDG-PET/CT in Patients with Laryngeal Cancer and Hypopharyngeal Cancer. J. Pers. Med. 2024, 14, 71. [Google Scholar] [CrossRef]
- Kudoh, T.; Haga, A.; Kudoh, K.; Takahashi, A.; Sasaki, M.; Kudo, Y.; Ikushima, H.; Miyamoto, Y. Radiomics analysis of [18F]-fluoro-2-deoxyglucose positron emission tomography for the prediction of cervical lymph node metastasis in tongue squamous cell carcinoma. Oral Radiol. 2023, 39, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.P.; Sharaf, K.; Zeevi, T.; Baumeister, P.; Reichel, C.; Forghani, R.; Kann, B.H.; Petukhova, A.; Judson, B.L.; Prasad, M.L.; et al. Prediction of post-radiotherapy locoregional progression in HPV-associated oropharyngeal squamous cell carcinoma using machine-learning analysis of baseline PET/CT radiomics. Transl. Oncol. 2021, 14, 100906. [Google Scholar] [CrossRef] [PubMed]
- Nakajo, M.; Kawaji, K.; Nagano, H.; Jinguji, M.; Mukai, A.; Kawabata, H.; Tani, A.; Hirahara, D.; Yamashita, M.; Yoshiura, T. The usefulness of machine learning-based evaluation of clinical and pretreatment [18F]-FDG-PET/CT radiomic features for predicting prognosis in hypopharyngeal cancer. Mol. Imaging Biol. 2023, 25, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Lafata, K.J.; Chang, Y.; Wang, C.; Mowery, Y.M.; Vergalasova, I.; Niedzwiecki, D.; Yoo, D.S.; Liu, J.G.; Brizel, D.M.; Yin, F.F. Intrinsic radiomic expression patterns after 20 Gy demonstrate early metabolic response of oropharyngeal cancers. Med. Phys. 2021, 48, 3767–3777. [Google Scholar] [CrossRef] [PubMed]
