Targeting Anterior Commissure Involvement with Hyperfractionated Radiotherapy for T1–T2 Squamous Cell Carcinoma of the Glottic Larynx
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Treatment
2.2. Radiotherapy
2.3. Salvage Surgery
2.4. Statistical Analyses
3. Results
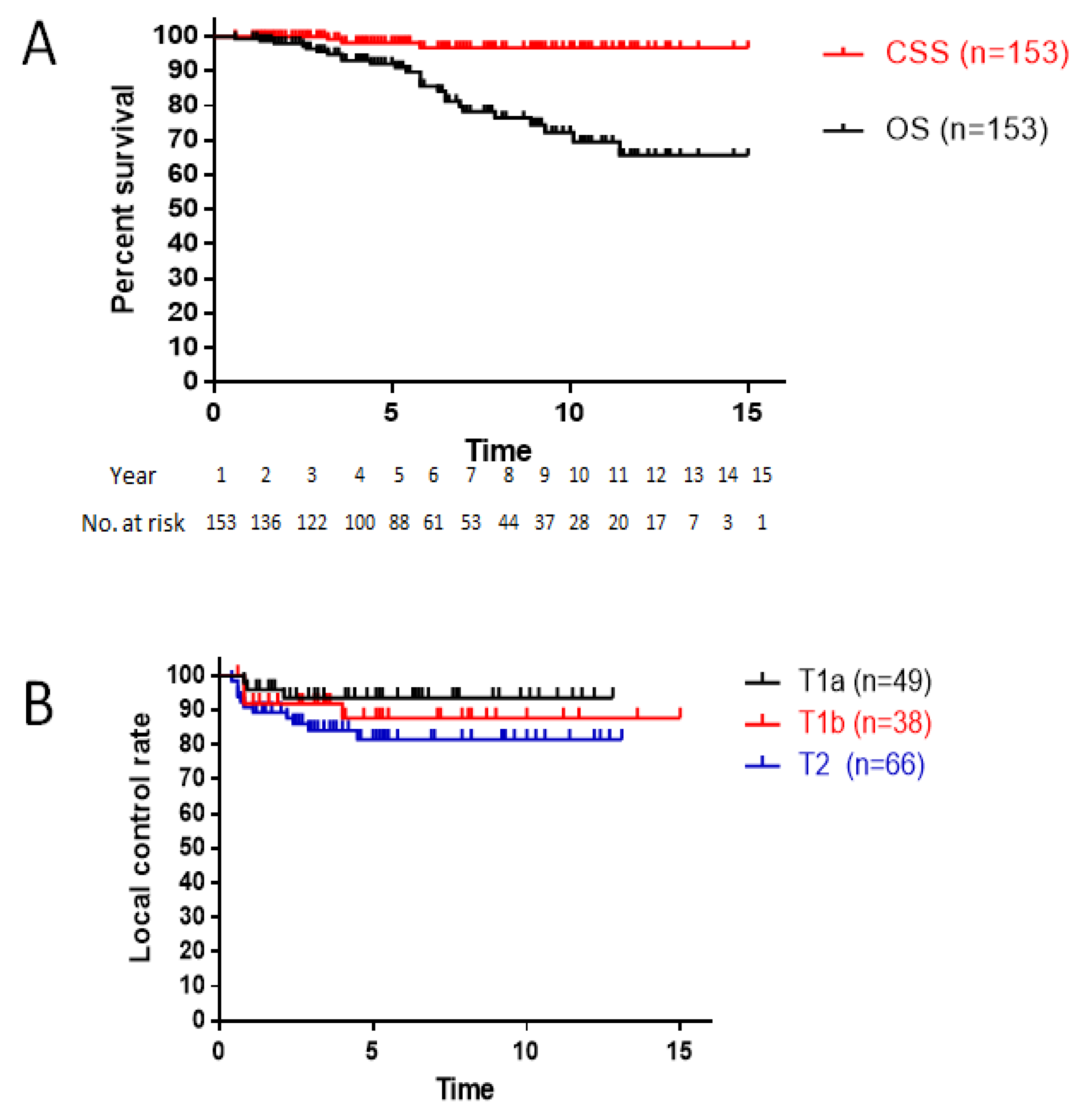
3.1. Survival
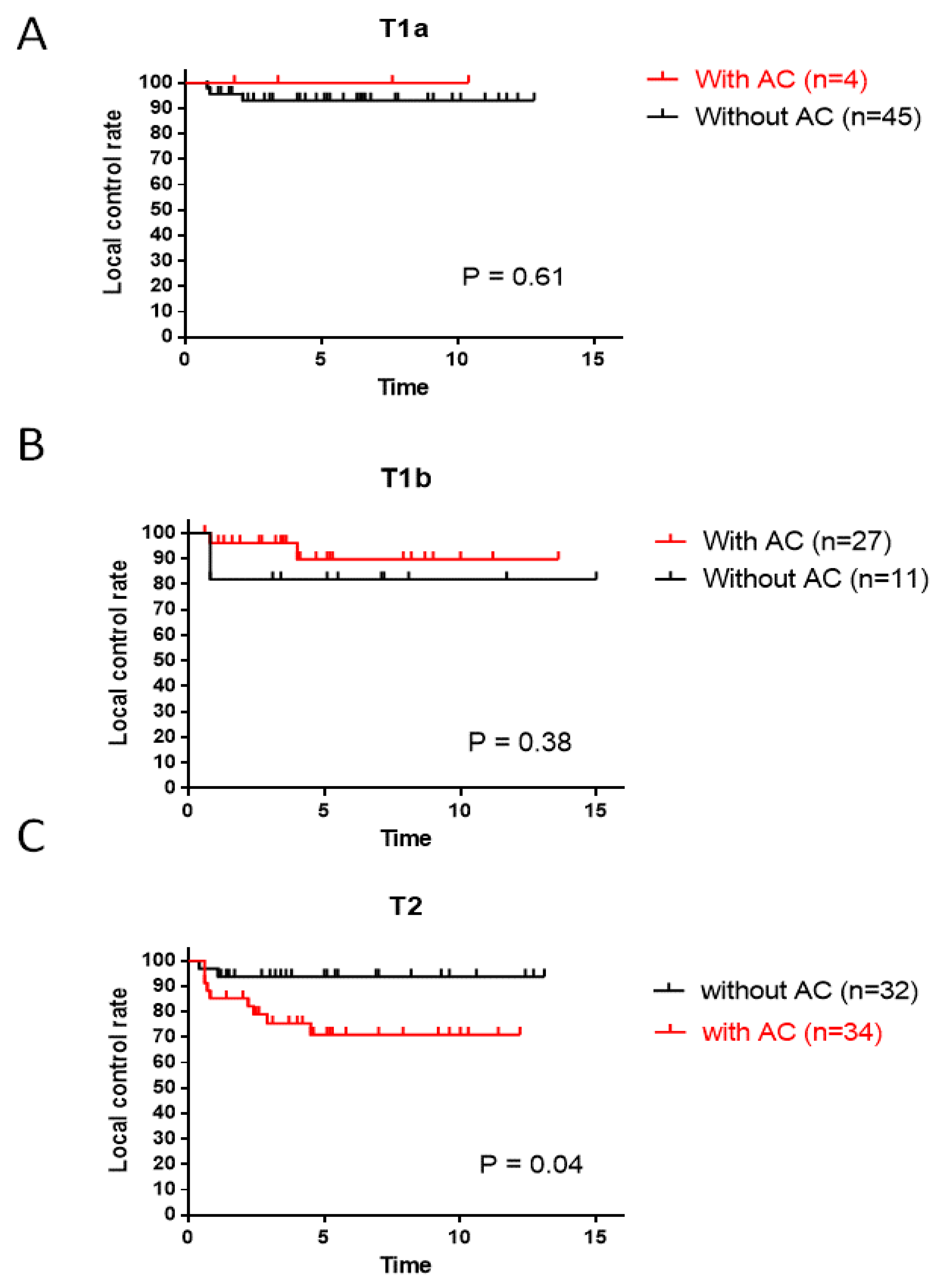
3.2. Local Control
3.3. Salvage Treatments and Laryngeal Preservation
3.4. Morbidity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Lyhne, N.M.; Johansen, J.; Kristensen, C.A.; Andersen, E.; Primdahl, H.; Andersen, L.J.; Bøje, C.R.; Jensen, A.R.; Overgaard, J. Pattern of failure in 5001 patients treated for glottic squamous cell carcinoma with curative intent–A population-based study from the DAHANCA group. Radiother. Oncol. 2016, 118, 257–266. [Google Scholar] [CrossRef]
- Chera, B.S.; Amdur, R.J.; Morris, C.G.; Kirwan, J.M.; Mendenhall, W.M. T1N0 to T2N0 squamous cell carcinoma of the glottic larynx treated with definitive radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 461–466. [Google Scholar] [CrossRef]
- Ermiş, E.; Teo, M.; Dyker, K.E.; Fosker, C.; Sen, M.; Prestwich, R.J. Definitive hypofractionated radiotherapy for early glottic carcinoma: Experience of 55Gy in 20 fractions. Radiat. Oncol. 2015, 10, 203. [Google Scholar] [CrossRef]
- Mendenhall, W.M.; Werning, J.W.; Hinerman, R.W.; Amdur, R.J.; Villaret, D.B. Management of T1–T2 glottic carcinomas. Cancer 2004, 100, 1786–1792. [Google Scholar] [CrossRef]
- Benninger, M.S.; Gillen, J.; Thieme, P.; Jacobson, B.; Dragovich, J. Factors associated with recurrence and voice quality following radiation therapy for T1 and T2 glottic carcinomas. Laryngoscope 1994, 104, 294–298. [Google Scholar] [CrossRef]
