And Yet It Moves: Clinical Outcomes and Motion Management in Stereotactic Body Radiation Therapy (SBRT) of Centrally Located Non-Small Cell Lung Cancer (NSCLC): Shedding Light on the Internal Organ at Risk Volume (IRV) Concept
Abstract
Simple Summary
Abstract
1. Introduction
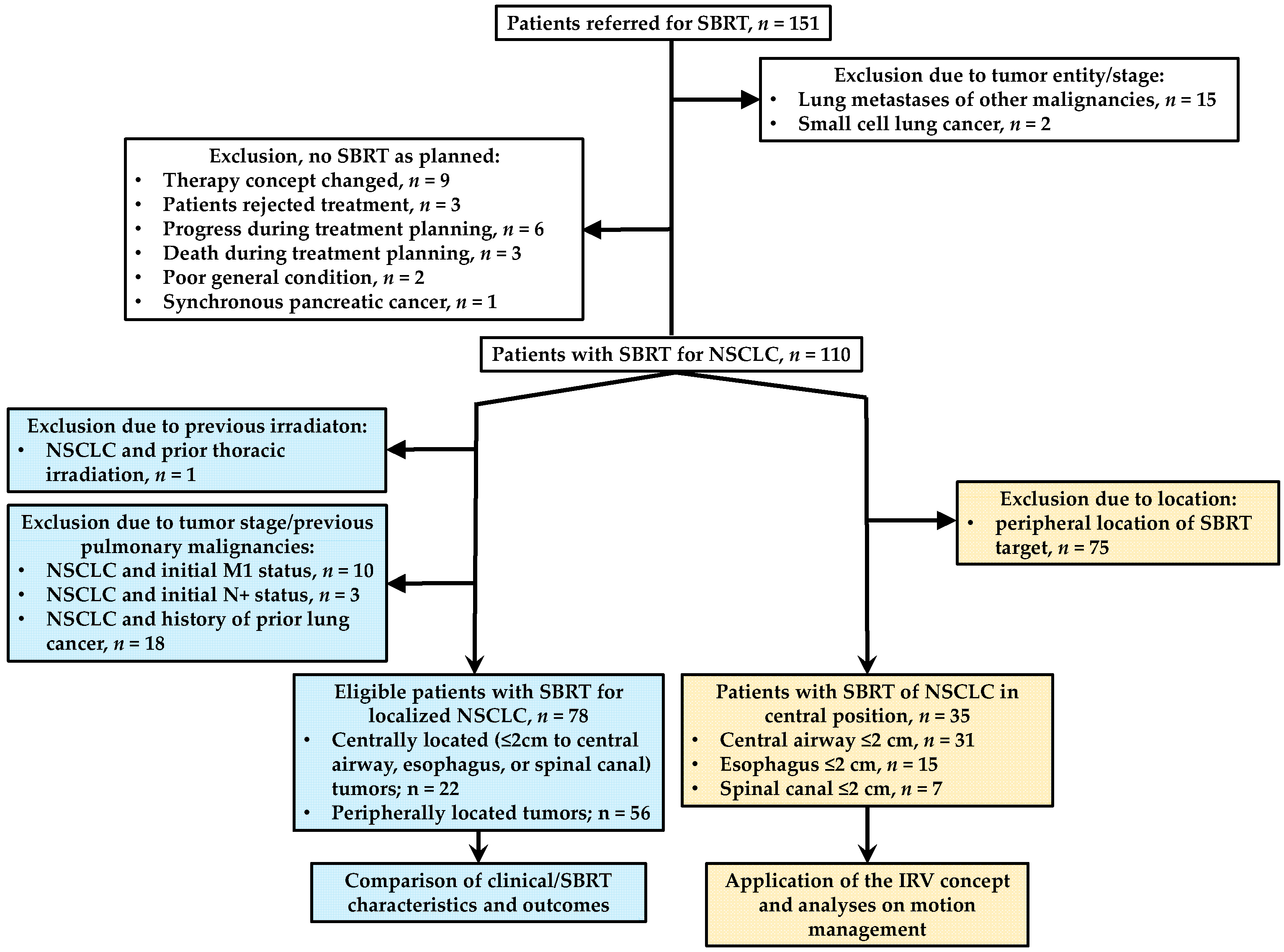
2. Patients and Methods
2.1. Study Design
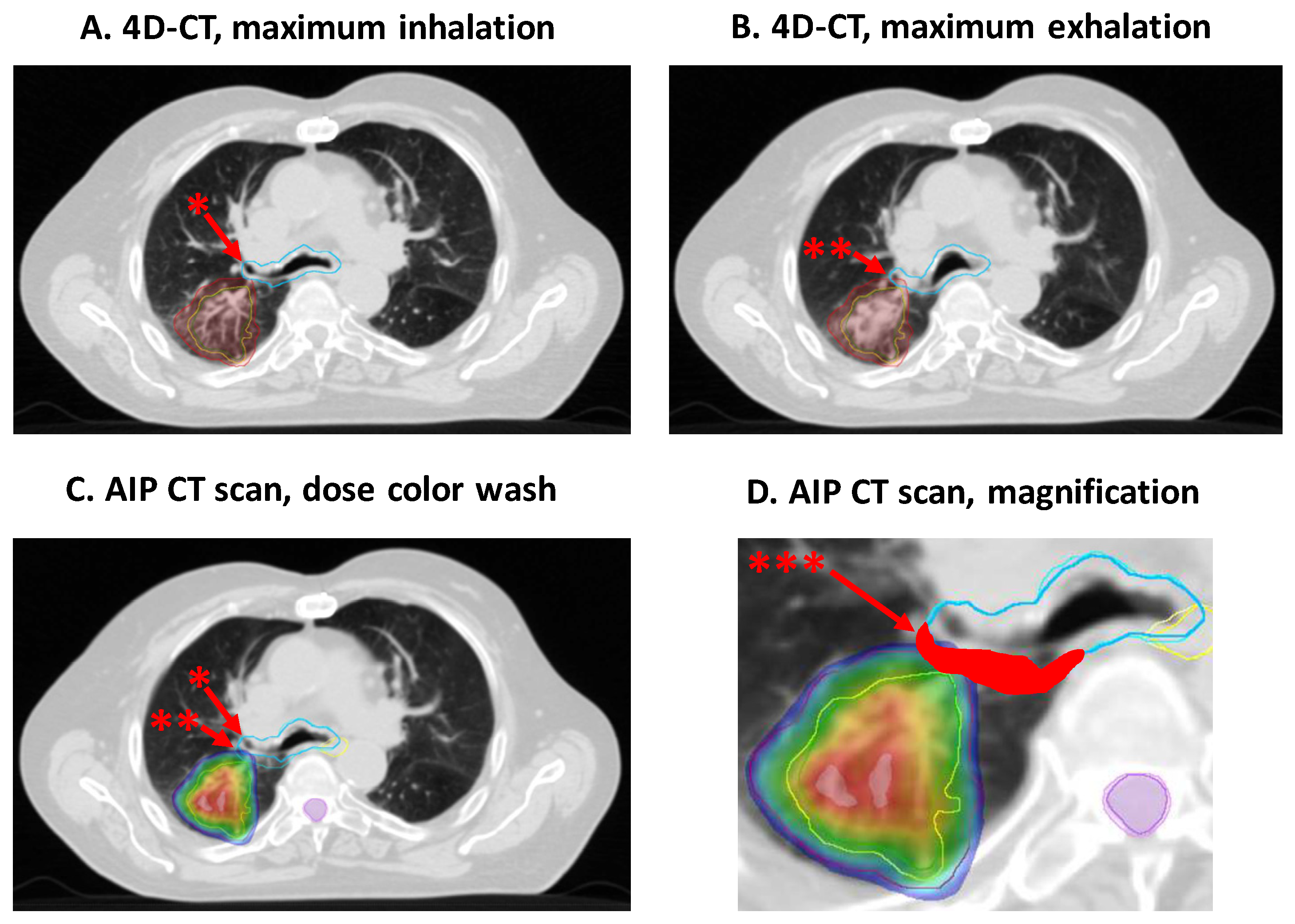
2.2. Radiotherapy, Planning and Delivery
2.2.1. Patient Cohort with Analysis of Clinical/SBRT Characteristics and Outcomes
2.2.2. Patient Cohort with Analyses on Motion Management
2.3. Endpoints and Statistical Methods
2.3.1. Patient Cohort with Analysis of Clinical/SBRT Characteristics and Outcomes
2.3.2. Patient Cohort with Analyses on Motion Management
3. Results
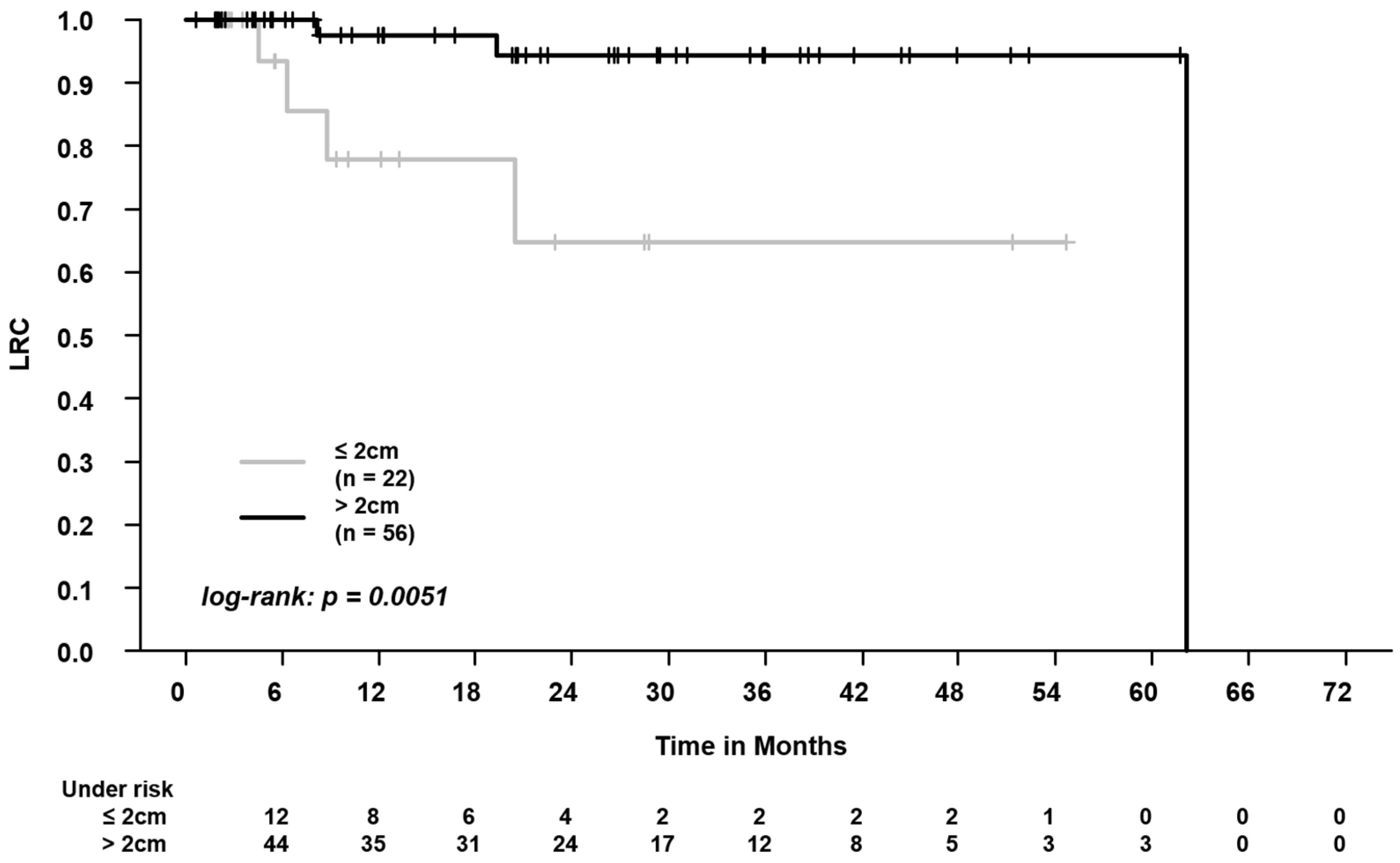
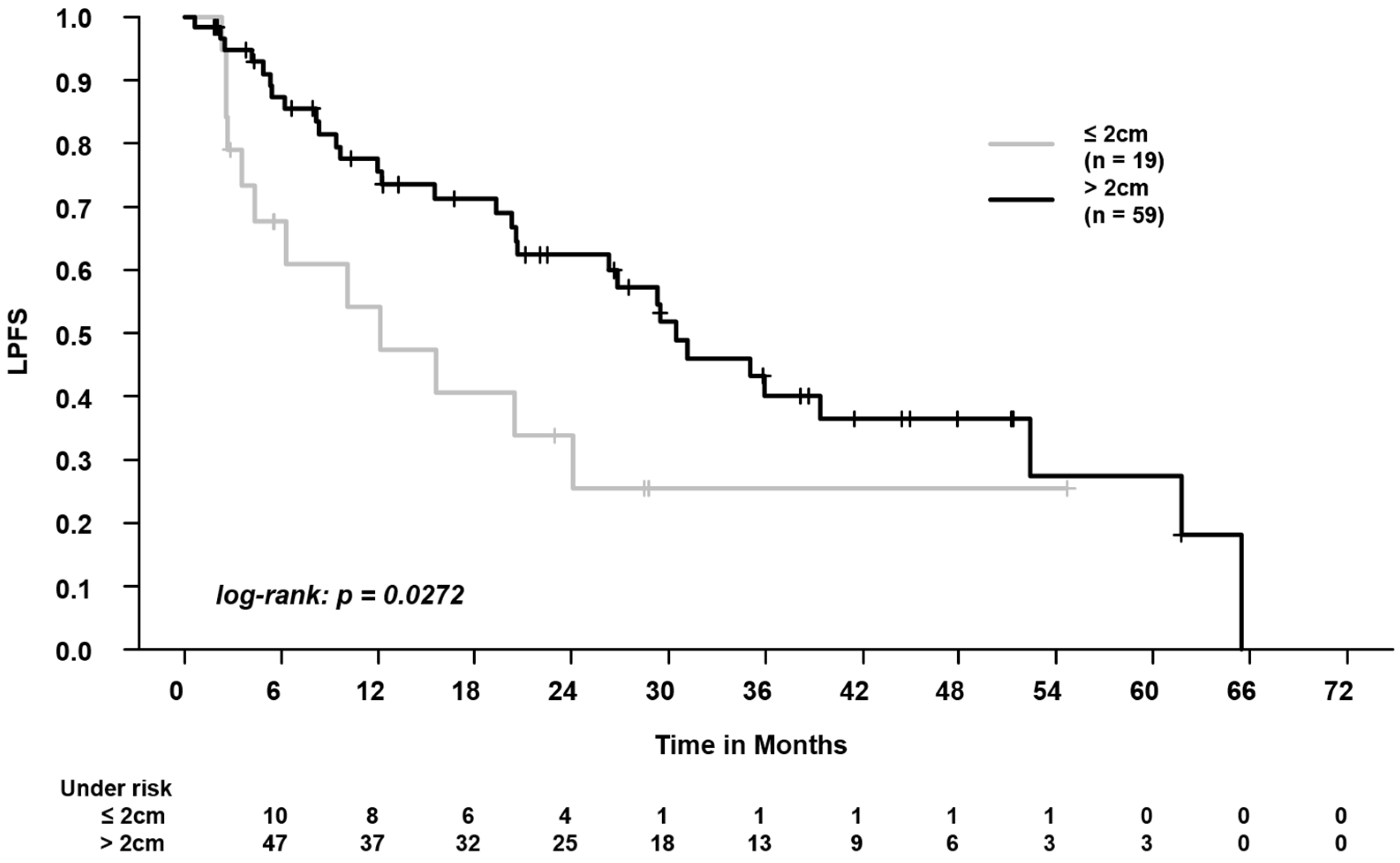
3.1. Clinical/SBRT Characteristics and Outcomes in Centrally vs. Peripherally Located Tumors
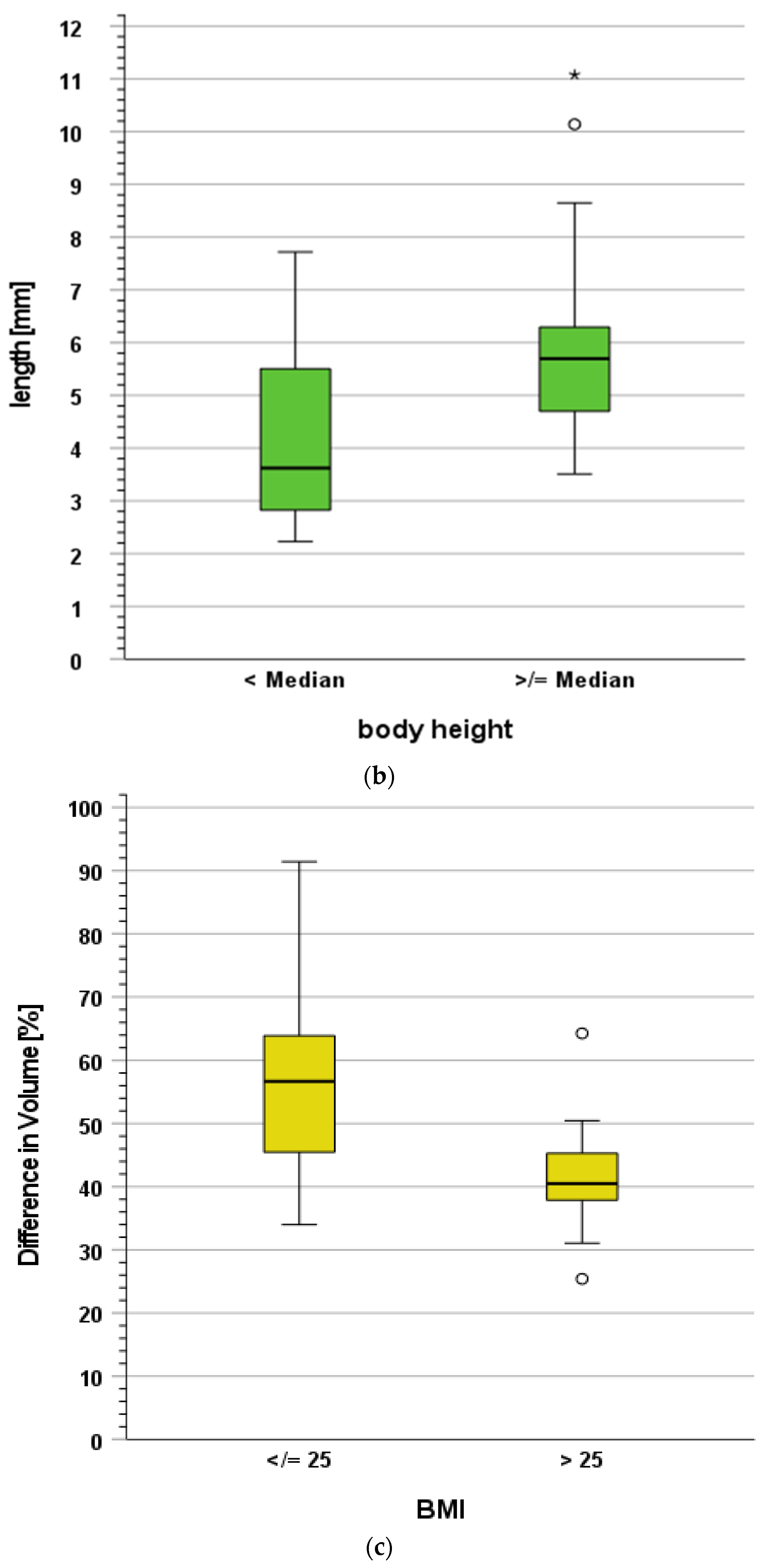
3.2. Structure Movement Amplitudes and Volumes
3.3. Influence of Clinical Characteristics on Structure Movement and Volume Changes
3.4. Influence of the Internal Organ at Risk Volume (IRV) Concept on Normal Tissue Complication Probabilities (NTCPs)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ceniceros, L.; Aristu, J.; Castañón, E.; Rolfo, C.; Legaspi, J.; Olarte, A.; Valtueña, G.; Moreno, M.; Gil-Bazo, I. Stereotactic body radiotherapy (SBRT) for the treatment of inoperable stage I non-small cell lung cancer patients. Clin. Transl. Oncol. 2016, 18, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Soldà, F.; Lodge, M.; Ashley, S.; Whitington, A.; Goldstraw, P.; Brada, M. Stereotactic radiotherapy (SABR) for the treatment of primary non-small cell lung cancer; Systematic review and comparison with a surgical cohort. Radiother. Oncol. 2013, 109, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kestin, L.; Grills, I.; Guckenberger, M.; Belderbos, J.; Hope, A.J.; Werner-Wasik, M.; Sonke, J.-J.; Bissonnette, J.-P.; Xiao, Y.; Yan, D. Dose–response relationship with clinical outcome for lung stereotactic body radiotherapy (SBRT) delivered via online image guidance. Radiother. Oncol. 