- Spielvogel, C.P.; Stoiber, S.; Papp, L.; Krajnc, D.; Grahovac, M.; Gurnhofer, E.; Trachtova, K.; Bystry, V.; Leisser, A.; Jank, B.; et al. Radiogenomic markers enable risk stratification and inference of mutational pathway states in head and neck cancer. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.P.; Zeevi, T.; Baumeister, P.; Reichel, C.; Sharaf, K.; Forghani, R.; Kann, B.H.; Judson, B.L.; Prasad, M.L.; Burtness, B.; et al. Potential added value of PET/CT radiomics for survival prognostication beyond AJCC 8th edition staging in oropharyngeal squamous cell carcinoma. Cancers 2020, 12, 1778. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Frood, R.; Brown, P.; Nelstrop, H.; Prestwich, R.; McDermott, G.; Currie, S.; Vaidyanathan, S.; Scarsbrook, A.F. Machine learning-based FDG PET-CT radiomics for outcome prediction in larynx and hypopharynx squamous cell carcinoma. Clin. Radiol. 2021, 76, 78.e9–78.e17. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Feng, H.; Lv, W.; Ashrafinia, S.; Yuan, Q.; Wang, Q.; Yang, W.; Feng, Q.; Chen, W.; Rahmim, A.; et al. Machine learning methods for optimal radiomics-based differentiation between recurrence and inflammation: Application to nasopharyngeal carcinoma post-therapy PET/CT images. Mol. Imaging Biol. 2020, 22, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Dong, D.; Fang, M.J.; Li, L.; Tang, L.L.; Chen, L.; Mao, Y.P.; Fan, W.; Liu, L.Z.; Tian, L.; et