- Krengli, M.; Policarpo, M.; Manfredda, I.; Aluffi, P.; Gambaro, G.; Panella, M.; Pia, F. Voice quality after treatment for T1a glottic carcinoma—Radiotherapy versus laser cordectomy. Acta Oncol. 2004, 43, 284–289. [Google Scholar] [CrossRef]
- Rifai, M.; Khattab, H. Anterior commissure carcinoma: I-histopathologic study. Am. J. Otolaryngol. 2000, 21, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Alkan, U.; Nachalon, Y.; Shkedy, Y.; Yaniv, D.; Shvero, J.; Popovtzer, A. T1 squamous cell carcinoma of the glottis with anterior commissure involvement: Radiotherapy versus transoral laser microsurgery. Head Neck 2017, 39, 1101–1105. [Google Scholar] [CrossRef]
- Rucci, L.; Gammarota, L.; Borghi Cirri, M.B. Carcinoma of the anterior commissure of the larynx: I. Embryological and anatomic considerations. Ann. Otol. Rhinol. Laryngol. 1996, 105, 303–308. [Google Scholar] [CrossRef]
- Prades, J.M.; Peoc’h, M.; Petcu, C.; Karkas, A.; Dumollard, J.M.; Gavid, M. The anterior commissure of the human larynx revisited. Surg. Radiol. Anat. 2017, 39, 871–876. [Google Scholar] [CrossRef]
- Rucci, L.; Romagnoli, P.; Casucci, A.; Ferlito, A. Embryological study of the glottic site and clinical implications. Oral Oncol. 2004, 40, 1017–1025. [Google Scholar] [CrossRef]
- Garden, A.S.; Forster, K.; Wong, P.F.; Morrison, W.H.; Schechter, N.R.; Ang, K.K. Results of radiotherapy for T2N0 glottic carcinoma: Does the “2” stand for twice–daily treatment? Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 322–328. [Google Scholar] [CrossRef]
- Cellai, E.; Frata, P.; Magrini, S.M.; Paiar, F.; Barca, R.; Fondelli, S.; Polli, C.; Livi, L.; Bonetti, B.; Vitali, E.; et al. Radical radiotherapy for early glottic cancer: Results in a series of 1087 patients from two Italian radiation oncology centers. I. The case of T1N0 disease. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 1378–1386. [Google Scholar] [CrossRef]