2014, 110, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Guckenberger, M.; Andratschke, N.; Alheit, H.; Holy, R.; Moustakis, C.; Nestle, U.; Sauer, O. Definition of stereotactic body radiotherapy: Principles and practice for the treatment of stage I non-small cell lung cancer. Strahlenther. Onkol. 2014, 190, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Harder, E.M.; Mancini, B.R.; Decker, R.H. Central versus Peripheral Tumor Location: Influence on Survival, Local Control, and Toxicity Following Stereotactic Body Radiotherapy for Primary Non–Small-Cell Lung Cancer. J. Thorac. Oncol. 2015, 10, 832–837. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoffman, D.; Dragojević, I.; Hoisak, J.; Hoopes, D.; Manger, R. Lung Stereotactic Body Radiation Therapy (SBRT) dose gradient and PTV volume: A retrospective multi-center analysis. Radiat. Oncol. 2019, 14, 162. [Google Scholar] [CrossRef]
- Kang, K.H.; Okoye, C.C.; Patel, R.B.; Siva, S.; Biswas, T.; Ellis, R.J.; Yao, M.; Machtay, M.; Lo, S.S. Complications from Stereotactic Body Radiotherapy for Lung Cancer. Cancers 2015, 7, 981–1004. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.J.; Williams, E.; Modh, A.; Foster, A.; Yorke, E.; Rimner, A.; Jackson, A. Dosimetric predictors of esophageal toxicity after stereotactic body radiotherapy for central lung tumors. Radiother. Oncol. 2014, 112, 267–271. [Google Scholar] [CrossRef]
- van Hoorn, J.E.; Dahele, M.; Daniels, J.M.A. Late Central Airway Toxicity after High-Dose Radiotherapy: Clinical Outcomes and a Proposed Bronchoscopic Classification. Cancers 2021, 13, 1313. [Google Scholar] [CrossRef]
- Timmerman, R.; McGarry, R.; Yiannoutsos, C.; Papiez, L.; Tudor, K.; DeLuca, J.; Ewing, M.; Abdulrahman, R.; DesRosiers, C.; Williams, M.; et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J. Clin. Oncol. 2006, 24, 4833–4839. [Google Scholar] [CrossRef]