al. Prognostic value of deep learning PET/CT-based radiomics: Potential role for future individual induction chemotherapy in advanced nasopharyngeal carcinoma. Clin. Cancer Res. 2019, 25, 4271–4279. [Google Scholar] [CrossRef]
- Nakajo, M.; Jinguji, M.; Ito, S.; Tani, A.; Hirahara, M.; Yoshiura, T. Clinical application of 18F-fluorodeoxyglucose positron emission tomography/computed tomography radiomics-based machine learning analyses in the field of oncology. Jpn. J. Radiol. 2024, 42, 28–55. [Google Scholar] [CrossRef]
- Nakajo, M.; Nagano, H.; Jinguji, M.; Kamimura, Y.; Masuda, K.; Takumi, K.; Tani, A.; Hirahara, D.; Kariya, K.; Yamashita, M.; et al. The usefulness of machine-learning-based evaluation of clinical and pretreatment 18F-FDG-PET/CT radiomic features for predicting prognosis in patients with laryngeal cancer. Br. J. Radiol. 2023, 96, 20220772. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lv, W.B.; Feng, H.; Du, D.Y.; Yuan, Q.Y.; Wang, Q.S.; Dai, Z.; Yang, W.; Feng, Q.; Ma, J.; et al. Subregional Radiomics Analysis of PET/CT Imaging with Intratumor Partitioning: Application to Prognosis for Nasopharyngeal Carcinoma. Mol. Imaging Biol. 2020, 22, 1414–1426. [Google Scholar] [CrossRef] [PubMed]
- Fujima, N.; Andreu-Arasa, V.C.; Meibom, S.K.; Mercier, G.A.; Salama, A.R.; Truong, M.T.; Sakai, O. Prediction of the treatment outcome using machine learning with FDG-PET image-based multiparametric approach in patients with oral cavity squamous cell carcinoma. Clin. Radiol. 2021, 76, 711.e1–711.e7. [Google Scholar] [CrossRef]