- Laccourreye, O.; Muscatello, L.; Laccourreye, L.; Naudo, P.; Brasnu, D.; Weinstein, G. Supracricoid partial laryngectomy with cricohyoidoepiglottopexy for “early” glottic carcinoma classified as T1-T2N0 invading the anterior commissure. Am. J. Otolaryngol. 1997, 18, 385–390. [Google Scholar] [CrossRef]
- Marshak, G.; Brenner, B.; Shvero, J.; Shapira, J.; Ophir, D.; Hochman, I.; Marshak, G.; Sulkes, A.; Rakowsky, E. Prognostic factors for local control of early glottic cancer: The Rabin Medical Center retrospective study on 207 patients. Int. J. Radiat. Oncol. Biol. Phys. 1999, 43, 1009–1013. [Google Scholar] [CrossRef]
- Smee, R.I.; Meagher, N.S.; Williams, J.R.; Broadley, K.; Bridger, G.P. Role of radiotherapy in early glottic carcinoma. Head Neck 2010, 32, 850–859. [Google Scholar] [CrossRef]
- Tulli, M.; Re, M.; Bondi, S.; Ferrante, L.; Dajko, M.; Giordano, L.; Gioacchini, F.M.; Galli, A.; Bussi, M. The prognostic value of anterior commissure involvement in T1 glottic cancer: A systematic review and meta-analysis. Laryngoscope 2020, 130, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Hoff, C.M. Importance of hemoglobin concentration and its modification for the outcome of head and neck cancer patients treated with radiotherapy. Acta Oncol. 2012, 51, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamgani, A.; van Rooij, P.H.; Mehilal, R.; Verduijn, G.M.; Tans, L.; Kwa, S.L. Radiotherapy for T1a glottic cancer: The influence of smoking cessation and fractionation schedule of radiotherapy. Eur. Arch. Otorhinolaryngol 2014, 271, 125–132. [Google Scholar] [CrossRef]
- Yamoah, K.; Showalter, T.N.; Ohri, N. Radiation therapy intensification for solid tumors: A systematic review of randomized trials. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 737–745. [Google Scholar] [CrossRef]