- Schmitt, D.; Blanck, O.; Gauer, T.; Fix, M.K.; Brunner, T.B.; Fleckenstein, J.; Loutfi-Krauss, B.; Manser, P.; Werner, R.; Wilhelm, M.-L.; et al. Technological quality requirements for stereotactic radiotherapy: Expert review group consensus from the DGMP Working Group for Physics and Technology in Stereotactic Radiotherapy. Strahlenther. Onkol. 2020, 196, 421–443. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.-G.; Kim, E.S. Efficient approach for determining four-dimensional computed tomography-based internal target volume in stereotactic radiotherapy of lung cancer. Radiat. Oncol. J. 2013, 31, 247–251. [Google Scholar] [CrossRef] [PubMed]
- De Ruysscher, D.; Faivre-Finn, C.; Moeller, D.; Nestle, U.; Hurkmans, C.W.; Le Péchoux, C.; Belderbos, J.; Guckenberger, M.; Senan, S. European Organization for Research and Treatment of Cancer (EORTC) recommendations for planning and delivery of high-dose, high precision radiotherapy for lung cancer. Radiother. Oncol. 2017, 124, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stroom, J.C.; Heijmen, B.J. Limitations of the planning organ at risk volume (PRV) concept. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 279–286. [Google Scholar] [CrossRef]
- Nardone, V.; Giugliano, F.M.; Reginelli, A.; Sangiovanni, A.; Mormile, M.; Iadanza, L.; Cappabianca, S.; Guida, C. 4D CT analysis of organs at risk (OARs) in stereotactic radiotherapy. Radiother. Oncol. 2020, 151, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Markova, S.; Garcia, A.; Huang, K.; Nie, X.; Choi, W.; Lu, W.; Wu, A.; Rimner, A.; Li, G. Evaluation of automatic contour propagation in T2-weighted 4DMRI for normal-tissue motion assessment using internal organ-at-risk volume (IRV). J. Appl. Clin. Med. Phys. 2018, 19, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Andruska, N.; Stowe, H.B.; Crockett, C.; Liu, W.; Palma, D.; Faivre-Finn, C.; Badiyan, S.N. Stereotactic Radiation for Lung Cancer: A Practical Approach to Challenging Scenarios. J. Thorac. Oncol. 2021, 16, 1075–1085. [Google Scholar] [CrossRef]
- Habermann, F.-N.O.J.; Schmitt, D.; Failing, T.; Fischer, J.; Ziegler, D.A.; Fischer, L.A.; Alt, N.J.; Muster, J.; Donath, S.; Hille, A.; et al. Patterns of Pretreatment Diagnostic Assessment in Patients Treated with Stereotactic Body Radiation Therapy (SBRT) for Non-Small Cell Lung Cancer (NSCLC): Special Characteristics in the COVID Pandemic and Influence on Outcomes. Curr. Oncol. 2022, 29, 1080–1092. [Google Scholar] [CrossRef]