- Fujima, N.; Andreu-Arasa, V.C.; Meibom, S.K.; Mercier, G.A.; Salama, A.R.; Truong, M.T.; Sakai, O. Deep learning analysis using FDG-PET to predict treatment outcome in patients with oral cavity squamous cell carcinoma. Eur. Radiol. 2020, 30, 6322–6330. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Chen, S.; Krauss, D.J.; Chen, P.Y.; Chinnaiyan, P.; Wilson, G.D. Tumor Voxel Dose-Response Matrix and Dose Prescription Function Derived Using 18F-FDG PET/CT Images for Adaptive Dose Painting by Number. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Reiazi, R.; Abbas, E.; Famiyeh, P.; Rezaie, A.; Kwan, J.Y.Y.; Patel, T.; Bratman, S.V.; Tadic, T.; Liu, F.F. The impact of the variation of imaging parameters on the robustness of Computed Tomography radiomic features: A review. Comput. Biol. Med. 2021, 133, 104400. [Google Scholar] [CrossRef] [PubMed]
- Larue, R.T.H.M.; van Timmeren, J.E.; de Jong, E.E.C.; Feliciani, G.; Leijenaar, R.T.H.; Schreurs, W.M.J.; Sosef, M.N.; Raat, F.H.P.J.; van der Zande, F.H.R.; Das, M.; et al. Influence of gray level discretization on radiomic feature stability for different CT scanners, tube currents and slice thicknesses: A comprehensive phantom study. Acta Oncol. 2017, 56, 1544–1553. [Google Scholar] [CrossRef]