- Lyhne, N.M.; Primdahl, H.; Kristensen, C.A.; Andersen, E.; Johansen, J.; Andersen, L.J.; Evensen, J.; Mortensen, H.R.; Overgaard, J. The DAHANCA 6 randomized trial: Effect of 6 vs 5 weekly fractions of radiotherapy in patients with glottic squamous cell carcinoma. Radiother. Oncol. 2015, 117, 91–98. [Google Scholar] [CrossRef]
- Yamazaki, H.; Nishiyama, K.; Tanaka, E.; Koizumi, M.; Chatani, M. Radiotherapy for early glottic carcinoma (T1N0M0): Results of prospective randomized study of radiation fraction size and overall treatment time. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 77–82. [Google Scholar] [CrossRef]
- Moon, S.H.; Cho, K.H.; Chung, E.J.; Lee, C.G.; Lee, K.C.; Chai, G.Y.; Kang, K.M.; Lee, J.Y.; Chung, W.K.; Park, W.Y.; et al. A prospective randomized trial comparing hypofractionation with conventional fractionation radiotherapy for T1-2 glottic squamous cell carcinomas: Results of a Korean Radiation Oncology Group (KROG-0201) study. Radiother. Oncol. 2014, 110, 98–103. [Google Scholar] [CrossRef]
- Trotti, A., 3rd; Zhang, Q.; Bentzen, S.M.; Emami, B.; Hammond, M.E.; Jones, C.U.; Morrison, W.H.; Sagar, S.M.; Ridge, J.A.; Fu, K.K.; et al. Randomized trial of hyperfractionation versus conventional fractionation in T2 squamous cell carcinoma of the vocal cord (RTOG 9512). Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 958–963. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 8th Ed. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Harada, A.; Sasaki, R.; Miyawaki, D.; Yoshida, K.; Nishimura, H.; Ejima, Y.; Kitajima, K.; Saito, M.; Otsuki, N.; Nibu, K. Treatment outcomes of the patients with early glottic cancer treated with initial radiotherapy and salvaged by conservative surgery. Jpn. J. Clin. Oncol. 2015, 45, 248–255. [Google Scholar] [CrossRef]