- Chang, J.Y.; Bezjak, A.; Mornex, F. Stereotactic ablative radiotherapy for centrally located early stage non-small-cell lung cancer: What we have learned. J. Thorac. Oncol. 2015, 10, 577–585. [Google Scholar] [CrossRef]
- Gross, A.; Ziepert, M.; Scholz, M. KMWin—A convenient tool for graphical presentation of results from Kaplan-Meier survival time analysis. PLoS ONE 2012, 7, e38960. [Google Scholar] [CrossRef]
- Kong, F.-M.S.; Quint, L.; Machtay, M.; Bradley, J. Atlases for Organs at Risk (OARs) in Thoracic Radiation Therapy. Available online: https://www.nrgoncology.org/Portals/0/Scientific%20Program/CIRO/Atlases/Lung%20Organs%20at%20Risk.ppt (accessed on 6 August 2021).
- Chang, J.H.; Gehrke, C.; Prabhakar, R.; Gill, S.; Wada, M.; Joon, D.L.; Khoo, V. RADBIOMOD: A simple program for utilising biological modelling in radiotherapy plan evaluation. Phys. Medica 2016, 32, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Duijm, M. Outcome and Toxicity Modelling after Stereoactic Radiotherapy of Central Lung Tumors. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjpi-3Y7MvyAhX2R_EDHUUNAb8QFnoECAQQAQ&url=https%3A%2F%2Frepub.eur.nl%2Fpub%2F134875%2FEmbargo-version-thesis-M-Duijm.pdf&usg=AOvVaw0yAt8B78bxLWtOomr_zQCw (accessed on 25 August 2021).
- Murrell, D.H.; Laba, J.M.; Erickson, A.; Millman, B.; Palma, D.A.; Louie, A.V. Stereotactic ablative radiotherapy for ultra-central lung tumors: Prioritize target coverage or organs at risk? Radiat. Oncol. 2018, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, K.; Grozman, V.; Karlsson, K.; Lindberg, S.; Lax, I.; Wersäll, P.; Persson, G.F.; Josipovic, M.; Khalil, A.A.; Moeller, D.S.; et al. The HILUS-Trial—A Prospective Nordic Multicenter Phase 2 Study of Ultracentral Lung Tumors Treated with Stereotactic Body Radiotherapy. J. Thorac. Oncol. 