- Bang, C.; Bernard, G.; Le, W.T.; Lalonde, A.; Kadoury, S.; Bahig, H. Artificial intelligence to predict outcomes of head and neck radiotherapy. Clin. Transl. Radiat. Oncol. 2023, 39, 100590. [Google Scholar] [CrossRef]
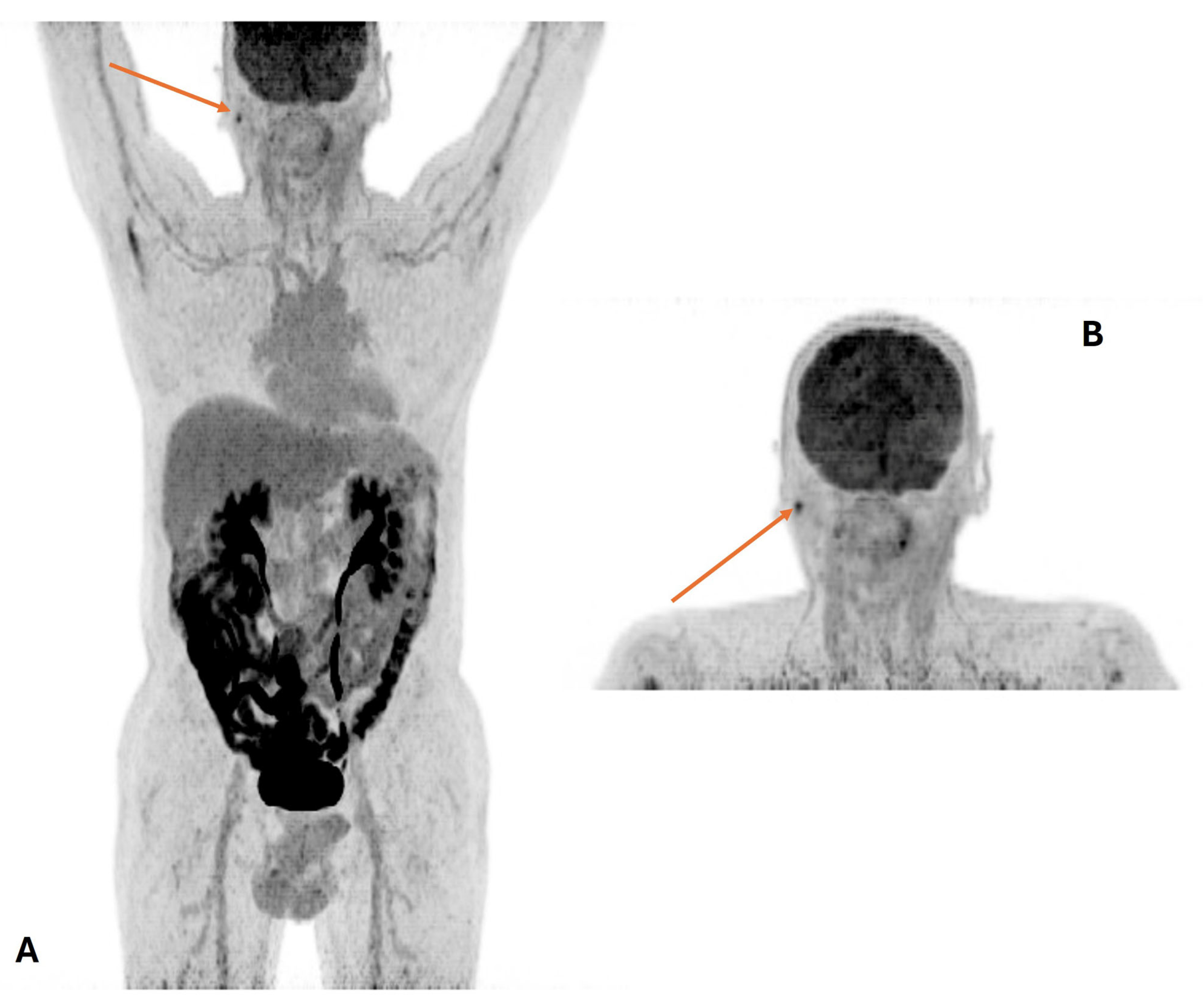
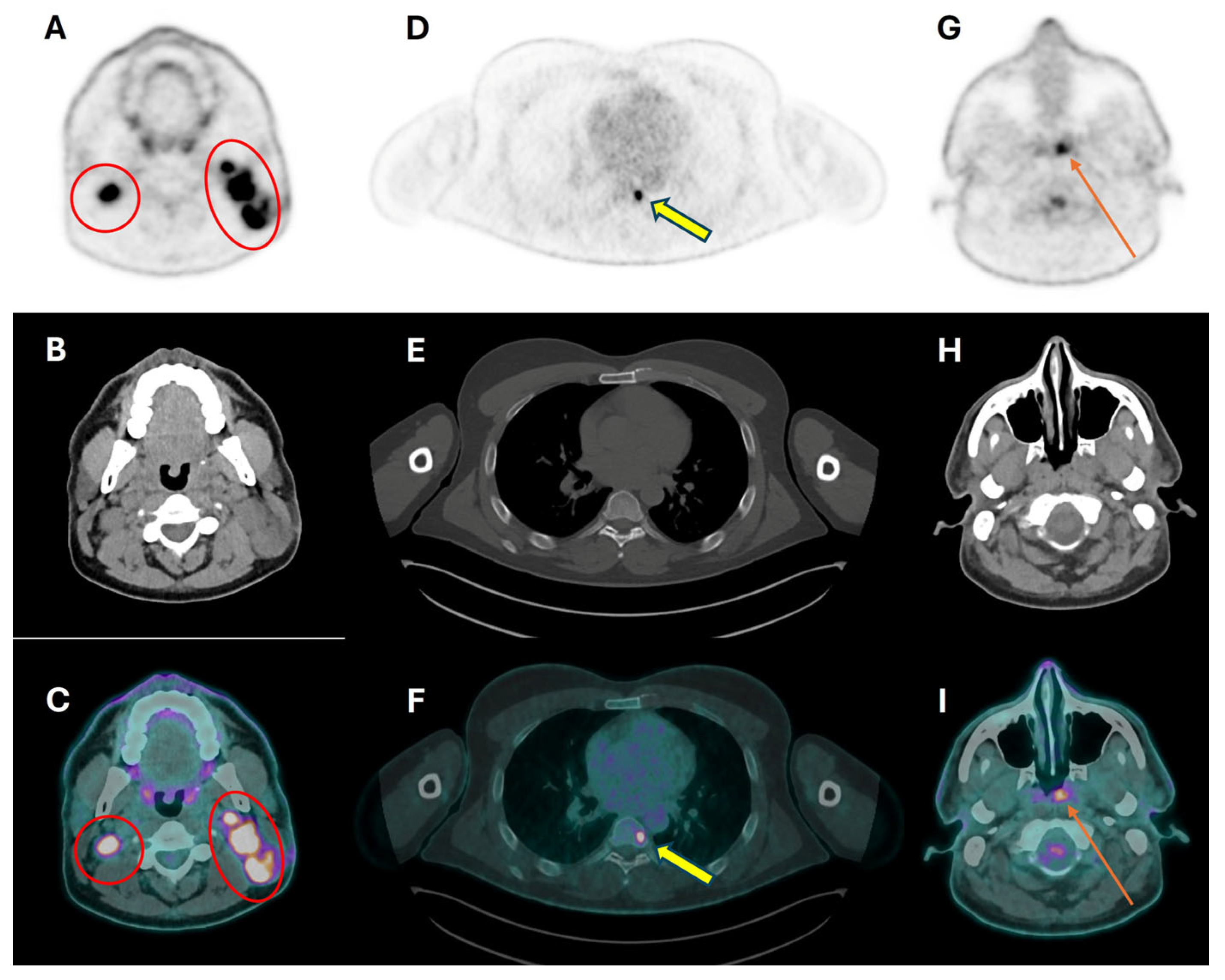
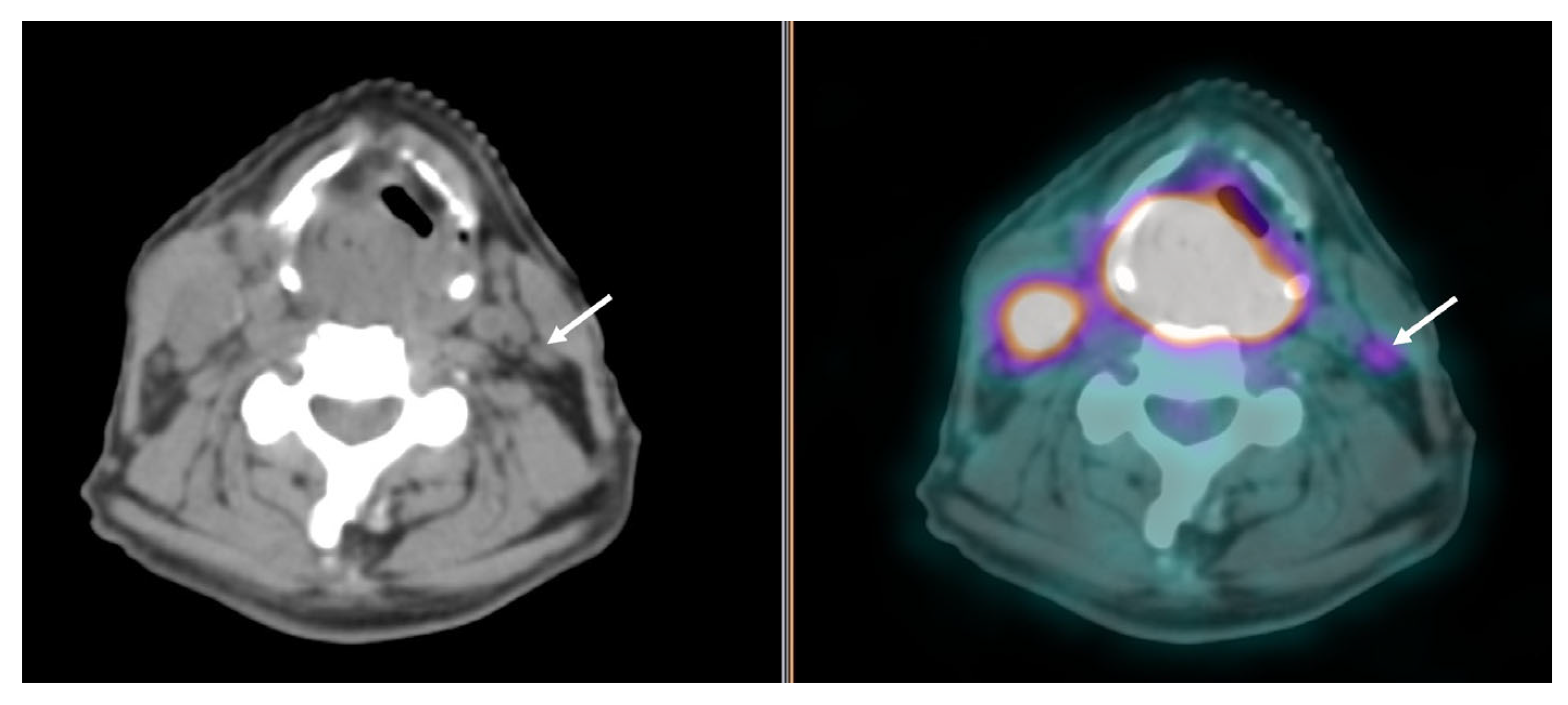
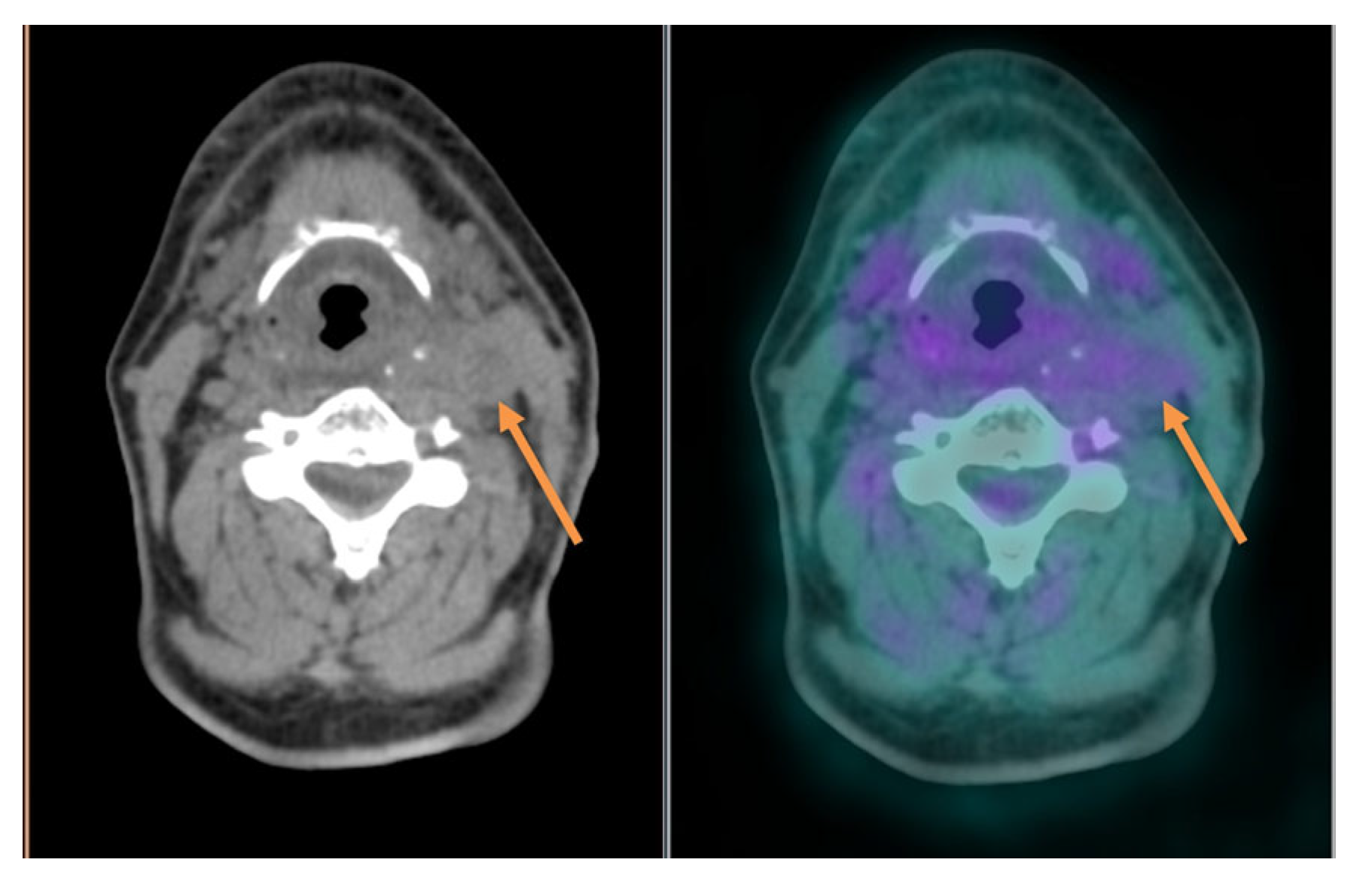
| PET/CT and Radiotherapy Planning | Clinical Examples |
|---|---|
| Patient selection and intended management | Treatment (local disease) versus non-treatment (distant metastases) |
| Goal of treatment | From curative to palliative and vice versa |
| Selection and delineation of GTV | - Detection of occult primary tumor (see text) - Tumor extension not defined on CT or MRI (see Figure 3) |
| Dose painting based on biological tumor features | Dose escalation to 18F-FDG avid or hypoxic sub-volumes |
| Adaptive radiotherapy | Escalation or de-escalation during treatment |
| Radiopharmaceutical | Molecular Target | Main Indications | Clinical Application |
|---|---|---|---|
| 18F-FMISO 18F-FAZA 18F-EF5 18F-FETNIM 18F-HX4 64Cu-ATSM | Hypoxia | Staging Response evaluation Adaptive Radiotherapy | Experimental |
| 68Ga-FAPi 18F-FAPi Al18F-NOTA-FAPi | Fibroblast Activating Protein (FAP) | Staging Unknown primary | Experimental |
| 11C-MET | Protein synthesis | Adaptive radiotherapy Response evaluation | Clinical |
| 18F-FET | Protein synthesis | Staging–Restaging | Experimental |
| 18F-FLT | Cell proliferation | Staging–Restaging Response evaluation Adaptive radiotherapy | Experimental |