- Reddy, S.P.; Mohideen, N.; Marra, S.; Marks, J.E. Effect of tumor bulk on local control and survival of patients with T1 glottic cancer. Radiother. Oncol. 1998, 47, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Biller, H.F.; Barnhill, F.R.; Ogura, J.H.; Perez, C.A. Hemilaryngectomy following radiation failure for carcinoma of the vocal cords. Laryngoscope 1970, 80, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Toma, M.; Nibu, K.; Nakao, K.; Matsuzaki, M.; Mochiki, M.; Yuge, T.; Terahara, A.; Sugasawa, M. Partial laryngectomy to treat early glottic cancer after failure of radiation therapy. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 909–912. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hendriksma, M.; Sjögren, E.V. Involvement of the anterior commissure in early glottic cancer (tis-T2): A review of the literature. Cancers 2019, 11, 1234. [Google Scholar] [CrossRef]
- Le, Q.T.X.; Fu, K.K.; Kroll, S.; Ryu, J.K.; Quivey, J.M.; Meyler, T.S.; Krieg, R.M.; Phillips, T.L. Influence of fraction size, total dose, and overall time on local control of T1-T2 glottic carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 1997, 39, 115–126. [Google Scholar] [CrossRef]
- Sapienza, L.G.; Ning, M.S.; Taguchi, S.; Calsavara, V.F.; Pellizzon, A.C.A.; Gomes, M.J.L.; Kowalski, L.P.; Baiocchi, G. Altered-fractionation radiotherapy improves local control in early-stage glottic carcinoma: A systematic review and meta-analysis of 1762 patients. Oral Oncol. 2019, 93, 8–14. [Google Scholar] [CrossRef]
- Jackson, S.M.; Weir, L.M.; Hay, J.H.; Tsang, V.H.Y.; Durham, J.S. A randomised trial of accelerated versus conventional radiotherapy in head and neck cancer. Radiother. Oncol. 1997, 43, 39–46. [Google Scholar] [CrossRef]
- Kaanders, J.H.; Van Der Kogel, A.J.; Ang, K.K. Altered fractionation: Limited by mucosal reactions? Radiother. Oncol. 1999, 50, 247–260. [Google Scholar] [CrossRef]