2021, 16, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Schanne, D.H.; Nestle, U.; Allgäuer, M.; Andratschke, N.; Appold, S.; Dieckmann, U.; Ernst, I.; Ganswindt, U.; Grosu, A.L.; Holy, R.; et al. Stereotactic body radiotherapy for centrally located stage I NSCLC: A multicenter analysis. Strahlenther Onkol. 2015, 191, 125–132. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Huang, Y.; Shi, S.; Hu, Y.; Zeng, Z. Comparison of Effects Between Central and Peripheral Stage I Lung Cancer Using Image-Guided Stereotactic Body Radiotherapy via Helical Tomotherapy. Technol. Cancer Res. Treat. 2015, 14, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Shin, I.-S.; Yoon, W.S.; Rim, C.H. Stereotactic Body Radiotherapy for Centrally Located Primary Non–Small-Cell Lung Cancer: A Meta-Analysis. Clin. Lung Cancer 2019, 20, e452–e462. [Google Scholar] [CrossRef] [PubMed]
- Roesch, J.; Panje, C.; Sterzing, F.; Mantel, F.; Nestle, U.; Andratschke, N.; Guckenberger, M. SBRT for centrally localized NSCLC—What is too central? Radiat. Oncol. 2016, 11, 157. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Ohashi, T.; Kunieda, E.; Sanuki, N.; Enomoto, T.; Takeda, T.; Oku, Y.; Shigematsu, N. Comparison of clinical, tumour-related and dosimetric factors in grade 0–1, grade 2 and grade 3 radiation pneumonitis after stereotactic body radiotherapy for lung tumours. Br. J. Radiol. 2012, 85, 636–642. [Google Scholar] [CrossRef]
- Welsh, J.; Thomas, J.; Shah, D.; Allen, P.K.; Wei, X.; Mitchell, K.; Gao, S.; Balter, P.; Komaki, R.; Chang, J.Y. Obesity increases the risk of chest wall pain from thoracic stereotactic body radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 91–96. [Google Scholar] [CrossRef]
- Thompson, M.; Rosenzweig, K.E. The evolving toxicity profile of SBRT for lung cancer. Transl. Lung Cancer Res. 2019, 8, 48–57. [Google Scholar] [CrossRef]
- Haseltine, J.M.; Rimner, A.; Gelblum, D.Y.; Modh, A.; Rosenzweig, K.E.; Jackson, A.; Yorke, E.D.; Wu, A.J. Fatal complications after stereotactic body radiation therapy for central lung tumors abutting the proximal bronchial tree. Pract. Radiat. Oncol. 2016, 6, e27–e33. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, S.; Grozman, V.; Karlsson, K.; Onjukka, E.; Lindbäck, E.; Al Jirf, K.; Lax, I.; Wersäll, P.; Persson, G.F.; Josipovic, M.; et al. Expanded HILUS Trial: A Pooled Analysis of Risk Factors for Toxicity from Stereotactic Body Radiation Therapy of Central and Ultracentral Lung Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2023. [Google Scholar] [CrossRef] [PubMed]