| 68Ga-DOTATOC 68Ga-DOTANOC 68Ga-DOTATATE | SSTR-expression | Staging–Restaging Response evaluation Targeted therapy | Clinical |
| 18F-DOPA | Neurotransmitter transportation | Staging–Restaging | Clinical |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caldarella, C.; De Risi, M.; Massaccesi, M.; Miccichè, F.; Bussu, F.; Galli, J.; Rufini, V.; Leccisotti, L. Role of 18F-FDG PET/CT in Head and Neck Squamous Cell Carcinoma: Current Evidence and Innovative Applications. Cancers 2024, 16, 1905. https://doi.org/10.3390/cancers16101905
Caldarella C, De Risi M, Massaccesi M, Miccichè F, Bussu F, Galli J, Rufini V, Leccisotti L. Role of 18F-FDG PET/CT in Head and Neck Squamous Cell Carcinoma: Current Evidence and Innovative Applications. Cancers. 2024; 16(10):1905. https://doi.org/10.3390/cancers16101905
Chicago/Turabian StyleCaldarella, Carmelo, Marina De Risi, Mariangela Massaccesi, Francesco Miccichè, Francesco Bussu, Jacopo Galli, Vittoria Rufini, and Lucia Leccisotti. 2024. "Role of 18F-FDG PET/CT in Head and Neck Squamous Cell Carcinoma: Current Evidence and Innovative Applications" Cancers 16, no. 10: 1905. https://doi.org/10.3390/cancers16101905
APA StyleCaldarella, C., De Risi, M., Massaccesi, M., Miccichè, F., Bussu, F., Galli, J., Rufini, V., & Leccisotti, L. (2024). Role of 18F-FDG PET/CT in Head and Neck Squamous Cell Carcinoma: Current Evidence and Innovative Applications. Cancers, 16(10), 1905. https://doi.org/10.3390/cancers16101905