- Okazaki, E.; Matsushita, N.; Tashiro, M.; Shimatani, Y.; Ishii, K.; Hosono, M.; Oishi, M.; Teranishi, Y.; Iguchi, H.; Miki, Y. Efficacy and toxicity profiles of two chemoradiotherapies for stage II laryngeal cancer—A comparison between late course accelerated hyperfractionation (LCAHF) and conventional fractionation (CF). Acta Otolaryngol. 2017, 137, 883–887. [Google Scholar] [CrossRef]
- Suzuki, G.; Yamazaki, H.; Aibe, N.; Masui, K.; Shimizu, D.; Kimoto, T.; Nishimura, T.; Kawabata, K.; Nagasawa, S.; Machida, K.; et al. Comparison of three fractionation schedules in radiotherapy for early glottic squamous cell carcinoma. In Vivo 2020, 34, 2769–2774. [Google Scholar] [CrossRef]
- Ligtenberg, H.; Jager, E.A.; Caldas-Magalhaes, J.; Schakel, T.; Pameijer, F.A.; Kasperts, N.; Willems, S.M.; Terhaard, C.H.; Raaijmakers, C.P.; Philippens, M.E. Modality-specific target definition for laryngeal and hypopharyngeal cancer on FDG-PET, CT and MRI. Radiother. Oncol. 2017, 123, 63–70. [Google Scholar] [CrossRef]
- Cergan, R.; Dumitru, M.; Vrinceanu, D.; Neagos, A.; Jeican, I.; Ciuluvica, R.C. Ultrasonography of the larynx: Novel use during the SARS-CoV-2 pandemic. Exp. Ther. Med. 2021, 21, 273. [Google Scholar] [CrossRef]


| Number (n = 153) | (%) | ||
|---|---|---|---|
| Age (range) | 72 (47–91) | ||
| Gender | Male | 136 | 89 |
| Female | 17 | 11 | |
| ECOG Performance Status | 0 | 63 | 41 |
| 1 | 70 | 46 | |
| 2 | 5 | 3 | |
| 3 | 1 | 1 | |
| unknown | 14 | 9 | |
| Stage | T1aN0M0 | 49 | 32 |
| T1bN0M0 | 38 | 25 | |
| T2N0M0 | 66 | 43 | |
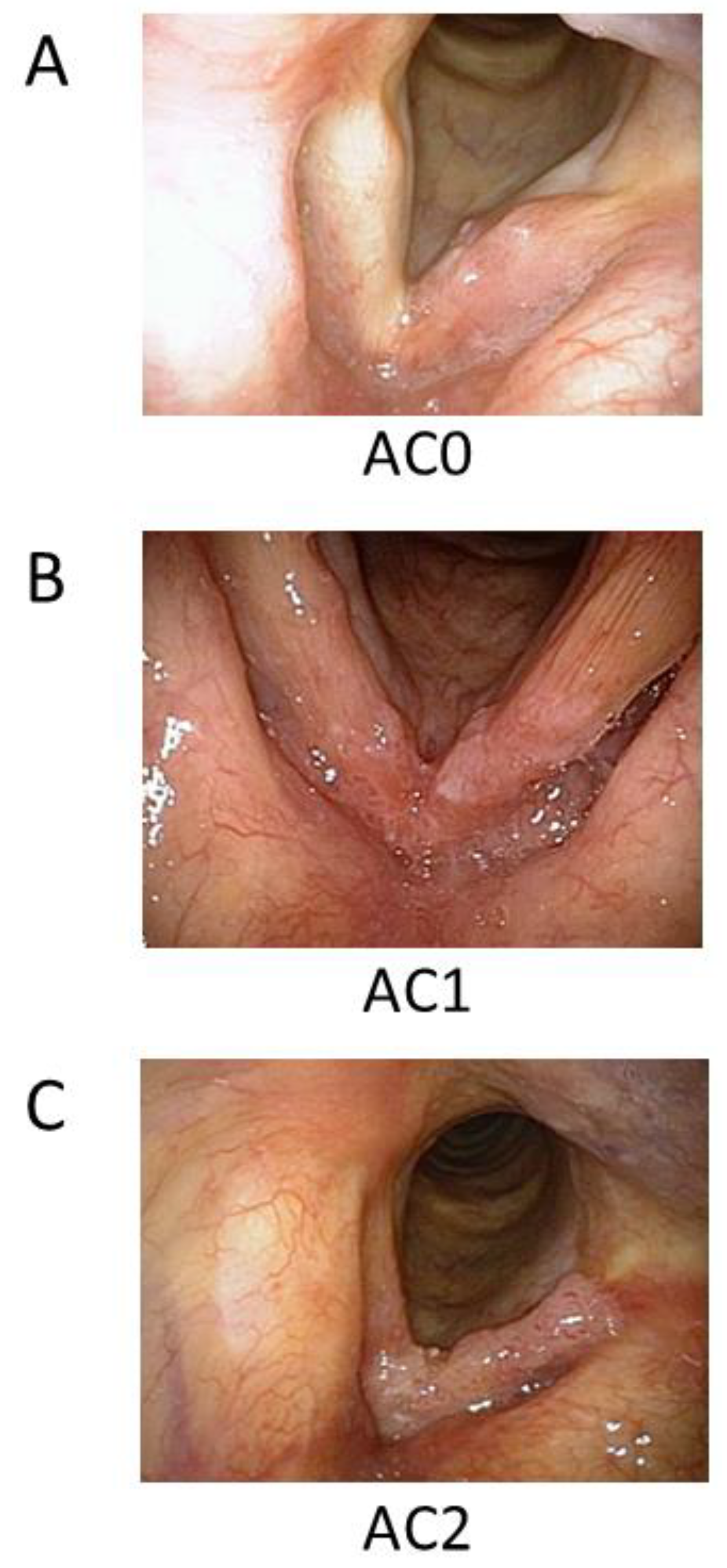
| Extent of AC involvement | AC0 | 88 | 58 |
| AC1 | 39 | 25 | |
| AC2 | 26 | 17 | |
| Radiation doses, fractions | 66 Gy/33 Fr | 34 | 22 |
| 70 Gy/35 Fr | 20 | 13 | |
| 74.4 Gy/62 Fr | 99 | 65 | |
| Total treatment days Median (days) | 48 (range: 43–64) | ||