- Noël, G.; Antoni, D. Organs at risk radiation dose constraints. Cancer Radiother. 2022, 26, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Almeida, P.F. And Yet It Moves. Biophys. J. 2017, 113, 759–761. [Google Scholar] [CrossRef]
- Rabe, M.; Thieke, C.; Düsberg, M.; Neppl, S.; Gerum, S.; Reiner, M.; Nicolay, N.H.; Schlemmer, H.; Debus, J.; Dinkel, J.; et al. Real-time 4DMRI-based internal target volume definition for moving lung tumors. Med. Phys. 2020, 47, 1431–1442. [Google Scholar] [CrossRef]
- Habatsch, M.; Schneider, M.; Requardt, M.; Doussin, S. Movement assessment of breast and organ-at-risks using free-breathing, self-gating 4D magnetic resonance imaging workflow for breast cancer radiation therapy. Phys. Imaging Radiat. Oncol. 2022, 22, 111–114. [Google Scholar] [CrossRef]
- Crockett, C.B.; Samson, P.; Chuter, R.; Dubec, M.; Faivre-Finn, C.; Green, O.L.; Hackett, S.L.; McDonald, F.; Robinson, C.; Shiarli, A.-M.; et al. Initial Clinical Experience of MR-Guided Radiotherapy for Non-Small Cell Lung Cancer. Front. Oncol. 2021, 11, 617681. [Google Scholar] [CrossRef]

| Parameter | Central Tumors (n = 22 Patients) | Peripheral Tumors (n = 56 Patients) | p-Value |
|---|---|---|---|
| Gender | 0.32 4 | ||
| Male | 16 (72.7) | 34 (60.7) | |
| Female | 6 (27.3) | 22 (39.3) | |
| ECOG (median, min, max) | 1 (0–2) | 1 (0–4) | 0.3 5 |
| UICC stages 1 | 0.96 4 | ||
| I | 17 (77.3) | 43 (76.8) | |
| II–III | 5 (22.7) | 13 (23.2) | |
| Planned dose 1 [Gy, median, min–max)] | 60 (44–60) | 55 (54–60) | 0.28 5 |
| Planned fractions 1,2 (median, min–max) | 8 (3–18) | 5 (3–18) | 0.002 5 |
| Planned dose 1 [biologically effective dose, α/β = 10 Gy, Gy, median, min–max] | 105 (68.2–151.2) | 115.5 (70.2–151.2) | 0.001 5 |
| Applied dose 1 [biologically effective dose, α/β = 10 Gy, Gy, median, min–max] | 105 (13.13–151.2) | 115.5 (70.2–151.2) | 0.001 5 |
| Applied dose < 100 Gy 1 [biologically effective dose, α/β = 10 Gy] | 6 (27.3) | 3 (5.4) | 0.006 4 |
| Radiotherapy technique 1 | 0.83 4 | ||
| VMAT/IMRT | 20 (90.9) | 50 (89.3) | |
| 3DCRT | 2 (9.1) | 6 (10.7) | |
| GTV volume 1 [cm3, median, min–max] | 32.55 (2.7–141.9) | 14.8 (1.4–119.4) | 0.17 5 |
| PTV volume 1 [cm3, median, min–max] | 90.26 (11.9–244.9) | 45.0 (12.8–329.8) | 0.046 5 |
| Lungs–GTV 1, Dmean [Gy] | 5.8 (1.24–11.3) | 3.7 (1.7–10.5) | 0.009 5 |
| Lungs–GTV 1, V5Gy [%] | 26.9 (4.9–56.2) | 17.3 (5.9–49.9) | 0.003 5 |
| Lungs–GTV 1, V20Gy [%] | 8.0 (0–15.6) | 5.5 (2.0–18.7) | 0.02 5 |
| Pneumonitis 3 | 5 (22.7) | 12 (21.4) | 0.90 4 |
| Parameter | Bronchial Tree | Trachea | Esophagus | Spinal Canal |
|---|---|---|---|---|
| Maximum difference in geometric centers, OAR (each respiratory phase), and IRV on AIP CT scan [mm] | 5.2 (2.2–11.1) | 4.2 (1.4–14.6) | 5.5 (2.0–13.2) | 4.3 (1.5–9.7) |
| OAR volumes on AIP CT scan [cm3] | 54.4 (34.7–85.4) | 40.5 (22.3–57.2) | 42.0 (25.7–74.2) | 57.2 (33.5–90.0) |
| IRV volumes on AIP CT scan [cm3] | 76.4 (46.8–116.1) | 48.3 (27.2–75.7) | 61.5 (37.4–103.9) | 65.4 (48.9–88.7) |
| Absolute difference, IRV–OAR volume [cm3] | 22.0 (10.5–35.5) | 7.8 (2.7–18.8) | 19.6 (11.4–33.7) | 8.2 (−1.3–20.2) |
| Relative difference, IRV–OAR volume [%] | 40.9 (26.9–60.4) | 19.4 (6.6–53.5) | 47.5 (25.4–91.4) | 16.1 (−1.4–57.4) |
| Structure | Patient No. | Distance between Tumor and Structure [cm] | NTCP of OAR [%] | NTCP of IRV [%] | NTCP, Absolute Increase [%] | NTCP, Relative Increase [%] |
|---|---|---|---|---|---|---|
| Bronchial Tree | 1 | 1.4 | 17.0 | 34.0 | 17.0 | 100.0 |
| 2 | 0.0 | 63.0 | 66.0 | 3.0 | 4.8 | |
| 3 | 0.3 | 26.0 | 37.5 | 11.5 | 44.2 | |
| 4 | 0.0 | 23.0 | 32.0 | 9.0 | 39.1 | |
| 5 | 0.0 | 45.0 | 48.0 | 3.0 | 6.7 | |
| 6 | 1.1 | 35.5 | 58.0 | 22.5 | 63.4 | |
| Esophagus | 3 | 2.4 | 2.1 | 5.9 | 3.8 | 181.0 |
| 7 | 0.3 | 1.5 | 4.0 | 2.5 | 166.7 | |
| Spinal Canal | 1 | 2.2 | 0.0 | 0.0 | 0.0 | 0.0 |
| 8 | 1.8 | <0.01 | <0.01 | 0.0 | 0.0 | |
| 9 | 4.1 | <0.01 | <0.01 | 0.0 | 0.0 | |
| Trachea | 6 | 4.1 | 4.0 | 5.0 | 1.0 | 25.0 |