| Grade | 0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| Acute | ||||||
| Mucositis | 1 (0.7%) | 68 (44%) | 78 (51%) | 6 (4%) | 0 | 0 |
| Dysphagia | 38 (25%) | 105 (69%) | 10 (7%) | 0 | 0 | 0 |
| Dermatitis | 0 | 107 (70%) | 42 (28%) | 4 (3%) | 0 | 0 |
| Late | ||||||
| Hypothyroidism | 127 (83%) | 15 (10%) | 11 (7%) | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seno, S.; Iwashita, K.; Kajiwara, A.; Sasaki, R.; Furukawa, T.; Teshima, M.; Shinomiya, H.; Kiyota, N.; Lynch, R.; Yoshida, K.; et al. Targeting Anterior Commissure Involvement with Hyperfractionated Radiotherapy for T1–T2 Squamous Cell Carcinoma of the Glottic Larynx. Cancers 2024, 16, 1850. https://doi.org/10.3390/cancers16101850
Seno S, Iwashita K, Kajiwara A, Sasaki R, Furukawa T, Teshima M, Shinomiya H, Kiyota N, Lynch R, Yoshida K, et al. Targeting Anterior Commissure Involvement with Hyperfractionated Radiotherapy for T1–T2 Squamous Cell Carcinoma of the Glottic Larynx. Cancers. 2024; 16(10):1850. https://doi.org/10.3390/cancers16101850
Chicago/Turabian StyleSeno, Satoshi, Kazuma Iwashita, Akifumi Kajiwara, Rie Sasaki, Tatsuya Furukawa, Masanori Teshima, Hirotaka Shinomiya, Naomi Kiyota, Rod Lynch, Kenji Yoshida, and et al. 2024. "Targeting Anterior Commissure Involvement with Hyperfractionated Radiotherapy for T1–T2 Squamous Cell Carcinoma of the Glottic Larynx" Cancers 16, no. 10: 1850. https://doi.org/10.3390/cancers16101850
APA StyleSeno, S., Iwashita, K., Kajiwara, A., Sasaki, R., Furukawa, T., Teshima, M., Shinomiya, H., Kiyota, N., Lynch, R., Yoshida, K., Ishihara, T., Miyawaki, D., Nibu, K.-i., & Sasaki, R. (2024). Targeting Anterior Commissure Involvement with Hyperfractionated Radiotherapy for T1–T2 Squamous Cell Carcinoma of the Glottic Larynx. Cancers, 16(10), 1850. https://doi.org/10.3390/cancers16101850