| 10 | 1.2 | 4.0 | 10.5 | 6.5 | 162.5 | |
| 11 | 3.5 | 4.5 | 5.5 | 1.0 | 22.2 | |
| 12 | 1.0 | 24.5 | 26.0 | 1.5 | 6.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habermann, F.-N.O.J.; Schmitt, D.; Failing, T.; Ziegler, D.A.; Fischer, J.; Fischer, L.A.; Guhlich, M.; Bendrich, S.; Knaus, O.; Overbeck, T.R.; et al. And Yet It Moves: Clinical Outcomes and Motion Management in Stereotactic Body Radiation Therapy (SBRT) of Centrally Located Non-Small Cell Lung Cancer (NSCLC): Shedding Light on the Internal Organ at Risk Volume (IRV) Concept. Cancers 2024, 16, 231. https://doi.org/10.3390/cancers16010231
Habermann F-NOJ, Schmitt D, Failing T, Ziegler DA, Fischer J, Fischer LA, Guhlich M, Bendrich S, Knaus O, Overbeck TR, et al. And Yet It Moves: Clinical Outcomes and Motion Management in Stereotactic Body Radiation Therapy (SBRT) of Centrally Located Non-Small Cell Lung Cancer (NSCLC): Shedding Light on the Internal Organ at Risk Volume (IRV) Concept. Cancers. 2024; 16(1):231. https://doi.org/10.3390/cancers16010231
Chicago/Turabian StyleHabermann, Felix-Nikolai Oschinka Jegor, Daniela Schmitt, Thomas Failing, David Alexander Ziegler, Jann Fischer, Laura Anna Fischer, Manuel Guhlich, Stephanie Bendrich, Olga Knaus, Tobias Raphael Overbeck, and et al. 2024. "And Yet It Moves: Clinical Outcomes and Motion Management in Stereotactic Body Radiation Therapy (SBRT) of Centrally Located Non-Small Cell Lung Cancer (NSCLC): Shedding Light on the Internal Organ at Risk Volume (IRV) Concept" Cancers 16, no. 1: 231. https://doi.org/10.3390/cancers16010231
APA StyleHabermann, F.-N. O. J., Schmitt, D., Failing, T., Ziegler, D. A., Fischer, J., Fischer, L. A., Guhlich, M., Bendrich, S., Knaus, O., Overbeck, T. R., Treiber, H., von Hammerstein-Equord, A., Koch, R., El Shafie, R., Rieken, S., Leu, M., & Dröge, L. H. (2024). And Yet It Moves: Clinical Outcomes and Motion Management in Stereotactic Body Radiation Therapy (SBRT) of Centrally Located Non-Small Cell Lung Cancer (NSCLC): Shedding Light on the Internal Organ at Risk Volume (IRV) Concept. Cancers, 16(1), 231. https://doi.org/10.3390/cancers16010231








